Introduction
Bladder cancer is a highly metastatic tumor
(1,2). It is also one of the most common
malignant tumors originates in the urinary system (3). The morbidity rate of bladder cancer is
second only to prostate cancer in Western countries, and in 2018,
there were ~549,400 new cases and 200,000 deaths (4). However, no significant symptoms of
bladder cancer at the early stage makes early diagnosis and
treatment more difficult (5).
Targeted therapy for bladder cancer has become an effective
treatment method (6,7). Therapeutic targets for bladder cancer
have already made some progress; however, it is still hard to meet
the therapeutic requirement (8,9). Thus,
novel and promising therapeutic targets are still required.
Lysosome-associated transmembrane protein 4β
(LAPTM4B) localizes on the late lysosomes by its lysosome
localization motif. It could affect several cellular functions by
regulating multiple signaling pathways (10,11).
LAPTM4B is involved in the process of autophagy (12). In addition, LAPTM4B could contribute
to the recruitment of the amino acid transporter to lysosomes;
therefore, promoting the uptake of lysosomal leucine (13). Mutations in LAPTM4B could lead to
serious genetic diseases, such as Myocardial Ischemia/Reperfusion
Injury (14).
LAPTM4B was firstly found to be highly expressed in
hepatocellular carcinoma (HCC), and has been associated with poor
prognosis in patients with HCC, breast cancer, gastric cancer, and
acute myeloid leukemia (15–18). LAPTM4B was also associated with the
susceptibility to non-small cell lung and ovarian cancers (18). In the development of HCC, LAPTM4B and
AP4 play synergistic roles (19).
LAPTM4B could also promote the development of gastric cancer via
EGFR over-activation, which was repressed by Beclin1 (20). Collectively, LAPTM4B could promote
the proliferation and invasion of cancer cells, induce autophagy,
apoptosis, and assist drug resistance (20). LAPTM4B affects the pathogenesis of
multiple types of tumor; however, its potential role in bladder
cancer progression remains unknown.
In the present study, it was found that LAPTM4B was
associated with the poor prognosis of patients with bladder cancer.
LAPTM4B knockdown notably inhibited cell proliferation, migration
and invasion, and suppressed tumor growth and metastasis in mice.
Thus, LAPTM4B could affect the progression of bladder cancer in
vitro and in vivo.
Materials and methods
Antibodies, primers and short hairpin
(sh) RNA plasmids
The anti-LAPTM4B rabbit polyclonal [for
immunohistochemistry (IHC), 1:100 dilution; for western blot
analysis, 1:1,000 dilution; cat. no. PA5-43047] antibody was
purchased from Thermo Fisher Scientific, Inc., and the anti-β-actin
mouse monoclonal (1:1,000 dilution; cat. no. ab8226) was purchased
from Abcam.
The following primers were used: LAPTM4B forward,
5′-GGAACTGCTACCGATACATCAA-3′ and reverse,
5′-TCACAGTGGCATCATCATACG-3′; β-actin forward,
5′-CAGCTCACCATGGATGATGATATC-3′ and reverse,
5′-AAGCCGGCCTTGCACAT-3′.
The pLVX shRNA plasmid of LAPTM4B (cat. no. 101111)
was purchased from OriGene Technologies, Inc., and has the
following targeted sequence: 5′-GGTCGCCTTCGGAGCGAAGGGTA-3′.
Human tissue samples and analysis
A total of 111 human bladder tumor tissues were
collected from patients (mean age 67.5; 71 males and 40 females)
with bladder cancer, and adjacent tissues (5 mm from the tumor
tissues) following surgery at Liaocheng People's Hospital
(Shandong, China) between September 2015 and June 2018. The
clinicopathological features, including age, sex, tumor stage,
tumor grade, lymph node metastasis and recurrence (tumor redevelops
at the original site) were respectively recorded and analyzed at
Liaocheng People's Hospital.
To further investigate the association between
LAPTM4B expression level and bladder cancer, IHC was performed. In
brief, the samples were fixed with 10% formalin for 24 h at 98°C,
embedded with resin (Epoxy resin; Sigma-Aldrich; Merck KGaA), and
divided into 5-µm thick sections. The sections were dewaxed with
xylene at 65°C, then rehydrated in a gradient ethanol series. The
samples were immersed in citrate buffer (pH, 6.0) at 98°C for 30
min and placed in a microwave for incubation for 10 min for antigen
retrieval. Then, hydrogen peroxide was added to block endogenous
peroxidase activity and the samples were incubated at room
temperature for 10 min, followed by blocking with 2% BSA
(Sigma-Aldrich; Merck KGaA) for 20 min at room temperature.
Subsequently, the samples were incubated with the LAPTM4B antibody
at room temperature for 2 h. Lastly, the samples were washed with
PBS, 4 times and incubated with the secondary antibody (rabbit;
1:200 dilution; cat. no. ab205718; Abcam). Diaminobenzidine was
used as a chromogen substrate. Images were captured using an
Olympus inverted fluorescence microscope (IX71; Zeiss AG).
The proportion of positive stained cells was scored
as follows: 0, negative; 1, 10–50% positive and 2, >50% positive
staining. The staining intensity was assessed on a score of 0
(negative level staining), 1 (low level staining), and 2 (high
level staining). The expression levels of LAPTM4B were further
examined based on a staining index: Staining intensity score plus
staining percentage score. Staining index, 0–2 indicated low
expression, while 3/4 indicated high expression. The Kaplan-Meier
survival analysis of overall survival (OS) and progression-free
survival (PFS) rates was performed between LAPTM4B low and high
expression groups. OS was defined as the time from recruitment into
the study to death from any cause. PFS was defined as the period
after treatment when the disease was stable and did not progress.
The development of the disease was followed up every 6 months.
Cell culture and transfection
The T24 and 5637 bladder tumor cell lines were
purchased from American Type Culture Collection. Both the cell
lines were maintained in RPMI-1640 medium, supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) and incubated at 37°C
in a humidified incubator with 5% CO2.
The shRNA plasmids of LAPTM4B were transfected into
the bladder cancer cell lines using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 4 h. The
scrambled control plasmid (5′-ATGGTACTGACCTCCAGAG-3′) was used as
the negative control (NC). A total of 1×105 cells per
well were seeded into 6-well plates and a total of 2 groups were
used: sh-LAPTM4B group (0.5 µg) and sh-NC group (0.5 µg). Knockdown
efficiency was measured by both reverse transcription-quantitative
PCR (RT-qPCR) and western blot analysis after 48 h. These cells
were used to assess the association between LAPTM4B expression
level and cellular processes. Subsequently the cells in which
LAPTM4B was stably knocked down were screened and used for the
in vitro and in vivo assays.
RT-qPCR
Total RNA was isolated from the T24 and 5637 cell
lines or tumor tissues using TRIzol® (Invitrogen; Thermo
Fisher Scientific, Inc.). Subsequently, the total RNA was reverse
transcribed using M-MLV reverse transcriptase at 42°C for 60 min
(Promega Corporation). qPCR was conducted using SYBR PrimeScript
RT-PCR kit II (cat. no. DRR083; Takara Biotechnology Co., Ltd.) and
the relative expression levels of LAPTM4B was normalized to the
mRNA expression levels of β-actin. The following thermocycling
conditions were used: Initial denaturation at 95°C for 3 min;
followed by 30 cycles of denaturation at 95°C for 30 sec, annealing
at 58°C for 30 sec and extension at 72°C for 30 sec. The
2−ΔΔCq method was used to quantify the results (21).
Western blot analysis
The bladder cancer cells or tissues were lyzed with
lysis buffer [60 mM Tris-HCl (pH 6.8), 2% SDS, 20% glycerol, 0.25%
bromophenol blue, 1.25% 2-mercaptoethanol and protease inhibitor
cocktail]. Protein determination was performed using the BCA
method. A total of 10 µg of each protein sample was loaded per lane
and separated using 10% SDS-PAGE. Then, the proteins were
transferred onto PVDF membranes (cat. no. IPSN07852;
MilliporeSigma) and the membranes were blocked with 5% skimmed milk
in TBS-Tween-20 (0.5%) buffer and subsequently incubated with the
primary antibodies for 2 h at room temperature. Following which,
the membranes were incubated with the secondary antibody (rabbit;
1:5,000 dilution; cat. no. ab205718; Abcam) for 45 min at room
temperature. Each blot was subsequently visualized with an ECL kit
(cat. no. RPN 2109; GE Healthcare).
Colony formation assay
Approximately 1,000 bladder cancer cells were added
into 6-well plates and cultured at 37°C for 48 h after
transfection. After 14 days, the cells were subsequently fixed with
4% paraformaldehyde for 30 min at room temperature and stained with
0.2% crystal violet at room temperature for 20 min, then washed
with PBS for 4 times. Colony numbers were manually counted using an
Olympus inverted fluorescence microscope and images captured (IX71;
Zeiss AG). A colony was counted when it included >100. The
number of colonies per visual field area visible under the
microscope was counted.
MTT assay
The cells, transfected with shRNA plasmids, were
added into 96-well plates for 3 days. Subsequently, the cells were
treated with MTT for 3 h, then 200 µl dimethyl sulfoxide was added
to dissolve the purple formazan and the OD value was measured using
a microplate reader at 570 nm (22).
Transwell invasion assay
The T24 and 5637 cells, transfected with shRNA
plasmid, were subsequently used for Transwell invasion assays. The
upper chambers were coated with 20% Matrigel in RPMI-1640 medium
and incubated at 37°C for 1 h. A total of 1×105 cells in
150 µl serum-free medium was then added into the upper chambers of
the inserts. Medium with 10% FBS was added into the bottom chamber
to stimulate cell invasion. After 24 h incubation at 37°C, the
cells were fixed with 4% paraformaldehyde for 25 min at room
temperature and stained with 0.2% crystal violet for 15 min at room
temperature. Then, images were captured and the number of invaded
cells were counted using an Olympus inverted fluorescence
microscope (IX71; Zeiss AG).
Wound healing assay
Both the T24 and 5637 cell lines were transfected
with shRNA plasmids and cultured to form confluent monolayers.
After the confluence reached 100%, the wound was created using a 20
µl pipette tip. Cell debris was washed with PBS 3 times. The
serum-free culture medium was added to the cells and the cells were
cultured. Images of the wounds were captured at 0 and 24 h, and the
extent of healing was measured. Migration ability was measured
using ImageJ software (v1.8.0; National Institutes of Health) and
quantified as a percentage of wound width (post-healing wound
width/pre-healing wound width).
Tumor growth and lung metastasis
analysis
All animal procedures were approved by the
Institutional Animal Care and Use Committee of Liaocheng People's
Hospital (Shandong, China). A total of 12 male nude BalB/c mice
(8-weeks-old; 18–22 g) were purchased from Beijing Vital River
Laboratory Animal Technology Co., Ltd., and fed with food and water
ad libitum and at specific pathogen-free conditions (20°C;
60% humidity and alternating 12-h light/dark cycles).
For the tumor growth assay, the T24 cell line was
stably transfected with shRNA plasmids, then 3×106 cells
were inoculated into the Balb/c nude mice and tumors formed 2 weeks
later. The tumor growth curves were analyzed 7 weeks later. The
tumor size was calculated using the following formula: Tumor
volume=length × width × width/2. The mice were euthanized with an
intraperitoneal injection of 120 mg/kg sodium pentobarbital before
the tumor was removed. The hearts of the mice were then monitored
and death was confirmed by cardiac arrest. There were six mice in
the control and LAPTM4B knockdown group.
To further detect the expression level of the
LAPTM4B in the tumor tissues from the mice, IHC was performed as
aforementioned. Subsequently, the staining intensity of LAPTM4B in
the tumor tissues was measured using ImageJ software (v1.8.0;
National Institutes of Health) and analyzed statistically using the
staining intensity, which was presented as the median ±
interquartile range, including the Q1/Q3 quartiles.
For the lung metastasis assay, 1×106 T24
cells, transfected with shRNA plasmids, were resuspended in 150 µl
PBS buffer and injected into the tail vein to stimulate lung
metastasis. After 7 weeks, all the mice were sacrificed and the
lungs were isolated and images were captured, and the metastasis
degree was measured.
Statistical analysis
GraphPad v6.0 software (GraphPad Software, Inc.) was
used for statistical analysis. The in vitro and in
vivo results were presented as the mean ± standard deviation.
The analysis between clinical features and LAPTM4B expression was
performed using a χ2 test. A Student's t-test was used
for statistical comparisons. The survival rates between cancer
progression and LAPTM4B expression was analyzed using the
Kaplan-Meier method and the log-rank test. IHC staining intensity
in animal experiments was presented as the median + interquartile
range and analyzed using Mann-Whitney U test. Each experiment was
repeated 3 times. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of LAPTM4B was associated
with the prognosis and clinical features of patients with bladder
cancer
To investigate the potential role of LAPTM4B in
bladder cancer progression, IHC, clinicopathological characteristic
analysis and Kaplan-Meier analysis were all conducted. The
expression level of LAPTM4B in human tumor tissues from patients
with bladder cancer, who underwent surgical resection was detected
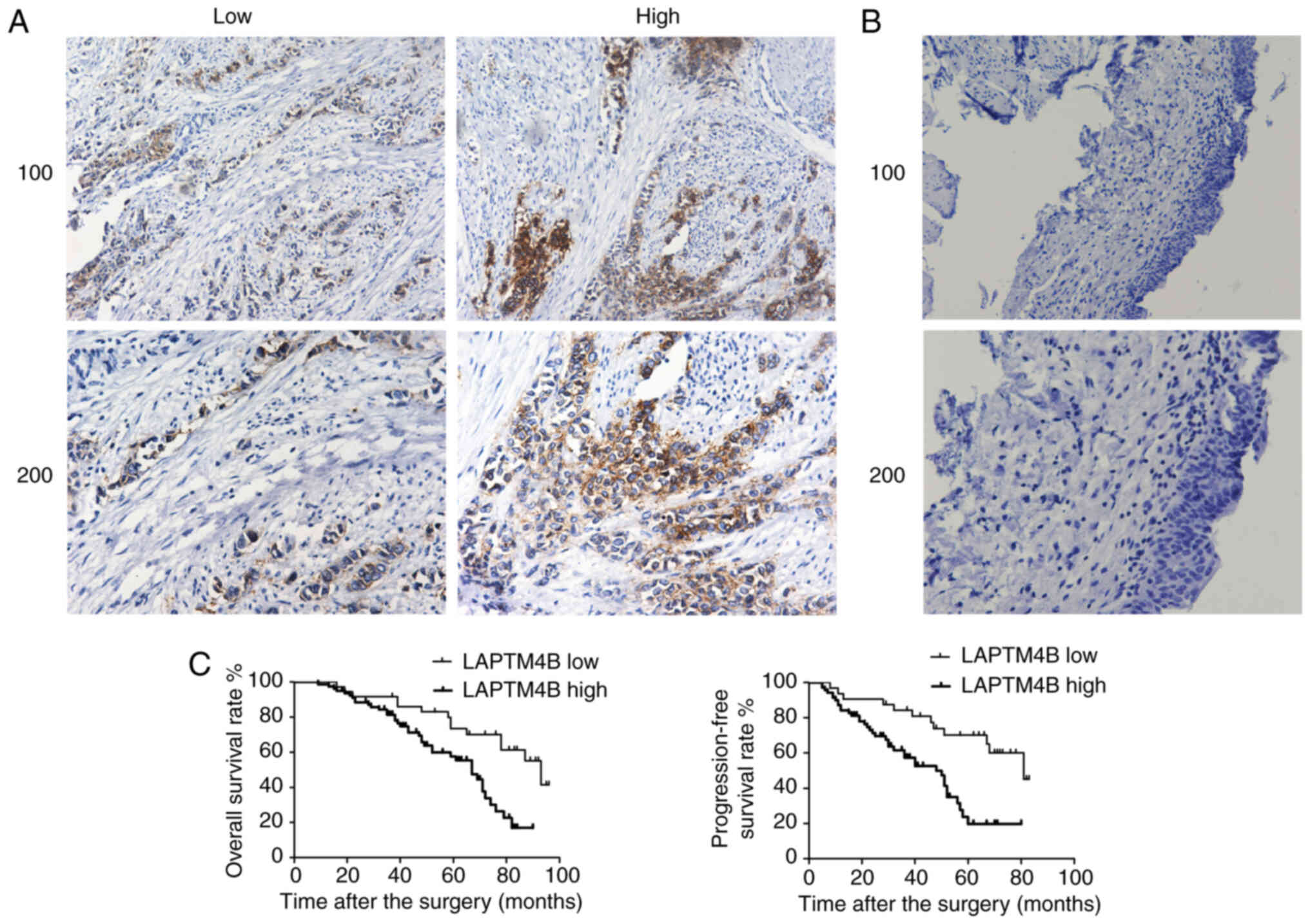
using IHC. According to the IHC staining results, LAPTM4B was
mainly expressed in the cytoplasm and membrane of human bladder
cancer tissues (Fig. 1A). A total
number of 111 surgical samples were classified into LAPTM4B low and
high-expression groups according to the staining intensity
(Fig. 1A). In comparison,
corresponding non-tumor tissues showed low expression level of
LAPTM4B (Fig. 1B). Based on the
expression level in bladder tumor tissues, 34 patients showed low
LAPTM4B expression, whereas 77 showed high expression (Table I).
 | Table I.Association between LAPTM4B and the
clinicopathological characteristics in 111 patients with bladder
cancer. |
Table I.
Association between LAPTM4B and the
clinicopathological characteristics in 111 patients with bladder
cancer.
|
|
| LAPTM4B
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | Total number | Low n=34 | High n=77 | χ2 | P-value |
|---|
| Age, years |
|
|
| 2.199 | 0.138 |
|
<55 | 44 | 17 | 27 |
|
|
|
≥55 | 67 | 17 | 50 |
|
|
| Sex |
|
|
| 0.933 | 0.334 |
|
Male | 71 | 24 | 47 |
|
|
|
Female | 40 | 10 | 30 |
|
|
| Tumor stage |
|
|
| 8.516 | 0.004 |
| T2 | 52 | 23 | 29 |
|
|
|
T3/T4 | 59 | 11 | 48 |
|
|
| Tumor grade |
|
|
| 1.321 | 0.250 |
|
Low | 31 | 12 | 19 |
|
|
|
High | 80 | 22 | 58 |
|
|
| Lymph node
metastasis |
|
|
| 3.074 | 0.080 |
|
Yes | 33 | 14 | 19 |
|
|
| No | 78 | 20 | 58 |
|
|
| Recurrence |
|
|
| 5.983 | 0.014 |
|
Yes | 52 | 10 | 42 |
|
|
| No | 59 | 24 | 35 |
|
|
The clinicopathological characteristics of patients
with bladder cancer were analyzed between the LAPTM4B low and high
expression level groups. Patient age, sex, tumor size, grade and
lymph node metastasis, were recorded and analyzed. Based on the
analysis results, no significant difference was found in these
features between the LAPTM4B low and high expression groups
(Table I). Notably, LAPTM4B
expression level in the bladder tumor tissues was significantly
associated with the tumor stage (P=0.004) and recurrence
(P=0.014).
Kaplan-Meier survival analysis was used to
investigate the association between LAPTM4B and the prognosis of
patients with bladder cancer. Data showed that LAPTM4B expression
was associated with OS and PFS rates (Fig. 1C). These results indicated that
LAPTM4B was associated with poor prognosis in patients with bladder
cancer.
LAPTM4B was associated with bladder
cancer cell proliferation and invasion in vitro
To further investigate the mechanism of LAPTM4B
regulation in the progression of bladder cancer, a shRNA targeting
LAPTM4B was transfected into the two types of bladder cancer cell
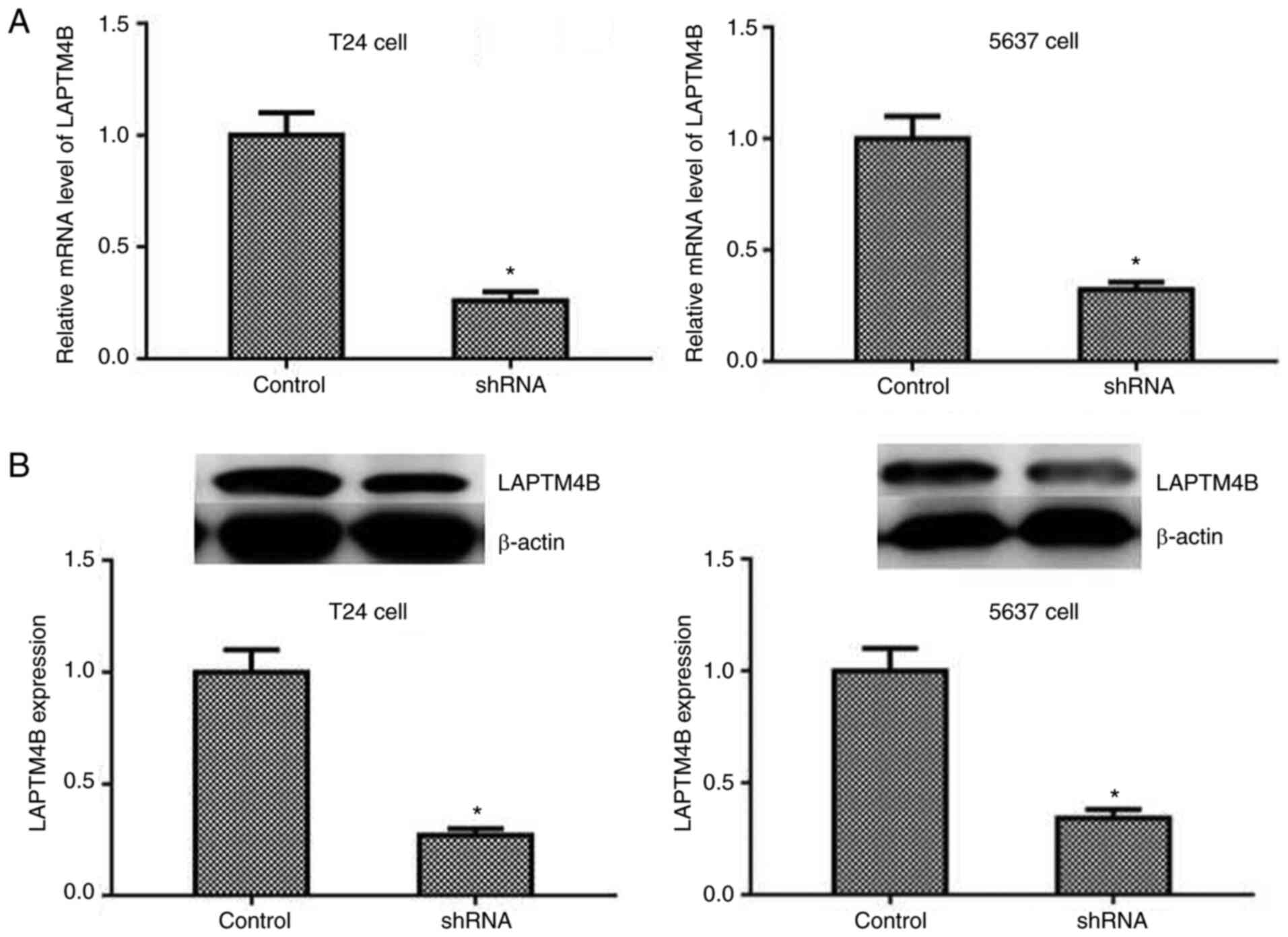
lines, T24 and 5637, to suppress the expression of LAPTM4B. qPCR
(Fig. 2A) and western blot analysis
(Fig. 2B) suggested that
transfection with LAPTM4B shRNA effectively knocked down LAPTM4B
expression in the T24 and 5637 cell lines.
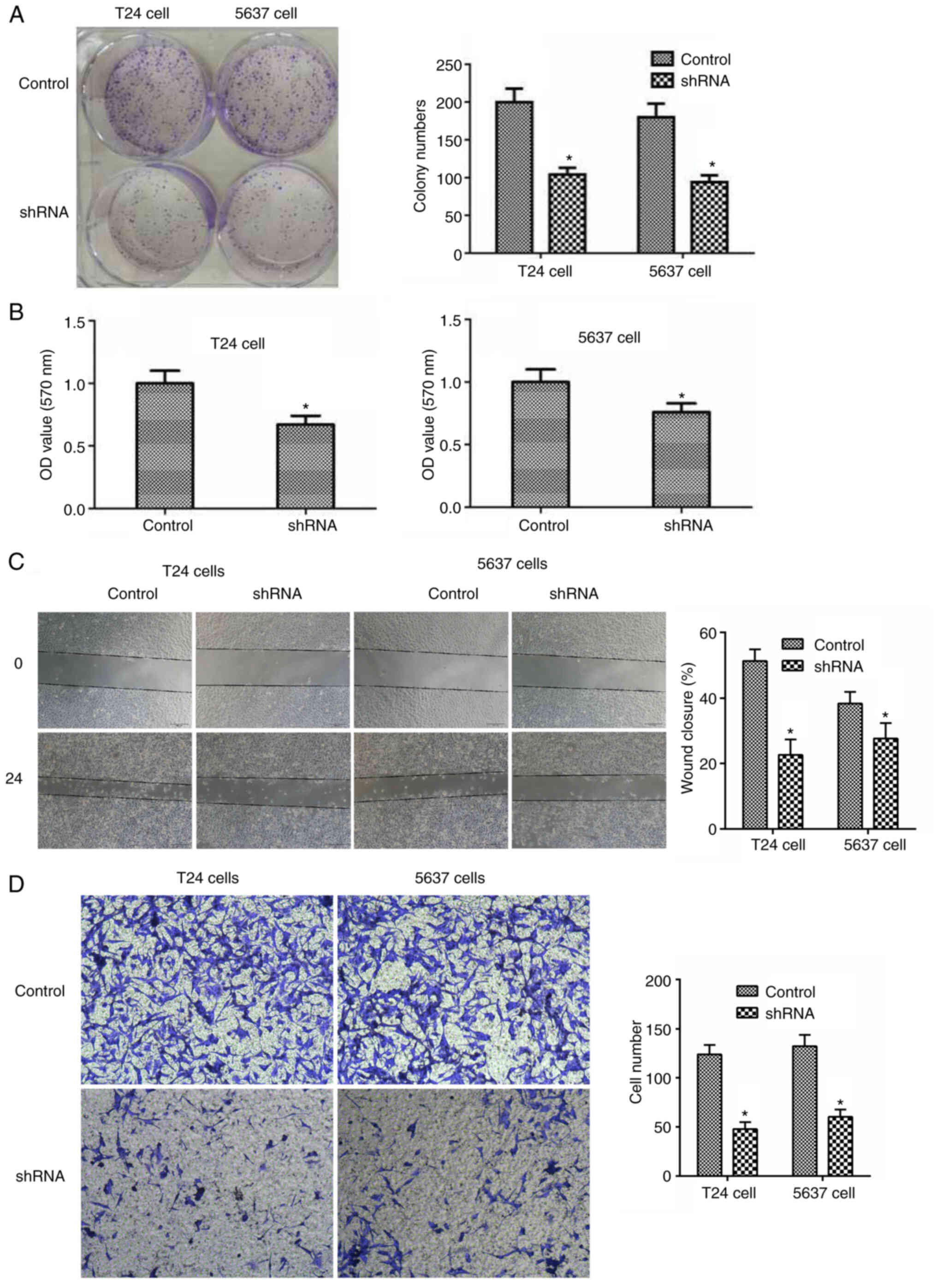
To investigate the potential effect of LAPTM4B on
the proliferation of bladder cancer, colony formation assays were
conducted. It was found that LAPTM4B knockdown markedly inhibited
the proliferation of the T24 and 5637 cell lines, from the decrease
in cell colony numbers (Fig. 3A).
Similarly, there was lower proliferation from the MTT assay, as
shown from the lower OD values in Fig.
3B.
The effects of LAPTM4B on the migration and invasion
of bladder cancer cells were also investigated. As expected,
knockdown of LAPTM4B also slowed down the extent of wound closure
in the two bladder cancer cell lines (Fig. 3C). In addition, T24 and 5637 cells
exhibited a significantly lower invasive ability following LAPTM4B
knockdown, with markedly decreased the number of invasive cell
(Fig. 3D).
Taken together, it was found that LAPTM4B was
associated with the regulation of bladder cancer cell
proliferation, and migration and invasion in vitro.
LAPTM4B promotes bladder tumor growth
and metastasis in mice
According to the in vitro findings, knockdown
of LAPTM4B reduced the proliferation, and migration and invasion of
bladder cancer cell lines; therefore, the role of LAPTM4B in the
growth and metastasis of bladder cancer was investigated further in
mice.
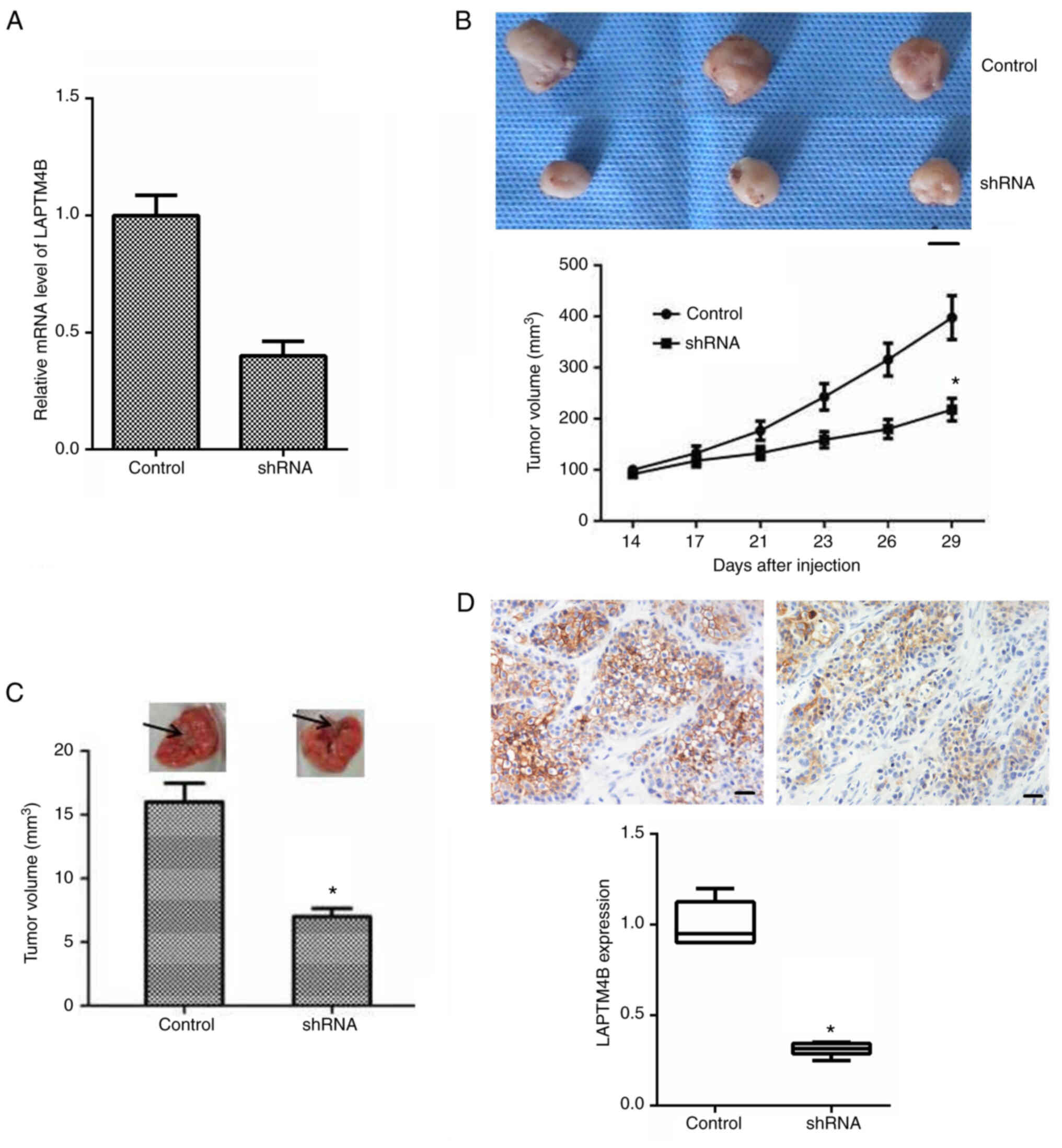
The T24 cell lines, transfected with control or
LAPTM4B shRNA plasmids, were inoculated into Balb/c nude mice and
tumor formation began 2 weeks later. The notable low mRNA
expression levels of LAPTM4B in the knockdown group (Fig. 4A), confirmed successful transfection.
Representative images of the tumors were captured and are shown in
Fig. 4B. The maximum volume of tumor
in mice was 10 mm. According to the results, the volume of the
tumors from the LAPTM4B knockdown mice was significantly smaller
compared with that in the control mice (Fig. 4B). Furthermore, lung metastasis assay
was performed in the mice and the volume of lung metastasis in the
mice with LAPTM4B-knockout T24 cells was markedly decreased
compared with that in the mice transfected with the control shRNA
(Fig. 4C).
IHC further confirmed the knockdown of LAPTM4B
expression in the tumor tissues (Fig.
4D). Therefore, all these data confirmed that LAPTM4B was
associated with the regulation of bladder cancer growth and
development in vivo.
Discussion
Over the past few decades, the mortality rate of
bladder cancer has increased (23).
With the clinical application of new treatment, the treatment
effect of bladder cancer has been significantly improved (3). However, targeted therapy is undoubtedly
the most promising choice, and highly effective therapeutic targets
for bladder cancer is still required (6,24). Some
gene mutations, such as in osteopontin and CTLA4, result in the
development of bladder cancer, which can be used as potential
targets for treatment (25). In
addition, a variety of new therapeutic targets, such as proteins or
long non-coding RNA, have been discovered, which provide
significant convenience for the study of tumor mechanism and
treatment (26–28). In the present study, LAPTM4B, a
regulator of multiple types of tumor (10–12,29,30), was
associated with the progression of bladder cancer. LAPTM4B has the
potential to be a novel therapeutic target for the treatment of
bladder cancer; however, the molecular mechanisms require further
investigation.
LAPTM4B was also associated with some of the
clinical features, including tumor stage and recurrence, further
suggesting that LAPTM4B might promote poor prognosis and distant
metastasis of bladder cancer, which is consistent with the results
from the in vitro experiments, that LAPTM4B promoted bladder
cancer proliferation, and migration and invasion. Further clinical
studies and experiments are required to investigate how LAPTM4B
precisely regulates the pathogenesis of bladder cancer.
LAPTM4B, as a transmembrane protein, has been
reported to be associated with poor prognosis in multiple types of
cancer, whereas its precise physiological function is not well
understood (10–12,29–31).
Previous studies confirmed that LAPTM4B could interact with
ceramide to promote its removal from late endosomal organelles;
therefore, regulating key sphingolipid-mediated cell death
processes (10,12,29). A
previous study also indicated that LAPTM4B was critical for
autophagic maturation (32). The
overexpression of LAPTM4B promoted autophagy, which led to cancer
cell proliferation (12). It was
found that LAPTM4B knockdown inhibited the proliferation of bladder
cancer cells; however, the association between LAPTM4B and
autophagy also requires further investigation. In addition, it was
reported that LAPTM4B, in cooperation with AP4, activated the
PI3K/AKT signaling pathway and the caspase-dependent pathway to
promote the proliferation and invasion of HCC (32). Notably, it was found that knockdown
of LAPTM4B significantly blocked proliferation and invasion of
bladder cancer in vitro and in mice, which might be partly
caused by the activation of these pathways.
In addition to the association between LAPTM4B and
bladder cancer found in the present study, LAPTM4B has been
associated with the growth and metastasis of several types of
tumor. Firstly, LAPTM4B knockdown markedly blocked the
proliferation and invasion of HeLa cells in vitro (33), which is consistent with the present
study. LAPTM4B activated the EGFR signaling pathway and further
promoted the development of gastric cancer, which was repressed by
Beclin1 (20). A report demonstrated
that overexpression of LAPTM4B induced cell proliferation,
migration, and simultaneous upregulation of vimentin and N-cadherin
to promote epithelial-mesenchymal transition (EMT) in breast cancer
cells (34). However, the effect of
LAPTM4B on EMT in bladder cancer requires further investigation.
LAPTM4B was also associated with tumor proliferation, angiogenesis,
and poor prognosis in patients with glioblastoma, which could be
used as a potential novel prognostic marker of glioblastoma to
improve its treatment (35).
Ethylglyoxal bisthiosemicarbazon, a specific inhibitor of LAPTM4B,
was found to have effective antitumor activity in HCC (36).
In conclusion, the role of LAPTM4B was identified in
bladder cancer progression using a range of different
experiments.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YY, YF, GY, and YD conceived the study, performed
the molecular biology and in vivo experiments, performed the
statistical analysis and drafted the manuscript. YY and YD confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All procedures performed in the current study were
approved by the Ethics Committee of Liaocheng People's Hospital
(Shandong, China). Written informed consent was provided by all the
patients or their families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yao X, Li D, Xiong DM, Li L, Jiang R and
Chen JX: A novel role of ribonuclease inhibitor in regulation of
epithelial-to-mesenchymal transition and ILK signaling pathway in
bladder cancer cells. Cell Tissue Res. 353:409–423. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang Y, Deng S, Zeng Q, Hu W and Chen T:
Highly stable selenadiazole derivatives induce bladder cancer cell
apoptosis and inhibit cell migration and invasion through the
activation of ROS-mediated signaling pathways. Dalton Trans.
45:18465–18475. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soria F, Krabbe LM, Todenhöfer T, Dobruch
J, Mitra AP, Inman BA, Gust KM, Lotan Y and Shariat SF: Molecular
markers in bladder cancer. World J Urol. 37:31–40. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Radosavljevic V and Belojevic G:
Shortcomings in bladder cancer etiology research and a model for
its prevention. Tumori. 100:1–8. 2014.PubMed/NCBI
|
|
6
|
Jordan EJ and Iyer G: Targeted therapy in
advanced bladder cancer: What have we learned? Urol Clin North Am.
42253–262. (ix)2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan CX, Zhang H, Tepper CG, Lin TY, Davis
RR, Keck J, Ghosh PM, Gill P, Airhart S, Bult C, et al: Development
and characterization of bladder cancer patient-derived xenografts
for molecularly guided targeted therapy. PLoS One. 10:e01343462015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou T, Zhou L, Wang L, Kazobinka G, Zhang
X and Chen Z: CLCA4 inhibits bladder cancer cell proliferation,
migration, and invasion by suppressing the PI3K/AKT pathway.
Oncotarget. 8:93001–93013. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ciccarese C, Massari F, Blanca A, Tortora
G, Montironi R, Cheng L, Scarpelli M, Raspollini MR, Vau N, Fonseca
J and Lopez-Beltran A: Tp53 and its potential therapeutic role as a
target in bladder cancer. Expert Opin Ther Targets. 21:401–414.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blom T, Li S, Dichlberger A, Bäck N, Kim
YA, Loizides-Mangold U, Riezman H, Bittman R and Ikonen E: LAPTM4B
facilitates late endosomal ceramide export to control cell death
pathways. Nat Chem Biol. 11:799–806. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang H, Qi S, Zhang T, Wang A, Liu R, Guo
J, Wang Y and Xu Y: miR-188-5p inhibits tumour growth and
metastasis in prostate cancer by repressing LAPTM4B expression.
Oncotarget. 6:6092–6104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Zhang Q, Tian R, Wang Q, Zhao JJ,
Iglehart JD, Wang ZC and Richardson AL: Lysosomal transmembrane
protein LAPTM4B promotes autophagy and tolerance to metabolic
stress in cancer cells. Cancer Res. 71:7481–7489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou K, Dichlberger A, Martinez-Seara H,
Nyholm TKM, Li S, Kim YA, Vattulainen I, Ikonen E and Blom T: A
ceramide-regulated element in the late endosomal protein LAPTM4B
controls amino acid transporter interaction. ACS Cent Sci.
4:548–558. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meng Y, Wang L, Chen D, Chang Y, Zhang M,
Xu JJ, Zhou R and Zhang QY: LAPTM4B: An oncogene in various solid
tumors and its functions. Oncogene. 35:6359–6365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang H, Xiong F, Qi R, Liu Z, Lin M, Rui
J, Su J and Zhou R: LAPTM4B-35 is a novel prognostic factor of
hepatocellular carcinoma. J Surg Oncol. 101:363–369. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao M, Jia S, Wang H, Wang J, Huang Y and
Li Z: Overexpression of LAPTM4B: An independent prognostic marker
in breast cancer. J Cancer Res Clin Oncol. 139:661–667. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang H, Tian B, Yu H, Yao H and Gao Z:
LAPTM4B-35 protein as a potential therapeutic target in gastric
cancer. Tumour Biol. 35:12737–12742. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lebovitz CB, Robertson AG, Goya R, Jones
SJ, Morin RD, Marra MA and Gorski SM: Cross-cancer profiling of
molecular alterations within the human autophagy interaction
network. Autophagy. 11:1668–1687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang L, Meng Y, Xu JJ and Zhang QY: The
transcription factor AP4 promotes oncogenic phenotypes and
cisplatin resistance by regulating LAPTM4B expression. Mol Cancer
Res. 16:857–868. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian M, Chen Y, Tian D, Qiao X, Ma Z and
Li J: Beclin1 antagonizes LAPTM4B-mediated EGFR overactivation in
gastric cancer cells. Gene. 626:48–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Y, Zhao Z, Xie C and Zhao Y:
Dual-targeting liposome modified by glutamic hexapeptide and folic
acid for bone metastatic breast cancer. Chem Phys Lipids.
228:1048822020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mahdavifar N, Ghoncheh M, Pakzad R,
Momenimovahed Z and Salehiniya H: Epidemiology, incidence and
mortality of bladder cancer and their relationship with the
development index in the world. Asian Pac J Cancer Prev.
17:381–386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jung HK, Kim S, Park RW, Park JY, Kim IS
and Lee B: Bladder tumor-targeted delivery of pro-apoptotic peptide
for cancer therapy. J Control Release. 235:259–267. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hussain SA, Palmer DH, Syn WK, Sacco JJ,
Greensmith RMD, Elmetwali T, Aachi V, Lloyd BH, Jithesh PV, Arrand
J, et al: Gene expression profiling in bladder cancer identifies
potential therapeutic targets. Int J Oncol. 50:1147–1159. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen MW, Wei XD, Shi X, Lu L, Zhang G,
Huang Y and Hou J: LncRNA HIF1A-AS2 accelerates malignant
phenotypes of renal carcinoma by modulating miR-30a-5p/SOX4 axis as
a ceRNA. Cancer Biol Med. 18:587–603. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen M, Zhuang C, Liu Y, Li J, Dai F, Xia
M, Zhan Y, Lin J, Chen Z, He A, et al: Tetracycline-inducible shRNA
targeting antisense long non-coding RNA HIF1A-AS2 represses the
malignant phenotypes of bladder cancer. Cancer Lett. 376:155–164.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun Z, Niu S, Xu F, Zhao W, Ma R and Chen
M: CircAMOTL1 promotes tumorigenesis through miR-526b/SIK2 axis in
cervical cancer. Front Cell Dev Biol. 8:5681902020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li S, Wang L, Meng Y, Chang Y, Xu J and
Zhang Q: Increased levels of LAPTM4B, VEGF and survivin are
correlated with tumor progression and poor prognosis in breast
cancer patients. Oncotarget. 8:41282–41293. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li L, Shan Y, Yang H, Zhang S, Lin M, Zhu
P, Chen XY, Yi J, McNutt MA, Shao GZ and Zhou RL: Upregulation of
LAPTM4B-35 promotes malignant transformation and tumorigenesis in
L02 human liver cell line. Anat Rec (Hoboken). 294:1135–1142. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Iglehart JD, Richardson AL and Wang
ZC: The amplified cancer gene LAPTM4B promotes tumor growth and
tolerance to stress through the induction of autophagy. Autophagy.
8:273–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meng Y, Wang L, Xu J and Zhang Q: AP4
positively regulates LAPTM4B to promote hepatocellular carcinoma
growth and metastasis, while reducing chemotherapy sensitivity. Mol
Oncol. 12:373–390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meng F, Chen X, Song H and Lou G: LAPTM4B
down regulation inhibits the proliferation, invasion and
angiogenesis of HeLa cells in vitro. Cell Physiol Biochem.
37:890–900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiao M, Yang S, Meng F, Qin Y, Yang Y, Jia
S, Cai X, Li C, Huang Y and Ning X: LAPTM4B predicts axillary lymph
node metastasis in breast cancer and promotes breast cancer cell
aggressiveness in vitro. Cell Physiol Biochem. 41:1072–1082. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dong X, Tamura K, Kobayashi D, Ando N,
Sumita K and Maehara T: LAPTM4B-35 is a novel prognostic factor for
glioblastoma. J Neurooncol. 132:295–303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li M, Zhou R, Shan Y, Li L, Wang L and Liu
G: Targeting a novel cancer-driving protein (LAPTM4B-35) by a small
molecule (ETS) to inhibit cancer growth and metastasis. Oncotarget.
7:58531–58542. 2016. View Article : Google Scholar : PubMed/NCBI
|