Introduction
The main treatment of early stage head and neck
squamous cell carcinoma (HNSCC) is surgery and/or radiotherapy
(1). Chemotherapy is often used in
various combinations with surgery and radiotherapy for patients in
late stage HNSCC to improve poor survival rate or increase organ
preservation (1–4). Increasing evidence suggest that
cisplatin/5-fluorouracil (PF)-based regimens are useful in
improving the clinical outcomes of patients in late stage HNSCC;
however, they are far from satisfactory (5–8). For
example, the 5-year survival rate for patients with oral cancer
remains at ~60% over the last few decades (9).
Paclitaxel (also known as taxol), a natural product
extracted from the bark of Pacific yew Taxus brevifolia, can
promote tubulin polymerization and inhibit microtubules
disassembly, causing cell death by disrupting the microtubule
dynamics required for cell division and vital interphase process
(10). Paclitaxel and docetaxel are
the prototypes of microtubule-targeting taxane drugs, and are
currently used as active chemotherapeutic agents against different
types of human cancer, including HNSCC (3,5,11,12).
Recent studies have demonstrated that taxane-containing triplets
(taxane/cisplatin/5 fluorouracil) are superior as an induction
regimen compared with the standard cisplatin/5 fluorouracil regimen
for locally advanced HNSCC, and may be superior as an induction
regimen followed by chemo-radiation compared with chemo-radiation
alone (13,14).
Although previous studies have investigated the
molecular mechanism of taxanes (15–18),
only a few have focused on paclitaxel-induced cell death in HNSCC
(19–21). Given that taxane-induced cell death
signaling pathways may be dependent on the genotype of cancer cells
and may be cell-type specific (22,23),
understanding paclitaxel-induced HNSCC cell death may be useful in
designing effective taxane-based regimens against HNSCC. It has
been reported that paclitaxel can significantly induce apoptosis in
most HNSCC cell lines, including FaDu, OC3 and OEC-M1 cells
(24). In addition, activation of
initiator caspases (caspase-8 and −9), downstream effector caspases
(caspase-3, −6 and −7), and poly-ADP-ribose polymerase cleavage
were also observed in these HNSCC cell lines (24), suggesting that activation of both
death receptors and mitochondria apoptotic pathways is a common
phenomenon in paclitaxel-treated HNSCC cell death.
The mitogen-activated protein kinase (MAPK)
superfamily is composed of extracellular signal-regulated kinases
(ERKs), c-Jun N-terminal kinases (JNKs) and p38 MAPKs (25). ERK, JNK and p38 MAPK have been
reported to play important roles in promoting the activation of
pro-apoptotic proteins (26–29). For example, ERK is involved in
promoting caspase-3 activation in cisplatin-induced apoptosis
(26). Additionally, JNK has been
reported to be involved in promoting caspase-9 and caspase-3
activation induced by gemcitabine (27), and it also contributes to Bax
activation, a pro-apoptotic Bcl-2 protein, following treatment with
sunitinib (28). In addition, p38
MAPK is associated with caspase-8 activation in TGFβ-mediated
apoptosis (29).
It has been reported that treatment of cancer cells
with the anticancer drugs decreased the mitochondrial membrane
potential (∆Ψm) (30), a phenomenon
reflecting that mitochondrial outer membrane permeabilization
(MOMP) is induced. Activation of several pro-apoptotic proteins,
such as Bax, Bak and Bid, has been demonstrated to contribute to
MOMP induction (31,32). Once MOMP is induced, cytochrome c is
released into the cytosol, which activates caspase-9 (33,34).
This in turn activates the downstream effector caspases, such as
caspase-3 or caspase-7, resulting in apoptosis (35). Thus, ∆Ψm may serve as an indicator of
apoptosis.
Although our previous study demonstrated that
paclitaxel can activate caspases and induce apoptosis in HNSCC
cells (24), the pivotal signaling
pathway for driving caspase activation and apoptosis induced by
paclitaxel remains unclear. Thus, the present study aimed to
investigate the underlying mechanism of paclitaxel-induced
apoptosis in HNSCC cells.
Materials and methods
Reagents and antibodies
Paclitaxel (Sigma-Aldrich; Merck KGaA) was
solubilized in dimethyl sulfoxide (DMSO), at a final concentration
of 1 mM. SP600125 was dissolved in DMSO as a 100 mM stock solution
and stored at −20°C. RNase A, propidium iodide (PI), BSA, DMSO,
penicillin/streptomycin, phenylmethylsulfonyl fluoride (PMSF),
dithiothreitol, copper sulfate,
K+-Na+-tartrate and MTT reagent were
purchased from Sigma-Aldrich; Merck KGaA). Fetal bovine serum
(FBS), RPMI-1640 medium and lyophilized trypsin-EDTA were purchased
from Gibco (Thermo Fisher Scientific, Inc.). Tween-20, sodium
hydroxide and hydrochloric acid were purchased from Merck KGaA.
SDS, acrylamide and Tris-base were purchased from J.T. Baker
(Avantor, Inc.). PD184352 (Enzo Life Sciences, Inc.) was dissolved
in DMSO as a 5 mM stock solution and stored at −20°C. SB202190
(Merck KGaA) was dissolved in DMSO as a 10 mM stock solution and
stored at −20°C.
Antibodies against cleaved caspase-3, cleaved
caspase-6, cleaved caspase-7, cleaved caspase-8, cleaved caspase-9,
phospho-JNK, JNK, phospho-ERK, ERK, phospho-p38, p38, β-actin and
Bid were purchased from Cell Signaling Technology, Inc. Caspase-8
inhibitor (Z-IETD-FMK) and caspase-9 inhibitor (Z-LEHD-FMK) were
purchased from R&D Systems, Inc. HEPES was purchased from
Avantor, Inc. Sodium bicarbonate, sodium carbonate and sodium
chloride were purchased from Riedel-de Haen (Honeywell
International, Inc.).
Cell line and cell culture
OEC-M1 is a cell line derived from a surgical
specimen of buccal mucosa squamous carcinoma from a Taiwanese, a
unique oral cancer indigenous in Taiwan, which was generously
gifted by Professor Kuo-Wei Chang at National Yang-Ming University
(Taipei, Taiwan) (36). Cells were
maintained and serially passaged in RPMI-1640 medium supplemented
with 10% FBS, 24 mM NaHCO3, 25 mM HEPES, 100 U/ml
penicillin and 100 µg/ml streptomycin, at pH 7.4, 37°C and in a
humidified atmosphere containing 95% air and 5% CO2.
MTT assay
Cell viability was assessed via the MTT assay, as
previously described (20). Briefly,
cells (1×104 cells/well) were seeded into a 96-well
plate. Following incubation for 24 h at 37°C with 5%
CO2, cells were treated with different concentrations of
paclitaxel for 48 h. Subsequently, MTT reagent was added to each
well (0.5 mg/ml final concentration). Following incubation for 4 h,
the medium was removed and the precipitate in each well was
dissolved in DMSO. The optical density (OD) values were measured at
a wavelength of 590 nm, using an ELISA reader (Dynex Opsys MR;
Aspect Scientific Ltd.). Each experiment was performed in
triplicate.
Flow cytometry
To demonstrate necrotic fractions of
paclitaxel-treated cells, cells (6×105) were seeded into
6-cm dishes and treated with different concentrations of paclitaxel
for 24 h. Following treatment, cells were trypsinized, washed with
PBS and stained with Annexin V for 15 min at room temperature in
the dark, then resuspended in staining solution containing RNase A
(100 µg/ml in PBS) and PI (40 µg/ml in PBS). Stained cells were
analyzed using a FACScan flow cytometer (Becton-Dickinson and
Companu), using CellQuest software (version 5.1) (Becton-Dickinson
and Company). Cells that were positively stained with Annexin V and
PI were considered necrotic cells (37).
Cell cycle analysis and DNA fragmentation were
assessed via flow cytometry using PI staining (38). For experiments involving JNK
inhibition, cells were treated with 50 nM paclitaxel for 24 h, in
the presence or absence of different concentrations of a specific
JNK inhibitor, SP600125. For experiments involving caspase-8 or
caspase-9 inhibition, cells were treated with caspase-8 inhibitor
(Z-IETD-FMK) 100 µM or caspase-9 inhibitor (Z-LEHD-FMK) 100 µM in
the presence of 50 nM paclitaxel for 24 h, respectively. Following
treatment, cells were trypsinized, washed with PBS and fixed in 70%
ethanol for 20 min at room temperature. The fixed cells were then
re-washed with PBS and resuspended in staining solution containing
RNase A (100 µg/ml in PBS) and PI (40 µg/ml in PBS) for 1 h at room
temperature. The fractions of cells in subG1,
G0/G1 and G2/M phases were
analyzed using a FACScan flow cytometer (Becton-Dickinson and
Company), using CellQuest software (Becton-Dickinson and Company).
To assess paclitaxel-induced ∆Ψm changes in OEC-M1 cells, a
fluorochrome (DiOC6, 3, 3-dihexyloxacarbocyanine) was
used, which exclusively emits with the spectrum of green light and
accumulates in the mitochondrial matrix under the influence of ∆Ψm
(39). Briefly, near-confluent cells
were treated with or without 50 nM paclitaxel in culture media for
12, 24, 36 or 48 h, collected and subsequently incubated with 40 nM
DiOC6 at 37°C for 20 min. ∆Ψm-related DiOC6
fluorescence was subsequently recorded using an FL1 photomultiplier
tube via a FACScan flow cytometer (Becton-Dickinson and Company).
Data from 10,000 cells were acquired for each sample. Analysis was
performed using CellQuest software (Becton-Dickinson and Company).
All experiments were performed at least three times
independently.
Western blotting
Cell lysates were harvested from adherent and
floating cells following drug treatment and lysed in lysis buffer
(50 mM Tris-base, 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5%
deoxychloride acid and 1 mM PMSF). Protein concentration was
determined via the Lowry assay (40), using BSA as standard. Proteins from
each sample (40 µg/lane) were separated via SDS-PAGE on a 12% gel
for caspases-8, −9, phospho-JNK, JNK, phospho-ERK, ERK, phospho-p38
MAPK, p38 MAPK and Bid, or on a 15% gel for caspase-3, −6 and −7.
Standard SDS-PAGE running buffer (24 mM Tris/HCl, 0.19 M glycine,
0.5% SDS, pH 8.3) was used as electrophoresis buffer to resolve
proteins, which were subsequently transferred onto polyvinylidene
difluoride membranes at 80 mA for 1.5 h in transfer buffer (20 mM
Tris/HCl, 150 mM glycine, 10% methanol, 0.01% SDS). The membranes
were blocked with 5% non-fat milk for 1 h at room temperature and
subsequently incubated with the following primary antibodies (all
from Cell Signaling Technology, Inc.) for 16–18 h at 4°C:
Anti-caspase-3 (cat. no. 9661; 1:1,000), anti-cleaved caspase-6
(cat. no. 9761; 1:1,000), anti-cleaved caspase-7 (cat. no. 8438;
1:1,000), anti-cleaved caspase-8 (cat. no. 9429; 1:1,000),
anti-cleaved caspase-9 (cat. no. 9509; 1:1,000), anti-phospho-JNK
(cat. no. 9251; 1:4,000), anti-JNK (cat. no. 9252; 1:1,000),
anti-phospho-ERK (cat. no. 9101; 1:4,000), anti-ERK (cat. no. 9102;
1:4,000), anti-phospho-p38 (cat. no. 9215; 1:1,000), p38 (cat. no.
9212; 1:4,000), anti-β-actin (cat. No. 58169; 1:5,000), and
anti-Bid (cat. no. 8762; 1:1,000). Following the primary
incubation, membranes were washed with TBS with Tween 20 (0.15 M
NaCl, 0.050 M Tris/HCl, 0.1% Tween-20, pH 7.6), and then incubated
with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG
(cat. no. 111-035-144; 1:5,000; Jackson ImmunoResearch, Inc.) or
HRP-conjugated goat anti-mouse IgG (cat. no. 111-035-146; 1:5,000;
Jackson ImmunoResearch, Inc.) secondary antibodies for 1 h at room
temperature. Protein bands were visualized using the enhanced
chemiluminescence detection kit (Amersham; Cytiva), and optical
densities were quantitated using a Quantity One (ProVision
Diagnostics, Inc.) computer-assisted image analysis system. All
experiments were performed at least three times independently.
ELISA
Cytokeratin 18 concentration in the cell culture
supernatants was determined using the SimpleStep ELISA kit (cat.
no. ab227896; Abcam), according to the manufacturer's
instructions.
Statistical analysis
All experiments were performed in triplicate and
data are presented as the mean ± standard error of the mean. The
statistical analysis was performed using SPSS software version 17.0
(SPSS, Inc.). Unpaired Student's t-test was used to compare
differences between two groups, while one-way ANOVA and Tukey's
post-hoc test were used to compare differences between multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
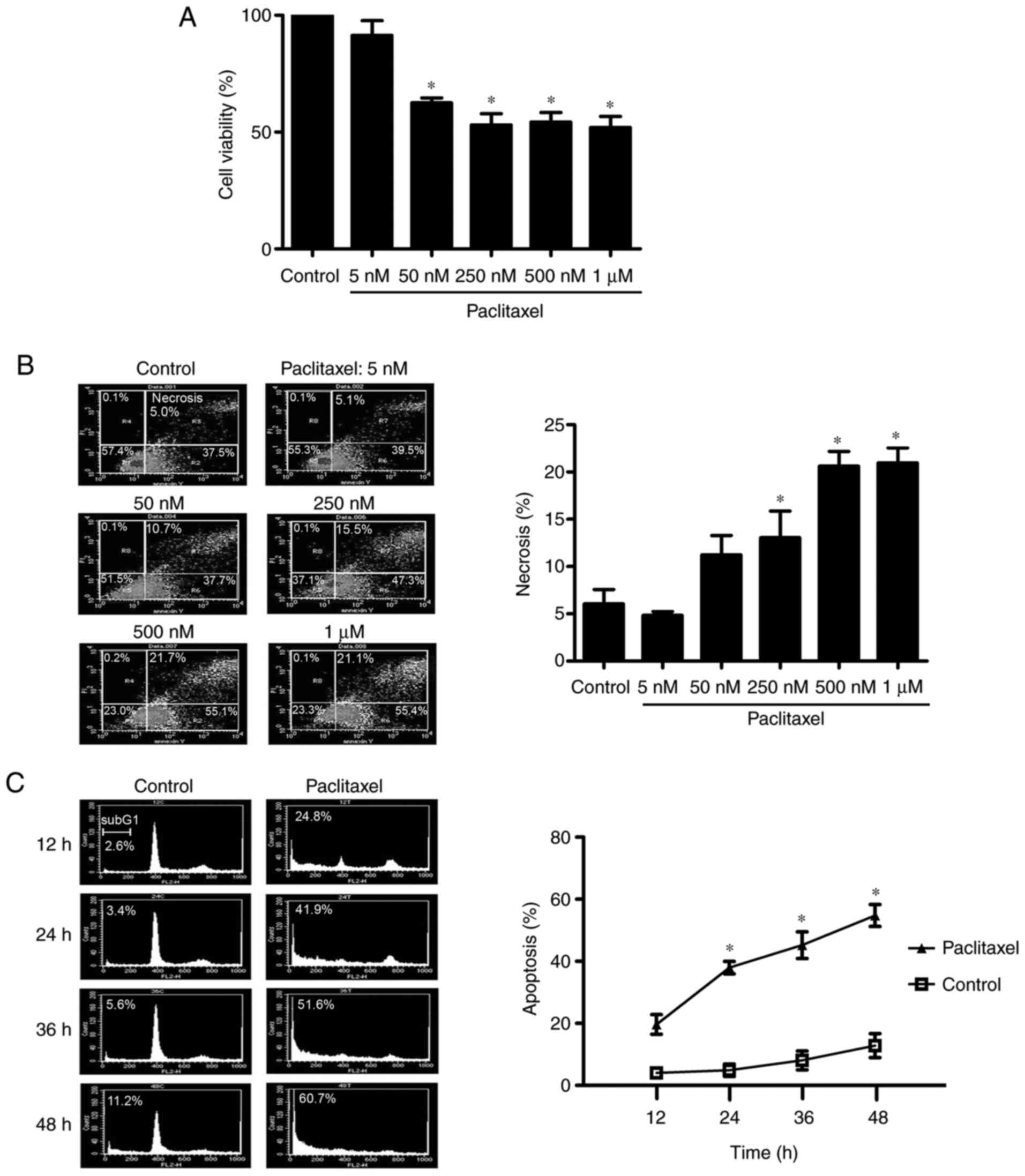
Paclitaxel (50 nM) induces apoptosis
but not necrosis in OEC-M1 cells
It has been reported that low-dose paclitaxel can
induce apoptosis, whereas high-dose paclitaxel induces necrosis
(41). To determine the optimal
experimental concentration of paclitaxel used in the present study,
OEC-M1 cells were treated with different concentrations of
paclitaxel for 48 h and the MTT assay was performed to assess cell
viability. As presented in Fig. 1A,
different doses of paclitaxel (50 nM to 1 µM) significantly
inhibited cell viability (P<0.05). The necrosis effect of these
doses was analyzed via flow cytometry. As presented in Fig. 1B, the necrotic fraction of OEC-M1
cells did not significantly increase following treatment with 50 nM
paclitaxel. However, a dose-dependent increase of necrotic cell
death was observed following treatment with 250 nM or higher
concentrations of paclitaxel (P<0.05). Given that 50 nM
paclitaxel induced apoptosis in OEC-M1 cells in a time-dependent
manner (P<0.05; Fig. 1C) and did
not significantly increase necrosis in cells (Fig. 1B), 50 nM paclitaxel was used as the
standard concentration to assess the apoptosis-inducing effect of
paclitaxel treatment on OEC-M1 cells.
JNK is activated in OEC-M1 cells
treated with low-dose paclitaxel
The MAPK superfamily governs the ERK, JNK and p38
MAPK pathways, which are distinctly modulated by
microtubule-targeting agents, such as paclitaxel (42). Thus, the present study investigated
which MAPK kinase pathway is more important in paclitaxel-induced
apoptosis of OEC-M1 cells. The JNK pathway was activated
(phosphorylated) following treatment with 50 nM paclitaxel at 12
and 24 h (P<0.05; Fig. 2A and B);
the ERK pathway was stimulated (phosphorylated) following treatment
with 50 nM paclitaxel at 6, 12, 24 and 36 h (P<0.05; Fig. 2A and C); and the p38 pathway was
induced (phosphorylated) following treatment with 50 nM paclitaxel
at 24 h (P<0.05; Fig. 2A and D)
in OEC-M1 cells, respectively.
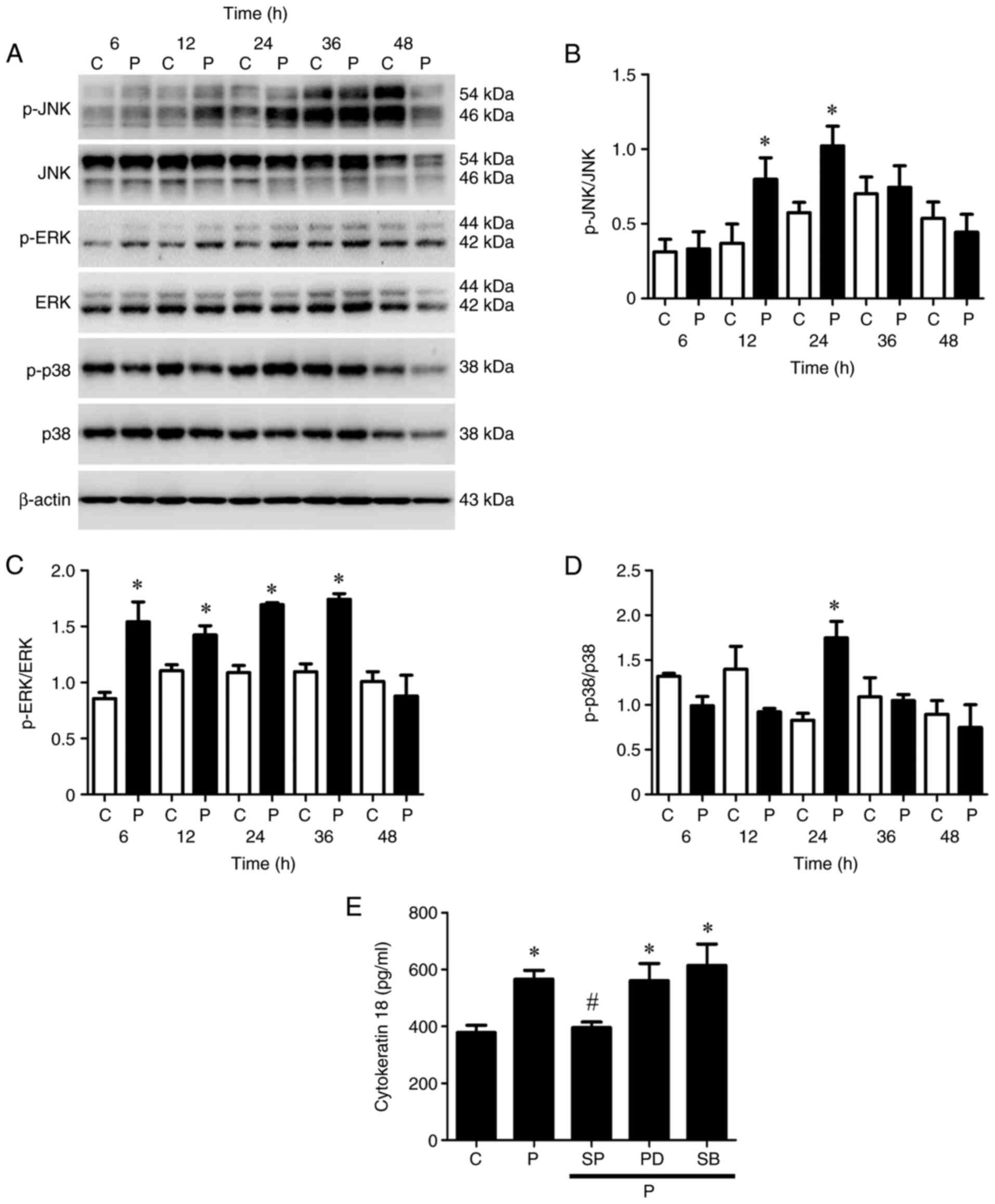 | Figure 2.Activation of the MAPK pathway in
low-dose paclitaxel-treated OEC-M1 cells. Cells were treated with
control or 50 nM paclitaxel at different time points. (A) Western
blot analysis was performed to detect the protein expression levels
of JNK, ERK and p38 MAPK pathways in OEC-M1 cells. Activation of
(B) JNK, (C) ERK and (D) p38 were quantified using Quantity One
image analysis system. OEC-M1 cells were treated with control or 50
nM paclitaxel, without or with 10 µM SP, 5 µM PD and 5 µM SB,
respectively, and (E) the expression levels of cytokeratin 18 were
determined via ELISA. *P<0.05 vs. control; #P<0.05
vs. paclitaxel alone treatment. MAPK, mitogen-activated protein
kinase; JNK, c-Jun N-terminal kinase; ERK, extracellular
signal-regulated kinase; C, control, P, paclitaxel; SP, JNK
inhibitor-SP600125; PD, ERK inhibitor-PD184352; SB, p38
inhibitor-SB202190. |
Caspase-cleaved cytokeratin 18, a biomarker of
apoptotic cell death, is released from epithelial cells during
apoptosis (43). The results of the
present study demonstrated that paclitaxel significantly promoted
the release of cytokeratin 18 from OEC-M1 cells compared with the
control group (P<0.05; Fig. 2E).
Treatment with JNK inhibitor, but not ERK inhibitor or p38
inhibitor, remarkably inhibited the paclitaxel-induced release of
cytokeratin 18 (P<0.05; Fig. 2E),
suggesting that the JNK pathway is the candidate MAPK pathway
mediating paclitaxel-induced apoptosis in OEC-M1 cells.
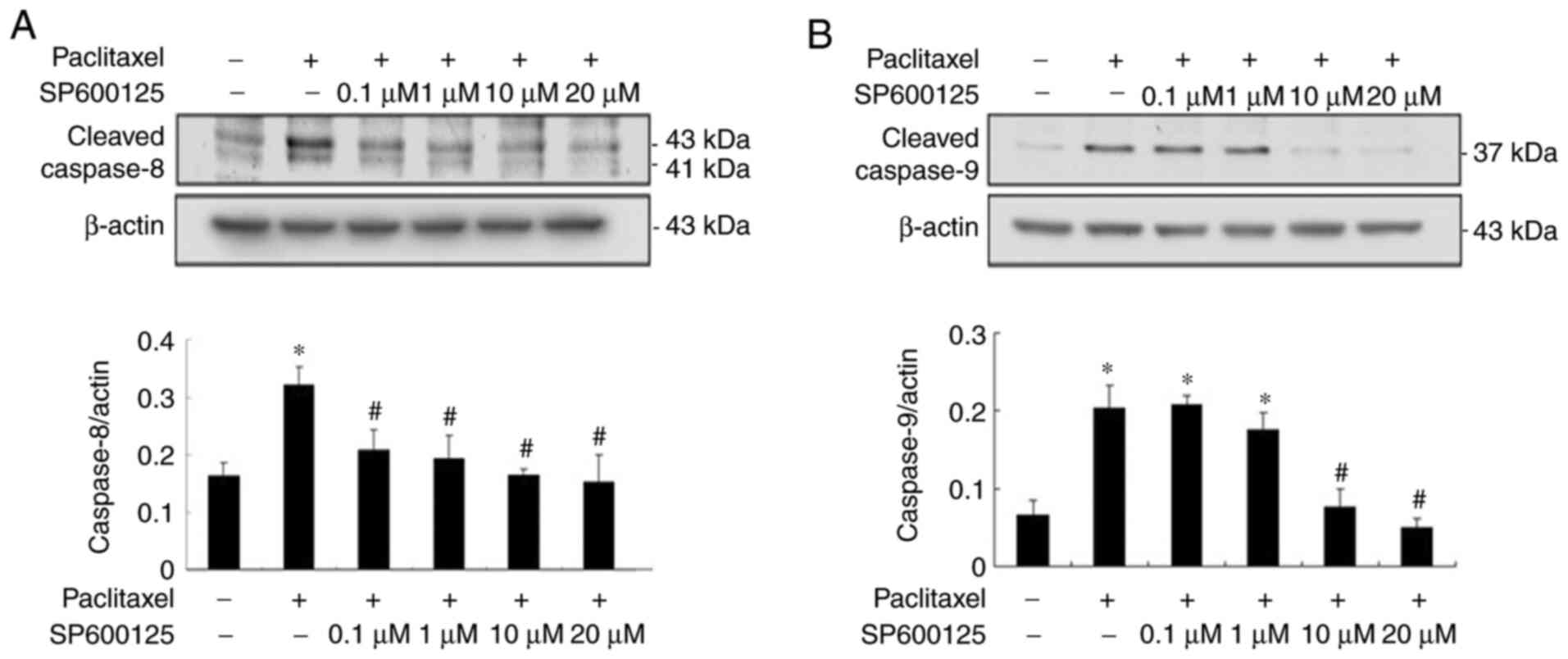
Inactivation of JNK effectively
prevents paclitaxel-induced apoptosis and caspase activation in
OEC-M1 cells
Our previous study demonstrated that paclitaxel can
induce apoptosis by activating caspases in the two HNSCC cell
lines, OEC-M1 and OC3, through the activation of initiator caspases
(caspase-8 and −9) and effector caspases (caspase-3, −6 and −7)
(24). The results of the present
study demonstrated that paclitaxel-activated JNK was important for
apoptosis in OEC-M1 cells (Fig. 2).
To determine the role of JNK activation in paclitaxel-induced
apoptosis, OEC-M1 cells were treated with 50 nM paclitaxel, with or
without different concentrations of JNK inhibitor, SP600125, for 24
h. Apoptosis was subsequently quantified by measuring the
subG1 fraction via flow cytometric analysis. As
presented in Fig. 3A, 10 µM SP600125
abolished JNK activation and cleaved caspase-3 expression to near
background level, respectively. The expression levels of cleaved
caspase-6 and −7 were also blocked following treatment with 10 µM
SP600125, respectively (Fig. 3B).
Inhibition of JNK activation with 10 µM SP600125 effectively
rescued paclitaxel-induced apoptosis in OEC-M1 cells (P<0.05;
Fig. 3C and D), suggesting that JNK
activation plays a central role in low-dose paclitaxel-induced
apoptosis of OEC-M1 cells. JNK inhibition also significantly
decreased the G0/G1 fraction (P<0.05;
Fig. 3C and E), and notably
increased the G2/M fraction (P<0.05; Fig. 3C and F) in paclitaxel-treated OEC-M1
cells.
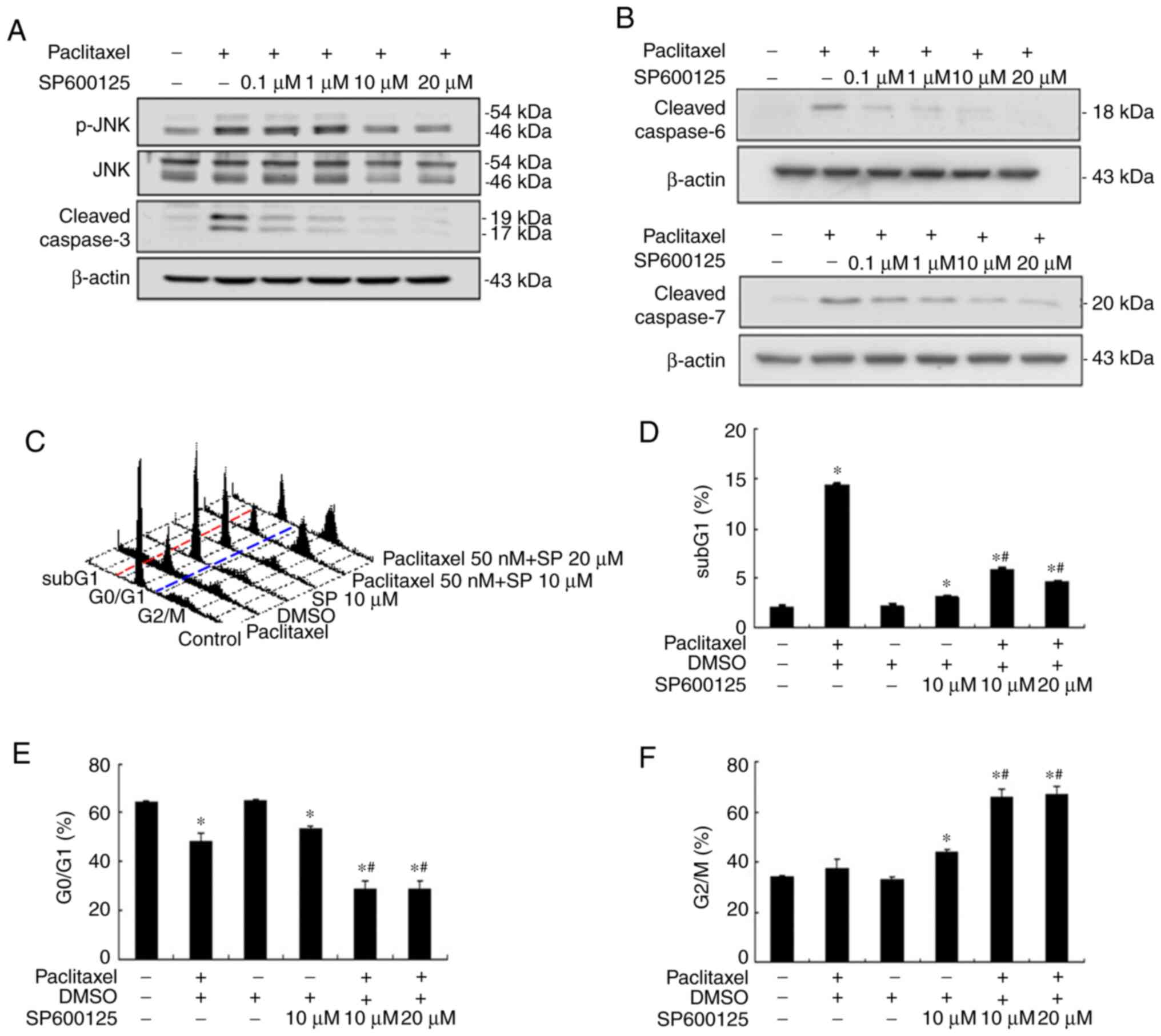 | Figure 3.JNK inhibitor (SP600125) inhibits
paclitaxel-induced apoptosis and caspases effectors in OEC-M1
cells. JNK inhibitor, SP600125, (0.1, 1, 10 and 20 µM) inhibited
paclitaxel-induced phosphorylation of (A) JNK and cleaved
caspase-3, and (B) cleaved caspase-6 and cleaved caspase-7. Cells
(6×105) were subsequently treated with 50 nM paclitaxel,
with or without different concentrations of SP600125 (10 and 20 µM)
for 24 h and fixed in 70% alcohol. (C) Following PI staining, cell
cycle events were measured via FACScan analysis. Fractions of (D)
subG1 phase (apoptosis), (E) G0/G1
phase and (F) G2/M phase were quantified using CellQuest
software. In (C) red and blue dotted lines were plotted to
illustrate the changes of subG1 (left to red line),
G0/G1 (between red and blue lines) and
G2/M phases (right to blue line) in the different
treatment groups. *P<0.05 vs. control; #P<0.05 vs.
paclitaxel alone treatment. JNK, c-Jun N-terminal kinase; PI,
propidium iodide. |
As presented in Fig.
4, paclitaxel-induced activation of initiator caspase-8 and −9
was significantly inhibited to near background level with JNK
inhibitors, respectively (P<0.05; Fig. 4A and B), suggesting that low-dose
paclitaxel-induced activation of caspases is mediated via the JNK
pathway.
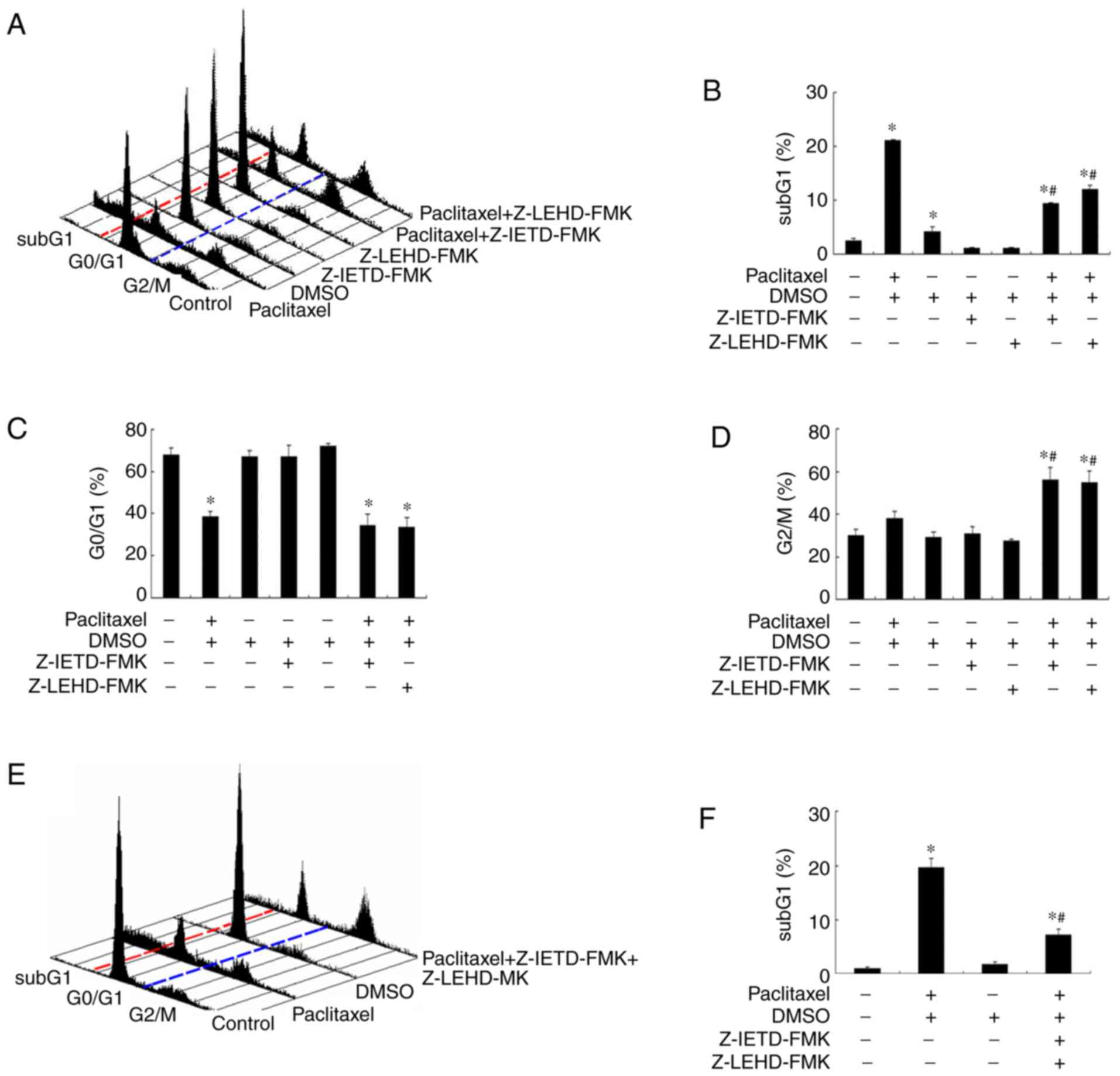
Inhibition of caspase-8 and −9
activation partially rescues paclitaxel-induced apoptosis in OEC-M1
cells
It has been demonstrated that microtubule-targeting
agents (MTA) preferentially activate different caspases in
different types of cancer (42,44–46).
Thus, the present study investigated whether caspase-8 and −9
activation is responsible for the apoptosis-inducing effect caused
by low-dose paclitaxel treatment in OEC-M1 cells. Notably, addition
of either caspase-8 inhibitor (Z-IETD-FMK) or caspase-9 inhibitor
(Z-LEHD-FMK) rescued about half of the apoptotic cells following
treatment with paclitaxel in OEC-M1 cells (P<0.05; Fig. 5A and B). Neither caspase inhibitors
affected the G0/G1 fraction of
paclitaxel-treated OEC-M1 cells (P<0.05; Fig. 5C). However, both caspase inhibitors
increased the G2/M fraction of paclitaxel-treated cells
(P<0.05; Fig. 5D), suggesting
that a significant proportion of paclitaxel-treated cells are
arrested in G2/M phase undergoing apoptosis through
activation of initiator caspase-8 and −9. In addition, a less than
additive effect was observed when the two caspase inhibitors were
used in combination (Fig. 5E and F).
Taken together, these results suggest that other caspases, or
caspase-independent apoptotic mechanism, may also be involved in
paclitaxel-induced apoptosis in OEC-M1 cells.
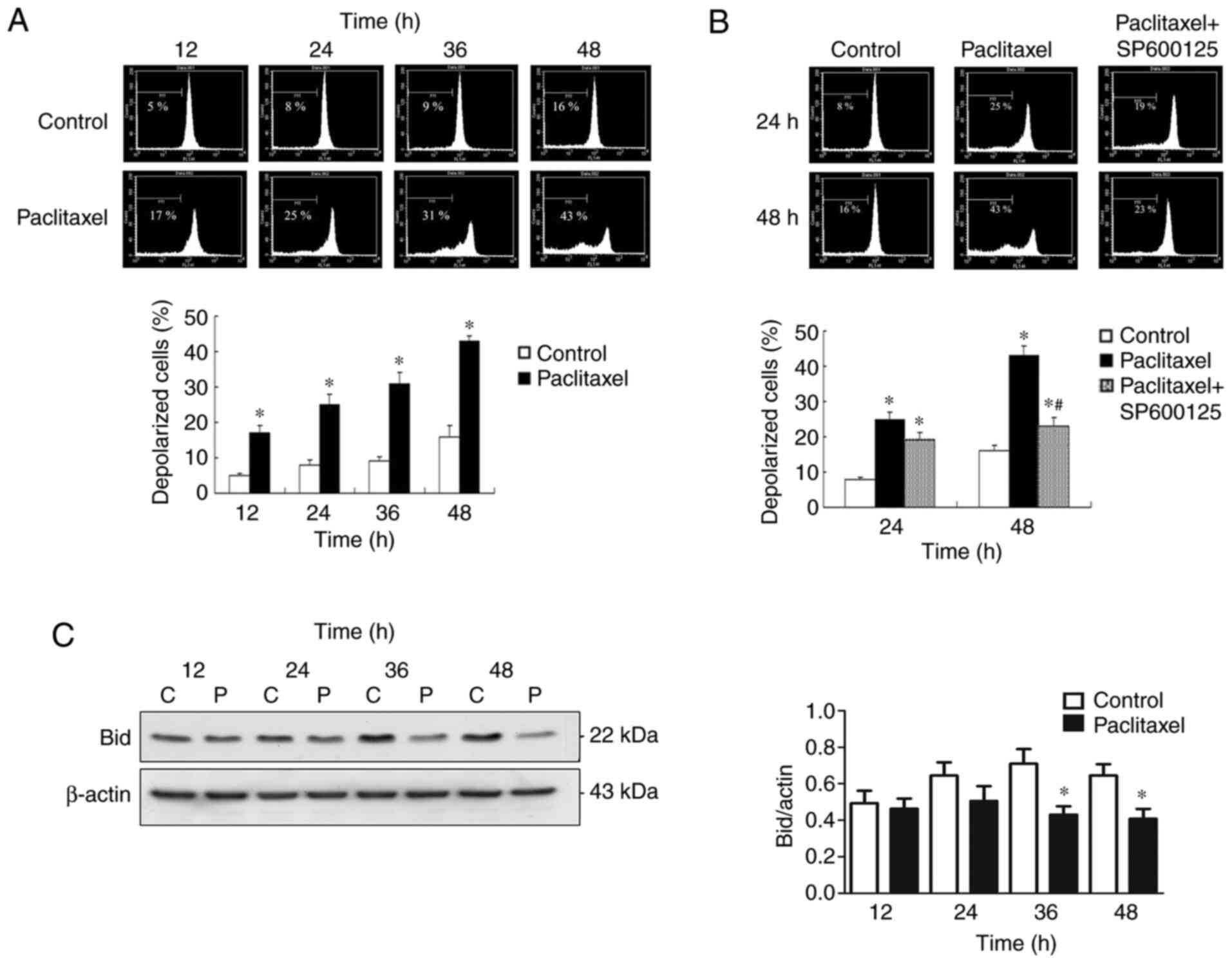
Inactivation of JNK inhibits
late-phase (48 h) ∆Ψm loss in OEC-M1 cells
The effectiveness of MTAs is a consequence of
caspase activation through the intrinsic mitochondrial apoptotic
pathway (42), which is closely
associated with ∆Ψm collapse (33).
To investigate the association between JNK activation and ∆Ψm
collapse, the preset study investigated whether low-dose paclitaxel
can affect ∆Ψm in OEC-M1 cells. As presented in Fig. 6A, 50 nM paclitaxel significantly
induced depolarization of ∆Ψm of OEC-M1 cells, in a time-dependent
manner (P<0.05). The present study also investigated whether the
∆Ψm loss is associated with JNK activation. Notably, inactivation
of JNK failed to reverse the early-phase (24 h) ∆Ψm loss of OEC-M1
cells, suggesting that JNK activation is dispensable for the
early-phase ∆Ψm loss (Fig. 6B).
However, inactivation of JNK effectively prevented
paclitaxel-induced late-phase (48 h) ∆Ψm loss (P<0.05; Fig. 6B), suggesting that JNK activation is
responsible for the late-phase (48 h) ∆Ψm collapse. The
JNK-dependent, late-phase ∆Ψm loss may, at least partially, be
explained by caspase-8-mediated truncation of Bid occurring at 36
and 48 h following treatment with low-dose paclitaxel (P<0.05;
Fig. 6C).
Schematic chart of paclitaxel action
on the apoptosis of OEC-M1 cells
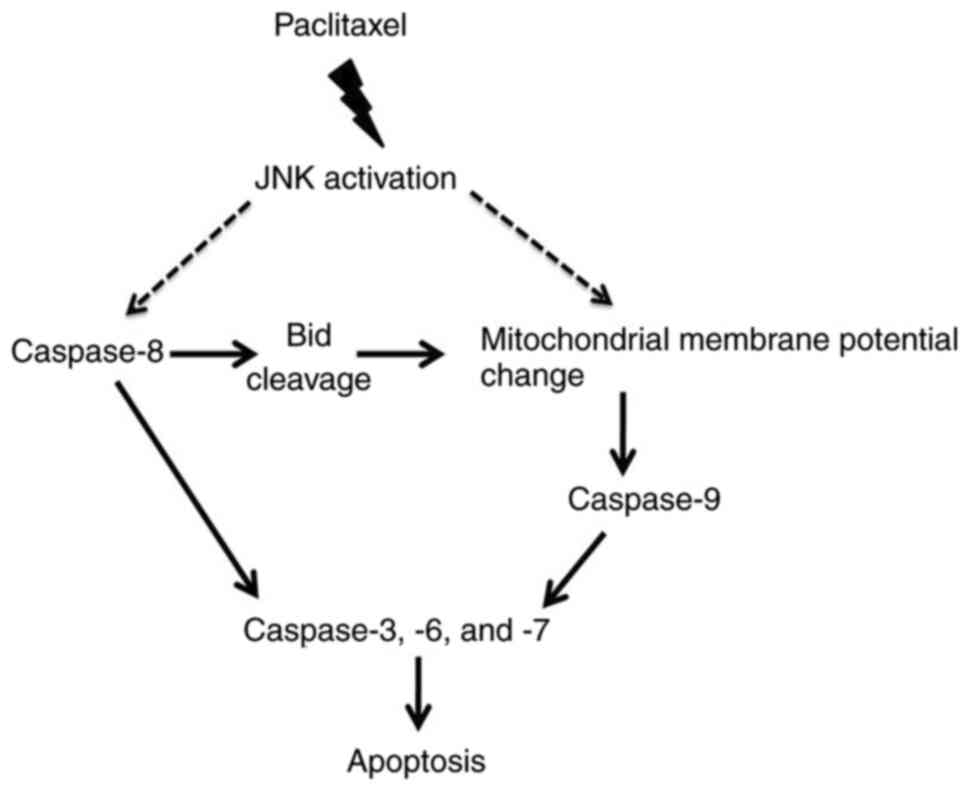
The potential signaling pathway of
paclitaxel-induced apoptosis in OEC-M1 cells is illustrated in
Fig. 7, which shows that JNK
activated by paclitaxel contributes to caspase-8 activation and ∆Ψm
reduction. Activation of caspase-8 results in Bid cleavage, which
may contribute to ∆Ψm loss (47).
The reduction of ∆Ψm results in activation of caspase-9 (33,34).
Activation of both caspase-8 and −9 promote activation of the
downstream caspases (caspase-3, −6 and −7) (48), resulting in apoptosis of OEC-M1
cells.
Discussion
The results of the present study demonstrated that
most of the cytotoxic effect of paclitaxel was achieved at a
relatively low concentration (50 nM) compared with high
concentration (1 µM) of paclitaxel in OEC-M1 cells. A similar
cytotoxic plateau has been reported in breast cancer cells and,
less remarkably, in OEC-M1 cells (41,49). The
cytotoxic plateau phenomenon observed in the present study may
largely be due to the apoptotic plateau in OEC-M1 cells treated
with different concentrations of paclitaxel (50 nM to 1 µM; data
not shown). Similar to the results of present study, an apoptotic
plateau has also been reported in paclitaxel-treated breast cancer
cells (41), as well as in
paclitaxel-treated HNSCC histocultures (46). These results suggest that most of the
apoptotic-inducing effect in HNSCC cells can be achieved with a
relative low dose of paclitaxel. In addition, the results of the
present study demonstrated that in OEC-M1 cells, high
concentrations of paclitaxel (250 and 500 nM and 1 µM) induced
necrosis, an unprogrammed form of cell death (50). It is well-known that necrotic cells
are able to release cellular cytoplasmic contents into
extracellular space, which evoke inflammatory reactions and
contribute to tumor progression (51). Given that high concentrations of
paclitaxel can induce necrosis and prolonged exposure to paclitaxel
concentrations exceeding the thresholds of 0.05 or 0.1 µM induce
side effects in patients with cancer, such as neutropenia and
peripheral neuropathy (52), in
terms of clinical usage, lower doses of paclitaxel are recommended
to minimize serious side effects while retaining most of the
apoptosis-inducing effect of this drug. This concept is supported
by a recent report demonstrating that, in treating patients with
metastatic breast cancer, low-dose weekly docetaxel/paclitaxel
infusion schedules can achieve overall response rates comparable to
several high dose infusion schedules, with fewer grade 3–4
toxicities (53).
It has been reported that paclitaxel not only causes
JNK activation but also causes ERK inactivation and a reduction of
basal p38 MAPK activity concomitantly in KB-3 human epidermoid
carcinoma cells (54). However, in
the present study, JNK, ERK and p38 MAPK were activated by
paclitaxel. This difference may be caused by the high paclitaxel
concentration used in the previous study (500 nM) compared with the
present study (50 nM). Furthermore, activation of specific MAPK
pathways may be cell-type specific.
The results of the present study demonstrated that
paclitaxel-induced JNK activation was responsible for most of the
apoptosis-inducing effect in OEC-M1 cells. JNK activation was also
responsible for paclitaxel-induced caspase activation. However, the
effect of inactivation of caspase-8 and/or caspase-9 in rescuing
paclitaxel-induced apoptosis was inferior compared with the effect
of JNK inhibition, suggesting that other caspase, such as
caspase-10 (55), or
caspase-independent apoptosis-like programmed cell death (56,57), may
also be involved in JNK-mediated, paclitaxel-induced apoptosis of
HNSCC cells.
Failure of JNK inactivation to prevent early-phase
(24 h) depolarization of OEC-M1 cells suggests that early-phase ∆Ψm
loss is not caused by JNK activation. The JNK-dependent, late-phase
(48 h) ∆Ψm loss can be explained, at least partially, by
caspase-8-induced truncation of Bid (a process of Bid activation),
which occurred at 36 and 48 h following treatment with paclitaxel.
Whether paclitaxel-induced JNK activation also causes activation of
other pro-apoptotic ‘BH-3 only’ proteins (58,59) and
contributes to the late-phase (48 h) ∆Ψm loss remain unknown.
The upstream events leading to JNK activation by
low-dose paclitaxel treatment are yet to be investigated. Reactive
oxygen species (ROS) production has been reported to be an early
and crucial step for paclitaxel-induced lung cancer cell death
(60). ROS production has also been
reported to mediate docetaxel-induced apoptosis of HNSCC cells
(61), and ROS is associated with
ASK1 activation, an upstream kinase of JNK (62). On the other hand, paclitaxel
treatment may also induce ceramide formation (63,64),
which can subsequently lead to both JNK activation (65) and ∆Ψm collapse (66,67). It
is speculated that paclitaxel may induce activation of JNK via the
ROS-ASK1 axis and ceramide formation. However, further studies are
required to verify these speculations.
p53 is able to upregulate several pro-apoptosis
related proteins, such as Bax and PUMA (68,69), or
promote activation of Bid and caspases (70,71).
Thus, p53 plays an important role in regulating apoptosis (72). It has been reported that JNK can
promote apoptosis by activating p53 (73). OEC-M1 is a cell line with p53
mutation, and our previous study demonstrated that paclitaxel can
induce apoptosis and activation of caspases in OEC-M1 cells
(24), suggesting that p53 is not
involved in paclitaxel-induced apoptosis. Apoptosis can be divided
into two pathways, death receptor apoptotic pathway (the extrinsic
pathway) and mitochondrial apoptotic pathway (the intrinsic
pathway) (48). The former,
activated by binding of Fas ligand to its receptor, Fas, leads to
formation of death-inducing signaling complex, which promotes
activation of initiator caspase-8 (48). The latter, activated by activation of
several pro-apoptotic Bcl-2 proteins, such as Bim and Bad, results
in formation of apoptosome, which promotes initiator caspase-9
activation (48). In addition, the
extrinsic pathway is involved in activating the intrinsic pathway
through activation of the caspase-8-Bid axis (48). The results of the present study
demonstrated that paclitaxel activated caspase-8, Bid and
caspase-9, and caused ∆Ψm loss, suggesting that both pathways are
activated in paclitaxel-induced apoptosis. Given that JNK can
upregulate Fas ligand (74) and
promote the activation of Bax and Bim (58,75), it
was speculated that during the progression of paclitaxel-induced
apoptosis, JNK may trigger activation of the extrinsic and the
intrinsic pathways to activate the initiator caspases (caspase-8
and −9) in OEC-M1 cells, which may contribute to activation of
downstream caspases (caspase-3, −6 and −7). Prospective studies are
required to investigate whether paclitaxel can upregulate Fas
ligand and activate Bax and Bim.
In conclusion, the results of the present study
demonstrated that paclitaxel-activated JNK is required for caspase
activation and loss of ∆Ψm to induce apoptosis of OEC-M1 cells. To
the best of our knowledge, the present study was the first to
demonstrate that JNK can activate caspases and reduce ∆Ψm, which is
important for paclitaxel-induced apoptosis of OEC-M1 cells. These
results may be applied to improve or enhance the therapeutic
efficacy of paclitaxel as a chemotherapeutic drug.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Ministry of
Science and Technology, Taiwan (grant no. MOST-107-2320-B-471-001
to YYL; grant no. MOST-109-2314-B-218-001 to HYC; and grant nos.
MOST-105-2320-B-006-028-MY3 and MOST-106-2320-B-006-001-MY3 to
BMH).
Availability of data and materials
The datasets used and analyzed in the present study
are available from the corresponding authors upon reasonable
request.
Authors' contributions
YYL, YHC, CL and BMH designed the present study and
analyzed the data. YYL, YHC, KLT, YTW and SCL performed the
experiments. YCC, CHW and HYC interpreted the results. YYL, YHC, CL
and YCC drafted the initial manuscript. YCC, CHW, HYC and BMH
revised the manuscript for important intellectual content. YYL, YCC
and BMH confirm the authenticity of all the raw data. All authors
read and approved the final manuscript and agreed to be accountable
for all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ERK
|
extracellular signal-regulated
kinase
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
JNK
|
c-Jun N-terminal kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MOMP
|
mitochondrial outer membrane
permeabilization
|
|
MTA
|
microtubule-targeting agents
|
|
ROS
|
reactive oxygen species
|
|
∆Ψm
|
mitochondrial membrane potential
|
References
|
1
|
Rades D, Seidl D, Wollenberg B, Schild SE
and Hakim SG: Radiochemotherapy with paclitaxel for recurrent
previously irradiated squamous cell carcinoma of the head and neck.
Anticancer Res. 36:5463–5468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forastiere AA, Goepfert H, Maor M, Pajak
TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et
al: Concurrent chemotherapy and radiotherapy for organ preservation
in advanced laryngeal cancer. N Engl J Med. 349:2091–2098. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Psyrri A, Kwong M, DiStasio S, Lekakis L,
Kassar M, Sasaki C, Wilson LD, Haffty BG, Son YH, Ross DA, et al:
Cisplatin, fluorouracil, and leucovorin induction chemotherapy
followed by concurrent cisplatin chemoradiotherapy for organ
preservation and cure in patients with advanced head and neck
cancer: Long-term follow-up. J Clin Oncol. 22:3061–3069. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lo Nigro C, Denaro N, Merlotti A and
Merlano M: Head and neck cancer: Improving outcomes with a
multidisciplinary approach. Cancer Manag Res. 9:363–371. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adamo V, Ferraro G, Pergolizzi S, Sergi C,
Laudani A, Settineri N, Alafaci E, Scimone A, Spano F and Spitaleri
G: Paclitaxel and cisplatin in patients with recurrent and
metastatic head and neck squamous cell carcinoma. Oral Oncol.
40:525–531. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Monnerat C, Faivre S, Temam S, Bourhis J
and Raymond E: End points for new agents in induction chemotherapy
for locally advanced head and neck cancers. Ann Oncol. 13:995–1006.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pignon JP, Bourhis J, Domenge C and
Designe L: Chemotherapy added to locoregional treatment for head
and neck squamous-cell carcinoma: Three meta-analyses of updated
individual data. MACH-NC Collaborative Group. Meta-analysis of
chemotherapy on head and neck cancer. Lancet. 355:949–955. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pendleton KP and Grandis JR:
Cisplatin-based chemotherapy options for recurrent and/or
metastatic squamous cell cancer of the head and neck. Clin Med
Insights Ther. 2013.10.4137/CMT.S10409, 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rowinsky EK and Donehower RC: Paclitaxel
(taxol). N Engl J Med. 332:1004–1014. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abu Samaan TM, Samec M, Liskova A, Kubatka
P and Busselberg D: Paclitaxel's mechanistic and clinical effects
on breast cancer. Biomolecules. 9:7892019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Habib S, Delourme J, Dhalluin X, Petyt G,
Tacelli N, Scherpereel A, Lafitte JJ and Cortot AB: Bevacizumab and
weekly paclitaxel for non-squamous non small cell lung cancer
patients: A retrospective study. Lung Cancer. 80:197–202. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Specenier P and Vermorken JB: The role of
taxanes and targeted therapies in locally advanced head and neck
cancer. Curr Opin Oncol. 19:195–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferrari D, Ghi MG, Franzese C, Codeca C,
Gau M and Fayette J: The slippery role of induction chemotherapy in
head and neck cancer: Myth and reality. Front Oncol. 10:72020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao P, Ma T, Zhou C, Xu Y, Liu Y and
Zhang H: Anticancer effect of docetaxel induces apoptosis of
prostate cancer via the cofilin-1 and paxillin signaling pathway.
Mol Med Rep. 13:4079–4084. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miller AV, Hicks MA, Nakajima W,
Richardson AC, Windle JJ and Harada H: Paclitaxel-induced apoptosis
is BAK-dependent, but BAX and BIM-independent in breast tumor. PLoS
One. 8:e606852013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han TD, Shang DH and Tian Y: Docetaxel
enhances apoptosis and G2/M cell cycle arrest by suppressing
mitogen-activated protein kinase signaling in human renal clear
cell carcinoma. Genet Mol Res. 15:2016. View Article : Google Scholar
|
|
18
|
Pan Z, Avila A and Gollahon L: Paclitaxel
induces apoptosis in breast cancer cells through different
calcium-regulating mechanisms depending on external calcium
conditions. Int J Mol Sci. 15:2672–2694. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu J, Zhang NA, Wang R, Huang F and Li G:
Paclitaxel induces apoptosis and reduces proliferation by targeting
epidermal growth factor receptor signaling pathway in oral cavity
squamous cell carcinoma. Oncol Lett. 10:2378–2384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nonaka M, Ikeda H, Fujisawa A, Uehara M
and Inokuchi T: Induction of apoptosis by paclitaxel in human oral
carcinoma cells. Int J Oral Maxillofac Surg. 35:649–652. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Holsinger FC, Doan DD, Jasser SA, Swan EA,
Greenberg JS, Schiff BA, Bekele BN, Younes MN, Bucana CD, Fidler IJ
and Myers JN: Epidermal growth factor receptor blockade potentiates
apoptosis mediated by Paclitaxel and leads to prolonged survival in
a murine model of oral cancer. Clin Cancer Res. 9:3183–3189.
2003.PubMed/NCBI
|
|
22
|
Ganansia-Leymarie V, Bischoff P, Bergerat
JP and Holl V: Signal transduction pathways of taxanes-induced
apoptosis. Curr Med Chem Anticancer Agents. 3:291–306. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang CY, Ju DT, Chang CF, Muralidhar
Reddy P and Velmurugan BK: A review on the effects of current
chemotherapy drugs and natural agents in treating non-small cell
lung cancer. Biomedicine (Taipei). 7:232017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsiao JR, Leu SF and Huang BM: Apoptotic
mechanism of paclitaxel-induced cell death in human head and neck
tumor cell lines. J Oral Pathol Med. 38:188–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Martindale JL and Holbrook NJ:
Requirement for ERK activation in cisplatin-induced apoptosis. J
Biol Chem. 275:39435–39443. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Teraishi F, Zhang L, Guo W, Dong F, Davis
JJ, Lin A and Fang B: Activation of c-Jun NH2-terminal kinase is
required for gemcitabine's cytotoxic effect in human lung cancer
H1299 cells. FEBS Lett. 579:6681–6687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Uzu M, Sato H, Shimizu A, Shibata Y, Ueno
K and Hisaka A: Connexin 43 enhances Bax activation via JNK
activation in sunitinib-induced apoptosis in mesothelioma cells. J
Pharmacol Sci. 134:101–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schrantz N, Bourgeade MF, Mouhamad S, Leca
G, Sharma S and Vazquez A: p38-mediated regulation of an
Fas-associated death domain protein-independent pathway leading to
caspase-8 activation during TGFbeta-induced apoptosis in human
Burkitt lymphoma B cells BL41. Mol Biol Cell. 12:3139–3151. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao H, Wu S, Li H, Duan Q, Zhang Z, Shen
Q, Wang C and Yin T: ROS/KRAS/AMPK signaling contributes to
gemcitabine-induced stem-like cell properties in pancreatic cancer.
Mol Ther Oncolytics. 14:299–312. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
James D, Parone PA, Terradillos O,
Lucken-Ardjomande S, Montessuit S and Martinou JC: Mechanisms of
mitochondrial outer membrane permeabilization. Novartis Found Symp.
287:170–176; discussion 176-182. 2007.PubMed/NCBI
|
|
32
|
Gahl RF, Dwivedi P and Tjandra N: Bcl-2
proteins bid and bax form a network to permeabilize the
mitochondria at the onset of apoptosis. Cell Death Dis.
7:e24242016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tait SW and Green DR: Mitochondrial
regulation of cell death. Cold Spring Harb Perspect Biol.
5:a0087062013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li P, Zhou L, Zhao T, Liu X, Zhang P, Liu
Y, Zheng X and Li Q: Caspase-9: Structure, mechanisms and clinical
application. Oncotarget. 8:23996–24008. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 7:a0267162015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Foo NP, Ko CL, Chu CY, Wang CY, So EC and
Huang BM: Arsenic compounds activate the MAPK and caspase pathways
to induce apoptosis in OEC-M1 gingival epidermal carcinoma. Oncol
Rep. 44:2701–2714. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Krysko DV, Vanden Berghe T, D'Herde K and
Vandenabeele P: Apoptosis and necrosis: Detection, discrimination
and phagocytosis. Methods. 44:205–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nicoletti I, Migliorati G, Pagliacci MC,
Grignani F and Riccardi C: A rapid and simple method for measuring
thymocyte apoptosis by propidium iodide staining and flow
cytometry. J Immunol Methods. 139:271–279. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Galluzzi L, Zamzami N, de La Motte Rouge
T, Lemaire C, Brenner C and Kroemer G: Methods for the assessment
of mitochondrial membrane permeabilization in apoptosis. Apoptosis.
12:803–813. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yeung TK, Germond C, Chen X and Wang Z:
The mode of action of taxol: Apoptosis at low concentration and
necrosis at high concentration. Biochem Biophys Res Commun.
263:398–404. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Esteve MA, Carre M and Braguer D:
Microtubules in apoptosis induction: Are they necessary? Curr
Cancer Drug Targets. 7:713–729. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ulukaya E, Karaagac E, Ari F, Oral AY,
Adim SB, Tokullugil AH and Evrensel T: Chemotherapy increases
caspase-cleaved cytokeratin 18 in the serum of breast cancer
patients. Radiol Oncol. 45:116–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lu KH, Lue KH, Chou MC and Chung JG:
Paclitaxel induces apoptosis via caspase-3 activation in human
osteogenic sarcoma cells (U-2 OS). J Orthop Res. 23:988–994. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mhaidat NM, Wang Y, Kiejda KA, Zhang XD
and Hersey P: Docetaxel-induced apoptosis in melanoma cells is
dependent on activation of caspase-2. Mol Cancer Ther. 6:752–761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Janssen K, Pohlmann S, Janicke RU,
Schulze-Osthoff K and Fischer U: Apaf-1 and caspase-9 deficiency
prevents apoptosis in a Bax-controlled pathway and promotes
clonogenic survival during paclitaxel treatment. Blood.
110:3662–3672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li H, Zhu H, Xu CJ and Yuan J: Cleavage of
BID by caspase 8 mediates the mitochondrial damage in the Fas
pathway of apoptosis. Cell. 94:491–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Baig S, Seevasant I, Mohamad J, Mukheem A,
Huri HZ and Kamarul T: Potential of apoptotic pathway-targeted
cancer therapeutic research: Where do we stand? Cell Death Dis.
7:e20582016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang GC, Liu SY, Lin MH, Kuo YY and Liu
YC: The synergistic cytotoxicity of cisplatin and taxol in killing
oral squamous cell carcinoma. Jpn J Clin Oncol. 34:499–504. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dhuriya YK and Sharma D: Necroptosis: A
regulated inflammatory mode of cell death. J Neuroinflammation.
15:1992018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee SY, Ju MK, Jeon HM, Jeong EK, Lee YJ,
Kim CH, Park HG, Han SI and Kang HS: Regulation of tumor
progression by programmed necrosis. Oxid Med Cell Longev.
2018:35374712018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mielke S: Individualized pharmacotherapy
with paclitaxel. Curr Opin Oncol. 19:586–589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Eniu A, Palmieri FM and Perez EA: Weekly
administration of docetaxel and paclitaxel in metastatic or
advanced breast cancer. Oncologist. 10:665–685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Stone AA and Chambers TC: Microtubule
inhibitors elicit differential effects on MAP kinase (JNK, ERK, and
p38) signaling pathways in human KB-3 carcinoma cells. Exp Cell
Res. 254:110–119. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Park SJ, Wu CH, Gordon JD, Zhong X, Emami
A and Safa AR: Taxol induces caspase-10-dependent apoptosis. J Biol
Chem. 279:51057–51067. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Broker LE, Kruyt FA and Giaccone G: Cell
death independent of caspases: A review. Clin Cancer Res.
11:3155–3162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Constantinou C, Papas KA and Constantinou
AI: Caspase-independent pathways of programmed cell death: The
unraveling of new targets of cancer therapy? Curr Cancer Drug
Targets. 9:717–728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Qu L, Liu FX, Cao XC, Xiao Q, Yang X and
Ren KQ: Activation of the apoptosis signal-regulating kinase
1/c-Jun N-terminal kinase pathway is involved in the
casticin-induced apoptosis of colon cancer cells. Exp Ther Med.
8:1494–1500. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Brockmann A, Bluwstein A, Kogel A, May S,
Marx A, Tschan MP and Brunner T: Thiazolides promote apoptosis in
colorectal tumor cells via MAP kinase-induced Bim and Puma
activation. Cell Death Dis. 6:e17782015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Alexandre J, Batteux F, Nicco C, Chéreau
C, Laurent A, Guillevin L, Weill B and Goldwasser F: Accumulation
of hydrogen peroxide is an early and crucial step for
paclitaxel-induced cancer cell death both in vitro and in vivo. Int
J Cancer. 119:41–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Taniguchi T, Takahashi M, Shinohara F,
Sato T, Echigo S and Rikiishi H: Involvement of NF-kappaB and
mitochondrial pathways in docetaxel-induced apoptosis of human oral
squamous cell carcinoma. Int J Mol Med. 15:667–673. 2005.PubMed/NCBI
|
|
62
|
Zhao Q, Liu Y, Zhong J, Bi Y, Liu Y, Ren
Z, Li X, Jia J, Yu M and Yu X: Pristimerin induces apoptosis and
autophagy via activation of ROS/ASK1/JNK pathway in human breast
cancer in vitro and in vivo. Cell Death Discov. 5:1252019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Asakuma J, Sumitomo M, Asano T, Asano T
and Hayakawa M: Selective Akt inactivation and tumor necrosis
actor-related apoptosis-inducing ligand sensitization of renal
cancer cells by low concentrations of paclitaxel. Cancer Res.
63:1365–1370. 2003.PubMed/NCBI
|
|
64
|
Swanton C, Marani M, Pardo O, Warne PH,
Kelly G, Sahai E, Elustondo F, Chang J, Temple J, Ahmed AA, et al:
Regulators of mitotic arrest and ceramide metabolism are
determinants of sensitivity to paclitaxel and other
chemotherapeutic drugs. Cancer Cell. 11:498–512. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lu S, Natarajan SK, Mott JL, Kharbanda KK
and Harrison-Findik DD: Ceramide induces human hepcidin gene
transcription through JAK/STAT3 pathway. PLoS One. 11:e01474742016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Villena J, Henriquez M, Torres V, Moraga
F, Díaz-Elizondo J, Arredondo C, Chiong M, Olea-Azar C, Stutzin A,
Lavandero S and Quest AF: Ceramide-induced formation of ROS and ATP
depletion trigger necrosis in lymphoid cells. Free Radic Biol Med.
44:1146–1160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kogot-Levin A and Saada A: Ceramide and
the mitochondrial respiratory chain. Biochimie. 100:88–94. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yamaguchi H, Chen J, Bhalla K and Wang HG:
Regulation of Bax activation and apoptotic response to
microtubule-damaging agents by p53 transcription-dependent and
-independent pathways. J Biol Chem. 279:39431–39437. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lee WT and Chang CW: Bax is upregulated by
p53 signal pathway in the SPE B-induced apoptosis. Mol Cell
Biochem. 343:271–279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Henry RE, Andrysik Z, Paris R, Galbraith
MD and Espinosa JM: A DR4:tBID axis drives the p53 apoptotic
response by promoting oligomerization of poised BAX. EMBO J.
31:1266–1278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shen YH, Utama B, Wang J, Raveendran M,
Senthil D, Waldman WJ, Belcher JD, Vercellotti G, Martin D,
Mitchelle BM and Wang XL: Human cytomegalovirus causes endothelial
injury through the ataxia telangiectasia mutant and p53 DNA damage
signaling pathways. Circ Res. 94:1310–1317. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Aubrey BJ, Kelly GL, Janic A, Herold MJ
and Strasser A: How does p53 induce apoptosis and how does this
relate to p53-mediated tumour suppression? Cell Death Differ.
25:104–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Buschmann T, Potapova O, Bar-Shira A,
Ivanov VN, Fuchs SY, Henderson S, Fried VA, Minamoto T,
Alarcon-Vargas D, Pincus MR, et al: Jun NH2-terminal kinase
phosphorylation of p53 on Thr-81 is important for p53 stabilization
and transcriptional activities in response to stress. Mol Cell
Biol. 21:2743–2754. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Dong Y, Shen X, He M, Wu Z, Zheng Q, Wang
Y, Chen Y, Wu S, Cui J and Zeng Z: Activation of the JNK-c-Jun
pathway in response to irradiation facilitates Fas ligand secretion
in hepatoma cells and increases hepatocyte injury. J Exp Clin
Cancer Res. 35:1142016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chu R, Upreti M, Ding WX, Yin XM and
Chambers TC: Regulation of Bax by c-Jun NH2-terminal kinase and
Bcl-xL in vinblastine-induced apoptosis. Biochem Pharmacol.
78:241–248. 2009. View Article : Google Scholar : PubMed/NCBI
|