Introduction
Colon cancer is one of the most commonly diagnosed
types of cancer worldwide and was ranked 4th among the most
commonly diagnosed cancers in 2018 (1). Thus, colon cancer represents a major
global public health concern. Conventional anticancer therapies,
including surgery and chemotherapy, remain the most effective
strategies for treatment; however, drug resistance develops in the
majority of patients receiving chemotherapy (2). Colon cancer has poor clinical outcomes.
Therefore, there is an urgent requirement to identify alternative
anticancer agents to improve future treatment.
Osthole is a natural coumarin-derivative and
bioactive compound extracted from the fruit of Cnidium
monnieri (3). Its chemical
formula is C15H16O3. It has been
reported that osthole exhibited a broad range of pharmacological
activities, including anti-osteoporotic, anti-inflammatory,
cardiovascular and neuroprotective properties (4–7), as well
as having anticancer effects, which have been demonstrated in
certain types of cancer cells, such as breast, ovarian and lung
cancer cells (3,8,9). In
addition, osthole induced cell death in human HCT116 and SW480
colon cancer cell lines (10).
Osthole exerts anticancer effects by inhibiting cell proliferation
and invasion, which may be associated with the induction of
apoptosis and cell cycle arrest (11). However, the target of osthole-induced
apoptosis of human HT-29 colorectal cancer cell line remains
unclear.
Substantial efforts have been made to determine the
molecular mechanisms that underlie cancer development and
progression. Apoptosis and autophagy are 2 types of programmed cell
death (12). Autophagy is induced in
response to various stresses that ultimately lead to apoptosis and
remove unnecessary or dysfunctional cytoplasmic components;
therefore, autophagy plays an important role in various cellular
functions, such as proliferation, apoptosis and
epithelial-mesenchymal transition (8). The endoplasmic reticulum (ER) is the
foremost intracellular compartment of the secretory pathway in
eukaryotic cells (13). Disruption
of ER homeostasis causes the accumulation of misfolded/unfolded
proteins in the ER lumen, which contributes to ER stress (ERS). The
unfolded protein response (UPR) is activated in response to
increased ERS, and orchestrates the recovery of homeostasis or
triggers apoptosis, depending on the degree and duration of damage
or stress (14–16). The UPR is governed by the action of 3
signaling proteins/transmembrane ERS sensors, namely
inositol-requiring enzyme 1α (IRE1α), protein kinase R (PKR)-like
ER kinase (PERK) and activating transcription factor 6 (ATF6)
(17). Persistent and severe ERS can
switch the cytoprotective functions of UPR and autophagy into cell
death programs (18).
As a potential anticancer agent, the effects of
osthole on the apoptosis of colorectal cancer cells and the
underlying mechanisms are poorly understood. The present study
aimed to investigate the effects of osthole treatment on HT-29
cells with respect to its possible role in ERS, autophagy and
apoptosis.
Materials and methods
Cell culture and treatments
The human HT-29 colorectal cancer cell line was
purchased from Procell Life Science & Technology Co., Ltd.
(cat. no. CL-0118), and authenticated using STR profiling. The
cells were cultured in Dulbeccos modified Eagle's medium (DMEM),
supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml
penicillin and 100 µg/ml streptomycin (all from Gibco; Thermo
Fisher Scientific, Inc.). Then, the cells were maintained at 37°C
in a humidified atmosphere with 5% CO2.
Osthole was purchased from Shanghai Aladdin
Biochemical Technology Co., Ltd., (cat. no. O101698) and dissolved
in DMSO (Sigma-Aldrich; Merck KGaA), then diluted in DMEM to the
desired final concentration (100, 50 and 25 µM). The HT-29 cell
line was randomly divided into 4 different treatment groups: i)
HT-29 cells were treated with 0.1% DMSO as the control and 100, 50
and 25 µM osthole for 24 h separately. All subsequent experiments
were performed using 50 µM osthole. ii) HT-29 cells were treated
with 10 mM 3-methyladenine (3MA; Sigma-Aldrich; Merck KGaA),
osthole and 3MA + osthole for 24 h separately, with 0.1% DMSO as
the control. iii) HT-29 cells were treated with 10 mM
4-phenylbutyric acid (4-PBA; Sigma-Aldrich; Merck KGaA), osthole
and 4-PBA + osthole for 24 h separately, with 0.1% DMSO as the
control. iv) HT-29 cells were treated with osthole, 3MA, 3MA +
osthole, 4-PBA, 4-PBA + osthole and 3MA + osthole + 4-PBA for 24 h
separately, with 0.1% DMSO as the control. The concentrations of
3MA, 4-PBA and osthole were referenced to the doses most commonly
used in the other experimental studies (19–21).
Colony formation assay
The effects of osthole on the proliferation of the
HT-29 cell line was measured using colony formation analysis. The
HT-29 cells, treated as aforementioned, were seeded into 6-well
culture plates separately, then cultured under normal conditions
for 7 days to form colonies. Subsequently, the colonies were fixed
with 4% paraformaldehyde (Sigma-Aldrich; Merck KGaA) for 30 min and
stained with 0.1% crystal violet (Sigma-Aldrich; Merck KGaA) for 20
min at room temperature. Images were captured with a digital camera
and colonies ~0.3–1.0 mm in size were counted.
Cell viability assay
The HT-29 cells were seeded into 96-well culture
plates, at a density of 5×103 per well. Following
treatment as aforementioned for the first 3 groups, cell viability
was detected using a Cell Counting Kit-8 (CCK-8; Beyotime Institute
of Biotechnology), according to the manufacturer's instructions; 10
µl CCK-8 solution was added and incubated for 1 h at 37°C. The
absorbance was measured at 450 nm using a microplate
spectrophotometer (cat. no. 1681150; Bio-Rad Laboratories, Inc.)
and cell viability was calculated using the following formula: Cell
viability
(%)=(Aexperimental-Ablank)/(Acontrol-Ablank)
× 100%.
Flow cytometry
The effects of osthole on the early and late
apoptosis of HT-29 cells was determined by flow cytometry.
Following all four different treatments, the HT-29 cells were
harvested, washed twice with PBS (Sigma-Aldrich; Merck KGaA),
centrifuged at 300 × g for 5 min at room temperature. The next
operation was performed using the apoptosis kit (Nanjing KeyGen
Biotech Co., Ltd.); the cells were gently resuspended in binding
buffer (500 µl) and incubated with Annexin V-APC (5 µl) and PI (5
µl) in the dark for 15 min. The cells were analyzed using a
FACSCalibur™ flow cytometer (BD Biosciences) and FlowJo software
version 10.6.2 (Tree Star, Inc.).
Western blot analysis
Following different treatments, the HT-29 cells were
collected. Total cellular protein was obtained by lysing the cells
with RIPA buffer (cat. no. R0020) and the protein concentration was
performed using a BCA Protein Assay kit (cat. no. PC0020) (both
from Beijing Solarbio Science & Technology Co., Ltd.). The
protein samples were denatured, and 50 µg total protein samples
were separated using 10% SDS-PAGE, and subsequently electroblotted
onto PVDF membranes (EMD Millipore). The membranes were blocked for
2 h at room temperature with TBS and 0.05% Tween-20 (TBST),
containing 5% skimmed milk, then the membranes were incubated
overnight at 4°C with anti-β-actin (1:1,000; cat. no. ab8227;
Abcam), anti-Bax (1:1,000; cat. no. ab53154; Abcam), anti-Bcl-2
(1:1,000; cat. no. ab59348; Abcam), anti-cleaved caspase-3
(1:1,000; cat. no. ab2302; Abcam), anti-p62 (1:500; cat. no.
ab155686; Abcam), anti-microtubule-associated protein light chain 3
(LC3; 1:1,000; cat. no. 12741; Cell Signaling Technology, Inc.),
anti-CHOP (1:1,000; cat. no. 2895; Cell Signaling Technology,
Inc.), anti-PERK (1:1,000; cat. no. 5683; Cell Signaling
Technology, Inc.), anti-phosphorylated (p)-PERK (1:1,000; cat. no.
3179; Cell Signaling Technology, Inc.), anti-eukaryotic initiation
factor 2 (eIF2)α (1:1,000; cat. no. 5324; Cell Signaling
Technology, Inc.), anti-p-eIF2α (1:1,000; cat. no. 3398; Cell
Signaling Technology, Inc.) and anti-78 kDa glucose-regulated
protein (GRP78; 1:1,000; ProteinTech Group, Inc.) antibodies. After
washing 3 times with TBST, the membranes were incubated with a
HRP-conjugated secondary antibody (1:2,000; cat. no. sc-2748; Santa
Cruz Biotechnology, Inc.) for 2 h at room temperature. The proteins
on the membranes were visualized with an enhanced chemiluminescence
detection kit (Bio-Rad Laboratories, Inc.) using a ChemiScope 6000
imaging system (Clinx Science Instruments Co., Ltd.). Densitometry
was performed using ImageJ software version 1.8.0 (National
Institutes of Health).
Statistical analysis
All experiments were independently repeated at least
3 times and all statistical analyses were performed using SPSS
software version 20.0 (IBM Corp.). All the data are presented as
the mean ± SD. Statistical comparisons were performed using one-way
ANOVA and Tukey's post hoc test for differences among multiple
groups and an unpaired t-test for differences between 2 groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
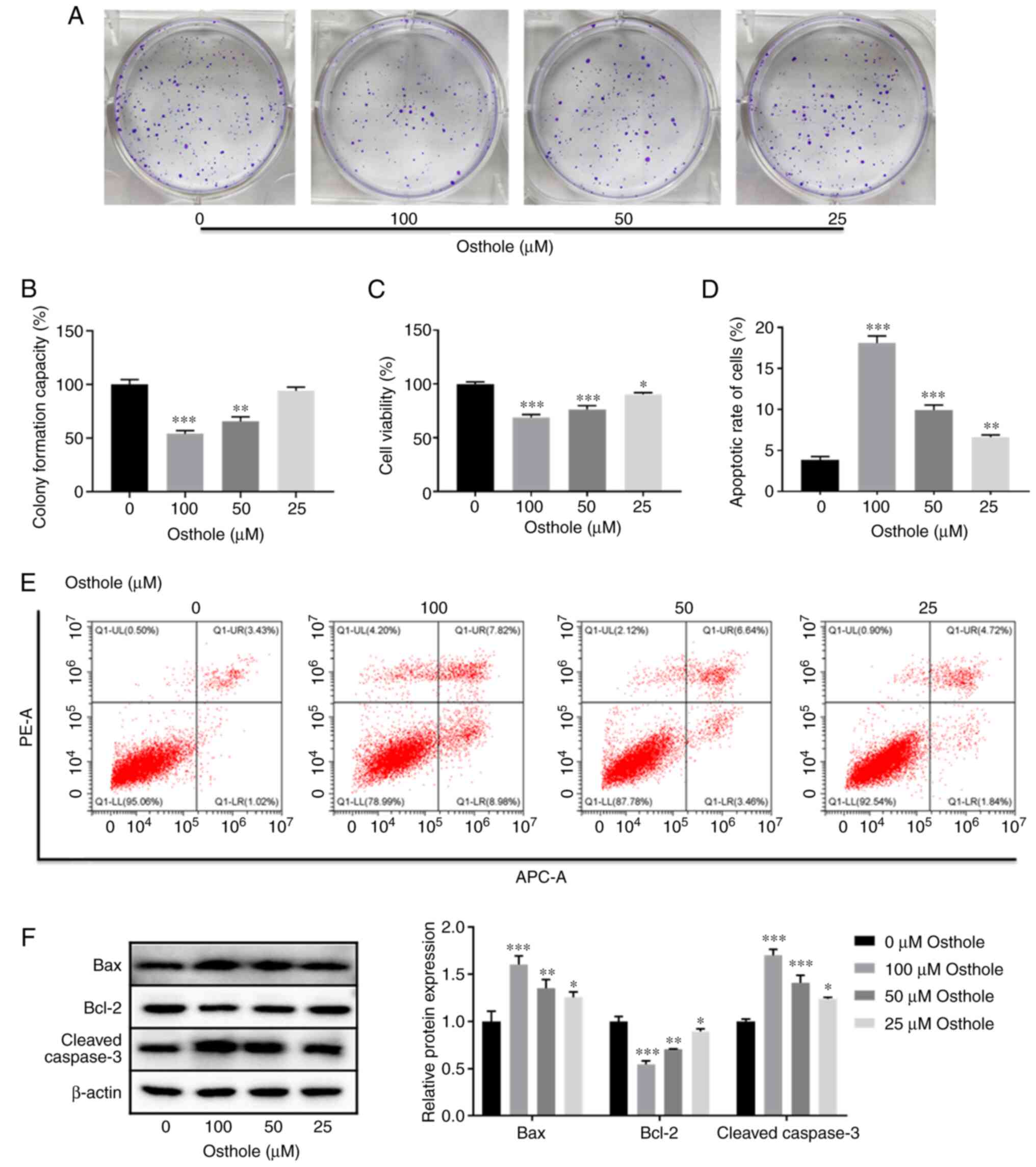
Osthole effectively suppresses
proliferation and induces apoptosis in the HT-29 cell line
Osthole has been reported to exert inhibitory
effects on several human cancer cells (3,8–9), and to have anticancer effects on human
HCT116 and SW480 colon cancer cells (10). To investigate whether osthole has a
similar inhibitory role in HT-29 colorectal cells, proliferation
and apoptosis were investigated. The results of the colony
formation assay revealed that osthole, at 50 µM, exerted a
significant inhibitory effect on the proliferation of the HT-29
cells and there was also a dose-dependent inhibitory effect
(P<0.001) (Fig. 1A and B).
Similarly, the CCK-8 assay also showed that osthole significantly
inhibited cell viability in a dose-dependent manner (P<0.05;
Fig. 1C).
As osthole treatment reduced the viability of the
HT-29 cells, it was subsequently investigated whether osthole could
induced apoptosis. The results of flow cytometry revealed that the
apoptotic rate of the HT-29 cell line was significantly increased,
in a dose-dependent manner (P<0.01) (Fig. 1D and E). This finding was further
confirmed following detection of the expression level of
apoptosis-related proteins using western blot analysis. It was
demonstrated that the protein expression levels of Bax and cleaved
caspase-3 were significantly increased, whereas the protein
expression level of Bcl-2 was significantly decreased, in a
dose-dependent manner (P<0.05; Fig.
1F). These results suggested that osthole inhibited the
proliferation and induced apoptosis in the HT-29 cell line.
Osthole activates autophagy and ERS in
the HT-29 cell line
Autophagy and ERS play important roles in cell
apoptosis (22,23); therefore, the effects of osthole on
autophagy and ERS were detected using western blot analysis. The
results demonstrated that the protein expression level of p62 was
significantly decreased and the protein expression level of
LC3-II/LC3-I was significantly increased following osthole
treatment, in a dose-dependent manner (P<0.05; Fig. 2A). In addition, the protein
expression level of GRP78 was significantly increased following
treatment with 25 µM osthole (P<0.05), and the protein
expression levels of p-PERK/PERK, p-elF2α/elF2α and CHOP were
significantly increased following treatment with 50 µM osthole
(P<0.05; Fig. 2B). These results
showed that osthole induced autophagy and ERS in the HT-29 cell
line.
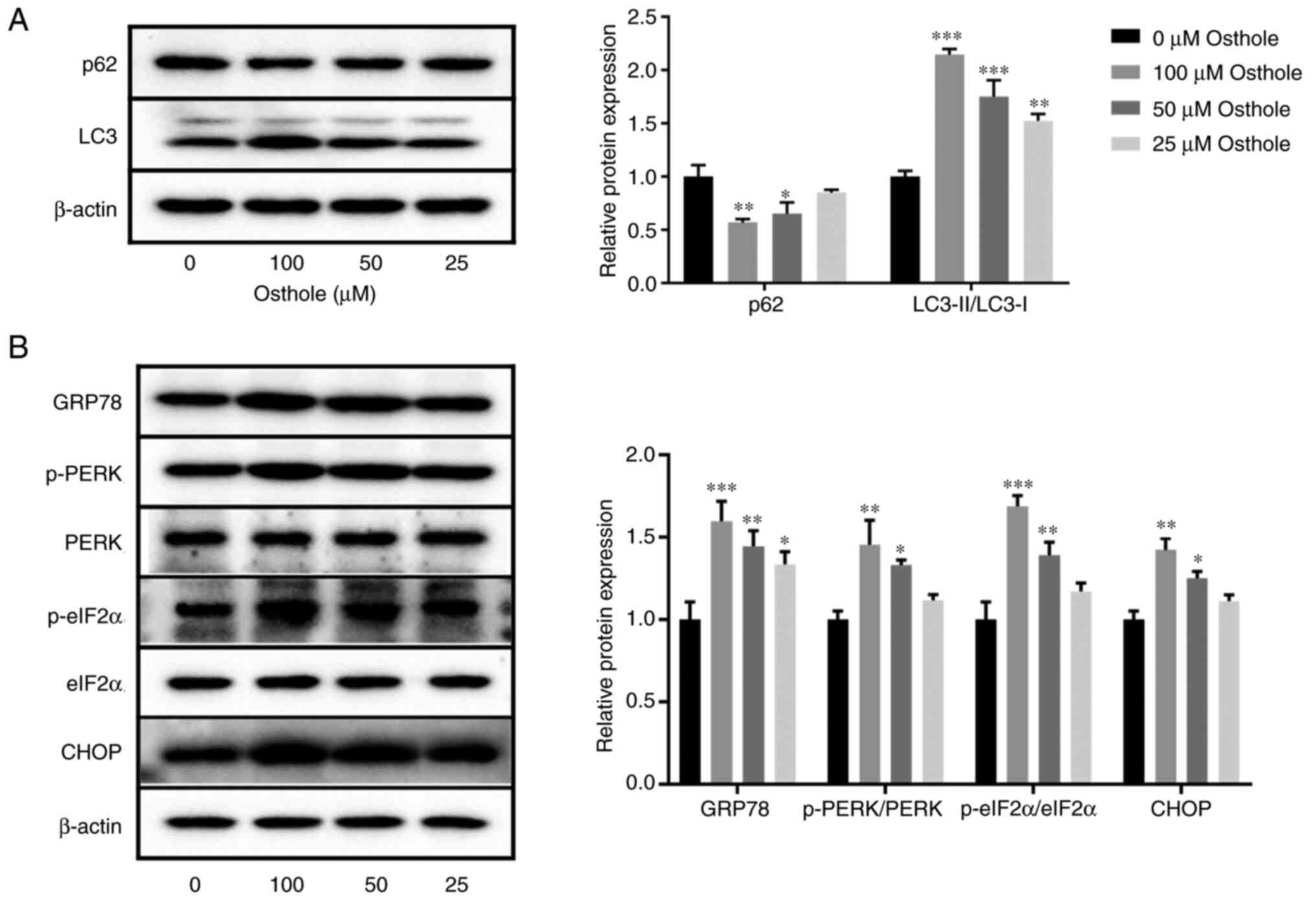 | Figure 2.Effects of osthole on the protein
expression levels of autophagy- and endoplasmic reticulum
stress-related markers in the HT-29 cell line. The protein
expression levels of (A) p62 and LC3-II/LC3-I, (B) GRP78,
p-PERK/PERK, p-elF2α/elF2α and CHOP were determined using western
blot analysis. β-actin was used as a loading control. *P<0.05,
**P<0.01, ***P<0.001 vs. control group (0 µM). LC3,
microtubule-associated protein light chain 3; GRP78, 78 kDa
glucose-regulated protein; p-, phosphorylated; PERK, protein kinase
R (PKR)-like endoplasmic reticulum kinase; elF2α, eukaryotic
initiation factor 2α. |
Effects of autophagy on
osthole-induced HT-29 cell apoptosis
To further determine the role of autophagy in
osthole-induced HT-29 cell apoptosis, the HT-29 cell line was
treated with osthole and the autophagy inhibitor, 3MA, and cellular
proliferation and viability was examined using colony formation and
CCK-8 assays, respectively. As shown in Fig. 3A and B, compared with control group,
osthole inhibited cell proliferation (P<0.05), whereas 3MA
increased the proliferation of HT-29 cells, but not significantly
(P>0.05). Notably, co-treatment with osthole and 3MA
significantly inhibited cell proliferation compared with that in
the 3MA group (P<0.05); however, there were no significant
differences compared with that in the osthole group (P>0.05).
Similarly, the CCK-8 assay also revealed that osthole significantly
inhibited cell viability compared with that in the control group
(P<0.001), and there was a significant decrease in cell
viability between the 3MA and 3MA + osthole groups (P<0.001).
However, co-treatment with osthole and 3MA did not significantly
inhibit cell viability compared with that in the osthole group
(P>0.05; Fig. 3C). Furthermore,
the apoptosis of the HT-29 cells was examined using flow cytometry
and it was found that the apoptosis of the HT-29 cells co-treated
with osthole and 3MA was significantly higher compared with that in
cells treated with 3MA alone (P<0.01), and significantly lower
compared with that in the osthole treatment alone group (P<0.01;
Fig. 3D and E), indicating that the
inhibition of autophagy by 3MA could attenuate osthole-induced cell
apoptosis.
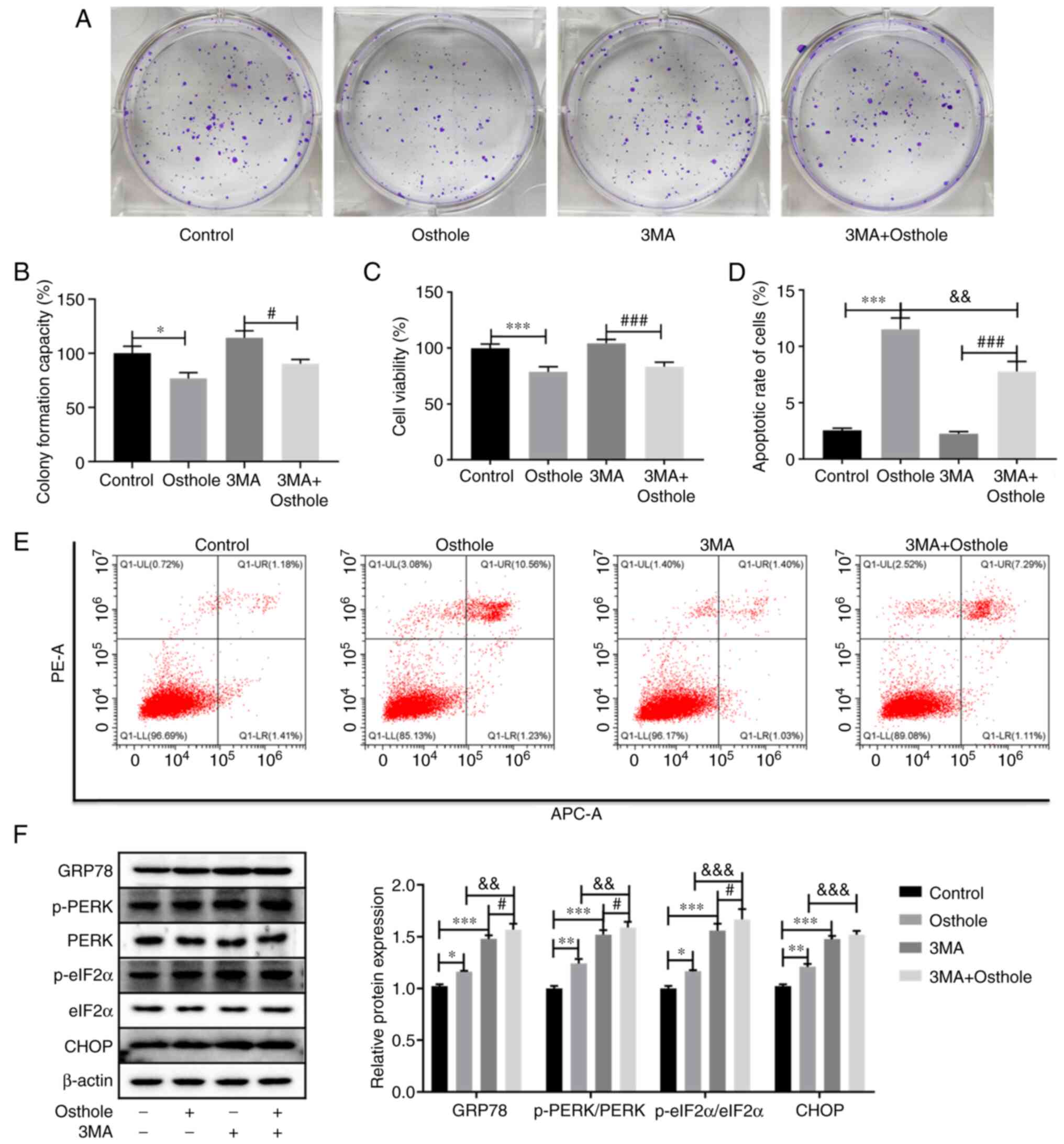 | Figure 3.Inhibition of autophagy by 3MA
modulates osthole-induced apoptosis and endoplasmic reticulum
stress in the HT-29 cell line. (A and B) Cell proliferation was
measured using a colony formation assay. (C) Cell viability was
measured using a Cell Counting Kit-8 assay. (D and E) Apoptosis
analysis was performed using flow cytometry. (F) The protein
expression levels of GRP78, p-PERK/PERK, p-elF2α/elF2α and CHOP
were determined using western blot analysis, and β-actin was used
as a loading control. *P<0.05, **P<0.01, ***P<0.001 vs.
control group. #P<0.05, ###P<0.001 vs.
3MA group. &&P<0.01,
&&&P<0.001 vs. osthole group. GRP78, 78
kDa glucose-regulated protein; p-, phosphorylated; PERK, protein
kinase R (PKR)-like endoplasmic reticulum kinase; elF2α, eukaryotic
initiation factor 2α; 3MA, 3-methyladenine. |
The present study also examined the changes of ERS
in the HT-29 cells treated with osthole and 3MA. The results
revealed that the protein expression levels of GRP78, p-PERK/PERK,
p-elF2α/elF2α and CHOP were significantly increased following
osthole or 3MA treatment alone, compared with that in the control
group (P<0.05). Furthermore, the HT-29 cells treated with
osthole and 3MA exhibited significantly increased protein
expression level of GRP78, p-PERK/PERK and p-elF2α/elF2α compared
with that in the osthole or 3MA alone treatment groups (P<0.01),
while the protein expression level of CHOP was significantly
increased compared with that in the osthole group (Fig. 3E). These results demonstrated that
inhibition of autophagy by 3MA may enhance osthole-induced ERS.
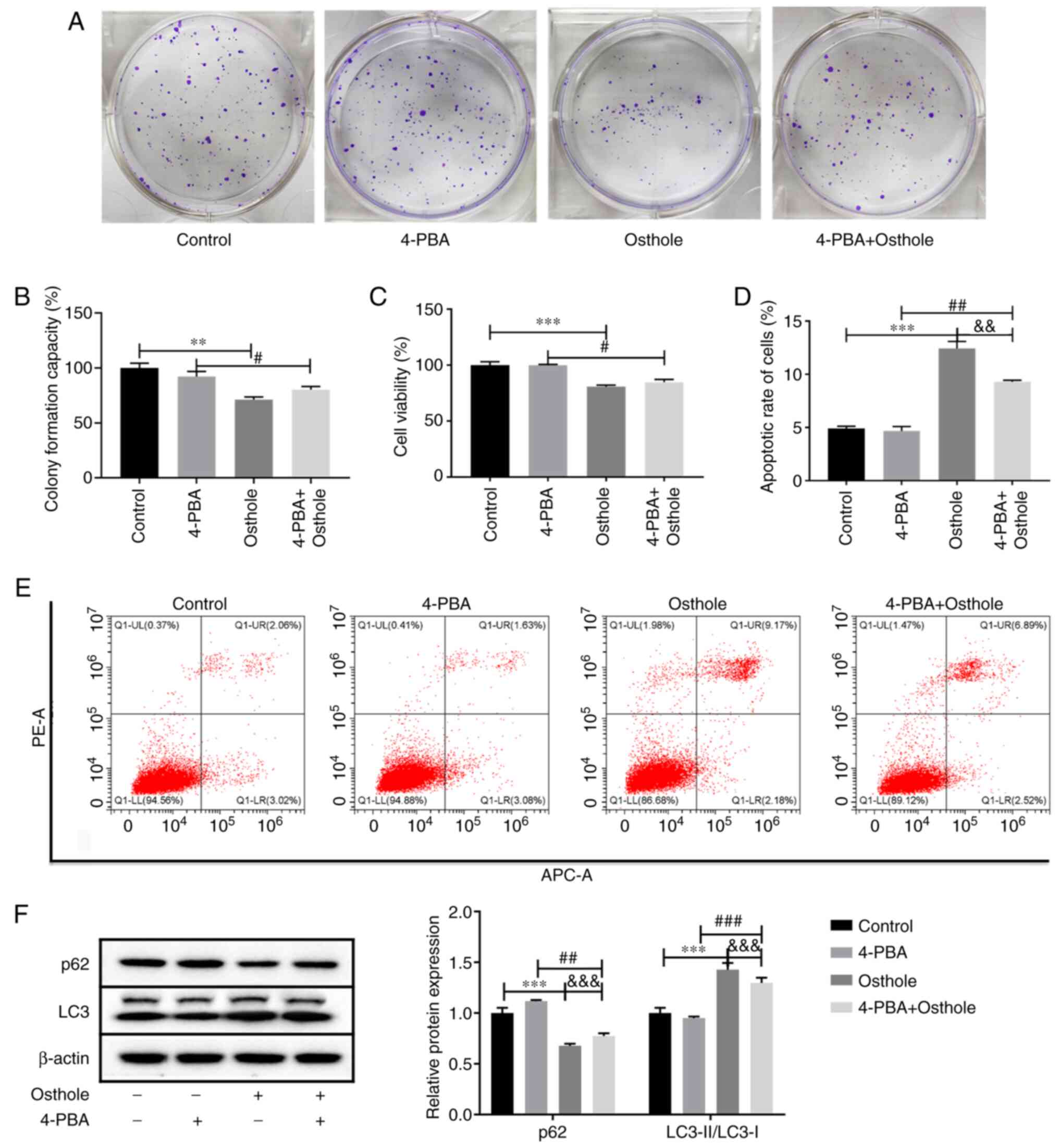
Effects of osthole combined with the
ERS inhibitor, 4-PBA, on the HT-29 cells
It was observed that inhibition of autophagy reduced
apoptosis and enhanced ERS induced by osthole. To further
characterize the role of ERS in osthole-induced apoptosis and
autophagy, the ERS inhibitor, 4-PBA, was used to treat the HT-29
cells, and colony formation (Fig. 4A and
B) and CCK-8 (Fig. 4C) assays
were performed to detect cell proliferation and viability,
respectively. The results revealed that 4-PBA had no significant
effects on cell proliferation and viability compared with that in
the control group, and the co-treatment of 4-PBA and osthole also
had no significant effects on cell proliferation and viability
compared with that in the osthole group (P>0.05). However, the
proliferation and viability of the HT-29 cells was significantly
decreased following osthole and 4-PBA co-treatment compared with
that in the 4-PBA group (P<0.05). In addition, the effects of
osthole and 4-PBA co-treatment on the apoptosis of the HT-29 cells
was examined using flow cytometry (Fig.
4D and E), and it was found that 4-PBA had no significant
effects on apoptosis, whereas cells co-treated with osthole and
4-PBA exhibited significantly higher levels of apoptosis compared
with that in the 4-PBA group and lower levels compared with that in
the osthole group (P<0.01). Furthermore, the protein expression
levels of autophagy-related proteins were detected using western
blot analysis (Fig. 4F). The results
demonstrated that 4-PBA had no significant effects on the protein
expression levels of p62 and LC3-II/LC3-I (P>0.05). However,
following co-treatment with osthole and 4-PBA, the protein
expression level of p62 was significantly lower compared with that
in the 4-PBA group and significantly higher compared with that in
the osthole group. Furthermore, the protein expression level of
LC3-II/LC3-I was significantly increased compared with that in the
4-PBA group and significantly decreased compared with that in the
osthole group (P<0.01), indicating that suppression of ERS with
4-PBA attenuated osthole-induced cell apoptosis and autophagy.
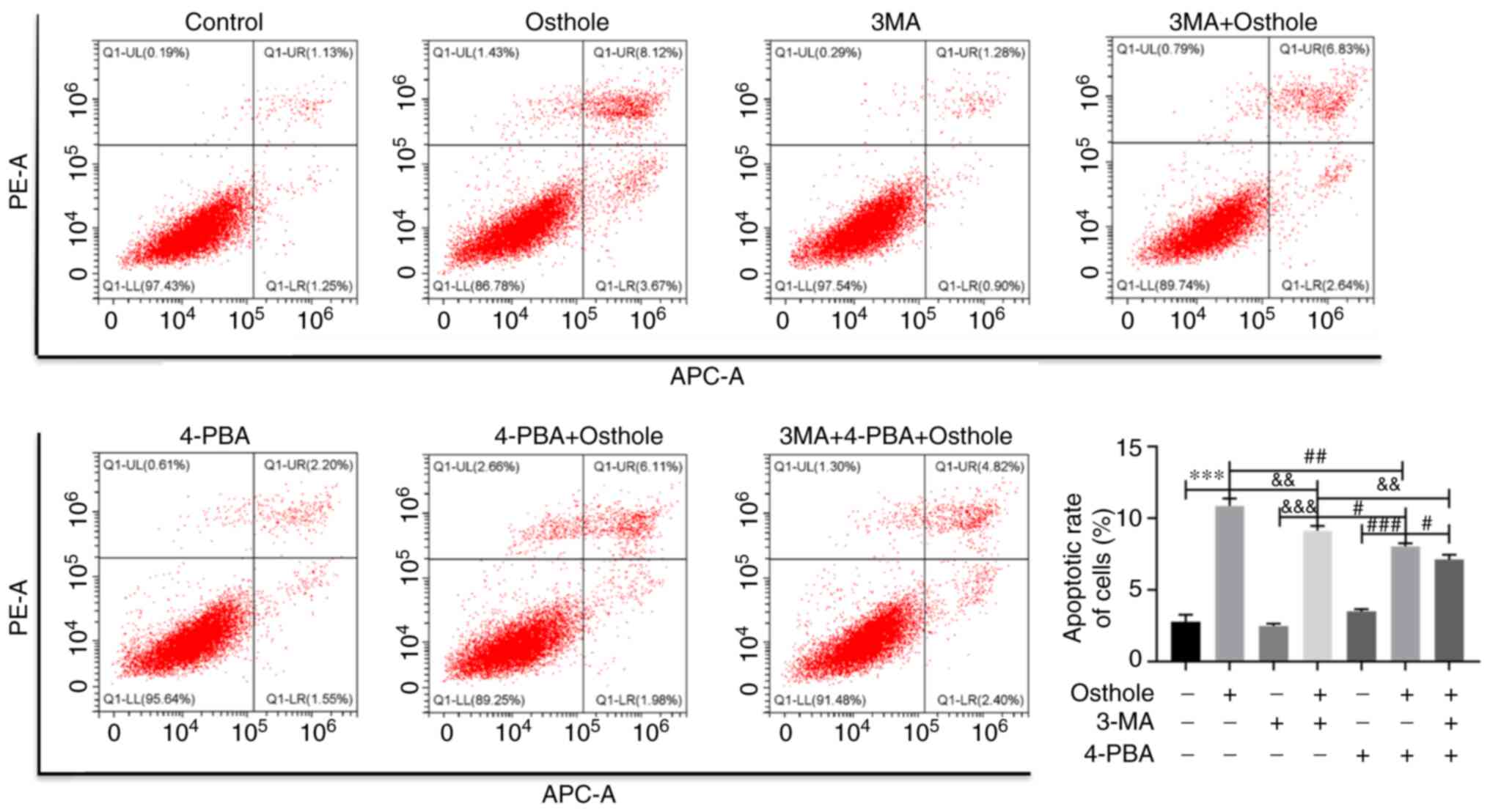
Combined effect of osthole, 3MA and
4-PBA on apoptosis in the HT-29 cells
Since the inhibition of autophagy and suppression of
ERS were found to attenuate osthole-induced cell apoptosis
separately, the present study further analyzed the combined effects
of osthole, 3MA and 4-PBA on the apoptosis of the HT-29 cells using
flow cytometry (Fig. 5). It was
found that cells co-treated with osthole, 3MA and 4-PBA exhibited
significantly lower levels of apoptosis compared with that in the
3MA and osthole or 4-PBA and osthole groups (P<0.05). These
results demonstrated that inhibition of autophagy and ERS, at the
same time, significantly attenuated osthole-induced colorectal
cancer cell apoptosis. Of note, 4-PBA and osthole treatment reduced
apoptosis more significantly compared with that in cells treated
with 3MA and osthole (P<0.05), suggesting that suppression of
ERS with 4-PBA played a more important role in alleviating
osthole-induced cell apoptosis.
Discussion
Due to its resistance to anticancer agents, colon
cancer has poor outcomes in the clinical setting (2). Therefore, identifying effective drugs
for the treatment of colon cancer is crucial. The ability to induce
apoptosis has been accepted as a mechanism of action for anticancer
drugs, and most of the conventional anticancer drugs are apoptosis
inducers, including cisplatin, oxaliplatin and cyclophosphamide
(24). Therefore, identifying new
agents that induce apoptosis in cancer cells offers potentially
useful approaches to improving patient responses to conventional
chemotherapy (9).
Accumulating evidence has shown that osthole exerts
anticancer effects in different cell lines (3,8,9), yet its preclinical significance and
biological role in colon cancer remains unclear. Huang et al
(10) found that osthole reduced the
viability of the human colon cancer cells, HCT116 and SW480.
Consistent with these results, in the present study, osthole
inhibited the proliferation and viability of the HT-29 cell line in
a dose-dependent manner. Apoptosis occurs via 2 signaling pathways:
The mitochondrial (intrinsic) pathway and the death receptor
(extrinsic) pathway. The Bcl-2 protein family regulates the
intrinsic pathway by controlling outer mitochondrial membrane
integrity (25). Bcl-2 and Bax are
important proteins in the Bcl-2 family, which exert anti- and
pro-apoptotic effects, respectively. Upregulation of Bax and
cleaved caspase-3, and downregulation of Bcl-2 play key roles in
inducing cell apoptosis (26,27).
Similarly, apoptosis was observed in osthole-treated HT-29 cells in
the present study. Furthermore, osthole reduced Bcl-2 and promoted
cleaved caspase-3 activation. Therefore, the results of the present
study suggested that osthole may induce apoptosis of the HT-29
cells via the intrinsic pathway, which has also been observed in
human breast cancer, lung cancer and colon carcinoma cells
(3,9,10).
Autophagy, or type II cell death, is an essential
cellular process responsible for the degradation of organelles,
proteins and other cytoplasmic components such as the cytoplasmic
membrane. It fulfils a dual role in all types of cancer, including
colon cancer, with both tumor-promoting and tumor-suppressing
properties (28,29). LC3 includes 2 forms (LC3-I and
LC3-II), in which LC3-II is the autophagosome-associated form,
which is converted from the cytosolic form, LC3-I (8). The turnover of LC3-II is often used as
a marker of autophagic activity (28). p62, also known as sequestosome 1, is
an adaptor protein that binds to LC3, which is also a substrate for
selective autophagy and mitophagy (30). Total p62 protein expression level is
negatively associated with autophagic flux (31). The accumulation of p62 is critical
for tumorigenesis and p62 has been found to be highly expressed in
colon cancer tissues (32,33). In the present study, it was found
that the ratio of LC3-II/LC3-I was significantly increased, whereas
the protein expression level of p62 was decreased following osthole
treatment. Osthole was shown to induce autophagy in human ovarian
cancer cells (8). The results of the
current study suggested that osthole promoted autophagy and p62
degradation in human colorectal cancer cells.
The UPR is governed by the action of 3 ER sensors:
PERK, IRE1α and ATF6. Under conditions of ERS, GRP78 is released
from these 3 sensors (34).
Dimerization and subsequent phosphorylation of PERK activates PERK,
so it can phosphorylate eIF2α and induce the translation of ATF4,
which consequently enhances the transcription of CHOP, a
proapoptotic transcription factor (18,35,36).
Osthole has been shown to activate the ERS signaling pathway in
normal human hepatocytes and breast cancer cells (21,37).
Similarly, the results of the present study demonstrated that the
ERS signaling proteins, GRP78, p-PERK/PERK, p-elF2α/elF2α and CHOP,
were upregulated following osthole treatment in the HT-29 cell
line.
Autophagy and the UPR are fundamental mechanisms
involved in the regulation of cellular responses to environmental
and genetic stresses. Both pathways are interconnected and regulate
cellular responses to apoptotic stimuli (38). ERS can induce autophagy via at least
2 UPR pathways, PERK-elF2α and IRE1α (17). The compound, 4-PBA, has been used as
a selective inhibitor of ERS. In the present study, autophagy was
found to be important for cell apoptosis due to pharmacological
ERS. Blockade of ERS induction via 4-PBA attenuated osthole-induced
apoptosis and autophagy. Consequently, these findings suggested
that osthole-induced ERS plays an important role in the crosstalk
between apoptosis and autophagy. Osthole could induce apoptosis in
colorectal cancer cells, and its effects are partly mediated by the
ERS pathway. Autophagy can alleviate ERS by degrading unfolded or
aggregated proteins (17,18). The compound, 3MA, is a known
inhibitor of autophagy. In the current study, inhibiting cell
autophagy with 3MA attenuated osthole-induced cell apoptosis, but
enhanced the expression levels of ERS-related proteins following
osthole treatment. These results indicated that autophagy played an
anti-tumorigenic role in apoptosis, which was induced by osthole.
Furthermore, the effects of autophagy inhibitors on alleviating
osthole-induced apoptosis was less prominent compared with that in
cells treated with ERS inhibitors, suggesting that the activation
of autophagy may be dependent on ERS during this process.
In conclusion, osthole inhibited the proliferation
and viability, and induced apoptosis of the HT-29 cell line via
activation of autophagy and the ERS pathway, and ERS plays an
important role in osthole-induced cell apoptosis. Thus, osthole may
be a promising candidate for the treatment of human colon
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81873073) and
Science and Technology Development Fund of Affiliated Hospital of
Chengdu University of Traditional Chinese Medicine (grant no.
18MZ27).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC conceived and designed the research. XHZ
performed most of the experiments. JK and ZDZ performed parts of
the experiments. XHZ, JK and ZDZ analyzed the data. XHZ and YC
wrote the manuscript. All authors read and approved the final
manuscript. YC and XHZ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu T, Li Z, Gao CY and Cho CH: Mechanisms
of drug resistance in colon cancer and its therapeutic strategies.
World J Gastroenterol. 22:6876–6889. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang L, Peng Y, Shi K, Wang H, Lu J, Li Y
and Ma C: Osthole inhibits proliferation of human breast cancer
cells by inducing cell cycle arrest and apoptosis. J Biomed Res.
29:132–138. 2015.PubMed/NCBI
|
|
4
|
Tang DZ, Hou W, Zhou Q, Zhang M, Holz J,
Sheu TJ, Li TF, Cheng SD, Shi Q, Harris SE, et al: Osthole
stimulates osteoblast differentiation and bone formation by
activation of beta-catenin-BMP signaling. J Bone Miner Res.
25:1234–1245. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bao Y, Meng X, Liu F, Wang F, Yang J, Wang
H and Xie G: Protective effects of osthole against inflammation
induced by lipopolysaccharide in BV2 cells. Mol Med Rep.
17:4561–4566. 2018.PubMed/NCBI
|
|
6
|
Li Y, Li Y, Shi F, Wang L, Li L and Yang
D: Osthole attenuates right ventricular remodeling via decreased
myocardial apoptosis and inflammation in monocrotaline-induced
rats. Eur J Pharmacol. 818:525–533. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu WB, Zhou J, Qu Y, Li X, Lu CT, Xie KL,
Sun XL and Fei Z: Neuroprotective effect of osthole on MPP+-induced
cytotoxicity in PC12 cells via inhibition of mitochondrial
dysfunction and ROS production. Neurochem Int. 57:206–215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang J, Zhou J, Xu Y, Huang X, Wang X,
Huang W and Li H: Osthole inhibits ovarian carcinoma cells through
LC3-mediated autophagy and GSDME-dependent pyroptosis except for
apoptosis. Eur J Pharmacol. 874:1729902020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu XM, Zhang ML, Zhang Y and Zhao L:
Osthole induces lung cancer cell apoptosis through inhibition of
inhibitor of apoptosis family proteins. Oncol Lett. 12:3779–3784.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang SM, Tsai CF, Chen DR, Wang MY and
Yeh WL: p53 is a key regulator for osthole-triggered cancer
pathogenesis. Biomed Res Int. 2014:1752472014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jarząb A, Grabarska A, Skalicka-Woźniak K
and Stepulak A: Pharmacological features of osthole. Postepy Hig
Med Dosw (Online). 71:411–421. 2017. View Article : Google Scholar
|
|
12
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019. View Article : Google Scholar
|
|
13
|
Oakes S: Endoplasmic reticulum stress
signaling in cancer cells. Am J Pathol. 190:934–946. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guzel E, Arlier S, Guzeloglu-Kayisli O,
Tabak MS, Ekiz T, Semerci N, Larsen K, Schatz F, Lockwood CJ and
Kayisli UA: Endoplasmic reticulum stress and homeostasis in
reproductive physiology and pathology. Int J Mol Sci. 18:7922017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Almanza A, Carlesso A, Chintha C,
Creedican S, Doultsinos D, Leuzzi B, Luís A, McCarthy N,
Montibeller L, More S, et al: Endoplasmic reticulum stress
signalling-from basic mechanisms to clinical applications. FEBS J.
286:241–278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karagöz GE, Acosta-Alvear D and Walter P:
The unfolded protein response: Detecting and responding to
fluctuations in the protein-folding capacity of the endoplasmic
Reticulum. Cold Spring Harbor Perspect Biol. 11:a0338862019.
View Article : Google Scholar
|
|
17
|
Sano R and Reed JC: ER stress-induced cell
death mechanisms. Biochim Biophys Acta. 1833:3460–3470. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Verfaillie T, Salazar M, Velasco G and
Agostinis P: Linking ER Stress to autophagy: Potential implications
for cancer therapy. Int J Cell Biol. 2010:9305092010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Latorraca LB, Feitosa WB, Mariano C, Moura
MT, Fontes PK, Nogueira MFG and Paula-Lopes FF: Autophagy is a
pro-survival adaptive response to heat shock in bovine
cumulus-oocyte complexes. Sci Rep. 10:137112020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Di S, Fan C, Ma Z, Li M, Guo K, Han D, Li
X, Mu D and Yan X: PERK/eIF-2α/CHOP pathway dependent ROS
generation mediates butein-induced non-small-cell lung cancer
apoptosis and G2/M phase arrest. Int J Biol Sci. 15:1637–1653.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park W, Park S, Song G and Lim W:
Inhibitory effects of osthole on human breast cancer cell
progression via induction of cell cycle arrest, mitochondrial
dysfunction, and ER stress. Nutrients. 11:27772019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song S, Tan J, Miao Y, Li M and Zhang Q:
Crosstalk of autophagy and apoptosis: Involvement of the dual role
of autophagy under ER stress. J Cell Physiol. 232:2977–2984. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and D'Orazi G: Apoptosis as anticancer mechanism: Function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging. 8:603–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chipuk JE, Moldoveanu T, Llambi F, Parsons
MJ and Green DR: The BCL-2 family reunion. Mol Cell. 37:299–310.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shokoohinia Y, Jafari F, Mohammadi Z,
Bazvandi L, Hosseinzadeh L, Chow N, Bhattacharyya P, Farzaei MH,
Farooqi AA, Nabavi SM, et al: Potential anticancer properties of
osthol: A comprehensive mechanistic review. Nutrients. 10:362018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Siddiqui WA, Ahad A and Ahsan H: The
mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update.
Arch Toxicol. 89:289–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Devenport SN and Shah YM: Functions and
implications of autophagy in colon cancer. Cells. 8:13492019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Ren S, Liu Y, Gao K, Liu Z and
Zhang Z: Inhibition of starvation-triggered endoplasmic reticulum
stress, autophagy, and apoptosis in ARPE-19 cells by taurine
through modulating the expression of calpain-1 and calpain-2. Int J
Mol Sci. 18:21462017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lei C, Zhao B, Liu L, Zeng X, Yu Z and
Wang X: Expression and clinical significance of p62 protein in
colon cancer. Medicine (Baltimore). 99:e187912020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang H, Zhang Y, Zhu X, Chen C, Zhang C,
Xia Y, Zhao Y, Andrisani O and Kong L: DEAD box protein 5 inhibits
liver tumorigenesis by stimulating autophagy via interaction with
p62/SQSTM1. Hepatology. 69:1046–1063. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gong J, Wang XZ, Wang T, Chen JJ, Xie XY,
Hu H, Yu F, Liu HL, Jiang XY and Fan HD: Molecular signal networks
and regulating mechanisms of the unfolded protein response. J
Zhejiang Univ Sci B. 18:1–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sisinni L, Pietrafesa M, Lepore S,
Maddalena F, Condelli V, Esposito F and Landriscina M: Endoplasmic
reticulum stress and unfolded protein response in breast cancer:
The balance between apoptosis and autophagy and its role in drug
resistance. Int J Mol Sci. 20:8572019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Woo KJ, Lee TJ, Lee SH, Lee JM, Seo JH,
Jeong YJ, Park JW and Kwon TK: Elevated gadd153/chop expression
during resveratrol-induced apoptosis in human colon cancer cells.
Biochem Pharmacol. 73:68–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shen Z, Chen J and Lu H: Osthole induced
apoptosis in human normal liver cells by regulating cell
proliferation and endoplasmic reticulum stress. Environ Toxicol.
34:768–776. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mokarram P, Albokashy M, Zarghooni M,
Moosavi MA, Sepehri Z, Chen QM, Hudecki A, Sargazi A, Alizadeh J,
Moghadam AR, et al: New frontiers in the treatment of colorectal
cancer: Autophagy and the unfolded protein response as promising
targets. Autophagy. 13:781–819. 2017. View Article : Google Scholar : PubMed/NCBI
|