Introduction
Oral cancer is a malignant tumor type that occurs in
the lips or mouth (1). 90% of oral
cancers are histologically originated from squamous cells (2) and oral cancer has traditionally been
defined as oral squamous cell carcinoma (OSCC) (3). According to US government statistics,
OSCC is the eighth most common cancer type among males and the 14th
most common cancer type among females (4). The development of OSCC is a complex
process modulated by genetic and environmental factors. The
accumulation of multiple genetic alterations is regulated by
hereditary predisposition, including sex and ethnicity. Despite
recent advances in imaging, surgery, radiation and systemic
therapy, the overall survival of patients with OSCC has not
significantly improved over the past 20 years (5). Therefore, OSCC remains a major clinical
challenge and there are currently no available biomarkers to guide
treatment decisions. Identifying reliable biomarkers and novel
molecular targets is critical to the stratification of patients to
develop precise and personalized treatment regimens, helping
physicians to deliver early and appropriate treatment regimens in a
timely manner that may reduce the risk of squamous cell cancer
recurrence (6). Numerous
biomolecules are involved in OSCC development and may provide
references for treatment and prognosis (6).
Toll-like receptor 4 (TLR4), a transmembrane protein
located on chromosome 9q32-q33, is a member of the TLR protein
family that has been well studied. TLR4 has a fundamental role in
various functions, including defense against pathogens, activation
of innate immunity and regulation of chronic inflammation. Previous
studies have indicated that stimulation of TLR4 expression on tumor
cells may induce chronic inflammation and promote tumor growth
(7). Overexpression of TLR4 has been
observed in several types of human cancer, such as breast (8), melanoma (9), colon (10), ovarian (11) and prostate cancers (10).
MyD88 is a central regulator of innate immunity; it
acts directly downstream of TLRs and cytokine receptors, while also
associated with carcinogenesis (12). MyD88 activates the TLRs or
interleukin-1 receptor (IL-1Rs) pathways autonomously, or perhaps
in relation to TNF receptor-associated factor 6 signaling, allowing
tumor cells to proliferate indefinitely (3). MyD88 is involved in oncogene-induced
inflammation and contributes to the development of cancers of the
skin, liver, pancreas and colon, as well as sarcoma formation
(13), and its expression is
associated with poor prognosis in colorectal cancer (14). Other studies have indicated that
MyD88, coupled with TLR4, has an essential role in skin tumor
promotion (3). Previous studies have
indicated that activation of the TLR4/MyD88-mediated signaling
pathway promotes tumor occurrence and metastasis (15,16).
MyD88 also has a mucosal protective effect, involving downstream
IL-18R for mucosal repair during oncogenic virus carcinogenesis or
during azomethane/sodium dextran sulfate-induced colon cancer
(8).
OSCC is a preventable disease and its risk factors
and clinical relevance have been well documented (4); effective prevention and treatment may
improve the prognosis of OSCC. The present study aimed to integrate
the clinical and histological features as well as the expression of
TLR4 and MyD88 in OSCC to verify the overexpression of TLR4 and
MyD88. Furthermore, it aimed to explore the association between the
development of oral cancer and the expression levels of TLR4 and
MyD88 to elucidate the biological mechanisms of OSCC.
Materials and methods
Patients
The electronic medical record system of the School
of Stomatology, Guangxi Medical University (Nanning, China), was
searched to retrieve information on the cases of OSCC encountered
at the Department of Oral and Maxillofacial Surgery from January
2020 to September 2020.
The inclusion criteria were as follows: i) The
patients were diagnosed with OSCC by pathological examination; ii)
patients underwent radical resection of oral cancer; iii) all
patients were initially treated and had no preoperative history of
biological immunotherapy or other malignant tumors. In addition,
the following exclusion criteria were applied: i) Patients with
another primary cancer or multiple primary cancers in the past or
present; ii) cases accompanied with immune system diseases; iii)
patients who objected to the use of their tumor specimens for the
present study; iv) patients accompanied by acute or chronic
infection, with long-term allergy. The degree of differentiation of
a patient's malignant tumor was derived from the patient's
discharge diagnosis, which was issued by two pathologists from the
Department of Pathology of the Stomatological Hospital Affiliated
with Guangxi Medical University (Nanning, China). Clinical and
follow-up data were recorded for all included patients. There were
39 males and 16 females, with a median age of 56 years (mean ±
standard deviation; 54.64 ± 12.87 years; range, 27–79 years). The
mean follow-up time was 4 months (range, 1–9 months). The present
study was approved by the Ethics Committee of the School of
Stomatology, Guangxi Medical University (Nanning, China) and all
patients enrolled provided written informed consent. Tumor growth
time can be inferred from the patient's clinical admission
record.
Specimen collection
For reverse-transcription quantitative PCR, the
removed tumor tissues and corresponding adjacent paracancerous
tissues from patients with OSCC who underwent surgery on the same
day were collected at the surgical operating room of the
Stomatological Hospital Affiliated to Guangxi Medical University
(Nanning, China) and were stored in RNA Keeper for later reverse
transcription-quantitative (RT-q)PCR analysis.
For immunohistochemistry, the histological specimens
of the 55 corresponding cases of OSCC were then formalin-fixed and
paraffin-embedded and stored at the Department of Pathology for
later analysis by immunohistochemistry. A retrospective
immunohistochemical analysis was performed on 55 formalin-fixed
paraffin-embedded specimens of clinically and histologically
confirmed cases of OSCC and corresponding adjacent non-tumoral
tissues that had been examined and stored at the Department of
Pathology, the Department of Oral and Maxillofacial Surgery,
College of Stomatology, Guangxi Medical University (Nanning,
China).
Immunohistochemistry
The tissue sections were first dewaxed in gradient
xylene and then rehydrated with an alcohol gradient. After rinsing
with tap water and soaking in pure water, the sections were
immersed in sodium citrate buffer (Beijing Zhongshan Golden Bridge
Biotechnology, Beijing, China) and microwave-heated for 5 min to
retrieve antigen. After the slices were cooled, they were rinsed
with pure water 3 times and then 0.3% hydrogen peroxide was used to
block endogenous peroxidase activity. Next, anti-TLR4 polyclonal
antibody and anti-MyD88 antibody (1:100 dilution; Bioss; cat. nos.
bs-1021R-50 and bs-1047R-50) were applied to the tissue sections
with incubation overnight in the refrigerator at 4°C. Biotinylated
goat anti-rabbit IgG (does not need to be diluted; ZSGB-BIO;
PV-6000) was then dripped on tissue sections and they were
incubated at 37°C for 20 min. The sections were washed with PBS
three times between all steps for 3 min each time. The sections
were then stained with diaminobenzidine for 2 min to visualize the
antibodies and counterstained with hematoxylin for 1 min. The
tissue sections were then dehydrated with ethanol and mounted with
glass slips.
The expression of TLR4 and MyD88 was examined in 5
different visual fields under a 20X enlargement factor microscope.
Each section stained was observed and evaluated by two independent
investigators. Sections were scored by cytoplasmic staining
intensity and distribution. The staining intensity was rated as
follows: 0, no staining; 1, weak staining; 2, moderate staining; 3,
strong staining. The staining distribution was divided into 4
grades according to the percentage of tumor cells stained: 0, 0%;
1, <25%; 2, 25–50%; 3, 51–75%; 4, >75% (17). The final staining scores were
calculated as follows: Staining intensity multiplied by the score
for the percentage of stained tumor cells. A final staining score
of >3 was considered to indicate positive protein
overexpression.
RT-qPCR analysis
Total RNA was isolated from human OSCC tissues and
paracancerous tissues using TRIzol® reagent (Takara Bio,
Inc.) according to the manufacturer's protocol. Total RNA (1 mg)
was reverse transcribed to cDNA with a PrimeScript RT reagent Kit
(Takara Bio, Inc.). The temperature and duration of reverse
transcription were 42°C for 2 min, followed by 37°C for 15 min and
85°C for 5 sec. qPCR was performed with the 2*RealStar Green Fast
Mixture (Genebrick) using the following thermocycling program:
Pre-denaturation at 95°C for 2 min, followed by 40 cycles of 95°C
for 15 sec, 60°C for 15 sec and 72°C for 45 sec, and a final
extension at 72°C for 30 sec. The primer sequences were as follows:
TLR4 forward, 5′-CCAAGAACCTGGACCTGAGCTTTA-3′ and reverse
5′-CCATCTTCAATTGTCTGGATTTCAC-3′; MyD88 forward,
5′-AGCCAGGCTGGAGCAAGGTA-3′ and reverse,
5′-GGCAGCTAAATGCCTCAACAAGA-3′; GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′.
Quantifications were performed using the ΔΔCq method (18), where GAPDH was used as a reference
gene for normalization of RNA expression.
Statistical analysis
SPSS 20 software (IBM Corporation) was used for
statistical analysis. To examine the association of the
immunohistochemical results with the pathological features and
clinical outcomes of patients, Pearson's χ2 test was
used (Table SI). For the RT-qPCR
results, the t-test was used to investigate the possible
association between mRNA expression and the pathological and
clinical results of the cases being studied (Table SII). For comparisons of >2 items,
single factor analysis of variance was used, bonferroni correction
was performed to mitigate variability when comparing multiple
samples. Spearman's bivariate correlation method was used to detect
the correlation between TLR4 and MyD88 expression in OSCC tissues
(Table I). P<0.05 was considered
to indicate statistical significance.
 | Table I.mRNA expression of MyD88 and TLR4 in
OSCC and pericarcinomatous tissue. |
Table I.
mRNA expression of MyD88 and TLR4 in
OSCC and pericarcinomatous tissue.
| Tissue type | N | Myd88 | t/F | P-value | TLR4 | t/F | P-value |
|---|
| OSCC | 55 | 3.941±9.344 | 2.327 | 0.024 | 2.401±4.068 | 2.545 | 0.014 |
| Pericarcinomatous
tissue | 55 | 1.012±0.036 |
|
| 1.005±0.006 |
|
|
Results
Clinical and pathological
features
All 55 patients were clinically and histologically
confirmed to have primary OSCC. The patients' age ranged from 27 to
79 years, with an average age of 54 years at first diagnosis. Among
the 55 patients, 39 were males and 16 were females. Sections were
examined independently by two pathologists. Well-differentiated
OSCC is similar to normal squamous epithelium, with varying numbers
of basal cells and squamous cells with intercellular bridge,
obvious keratosis, a small amount of mitotic figures, low frequency
of atypical mitotic figures and multinucleated cells and no obvious
pleomorphism of nuclei and cells. Moderately-differentiated OSCC
has a unique nuclear pleomorphism and mitotic features, including
abnormal mitotic features, infrequent keratosis and inconspicuous
intercellular bridging. Poorly-differentiated OSCC is dominated by
immature cells with a large number of normal or abnormal mitotic
images, a low frequency of keratinization and almost no
intercellular bridging (18).
Histologically, the 55 patients were classified as well and
moderately differentiated (51 cases) and poorly differentiated (4
cases). TNM staging was performed according to the Union for
International Cancer Control, according to which 6 cases were stage
I, 16 cases were stage II, 20 cases were stage III and 13 cases
were stage IV (19). No lymph node
metastasis was detected in 29 patients and lymph node metastasis
was confirmed in 26 patients. The last follow-up ranged from 1 to 9
months (mean, 4 months). Recurrence was reported in 2 cases. A
total of 12 cases were basic disease-negative and 43 cases were
basic disease-positive. Underlying diseases mainly included
diabetes, hypertension and heart disease.
Histological localization of TLR4 and
MyD88
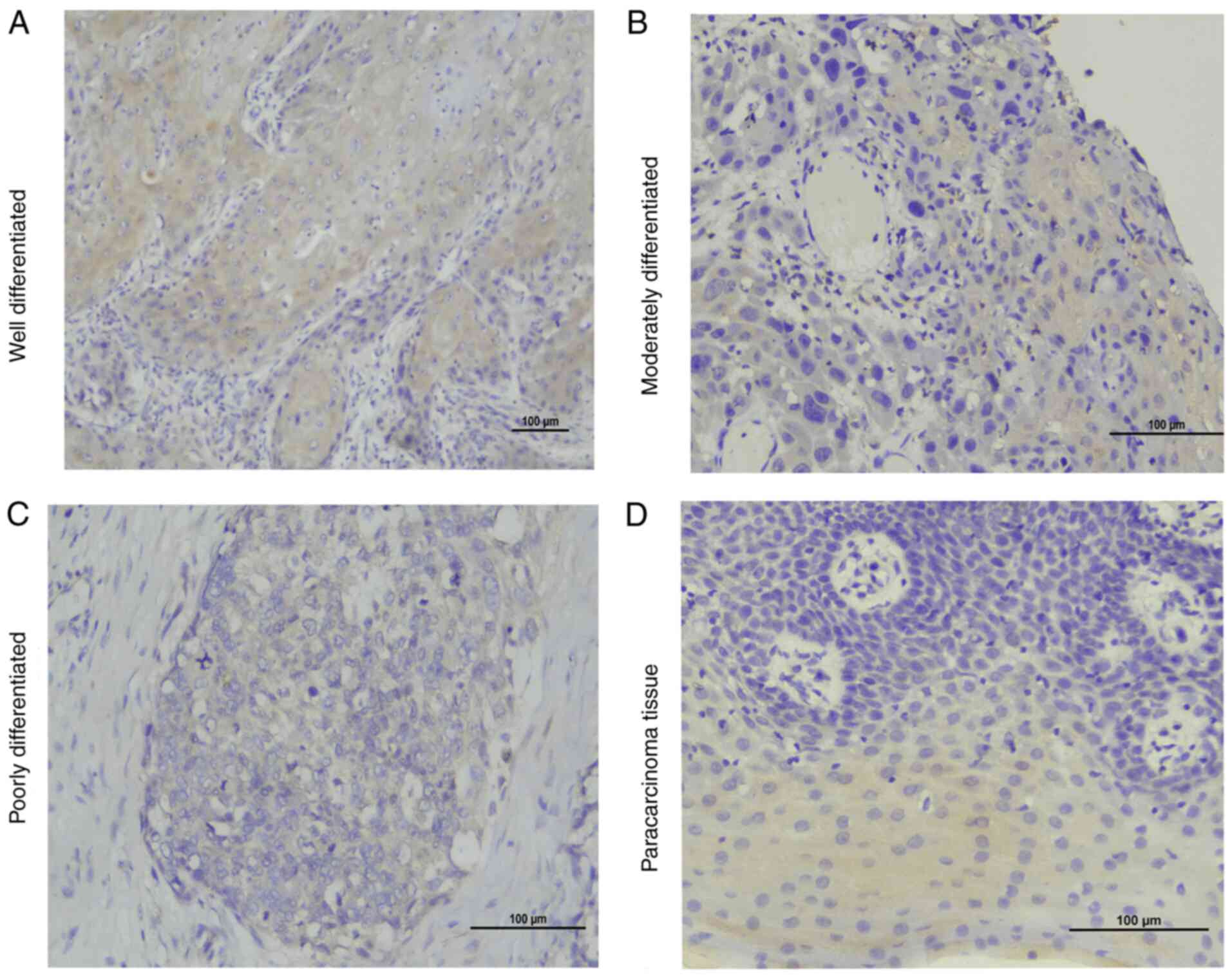
Immunohistochemical staining of intact paraffin
sections of OSCC tissues and paracancerous tissues was performed
using standard procedures. In oral squamous cells, it was observed
that TLR4 was localized in the membrane and cytoplasm, while MyD88
was localized only in the cytoplasm. TLR4 and MyD88 were indicated
to be expressed in paracancerous tissues but the expression was
weak. In addition, it was revealed that TLR4 and MyD88 were highly
expressed in inflammatory cells and duct epithelium.
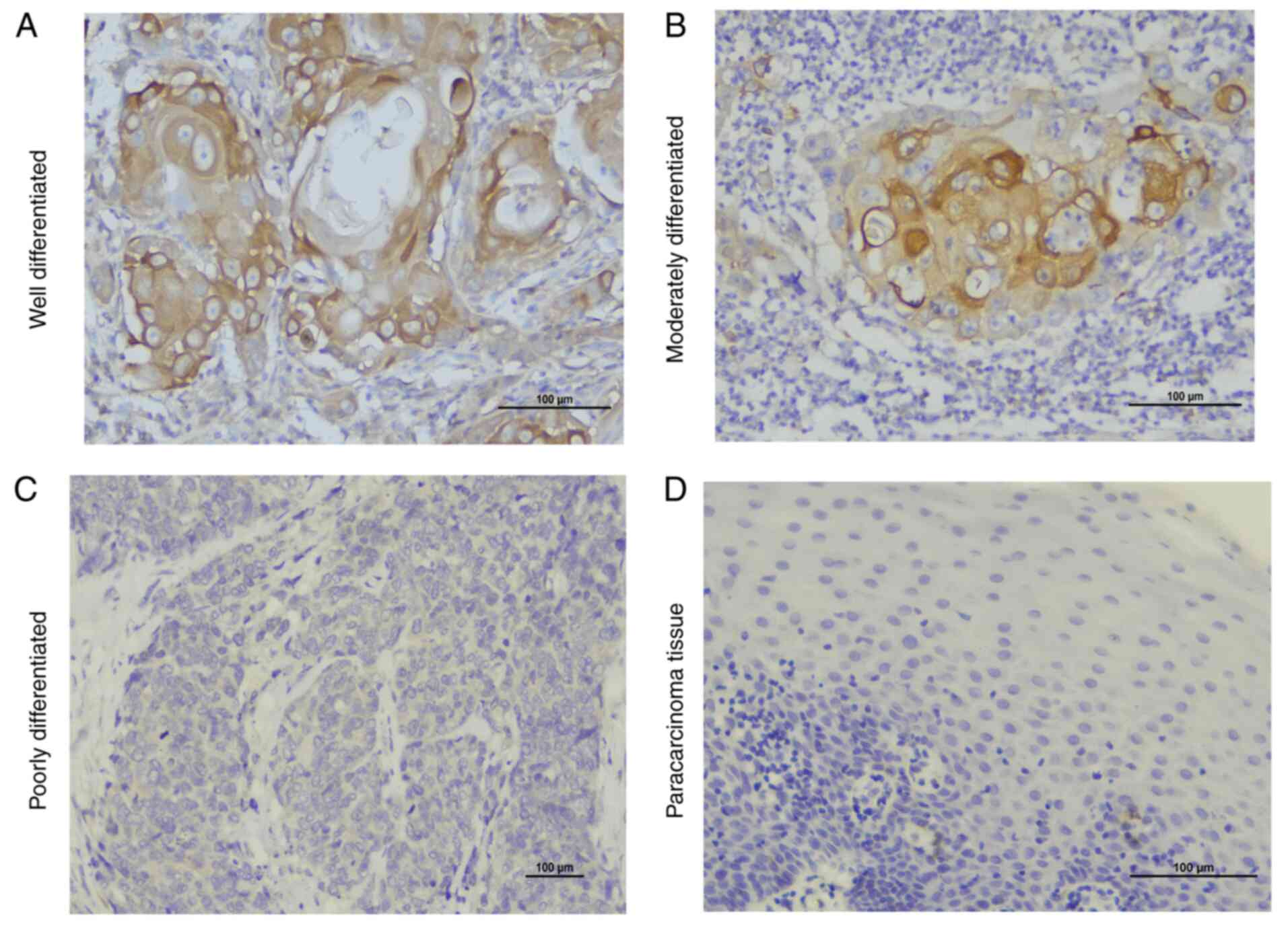
TLR4 and MyD88 are highly expressed in
OSCC
Two pathologists separately evaluated all specimens
using the same staining scoring criteria. TLR4 and MyD88 protein
expression was analyzed in all 55 samples. The expression results
of TLR4 and MyD88 were as follows: TLR4 and MyD88 were rarely
expressed in paracancerous tissues, while positive staining was
observed in most of the 55 OSCC specimens. In the OSCC area,
staining for TLR4 was positive in 49 cases and negative in 6 cases.
Staining for TLR4 in their corresponding adjacent tissues was
positive in 2 cases and negative in 53 cases and the difference
from the tumor tissues was statistically significant
(χ2=80.754, P<0.001). In OSCC, staining for MyD88 was
positive in 46 cases and negative in 9 cases. In normal adjacent
tissues, staining for MyD88 was positive in 6 cases and negative in
49 cases and the difference from the tumor tissues was
statistically significant (χ2=58.355, P<0.001;
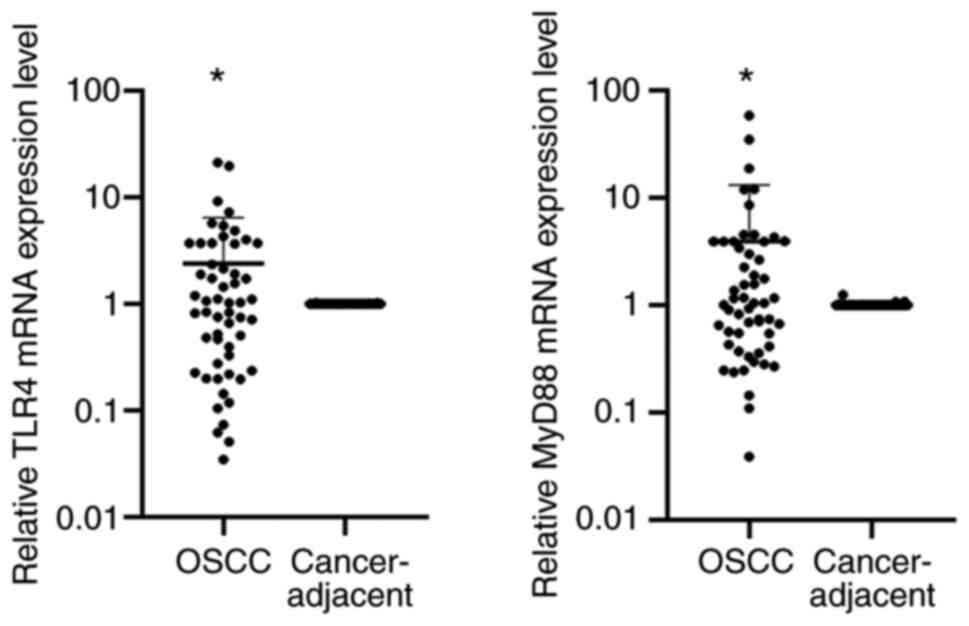
Table II). The RT-qPCR analysis
also revealed elevated expression of TLR4 and MyD88 in OSCC
(Table I). The results of the
RT-qPCR analysis were consistent with those of the
immunohistochemical detection in terms of TLR4 and MyD88 being
highly expressed in OSCC, while their levels in the corresponding
tissue adjacent to carcinoma of TLR4 and MyD88 were low (Table I, Fig.
1,Fig. 2,Fig. 3). Therefore, it was concluded that
the expression levels of TLR4 and MyD88 in OSCC were higher than
the corresponding paracancerous tissues.
 | Table II.Protein expression of MyD88 and TLR4
in OSCC and pericarcinomatous tissue. |
Table II.
Protein expression of MyD88 and TLR4
in OSCC and pericarcinomatous tissue.
|
| Myd88 |
|
| TLR4 |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Tissue type | − | + | χ2 | P-value | − | + | χ2 | P-value |
|---|
| OSCC | 9
(16.3) | 46 (83.7) | 58.355 | <0.001 | 6
(10.9) | 49 (89.1) | 80.754 | <0.001 |
| Pericarcinomatous
tissue | 49 (89.1) | 6
(10.9) |
|
| 53 (96.4) | 2 (3.6) |
|
|
MyD88 is highly expressed in OSCC with
a high and intermediate degree of differentiation compared with
that in poorly differentiated OSCC
The positive expression rate of MyD88 in OSCC was
compared among different histological grades. It was revealed that
the expression rate of MyD88 was higher in well and moderately
differentiated tumors than in poorly differentiated tumors (P=0.01;
Table III, Fig. 2). This result suggested that
overexpression of MyD88 may be related to a higher degree of
differentiation.
 | Table III.Association between the
clinicopathological features and the protein expression of TLR4 and
MyD88. |
Table III.
Association between the
clinicopathological features and the protein expression of TLR4 and
MyD88.
|
|
| Myd88 |
|
| TLR4 |
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Characteristic | N | − | + | χ2 | P-value | − | + | χ2 | P-value |
|---|
| Pathological
differentiation grade |
|
|
| 6.709 | 0.01 |
|
| 0.000 | 1.000 |
|
Well-moderate | 51 | 6
(11.8) | 45 (88.2) |
|
| 6
(11.8) | 45 (88.2) |
|
|
|
Poor | 4 | 3
(75.0) | 1
(25.0) |
|
| 0 (0.0) | 4
(100) |
|
|
| Underlying
disease |
|
|
| 5.010 | 0.025 |
|
| 0.718 | 0.153 |
| No | 12 | 5
(41.7) | 7
(58.3) |
|
| 0 (0.0) | 12
(100.0) |
|
|
|
Yes | 43 | 4 (9.3) | 39 (90.7) |
|
| 6
(14.0) | 37 (86.0) |
|
|
| Recurrence |
|
|
| 5.214 | 0.022 |
|
| 0.000 | 1.000 |
| No | 53 | 7
(13.2) | 46 (86.8) |
|
| 6
(11.3) | 47 (88.7) |
|
|
|
Yes | 2 | 2
(100.0) | 0 (0.0) |
|
| 0 (0.0) | 2
(100.0) |
|
|
MyD88 is highly expressed in patients
without short-term recurrence
The expression of MyD88 was compared between OSCC
tissues of patients with or without short-term recurrence (within a
year). The expression of MyD88 was high in patients without
short-term recurrence, while it was low in patients with recurrence
(P=0.022; Table III). However,
there were only 2 cases of recurrence, which was not sufficient to
signify that the overexpression of MyD88 is related to the
prognosis of OSCC.
MyD88 is highly expressed in patients
with underlying disease
The expression of MyD88 in OSCC tissues of patients
with or without underlying diseases was then compared. It was
revealed that the expression of MyD88 was high in patients with
underlying disease, while it was low in patients without underlying
disease (P=0.025; Table III). This
result indicated that overexpression of MyD88 may be related to
underlying disease. The protein and mRNA expression levels of MyD88
in OSCC tissues were not associated with to sex, age, ethnicity,
smoking, alcohol consumption, tumor stage, nodal status, tumor
site, TNM stage, contralateral lymph node metastasis and
diabetes(Tables SI and SII).
TLR4 expression is significantly
associated with tumor growth time
The RT-qPCR results in Table IV indicated that the expression
level of TLR4 was significantly associated with the tumor growth
time (P=0.001). A longer growth time of OSCC was associated with a
higher expression of TLR4 protein; however, MyD88 was not
associated with tumor growth time. The protein and the mRNA
expression of TLR4 in OSCC tissues of patients are unconcerned with
gender, age, nationality, smoking or drinking and so on (Tables SI and SII).
 | Table IV.Association between the
clinicopathological features and the mRNA expression of TLR4 and
MyD88. |
Table IV.
Association between the
clinicopathological features and the mRNA expression of TLR4 and
MyD88.
| Tumor growth time
(months) | N | Myd88 | t/F | P-value | TLR4 | t/F | P-value |
|---|
| ≤6 (A) | 42 |
3.930±10.310 | 0.011 | 0.989 | 1.430±1.850 | 7.688 | 0.001 |
| 6–12 (B) | 4 | 3.380±1.800 |
|
| 2.920±2.270 |
|
|
| 12–18 (C) | 9 | 4.220±6.590 |
|
| 6.650±8.150 |
|
|
TLR4 is positively correlated with
MyD88 expression in OSCC
Spearman's correlation analysis was performed to
calculate the correlation between the protein and mRNA expression
levels of TLR4 and MyD88. Analysis of the immunohistochemical
results indicated a positive correlation between TLR4 and MyD88
protein expression levels in OSCC (r=0.653, P<0.001).
Furthermore, the RT-qPCR results also revealed a positive
correlation between TLR4 and MyD88 mRNA expression levels (r=0.431,
P=0.001). In addition, the expression of MyD88 in OSCC was higher
than that in TLR4 (t=7.361, P<0.001). These statistical results
indicated that because MyD88 is downstream of TLR4 (20), upregulation of TLR4 expression
promoted the upregulation of MyD88 expression.
Discussion
OSCC is a global public health issue and a
particular challenge for oral physicians. The major complaints
associated with OSCC are impairment of speech, swallowing and
chewing functions, with pain as the major symptom. According to the
International Agency for Research on Cancer, OSCC has a high
incidence rate globally, with >300,000 cases diagnosed each year
and ~145,000 deaths annually (21).
Approximately one-third of patients with OSCC are diagnosed with
stage I/II disease (22). These
patients have a good prognosis, with a cure rate of ~80% (stage I)
and 65% (stage II) (23). However,
two-thirds of OSCC cases are diagnosed at the late stages of the
disease (stage III or IV) (24),
with a 5-year survival rate of <50% and a cure rate of 30%
(25). Therefore, early diagnosis
and active treatment may effectively improve their prognosis.
Current treatment options for OSCC include surgery, chemotherapy,
biotherapy and radiation therapy (26). However, there are currently no
reliable biomarkers to stratify patients and optimize the treatment
for OSCC. Therefore, identifying reliable biomarkers and novel
molecular targets is critical for stratifying patients to develop
precise and personalized treatment regimens. Chronic inflammation
is related to the occurrence and development of cancer and may
promote cancer progression by activating oncogenic signaling
pathways (27).
Wang et al (13) indicated that high expression of TLR4
is conducive to immune escape of tumor cells and promotes the
proliferation, invasion and metastasis of tumor cells. MyD88 is an
adaptor for the IL-1 and TLR family of downstream inflammatory
signaling pathways. In addition, MyD88 signaling has an important
role in regulating inflammation during bacterial infection and
cancer development (28). Signaling
molecules downstream of TLR4/MyD88, for instance NF-κB and
IKK/IκB/MAPK, as well as atypical Akt signaling pathways, are
involved in tumor-cell over proliferation (13). According to previous reports that
TLR4/MyD88 signaling is dependent on cyclooxygenase, epidermal
growth causes excessive proliferation of tumor cells. Activation of
TLR4 and MyD88 may increase the expression of prostaglandin E2 and
cyclooxygenase-2, enhance EGFR signaling and promote the occurrence
and development of inflammation-related cancer (29).
In various studies, different tumor models have been
previously used to reach contrasting conclusions. Of note,
experimental data associated with TLRS/MyD88 signaling may be
contradictory. TLRs have dual functions in tumor development: On
the one hand, they may activate the death signal of the tumor; on
the other hand, they facilitate proliferation, invasion and
migration (30). TLR promotes
inflammation, primarily through the previously mentioned TLRS/MyD88
signaling pathway. TLR has a tumor-promoting effect when it acts as
a proinflammatory factor and has an anti-apoptotic effect. First,
TLRs/MyD88 acts as an upstream signaling pathway to regulate the
inflammatory pathway. Individuals with chronic inflammation over a
long period of time are at a higher risk of developing cancer
(31). Furthermore, the TLRs/MyD88
signaling pathway activates NF-κB, which controls the expression of
anti-apoptotic genes and has a strong anti-apoptotic effect
(32). High expression of TLR and
MyD88 contribute to proliferation, invasion and metastasis
(33). Another study suggested that
genetically engineered TLR4 overexpression increases the
susceptibility of mice to inflammation-induced neoplasia (15).
Furthermore, the TLRs/MyD88 signaling pathway has
been reported to have antitumor effects. In clinical trials,
patients with high TLR4 expression had a 36.9% higher decreased
risk of cancer-associated death within 5 years (32). Studies have indicated that TLRs and
the promotion of downstream mediators may transform the
immunomodulatory effects of TLRs/MyD88 into anti-tumor effects
(34,35).
The role of TLR as an anti-tumor or pro-tumor agent
depends on the type of TLR, different tumor subtypes and the
environment of tumor cells. Activation of the TLRs/MyD88 signaling
pathway promotes the secretion of IFN and pro-inflammatory
cytokines, enhances the antigen presentation ability of dendritic
cells (DC) and has anticancer effects (36). Pro-inflammatory cytokines and the
enhancement of the antigen presentation ability of DC have
anticancer effects, which eventually lead to the maturation of DCs
and the enhancement of the antigen presentation ability of DCs
(37).
In the present study, a significant increase in the
expression of TLR4 and MyD88 in OSCC was observed. This result is
consistent with the results of Sharma and Bala (38). The TLR4/MyD88 signaling pathway
activates the expression of NF-κB (39) and secondarily promotes the expression
of pro-inflammatory genes, thereby triggering the inflammatory
response of the host. When activated by TLR4 through stress,
injury, death of cells or degradation of extracellular matrix, two
signaling pathways are activated, of which the MyD88-dependent
pathway is activated in a series of ways, and finally, the NF-κB
signaling pathway is activated. When cells are exposed to the
outside stimulation, such as tissue injury or infection (40), the NF-κB will be transferred from the
cytoplasm into the nucleus to relate gene transcription, thus
promote inflammation reaction and immune response (41).
To clarify the specific effects of TLR4 and MyD88 in
OSCC, the association between biomolecular expression and
clinicopathological features was further examined. The expression
of MyD88 was greater in highly differentiated and moderately
differentiated OSCC than in poorly differentiated OSCC. This
suggests that overexpression of MyD88 is involved in tumor
differentiation. The results are consistent with the results of
Sharma and Bala (38), who also
reported that high expression of TLR4 was associated with deep
tumor invasion. He et al (42) indicated that activation of the
TLR4-MyD88-NF-κB signaling pathway may stimulate
epithelial-mesenchymal transition (EMT), which allows cancer cells
to detach from the primary site and then invade lymphatic and/or
blood vessels, resulting in loss of epithelial adhesion and
polarity of cancer cells and promote tumor metastasis. Multiple
biomarkers involved in EMT induction may be future therapeutic
targets for oral cancer (43).
Studies have suggested that blocking MyD88 signaling significantly
improves anorexia and fatigue, decreases weight loss, reduces
muscle catabolism and atrophy, decreases systemic and central
nervous system inflammation, and ultimately improves survival
(44–46).
In addition, high expression of MyD88 was observed
in patients with short-term relapse in the present study, but the
number of patients with short-term relapse was too low (2 cases);
therefore, further study is required to confirm this conclusion. To
the best of our knowledge, the present study is the first to
demonstrate that MyD88 is highly expressed in patients with OSCC,
with underlying disease. However, MyD88 expression is not
associated with diabetes (P=0.286; Table SI).
It has been established that the immune system has a
leading role in the initiation and maintenance of hypertension.
However, the process by which the immune response is initiated has
remained to be fully elucidated (47). The innate immune system activates the
adaptive immune system through direct molecular interactions and
the release of immune mediators (48); components of innate immunity may be
involved in the initiation of hypertension. Certain TLRs are
associated with hypertension (49).
These TLRs utilize MyD88 for intracellular signal transduction and
activate a pro-inflammatory cascade. It has been indicated that
TLR4 is an important receptor for signal transduction in the innate
immune system and may influence various cardiovascular diseases,
such as heart failure and hypertension (47).
Angiotensin II (Ang II) is the major effector
peptide of the renin-angiotensin system. Ang II type 1 receptor
promotes blood pressure regulation by interacting with Ang II
(50). Singh et al (47) observed that TLR3 and TLR4
differentially induced hypertension and cardiac hypertrophy by Ang
II and MyD88 inhibited the pro-inflammatory hypertensive effects of
Ang II.
In the TLR4 signaling pathway, the MyD88-dependent
signaling pathway is an important activator of NF-κB and its
subsequent regulation (51). Agarwal
et al (52) demonstrated that
the increase of various pro-inflammatory cytokines activates the
NF-κB signaling pathway and leads to increased production of
extracellular reactive oxygen species, resulting in intracellular
redox state transfer leading to NF-κB activation and enhancement of
the NF-κB signaling pathway. The activation of NF-κB was further
upregulated. This vicious cycle eventually leads to the development
of hypertension.
Hypertension is frequently associated with impaired
glucose tolerance, insulin resistance and obesity. Consequently,
numerous individuals with hypertension develop diabetes (53). Hypertension is a recognized risk
factor for cardiovascular disease, regardless of diabetes (54). Hypertension is more common in
patients with type 2 diabetes, and those who have both hypertension
and diabetes are at higher risk of developing cardiovascular
disease than those who have either disease alone (55).
The TLR pathway is thought to have a key role in the
mechanisms leading to diabetes in human and animal models (56). Duparc et al (57) reported that MyD88 has a role in
developing glucose intolerance and hepatic steatosis. Androulidaki
et al (58) identified MyD88
signaling as an important pathogenic factor in autoimmune diabetes.
The MyD88 signaling pathway promotes the development of diabetes in
non-obese diabetic mice by accelerating the onset of diabetes.
A clear and unambiguous link exists between diabetes
and the incidence of coronary artery disease (58). Given the high incidence of coronary
artery disease among patients with diabetes, it is not surprising
that diabetic patients are at high risk of developing ischemic
cardiomyopathy and heart failure. Although the majority of cases of
heart failure in patients with diabetes are caused by progression
from coronary artery disease to ischemic cardiomyopathy (59), the long-term metabolic disorders of
diabetes may also be toxic to the heart muscle (60). Diabetes mellitus is widely recognized
as an important risk factor for the development of heart failure
and is an independent risk factor for increased mortality in
patients with heart failure (61).
Dong et al (62) reported
that the TLR4 signaling pathway may lead to myocardial injury in
patients with diabetes. Type 2 diabetes and congestive heart
failure occur simultaneously due to common risk factors, such as
coronary artery disease, and the direct cardiotoxic effects of type
2 diabetes (62). Follow-up data
from the Framingham study collected over 20 years indicated that
diabetes is an independent risk factor for the development of heart
failure (62).
Myocardial inflammation caused by coronary
microembolism (CME) is the major cause of cardiac injury (39). Su et al (39) indicated that TLR4/MyD88/NF-κB
signaling is involved in myocardial inflammation after CME,
activating the NOD-like receptor protein 3 inflammasome, promoting
the inflammatory cascade and aggravating myocardial injury.
Numerous studies have indicated that the TLR4/MyD88/NF-κB signaling
pathway controls the production of pro-inflammatory factors and
induces the inflammatory response of myocardial tissue, which is
the major cause of myocardial tissue injury (39). The role of MyD88 (39) and TLR4 (63) has been demonstrated in myocardial
infarction and aortic band-induced cardiac hypertrophy.
To sum up, hypertension, heart disease and diabetes
affect each other and the TLR4/MyD88 pathway has an important role
in the occurrence and development of hypertension, heart disease
and diabetes, which may explain the high expression of MyD88 in
basic diseases.
Furthermore, the present study also suggested that
TLR4 expression levels were significantly associated with tumor
growth time; a longer tumor growth time was linked to a higher TLR4
expression level. These results prove that TLR4 is closely related
to the occurrence and development of OSCC. TLR4 may be involved in
and promote the development of cancer, and its high expression may
indicate a poor prognosis for OSCC. These results further confirm
the previous assumption that TLR4 and MyD88 are related to tumor
progression.
A study by Park et al (64) on inflammation suggested that
lipopolysaccharide was able to regulate the oligomerization of
TLR4. It binds to five TIR domains, including MyD88 (65). Among them, the combination of TLR4
and MyD88 is able to initiate NF-κB and thus upregulate
pro-inflammatory genes to activate the inflammatory response to
infection (66). In the present
study, the expression levels of TLR4 and MyD88 were determined to
be closely correlated in OSCC. Combined with those of previous
studies, the present results indicated that TLR4 also regulates
MyD88 in oral cancer.
Existing studies reported on numerous inhibitors
targeting TLR4 and MyD88. Eritoran (E5564) (67) is an investigational drug for treating
severe sepsis and acts as an inhibitor of TLR4. Curcumin, a
polyphenol, suppresses the TLR4/MAPK/NF-κB pathway to inhibit
excessive inflammatory mediators (68). Reversed retinoic acid targets TNF-α
and hydrogen oxide synthase 2 inhibitors through TLR4/NF-κB
signaling pathways. This may resemble a novel treatment strategy
for oral cancer (69).
In the present study, TLR4 and MyD88 were only
assessed at the tissue and molecular levels and the analysis of
TLR4 and MyD88 remains insufficient. Both of them involve complex
networks and various pathways, which should be improved by
experiments at the cellular and animal levels, which is a goal of
future research by our group. The results of the present study are
based on 2D cross-sections, while 3D organotypic models are
receiving increasing attention due to their ability to reconstruct
precise tissue. Human physiology is profoundly different from the
mouse model system. Common drugs such as ibuprofen (70) and warfarin (71), which are metabolized differently in
the livers of humans and rodents, where ibuprofen is used to treat
pain, fever and inflammation and warfarin is used as an
anticoagulant, are both toxic to mice. It is not surprising that
there are large metabolic differences between human and laboratory
models. Most drug candidates do not enter the market due to
unrepresentative two-dimensional preclinical models and poor
correlation between preclinical and clinical trial results. Even
when drugs are approved for clinical use, certain products may be
recalled due to serious adverse reactions. 3D tissue cultures may
produce samples suitable for low-cost, high-throughput drug
screening and predict human drug responses in vitro in a
timely manner.
Organ on a chip is a promising tool for the
preclinical testing of drugs. Tumors have a complex
microenvironment, including a dense extracellular matrix and a wide
variety of stromal cells and immune cells (72). Irregular blood vessels and limited
perfusion of nutrients may significantly affect the efficacy of
administration therapy. By combining 3D organic culture systems
with microfluidics to form an ‘organ on a chip’, in vitro
controlled microfluidics are able to stimulate blood flow within
the organ and reproduce the dynamic distribution of nutrients
(73). By connecting different
organs on a chip, microfluidic systems may simulate complex
multi-organ metabolism and pharmacokinetics of drugs. Cellular
behavior, particularly drug responses, may be easily monitored. The
organ-on-a-chip approach has been used to model various healthy and
diseased organs, such as kidney (74) and lung (75).
The quasi-human organ is a three-dimensional culture
system derived from stem cells that have the potential to
reconstruct the structure and physiology of human organs in
remarkable detail. Human organoids are already being used to study
infectious diseases, genetic diseases and cancer through genetic
engineering of human stem cells, and may also be generated directly
from a patient's biopsy sample (76).
3D culture systems are highly similar to actual
human organs and in certain cases, they are histologically
indistinguishable. A feature common to all organoids is that they
are composed of pluripotent stem cells or adult stem cells (also
known as tissue stem cells), mimicking human development or organ
regeneration in vitro. Thus, analysis of the formation of
organ-like systems may provide valuable information on the
mechanisms of human development and organ regeneration,
highlighting its value in basic biological research and its
potential applications in drug testing and molecular medicine.
Establishing an animal model for a particular
disease requires prior knowledge of the pathogenic conditions or
genes involved. Organ models may be generated directly from
affected patients without prior knowledge of the specific genes
involved. This is particularly relevant to polygenetic diseases
(such as inflammatory bowel disease, if the pathology is caused by
the affected epithelial cells) and cancer, where cancer-like organs
may be isolated directly from the patient (77). One drawback of organ-like systems is
the lack of inter-organ communication. There is the possibility of
generating a cavity device that combines organic research with
organ-on-a-chip, allowing different organ-like types to be
cultivated separately, thereby preventing organ-like uncontrolled
fusion while allowing organ-like communication (78,79).
Human organ-like systems will offer unprecedented opportunities to
improve human health.
In conclusion, the present study confirmed
differences in TLR4 and MyD88 expression between OSCC and
paracancerous tissues and analyzed the relationship between TLR4
and MyD88, as well as clinical pathology parameters and patient
prognosis. It was demonstrated for the first time that MyD88 is
overexpressed in patients with underlying conditions, such as
hypertension and diabetes, and may have an important role in
long-term prognosis. Furthermore, TLR4 expression levels were
significantly correlated with the tumor growth time. These gene
expression experiments verified that MyD88 and TLR4 may be used as
biomarkers, that may provide guidance for decision making regarding
the treatment of OSCC.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Danping Li
(Pathology of the School of Stomatology, Guangxi Medical
University, Guangxi, China) for helping prepare the tissue
sections. The authors would also like to thank Mr. Qincao Tang
(Department of Oral and Maxillofacial Surgery, Guangxi Medical
University, Guangxi, China) for technical support with RT-qPCR.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81560187).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
LL was involved in designing the study and
participated in the collection of specimens, performing the
experiments, data analysis, interpretation of the data and drafting
and revising the manuscript. ZZ and KM assisted in interpreting
experimental results and participated in manuscript writing. PL
assisted in performing the experiments and the completion of
interpreting the research results and assisted in revising the
manuscript. ZW and YW assisted in the collection of specimens and
revision of the manuscript. YC and XM provided assistance in
performing the experiments. TZ assisted with the data analysis and
revision of the manuscript. DW conceived and directed the study and
assisted with interpreting the study results and the revision of
the manuscript. LL and ZZ authenticated all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the College of Stomatology, Guangxi Medical University
(Nanning, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
de Groen RAL, Schrader AMR, Kersten MJ,
Pals ST and Vermaat JSP: MYD88 in the driver's seat of B-cell
lymphomagenesis: From molecular mechanisms to clinical
implications. Haematologica. 104:23372019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ali J, Sabiha B, Jan HU, Haider SA, Khan
AA and Ali SS: Genetic etiology of oral cancer. Oral Oncol.
70:23–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oh HN, Seo JH, Lee MH, Kim C, Kim E, Yoon
G, Cho SS, Cho YS, Choi HW, Shim JH and Chae JI: Licochalcone C
induced apoptosis in human oral squamous cell carcinoma cells by
regulation of the JAK2/STAT3 signaling pathway. J Cell Biochem.
119:10118–10130. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rivera C: Essentials of oral cancer. Int J
Clin Exp Pathol. 8:11884–11894. 2015.PubMed/NCBI
|
|
5
|
Edwards BK, Brown ML, Wingo PA, Howe HL,
Ward E, Ries LA, Schrag D, Jamison PM, Jemal A, Wu XC, et al:
Annual report to the nation on the status of cancer, 1975–2002,
featuring population-based trends in cancer treatment. J Natl
Cancer Inst. 97:1407–1427. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lindemann A, Takahashi H, Patel AA, Osman
AA and Myers JN: Targeting the DNA damage response in OSCC with TP
53 mutations. J Dent Res. 97:635–644. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roi A, Roi CI, Negruțiu ML, Riviș M,
Sinescu C and Rusu LC: The challenges of OSCC diagnosis: Salivary
cytokines as potential biomarkers. J Clin Med. 9:28662020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen R, Alvero AB, Silasi DA and Mor G:
Inflammation, cancer and chemoresistance: Taking advantage of the
toll-like receptor signaling pathway. Am J Reprod Immunol.
57:93–107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mehmeti M, Allaoui R, Bergenfelz C, Saal
LH, Ethier SP, Johansson ME, Jirström K and Leandersson K:
Expression of functional toll like receptor 4 in estrogen
receptor/progesterone receptor-negative breast cancer. Breast
Cancer Res. 17:1302015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Semlali A, Reddy Parine N, Arafah M,
Mansour L, Azzi A, Al Shahrani O, Al Amri A, Shaik JP, Aljebreen
AM, Alharbi O, et al: Expression and polymorphism of toll-like
receptor 4 and effect on NF-κB mediated inflammation in colon
cancer patients. PLoS One. 11:e01463332016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takazawa Y, Kiniwa Y, Ogawa E, Uchiyama A,
Ashida A, Uhara H, Goto Y and Okuyama R: Toll-like receptor 4
signaling promotes the migration of human melanoma cells. Tohoku J
Exp Med. 234:57–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo XZ, He QZ and Wang K: Expression of
toll-like receptor 4 in ovarian serous adenocarcinoma and
correlation with clinical stage and pathological grade. Int J Clin
Exp Med. 8:14323–14327. 2015.PubMed/NCBI
|
|
13
|
Wang JQ, Jeelall YS, Ferguson LL and
Horikawa K: Toll-like receptors and cancer: MYD88 mutation and
inflammation. Front Immunol. 5:3672014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deguine J and Barton GM: MyD88: A central
player in innate immune signaling. F1000Prime Rep. 6:972014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang EL, Qian ZR, Nakasono M, Tanahashi T,
Yoshimoto K, Bando Y, Kudo E, Shimada M and Sano T: High expression
of toll-like receptor 4/myeloid differentiation factor 88 signals
correlates with poor prognosis in colorectal cancer. Br J Cancer.
102:908–915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang HY, Zhang ZJ, Cao CB, Wang N, Liu
FF, Peng JQ, Ren XJ and Qian J: The TLR4/NF-κB signaling pathway
mediates the growth of colon cancer. Eur Rev Med Pharmacol Sci.
18:3834–3843. 2014.PubMed/NCBI
|
|
17
|
Chen X, Zhao F, Zhang H, Zhu Y, Wu K and
Tan G: Significance of TLR4/MyD88 expression in breast cancer. Int
J Clin Exp Pathol. 8:7034–7039. 2015.PubMed/NCBI
|
|
18
|
Mills SE, Carter D, Greenson JK, Reuter VE
and Stoler MH: Sternberg's diagnostic surgical pathology, 5th
edition. 2-2. Lippincott Williams & Wilkins; Philidelphia, PA,
USA: pp. 2348. 2012
|
|
19
|
The Union for International Cancer Control
(UICC), . TNM History, Evolution and Milestones. https://www.uicc.org/sites/main/files/atoms/files/TNM-History-2021.pdfSeptember
11–2021
|
|
20
|
Lin SC, Lo YC and Wu H: Helical assembly
in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature.
465:885–890. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song Y, Zhou M, Cao Y, Qi J, Geng J and
Liu X: Expression of GLP-1 receptor and CD26 in human thyroid
C-cells: The association of thyroid C-cell tumorigenesis with
incretin-based medicine. Oncol Lett. 13:2684–2690. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
González-Moles MÁ, Warnakulasuriya S,
González-Ruiz I, González-Ruiz L, Ayén Á, Lenouvel D, Ruiz-Ávila I
and Ramos-García P: Clinicopathological and prognostic
characteristics of oral squamous cell carcinomas arising in
patients with oral lichen planus: A systematic review and a
comprehensive meta-analysis. Oral Oncol. 106:1046882020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Warnakulasuriya S, Johnson NW and Van der
Waal I: Nomenclature and classification of potentially malignant
disorders of the oral mucosa. J Oral Pathol Med. 36:575–580. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Güneri P and Epstein JB: Late stage
diagnosis of oral cancer: Components and possible solutions. Oral
Oncol. 50:1131–1136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng YS, Rees T and Wright J: A review of
research on salivary biomarkers for oral cancer detection. Clin
Transl Med. 3:32014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rivera C: The challenge of the state of
susceptibility to oral cancer. J Oral Res. 4:8–9. 2015. View Article : Google Scholar
|
|
28
|
Sakurai T, Kashida H, Watanabe T, Hagiwara
S, Mizushima T, Iijima H, Nishida N, Higashitsuji H, Fujita J and
Kudo M: Stress response protein cirp links inflammation and
tumorigenesis in colitis-associated cancer. Cancer Res.
74:6119–6128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oshima H and Oshima M: The inflammatory
network in the gastrointestinal tumor microenvironment: Lessons
from mouse models. J Gastroenterol. 47:97–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang L, Zhu R, Huang Z, Li H and Zhu H:
Lipopolysaccharide-induced toll-like receptor 4 signaling in cancer
cells promotes cell survival and proliferation in hepatocellular
carcinoma. Dig Dis Sci. 58:2223–2236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fukata M, Chen A, Klepper A, Krishnareddy
S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ
and Abreu MT: Cox-2 is regulated by toll-like receptor-4 (TLR4)
signaling: Role in proliferation and apoptosis in the intestine.
Gastroenterology. 131:862–877. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sahu U, Choudhury A, Parvez S, Biswas S
and Kar S: Induction of intestinal stemness and tumorigenicity by
aberrant internalization of commensal non-pathogenic E. coli. Cell
Death Dis. 8:e26672017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Paarnio K, Väyrynen S, Klintrup K, Ohtonen
P, Mäkinen MJ, Mäkelä J and Karttunen TJ: Divergent expression of
bacterial wall sensing toll-like receptors 2 and 4 in colorectal
cancer. World J Gastroenterol. 23:4831–4838. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu L, Wang L and Chen S: Dual character of
toll-like receptor signaling: Pro-tumorigenic effects and
anti-tumor functions. Biochim Biophys Acta. 1835:144–154.
2013.PubMed/NCBI
|
|
36
|
Mikulandra M, Pavelic J and Glavan TM:
Recent findings on the application of toll-like receptors agonists
in cancer therapy. Curr Med Chem. 24:2011–2032. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kubo T, Hatton RD, Oliver J, Liu X, Elson
CO and Weaver CT: Regulatory T cell suppression and anergy are
differentially regulated by proinflammatory cytokines produced by
TLR-activated dendritic cells. J Immunol. 173:7249–7258. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sharma Y and Bala K: Role of toll like
receptor in progression and suppression of oral squamous cell
carcinoma. Oncol Rev. 14:4562020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Su Q, Li L, Sun Y, Yang H, Ye Z and Zhao
J: Effects of the TLR4/Myd88/NF-κB signaling pathway on NLRP3
inflammasome in coronary microembolization-induced myocardial
injury. Cell Physiol Biochem. 47:1497–1508. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qin DP, Sun PN, Zhou YJ, Chen FM, Zhang
CL, Han JX and Yang XJ: Effect of tripterygium wilfordii polycoride
upon inflammation and TLR4/MyD88 signaling pathway in ulcerative
colitis rats model. Zhonghua Yi Xue Za Zhi. 96:1444–1449. 2016.(In
Chinese). PubMed/NCBI
|
|
42
|
He Z, Deng R, Huang X, Ni Y, Yang X, Wang
Z and Hu Q: Lipopolysaccharide enhances OSCC migration by promoting
epithelial-mesenchymal transition. J Oral Pathol Med. 44:685–692.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Todosi AM, Gavrilescu MM, Aniţei GM, Filip
B and Scripcariu V: Colon cancer at the molecular level-usefulness
of epithelial-mesenchymal transition analysis. Rev Med Chir Soc Med
Nat Iasi. 116:1106–1111. 2012.PubMed/NCBI
|
|
44
|
Zhu X, Burfeind KG, Michaelis KA, Braun
TP, Olson B, Pelz KR, Morgan TK and Marks DL: MyD88 signalling is
critical in the development of pancreatic cancer cachexia. J
Cachexia Sarcopenia Muscle. 10:378–390. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Y and Zeng Y: Curcumin reduces
inflammation in knee osteoarthritis rats through blocking
TLR4/MyD88/NF-κB signal pathway. Drug Dev Res. 80:353–359. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ju M, Liu B, He H, Gu Z, Liu Y, Su Y, Zhu
D, Cang J and Luo Z: MicroRNA-27a alleviates LPS-induced acute lung
injury in mice via inhibiting inflammation and apoptosis through
modulating TLR4/MyD88/NF-κB pathway. Cell Cycle. 17:2001–2018.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Singh MV, Cicha MZ, Nunez S, Meyerholz DK,
Chapleau MW and Abboud FM: Angiotensin II-induced hypertension and
cardiac hypertrophy are differentially mediated by TLR3- and
TLR4-dependent pathways. Am J Physiol Heart Circ Physiol.
316:H1027–H1038. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jain A and Pasare C: Innate control of
adaptive immunity: Beyond the three-signal paradigm. J Immunol.
198:3791–3800. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dange RB, Agarwal D, Teruyama R and
Francis J: Toll-like receptor 4 inhibition within the
paraventricular nucleus attenuates blood pressure and inflammatory
response in a genetic model of hypertension. J Neuroinflammation.
12:312015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kang YM, Zhang DM, Yu XJ, Yang Q, Qi J, Su
Q, Suo YP, Yue LY, Zhu GQ and Qin DN: Chronic infusion of
enalaprilat into hypothalamic paraventricular nucleus attenuates
angiotensin II-induced hypertension and cardiac hypertrophy by
restoring neurotransmitters and cytokines. Toxicol Appl Pharmacol.
274:436–444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhu HT, Bian C, Yuan JC, Chu WH, Xiang X,
Chen F, Wang CS, Feng H and Lin JK: Curcumin attenuates acute
inflammatory injury by inhibiting the TLR4/MyD88/NF-κB signaling
pathway in experimental traumatic brain injury. J
Neuroinflammation. 11:592014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Agarwal D, Welsch MA, Keller JN and
Francis J: Chronic exercise modulates RAS components and improves
balance between pro- and anti-inflammatory cytokines in the brain
of SHR. Basic Res Cardiol. 106:1069–1085. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gress TW, Nieto FJ, Shahar E, Wofford MR
and Brancati FL: Hypertension and antihypertensive therapy as risk
factors for type 2 diabetes mellitus. Atherosclerosis risk in
communities study. N Engl J Med. 342:905–912. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bensimhon HF and Cavender MA: Hypertension
treatment in diabetes: Focus on heart failure prevention. Heart
Fail Clin. 15:551–563. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Stamler J, Vaccaro O, Neaton JD and
Wentworth D: Diabetes, other risk factors, and 12-yr cardiovascular
mortality for men screened in the multiple risk factor intervention
trial. Diabetes Care. 16:434–444. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chervonsky A: Innate receptors and
microbes in induction of autoimmunity. Curr Opin Immunol.
21:641–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Duparc T, Plovier H, Marrachelli VG, Van
Hul M, Essaghir A, Ståhlman M, Matamoros S, Geurts L, Pardo-Tendero
MM, Druart C, et al: Hepatocyte MyD88 affects bile acids, gut
microbiota and metabolome contributing to regulate glucose and
lipid metabolism. Gut. 66:620–632. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Androulidaki A, Wachsmuth L, Polykratis A
and Pasparakis M: Differential role of MyD88 and TRIF signaling in
myeloid cells in the pathogenesis of autoimmune diabetes. PLoS One.
13:e01940482018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Marwick TH: Diabetic heart disease.
Postgrad Med J. 84:188–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Marwick TH, Ritchie R, Shaw JE and Kaye D:
Implications of underlying mechanisms for the recognition and
management of diabetic cardiomyopathy. J Am Coll Cardiol.
71:339–351. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mitchell JB, Bubolz T, Paul JE, Pashos CL,
Escarce JJ, Muhlbaier LH, Wiesman JM, Young WW, Epstein RS and
Javitt JC: Using medicare claims for outcomes research. Med Care.
32 (Suppl 7):JS38–JS51. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dong B, Qi D, Yang L, Huang Y, Xiao X, Tai
N, Wen L and Wong FS: TLR4 regulates cardiac lipid accumulation and
diabetic heart disease in the nonobese diabetic mouse model of type
1 diabetes. Am J Physiol Heart Circ Physiol. 303:H732–H742. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jiang DS, Zhang XF, Gao L, Zong J, Zhou H,
Liu Y, Zhang Y, Bian ZY, Zhu LH, Fan GC, et al: Signal regulatory
protein-α protects against cardiac hypertrophy via the disruption
of toll-like receptor 4 signaling. Hypertension. 63:96–104. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Park BS, Song DH, Kim HM, Choi BS, Lee H
and Lee JO: The structural basis of lipopolysaccharide recognition
by the TLR4-MD-2 complex. Nature. 458:1191–1195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
O'Neill LA and Bowie AG: The family of
five: TIR-domain-containing adaptors in toll-like receptor
signalling. Nat Rev Immunol. 7:353–364. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Blauvelt A, Lebwohl MG and Bissonnette R:
IL-23/IL-17A dysfunction phenotypes inform possible clinical
effects from anti-IL-17A therapies. J Invest Dermatol.
135:1946–1953. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Shirey KA, Lai W, Scott AJ, Lipsky M,
Mistry P, Pletneva LM, Karp CL, McAlees J, Gioannini TL, Weiss J,
et al: The TLR4 antagonist Eritoran protects mice from lethal
influenza infection. Nature. 497:498–502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xu Z, Ren T, Xiao C, Li H and Wu T: Nickel
promotes the invasive potential of human lung cancer cells via
TLR4/MyD88 signaling. Toxicology. 285:25–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Feng X, Tan W, Cheng S, Wang H, Ye S, Yu
C, He Y, Zeng J, Cen J, Hu J, et al: Upregulation of microRNA-126
in hepatic stellate cells may affect pathogenesis of liver fibrosis
through the NF-κB pathway. DNA Cell Biol. 34:470–480. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sanoh S, Horiguchi A, Sugihara K, Kotake
Y, Tayama Y, Uramaru N, Ohshita H, Tateno C, Horie T, Kitamura S
and Ohta S: Predictability of metabolism of ibuprofen and naproxen
using chimeric mice with human hepatocytes. Drug Metab Dispos.
40:2267–2272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Inoue T, Nitta K, Sugihara K, Horie T,
Kitamura S and Ohta S: CYP2C9-catalyzed metabolism of S-warfarin to
7-hydroxywarfarin in vivo and in vitro in chimeric mice with
humanized liver. Drug Metab Dispos. 36:2429–2433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sherman MH, Yu RT, Tseng TW, Sousa CM, Liu
S, Truitt ML, He N, Ding N, Liddle C, Atkins AR, et al: Stromal
cues regulate the pancreatic cancer epigenome and metabolome. Proc
Natl Acad Sci USA. 114:1129–1134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sun W, Luo Z, Lee J, Kim HJ, Lee K, Tebon
P, Feng Y, Dokmeci MR, Sengupta S and Khademhosseini A:
Organ-on-a-chip for cancer and immune organs modeling. Adv Healthc
Mater. 8:18013632019. View Article : Google Scholar
|
|
74
|
Musah S, Mammoto A, Ferrante TC, Jeanty
SSF, Hirano-Kobayashi M, Mammoto T, Roberts K, Chung S, Novak R,
Ingram M, et al: Mature induced-pluripotent-stem-cell-derived human
podocytes reconstitute kidney glomerular-capillary-wall function on
a chip. Nat Biomed Eng. 1:00692017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Huh D, Matthews BD, Mammoto A,
Montoya-Zavala M, Hsin HY and Ingber DE: Reconstituting organ-level
lung functions on a chip. Science. 328:1662–1668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kim J, Koo BK and Knoblich JA: Human
organoids: Model systems for human biology and medicine. Nat Rev
Mol Cell Biol. 21:571–584. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Dotti I, Mora-Buch R, Ferrer-Picón E,
Planell N, Jung P, Masamunt MC, Leal RF, Martín de Carpi J, Llach
J, Ordás I, et al: Alterations in the epithelial stem cell
compartment could contribute to permanent changes in the mucosa of
patients with ulcerative colitis. Gut. 66:2069–2079. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Gonneaud A, Asselin C, Boudreau F and
Boisvert FM: Phenotypic analysis of organoids by proteomics.
Proteomics. 17:2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Akhtar AA, Sances S, Barrett R and Breunig
JJ: Organoid and organ-on-a-chip systems: New paradigms for
modeling neurological and gastrointestinal disease. Curr Stem Cell
Rep. 3:98–111. 2017. View Article : Google Scholar : PubMed/NCBI
|