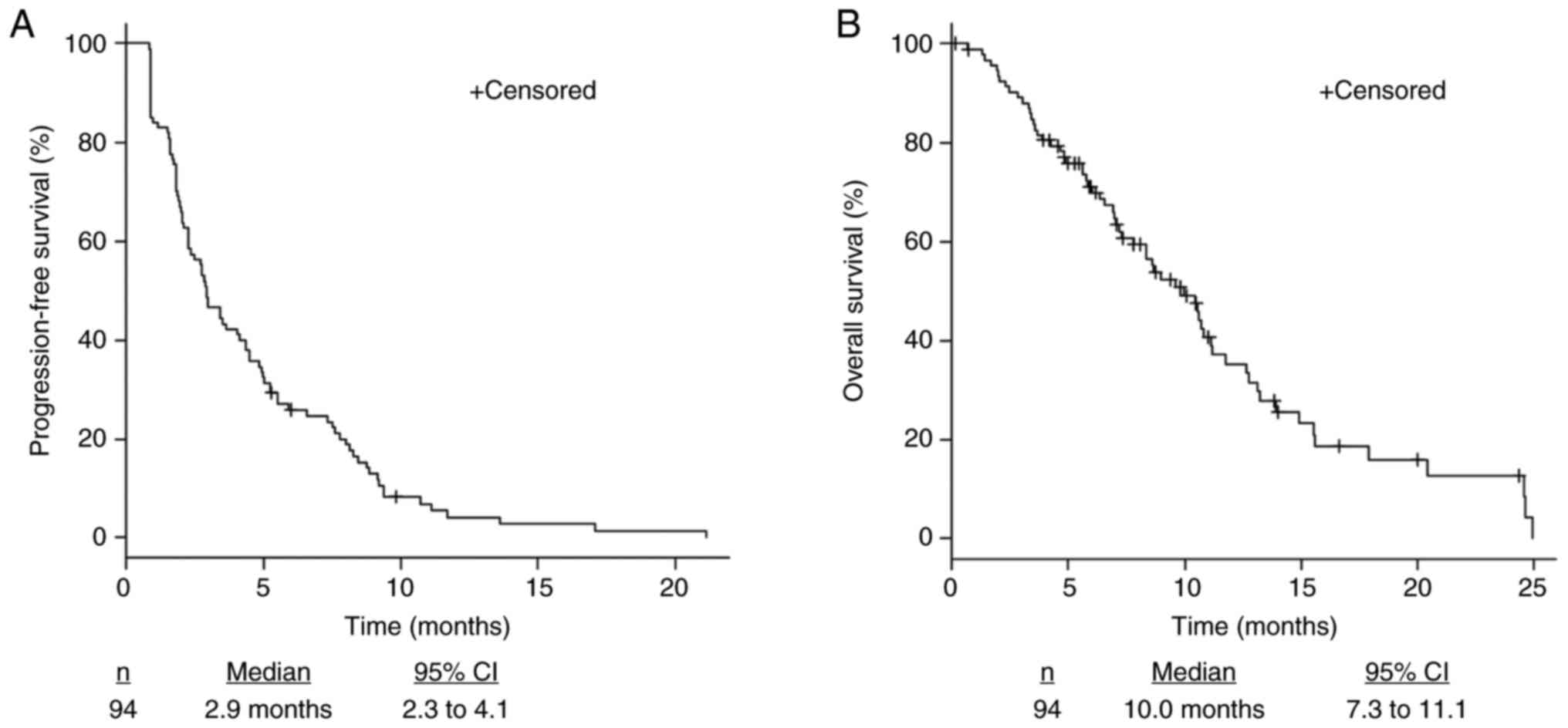
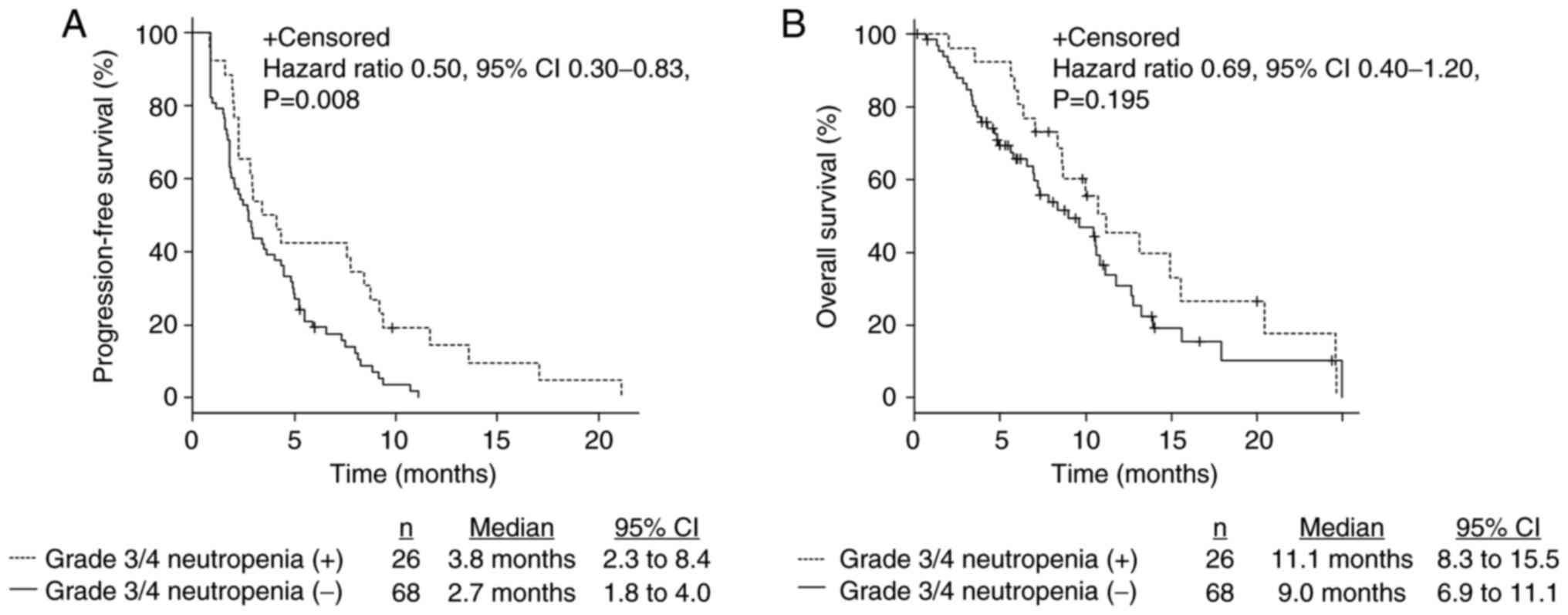
|
1
|
Tanaka N, Sakamoto K, Okabe H, Fujioka A,
Yamamura K, Nakagawa F, Nagase H, Yokogawa T, Oguchi K, Ishida K,
et al: Repeated oral dosing of TAS-102 confers high trifluridine
incorporation into DNA and sustained antitumor activity in mouse
models. Oncol Rep. 32:2319–3226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heidelberger C, Parsons DG and Remy DC:
Syntheses of 5-trifluoromethyluracil and
5-trifluoromethyl-2′-deoxyuridine. J Med Chem. 7:1–5. 1964.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fujiwara Y, Oki T and Heidelberger C:
Fluorinated pyrimidines. XXXVII. Effects of
5-trifluoromethyl-2′-deoxyuridine on the synthesis of
deoxyribonucleic acid of mammalian cells in culture. Mol Pharmacol.
6:273–280. 1970.PubMed/NCBI
|
|
4
|
Emura T, Suzuki N, Fujioka A, Ohshimo H
and Fukushima M: A novel combination antimetabolite, TAS-102,
exhibits antitumor activity in FU-resistant human cancer cells
through a mechanism involving FTD incorporation in DNA. Int J
Oncol. 25:571–578. 2004.PubMed/NCBI
|
|
5
|
Mayer RJ, Van Cutsem E, Falcone A, Yoshino
T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero
J, Komatsu Y, et al: Randomized trial of TAS-102 for refractory
metastatic colorectal cancer. N Engl J Med. 372:1909–1919. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu J, Kim TW, Shen L, Sriuranpong V, Pan
H, Xu R, Guo W, Han SW, Liu T, Park YS, et al: Results of a
randomized, double-blind, placebo-controlled, phase iii trial of
trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with
previously treated metastatic colorectal cancer: The TERRA study. J
Clin Oncol. 36:350–358. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hashiguchi Y, Muro K, Saito Y, Ito Y,
Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M,
et al: Japanese Society for Cancer of the Colon and Rectum (JSCCR)
guidelines 2019 for the treatment of colorectal cancer. Int J Clin
Oncol. 25:1–42. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Benson AB, Venook AP, Al-Hawary MM, Arain
MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, et
al: Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines
in Oncology. J Natl Compr Canc Netw. 19:329–359. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshino T, Cleary JM, Van Cutsem E, Mayer
RJ, Ohtsu A, Shinozaki E, Falcone A, Yamazaki K, Nishina T,
Garcia-Carbonero R, et al: Neutropenia and survival outcomes in
metastatic colorectal cancer patients treated with
trifluridine/tipiracil in the RECOURSE and J003 trials. Ann Oncol.
31:88–95. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuboki Y, Nishina T, Shinozaki E, Yamazaki
K, Shitara K, Okamoto W, Kajiwara T, Matsumoto T, Tsushima T,
Mochizuki N, et al: TAS-102 plus bevacizumab for patients with
metastatic colorectal cancer refractory to standard therapies
(C-TASK FORCE): An investigator-initiated, open-label, single-arm,
multicentre, phase 1/2 study. Lancet Oncol. 18:1172–1181. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Satake H, Kato T, Oba K, Kotaka M, Kagawa
Y, Yasui H, Nakamura M, Watanabe T, Matsumoto T, Kii T, et al:
Phase Ib/II Study of biweekly TAS-102 in combination with
bevacizumab for patients with metastatic colorectal cancer
refractory to standard therapies (BiTS Study). Oncologist.
25:e1855–e1863. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Van Cutsem E, Danielewicz I, Saunders MP,
Pfeiffer P, Argilés G, Borg C, Glynne-Jones R, Punt CJA, Van de
Wouw AJ, Fedyanin M, et al: Trifluridine/tipiracil plus bevacizumab
in patients with untreated metastatic colorectal cancer ineligible
for intensive therapy: The randomized TASCO1 study. Ann Oncol.
31:1160–1168. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pfeiffer P, Yilmaz M, Möller S, Zitnjak D,
Krogh M, Petersen LN, Poulsen LØ, Winther SB, Thomsen KG and
Qvortrup C: TAS-102 with or without bevacizumab in patients with
chemorefractory metastatic colorectal cancer: An
investigator-initiated, open-label, randomised, phase 2 trial.
Lancet Oncol. 21:412–420. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nose Y, Kagawa Y, Hata T, Mori R, Kawai K,
Naito A, Sakamoto T, Murakami K, Katsura Y, Ohmura Y, et al:
Neutropenia is an indicator of outcomes in metastatic colorectal
cancer patients treated with FTD/TPI plus bevacizumab: A
retrospective study. Cancer Chemother Pharmacol. 86:427–433. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kotani D, Kuboki Y, Horasawa S, Kaneko A,
Nakamura Y, Kawazoe A, Bando H, Taniguchi H, Shitara K, Kojima T,
et al: Retrospective cohort study of trifluridine/tipiracil
(TAS-102) plus bevacizumab versus trifluridine/tipiracil
monotherapy for metastatic colorectal cancer. BMC Cancer.
19:12532019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsuhashi N, Takahashi T, Fujii H,
Suetsugu T, Fukada M, Iwata Y, Tokumaru Y, Imai T, Mori R,
Tanahashi T, et al: Combination chemotherapy with TAS-102 plus
bevacizumab in salvage-line treatment of metastatic colorectal
cancer: A single-center, retrospective study examining the
prognostic value of the modified Glasgow Prognostic Score in
salvage-line therapy of metastatic colorectal cancer. Mol Clin
Oncol. 11:390–396. 2019.PubMed/NCBI
|
|
19
|
Fujii H, Matsuhashi N, Kitahora M,
Takahashi T, Hirose C, Iihara H, Yamada Y, Watanabe D, Ishihara T,
Suzuki A and Yoshida K: Bevacizumab in combination with TAS-102
improves clinical outcomes in patients with refractory metastatic
colorectal cancer: A retrospective study. Oncologist. 25:e469–e476.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shibutani M, Nagahara H, Fukuoka T, Iseki
Y, Wang EN, Okazaki Y, Kashiwagi S, Maeda K, Hirakawa K and Ohira
M: Combining bevacizumab with trifluridine/thymidine phosphorylase
inhibitor improves the survival outcomes regardless of the usage
history of bevacizumab in front-line treatment of patients with
metastatic colorectal cancer. Anticancer Res. 40:4157–4163. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshino T, Mizunuma N, Yamazaki K, Nishina
T, Komatsu Y, Baba H, Tsuji A, Yamaguchi K, Muro K, Sugimoto N, et
al: TAS-102 monotherapy for pretreated metastatic colorectal
cancer: A double-blind, randomised, placebo-controlled phase 2
trial. Lancet Oncol. 13:993–1001. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arita S, Shirakawa T, Matsushita Y,
Shimokawa HK, Hirano G, Makiyama A, Shibata Y, Tamura S, Esaki T,
Mitsugi K, et al: Efficacy and safety of TAS-102 in clinical
practice of salvage chemotherapy for metastatic colorectal cancer.
Anticancer Res. 36:1959–1966. 2016.PubMed/NCBI
|
|
23
|
Masuishi T, Taniguchi H, Hamauchi S,
Komori A, Kito Y, Narita Y, Tsushima T, Ishihara M, Todaka A,
Tanaka T, et al: Regorafenib versus trifluridine/tipiracil for
refractory metastatic colorectal cancer: A retrospective
comparison. Clin Colorectal Cancer. 16:e15–e22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kotani D, Shitara K, Kawazoe A, Fukuoka S,
Kuboki Y, Bando H, Okamoto W, Kojima T, Doi T, Ohtsu A, et al:
Safety and efficacy of trifluridine/tipiracil monotherapy in
clinical practice for patients with metastatic colorectal cancer:
Experience at a single institution. Clin Colorectal Cancer.
15:e109–e115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sueda T, Sakai D, Kudo T, Sugiura T,
Takahashi H, Haraguchi N, Nishimura J, Hata T, Hayashi T, Mizushima
T, et al: Efficacy and safety of regorafenib or TAS-102 in patients
with metastatic colorectal cancer refractory to standard therapies.
Anticancer Res. 36:4299–4306. 2016.PubMed/NCBI
|
|
26
|
Kwakman JJM, Vink G, Vestjens JH,
Beerepoot LV, de Groot JW, Jansen RL, Opdam FL, Boot H, Creemers
GJ, van Rooijen JM, et al: Feasibility and effectiveness of
trifluridine/tipiracil in metastatic colorectal cancer: Real-life
data from the netherlands. Int J Clin Oncol. 23:482–489. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cremolini C, Rossini D, Martinelli E,
Pietrantonio F, Lonardi S, Noventa S, Tamburini E, Frassineti GL,
Mosconi S, Nichetti F, et al: Trifluridine/tipiracil (TAS-102) in
refractory metastatic colorectal cancer: A multicenter register in
the frame of the Italian compassionate use program. Oncologist.
23:1178–1187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moriwaki T, Fukuoka S, Taniguchi H,
Takashima A, Kumekawa Y, Kajiwara T, Yamazaki K, Esaki T, Makiyama
C, Denda T, et al: Propensity score analysis of regorafenib versus
trifluridine/tipiracil in patients with metastatic colorectal
cancer refractory to standard chemotherapy (REGOTAS): A Japanese
Society for Cancer of the Colon and Rectum multicenter
observational study. Oncologist. 23:7–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsukihara H, Nakagawa F, Sakamoto K,
Ishida K, Tanaka N, Okabe H, Uchida J, Matsuo K and Takechi T:
Efficacy of combination chemotherapy using a novel oral
chemotherapeutic agent, TAS-102, together with bevacizumab,
cetuximab, or panitumumab on human colorectal cancer xenografts.
Oncol Rep. 33:2135–2142. 2015.PubMed/NCBI
|
|
30
|
Kasi PM, Kotani D, Cecchini M, Shitara K,
Ohtsu A, Ramanathan RK, Hochster HS, Grothey A and Yoshino T:
Chemotherapy induced neutropenia at 1-month mark is a predictor of
overall survival in patients receiving TAS-102 for refractory
metastatic colorectal cancer: A cohort study. BMC Cancer.
16:4672016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hamauchi S, Yamazaki K, Masuishi T, Kito
Y, Komori A, Tsushima T, Narita Y, Todaka A, Ishihara M, Yokota T,
et al: Neutropenia as a predictive factor in metastatic colorectal
cancer treated with TAS-102. Clin Colorectal Cancer. 16:51–57.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Makihara K, Fukui R, Uchiyama H, Shigeoka
Y and Toyokawa A: Decreased percentage of neutrophil is a predict
factor for the efficacy of trifluridine and tipiracil hydrochloride
for pretreated metastatic colorectal cancer. J Gastrointest Oncol.
10:878–885. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Bock K, Mazzone M and Carmeliet P:
Anti-angiogenic therapy, hypoxia, and metastasis: Risky liaisons,
or not? Nat Rev Clin Oncol. 8:393–404. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schutz FA, Jardim DL, Je Y and Choueiri
TK: Haematologic toxicities associated with the addition of
bevacizumab in cancer patients. Eur J Cancer. 47:1161–1174. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bennouna J, Sastre J, Arnold D, Österlund
P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C,
et al: Continuation of bevacizumab after first progression in
metastatic colorectal cancer (ML18147): A randomised phase 3 trial.
Lancet Oncol. 14:29–37. 2013. View Article : Google Scholar : PubMed/NCBI
|