Introduction
Mantle cell lymphoma (MCL), which is a non-Hodgkin's
lymphoma type, is a refractory hematological cancer with a poor
prognosis (1–3) due to its high drug resistance and it
has a survival period of 1–2 years after relapse (4,5).
Therefore, effective and highly safe therapies that specifically
target molecular MCL are imperative (6).
Sex-determining region Y-box 11 (SOX11), which
belongs to the SOX family, is an intron-lacking gene that is highly
expressed in various types of cancer, including breast cancer,
ovarian cancer and MCL (7–11). It promotes angiogenesis and tumor
cell migration and metastasis by mediating the expression of
platelet-derived growth factor α (12,13).
Recently, the targeting roles of microRNAs (miRNAs/miRs) have
attracted great attention. miRNAs can regulate mRNAs by binding to
the 3′-untranslated region of the target mRNA, thereby regulating
gene expression, and miRNA dysregulation often leads to cancer
(14,15). miR-212-3p is a miRNA that inhibits
the proliferation of blood cancer cells; however, it is minimally
expressed in adult T-cell leukemia/lymphoma and plasmablastic
lymphoma (16,17). It has been revealed that miR-212-3p
acts as a tumor suppressor in numerous types of cancer, such as
osteosarcoma (18), glioblastoma
(19) and intrahepatic
cholangiocarcinoma (20). Overall,
SOX11 seems to have a promoting effect on the growth of lymphoma,
whereas miR-212-3p expression is low in various cancer cells.
However, to the best of our knowledge, the effect of miR-212-3p on
SOX11 expression has not been previously investigated, and how this
effect affects cell proliferation and migration in lymphoma remains
unclear. Therefore, the present study aimed to assess the
association between miR-212-3p and SOX11, and the effects of
miR-212-3p on cell proliferation and migration in MCL.
Materials and methods
Tissue collection
Cancerous and corresponding paracancerous tissues (5
mm distance from cancerous tissue) were collected from 65 patients
with MCL who underwent surgery at Huai'an Hospital Affiliated to
Xuzhou Medical College and Huai'an Second People's Hospital
(Huai'an, China) between November 2016 and November 2019. The
cancerous tissues of the patients were all taken from the lymph
node tissues, and the adjacent tissues were dominated by normal B
lymphocytes (such as tunicular lymphocytes) (Table I). Patients diagnosed with MCL at the
aforementioned hospital, without other major diseases, and with
relatively complete clinical data were included in the present
study. Conversely, patients who had received any radiotherapy and
chemotherapy before the study, or who presented with diseases that
could affect the study results or with severe hepatic dysfunction
were excluded. The Ethics Committee of Huai'an Hospital Affiliated
to Xuzhou Medical College and Huai'an Second People's Hospital
approved the present study. All patients and their families were
informed of the study, and they signed informed consent forms
before the commencement of the study.
 | Table I.Clinical characteristics of the
patients with mantle cell lymphoma (n=65). |
Table I.
Clinical characteristics of the
patients with mantle cell lymphoma (n=65).
|
Characteristics | Values |
|---|
| Sex, n (%) |
|
|
Male | 35 (53.85) |
|
Female | 30 (46.15) |
| Mean age ± SD,
years | 45.23±12.31 |
| Mean BMI ± SD,
kg/m2 | 23.23±0.87 |
| Lymphoma and the
location of adjacent tissues, n (%) |
|
|
Colorectal | 18 (27.70) |
|
Stomach | 17 (26.15) |
| Torso
skin | 20 (30.77) |
|
Marrow | 10 (15.38) |
Main reagents and instruments
Human MCL cells (JeKo-1 and Z-138 cells) and DMEM
were obtained from Hunan Fenghui Biotechnology Co., Ltd. Fetal
bovine serum (FBS) was obtained from Thermo Fisher Scientific, Inc.
Cell apoptosis assay kit and Lipofectamine® 3000 were
obtained from Sigma-Aldrich (Merck KGaA). TRIzol® was
obtained from Invitrogen; Thermo Fisher Scientific, Inc. Transwell
assay kit was obtained from ElabScience Biotechnology, Co., Ltd.
Primers, transfection plasmids of miR-212-3p and SOX11, and
internal references (U6 and GAPDH) were designed and synthesized by
Sangon Biotech Co., Ltd. The mimic control (scrambled) was
purchased from Shanghai GenePharma Co., Ltd. The sequences of the
mimics were as follows: miR-212-3p mimic sense,
5′-UAACAGUCUCCAGUCACGGGG-3′ and antisense,
5′-CCGUGACUGGAGACUGUUAUU-3′; and mimic control (miR-NC) sense,
5′-UUCUCCGAACGCGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. The equipment included an ultraviolet
spectrophotometer (Thermo Fisher Scientific Inc.), a flow cytometer
(CoulterCytoFLEX; Beckman Coulter, Inc.), an ABI 7500 FAST
Real-Time PCR instrument (ABI 7500; Applied Biosystems; Thermo
Fisher Scientific, Inc.).
Determination of miR-212-3p and SOX11
mRNA expression
Total RNA was extracted from patient cancerous
tissues and transfected cells in each group using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the
manufacturer's instructions. cDNA was synthesized using the
PrimeScript® RT reagent kit according to the
manufacturer's protocol. (Takara Bio, Inc.). RT-qPCR was performed
using SYBR Premix ExTaq™ (Takara Bio, Inc.) on the platform of
Applied Biosystems 7500 (Applied Biosystems; Thermo Fisher
Scientific, Inc.). U6 and GAPDH were used as internal controls for
miRNA and mRNA, respectively. The reverse universal miR qPCR
primers were included in the PrimeScript™ miRNA RT-PCR kit (cat.
no. RR716; Takara Biotechnology Co., Ltd.). The total reaction
system was 20 µl in volume, containing 1 µl cDNA, 10 µl SYBR Premix
EX Taq, 1 µl each of the primers (10 µM) and 7 µl ddH2O.
The PCR program was as follows: 95°C for 3 min, followed by 40
cycles of 95°C for 10 sec and 60°C for 30 sec. Primers were
synthesized by Sangon Biotech Co., Ltd., as follows: miR-221-3p
forward, 5′-CGGCTACATTGTCTGCCTG-3′ and reverse,
5′-CAGTGCGTGTCGTGGAGT-3′; SOX11 forward, 5′-AGCAAGAAATGCGGCAAGC-3′
and reverse, 5′-ATCCAGAAACACGCACTTGAC-3′; GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′; and U6 forward,
5′-CGCTTCGGCAGCACATATAC-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′.
The relative expression levels of miR-221-3p and SOX11 were
calculated using the 2−ΔΔCq method (21). All experiments were conducted in
triplicate.
Determination of SOX11 protein
expression
Total protein from patient cancerous tissues and
transfected cells was lysed with RIPA buffer (Invitrogen; Thermo
Fisher Scientific, Inc.), and protein concentration was measured
using a BCA kit (Beyotime Institute of Biotechnology). Total
protein (50 µg/lane) was separated by 10% SDS-PAGE and blotted onto
polyvinylidene fluoride membranes. After blocking in 5% skimmed
milk for 1 h at room temperature, the membranes were incubated
overnight at 4°C with primary antibodies against SOX2 (1:1,000;
cat. no. ab170916; Abcam) and β-actin (1:1,000; cat. no. ab6276;
Abcam). Subsequently, secondary horse-radish peroxidase
(HRP)-rabbit antibodies were incubated with the membranes at room
temperature for 2 h (1:5,000; cat. no. ab6858; Abcam). The bands
were visualized using an ECL kit (Beyotime Institute of
Biotechnology), and the gray values of the bands were calculated
automatically using the ImageJ software version k1.45 (National
Institutes of Health). SOX11/GAPDH ratios represented relative
protein expression.
Cell culture and transfection
JeKo-1 and Z-138 cells were sub-cultured routinely
in high-sugar DMEM containing 10% FBS in a 5% CO2 cell
incubator at 37°C. After culture, the cells were divided into the
miR-NC and miR-212-3p mimic groups before being seeded into a
96-well plate, and cells were then transfected with the miR-NC or
miR-212-3p mimic at a concentration of 80 nmol/ml using
Lipofectamine 3000® at 37°C for 24 h. miR-212-3p
expression in each group was detected 24 h after transfection.
Cell proliferation assay
Transfected JeKo-1 and Z-138 cells in the two groups
were seeded (4×103/well) into a 96-well plate
separately, and each sample was tested in three duplicate wells.
Subsequently, the plate was cultured for 24, 48 and 72 h. A total
of 10 µl CCK8 reagent from the cell proliferation colorimetric
assay kit (CCK8; Shanghai Xiangsheng Biological Technology Co.,
Ltd.) was added to each well 2 h before the end of culture period.
After addition of the reagent at each time-point, the plate was
cultured in a 5% CO2 cell incubator at 37°C for 2 h, and
the optical density of each well was measured at 490 nm using an
automatic microplate reader to analyze cell proliferation.
Cell invasion assay
To detect cell migration, the Transwell assay was
performed. First, matrix gelatin (10 µg/µl) was melted at 4°C,
diluted to 0.25 µg/µl with DMEM, and stored in an ice box for
future use. Subsequently, 100 µl of diluted matrix gelatin was
added to each well of the 24-well Transwell insert, and the insert
was cultured in a 5% CO2 incubator at 37°C for 1 h.
After the matrix gel solidified to form the mechanical barrier
layer, the remaining uncured liquid was absorbed using filter
paper. Subsequently, cells in each group were transferred to the
insert. DMEM (100 µl) with 10% FBS was added to the upper chamber
and DMEM (600 µl) with 20% FBS was added to the lower chamber
(22). The upper chamber (coated
with Matrigel) was filled with cells (4×103 cells/well).
Finally, the cells were incubated in a 5% CO2 incubator
at 37°C for 1 day. Paraformaldehyde (4%) was used to fix the cells
at 4°C for 20 min that were being moved via the membrane and
crystal violet hydrate solution was used to dye them (Shanghai
Yuanye Biological Technology Co., Ltd.), and counted under a light
microscope (Leica Microsystems) (magnification, ×100) after 24 h
incubation period at room temperate.
Apoptosis assay
Cells were transfected for 48 h and were stained
with Annexin V and PI (cat. no. V13241; BD Biosciences) in a 6-well
plate for 30 min at 4°C. Subsequently, cells were detected using a
CoulterCytoFLEX flow cytometer (Beckman Coulter, Inc.), and the
assay was repeated three times. FlowJo v.10 (Flowjo LLC) was used
for analysis.
Bioinformatics analysis
To identify the putative miRNA target, the online
miRNA target analysis tools TargetScanHuman v.7.2 (http://www.targetscan.org/vert_70/) was used.
Verification of the targeting
association between miR-212-3p and SOX11 using a dual luciferase
reporter gene assay
JeKo-1 and Z-138 cells (4×103 cells/well)
were seeded into a 96-well plate, The SOX11 mutated and SOX11
wild-type (WT) 3′UTR was also added to the Luciferase miRNA
expression reporter (Promega Inc.) and miR-212-3p mimics, SOX11
mutated and SOX11 wild-type (WT) were transfected into the cells
using Lipofectamine 3000, as aforementioned. After 48 h of
transfection Firefly and Renilla luciferase activities in
the cells were determined using a dual luciferase reporter assay
kit (Promega Corporation). The Firefly luciferase activities were
normalized to Renilla luciferase activity and the relative
luciferase activity of Firefly (to Renilla) were reported in
the present study. Each assay was repeated thrice.
Statistical analysis
Statistical analyses were performed using SPSS 19.0
(IBM Corp.). Comparisons between tumor and normal tissues were
analyzed using a paired t-test, while comparisons between
transfection groups were analyzed using an unpaired t-test and
one-way or two-way ANOVA with Bonferroni correction as the post hoc
test. The values were expressed as the mean ± SE. Each experiment
was performed independently at least 3 times. Count data were
analyzed using the χ2 test. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-212-3p and SOX11 expression in
paracancerous and cancerous tissues
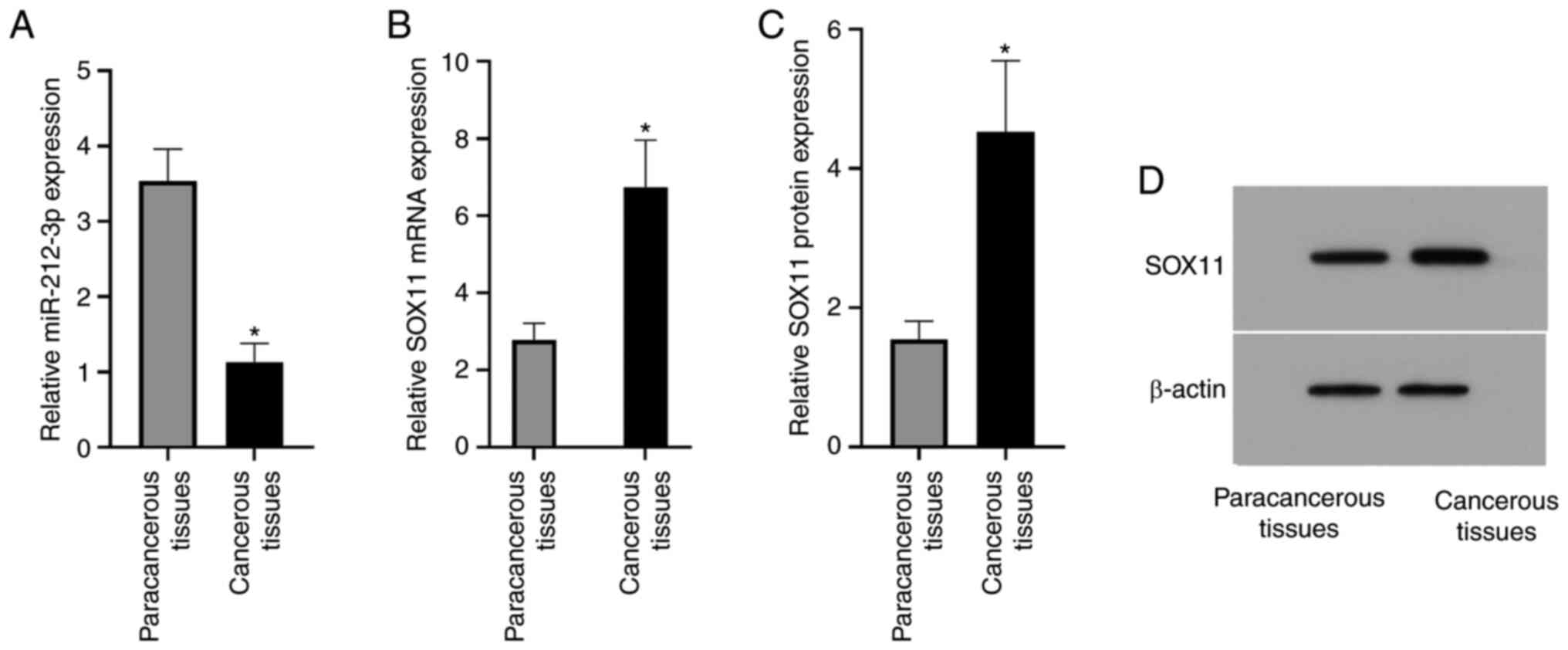
miR-212-3p expression in the paracancerous tissues
was significantly higher than that in the cancerous tissues
(3.54±0.42 vs. 1.13±0.25; P<0.0015; Fig. 1). The relative SOX11 mRNA expression
in the paracancerous and cancerous tissues was 2.78±0.43 and
6.74±1.22, respectively, and the relative SOX11 protein expression
was 1.55±0.26 and 4.53±1.02, respectively; thus, relative SOX11
mRNA and protein expression in the cancerous tissues was
significantly higher than that in the paracancerous tissues (both
P<0.05; Fig. 1). The current
results indicated that miR-212-3p expression may be negatively
associated with lymphoma, suggesting that miR-212-3p may have an
inhibitory effect on lymphoma. Both mRNA and protein expression
levels of SOX11 were high in the cancerous tissues, suggesting that
SOX11 may serve an important role in lymphoma.
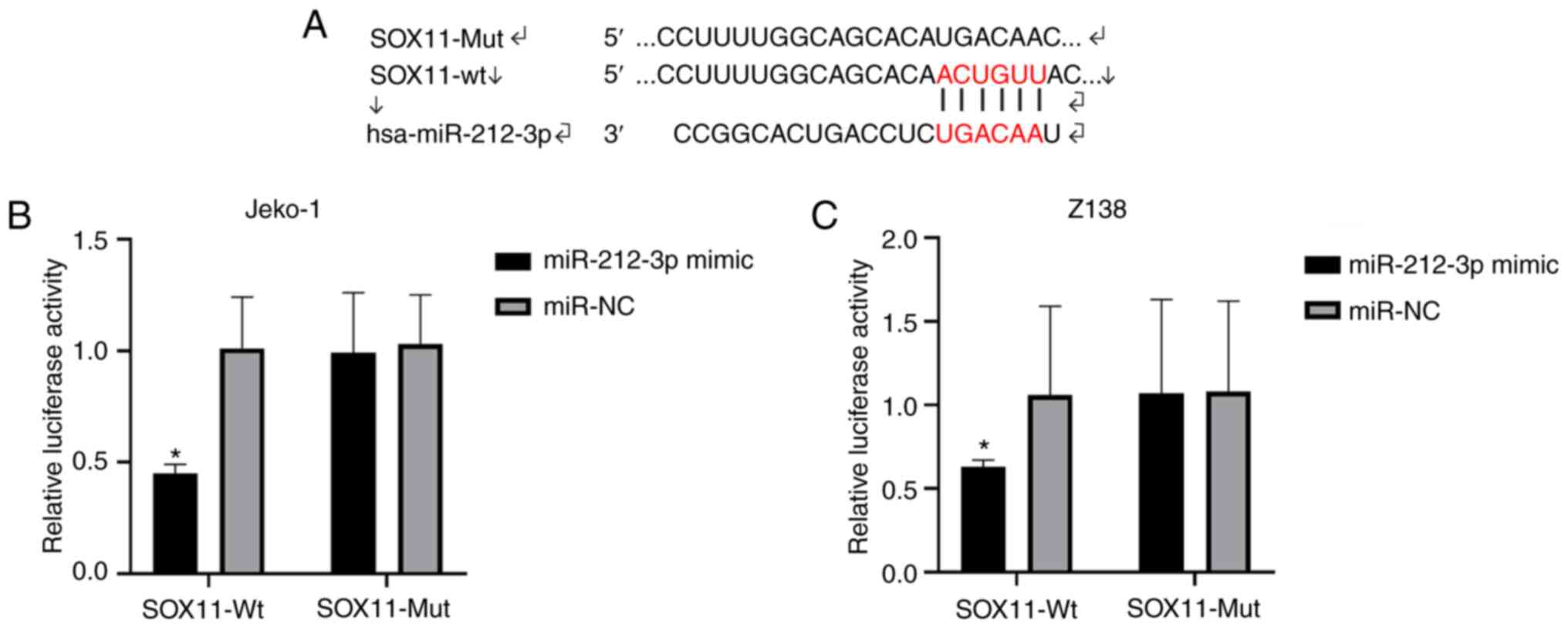
Targeting association between
miR-212-3p and SOX11
As predicted with TargetScanHuman v.7.2, binding
loci were found between miR-212-3p and SOX11 (Fig. 2A). The binding between SOX11 and
miR-212-3p significantly decreased the luciferase activity in the
SOX11-Wt and miR-212-3p mimic group (P<0.05; Fig. 2B and C). Therefore, miR-212-3p
targeted regulation of SOX11, and it was speculated that miR-212-3p
may serve an important role in lymphoma by regulating SOX11.
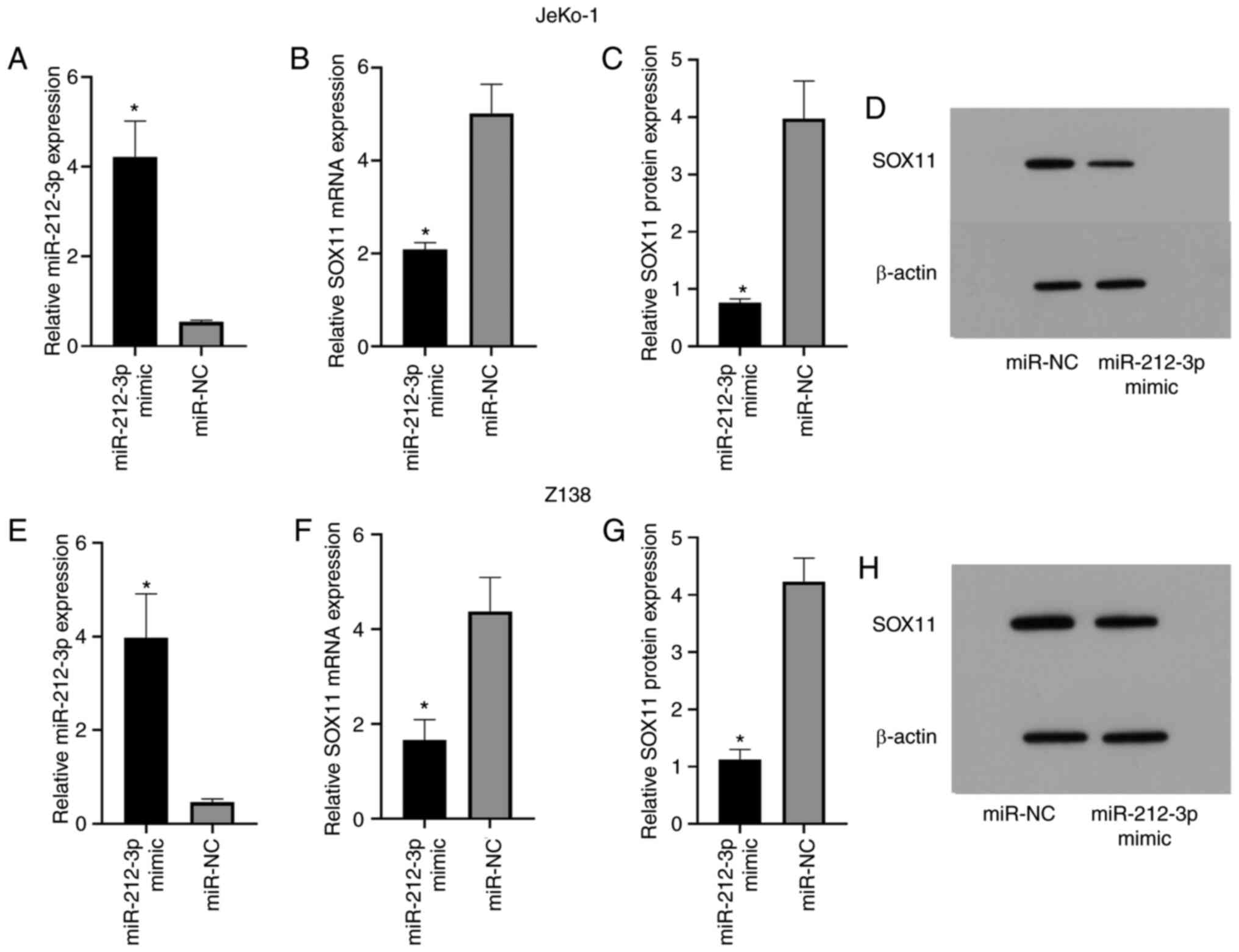
Elevated SOX11 expression after
miR-212-3p overexpression
As shown in the experimental results, the miR-212-3p
mimic group exhibited significantly higher miR-212-3p expression
than the miR-NC group (JeKo-1, 4.22±0.80 vs. 0.54±0.04; Z-138,
3.97±0.94 vs. 0.46±0.07; P<0.05; Fig.
3A and E). Additionally, SOX11 mRNA expression in the
miR-212-3p mimic group was significantly lower than that in the
miR-NC group (JeKo-1, 2.08±0.15 vs. 5.01±0.63; Z-138, 1.66±0.43 vs.
4.37±0.72; P<0.05; Fig. 3B and
F). Similarly, SOX11 protein expression in the miR-212-3p mimic
group was significantly lower than that in the miR-NC group
(JeKo-1, 0.76±0.07 vs. 3.97±0.66; Z-138, 1.12±0.18 vs. 4.23±0.41;
P<0.05; Fig. 3C, D, G and H). The
current results indicated that miR-212-3p may have an inhibitory
effect on SOX11.
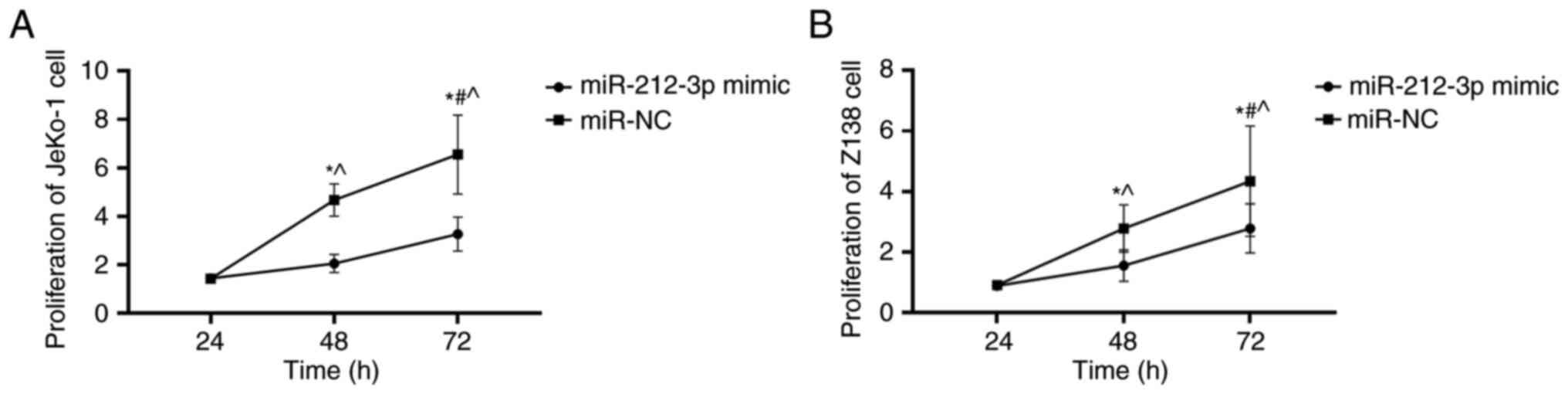
miR-212-3p overexpression decreases
the proliferation of JeKo-1 and Z-138 cells
At 24 h, cell proliferation in the miR-212-3p mimic
group was not significantly different from that in the miR-NC group
(P>0.05; Fig. 4). However, at 48
and 72 h, the miR-212-3p mimic group exhibited significantly
decreased cell proliferation than the miR-NC group (P<0.05;
Fig. 4). The proliferation of
lymphatic cancer cells was slowed down, indicating that miR-212-3p
may affect the proliferation of lymphatic cancer cells by
inhibiting SOX11.
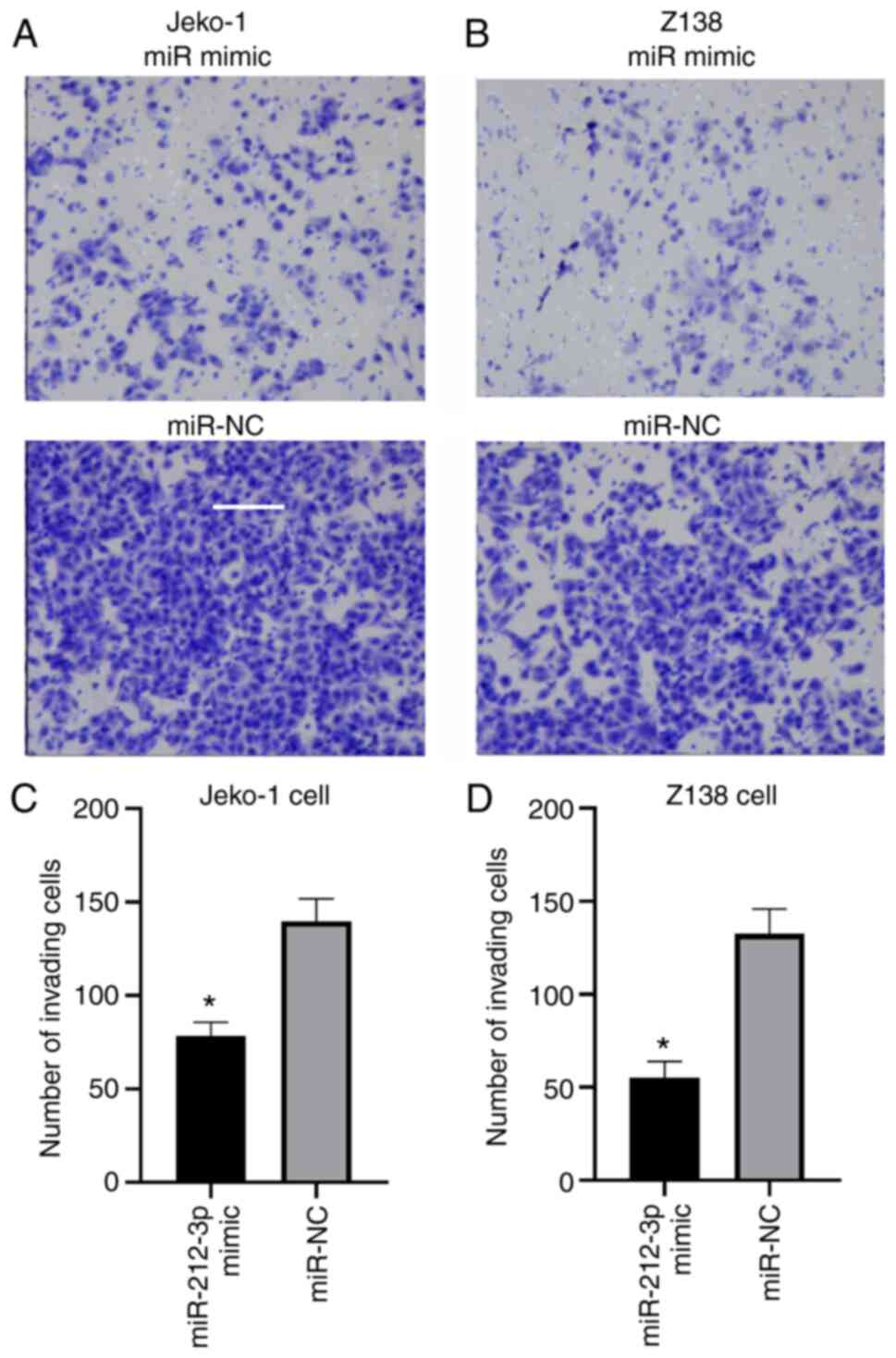
miR-212-3p overexpression decreases
the migration of JeKo-1 and Z-138 cells
The number of migrating cells in the miR-212-3p
mimic group was significantly lower than that in the miR-NC group
(JeKo-1, 78.56±7.25 vs. 139.78±11.98 cells; Z-138, 55.23±8.67 vs.
132.55±13.36; P<0.05; Fig. 5).
The slowing down of the migration rate of the lymphatic cancer
JeKo-1 and Z-138 cells indicated that miR-212-3p may affect the
migration of lymphatic cancer cells by inhibiting SOX11.
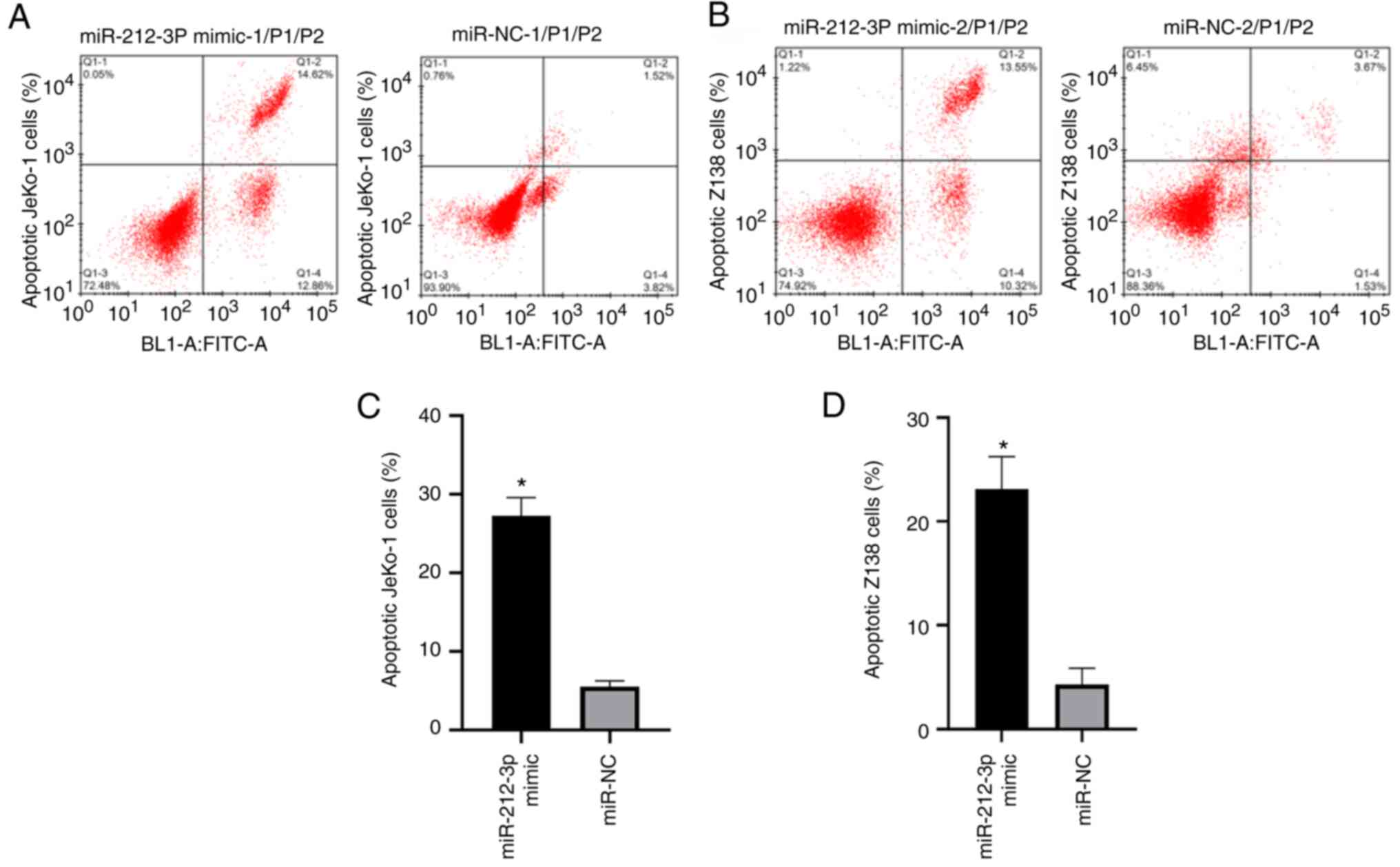
miR-212-3p overexpression increased
the apoptosis of JeKo-1 and Z-138 cells
The miR-212-3p mimic group exhibited a significantly
higher apoptotic rate than the miR-NC group (JeKo-1, 27.25±2.34%
vs. 5.51±0.74%; Z-138, 23.12±3.11% vs. 4.32±1.55%; P<0.001;
Fig. 6). The increase in the
apoptotic rate of the lymphatic cancer JeKo-1 and Z-138 cells
indicated that miR-212-3p may affect the apoptosis of lymphatic
cancer cells by inhibiting SOX11.
Discussion
At present, chemotherapy and immunotherapy for MCL
have greatly improved; however, an increasing number of patients
has been estimated to die of MCL recurrence after surgery (23). Thus, the treatment methods and
prognosis for patients with MCL should be urgently improved.
Targeted therapy against SOX11 may be a good method for MCL
management (24); therefore, the
present study aimed to explore the association between miR-212-3p
and SOX11.
In some lymphomas compared with normal tissue,
miR-212-3p expression is downregulated, whereas in MCL, SOX11
expression is upregulated (17,25).
Therefore, miR-212-3p and SOX11 may be negatively associated in
MCL. The results of the current study revealed that miR-212-3p
expression in cancerous tissues was significantly lower than that
in the paracancerous tissues, while SOX11 expression was
significantly increased in cancerous tissues compared with in
paracancerous tissues, suggesting that SOX11 and miR-212-3p may be
negatively associated.
According to previous studies, miR-212-3p hinders
the progression of some types of cancer, including pancreatic and
bladder cancer (19,26). The present study suggested a negative
association between SOX11 and miR-212-3p expression, although
whether this association was targeted was unclear. A study on
epilepsy has revealed that miR-212-3p inhibits further
deterioration of the disease by targeting SOX11 (27). Therefore, SOX11 expression was
detected in cells transfected with miR-212-3p mimics, revealing
that SOX11 expression was decreased following miR-212-3p
overexpression. Therefore, miR-212-3p and SOX11 exhibited a
targeting inhibitory association.
SOX11 expression has different roles in various
types of cancer (28). SOX11
overexpression inhibits the proliferation, invasion and apoptosis
of ovarian and prostate cancer cells (10,29).
However, SOX11 overexpression promotes the proliferation and
invasion in different head cell carcinoma cells, such as thyroid
carcinoma and lymphoma cells, such as MCL (30). By contrast, SOX11 inhibition leads to
the opposite effects (9,31,32).
Therefore, modulation of SOX11 expression has different effects in
various types of cancer. Moreover, according to the current
results, miR-212-3p inhibited SOX11 in a targeted manner. For
verification, the present study determined and analyzed the
targeted inhibition of SOX11 by miR-212-3p, and analyzed the
effects of miR-212-3p on the proliferation, migration and apoptosis
of lymphoma cells. Consequently, cells in the miR-212-3p mimic
group exhibited lower proliferation and migration, and higher
apoptotic rates than those in the miR-NC group. According to the
current results miR-212-3p may serve a targeted inhibitory role
against SOX11. miR-212-3p overexpression resulted in decreased
SOX11 expression, leading to decreased proliferation and migration
of lymphoma cells and elevated apoptosis. miR-212-3p specifically
inhibited SOX11. The current findings provide insights into novel
molecular therapy schemes. However, although high SOX11 expression
was demonstrated in cancerous tissues compared with in
paracancerous tissues, due to constraints in time and technologies,
experiments analyzing SOX11 expression under miR-212-3p inhibition,
as well as experiments to prove changes in cell proliferation,
migration and apoptosis under SOX11 overexpression were not
performed in the present study. Therefore, further studies should
be performed in the future to confirm the current results.
In conclusion, miR-212-3p inhibited cell
proliferation and migration, and promoted the apoptosis of lymphoma
cells by specifically regulating SOX11; thus, miR-212-3p may serve
as a novel therapeutic target and marker for lymphoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Research
Project of Xuzhou Medical College (grant no. 2018KJ11), the Natural
Science Foundation of Huai'an, Jiangsu (grant no. HAB201814),
Jiangsu Provincial Commission of Health and Family Planning (grant
no. H201556), the Six Top Talent Peak Projects in Jiangsu (grant
no. WSN-019) and the 333 Talent Project of Jiangsu Province (grant
no. BRA2017246).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YT, LW and GL conceived and designed the research,
and interpreted the results of the experiments. YZ and YF performed
the experiments. LL and XZ analyzed the data. GL prepared the
figures and drafted, edited and revised the manuscript. YT and LW
confirmed the authenticity of all the raw data. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Huai'an Hospital Affiliated
to Xuzhou Medical College and Huai'an Second People's Hospital
(Huai'an, China) approved the present study. All patients and their
families were informed of the study, and they signed informed
consent forms before the commencement of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cheah CY, Seymour JF and Wang ML: Mantle
cell lymphoma. J Clin Oncol. 34:1256–1269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Robak T, Huang H, Jin J, Zhu J, Liu T,
Samoilova O, Pylypenko H, Verhoef G, Siritanaratkul N, Osmanov E,
et al: Bortezomib-based therapy for newly diagnosed mantle-cell
lymphoma. N Engl J Med. 372:944–953. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vose JM: Mantle cell lymphoma: 2013 Update
on diagnosis, risk-stratification, and clinical management. Am J
Hematol. 88:1082–1088. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pérez-Galán P, Dreyling M and Wiestner A:
Mantle cell lymphoma: Biology, pathogenesis, and the molecular
basis of treatment in the genomic era. Blood. 117:26–38. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mohanty A, Sandoval N, Das M, Pillai R,
Chen L, Chen RW, Amin HM, Wang M, Marcucci G, Weisenburger DD, et
al: CCND1 mutations increase protein stability and promote
ibrutinib resistance in mantle cell lymphoma. Oncotarget.
7:73558–73572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dreyling M and Ferrero S; European Mantle
Cell Lymphoma Network, : The role of targeted treatment in mantle
cell lymphoma: Is transplant dead or alive? Haematologica.
101:104–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beà S and Amador V: Role of SOX11 and
genetic events cooperating with cyclin D1 in mantle cell lymphoma.
Curr Oncol Rep. 19:432017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lord M, Wasik AM, Christensson B and
Sander B: The utility of mRNA analysis in defining SOX11 expression
levels in mantle cell lymphoma and reactive lymph nodes.
Haematologica. 100:e369–e372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Palomero J, Vegliante MC, Eguileor A,
Rodriguez ML, Balsas P, Martinez D, Campo E and Amador V: SOX11
defines two different subtypes of mantle cell lymphoma through
transcriptional regulation of BCL6. Leukemia. 30:1596–1599. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang G, Liu J, Wang Q, Huang X, Yang R,
Pang Y and Yang M: MicroRNA-223-3p regulates ovarian cancer cell
proliferation and invasion by targeting SOX11 expression. Int J Mol
Sci. 18:12082017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shepherd JH, Uray IP, Mazumdar A,
Tsimelzon A, Savage M, Hilsenbeck SG and Brown PH: The SOX11
transcription factor is a critical regulator of basal-like breast
cancer growth, invasion, and basal-like gene expression.
Oncotarget. 7:13106–13121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Palomero J, Vegliante MC, Rodríguez ML,
Eguileor Á, Castellano G, Planas-Rigol E, Jares P, Ribera-Cortada
I, Cid MC, Campo E and Amador V: SOX11 promotes tumor angiogenesis
through transcriptional regulation of PDGFA in mantle cell
lymphoma. Blood. 124:2235–2247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Balsas P, Palomero J, Eguileor Á,
Rodríguez ML, Vegliante MC, Planas-Rigol E, Sureda-Gómez M, Cid MC,
Campo E and Amador V: SOX11 promotes tumor protective
microenvironment interactions through CXCR4 and FAK regulation in
mantle cell lymphoma. Blood. 130:501–513. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, Gee H, Rose B, Lee CS, Clark J,
Elliott M, Gamble JR, Cairns MJ, Harris A, Khoury S and Tran N:
Regulation of the tumour suppressor PDCD4 by miR-499 and miR-21 in
oropharyngeal cancers. BMC Cancer. 16:862016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Guo Q, Yang P and Long G:
Restoration of microRNA-212 causes a G0/G1 cell cycle arrest and
apoptosis in adult T-cell leukemia/lymphoma cells by repressing
CCND3 expression. J Investig Med. 65:82–87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ambrosio MR, Mundo L, Gazaneo S,
Picciolini M, Vara PS, Sayed S, Ginori A, Lo Bello G, Del Porro L,
Navari M, et al: MicroRNAs sequencing unveils distinct molecular
subgroups of plasmablastic lymphoma. Oncotarget. 8:107356–107373.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ju C, Zhou R, Sun J, Zhang F, Tang X, Chen
KK, Zhao J, Lan X, Lin S, Zhang Z and Lv XB: LncRNA SNHG5 promotes
the progression of osteosarcoma by sponging the miR-212-3p/SGK3
axis. Cancer Cell Int. 18:1412018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu H, Li C, Shen C, Yin F, Wang K, Liu Y,
Zheng B, Zhang W, Hou X, Chen X, et al: miR-212-3p inhibits
glioblastoma cell proliferation by targeting SGK3. J Neurooncol.
122:431–439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hou P, Kang Y and Luo J: Hypoxia-mediated
miR-212-3p downregulation enhances progression of intrahepatic
cholangiocarcinoma through upregulation of Rab1a. Cancer Biol Ther.
19:984–993. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan S, Dong Y, Peng L, Yang M, Niu L, Liu
Z and Xie J: Tumor-associated macrophages affect the biological
behavior of lung adenocarcinoma A549 cells through the PI3K/AKT
signaling pathway. Oncol Lett. 18:1840–1846. 2019.PubMed/NCBI
|
|
23
|
Danilov AV and Persky DO: Incorporating
acalabrutinib, a selective next-generation Bruton tyrosine kinase
inhibitor, into clinical practice for the treatment of
haematological malignancies. Br J Haematol. 193:15–25. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuo PY, Leshchenko VV, Fazzari MJ, Perumal
D, Gellen T, He T, Iqbal J, Baumgartner-Wennerholm S, Nygren L,
Zhang F, et al: High-resolution chromatin immunoprecipitation
(ChIP) sequencing reveals novel binding targets and prognostic role
for SOX11 in mantle cell lymphoma. Oncogene. 34:1231–1240. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beekman R, Amador V and Campo E: SOX11, a
key oncogenic factor in mantle cell lymphoma. Curr Opin Hematol.
25:299–306. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia L, Li D, Lin C, Ou S, Li X and Pan S:
Comparative study of joint bioinformatics analysis of underlying
potential of ‘neurimmiR’, miR-212-3P/miR-132-3P, being involved in
epilepsy and its emerging role in human cancer. Oncotarget.
8:40668–40682. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haenisch S, Zhao Y, Chhibber A,
Kaiboriboon K, Do LV, Vogelgesang S, Barbaro NM, Alldredge BK,
Lowenstein DH, Cascorbi I and Kroetz DL: SOX11 identified by target
gene evaluation of miRNAs differentially expressed in focal and
non-focal brain tissue of therapy-resistant epilepsy patients.
Neurobiol Dis. 77:127–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Z, Jiang S, Lu C, Ji T, Yang W, Li T,
Lv J, Hu W, Yang Y and Jin Z: SOX11: Friend or foe in tumor
prevention and carcinogenesis? Ther Adv Med Oncol.
11:17588359198534492019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yao Z, Sun B, Hong Q, Yan J, Mu D, Li J,
Sheng H and Guo H: The role of tumor suppressor gene SOX11 in
prostate cancer. Tumour Biol. 36:6133–6138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsang SM, Oliemuller E and Howard BA:
Regulatory roles for SOX11 in development, stem cells and cancer.
Semin Cancer Biol. 67:3–11. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang J, Ji EH, Zhao X, Cui L, Misuno K,
Guo M, Huang Z, Chen X and Hu S: Sox11 promotes head and neck
cancer progression via the regulation of SDCCAG8. J Exp Clin Cancer
Res. 38:1382019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang L, Shen YF, Shi ZM, Shang XJ, Jin DL
and Xi F: Overexpression miR-211-5p hinders the proliferation,
migration, and invasion of thyroid tumor cells by downregulating
SOX. J Clin Lab Anal. 32:e222932018. View Article : Google Scholar : PubMed/NCBI
|