Overview of NK cells
Natural killer (NK) cells are considered to be a
subpopulation of lymphocytic cells and, due to their granularity
and size, are also regarded as long granular lymphocytes; however,
NK cells are a morphologically homogeneous population
indistinguishable from the total lymphocyte pool. NK cells
constitute 5–15% of all mononuclear cells in the peripheral blood
(1), and were initially thought to
be generated only from CD34+ lymphoid precursors in the
bone marrow (2). However, studies in
mice and humans suggest that NK cells are also able to develop and
mature in secondary lymphoid tissues (3).
Phenotypically, NK cells are primarily identified by
the expression of CD56 (neural cell adhesion molecule) (4) and CD16 (human IgG Fc receptor III,
FcγRIII), as well as the lack of CD3, T-cell receptors and CD19,
typical of T and B lymphocytes (5).
NK cells have recently been categorized as group 1 innate lymphoid
cells, due to their ability to produce IFN-γ and selectively
express the T-box eomesodermin transcription factor, which is
necessary for their development and function (6).
Functionally, NK cells play an important role in
immunosurveillance, since they participate in innate resistance
against infected cells without prior sensitization or antigenic
presentation, and also exert natural cytotoxic activity against
tumor cells (7). The activation of
NK cells favors the maintenance of an inflammatory environment,
since they produce a number of inflammatory cytokines that recruit
other immune cells (8). Similarly,
NK cells serve an important role in the expansion of Th1-type
responses, and are involved in hematopoiesis-associated processes
(9).
The existence of a population of memory NK cells has
been suggested. However, despite evidence in non-human primates
(10), this NK cell function has not
yet been verified in humans, and requires further investigation
(11).
Origin of NK cells
NK cells are derived from precursors that originate
from hematopoietic stem cells in the bone marrow; when stimulated
with interleukin (IL)-2, IL-15 and IL-7, these precursors develop
into mature NK cells (12). However,
these NK precursors (NKPs) can also originate from an early thymus
lymphoid precursor (13). NKPs are
able to circulate between different organs, maturing into
functional NK cells in the bone marrow, thymus, liver, spleen and
lymph nodes (12).
On reaching immune competence, NK cells leave their
sites of origin and migrate to target tissues, including the lung,
mucosa, intestine, liver, skin and uterus. At these sites, NK cells
differentiate according to their resting or activated phenotypes,
which express stimulation markers and exhibit unique functional
properties (14).
NK cell diversification
Due to the various effector functions exhibited by
NK cells in humans, the existence of subpopulations with
specialized functions has been suggested. The use of monoclonal
antibodies and flow cytometry has enabled the classification of NK
cells according to the expression density of surface markers and
their association with characteristic functions.
Based on the surface expression density of CD56, NK
cells can be classified as ‘bright’, with high CD56 expression
density, and ‘dim’, with low CD56 expression density. In the
peripheral blood, ~90% of NK cells are CD56dim, while
the other 10% are CD56bright (15). Furthermore, according to the relative
co-expression of CD56 and CD16, NK cells can also be classified
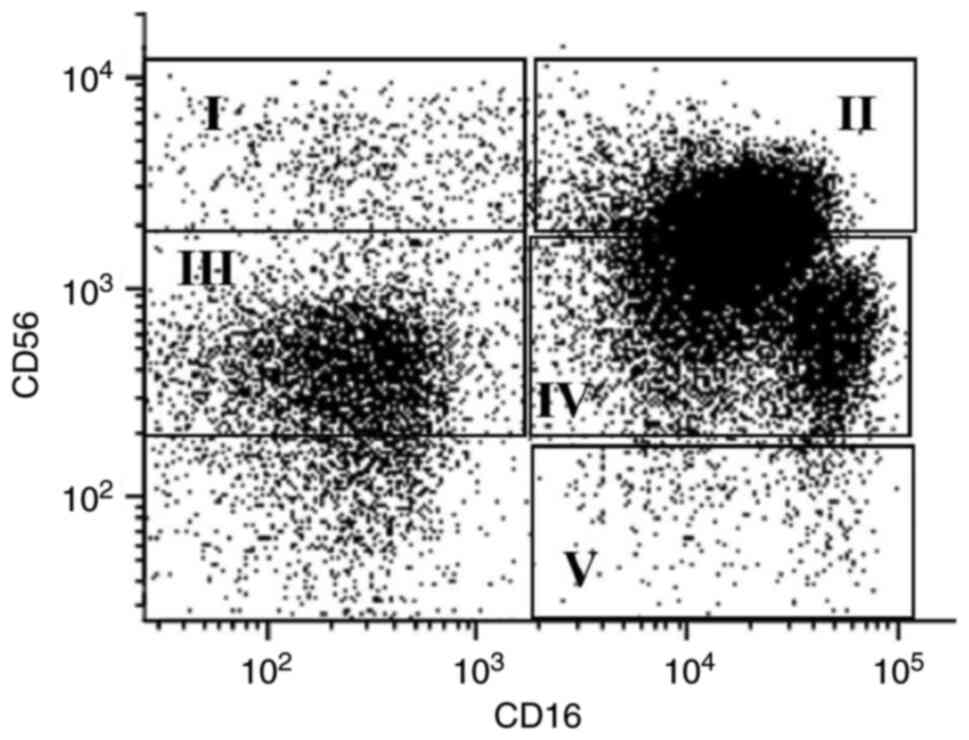
into the following five subpopulations: i)
CD56bright/CD16−, constituting 50–70% of the
total CD56bright population; ii)
CD56bright/CD16+, constituting 30–50% of
total CD56bright cells; iii)
CD56dim/CD16−; iv)
CD56dim/CD16+; and v)
CD56−/CD16+ (16). These populations detected in the
blood of a healthy donor by flow cytometry (Appendix S1) are presented in Fig. 1.
CD56bright NK cells are considered to be
immunoregulatory, as they have the ability to secrete
immunomodulatory cytokines such as IFN-γ, IL-10, IL-13, TNF-β and
granulocyte and monocyte colony stimulating factor at rest, with
increased secretion following activation. However, the quantity and
quality of these cytokines varies according to the stimulus, since
interleukins such as IL-2, IL-15 and IL-18 are capable of
increasing the secretion of IFN-γ, IL-10 and TNF-β (17). NK CD56bright cells have a
low density of perforin granules and granzymes, and exert decreased
cytotoxic activity compared with CD56dim cells (18). Furthermore, CD56bright
cells are the only NK cells that constitutively express the
high-affinity IL-2 receptor, which is able to induce the
proliferation of NK cells at low (picomolar) concentrations
(19). CD56bright cells
are also capable of proliferation and self-maintenance in the
presence of IL-15, without co-stimulation (20).
The expression of adhesion molecules such as CD62L
(L-selectin) (21), as well as
chemokine receptors, namely C-C chemokine receptor type (CCR) 7,
CCR4 and C-X-C chemokine receptor type (CXCR) 3, on the surface of
CD56bright NK cells enables them to preferentially
migrate to secondary lymphoid organs such as the tonsils and lymph
nodes (22). Furthermore, the
expression of CXCR1 and CXCR3 confers different migratory
properties to CD56bright cells, causing them to be
located primarily in the spleen and bone marrow, as well as at
sites of acute inflammation (16).
CD56bright NK cells have been suggested
to be precursors of their CD56dim counterparts as, under
the influence of certain cytokines, the former can decrease their
expression of CD56 and increase that of CD16 (23). Also, the CD56bright
subpopulation is the first to appear during reconstitution of the
immune system after bone marrow transplantation. In the
NKbright population,
CD56bright/CD16− cells are considered
immunomodulatory due to their interaction with dendritic cells in
the lymph nodes, and elevated production of IFN-γ (16). By contrast, the
CD56bright/CD16+ subpopulation is considered
to be transitional, with minimal IL-2-induced proliferation and
cytotoxic activity (24).
Approximately 90% of peripheral blood NK cells are
NKdim, of which the predominant phenotype is
CD56dim/CD16bright (95%), with lower numbers
of CD56dim/CD16− and
CD56−/CD16bright cells (16). Unlike their bright counterparts,
NKdim cells are poor cytokine producers (17). However, when activated by contact
with target cells, they are capable of producing IFN-γ, TNF-α,
macrophage inflammatory protein (MIP)-1α and MIP-1β, sometimes in
higher concentrations than those generated by CD56bright
cells (8).
NKdim cells express low surface levels of
the IL-2 receptor and, therefore, have little proliferative
capacity in vitro, even when stimulated with high
concentrations of IL-2 (19) and
IL-15 (20). NKdim cells
generally possess high cytotoxic activity due to their high
expression levels of perforins, granzymes and cytolytic granules
(25). Furthermore, NKdim
cells express high quantities of receptors that enhance their
ability to respond to abnormal cells, and a high surface density of
CD16 that allows them to carry out antibody-mediated cytotoxicity
functions (25).
Unlike CD8 T cells, the cytotoxic activity of NK
cells against target cells does not require prior activation and is
independent of the expression of major histocompatibility complex
(MHC) molecules. This activity can be initiated by different
processes, including degranulation and antibody-dependent
cytotoxicity, as well as the binding of ligands to cellular death
receptors (26).
The molecular mechanisms that regulate cellular
cytotoxicity may be divided into three categories: i) Recognition
of the target cell; ii) formation of the immune synapse; and iii)
NK-induced cell death. Following recognition and the formation of
the immune synapse, cell death is induced by two primary mechanisms
(27). The first mechanism involves
the activation of cell death receptors present on the surface of
the target cell. These receptors include the TNF-related
apoptosis-inducing ligand receptor (TRAIL-R) and Fas (CD95), which
are activated by TRAIL and Fas ligand (CD95L), respectively
(26). The stimulation of these
receptors induces activation of the caspase 8 and 10 pathways,
resulting in apoptotic cell death (28). However, the predominant mechanism of
cytotoxicity involves the direct release of lytic granules towards
the target cell. This requires reorganization of the cytoskeleton,
as well as the polarization of NK cell microtubules. NK cells also
contain perforins, enzymes that when integrated into the target
cell membrane form a pore through which water can enter the cell,
promoting osmotic lysis (29).
Granzyme B is a critical component of NK cells, as
it is a protein capable of inducing apoptosis by promoting the
breakdown of peptides with aspartic acid residues (26). Once inside the target cell, granzyme
B induces caspase-dependent and -independent apoptosis, which is
itself dependent on initiation by the direct cleavage of caspase 8
or 3 (30). Caspase-independent
apoptosis is achieved by the induction of cytochrome release from
the mitochondria, and the cleavage of the pro-apoptotic protein Bid
(31). These natural cytotoxic
activities are reportedly most effectively by
CD56dim/CD16− cells since upon activation,
CD56dimCD16bright cells have been shown to
lose their CD16 expression through metalloprotease-mediated
cleavage to become CD56dimCD16−, and express
increased levels of CD107a, which has been described as a marker of
degranulation (32).
As aforementioned, NK cells are capable of
participating in antibody-mediated cytotoxicity, an immune
mechanism by which NK cells induce the death of antibody-opsonized
cells (33). Since
CD56dim/CD16bright NK cells express a high
density of CD16, this subpopulation of NKdim cells is
the most capable of performing this process (18). Once the target cell has been
opsonized and CD16 recognizes the Fc region of immunoglobulins,
cell death is induced by one of the aforementioned mechanisms
(26).
NK cell receptors
NK cells express a variety of different receptors
that allow them to interact with diverse cell subpopulations, and
thus trigger different effector functions. Since NK cells do not
express clonal receptors, the receptor repertoire of these cells
comprises germline receptors, which have traditionally been
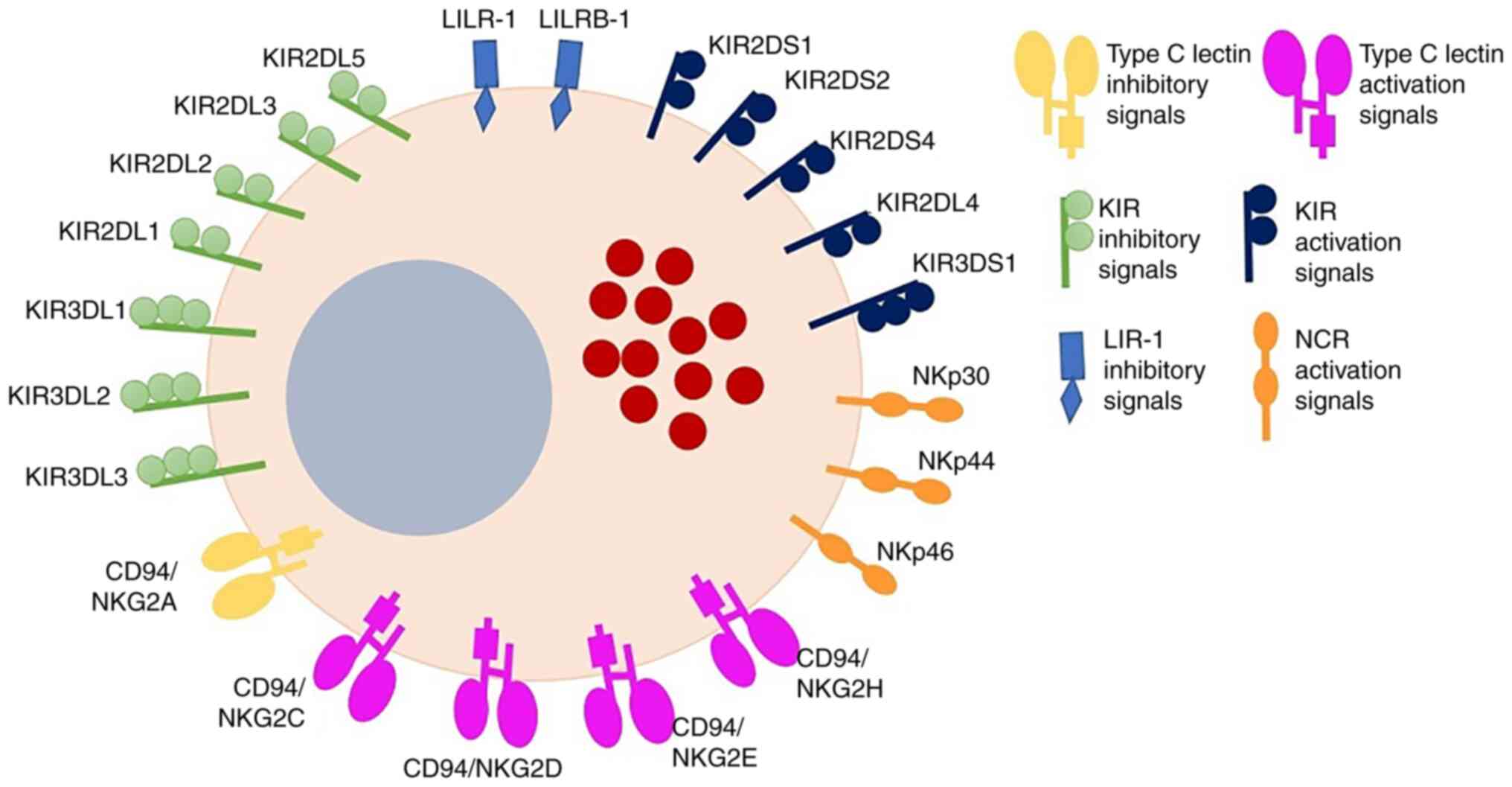
classified as inhibitors or activators (15) (Fig.
2). These germline receptors include pattern recognition
receptors (PRRs), which are discussed in more detail below.
The inhibitory receptors comprise two NK receptor
superfamilies. The first family comprises the killer cell
immunoglobulin-like receptors (KIRs) KIR2DL1-5 and KIR3DL1-3, which
recognize classical MHC-I molecules. The other superfamily
comprises lectin C-type receptors, which are heterodimers such as
CD94/natural killer group 2 member A (NKG2A) that primarily
recognize non-classical MHC molecules, including human leukocyte
antigen (HLA)-E (34). When these
receptors interact with their ligands, immunoreceptor
tyrosine-based inhibitory motifs initiate signaling cascades that
prevent NK-cell activation (35). NK
cells also express subfamily B member 1 of the inhibitory leukocyte
immunoglobulin-like receptor family (LILRB1), which binds
non-classical MHC molecules such as HLA-G (36). Under physiological conditions, NK
cells are inhibited by the interaction between the aforementioned
receptors and their ligands, which are present on the surface of
healthy cells. By contrast, when infected with intracellular
pathogens or undergoing malignant transformation, cells frequently
experience a reduction or loss of MHC-I expression, and therefore,
become potential NK-cell targets. This phenomenon is known as
missing self-recognition (37).
However, the absence of MHC-I molecules on the surface of target
cells is not sufficient to trigger the activation of NK cells, as
the correct balance between inhibitory and activating receptor
signals is required (38).
Activating receptors, such as KIR2DS, KIR3DS, NKG2D
and NKG2E, are also capable of recognizing classical MHC-I
molecules, while NKG2D receptors also bind non-classical MHC-I
(36). These receptors possess
immunoreceptor tyrosine-based activating motifs that transduce
activation signals (39).
Furthermore, NK cells express natural cytotoxicity
receptors, which are involved in the preferential activation of NK
cells against tumor cells (15).
Among these receptors, NKp30 (CD337) and NKp46 (CD335) are
expressed by resting and activated NK cells, while NKp44 (CD336) is
only expressed by activated NK cells (34).
NK cells also express co-stimulation ligands,
including CD40L (CD154) (40) and
OX40L, which allow them to impart co-stimulatory signals to T or B
cells (41). Thus, NK cells serve as
a bridge between innate and adaptive immunity. Dendritic cells
stimulate NK cells to provide co-stimulatory signals to T or B
cells, allowing for an optimal immune response (42).
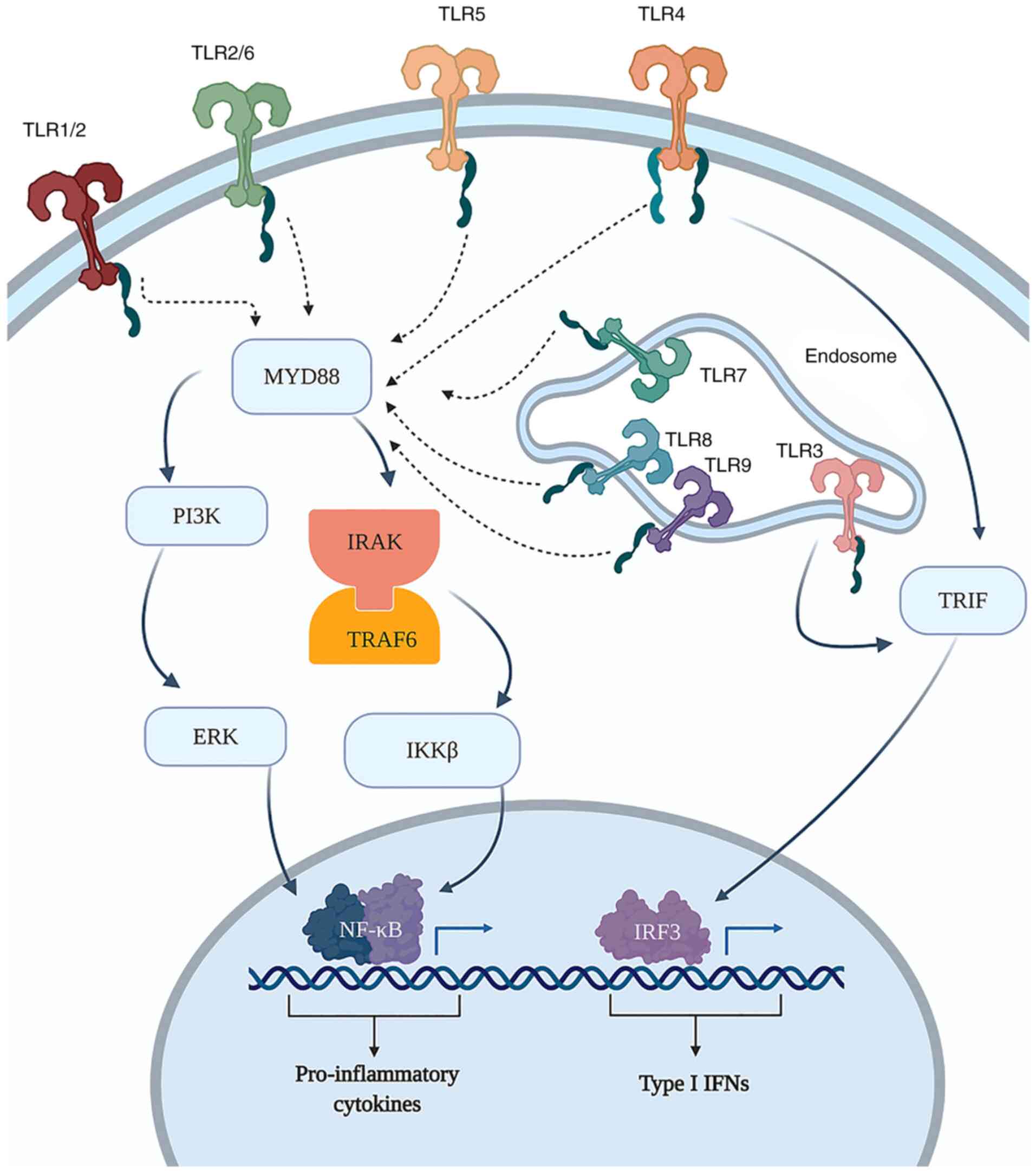
Additionally, NK cells express PRRs through which
they recognize pathogen-associated molecular patterns, as well as
damage-associated molecular patterns (DAMPs). PRRs include
Toll-like receptors (TLRs), NOD-like receptors (NLRs) (43) and RIG-I-like receptors (RLRs)
(44). From the NLR family, NK cells
have been shown to express NOD2 and NLRP3; when activated, these
NLRs increase the cytotoxicity of NK cells against tumor cells, and
upregulate the production of cytokines such as TNF-α and IFN-γ
(45). With respect to RLRs, NK
cells have been shown to express RIG-I and melanoma
differentiation-associated protein 5 receptors, which are of great
importance in antiviral defense. These receptors have been shown to
serve an important role in human NK-cell functioning, increasing
their production of IFN-γ as well as their antitumor activity
(44).
Several studies have demonstrated the functional
responses of human NK cells to stimulation with various TLR ligands
(46,47). The majority of studies indicate that
NK cells require the concurrent presence of pro-inflammatory
cytokines to respond to TLR agonists (48).
Toll-like receptor expression in NK
cells
Initially, the identification of TLR expression in
NK cells was based on mRNA detection only. In NK cells isolated
from human peripheral blood, the constitutive expression of TLR1-8
mRNA was observed, of which TLR2 and 3 were the most abundantly
expressed. By contrast, the expression of TLR9 and 10 mRNA was
found to be insignificant in CD56bright and
CD56dim NK-cell populations (43). Furthermore, TLR2 mRNA was reported to
be highly expressed, while TLR4 and 3 mRNAs were weakly expressed,
and TLR8 mRNA was undetectable in resting human NK cells (49). Additionally, the mRNA levels of these
four TLRs increased considerably following exposure to viral
particles (49). In general, it has
been observed that TLR2, 3, 5 and 6 are more frequently expressed
than TLR4, 7, 8 and 9 by NK cells (50).
Regarding the responses generated by the activation
of these TLRs, variations between different TLRs have been observed
in NK cells in vitro and in samples from healthy donors.
Becker et al (51) observed
that when activated via TLR2, using lipophosphoglycan purified from
leishmania, NK cells exhibited increased levels of IFN-γ, as well
as the increased expression of cell surface TLR2. In 2004, Schmidt
et al (52) determined that
poly-inosinic-cytidylic acid [poly (I:C)] activates human NK cells
via TLR3. In the same year, Sivori et al (53) demonstrated that TLR9 activation
induces human NK cells to secrete IFN-γ and TNF-α.
Lauzon et al (50) revealed that human NK cells express
TLR1-10 mRNA, and that the ligand binding of TLR2, 3, 5 and 9
stimulates the secretion of IFN-γ. In the same year, Gorski et
al (54) concluded that TLR7 and
8 activation increases the production of IFN-γ by human NK cells.
In 2007, Alter et al (55)
observed that TLR7 and 8 activation increased the production of
pro-inflammatory cytokines by human NK cells from patients with
HIV-1. In 2010, Mian et al (56) revealed that the production of IFN-γ
and TNF-α was increased via TLR4 activation in humans and mice, and
in 2013, He et al (57)
demonstrated that human and mouse NK cells could be activated via
the binding of TLR1 with several miRNAs.
These observations are generalized throughout the NK
subpopulations. However, relative TLR expression varies according
to NK-cell phenotype. For example, TLR2 is preferentially expressed
by CD56bright NK cells, and TLR3 by CD56dim
cells (48,58). These variations suggest that
stimulation with TLR ligands imparts a cytotoxic or
immunomodulatory response, depending on the ligand.
The knowledge that TLRs are expressed by NK cells
(Fig. 3) has increased interest in
their immune response against viral and bacterial infections, as
well as their antitumor activity. However, it has been observed
that the DAMP-induced activation of NK cells can only occur via
complex interaction with other cells of the immune system and
within the cellular microenvironment (59).
NK cells in cancer
As aforementioned, NK cells are the primary
mediators of immunosurveillance against tumor cells (60), as they can detect changes in the
expression of MHC-I molecules and eliminate cells that have
undergone malignant transformation (29). Mutations that arise during malignant
transformation are reflected via alterations in MHC-I expression
(61), as well as the overexpression
of stress molecules that can be recognized by NKG2D receptors
(62). However, neoplastic cells use
various mechanisms to evade antitumor activity. In an 11-year
follow-up study, an association was found between the low
cytotoxicity of peripheral blood NK cells and an increased risk of
cancer (63). Although the
infiltration of NK cells has been shown to favor tumor elimination
in various carcinomas, including colorectal (64), gastric (65) and lung cancers (66), these cells are found in small
quantities within the tumor (67)
and exhibit changes in the expression of activating receptors
(68); additionally, the tumor
microenvironment favors immunosuppression (69,70),
which may also explain the small number of NK cells found within
the tumor itself.
NK cells in acute lymphoblastic
leukemia
With regard to hematological cancers such as
leukemia, little is known of how these disorders affect the
origination of NK cells, since both the neoplasia and NK cells
originate from the bone marrow. On the basis of studies of patients
with chronic myeloid leukemia (71,72),
impaired or abnormal NK-mediated cytotoxicity, as well as the
aberrant expression of NK receptors, constitute fundamental factors
in the progression of the neoplasm (73).
A study of NK cells in the peripheral blood of
patients when diagnosed with acute lymphoblastic leukemia (ALL)
type B revealed that they exhibit TGF-β1-mediated compromised
cytotoxicity towards K562 cells and autologous blasts. Furthermore,
the NK cells were found to have an inhibitory phenotype,
represented by altered cell surface expression of NKp46 and NKG2A,
compared with those from healthy control subjects of the same age
(74). The study also demonstrated
that after remission, NK cell-mediated cytotoxicity towards K562
was recovered, but not that towards autologous blasts, suggesting
that blasts undergo successful immune editing (74).
A recent study in Mexican patients with ALL reported
a reduction in the percentage of NK cells at diagnosis, as well as
impaired cytotoxicity in patients with B- and T-ALL (75). Furthermore, in B-ALL, NK
cell-mediated cytotoxicity in patients with leukocyte counts of
>50,000/mm3 was observed to be impaired compared with
that in cases with lower counts. These data suggest that the
abnormal effector function of NK cells is not observed equally in
all pediatric patients with B-ALL, and that there may be other
contributing factors to NK cell-mediated cytotoxicity (75).
Chemotherapeutics against leukemia have been shown
to decrease the number and activity of NK cells during treatment.
However, upon the discontinuation of treatment, it is possible that
normal NK-cell levels slowly recover, although their cytotoxicity
does not (76). Evidence of the
importance of NK cells in tumor regulation has primarily been based
on clinical studies of patients with leukemia who have undergone
allogeneic hematopoietic stem cell transplantation (cell therapy)
(77). This is a safe therapy, which
avoids graft-vs.-host rejection disease (GvHD) (78).
Cellular immunotherapy, also known as adoptive cell
therapy, has been accomplished by the use of engineered cells,
among which chimeric antigenic receptor T (CAR-T) cells have
achieved successful outcomes for B-cell lymphoblastic leukemia; a
cohort study revealed that a single infusion of this therapy
provided durable remission with long-term persistence in pediatric
and young adult patients with relapsed or refractory B-cell ALL
(79). However, the application of
CAR-T cells has faced obstacles, two of which are: Patients who
experience low CAR-T cell persistence or disease relapse cannot be
reinfused with CAR-T cells; and the majority of patients experience
cytotoxic effects (80). The
development of CAR-NK cells could be an attractive solution to
those issues.
As NK cells lack T-cell receptors that could cause
GvHD and do not require HLA matching to be cytotoxic, CAR-NK cells
have potential as cellular therapeutics. Pre-clinical studies with
CAR-expressing NK cells have focused on anti-CD19 and anti-CD20
CARs targeting B cell malignancies, and have observed that CAR-NK
cells are capable of increasing IFN-γ expression in response to
CD19+ cells as well as increasing their specific
cytotoxicity in xenograft models of B-ALL (81,82).
Despite their advantages over CAR T cells, CAR-NK
cell-based therapies have made limited clinical progress (83). Currently, there are 19 studies
registered in the clinicaltrials.gov database for the use of CAR-NK
cells in patients with cancer; 12 are trials in phase I/II that are
recruiting, and two have already been completed. In the majority of
these trials, the CAR-NK cells target CD19, CD22, CD33 or CD7 on
hematopoietic malignancies (83).
The first large-scale trial of CAR-NK cells used
CAR-NK cells derived from cord blood to treat high-risk
CD19+ B cell malignancies (84). An anti-CD19-CD28-CD3ζ CAR was used
for transduction of the cells, in addition to an IL-15 gene and
inducible caspase 9 as a safety switch. In that study, 7/11
patients achieved complete remission, and showed an early expansion
of CAR-NK cells compared with the non-responders. This response was
achieved with no serious side effects, such as cytokine release
syndrome, neurotoxicity and GvHD, even in the 5 patients with a
KIR-ligand mismatch (84). Overall,
the study demonstrated the initial efficacy and safety profile of
this CAR-NK cell therapy. Although CAR-NK cells have multiple
advantages compared with CAR-T cells, they have several challenges
to overcome, such as loss of targeted antigen and tumor
heterogeneity (83). Therefore,
novel strategies should be sought to optimize the efficacy of
CAR-based NK cell therapy.
TLRs' importance against acute lymphoblastic
leukemia
Considering that poor cytotoxic activity of NK cells
suggests an alteration in their activation, immunological
strategies designed to improve the sensitivity of neoplastic cells
to NK cell-mediated cytotoxicity should focus on the mechanisms by
which NK cells are activated. Such mechanisms include the use of
specific TLR ligands to restore NK-cell activation and
cytotoxicity.
However, little information is available regarding
the possibility of reactivating or modulating the functions of NK
cells in vivo. Although ligands exist for the stimulation of
these receptors in vitro, it is not yet known with certainty
whether they can be used as therapeutic agents in leukemia, where a
reduction in the expression of TLRs has been reported in peripheral
blood mononuclear cells (85).
A preclinical study demonstrated that TLR ligands
can effectively activate donor NK cells to induce blast lysis
(86). Therefore, they have been
suggested as potential boosters for stimulating the immunological
effector function of NK cells in leukemia immunotherapy. Among
these ligands, the TLR3 agonist poly (I:C) demonstrated the ability
to activate NK cells in vivo, and improved the therapeutic
activity of a monoclonal antibody against human CD7 in a xenograft
model of T-ALL (87). The TLR3
agonist was also demonstrated to cause changes in the expression
levels of CD2, CD16/32 (FcRII/RIII), CD161 (NK1.1) and F4/80 in the
host splenocyte population. It was suggested that the mechanism
underling the boost in NK cells following their activation via TLR3
in the ALL model is antibody-dependent cytotoxicity (ADCC), based
on the previous observation that when cancer cells are coated by an
antibody, the cell lysis is increased compared with that of cells
not coated with antibody (87).
There is evidence that the TLR7 agonist R848 can
boost the efficacy of obinutuzumab in CD-20+ lymphoma,
since it has been shown to enhance non-specific NK-cell
cytotoxicity (88). Since R848 and
other TLR7 agonists can raise the levels of activating FcγR and
reduce those inhibitory FcγRIIb, this supports the suggestion that
R848 triggers ADCC as an effector mechanism. Notably, the
interaction of NK cells with R848 in vivo induced the
production of IFN-γ by the NK cells upon engagement of the Fc
receptors by obinutuzumab. Furthermore, the study demonstrated that
the systemic administration of R848 combined with obinutuzumab
therapy enhanced the long-term survival of patients with lymphoma
(88). A more recent study with an
encapsulated TLR7/8 agonist also demonstrated positive
immunotherapeutic effects, since strong in vivo cytotoxicity
against the K562 leukemia cell line was achieved through NK
degranulation and the release of granzyme B, and the activation of
NK cells was prolonged. In addition, the TLR7/8 agonist enhanced
the in vitro and in vivo ADCC of the epidermal growth
factor receptor-targeting antibody cetuximab. These findings
indicate the potential of the TLR7/8 agonist as a potent
immunostimulatory adjuvant for antibody-based cancer immunotherapy,
which acts via the promotion of NK-cell activation (89).
Although the aforementioned studies confirm that it
is possible to activate NK cells through their TLRs to favor the
lysis of carcinogenic cells, it is not yet possible to affirm the
effects of this method in NK cells from hematological disorders
such as ALL. Although there are existing clinical trials evaluating
the effects of TLR stimulation on leukemia, with the clinicaltrials.gov database listing four completed
clinical trials and two in the recruitment phase, only one of these
has been performed in patients with ALL, with one NK-associated
observation (90). This trial,
reported by Ronsley et al (90), was a phase I pilot study, in which
the regulatory effect of the TLR9 ligand GNKG168 on various genes
was evaluated in three pediatric patients with ALL with residual
disease following conventional therapy. GNKG168 was concluded to
induce immunological changes in the mononuclear cells of patients
via the negative regulation of genes (mRNAs) that participate in
the antitumor response, such as single Ig and TIR domain
containing, IL-1 receptor ligand 1, CCR8, IL-7 receptor (IL7R),
CD8B and CD3D. These findings suggest that inhibiting IL7R may also
act as a checkpoint inhibitor of IL7R expression in the CD56
bright NK population, which is important for modulating
the post-transplant response.
Conclusions
NK cells play an important role in the antitumor
response. Due to the discovery that TLRs can induce the
cytotoxicity and cytokine production of NK cells, TLR agonists have
been proposed as potential stimulators of the antitumor effector
functions of NK cells. However, further studies are required to
elucidate the antitumor effects of NK cells, and understand the
activation mechanisms of TLRs therein, in order to improve NK
cell-mediated immunotherapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Children's Hospital of Mexico Federico Gómez (grant
no. HIM/2019/035 SSA 1618).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JGZ reviewed the literature and prepared drafts of
the manuscript. CMB assisted in evaluation of the literature and
finalizing manuscript for submission. All authors have read and
approved the final version of this manuscript.
Ethics approval and consent to
participate
The study was ethically approved by the Research,
Research Ethics and Biosafety Committees of the Children's Hospital
of Mexico Federico Gómez (protocol no. HIM/2019/035). Patient
consent was not required because waste blood from the blood bank of
the Children's Hospital of Mexico Federico Gomez was used.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Timonen BYT, Ortaldo JR and Herberman RB:
Characteristics of human large granular lymphocytes and
relationship to natural killer and K cells. J Exp Med. 153:569–582.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galy A, Travis M, Cen D and Chen B: Human
T, B, natural killer, and dendritic cells arise from a common bone
marrow progenitor cell subset. Immunity. 3:459–473. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scoville SD, Freud AG and Caligiuri MA:
Modeling human natural killer cell development in the era of innate
lymphoid cells. Front Immunol. 8:3602017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nitta T, Yagita H, Sato K and Okumura K:
Involvement of CD56 (NKH-1/Leu-19 antigen) as an adhesion molecule
in natural killer-target cell interaction. J Exp Med.
170:1757–1761. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lanier LL, Le AM, Civin CI, Loken MR and
Phillips JH: The relationship of CD16 (Leu-11) and Leu-19 (NKH-1)
antigen expression on human peripheral blood NK cells and cytotoxic
T lymphocytes. J Immunol. 136:4480–4486. 1986.PubMed/NCBI
|
|
6
|
Vivier E, Artis D, Colonna M, Diefenbach
A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ,
Mebius RE, et al: Innate lymphoid cells: 10 years on. Cell.
174:1054–1066. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiorean EG and Miller JS: The biology of
natural killer cells and implications for therapy of human disease.
J Hematotherapy Stem Cell Res. 10:451–463. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fauriat C, Long EO, Ljunggren HG and
Bryceson YT: Regulation of human NK-cell cytokine and chemokine
production by target cell recognition. Blood. 115:2167–2176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lotze MT and Thomson AW: Natural killer
cells: Basic science and clinical application. Academic Press;
2009
|
|
10
|
Reeves RK, Li H, Jost S, Blass E, Li H,
Schafer JL, Varner V, Manickam C, Eslamizar L, Altfeld M, et al:
Antigen-specific NK cell memory in rhesus macaques. Nat Immunol.
16:927–932. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nikzad R, Angelo LS, Aviles-Padilla K, Le
DT, Singh VK, Bimler L, Vukmanovic-Stejic M, Vendrame E, Ranganath
T, Simpson L, et al: Human natural killer cells mediate adaptive
immunity to viral antigens. Sci Immunol. 4:eaat81162019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Freud AG, Yokohama A, Becknell B, Lee MT,
Mao HC, Ferketich AK and Caligiuri MA: Evidence for discrete stages
of human natural killer cell differentiation in vivo. J Exp Med.
203:1033–1043. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rosmaraki EE, Douagi I, Roth C, Colucci F,
Cumano A and Di Santo JP: Identification of committed NK cell
progenitors in adult murine bone marrow. Eur J Immunol.
31:1900–1909. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huntington ND, Vosshenrich CAJ and Di
Santo JP: Developmental pathways that generate natural-killer-cell
diversity in mice and humans. Nat Rev Immunol. 7:703–714. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cooper MA, Fehniger TA and Caligiuri MA:
The biology of human natural killer-cell subsets. Trends Immunol.
22:633–640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Poli A, Michel T, Thérésine M, Andrès E,
Hentges F and Zimmer J: CD56bright natural killer (NK) cells: An
important NK cell subset. Immunology. 126:458–465. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cooper MA, Fehniger TA, Turner SC, Chen
KS, Ghaheri BA, Carson WE and Caligiuri MA: Human natural killer
cells: A unique innate immunoregulatory role for the CD56bright
subset. Blood. 96:3146–3151. 2000.
|
|
18
|
Nagler A, Lanier LL, Cwirla S and Phillips
JH: Comparative studies of human FcRIII-positive and negative
natural killer cells. J Immunol. 143:3183–3191. 1989.PubMed/NCBI
|
|
19
|
Caligiuri MA, Murray C, Robertson MJ, Wang
E, Cochran K, Cameron C, Schow P, Ross ME, Klumpp TR, Soiffer RJ,
et al: Selective modulation of human natural killer cells in vivo
after prolonged infusion of low dose recombinant interleukin 2. J
Clin Invest. 91:123–132. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mrózek E, Anderson P and Caligiuri MA:
Role of interleukin-15 in the development of human CD56+ natural
killer cells from CD34+ hematopoietic progenitor cells. Blood.
87:2632–2640. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frey M, Packianathan NB, Fehniger TA, Ross
ME, Wang WC, Stewart CC, Caligiuri MA and Evans SS: Differential
expression and function of L-selectin on CD56bright and CD56dim
natural killer cell subsets. J Immunol. 161:400–408.
1998.PubMed/NCBI
|
|
22
|
Berahovich RD, Lai NL, Wei Z, Lanier LL
and Schall TJ: Evidence for NK cell subsets based on chemokine
receptor expression. J Immunol. 177:7833–7840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chan A, Hong DL, Atzberger A, Kollnberger
S, Filer AD, Buckley CD, McMichael A, Enver T and Bowness P:
CD56bright human NK cells differentiate into CD56dim cells: Role of
contact with peripheral fibroblasts. J Immunol. 179:89–94. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Béziat V, Duffy D, Quoc SN, Le
Garff-Tavernier M, Decocq J, Combadière B, Debré P and Vieillard V:
CD56brightCD16+ NK cells: A functional intermediate stage of NK
cell differentiation. J Immunol. 186:6753–6761. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vivier E, Tomasello E, Baratin M, Walzer T
and Ugolini S: Functions of natural killer cells. Nat Immunol.
9:503–510. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smyth MJ, Cretney E, Kelly JM, Westwood
JA, Street SE, Yagita H, Takeda K, van Dommelen SL, Degli-Esposti
MA and Hayakawa Y: Activation of NK cell cytotoxicity. Mol Immunol.
42:501–510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krzewski K and Strominger JL: The killer's
kiss: The many functions of NK cell immunological synapses. Curr
Opin Cell Biol. 20:597–605. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guicciardi ME and Gores GJ: Life and death
by death receptors. FASEB J. 23:1625–1637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abel AM, Yang C, Thakar MS and Malarkannan
S: Natural killer cells: Development, maturation, and clinical
utilization. Front Immunol. 9:1–23. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barry M, Heibein JA, Pinkoski MJ, Lee SF,
Moyer RW, Green DR and Bleackley RC: Granzyme B short-circuits the
need for caspase 8 activity during granule-mediated cytotoxic
T-lymphocyte killing by directly cleaving Bid. Mol Cell Biol.
20:3781–3794. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pinkoski MJ, Waterhouse NJ, Heibein JA,
Wolf BB, Kuwana T, Goldstein JC, Newmeyer DD, Bleackley RC and
Green DR: Granzyme B-mediated apoptosis proceeds predominantly
through a Bcl-2-inhibitable mitochondrial pathway. J Biol Chem.
276:12060–12067. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amand M, Iserentant G, Poli A, Sleiman M,
Fievez V, Sanchez IP, Sauvageot N, Michel T, Aouali N, Janji B, et
al: Human CD56dimCD16dim cells as an
individualized natural killer cell subset. Front Immunol.
8:6992017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iannello A and Ahmad A: Role of
antibody-dependent cell-mediated cytotoxicity in the efficacy of
therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis
Rev. 24:487–499. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zambello R, Falco M, Della Chiesa M,
Trentin L, Carollo D, Castriconi R, Cannas G, Carlomagno S,
Cabrelle A, Lamy T, et al: Expression and function of KIR and
natural cytotoxicity receptors in NK-type lymphoproliferative
diseases of granular lymphocytes. Blood. 102:1797–1805. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bryceson YT, March ME, Ljunggren HG and
Long EO: Activation, coactivation, and costimulation of resting
human natural killer cells. Immunol Rev. 214:73–91. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li NL, Davidson CL, Humar A and Burshtyn
DN: Modulation of the inhibitory receptor leukocyte Ig-like
receptor 1 on human natural killer cells. Front Immunol.
2:462011.PubMed/NCBI
|
|
37
|
Ljunggren HG and Kärre K: In search of the
‘missing self’: MHC molecules and NK cell recognition. Immunol
Today. 11:237–244. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tomasello E, Blery M, Vely E and Vivier E:
Signaling pathways engaged by NK cell receptors: Double concerto
for activating receptors, inhibitory receptors and NK cells. Semin
Immunol. 12:139–147. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lanier LL: Up on the tightrope: Natural
killer cell activation and inhibition. Nat Immunol. 9:495–502.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Blanca IR, Bere EW, Young HA and Ortaldo
JR: Human B cell activation by autologous NK cells is regulated by
CD40-CD40 ligand interaction: Role of memory B cells and CD5+ B
cells. J Immunol. 167:6132–6139. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zingoni A, Sornasse T, Cocks BG, Tanaka Y,
Santoni A and Lanier LL: Cross-talk between activated human NK
cells and CD4+ T cells via OX40-OX40 ligand interactions. J
Immunol. 173:3716–3724. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Orange JS and Ballas ZK: Natural killer
cells in human health and disease. Clin Immunol. 118:1–10. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chalifour A, Jeannin P, Gauchat JF,
Blaecke A, Malissard M, N'Guyen T, Thieblemont N and Delneste Y:
Direct bacterial protein PAMP recognition by human NK cells
involves TLRs and triggers α-defensin production. Blood.
104:1778–1783. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Perrot I, Deauvieau F, Massacrier C,
Hughes N, Garrone P, Durand I, Demaria O, Viaud N, Gauthier L,
Blery M, et al: TLR3 and Rig-like receptor on myeloid dendritic
cells and Rig-like receptor on human NK cells are both mandatory
for production of IFN-gamma in response to double-stranded RNA. J
Immunol. 185:2080–2088. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qiu F, Maniar A, Quevedo Diaz M, Chapoval
AI and Medvedev AE: Activation of cytokine-producing and antitumor
activities of natural killer cells and macrophages by engagement of
Toll-like and NOD-like receptors. Innate Immun. 17:375–387. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sivori S, Carlomagno S, Moretta L and
Moretta A: Comparison of different CpG oligodeoxynucleotide classes
for their capability to stimulate human NK cells. Eur J Immunol.
36:961–967. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Girart MV, Fuertes MB, Domaica CI, Rossi
LE and Zwirner NW: Engagement of TLR3, TLR7, and NKG2D regulate
IFN-gamma secretion but not NKG2D-mediated cytotoxicity by human NK
cells stimulated with suboptimal doses of IL-12. J Immunol.
179:3472–3479. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sivori S, Carlomagno S, Pesce S, Moretta
A, Vitale M and Marcenaro E: TLR/NCR/KIR: Which one to use and
when? Front Immunol. 5:1052014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Saikh KU, Lee JS, Kissner TL, Dyas B and
Ulrich RG: Toll-like receptor and cytokine expression patterns of
CD56+ T cells are similar to natural killer cells in response to
infection with Venezuelan equine encephalitis virus replicons. J
Infect Dis. 188:1562–1570. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lauzon NM, Mian F, MacKenzie R and Ashkar
AA: The direct effects of Toll-like receptor ligands on human NK
cell cytokine production and cytotoxicity. Cell Immunol.
241:102–112. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Becker I, Salaiza N, Aguirre M, Delgado J,
Carrillo-Carrasco N, Kobeh LG, Ruiz A, Cervantes R, Torres AP,
Cabrera N, et al: Leishmania lipophosphoglycan (LPG) activates NK
cells through toll-like receptor-2. Mol Biochem Parasitol.
130:65–74. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Schmidt KN, Leung B, Kwong M, Zarember KA,
Satyal S, Navas TA, Wang F and Godowski PJ: APC-independent
activation of NK cells by the toll-like receptor 3 agonist
double-stranded RNA. J Immunol. 172:138–143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sivori S, Falco M, Della Chiesa M,
Carlomagno S, Vitale M, Moretta L and Moretta A: CpG and
double-stranded RNA trigger human NK cells by toll-like receptors:
Induction of cytokine release and cytotoxicity against tumors
dendritic cells. Proc Natl Acad Sci USA. 101:10116–10121. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gorski KS, Waller EL, Bjornton-Severson J,
Hanten JA, Riter CL, Kieper WC, Gorden KB, Miller JS, Vasilakos JP,
Tomai MA and Alkan SS: Distinct indirect pathways govern human
NK-cell activation by TLR-7 and TLR-8 agonists. Int Immunol.
18:1115–1126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Alter G, Suscovich TJ, Teigen N, Meier A,
Streeck H, Brander C and Altfeld M: Single-stranded RNA derived
from HIV-1 serves as a potent activator of NK cells. J Immunol.
178:7658–7666. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mian MF, Lauzon NM, Andrews DW, Lichty BD
and Ashkar AA: FimH can directly activate human and murine natural
killer cells via TLR4. Mol Ther. 18:1379–1388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
He S, Chu J, Wu LC, Mao H, Peng Y,
Alvarez-Breckenridge CA, Hughes T, Wei M, Zhang J, Yuan S, et al:
MicroRNAs activate natural killer cells through Toll-like receptor
signaling. Blood. 121:4663–4671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Guo Q and Zhang C: Critical role of
Toll-like receptor signaling in NK cell activation. Chinese Sci
Bull. 57:3192–3202. 2012. View Article : Google Scholar
|
|
59
|
Adib-Conquy M, Scott-Algara D, Cavaillon
JM and Souza-Fonseca-Guimaraes F: TLR-mediated activation of NK
cells and their role in bacterial/viral immune responses in
mammals. Immunol Cell Biol. 92:256–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ljunggren HG and Malmberg KJ: Prospects
for the use of NK cells in immunotherapy of human cancer. Nat Rev
Immunol. 7:329–339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Algarra I, García-Lora A, Cabrera T,
Ruiz-Cabello F and Garrido F: The selection of tumor variants with
altered expression of classical and nonclassical MHC class I
molecules: Implications for tumor immune escape. Cancer Immunol
Immunother. 53:904–910. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Samarakoon A, Chu H and Malarkannan S:
Murine NKG2D ligands:‘Double, double toil and trouble.’. Mol
Immunol. 46:1011–1019. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Imai K, Matsuyama S, Miyake S, Suga K and
Nakachi K: Natural cytotoxic activity of peripheral-blood
lymphocytes and cancer incidence: An 11-year follow-up study of a
general population. Lancet. 356:1795–1799. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Coca S, Perez-Piqueras J, Martinez D,
Colmenarejo A, Saez MA, Vallejo C, Martos JA and Moreno M: The
prognostic significance of intratumoral natural killer cells in
patients with colorectal carcinoma. Cancer. 79:2320–2328. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ishigami S, Natsugoe S, Tokuda K, Nakajo
A, Che X, Iwashige H, Aridome K, Hokita S and Aikou T: Prognostic
value of intratumoral natural killer cells in gastric carcinoma.
Cancer. 88:577–583. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Villegas FR, Coca S, Villarrubia VG,
Jiménez R, Chillón MJ, Jareño J, Zuil M and Callol L: Prognostic
significance of tumor infiltrating natural killer cells subset CD57
in patients with squamous cell lung cancer. Lung Cancer. 35:23–28.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Habif G, Crinier A, André P, Vivier E and
Narni-Mancinelli E: Targeting natural killer cells in solid tumors.
Cell Mol Immunol. 16:415–422. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Guillerey C, Huntington ND and Smyth MJ:
Targeting natural killer cells in cancer immunotherapy. Nat
Immunol. 17:1025–1036. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Vitale M, Cantoni C, Pietra G, Mingari MC
and Moretta L: Effect of tumor cells and tumor microenvironment on
NK-cell function. Eur J Immunol. 44:1582–1592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mushtaq MU, Papadas A, Pagenkopf A,
Flietner E, Morrow Z, Chaudhary SG and Asimakopoulos F: Tumor
matrix remodeling and novel immunotherapies: The promise of
matrix-derived immune biomarkers. J Immunother Cancer. 6:652018.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Diermayr S, Himmelreich H, Durovic B,
Mathys-Schneeberger A, Siegler U, Langenkamp U, Hofsteenge J,
Gratwohl A, Tichelli A, Paluszewska M, et al: NKG2D ligand
expression in AML increases in response to HDAC inhibitor valproic
acid and contributes to allorecognition by NK-cell lines with
single KIR-HLA class I specificities. Blood. 111:1428–1436. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chang MC, Cheng HI, Hsu K, Hsu YN, Kao CW,
Chang YF, Lim KH and Chen CG: NKG2A down-regulation by dasatinib
enhances natural killer cytotoxicity and accelerates effective
treatment responses in patients with chronic myeloid leukemia.
Front Immunol. 9:31522018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sanchez-Correa B, Morgado S, Gayoso I,
Bergua JM, Casado JG, Arcos MJ, Bengochea ML, Duran E, Solana R and
Tarazona R: Human NK cells in acute myeloid leukaemia patients:
Analysis of NK cell-activating receptors and their ligands. Cancer
Immunol Immunother. 60:1195–1205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Rouce RH, Shaim H, Sekine T, Weber G,
Ballard B, Ku S, Barese C, Murali V, Wu MF, Liu H, et al: The
TGF-β/SMAD pathway is an important mechanism for NK cell immune
evasion in childhood B-acute lymphoblastic leukemia. Leukemia.
30:800–811. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Valenzuela-Vazquez L, Núñez-Enríquez JC,
Sánchez-Herrera J, Jiménez-Hernández E, Martín-Trejo JA,
Espinoza-Hernández LE, Medina-Sanson A, Flores-Villegas LV,
Peñaloza-González JG, Refugio Torres-Nava J, et al: Functional
characterization of NK cells in Mexican pediatric patients with
acute lymphoblastic leukemia: Report from the Mexican
Interinstitutional Group for the Identification of the Causes of
Childhood Leukemia. PLoS One. 15:e02273142020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jarosz M, Hak Ł, Więckiewicz J, Balcerska
A and Myśliwska J: Clinical immunology NK cells in children with
acute lymphoblastic leukemia and non-Hodgkin lymphoma after
cessation of intensive chemotherapy. Cent Eur J Immunol. 34:94–99.
2009.
|
|
77
|
Hsu KC, Keever-Taylor CA, Wilton A, Pinto
C, Heller G, Arkun K, O'Reilly RJ, Horowitz MM and Dupont B:
Improved outcome in HLA-identical sibling hematopoietic stem-cell
transplantation for acute myelogenous leukemia predicted by KIR and
HLA genotypes. Blood. 105:4878–4884. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Fei F, Lim M, George AA, Kirzner J, Lee D,
Seeger R, Groffen J, Abdel-Azim H and Heisterkamp N: Cytotoxicity
of CD56-positive lymphocytes against autologous B-cell precursor
acute lymphoblastic leukemia cells. Leukemia. 29:788–797. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Maude SL, Laetsch TW, Buechner J, Rives S,
Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers
GD, et al: Tisagenlecleucel in children and young adults with
B-cell lymphoblastic leukemia. N Engl J Med. 378:439–448. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Park JH, Rivière I, Gonen M, Wang X,
Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, et
al: Long-term follow-up of CD19 CAR therapy in acute lymphoblastic
leukemia. N Engl J Med. 378:449–459. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Li L, Liu LN, Feller S, Allen C,
Shivakumar R, Fratantoni J, Wolfraim LA, Fujisaki H, Campana D,
Chopas N, et al: Expression of chimeric antigen receptors in
natural killer cells with a regulatory-compliant non-viral method.
Cancer Gene Ther. 17:147–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Shimasaki N, Fujisaki H, Cho D, Masselli
M, Lockey T, Eldridge P, Leung W and Campana D: A clinically
adaptable method to enhance the cytotoxicity of natural killer
cells against B-cell malignancies. Cytotherapy. 14:830–840. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Xie G, Dong H, Liang Y, Ham JD, Rizwan R
and Chen J: CAR-NK cells: A promising cellular immunotherapy for
cancer. EBioMedicine. 59:1029752020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liu E, Marin D, Banerjee P, Macapinlac HA,
Thompson P, Basar R, Nassif Kerbauy L, Overman B, Thall P, Kaplan
M, et al: Use of CAR-transduced natural killer cells in
CD19-positive lymphoid tumors. N Engl J Med. 382:545–553. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sánchez-Cuaxospa M, Contreras-Ramos A,
Pérez-Figueroa E, Medina-Sansón A, Jiménez-Hernández E, Torres-Nava
JR, Rojas-Castillo E and Maldonado-Bernal C: Low expression of
Toll-like receptors in peripheral blood mononuclear cells of
pediatric patients with acute lymphoblastic leukemia. Int J Oncol.
49:675–681. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Samudio I, Rezvani K, Shaim H, Hofs E,
Ngom M, Bu L, Liu G, Lee JT, Imren S, Lam V, et al: UV-inactivated
HSV-1 potently activates NK cell killing of leukemic cells. Blood.
127:2575–2586. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Flavell DJ, Holmes SE, Warnes SL and
Flavell SU: The TLR3 agonist poly inosinic: Cytidylic acid
significantly augments the therapeutic activity of an anti-CD7
immunotoxin for human T-cell leukaemia. Biomedicines. 7:132019.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cheadle EJ, Lipowska-Bhalla G, Dovedi SJ,
Fagnano E, Klein C, Honeychurch J and Illidge TM: A TLR7 agonist
enhances the antitumor efficacy of obinutuzumab in murine lymphoma
models via NK cells and CD4 T cells. Leukemia. 31:1611–1621. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kim H, Khanna V, Kucaba TA, Zhang W,
Sehgal D, Ferguson DM, Griffith TS and Panyam J: TLR7/8 agonist
loaded nanoparticles augment NK Cell-mediated Antibody-based cancer
immunotherapy. Mol Pharm. 17:2109–2124. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ronsley R, Kariminia A, Ng B, Mostafavi S,
Reid G, Subrt P, Hijiya N and Schultz KR: The TLR9 agonist
(GNKG168) induces a unique immune activation pattern in vivo in
children with minimal residual disease positive acute leukemia:
Results of the TACL T2009-008 phase I study. Pediatr Hematol Oncol.
36:468–481. 2019. View Article : Google Scholar : PubMed/NCBI
|