Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
common type of B-cell non-Hodgkin lymphoma in adults, and is known
to be heterogeneous in clinical manifestations, tissue morphology,
immune typing and prognosis (1,2). The
sub-classification of DLBCL, which is based on cell-of-origin by
using the Hans algorithm (3),
includes germinal center B-cell like (GCB) and non-GCB DLBCL
subtypes. The 5-year overall survival rate for patients with DLBCL
is 60–70% with standard chemotherapy of rituximab plus
cyclophosphamide, doxorubicin, vincristine and prednisone (4). However, DLBCL has a recurrence rate of
30–50% shortly after treatment, and commonly progresses to the
advanced stage (4). Previous studies
(5,6)
have reported that multiple targets and abnormal signaling pathways
lead to DLBCL development, recurrence and drug resistance; thus,
potential therapeutic targets associated with signaling pathways
are key to improve outcomes for patients. The pathogenesis of DLBCL
and effective therapeutic drugs for the disease are currently being
investigated.
It is well known that abnormally expressed proteins
lead to diseases or tumors. Therefore, targeting abnormal proteins
has become a research hotspot. However, without changing the
regulation of gene expression, abnormal proteins will still be
produced. Since DNA is usually not easy to change, RNA has become
the target of regulation. There are numerous non-coding RNAs
(ncRNAs), which serve numerous important roles (7).
MicroRNAs (miRNAs/miRs) form a large family of
ncRNAs with a length of 19–22 nucleotides, and dysregulation of
miRNAs is involved in cancer development and progression (8). Since the first miRNA (lin-4) was
identified by Lee et al (9)
in 1993, the role of miRNAs in tumors has been widely studied, and
it has been reported that miRNAs can regulate ~1/3 of all mammalian
expressing genes at the post-transcriptional level, as well as
inhibiting translation and/or degrading their targeted mRNAs
(8).
The pathogenesis of DLBCL is multifactorial and
complex; understanding the molecular mechanisms involved in DLBCL
is important to identify new therapeutic targets. The present study
aimed to explore the possible pathogenic factors responsible for
the occurrence and development of DLBCL at the miRNA, mRNA and
protein levels, in order to expand the understanding of the
development of DLBCL and provide therapeutic targets for this
disease. Our previous study (5) used
proteomics methods isobaric tags for relative and absolute
quantification (iTRAQ) to explore differentially expressed proteins
in DLBCL. A total of 335 differentially expressed proteins were
identified. Through Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway enrichment analysis, it was found that pathways in cancer,
PI3K/AKT signaling and alcoholism were significantly changed. The
PI3K/AKT signaling pathway was associated with a large number of
differentially expressed proteins, suggesting that DLBCL may be
associated with PI3K/AKT signaling, which may help to develop a
comprehensive treatment of DLBCL and provides new insights into the
pathogenesis of the disease (5).
Furthermore, formalin-fixed paraffin-embedded DLBCL tissue samples
were subjected to immunohistochemistry to verify the expression
levels of the differentially expressed proteins identified by iTRAQ
and associated with the PI3K/AKT signaling pathway (6). The results revealed that this signaling
pathway serves an essential regulatory role in DLBCL (6).
In the current study, microarray methods involving
Agilent Human miRNA Array were employed to detect differentially
expressed miRNAs between DLBCL and lymph node reactive hyperplasia
(LRH) control groups. A total of 10 fresh tissue samples (5 DLBCL
and 5 LRH) were analyzed. Databases were used to predict the
potential target genes of the differentially expressed miRNAs. The
potential target genes were then analyzed by Gene Ontology (GO) and
KEGG pathway enrichment analyses. The results of proteomics and
miRNA array were comprehensively analyzed, and the expression
levels of 8 differentially expressed miRNAs, whose potential target
genes may be regulated by the PI3K/AKT signaling pathway, were
verified by reverse transcription-quantitative PCR (RT-qPCR). The
present study screened and identified differentially expressed
miRNAs, and predicted the potential target genes of the
differentially expressed miRNAs and their key signaling pathways.
The present results provide a basis for further studies on the
causes, underlying molecular mechanisms and molecular biomarkers
for the diagnosis, prevention and effective treatment of DLBCL.
Materials and methods
Patient samples
A total of 30 fresh tissues were collected from
untreated patients who did not receive radiation or chemotherapy
prior to surgery at The First Affiliated Hospital of Xinjiang
Medical University (Ürümqi, China) between January 2012 and
December 2019, including 15 cases of DLBCL (5 GCB and 10 non-GCB)
and 15 cases of LRH. There were 21 males and 11 females patients
with an age range of 3 to 83 years and a median age of 41.5. The
exclusion criteria comprised patients with other lymphoid diseases
and patients undergoing chemotherapy. The inclusion criteria were
fresh lymph node tissue from patients with DLBCL before any
treatment. All fresh lymph node tissues were collected according to
standard operating procedures during surgery, and the samples were
washed with isotonic saline. The samples were slowly frozen in
liquid nitrogen within 8 min, and then stored in a refrigerator at
−80°C. The complete clinical and pathological data, hematoxylin and
eosin sections and paraffin-embedded tissue samples of all patients
were obtained, and the histological diagnosis of the tissues was
confirmed and classified by two senior hematologists. The
pathological classification was based on the 2016-revised 4th
edition of the World Health Organization classification (10), and was further subtyped using the
Hans algorithm (3) by senior
hematopathologists. In total, 10 fresh frozen tissues from the
aforementioned 30 fresh frozen samples were randomly selected and
divided into two groups: 5 DLBCL (2 GCB and 3 non-GCB) tissues as
the experimental group and 5 LRH tissues as the control group. The
samples were subjected to miRNA microarray analysis to screen
differential miRNAs. All the aforementioned 30 fresh frozen tissues
were divided into two groups: 15 DLBCL (5 GCB and 10 non-GCB)
tissues as the experimental group and 15 LRH tissues as the control
group. The samples were subjected to RT-qPCR for detecting the
expression levels of the differentially expressed miRNAs. Written
informed consent was obtained from all patients. All procedures
involving human participants in the present study comply with the
ethical standards of institutions and/or national research
councils, as well as with the Declaration of Helsinki and its
subsequent amendments or similar ethical standards. The present
study was approved by the Ethics Committee of the Department of
Medicine of The First Affiliated Hospital of Xinjiang Medical
University (approval no. 20160218-13). The series record GSE173080
provides access to all of the current data (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE173080).
Screening and identification of
differentially expressed miRNAs RNA extraction
Total RNA was extracted from fresh frozen tissues
using the mirVana™ RNA Isolation kit (cat. no. AM1561; Thermo
Fisher Scientific Inc.) according to the manufacturer's
instructions. Total RNA was quantified by NanoDrop ND-2000 (Thermo
Fisher Scientific, Inc.) and the RNA integrity was assessed using
Agilent Bioanalyzer 2100 (Agilent Technologies, Inc.).
miRNA microarray
The Agilent Human miRNA Array (cat. no. 8×60 K;
Design ID: 070156; Agilent Technologies, Inc.) was used for
analysis of the fresh frozen samples. Sample labeling, microarray
hybridization and washing were performed according to the
manufacturer's standard protocols. Briefly, total RNA was
dephosphorylated, denatured and labeled with cyanine-3-CTP(Cy3).
After purification, the labeled RNAs were hybridized onto the
microarray. After washing, the arrays were scanned with the Agilent
Scanner G2505C (Agilent Technologies, Inc.).
miRNA microarray data analysis
Feature Extraction software (version 10.7.1.1;
Agilent Technologies, Inc.) was used to analyze array images to
obtain the raw data. Next, GeneSpring software (version 12.5;
Agilent Technologies, Inc.) was employed for basic analysis of the
raw data. First, the raw data were normalized with the quantile
algorithm. Probes that had ≥75% of samples in any one condition out
of two conditions with flags in ‘Detected’ were selected for
further data analysis. Differentially expressed miRNAs were
identified by their fold-change and P-value calculated using the
unpaired Student's t-test. The thresholds set for upregulated and
downregulated genes were fold-change ≥2.0 and P≤0.05 (11). Target genes of differentially
expressed miRNAs were the intersection predicted with three
databases [TargetScan, www.targetscan.org; microRNA.org, www.microrna.org; and PITA, http://genie.weizmann.ac.il/pubs/mir07/mir07_dyn_data.html).
GO and KEGG analyses were applied to determine the roles of these
target genes (12). Hierarchical
clustering was performed to show the distinguishable miRNA
expression pattern among samples.
RT-qPCR
Nine differentially expressed proteins which
associated with the PI3K/AKT signaling pathway were screened by the
proteomics method in DLBCL in our previous study (5). A total of 204 differentially expressed
miRNAs were screened by miRNA microarray in the present study, and
miRNA-mRNA prediction was conducted between 204 differentially
expressed miRNAs and the aforementioned 9 differentially expressed
genes, and it was found that the 8 differential miRNAs had
targeting relationships with the 9 differentially expressed genes.
Based on the array and miRNA-mRNA prediction results, 8
differentially expressed miRNAs were selected, including
miR-193a-3p, miR-19a-3p, miR-19b-3p, miR-370-3p, miR-1275,
miR-490-5p, miR-630 and miR-665, whose potential target genes may
regulate the PI3K/AKT signaling pathway, were verified by RT-qPCR
in DLBCL and LRH tissues. Total RNA was isolated from tissues using
TRIzol® reagent (cat. no. 15596026; Thermo Fisher
Scientific, Inc.) and reversely transcribed into cDNA using the
miRNA First Strand cDNA kit (cat. no. B532451; Sangon Biotech Co.,
Ltd.) according to the manufacturer's protocol. The sequences of
the forward primers for miRNAs are shown in Table I, and the reverse primer was provided
in the miRNA Fluorescence Quantitative PCR kit (SYBR Green dye
method) (cat. no. B532461; Sangon Biotech Co., Ltd.). qPCR was
performed with the Applied Biosystems 7500 Fast PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using the miRNA
Fluorescence Quantitative PCR kit. U6 was used as an internal
control gene (13). The miRNA
expression levels were determined by RT-qPCR and calculated using
the 2−ΔΔCq method (14,15). The
thermocycling conditions for the PCR were 95°C for 30 sec, followed
by 40 cycles of 95°C for 5 sec and 60°C for 30 sec.
 | Table I.Sequences of the miRNA primers used
for reverse transcription-quantitative PCR. |
Table I.
Sequences of the miRNA primers used
for reverse transcription-quantitative PCR.
| miRNA | Sequence
(5′-3′) |
|---|
|
hsa-miR-193a-3p-F |
GCAGAACTGGCCTACAAAG |
|
hsa-miR-19a-3p-F |
GCAGTGTGCAAATCTATGCAA |
|
hsa-miR-19b-3p-F |
AGTGTGCAAATCCATGCAA |
|
hsa-miR-370-3p-F |
CCTGCTGGGGTGGAA |
| hsa-miR-1275-F |
GGTGGGGGAGAGGCT |
|
hsa-miR-490-5p-F |
GCAGCCATGGATCTCC |
| hsa-miR-630-F |
CAGAGTATTCTGTACCAGGGAA |
| hsa-miR-665-F |
ACCAGGAGGCTGAGG |
| U6-F |
GCTTCGGCAGCACATATACTAAAAT |
Statistical analysis
All quantitative data are expressed as the mean ± SD
of 3 experiments and were analyzed with SPSS 18.0 software (SPSS,
Inc.). Unpaired Student's t-test and one-way ANOVA followed by the
least significance difference post-hoc test were used for
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Differential miRNAs screening
A total of 2,550 miRNAs were detected by Agilent
Human miRNA Array. For the comparison between the experimental
group and the control group, the probes marked with 75% samples
‘Detected’ in at least one group were selected for the second step
of differential screenings. For the group with biological repeats,
fold-change ≥2 and P≤0.05 were used as the screening criteria. In
total, 204 differential miRNAs were screened (Table SI), among which, 54 were upregulated
and 150 were downregulated in DLBCL compared with in LRH
tissues.
Target gene prediction
Target genes of differentially expressed miRNAs were
the intersection predicted with three databases (TargetScan,
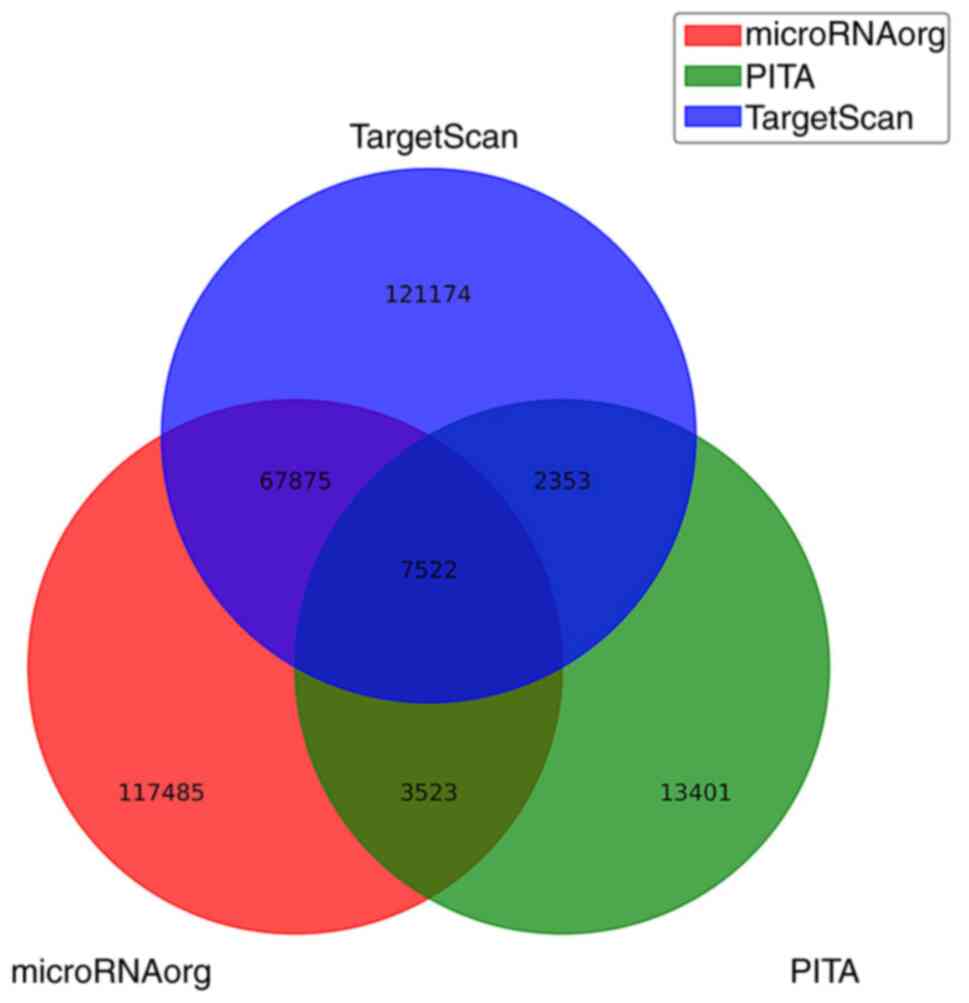
microRNA.org and PITA). A total of 121,174 target genes were
predicted only in TargetScan, 13,401 target genes were predicted
only in PITA and 117,485 target genes were predicted only in
microRNA.org. Additionally, 67,875 overlapping genes (between
microRNA.org and TargetScan), 3,523 overlapping genes (between
microRNA.org and PITA) and 2,353 overlapping genes (between
TargetScan and PITA) were identified. The intersection of the
target genes predicted by all three databases consisted of 7,522
target genes (Fig. 1).
GO functional enrichment analysis
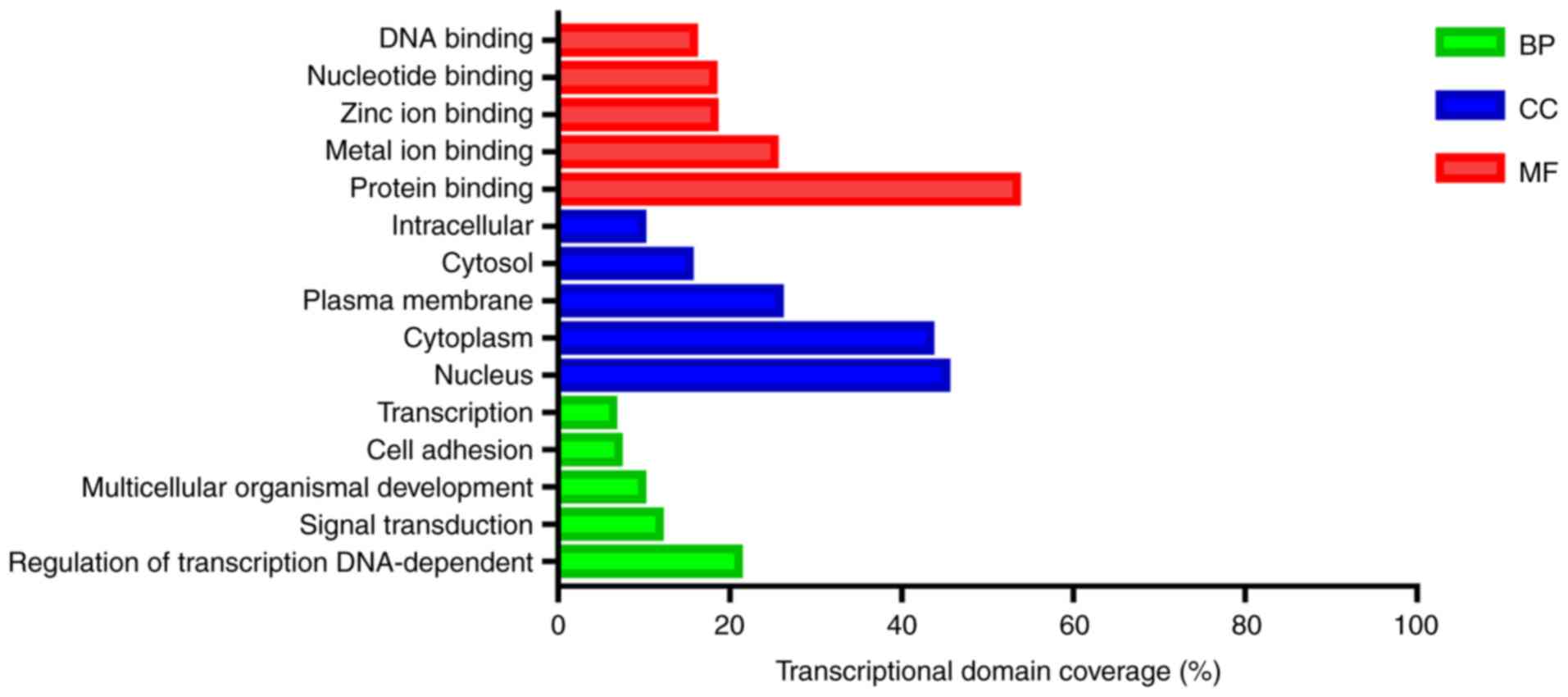
The target genes of differentially expressed miRNAs
were particularly enriched in molecular functions, especially in
‘protein binding’, ‘metal ion binding’ and ‘zinc ion binding’
(Table II and Fig. 2). For biological processes, the
target genes of differentially expressed miRNAs were enriched in
‘regulation of transcription, DNA-dependent’, ‘signal transduction’
and ‘multicellular organismal development’ (Table II and Fig. 2). In addition, GO cell component
analysis indicated that the target genes were enriched in
‘nucleus’, ‘cytoplasm’ and ‘plasma membrane’ (Table II and Fig. 2).
 | Table II.GO functional enrichment analysis of
target genes. |
Table II.
GO functional enrichment analysis of
target genes.
| A, Biological
process |
|---|
|
|---|
| Rank | ID | Term | List hits | List total | Population
hits | Population
total | P-value |
|---|
| 1 | GO:0006355 | Regulation of
transcription, DNA-dependent |
607 | 3,540 | 1,900 | 14,200 |
6.83×10−14 |
| 2 | GO:0007165 | Signal
transduction |
341 | 3,540 | 1,146 | 14,200 |
6.18×10−5 |
| 3 | GO:0007275 | Multicellular
organismal development |
282 | 3,540 |
913 | 14,200 |
1.49×10−5 |
| 4 | GO:0007155 | Cell adhesion |
204 | 3,540 |
542 | 14,200 |
1.62×10−11 |
| 5 | GO:0006351 | Transcription,
DNA-dependent |
203 | 3,540 |
538 | 14,200 |
1.40×10−11 |
| 6 | GO:0006468 | Protein
phosphorylation |
186 | 3,540 |
487 | 14,200 |
3.13×10−11 |
| 7 | GO:0045944 | Positive regulation
of transcription from RNA polymerase II promoter |
183 | 3,540 |
470 | 14,200 |
6.66×10−12 |
| 8 | GO:0007399 | Nervous system
development |
169 | 3,540 |
387 | 14,200 |
2.83×10−16 |
| 9 | GO:0006915 | Apoptosis |
168 | 3,540 |
582 | 14,200 |
1.51×10−2 |
| 10 | GO:0030154 | Cell
differentiation |
158 | 3,540 |
496 | 14,200 |
2.40×10−4 |
|
| B, Cellular
component |
|
| Rank | ID | Term | List
hits | List
total | Population
hits | Population
total | P-value |
|
| 1 | GO:0005634 | Nucleus | 1,537 | 3,899 | 5,241 | 16,399 |
7.40×10−30 |
| 2 | GO:0005737 | Cytoplasm | 1,471 | 3,899 | 4,954 | 16,399 |
4.45×10−31 |
| 3 | GO:0005886 | Plasma
membrane |
876 | 3,899 | 3,398 | 16,399 |
1.18×10−3 |
| 4 | GO:0005829 | Cytosol |
562 | 3,899 | 1,980 | 16,399 |
2.54×10−7 |
| 5 | GO:0005622 | Intracellular |
521 | 3,899 | 1,939 | 16,399 |
4.15×10−4 |
| 6 | GO:0005730 | Nucleolus |
334 | 3,899 | 1,097 | 16,399 |
9.81×10−8 |
| 7 | GO:0005794 | Golgi
apparatus |
294 | 3,899 |
885 | 16,399 |
3.72×10−11 |
| 8 | GO:0005783 | Endoplasmic
reticulum |
248 | 3,899 |
952 | 16,399 |
4.95×10−2 |
| 9 | GO:0005654 | Nucleoplasm |
240 | 3,899 |
813 | 16,399 |
6.67×10−5 |
| 10 | GO:0005856 | Cytoskeleton |
239 | 3,899 |
773 | 16,399 |
2.03×10−6 |
|
| C, Molecular
function |
|
| Rank | ID | Term | List
hits | List
total | Population
hits | Population
total | P-value |
|
| 1 | GO:0005515 | Protein
binding | 1,675 | 3,673 | 5,297 | 14,962 |
3.30×10−49 |
| 2 | GO:0046872 | Metal ion
binding |
790 | 3,673 | 2,879 | 14,962 |
3.88×10−5 |
| 3 | GO:0008270 | Zinc ion
binding |
571 | 3,673 | 1,988 | 14,962 |
2.68×10−6 |
| 4 | GO:0000166 | Nucleotide
binding | 569 | 3,673 | 1,985 | 14,962 |
3.65×10−6 |
| 5 | GO:0003677 | DNA binding | 497 | 3,673 | 1,775 | 14,962 |
2.09×10−4 |
| 6 | GO:0005524 | ATP binding | 402 | 3,673 | 1,494 | 14,962 |
1.44×10−2 |
| 7 | GO:0003700 | Sequence-specific
DNA binding transcription factor activity | 337 | 3,673 |
936 | 14,962 |
4.15×10−16 |
| 8 | GO:0005509 | Calcium ion
binding | 206 | 3,673 |
649 | 14,962 |
1.36×10−5 |
| 9 | GO:0043565 | Sequence-specific
DNA binding | 205 | 3,673 |
548 | 14,962 |
6.39×10−12 |
| 10 | GO:0003723 | RNA binding | 170 | 3,673 |
608 | 14,962 |
2.69×10−2 |
KEGG pathway functional enrichment
analysis
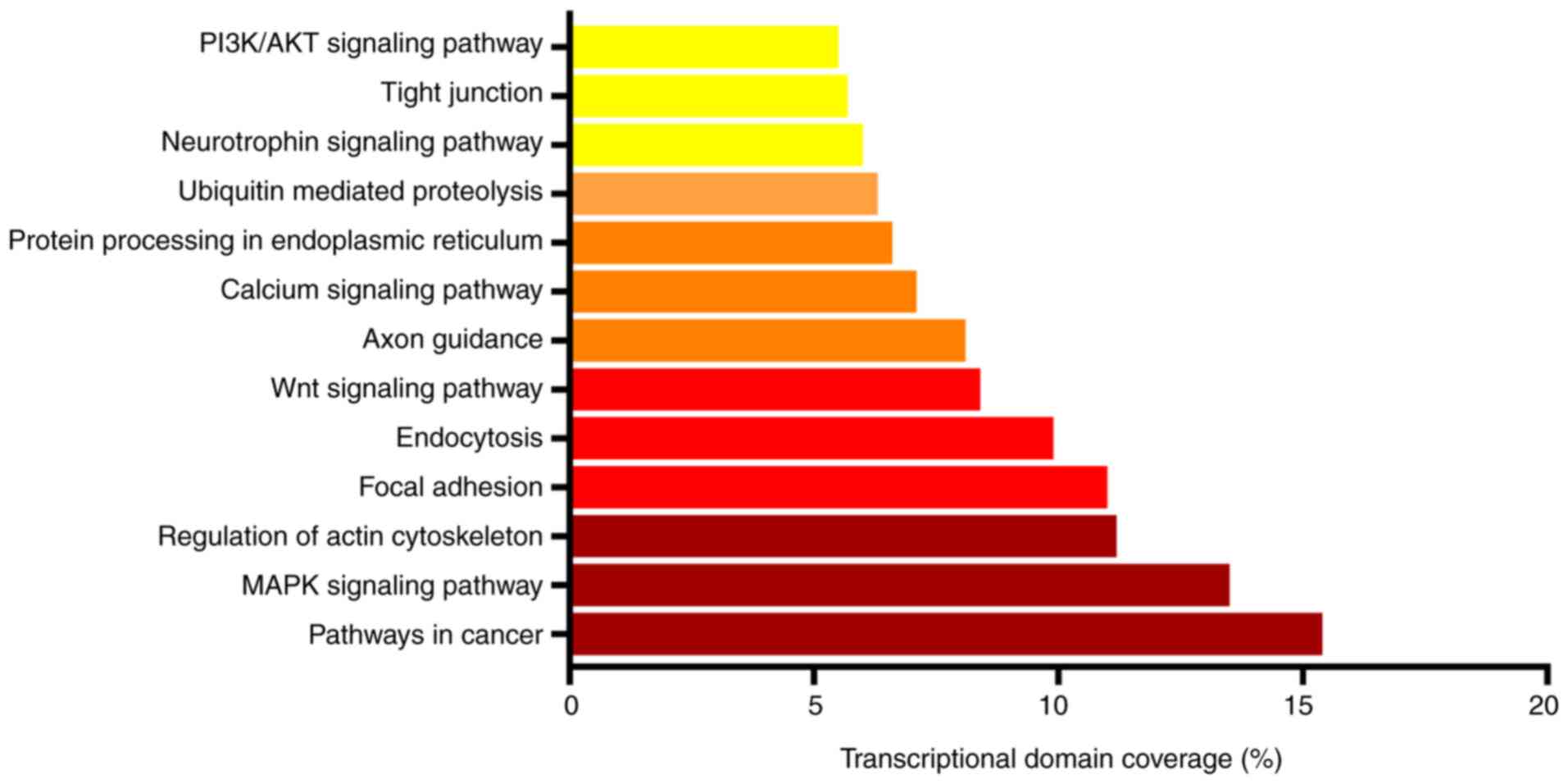
According to the results of KEGG enrichment
analysis, the target genes of differentially expressed miRNAs were
enriched in ‘pathways in cancer’, ‘MAPK signaling pathway’,
‘regulation of actin cytoskeleton’, ‘focal adhesion’,
‘endocytosis’, ‘Wnt signaling pathway’, ‘axon guidance’, ‘calcium
signaling pathway’ and ‘PI3K/AKT signaling pathway’ (Table III and Fig. 3).
 | Table III.Kyoto Encyclopedia of Genes and
Genomes pathway functional enrichment analysis. |
Table III.
Kyoto Encyclopedia of Genes and
Genomes pathway functional enrichment analysis.
| Rank | ID | Term | List hits | List total | Population
hits | Population
total | P-value |
|---|
| 1 | 5200 | Pathways in
cancer | 131 | 1,399 | 327 | 5,981 |
3.51×10−12 |
| 2 | 4010 | MAPK signaling
pathway | 115 | 1,399 | 268 | 5,981 |
3.68×10−13 |
| 3 | 4810 | Regulation of actin
cytoskeleton | 95 | 1,399 | 214 | 5,981 |
4.37×10−12 |
| 4 | 4510 | Focal adhesion | 94 | 1,399 | 200 | 5,981 |
8.62×10−14 |
| 5 | 4144 | Endocytosis | 84 | 1,399 | 203 | 5,981 |
5.40×10−9 |
| 6 | 4310 | Wnt signaling
pathway | 71 | 1,399 | 151 | 5,981 |
1.02×10−10 |
| 7 | 4360 | Axon guidance | 69 | 1,399 | 130 | 5,981 |
1.27×10−13 |
| 8 | 4020 | Calcium signaling
pathway | 60 | 1,399 | 177 | 5,981 |
8.32×10−4 |
| 9 | 4141 | Protein processing
in endoplasmic reticulum | 56 | 1,399 | 167 | 5,981 |
1.63×10−3 |
| 10 | 4120 | Ubiquitin mediated
proteolysis | 53 | 1,399 | 139 | 5,981 |
5.85×10−5 |
| 11 | 4722 | Neurotrophin
signaling pathway | 51 | 1,399 | 127 | 5,981 |
1.56×10−5 |
| 12 | 4530 | Tight junction | 48 | 1,399 | 133 | 5,981 |
5.75×10−4 |
| 13 | 4151 | PI3K/AKT signaling
pathway | 46 | 1,399 | 138 | 5,981 |
4.63×10−3 |
| 14 | 4514 | Cell adhesion
molecules (CAMs) | 42 | 1,399 | 136 | 5,981 |
2.61×10−2 |
| 15 | 4660 | T cell receptor
signaling pathway | 42 | 1,399 | 108 | 5,981 |
2.03×10−4 |
Hierarchical clustering
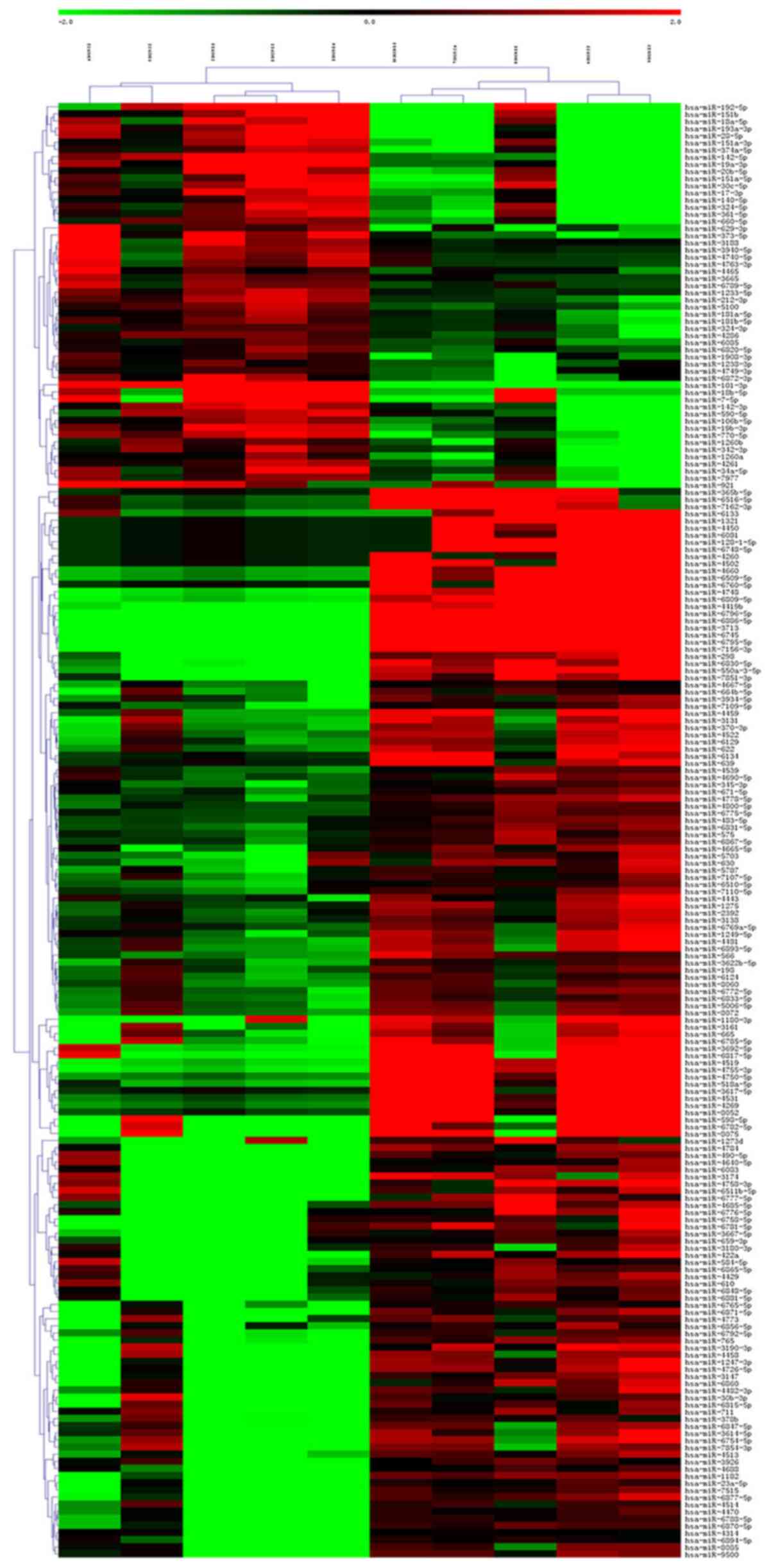
Through hierarchical clustering analysis, it was
found that the same samples can appear in the same cluster, and
miRNAs clustered in the same cluster may have similar biological
functions (Fig. 4).
Validation of the differentially
expressed miRNAs
Only statistically significant different miRNAs
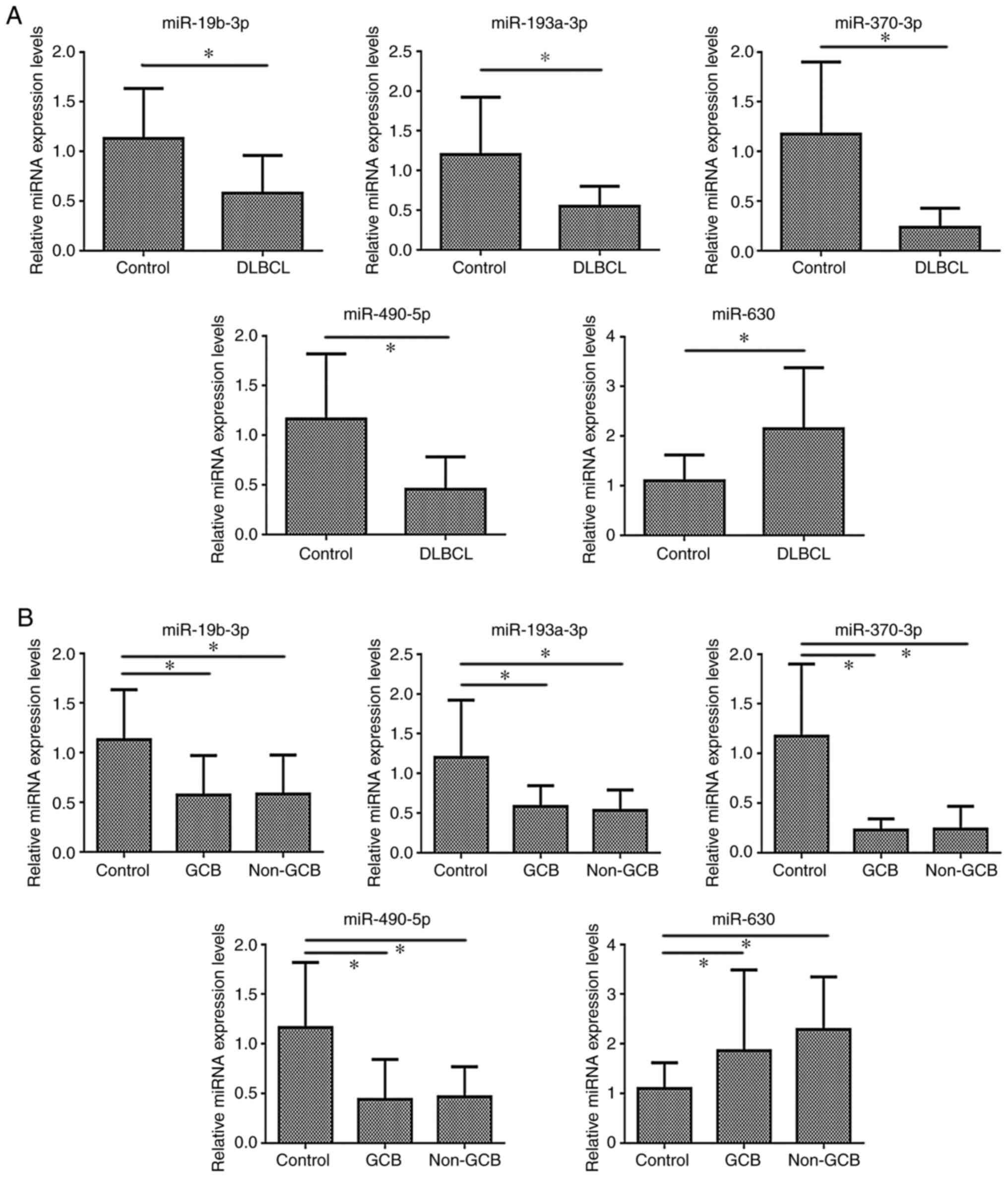
RT-qPCR results were shown. Compared with those in the control
group, the expression levels of miR-19b-3p, miR-193a-3p, miR-370-3p
and miR-490-5p were significantly lower in the DLBCL group
(P<0.05), while miR-630 expression was significantly higher in
DLBCL (P<0.05) (Table IV and
Fig. 5A).
 | Table IV.Analysis of miRNA expression levels
in DLBCL vs. control groups (mean±SD). |
Table IV.
Analysis of miRNA expression levels
in DLBCL vs. control groups (mean±SD).
| Groups | miR-19a-3p | miR-19b-3p | miR-193a-3p | miR-370-3p | miR-1275 | miR-490-5p | miR-630 | miR-665 |
|---|
| Control (LRH)
(n=15) | 1.134±0.576 | 1.132±0.503 | 1.200±0.722 | 1.172±0.728 | 1.186±0.788 | 1.164±0.655 | 1.095±0.524 | 1.085±0.430 |
| DLBCL (n=15) | 0.882±0.648 | 0.578±0.382 | 0.548±0.252 | 0.236±0.192 | 1.299±0.753 | 0.456±0.326 | 2.145±1.235 | 1.440±0.948 |
| T value | 1.126 | 3.397 | 3.299 | 4.817 | −0.401 | 3.742 | −3.030 | −1.319 |
| P-value | 0.270 | 0.002 | 0.004 | 0.000 |
0.692 | 0.001 |
0.007 |
0.198 |
As shown in Table V,
the GCB and non-GCB groups exhibited significantly lower expression
levels of miR-19b-3p, miR-193a-3p, miR-370-3p and miR-490-5p
(P<0.05), and significantly higher expression levels of miR-630
compared with the LRH group. Additionally, there was no significant
differences in miRNA expression between the GCB and non-GCB groups
(Fig. 5B).
 | Table V.Analysis of miRNA expression levels
in GCB, non-GCB DLBCL subtypes and control groups (mean±SD). |
Table V.
Analysis of miRNA expression levels
in GCB, non-GCB DLBCL subtypes and control groups (mean±SD).
| Groups | miR-19a-3p | miR-19b-3p | miR-193a-3p | miR-370-3p | miR-1275 | miR-490-5p | miR-630 | miR-665 |
|---|
| Control (LRH)
(n=15) | 1.134±0.576 | 1.132±0.503 | 1.269±0.904 | 1.172±0.728 | 1.186±0.788 | 1.164±0.655 | 1.095±0.524 | 1.085±0.430 |
| GCB (n=5) | 0.682±0.308 |
0.573±0.397a |
0.582±0.263a |
0.230±0.111a | 0.975±0.568 |
0.437±0.406a |
1.860±1.628a | 0.949±0.372 |
| Non-GCB (n=10) | 0.982±0.760 |
0.580±0.396a |
0.531±0.259a |
0.239±0.228a | 1.461±0.807 |
0.466±0.303a |
2.287±1.061a | 1.685±1.067 |
Discussion
DLBCL is a common lymphoid malignancy in adults.
Despite the improvements in therapeutic options and survival of
patients, treatment resistance is a major clinical challenge in
DLBCL, and ~40% of patients have refractory disease or relapse
(16). Therefore, it is urgent to
find novel treatment targets and effective therapeutic drugs to
improve the survival of patients with DLBCL.
Microarray analysis has become a widely used tool
for generating gene expression data on a genomic scale and has
emerged as a promising and efficient tool for screening significant
genetic or epigenetic alterations in carcinogenesis (17). miRNAs are known as one of the main
regulators of gene expression in important biological and
physiological processes, such as cell proliferation, apoptosis,
proliferation, differentiation, cell motility and angiogenesis, as
well as in disease initiation and progression, being particularly
important in cancer cell invasion, migration and metastasis
(18). Additionally, miRNAs are
involved in B-cell development, B-cell receptor, B-cell
migration/adhesion and production of follicles, plasma cells and
memory B cells (19). Therefore,
miRNAs are expected to receive increased attention as molecular
targets for the diagnosis, prediction of prognosis and treatment of
DLBCL. Beheshti et al (20)
identified a circulating miRNA signature in a Smurf2-deficient
mouse model that spontaneously develops DLBCL. By using cut-points
from recursive partitioning analysis, they derived a 5-miRNA
signature (let-7b, let-7c, miR-18a, miR-24, and miR-15a) with a
classification rate of 91% for serum from patients with DLBCL vs.
normal controls. These circulating miRNAs were able to distinguish
between DLBCL subtypes and disease characteristics for
clinicopathological diagnosis. Ting et al (21) found that miR-155, miR-17/92, miR-21,
miR-224 and mir-146b-5p have value in predicting treatment response
to chemotherapy in DLBCL. It has been suggested that miRNAs can be
employed as an indicator to predict relapse or refractoriness after
treatment of DLBCL (21). In the
present study, 204 differential miRNAs were screened in DLBCL,
which may provide a basis for researchers to identify the
pathogenesis of DLBCL, and may serve in the future as reliable
biomarkers for precise diagnosis and as therapeutic targets for
improvement of treatment efficacy in DLBCL.
miRNAs are important regulators of gene expression,
since they eventually lead to a decrease in the observed mRNA
expression levels of target genes (22). Therefore, the present study predicted
the target genes of the 204 differentially expressed miRNAs with
three databases (TargetScan, microRNA.org and PITA). In total,
7,522 overlapping target genes of the 204 differential miRNAs were
identified. The current results may provide a theoretical basis for
future studies on the occurrence and development of DLBCL.
miRNAs regulate target genes or themselves to
activate or inhibit signaling pathways, and have become a research
hotspot for tumor development and therapeutic targets in DLBCL
(23). In the present study, KEGG
analysis was implemented to determine the roles of these target
genes. It was found that the target genes were enriched in
‘pathways in cancer’, ‘MAPK signaling pathway’, ‘regulation of
actin cytoskeleton’, ‘focal adhesion’, ‘endocytosis’, ‘Wnt
signaling pathway’, ‘axon guidance’, ‘calcium signaling pathway’
and ‘PI3K/AKT signaling pathway’. Shim et al (24) has reported that miR-124 acts as a
tumor suppressor by targeting NF-κB p65 in B-cell lymphoma. Zhao
et al (25) has indicated
that SMAD5 antisense RNA 1 inhibits DLBCL cell proliferation by
sponging miR-135b-5p to upregulate adenomatous polyposis coli
expression and inactivate the classic Wnt/β-catenin signaling
pathway. Yoon et al (23)
found that the PI3K/AKT signaling pathway is strongly enriched with
targets of miR-29 in DLBCL. In the present study, certain signaling
pathways, such as pathways in cancer, and the MAPK, Wnt and
PI3K/AKT signaling pathways, were identified, which is consistent
with the results of previous studies on DLBCL (5,23). In
addition, a number of novel signaling pathways were identified in
the present study, such as regulation of actin cytoskeleton, focal
adhesion, endocytosis, axon guidance and the calcium signaling
pathway, which may therefore be associated with the occurrence and
development of DLBCL. The role of these novel signaling pathways in
DLBCL needs to be further studied.
According to our previous studies (5,6) the
PI3K/AKT signaling pathway serves an important regulatory role in
the occurrence and progression of DLBCL. A previous proteomic study
demonstrated that there were 9 differential proteins in DLBCL that
regulated the PI3K/AKT signaling pathway (5), hence miRNA-mRNA prediction was
conducted with these 9 miRNAs in the present study. Thus, eight
differentially expressed miRNAs in DLBCL and LRH, whose potential
target genes may regulate the PI3K/AKT signaling pathway, were
verified by RT-qPCR. It was revealed that miR-19b-3p, miR-193a-3p,
miR-370-3p and miR-490-5p exhibited low expression in DLBCL
(P<0.05), while miR-630 expression in DLBCL was high
(P<0.05). These differentially expressed miRNAs serve an
important regulatory role in a variety of tumors; however, there
are limited studies on DLBCL.
miR-19b-3p has been reported to be associated with
favorable or unfavorable events in several types of cancer. Its
role is controversial depending on the tumor, and it may be a good
non-invasive biomarker for cancer detection. Tang et al
(26) has reported that miR-19b-3p
promotes intrahepatic cholangiocarcinoma (ICC) cell proliferation
and epithelial-mesenchymal transition (EMT), and inhibits
apoptosis, while knockdown of coiled-coil domain containing 6
(CCDC6) reverses these effects. These results suggest that serum
miR-19b-3p expression may be an important biomarker for ICC
diagnosis, and targeting the miR-19b-3p-CCDC6 axis may be a
promising strategy for ICC treatment (26). Park et al (27) has reported that miR-19b-3p can
inhibit the migration of breast cancer cells through
exosome-mediated delivery by targeting aquaporin-5. Marcuello et
al (28) has demonstrated that a
plasma 6-miRNA signature (miR-15b-5p, miR-18a-5p, miR-29a-3p,
miR-335-5p, miR-19a-3p and miR-19b-3p) can be used to distinguish
between colorectal cancer (CRC) or advanced adenoma and healthy
individuals. The aforementioned serum 6-miRNA signature may be a
useful strategy to improve the diagnostic performance of current
CRC screening programs. Although miR-19b-3p has been implicated in
certain types of cancer, its role in cancer remains controversial,
and there are no relevant studies on its role in DLBCL.
Numerous studies (29,30) have
revealed the crucial role of the miR-193 family, which comprises
miR-193a-3p, miR-193a-5p, miR-193b-3p and miR-193b-5p, in health
and disease-associated biological processes by interacting with
specific target genes and signaling pathways, mainly acting as a
tumor suppressor. Wang et al (29) has demonstrated that miR-193a-3p is a
tumor suppressor gene that serves an important role in the biology
of health and disease by interacting with specific targets and
signals, and it also inhibits the proliferation and facilitates the
apoptosis of hepatocellular carcinoma (HCC) cells. It has been
suggested that miR-193a-3p may be used as a promising biomarker for
the diagnosis of HCC and as a therapeutic target for HCC in the
future (29). Lin et al
(30) has revealed that the
expression levels of miR-193a-3p in human CRC cell lines are
significantly decreased compared with those in a normal colonic
epithelium cell line. Overexpression of miR-193a-3p inhibits the
proliferation, migration and angiogenesis of CRC cells, while
forced expression of plasminogen activator urokinase rescues these
inhibitory effects (30). Chen et
al (31) has demonstrated that
miR-193a-3p expression in pancreatic ductal adenocarcinoma (PDAC)
tissues is significantly lower than that in non-cancerous tissues.
When overexpressing miR-193a-3p in PDAC cells, their proliferative
ability was significantly inhibited, the apoptosis rate was
accelerated, and the cell cycle was blocked in the G1
and G2/M phases (31). In
the present study, it was found that miR-193a-3p exhibited low
expression in DLBCL (P<0.05). It was speculated that the
functional role of this miRNA in DLBCL may be considered as a tumor
suppressor. However, to the best of our knowledge, there are no
relevant studies on the role of miR-193a-3p in DLBCL.
miR-370-3p serves an important regulatory role in a
variety of tumors, and increasing evidence has suggested that it is
downregulated and acts as a tumor suppressor in numerous types of
cancer. Several studies (32–34) have
indicated that it serves a regulatory role in tumors through the
regulation of target genes, it can increase the sensitivity to
chemotherapy drugs and it can be used as a biomarker or a
therapeutic target in tumors. Li et al (32) has found that miR-370-3p inhibits
chronic myeloid leukemia cell proliferation and induces apoptosis
by suppressing PDZ and LIM domain protein 1/Wnt/β-catenin
signaling. Nadaradjane et al (33) has indicated that
miR-370-3p/temozolomide (TMZ) treatment is 2-fold more efficient
than TMZ treatment alone in decreasing the volume of glioblastoma
multiforme in mice. It has been suggested that miR-370-3p may be
used as a therapeutic tool for anti-glioblastoma multiforme therapy
(33). Leivonen et al
(34) has profiled the miRNAs of
matched primary and relapsed DLBCL by next-generation sequencing
and has revealed that miR-370-3p is markedly downregulated in the
majority of relapsed DLBCL samples, while overexpression of
miR-370-3p regulates the target genes MAP3K8, PIK3R1, PIK3CG,
PI3KCD and SYK, resulting in downregulated mRNA expression levels.
It has been demonstrated that miR-370-3p downregulates genes
involved in the PI, MAPK and BCR signaling pathways, and enhances
the chemosensitivity of DLBCL cells in vitro (34). The present study indicated that
miR-370-3p expression was downregulated in DLBCL, suggesting that
it may inhibit the occurrence and development of DLBCL. Research on
miR-370-3p in patients with DLBCL is rare; thus, further studies
are required.
Recent studies have found that miR-490-5p is
associated with the occurrence and development of tumors, and
serves an important role in a variety of tumors. Wang et al
(35) has revealed that miR-490-5p
expression is markedly downregulated in neuroblastoma (NB) tissues
and cell lines. Overexpression of miR-490-5p suppresses cell
proliferation, migration and invasion, and induces cell cycle
G0/G1 arrest and apoptosis in NB cell lines
(35). The aforementioned results
have demonstrated for the first time that miR-490-5p may function
as a tumor suppressor in NB by targeting a myeloma overexpressed
gene (35). Xiang et al
(36) has demonstrated that
miR-490-5p promotes the proliferation of bladder cancer cells and
inhibits their apoptosis. In the present study, low miR-490-5p
expression was observed in DLBCL. It was speculated that miR-490-5p
may be involved in the occurrence and development of DLBCL, but no
relevant studies on its regulatory mechanism in DLBCL have been
conducted thus far.
Previous evidence has demonstrated that miR-630 is
involved in multiple processes during cancer development and
progression. Valera et al (37) has performed miRNA profiling in young
patients with prostate cancer (PCa) and compared the results with
those of PCa in older men. Their results have revealed that,
compared with its expression in PCa and its normal counterpart,
miR-630 expression is significantly upregulated in PCa (37). Differentially expressed miRNAs
provide insights into the molecular mechanisms involved in this PCa
subtype. Pan et al (38) has
provided the first evidence that miR-630 inhibits papillary thyroid
carcinoma (PTC) cell growth, metastasis and EMT by suppressing the
JAK2/STAT3 signaling pathway, and indicated that a potential
therapeutic strategy through enhancing miR-630 expression may
benefit patients with PTC. The present study indicated that miR-630
was a differentially expressed miRNA with high expression in DLBCL.
It was hypothesized that miR-630 may be involved in the occurrence
or development of DLBCL, but no relevant studies have been
conducted on DLBCL to date. However, since it serves an important
role in other tumors, it is worth further studying the role of
miR-630 in DLBCL.
In summary, the present study screened 204 miRNAs
that exhibited differential expression between DLBCL and control
groups via miRNA microarray. Altered expression levels of five
miRNAs, including miR-19b-3p, miR-193a-3p, miR-370-3p, miR-490-5p
and miR-630, may contribute to the development of DLBCL. The
current findings provide valuable information to understand the
pathogenesis of DLBCL, and may lead to the development of
therapeutic strategies involving the use of miRNAs for the
treatment of patients with DLBCL. Further exploration of the
biological role of these differentially expressed miRNAs in DLBCL
will strengthen the conclusions of the present study. For example,
future studies should be performed to analyze these differentially
expressed miRNAs in DLBCL cell lines.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Chinese
Society of Clinical Oncology (CSCO)-Roche Solid Tumor Research Fund
(grant no. Y-Roche2019/2-0061) and the Non-profit Central Research
Institute Fund of the Chinese Academy of Medical Sciences (grant
no. 2020-PT330-003).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the Gene Expression Omnibus
repository (GSE173080) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE173080).
Authors' contributions
All authors participated in the preparation of the
manuscript. HXG wrote the first draft of the manuscript and edited
the original figures and tables. XXL conceived and designed the
study. HXG, SJL and MBW conducted the experiments and contributed
to data analysis. XXL and HXG confirmed the authenticity of all the
raw data. XXL, WZ and WLC conceived and designed the study and
revised and reviewed this article for important intellectual
content. SFY, ZPM, JX and WS collected the paraffin-embedded tissue
samples, fresh tissue samples and clinicopathological information.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients. All procedures involving human participants in the
present study comply with the ethical standards of institutions
and/or national research councils, as well as with the Declaration
of Helsinki and its subsequent amendments or similar ethical
standards. The present study was approved by the Ethics Committee
of the Department of Medicine of The First Affiliated Hospital of
Xinjiang Medical University (Ürümqi, China) (approval no.
20160218-13).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DLBCL
|
diffuse large B-cell lymphoma
|
|
LRH
|
lymph node reactive hyperplasia
|
|
miRNAs/miRs
|
microRNAs
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
GCB
|
germinal center B-cell like
|
|
ncRNAs
|
non-coding RNAs
|
|
iTRAQ
|
isobaric tags for relative and
absolute quantification
|
|
ICC
|
intrahepatic cholangiocarcinoma
|
|
CCDC6
|
coiled-coil domain containing 6
|
|
EMT
|
epithelial-mesenchymal transition
|
|
HCC
|
hepatocellular carcinoma
|
|
CRC
|
colorectal cancer
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
TMZ
|
temozolomide
|
|
NB
|
neuroblastoma
|
|
PCa
|
prostate cancer
|
|
PTC
|
papillary thyroid carcinoma
|
References
|
1
|
Li S, Young KH and Medeiros LJ: Diffuse
large B-cell lymphoma. Pathology. 50:74–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roschewski M, Staudt LM and Wilson WH:
Diffuse large B-cell lymphoma-treatment approaches in the molecular
era. Nat Rev Clin Oncol. 11:12–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coiffier B and Sarkozy C: Diffuse large
B-cell lymphoma: R-CHOP failure-what to do? Hematology Am Soc
Hematol Educ Program. 2016:366–378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao HX, Nuerlan A, Abulajiang G, Cui WL,
Xue J, Sang W, Li SJ, Niu J, Ma ZP, Zhang W and Li XX: Quantitative
proteomics analysis of differentially expressed proteins in
activated B-cell-like diffuse large B-cell lymphoma using
quantitative proteomics. Pathol Res Pract. 215:1525282019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao HX, Li SJ, Niu J, Ma ZP, Nuerlan A,
Xue J, Wang MB, Cui WL, Abulajiang G, Sang W, et al: TCL1 as a hub
protein associated with the PI3K/AKT signaling pathway in diffuse
large B-cell lymphoma based on proteomics methods. Pathol Res
Pract. 216:1527992020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Z, Wang Y, Shu S, Cai J, Tang C and
Dong Z: Non-coding RNAs in kidney injury and repair. Am J Physiol
Cell Physiol. 317:C177–C188. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baronti L, Guzzetti I, Ebrahimi P, Friebe
Sandoz S, Steiner E, Schlagnitweit J, Fromm B, Silva L, Fontana C,
Chen AA and Petzold K: Base-pair conformational switch modulates
miR-34a targeting of Sirt1 mRNA. Nature. 583:139–144. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM,
et al: WHO classification of tumours of haematopoetic and lymphoid
tissues. 4th edition. IARC Press; Lyon: 2017
|
|
11
|
Felix TF, Lopez Lapa RM, de Carvalho M,
Bertoni N, Tokar T, Oliveira RA, M Rodrigues MA, Hasimoto CN,
Oliveira WK, Pelafsky L, et al: MicroRNA modulated networks of
adaptive and innate immune response in pancreatic ductal
adenocarcinoma. PLoS One. 14:e02174212019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xue J, Gao HX, Sang W, Cui WL, Liu M, Zhao
Y, Wang MB, Wang Q and Zhang W: Identification of core
differentially methylated genes in glioma. Oncol Lett.
18:6033–6045. 2019.PubMed/NCBI
|
|
13
|
Hu S, Zheng Q, Wu H, Wang C, Liu T and
Zhou W: miR-532 promoted gastric cancer migration and invasion by
targeting NKD1. Life Sci. 177:15–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kakurina GV, Kolegova ES, Shashova EE,
Cheremisina OV, Choynzonov EL and Kondakova IV: Relationship
between the mRNA expression levels of calpains 1/2 and proteins
involved in cytoskeleton remodeling. Acta Naturae. 12:110–113.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Due H, Brøndum RF, Young KH, Bøgsted M and
Dybkær K: MicroRNAs associated to single drug components of R-CHOP
identifies diffuse large B-cell lymphoma patients with poor outcome
and adds prognostic value to the international prognostic index.
BMC Cancer. 20:2372020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li L, Lei Q, Zhang S, Kong L and Qin B:
Screening and identification of key biomarkers in hepatocellular
carcinoma: Evidence from bioinformatic analysis. Oncol Rep.
38:2607–2618. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khordadmehr M, Shahbazi R, Sadreddini S
and Baradaran B: miR-193: A new weapon against cancer. J Cell
Physiol. 234:16861–16872. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Musilova K and Mraz M: MicroRNAs in B-cell
lymphomas: How a complex biology gets more complex. Leukemia.
29:1004–1017. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beheshti A, Stevenson K, Vanderburg C,
Ravi D, McDonald JT, Christie AL, Shigemori K, Jester H, Weinstock
DM and Evens AM: Identification of circulating serum multi-MicroRNA
signatures in human DLBCL models. Sci Rep. 9:171612019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ting CY, Liew SM, Price A, Gan GG, Bee-Lan
Ong D, Tan SY and Bee PC: Clinical significance of aberrant
microRNAs expression in predicting disease relapse/refractoriness
to treatment in diffuse large B-cell lymphoma: A meta-analysis.
Crit Rev Oncol Hematol. 144:1028182019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoon S, Nguyen HCT, Jo W, Kim J, Chi SM,
Park J, Kim SY and Nam D: Biclustering analysis of transcriptome
big data identifies condition-specific microRNA targets. Nucleic
Acids Res. 47:e532019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shim H, Nam J and Kim SW: NF-κB p65
represses microRNA-124 transcription in diffuse large B-cell
lymphoma. Genes Genomics. 42:543–551. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao CC, Jiao Y, Zhang YY, Ning J, Zhang
YR, Xu J, Wei W and Kang-Sheng G: Lnc SMAD5-AS1 as ceRNA inhibit
proliferation of diffuse large B cell lymphoma via Wnt/β-catenin
pathway by sponging miR-135b-5p to elevate expression of APC. Cell
Death Dis. 10:2522019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang Y, Yang J, Wang Y, Tang Z, Liu S and
Tang Y: MiR-19b-3p facilitates the proliferation and
epithelial-mesenchymal transition, and inhibits the apoptosis of
intrahepatic cholangiocarcinoma by suppressing coiled-coil domain
containing 6. Arch Biochem Biophys. 686:1083672020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park EJ, Jung HJ, Choi HJ, Jang HJ, Park
HJ, Nejsum LN and Kwon TH: Exosomes co-expressing AQP5-targeting
miRNAs and IL-4 receptor-binding peptide inhibit the migration of
human breast cancer cells. FASEB J. 34:3379–3398. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marcuello M, Duran-Sanchon S, Moreno L,
Lozano JJ, Bujanda L, Castells A and Gironella M: Analysis of A
6-mirna signature in serum from colorectal cancer screening
participants as non-invasive biomarkers for advanced adenoma and
colorectal cancer detection. Cancers (Basel). 11:15422019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang SS, Huang ZG, Wu HY, He RQ, Yang LH,
Feng ZB, Dang YW, Lu HP, Fang YY and Chen G: Downregulation of
miR-193a-3p is involved in the pathogenesis of hepatocellular
carcinoma by targeting CCND1. PeerJ. 8:e84092020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin M, Zhang Z, Gao M, Yu H, Sheng H and
Huang J: MicroRNA-193a-3p suppresses the colorectal cancer cell
proliferation and progression through downregulating the PLAU
expression. Cancer Manag Res. 11:5353–5363. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen ZM, Yu Q, Chen G, Tang RX, Luo DZ,
Dang YW and Wei DM: MiR-193a-3p inhibits pancreatic ductal
adenocarcinoma cell proliferation by targeting CCND1. Cancer Manag
Res. 11:4825–4837. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li LM, Luo FJ and Song X: MicroRNA-370-3p
inhibits cell proliferation and induces chronic myelogenous
leukaemia cell apoptosis by suppressing PDLIM1/Wnt/β-catenin
signaling. Neoplasma. 67:509–518. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nadaradjane A, Briand J, Bougras-Cartron
G, Disdero V, Vallette FM, Frenel JS and Cartron PF: miR-370-3p is
a therapeutic tool in anti-glioblastoma therapy but is not an
intratumoral or cell-free circulating biomarker. Mol Ther Nucleic
Acids. 13:642–650. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leivonen SK, Icay K, Jäntti K, Siren I,
Liu C, Alkodsi A, Cervera A, Ludvigsen M, Hamilton-Dutoit SJ,
d'Amore F, et al: MicroRNAs regulate key cell survival pathways and
mediate chemosensitivity during progression of diffuse large B-cell
lymphoma. Blood Cancer J. 7:6542017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang J, Zhang X, Yao H, Le Y, Zhou W, Li
J, Lu L, Chen M and Li X: MiR-490-5p functions as tumor suppressor
in childhood neuroblastoma by targeting MYEOV. Hum Cell.
33:261–271. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiang M, Yuan W, Zhang W and Huang J:
Expression of miR-490-5p, miR-148a-3p and miR-608 in bladder cancer
and their effects on the biological characteristics of bladder
cancer cells. Oncol Lett. 17:4437–4442. 2019.PubMed/NCBI
|
|
37
|
Valera VA, Parra-Medina R, Walter BA,
Pinto P and Merino MJ: microRNA expression profiling in young
prostate cancer patients. J Cancer. 11:4106–4114. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pan XM, He XY, Yang YL, Jia WJ, Yang ZQ,
Yan D and Ma JX: MiR-630 inhibits papillary thyroid carcinoma cell
growth, metastasis, and epithelial-mesenchymal transition by
suppressing JAK2/STAT3 signaling pathway. Eur Rev Med Pharmacol
Sci. 23:2453–2460. 2019.PubMed/NCBI
|