Introduction
For patients with acute lymphoblastic leukemia
(ALL), the accompanying risk of central nervous system leukemia
(CNSL) is more severe compared with that of patients with acute
myeloid leukemia (1,2). CNSL is one of the primary causes of
relapse in patients with acute leukemia and is associated with a
less favorable prognosis (3).
Initially, patients may achieve CNSL remission in the short term
using established treatments, such as chemotherapy, intrathecal
chemotherapy and radiation therapy (4).
The second generation of CD19-directed chimeric
antigen receptors (CAR) results in a more pronounced activation and
expansion of T cells to eliminate malignant B cells in vitro
and in vivo (5). The use of
anti-CD19 chimeric antigen receptor modified T (CAR-T) cells has
achieved 70–88% CR and CRi in recurrent or refractory (R/R) B-ALL
(6,7). Although for cases of R/R B-cell ALL,
CD19 CAR-T therapy induces rapid and effective responses, it is
associated with acute toxicity, including cytokine-release syndrome
(CRS) and CAR-T cell-related encephalopathy syndrome (CRES), which
are severe or even fatal (8). CRS is
characterized by a high fever, hypotension, hypoxia and/or
multiorgan toxicity, whereas CRES is typically characterized by
confusion, delirium and cerebral edema (9–12).
Therefore, CRES is likely to be the more toxic side effect of CD19
CAR-T therapy in patients with CNSL arising from B-ALL.
At present, targeted CD19 CAR-T therapy for R/R
B-cell malignant hematopathy is primarily attributed to the
single-chain Fvs (scFvs) in CAR structures, which are derived from
murine FMC63 or SJ25C1, which are the clone IDs for monoclonal
antibodies targeting CD19 (6,13,14).
In the majority of patients, immune responses are induced by the
invasion of mouse-derived scFv structures to CD19 CAR-T cells
following therapy (15). Although
the effects are significant in the short term, survival of CAR-T
cells in the peripheral blood following treatment for a short
amount of time may assist the maintenance of the long term
therapeutic effect (15). It has
been reported that 93% of patients with R/R ALL exhibit a complete
response (CR) to B cells following CD19 CAR-T cell therapy
(7).
In a clinical study, 32 patients with ALL were
treated with murine CD19 CAR-T therapy, and 5 patients relapsed;
after receiving the second infusion, all of them did not respond to
any curative effects after reinfusion of murine CD19 CAR-T cells
(16). Immunogenicity may be an
important factor affecting the survival of CAR-T cells in
vivo and the curative effect of murine CD19 CAR-T therapy when
used more than once (6). Therefore,
humanized CD19 CAR-T cells have been constructed by isolation of
anti-human CD19 scFvs from human DNA libraries and replaced with
murine FMC63-derived scFv which possesses similar binding
characteristics (17). A previous
study has demonstrated that host immune responses can recognize the
epitopes of the murine scFv and render subsequent infusions
ineffective (18). Humanized CD19
CAR-T cells exhibit improved effects against tumor cell lines
compared with murine CD19 CAR-T cells in vitro (19). Research on humanized CD19 CAR-T cells
is being performed at several centers around the world; however,
there are relatively few reports of humanized CD19 CAR-T therapy in
clinical trials. The aim of the present study was to compare the
differences between humanized CD19 and murine CD19 CAR-T cell
therapy in recurrent B-ALL.
Materials and methods
Medical history presentation
History prior to murine CD19 CAR-T therapy: A
62-year-old male patient was admitted to Tianjin First Central
Hospital (Tianjin, China) due to leukocytosis in September 2013.
After bone marrow puncture and flow cytometry, the patient was
diagnosed with B-ALL (BCR/ABL+). The patient received
VDCP (vincristine, daunorubicin, cyclophosphamide and
dexamethasone) chemotherapy and oral imatinib treatment.
Subsequently, the first CR (CR1) was achieved in October 2013. In
the following two courses of consolidation chemotherapy with VDCP,
leukemia cells were found in the cerebrospinal fluid (CSF). The
minimal residual disease and the BCR-ABL in the bone marrow was
negative at this time. Therefore, the patient was diagnosed with
CNSL and received two courses of high dose methotrexate (MTX)
combined with intrathecal chemotherapy. CR2 was achieved in May
2014 and maintained with imatinib therapy for the following 17
months. In September 2016, the patient was readmitted with
dizziness and tinnitus and diagnosed as relapsed CNSL. The patient
received high dose MTX combined with several courses of intrathecal
chemotherapy; however, CR was not achieved again.
Murine CD19 CAR-T therapy as a
first-time salvage therapy
According to the examination, 57.51% of leukemia
cells were found in the CSF, the patient's minimal residual disease
in the bone marrow was 0.24% and P210 was 0.81% in February 2017.
The patient was enrolled in a clinical trial at the Department of
Hematology at Tianjin First Central Hospital (Tianjin, China) for
treatment with autologous CAR-T 19 cells expressing murine
anti-CD19 scFv and 4-1BB-CD3ζ costimulatory-activation domains
(ChiCTR-ONN-16009862) following relapsed CNSL. Lymphodepleting
chemotherapy with fludarabine (30 mg/m2) and
cyclophosphamide (400 mg/m2) was administered daily
between 4 and 2 days prior to initiation of the trial therapy. The
patient received four courses of intrathecal chemotherapy to reduce
the number of leukemia cells in the CSF from 57.51 to 5.26% prior
to infusion of CAR-T 19 cells. Autologous murine CAR-T 19 cells
were infused (1×106 cells/kg on day 1 and
6×106 cells/kg on day 2). The transduction efficiency of
CD19 CAR and amplification of CD19 CAR-T cells were analyzed by
flow cytometry (FCM) (data not shown).
Humanized CD19 CAR-T therapy as a
second-time salvage therapy
The patient maintained a stable condition for the
following 15 months after treatment with murine CD19 CAR-T therapy
combined with oral imatinib. In May 2018, the dizziness, tinnitus
and deafness progressed, and the patient was diagnosed with
relapsed CNSL again. The patient received high dose MTX combined
with several courses of intrathecal chemotherapy as salvage therapy
for 6 months. In November 2018, 79.28% leukemia cells were found in
the CSF, while the patient's minimal residual disease in the bone
marrow was 3.58% and P210 was 32.04%. The patient was given six
courses of intrathecal chemotherapy, after which the patient was
enrolled in a clinical trial for treatment with autologous CAR-T 19
cells expressing humanized anti-CD19 scFv and 4-1BB-CD3ζ
costimulatory-activation domains (ChiCTR1800019622) as second-time
relapsed CNSL. The patient received lymphodepleting chemotherapy in
the same manner as the first time. After six courses of intrathecal
chemotherapy, the quantity of leukemia cells in the CSF was reduced
to 10.25% prior to CAR-T 19 cell infusion. Autologous CAR-T 19
cells were infused (5×105 cells/kg on day 1 and
5×105 cells/kg on day 2). Since the patient began to
exhibit pyrexia 24 h after the first infusion, the total number of
autologous humanized CAR-T 19 cells was reduced to 1×106
cells/kg compared with 7×106 cells/kg in the murine CD19
CAR-T therapy. The transduction efficiency of CD19 CAR and
amplification of CD19 CAR-T cells were analyzed by FCM (data not
shown).
Isolation of peripheral blood
mononuclear cells (PBMCs) and transduction of T cells with murine
and humanized CD19 CAR
PBMCs were isolated by Ficoll density gradient
centrifugation at 400 ×g at 37°C for 20 min. CD3+ T
cells were selected from the PBMCs by MACS using CD3 microbeads
(Miltenyi Biotec, Inc.). CD3+ T cells were stimulated by
anti-CD3/anti-CD28 mAb-coated Human T-Expander beads (cat. no.
11141D; Thermo Fisher Scientific, Inc.) and cultured in T-cell
medium X–Vivo 15 (Lonza Group, Ltd.) supplemented with 250 IU/ml
IL-2 (Proluekin; Novartis International AG). After the 4th day of
culturing, at which point the CD19+ leukaemia cells
could not be detected in the culture by FCM (BD Biosciences), T
cells (3×106) were transduced with a lentiviral vector
encoding CD19-CAR constructs [10 µg; lenti-CD19-2rd-CAR
(murine/humanized); Shanghai Genbase Biotechnology Co., Ltd.] and
cultured in medium containing recombinant human IL-2 (250 U/ml;
Proleukin; Novartis International AG) at 37°C for 12 days. In the
lentivirus packaging process, ~2.5–3.5 µg lentiviral vector was
packaged in 293T cells (1×107). The multiplicity of
infection (MOI) was 10 in murine CAR-T time and 1 in humanized
CAR-T time. After 12 days of cultivation, the transduction
efficiencies of anti-CD19 CAR (Shanghai Genbase Biotechnology Co.,
Ltd.) were analyzed by FCM. A total of 2×105 cells were
added into each centrifuge tube, 100 µl biotin-labeled CD19 protein
(cat. no. CD9-H8259; ACROBiosystems) was added into each tube, and
then incubated at 4°C for 1 h. The final concentration of
biotin-labeled CD19 protein was 10 µg/ml. After centrifugation at
300 × g at 37°C for 5 min, the supernatant was discarded and the
cells were washed with PBS buffer once. A total of 100 µl diluted
FITC-labeled streptavidin (cat. no. 405201; 1:500; BioLegend, Inc.)
was added to each tube. After mixing, the tubes were incubated at
4°C for 1 h. After centrifugation at 300 × g at 37°C for 5 min, the
supernatant was discarded and the cells were washed three times
with PBS buffer. A total of 100 µl PBS suspension containing cells
were added to each tube. All data were acquired using a BD Fortessa
flow cytometer (BD Biosciences) and analyzed using FlowJo software
(v10.0.7; FlowJo LLC). The CD19 CAR transfection rate was
determined according to the instruction of CARTTEST-19 assay kit
(Shanghai Genbase Biotechnology Co., Ltd.).
Primary cells and Nalm-6 cells (American Type
Culture Collection) were cultured at 37°C in a humidified incubator
with 4% CO2 in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 50 IU/ml penicillin/streptomycin (Gibco;
Thermo Fisher Scientific, Inc.). The human embryonic kidney 293T
(Lenti-X-293T) cells (American Type Culture Collection) were
maintained at 37°C in a humidified incubator with a 4%
CO2 atmosphere in DMEM (Sigma-Aldrich; Merck KGaA)
supplemented with 10% FBS and 50 UI/ml penicillin/streptomycin.
Murine and humanized CD19 CAR-T cell
proliferation
After the CAR-T cells were harvested, the viability
of murine and humanized CD19 CAR-T cells co-cultured with Nalm-6
cells (effect to target proportion was 1:1) in vitro was detected
using a Cell Counting Kit-8 (Dojindo Molecular Technologies, Inc.).
A total of 100 µl cell suspension (5,000 cells/well) in a 96-well
plate were cultured for 0, 24 and 48 h in a humidified incubator at
37°C. Subsequently, 10 µl CCK-8 solution was added to each well of
the plate, and the plate was incubated for 4 h at 37°C. The
absorbance was measured at 450 nm using a microplate reader.
Optical density (OD)450 values were achieved using the Synergy H1
Hybrid Reader (BioTek China) after incubation and analyzed with
Gen5 software.
In vitro cytotoxicity to Nalm-6 cells
and cytokine-release assays
Two groups of CD19 CAR-T cells were co-cultured at
37°C with Nalm-6 cells at a ratio of 4:1 for 48 h in the absence of
supplemented cytokines. Cytotoxicity was detected at 0, 24 and 48 h
using a lactate dehydrogenase cytotoxicity test kit (cat. no.
C0017; Beyotime Institute of Biotechnology) at according to the
manufacturer's instructions at 490 nm. All assays were performed in
duplicate or triplicate.
The release of TNF-α and IFN-γ from cells was
detected using ELISA kits (cat. nos. 555268 and 550612,
respectively; BD Biosciences). The absorbance value at 450 nm was
detected at 0, 12, 24 and 48 h.
Mouse tumor model establishment and
experiment
The mouse tumor model was established based on a
previous study by Demehri et al (20). The method of euthanasia was carbon
dioxide anesthesia. The animals were placed in a 10-liter
euthanasia chamber with a carbon dioxide to oxygen ratio of 6:4.
After the animals gradually lost consciousness, the chambers were
filled with 100% carbon dioxide, and the flow rate was 2 l/min (20%
carbon dioxide/min). Carbon dioxide was increased to 100% and kept
for 10 min to determine the death of animals (21). Six to eight-week old female
CAnN.Cg-Foxn1nu/CrlVR (BALB/c) mice weighing 20.31±1.39 g (n=30;
Charles River Laboratories, Inc.) were used in the present study.
BALB/c mice were raised in SPF conditions at 20–26°C, relative
humidity 40–70% and light and dark alternated 10/14 h cycle. They
were fed with food and water sterilized by high temperature and
high pressure steam. Mice were injected subcutaneously with
5×106 Nalm-6 cells transduced with luciferase (AmyJet
Scientific). Mice were monitored with bioluminescence imaging (BLI)
for disease progression twice a week following intraperitoneal
injection with D-luciferin (150 mg/kg) (AmyJet Scientific) 10 min
before scanning. Before imaging, mice were anesthetized via a nose
cone with 2% isoflurane medical oxygen (Zoetis) and maintained
under continuous inhalational anesthesia. All mice were sacrificed
wwhen the tumor volume exceeded 20 mm3 or when the
experimental study has been completed. The mice were divided into
three groups. The first group was given no treatment, the second
group was given murine CD19 CAR-T, and the third group was given
humanized CD19 CAR-T. The survival time of mice was assessed. The
release of IFN-γ from peripheral blood was detected by ELISA (cat.
no. E4593-100; AmyJet Scientific, Inc.). A total of 500 µl of blood
was collected from the inner canthus of the three groups on days 7,
14, 21 and 28. Survival time of mice was the time when mice died or
were killed for ethical reasons during the experiment.
Statistical analysis
All experiments were performed independently at
least three times and data are presented as the mean ± SD.
Statistical analyses were performed using SPSS 18.0 software (SPSS,
Inc.). Comparisons between two groups were made using the
independent sample t-test. Comparisons among >2 groups were made
using one-way ANOVA followed by Fisher's LSD post-hoc test.
Survival curves were plotted using the Kaplan-Meier method, and the
difference in OS survival rates was assessed using the log-rank
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expansion of anti-CD19-CAR T-cells
during therapy
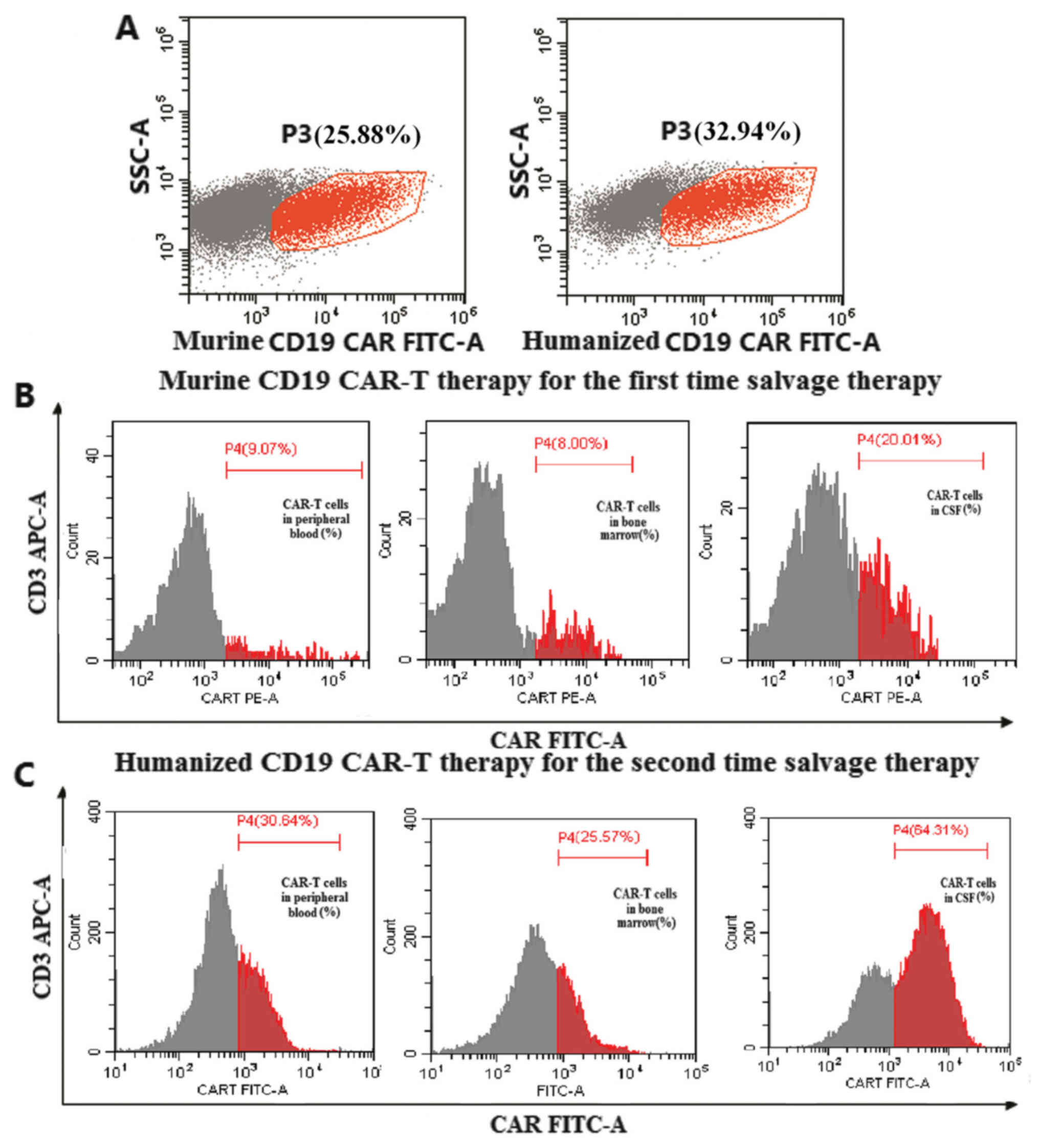
The transduction efficiency of murine CD19 CAR was
25.88% (Fig. 1A). Peak levels of
expansion of CAR-T cells were observed on day 7 in CSF accounting
for 20.01% of cells, 9.07% in the peripheral blood and 8.00% in
bone marrow (Fig. 1B). The time
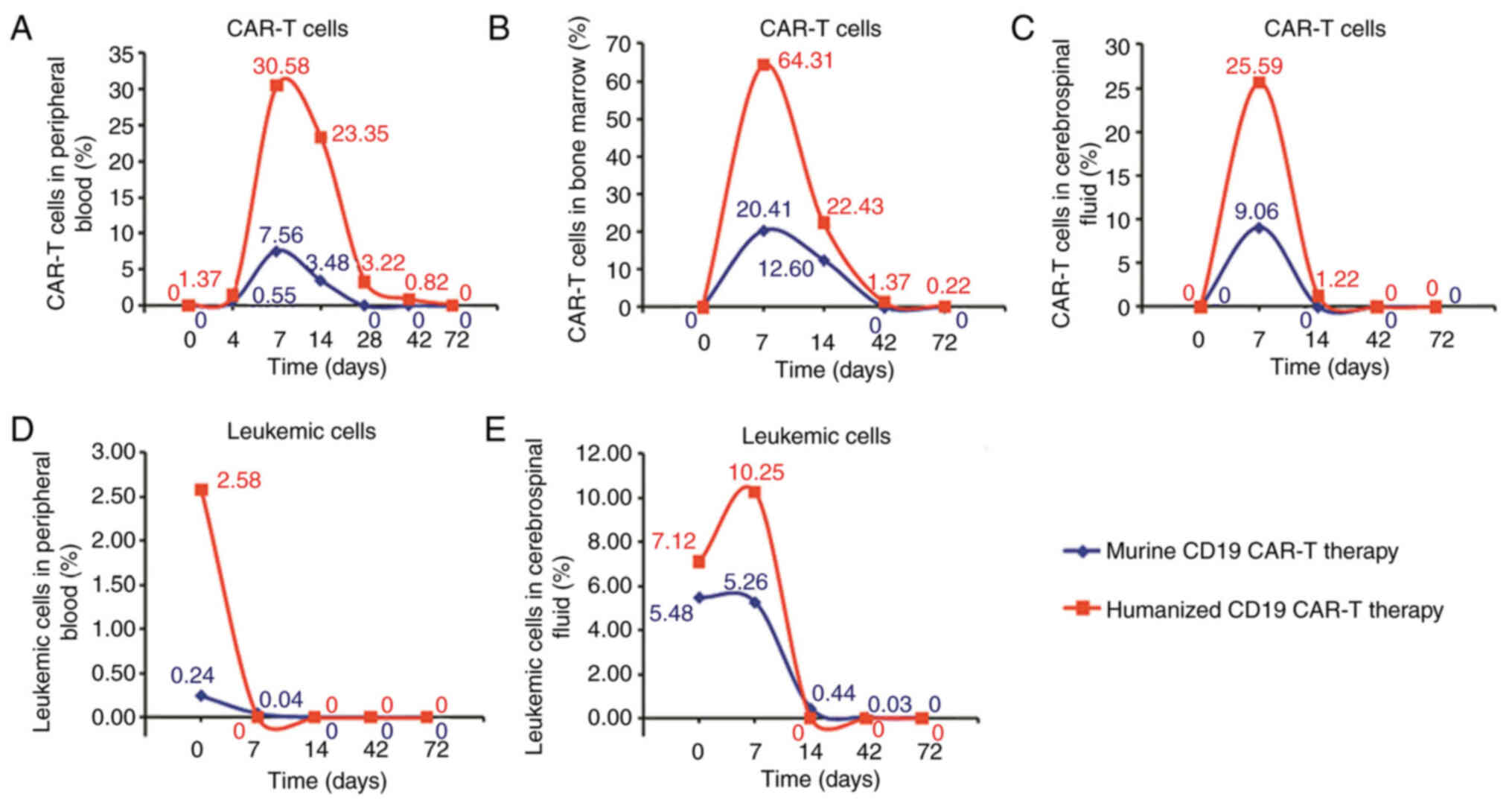
period before all murine CAR-T cells had disappeared was ~14 days
in the CSF, 28 days in the peripheral blood and 42 days in the bone
marrow (Fig. 2A-C).
The transduction efficiency of humanized CD19 CAR
was 32.94% (Fig. 1A). Peak expansion
of humanized CAR-T cells was observed on day 7 in the CSF
accounting for 64.31% of cells, 30.64% in the peripheral blood and
25.57% in the bone marrow (Fig. 1C).
The time period before all humanized CAR-T cells had disappeared
was ~72 days in the peripheral blood and 72 days in the bone
marrow. Humanized CAR-T 19 cells in the CSF were still present
after 42 days of CAR-T 19 cell infusion (Fig. 2A-C).
Adverse events following
treatment
The infusions were well tolerated following murine
CD19 CAR-T therapy. Notable adverse events were Grade 1 CRS and
Grade 1 CRES (6,9). The adverse events manifested as pyrexia
(39.7°C fever) with chills from days 3–5 following murine CAR-T 19
infusion, accompanied by a headache, dizziness, muscle ache,
cognitive impairment, confusion and mild hypotension (Table I). Following murine CD19 CAR-T
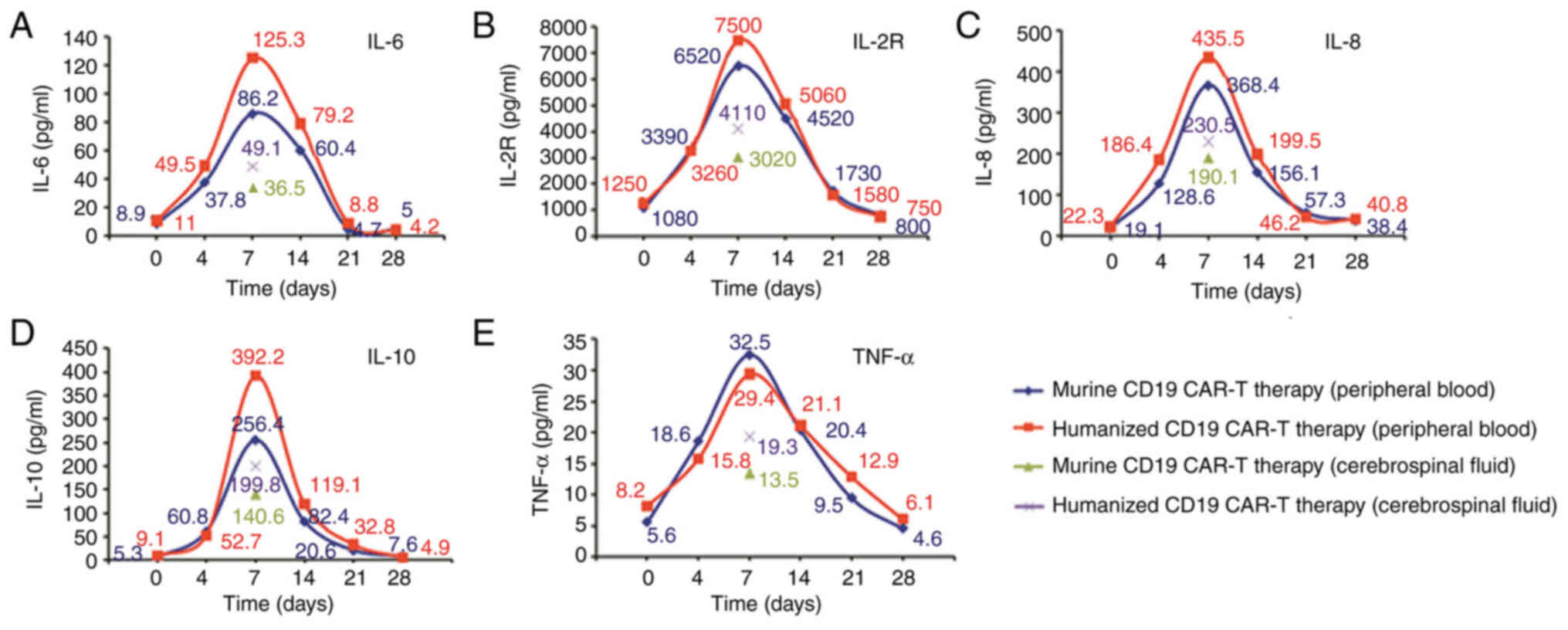
therapy, the levels of cytokines, including IL-2R, IL-6 and IL-10
were assessed. The highest level of IL-6 in serum was 86.2 pg/ml on
day 7 after infusion (Fig. 3A).
IL-2R, IL-8, IL-10 and TNF-α levels peaked on day 7 after infusion
of murine CAR-T 19 cells (Fig 3B-E).
The levels of cytokines in the CSF were detected by lumbar puncture
on day 7 after infusion (Fig. 3).
The level of cytokines increased gradually after CAR-T infusion,
and the level of cytokines in CSF were higher compared with in
peripheral blood from the fourth day after CAR-T infusion (Fig. 3). The patient was given an
acetaminophen and diphenhydramine to overcome the adverse
events.
 | Table I.Adverse events in murine and
humanized CD19 CAR-T cell therapy. |
Table I.
Adverse events in murine and
humanized CD19 CAR-T cell therapy.
| Event | Murine CD19
CAR-T | Humanized CD19
CAR-T |
|---|
| Fever | Grade 1-CRS | Grade 1-CRS |
| Chills | Grade 1-CRS | Grade 1-CRS |
| Neutropenia | No | No |
| Lymphopenia | No | No |
| Anemia | No | No |
|
Thrombocytopenia | No | No |
| Fatigue | Grade 1-CRS | Grade 1-CRS |
| Headache | Grade 1-CRS | Grade 1-CRS |
| Dizziness | Grade 1-CRES | Grade 1-CRES |
| Nausea | No | Grade 2-CRS |
| Vomiting | No | No |
| Somnolence | No | No |
| Agitation | No | Grade 2-CRS |
| Conscious
disturbance | Grade 1-CRES | Grade 2-CRES |
| Diminished
attention | No | No |
| Language
disturbance | No | No |
| Seizures | No | No |
| Ataxia | No | Grade 2-CRS |
| Anorexia | No | No |
| Dyspnea | No | No |
| Delirium | No | Grade 2-CRES |
| Muscle
soreness | Grade 1-CRS | Grade 1-CRS |
| Muscle
weakness | Grade 1-CRS | Grade 1-CRS |
| Hypotension | Grade 1-CRS | Grade 1-CRS |
| Hypoxemia | No | No |
|
Hypoalbuminemia | No | No |
| Transaminase
increased | No | No |
| Blood bilirubin
increased | No | No |
| Serum creatinine
increased | No | No |
The adverse events observed following humanized
CAR-T cell therapy were Grade 1 CRS and Grade 2 CRES. Pyrexia
(39.7°C fever) with chills from days 2–6 after infusion with
humanized CD19 CAR-T cells, and headache, dizziness, muscle ache,
fatigue, nausea, agitation, confusion and delirium were also
experienced by the patient (Table
I). The highest level of IL-6 in the serum was 125.3 pg/ml (on
day 7 after infusion (Fig. 3A). The
levels of IL-6, IL-8, IL-2R and IL-10 assessed peaked on day 7
after infusion of humanized CD19 CAR-T and were higher compared
with murine CD19 CAR-T infusion. However, the change in TNF-α was
not obvious. (Fig. 3B-E). The peak
levels of all cytokines in the CSF on day 7 after infusion were
lower compared with those in the peripheral blood. In the CSF, the
cytokine levels at 7 days in the CSF when treated with humanized
CD19 CAR-T therapy were higher compared with those for murine CD19
CAR-T therapy (Fig. 3).
Therapeutic response to CD19-CAR-T
cell therapy
The adverse events associated with murine CAR-T cell
therapy were relieved after 10 days of infusion and the patient
achieved CR after the murine CD19 CAR-T therapy. The disappearance
time of leukemic cells from peripheral blood was 14 days after the
treatment with murine CD19 CAR-T and 7 days after the treatment
with humanized CD19 CAR-T (Fig. 2D).
The disappearance time of leukemic cells from CSF was 72 days after
the treatment with murine CD19 CAR-T and 14 days after the
treatment with humanized CD19 CAR-T (Fig. 2E).
The patient was followed up in the outpatient
department and underwent bone marrow biopsy every 2–3 months. He
remained in CR condition for the following 18 months after
treatment with humanized CD19 CAR-T therapy combined with oral
imatinib. Subsequently, the patient relapsed again and declined
further treatment.
Effects of murine CD19 CAR-T and
humanized CD19 CAR-T on proliferation and cytotoxicity in
vitro
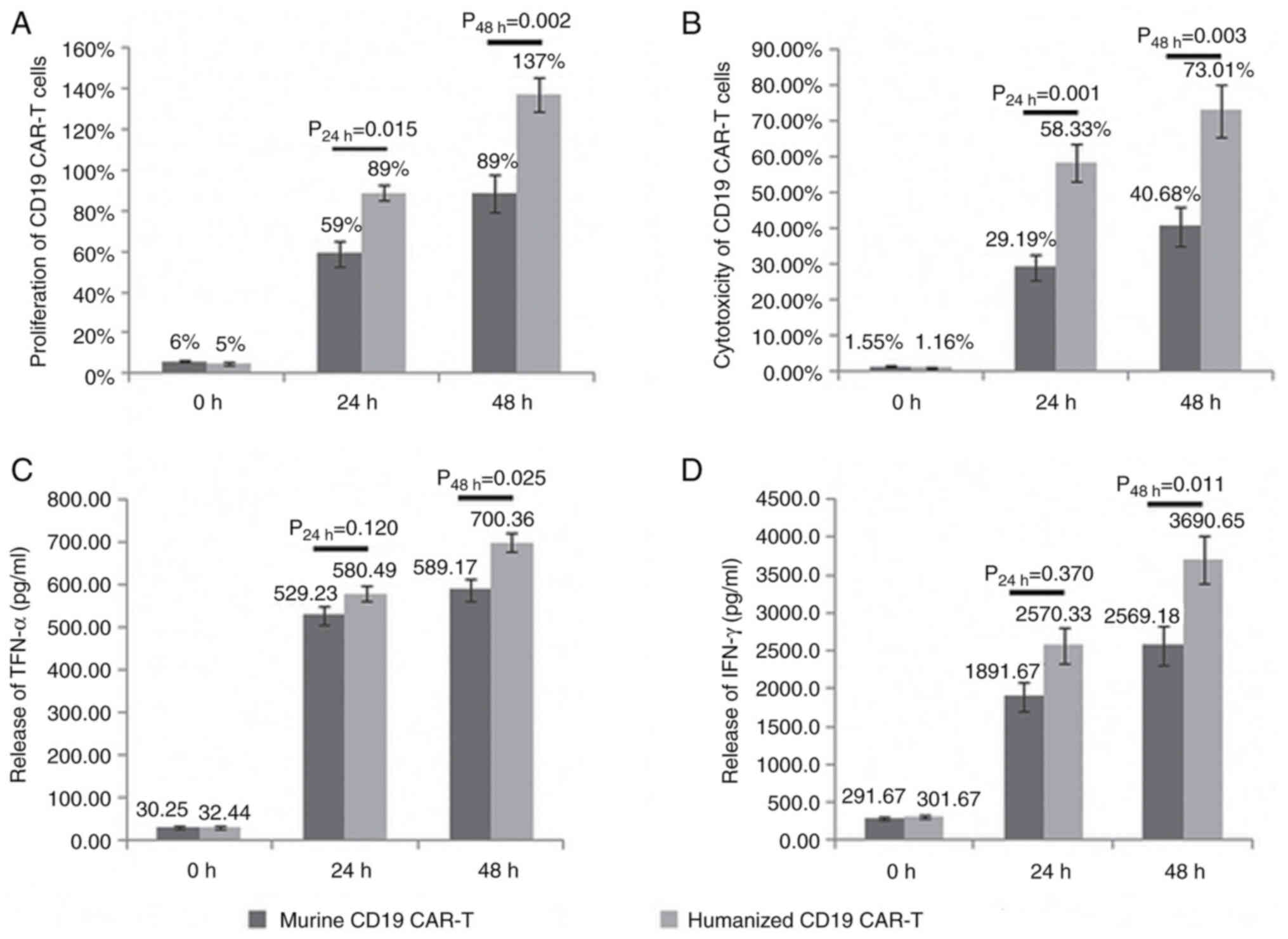
The proliferation of humanized CD19 CAR-T cells was
higher compared with that of murine CD19 CAR-T cells (24 h,
P=0.015; 48 h, P=0.002; Fig. 4A).
The cytotoxicity of humanized CD19 CAR-T cells was higher compared
with the murine CD19 CAR-T cells (24 h, P=0.001; 48 h, P=0.003;
Fig. 4B).
There was no significant difference between the two
groups in terms of the release of TNF-α and IFN-γ after 24 h.
However, the release of TNF-α and IFN-γ following treatment with
humanized CD19 CAR-T cells was higher compared with that following
treatment with murine CD19 CAR-T cells after 48 h (TNF-α, 24 h,
P=0.12 and 48 h, P=0.025; Fig. 4C;
IFN-γ, 24 h, P=0.37 and 48 h, P=0.011; Fig. 4D).
Comparison between the murine and
humanized CD19 CAR-T therapy in mice
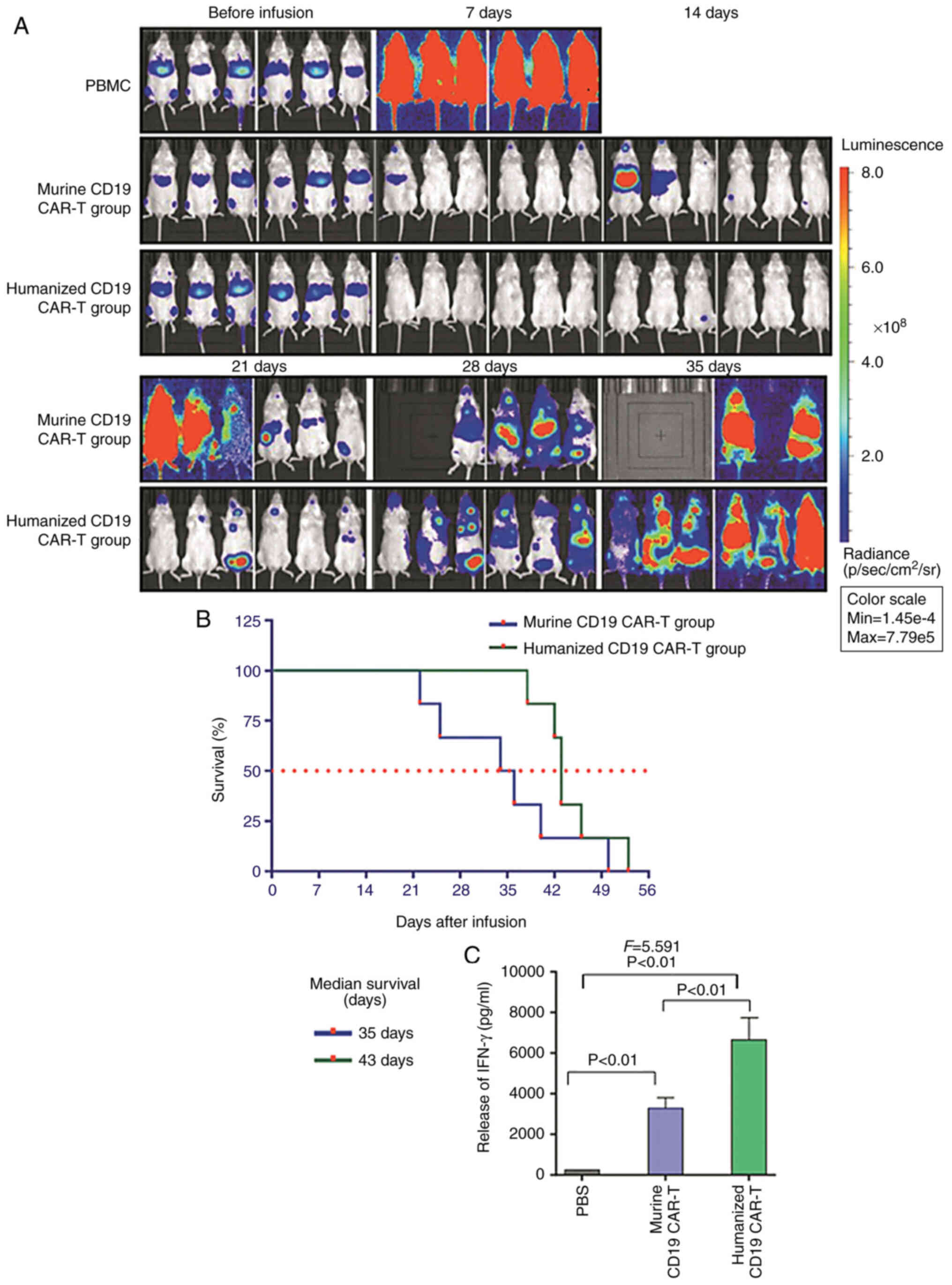
All mice in the PBMCs group died at 7 days after
therapy. Tumor fluorescence was barely present in the murine and
humanized CD19 CAR-T groups at 7 days after infusion of CAR-T
cells, and began to appear 14 days after infusion in the murine
CD19 CAR-T group. In the humanized CD19 CAR-T group, fluorescence
began to appear at 21 days after infusion. Tumor fluorescence in
the murine CD19 CAR-T group rapidly increased in mice treated with
murine CD19 CAR-T cells, and the mice began to die 21 days after
infusion. Although the tumor fluorescence was also enhanced in the
humanized CD19 CAR-T group, the mice survived until 35 days after
infusion (Fig. 5A). The median
survival time of mice in the murine CD19 CAR-T group was 35 days,
and this was 43 days in the humanized CD19 CAR-T group (Fig. 5B). The release of IFN-γ in the
humanized CD19 CAR-T cells group was increased compared with that
in the murine CD19 CAR-T group (P<0.001; Fig. 5C)
Discussion
The present study investigated the safety and
efficacy of humanized CD19 CAR-T therapy as a salvage treatment
following murine CD19 CAR-T therapy. The transduction efficiency,
proliferation, efficacy, survival time and the release of cytokines
were compared between treatment with murine and humanized CD19
CAR-T cells in vitro and in mice.
CRES is a severe and potentially fatal acute
toxicity of CD19 CAR-T therapy for patients with R/R B-cell ALL,
despite the promising results of the therapy (22). It has been reported that CAR-T cells
are detectable in the CSF of patients who received CD19 CAR-T
therapy, indicating severe neurotoxicity (13,23).
Another study has demonstrated that the numbers of CAR-T cells in
the CSF from patients with severe CRS or CRES were significantly
higher compared with those in patients without neurotoxicity
(8). The number of CAR-T cells in
the peripheral blood also tends to be higher in patients who
develop neurotoxicity compared with those who do not (13). In the present study, it was observed
that the peak number of CAR-T cells in the CSF was higher compared
with that in the peripheral blood or in bone marrow. Therefore,
toxicity, and in particular neurotoxicity, may be a severe side
effect of the process of CD19 CAR-T therapy for the treatment of
patients with CNSL of B-cell ALL.
For patients with significant tumor burdens,
particularly patients with ALL, the possibility of serious CRS or
CRES is increased (8,11,24).
Therefore, reducing the tumor burden may be an important means to
prevent the manifestation of the more severe side effects. In the
present study, the case of a patient who received several courses
of high dose MTX combined with intrathecal chemotherapy to reduce
the number of leukemia cells in the CSF before the infusion of
CAR-T 19 cells is reported. The notable adverse events were Grade 1
CRS and Grade 2 CRES. Life threatening cerebral edema is the most
serious neurotoxic symptoms observed in patients treated with CAR-T
cell therapy (13,25–27),
since there is a resultant increase to the permeability of the
blood-brain barrier and increased levels of cytokines caused by the
activation of central nervous system endothelial cells in CD19
CAR-T therapy (28,29). In studies using CAR-T, neurologic
adverse events were reported in 40% of children and young adults
with ALL (13% severe) and 50% of adults with ALL (50% severe)
(16,30). Therefore, reducing the tumor burden
prior to initiation of CD19 CAR-T therapy may be an effective
method for the prevention of life-threatening cerebral edema. For
the patient reported on in the present study, there was no cerebral
edema reported for treatment with CD19 CAR-T therapy due to the
several courses of high dose MTX combined with intrathecal
chemotherapy prior to administration of CD19 CAR-T therapy.
Although the most notable therapeutic effect is
observed following application of CD19 CAR-T therapy for R/R B cell
malignancies, the remission achieved by this therapy is a
reflection of its specificity, potency and the persistence of CAR-T
cells (26,31). Human leukocyte antigen restricts the
T-cell-mediated immune response to epitopes derived from the murine
scFv, which may affect the survival time of CAR-T cells in
vivo (32). It has been reported
that patients treated with murine CD19 CAR-T therapy develop an
immune response specific to the murine scFv, and thus exhibit
subsequent failure to respond to murine CD19 CAR-T therapy
(15). Taking advantage of humanized
scFvs may reduce the immunogenicity of murine CD19 CAR-T cells and
improve the longevity of CAR-T cells in patients (17,33–35). In
the present study, humanized CD19 CAR-T cells exhibited higher
efficiency towards tumor cell lines in vitro and in mice
compared with murine CD19 CAR-T cells. Additionally, the
transduction efficiency, proliferation, cytotoxicity to Nalm-6
cells and cytokine-release of humanized CAR-T cells were higher
compared with those of murine CAR-T cells in vitro and in
mice. Based on the aforementioned experimental results, the number
of CD19 CAR-T cells infused in the second therapy was reduced. The
total number of autologous humanized CAR-T 19 cells infused was
1×106 cells/kg in the second therapy compared with
7×106 cells/kg in the murine CD19 CAR-T therapy. The
second therapy had a complete curative effect and the severity of
side effects was the same as for the first therapy. Another
promising result observed in the present study was that the
survival times of humanized CAR-T 19 cells in the CSF, peripheral
blood and bone marrow were longer compared with those of murine
CAR-T 19 cells. At this point, the patient remained in remission
for >9 months after treatment with humanized CD19 CAR-T therapy.
Longer remission was achieved and the risk of recurrence was
reduced with the humanized CD19 CAR-T therapy. After successfully
treating the patient and following recurrence, the patient was
entered into another clinical trial for CD19 CAR-T therapy
(ChiCTR1800019622). To establish a salvage therapy based on
humanized CD19 CAR-T cell therapy for treatment of R/R B-cell
hematologic malignancy to prolong survival, including patients who
failed to respond or relapsed when treated with murine CD19 CAR-T
therapy. The advantage of humanized CD19 CAR-T over murine CD19
CAR-T is due to a different humanized scFv sequence, the reversed
order of heavy chain and light chain, and/or incorporation of the
selective short peptide, which might have contributed to a change
in the three-dimensional configuration of the antigen-recognizing
domain of CAR (18).
In conclusion, the present study investigated the
efficacy of humanized CD19 CAR-T as a salvage therapy for recurrent
CNS B-ALL following murine CD19 CAR-T therapy. The prevention of
life-threatening neurotoxicity, such as cerebral edema, following
treatment with humanized CD19 CAR-T therapy may be achieved by
reducing the initial tumor burden prior to initiation of
treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QD conceived and designed the study. XL, ZXY and NM
performed the experiments. MJL and JM contributed to acquisition of
data. HBZ and JW contributed to analysis and interpretation of
data. XL wrote, reviewed and revised the manuscript. XL and QD
confirmed the authenticity of all the raw data. All authors read
and approved the manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tianjin First Central Hospital (Tianjin, China), and
written informed consent was obtained from the enrolled patient.
Additionally, the patient data were treated in accordance with the
local privacy regulations. All animal procedures were approved by
the institutional animal and care use committee of Tianjin First
Central Hospital (Tianjin, China).
Patient consent for publication
The patient provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rozovski U, Ohanian M, Ravandi F,
Garcia-Manero G, Faderl S, Pierce S, Cortes J and Estrov Z:
Incidence of and risk factors for involvement of the central
nervous system in acute myeloid leukemia. Leuk Lymphoma.
56:1392–1397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aoki J, Ishiyama K, Taniguchi S, Fukuda T,
Ohashi K, Ogawa H, Kanamori H, Eto T, Iwato K, Sakamaki H, et al:
Outcome of allogeneic hematopoietic stem cell transplantation for
acute myeloid leukemia patients with central nervous system
involvement. Biol Blood Marrow Transplant. 20:2029–2033. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krishnan S, Wade R, Moorman AV, Mitchell
C, Kinsey SE, Eden TO, Parker C, Vora A, Richards S and Saha V:
Temporal changes in the incidence and pattern of central nervous
system relapses in children with acute lymphoblastic leukaemia
treated on four consecutive Medical Research Council trials,
1985–2001. Leukemia. 24:450–459. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin MW, Xu SM and An Q: Central nervous
disease in pediatric patients during acute lymphoblastic leukemia
(ALL): A review. Eur Rev Med Pharmacol Sci. 22:6015–6019.
2018.PubMed/NCBI
|
|
5
|
Irving BA and Weiss A: The cytoplasmic
domain of the T cell receptor zeta chain is sufficient to couple to
receptor-associated signal transduction pathways. Cell. 64:891–901.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davila ML, Riviere I, Wang X, Bartido S,
Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska
M, et al: Efficacy and toxicity management of 19–28z CAR T cell
therapy in B cell acute lymphoblastic leukemia. Sci Transl Med.
6:224ra252014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee DW, Kochenderfer JN, Stetler-Stevenson
M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M,
Shah NN, et al: T cells expressing CD19 chimeric antigen receptors
for acute lymphoblastic leukaemia in children and young adults: A
phase 1 dose-escalation trial. Lancet. 385:517–528. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Neelapu SS, Tummala S, Kebriaei P, Wierda
W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, et
al: Chimeric antigen receptor T-cell therapy - assessment and
management of toxicities. Nat Rev Clin Oncol. 15:47–62. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee DW, Gardner R, Porter DL, Louis CU,
Ahmed N, Jensen M, Grupp SA and Mackall CL: Current concepts in the
diagnosis and management of cytokine release syndrome. Blood.
124:188–195. 2014.Erratum in: Blood 128: 1533, 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brudno JN and Kochenderfer JN: Toxicities
of chimeric antigen receptor T cells: Recognition and management.
Blood. 127:3321–3330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maude SL, Barrett D, Teachey DT and Grupp
SA: Managing cytokine release syndrome associated with novel T
cell-engaging therapies. Cancer J. 20:119–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Y, Sun J, Wu Z, Yu J, Cui Q, Pu C,
Liang B, Luo Y, Shi J, Jin A, et al: Predominant cerebral cytokine
release syndrome in CD19-directed chimeric antigen
receptor-modified T cell therapy. J Hematol Oncol. 9:702016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lamers CH, Willemsen R, van Elzakker P,
van Steenbergen-Langeveld S, Broertjes M, Oosterwijk-Wakka J,
Oosterwijk E, Sleijfer S, Debets R and Gratama JW: Immune responses
to transgene and retroviral vector in patients treated with ex
vivo-engineered T cells. Blood. 117:72–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grupp SA, Kalos M, Barrett D, Aplenc R,
Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et
al: Chimeric antigen receptor-modified T cells for acute lymphoid
leukemia. N Engl J Med. 368:1509–1518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mulazzani M, Fräßle SP, von Mücke-Heim I,
Langer S, Zhou X, Ishikawa-Ankerhold H, Leube J, Zhang W, Dötsch S,
Svec M, et al: Long-term in vivo microscopy of CAR T cell dynamics
during eradication of CNS lymphoma in mice. Proc Natl Acad Sci USA.
116:24275–24284. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Turtle CJ, Hanafi LA, Berger C, Gooley TA,
Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto
TM, et al: CD19 CAR-T cells of defined
CD4+:CD8+ composition in adult B cell ALL
patients. J Clin Invest. 126:2123–2138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sommermeyer D, Hill T, Shamah SM, Salter
AI, Chen Y, Mohler KM and Riddell SR: Fully human CD19-specific
chimeric antigen receptors for T-cell therapy. Leukemia.
31:2191–2199. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song DG, Ye Q, Poussin M, Liu L, Figini M
and Powell DJ Jr: A fully human chimeric antigen receptor with
potent activity against cancer cells but reduced risk for off-tumor
toxicity. Oncotarget. 6:21533–21546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao Y, Liu Z, Wang X, Wu H, Zhang J, Yang
J, Zhang F, Liu L, Long J, Lu P, et al: Treatment with humanized
selective CD19CAR-T cells shows efficacy in highly treated B-ALL
patients who have relapsed after receiving murine-based CD19CAR-T
therapies. Clin Cancer Res. 25:5595–5607. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Demehri S, Corbin A, Loriaux M, Druker BJ
and Deininger MW: Establishment of a murine model of aggressive
systemic mastocytosis/mast cell leukemia. Exp Hematol. 34:284–288.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsukahara T, Ohmine K, Yamamoto C,
Uchibori R, Ido H, Teruya T, Urabe M, Mizukami H, Kume A, Nakamura
M, et al: CD19 target-engineered T-cells accumulate at tumor
lesions in human B-cell lymphoma xenograft mouse models. Biochem
Biophys Res Commun. 438:84–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu D and Zhao J: Cytokine release
syndrome: Grading, modeling, and new therapy. J Hematol Oncol.
11:1212018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maude SL, Frey N, Shaw PA, Aplenc R,
Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et
al: Chimeric antigen receptor T cells for sustained remissions in
leukemia. N Engl J Med. 371:1507–1517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kochenderfer JN, Dudley ME, Carpenter RO,
Kassim SH, Rose JJ, Telford WG, Hakim FT, Halverson DC, Fowler DH,
Hardy NM, et al: Donor-derived CD19-targeted T cells cause
regression of malignancy persisting after allogeneic hematopoietic
stem cell transplantation. Blood. 122:4129–4139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Turtle CJ, Hay KA, Hanafi LA, Li D,
Cherian S, Chen X, Wood B, Lozanski A, Byrd JC, Heimfeld S, et al:
Durable molecular remissions in chronic lymphocytic leukemia
treated with CD19-specific chimeric antigen receptor modified T
cells after failure of ibrutinib. J Clin Oncol. 35:3010–3020. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Johnson LA and June CH: Driving
gene-engineered T cell immunotherapy of cancer. Cell Res. 27:38–58.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lowe KL, Mackall CL, Norry E, Amado R,
Jakobsen BK and Binder G: Fludarabine and neurotoxicity in
engineered T-cell therapy. Gene Ther. 25:176–191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jain MD, Bachmeier CA, Phuoc VH and Chavez
JC: Axicabtagene ciloleucel (KTE-C19), an anti CD19 CAR T therapy
for the treatment of relapsed/refractory aggressive B cell non
Hodgkin's lymphoma. Ther Clin Risk Manag. 14:1007–1017. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gust J, Hay KA, Hanafi LA, Li D, Myerson
D, Gonzalez-Cuyar LF, Yeung C, Liles WC, Wurfel M, Lopez JA, et al:
Endothelial activation and bloodbrain barrier disruption in
neurotoxicity after adoptive immunotherapy with CD19 CAR T cells.
Cancer Discov. 7:1404–1419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gardner RA, Finney O, Annesley C, Brakke
H, Summers C, Leger K, Bleakley M, Brown C, Mgebroff S,
Kelly-Spratt KS, et al: Intent-to-treat leukemia remission by CD19
CAR T cells of defined formulation and dose in children and young
adults. Blood. 129:3322–3331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brentjens RJ, Rivière I, Park JH, Davila
ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda
O, et al: Safety and persistence of adoptively transferred
autologous CD19-targeted T cells in patients with relapsed or
chemotherapy refractory B-cell leukemias. Blood. 118:4817–4828.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mirzaei HR, Pourghadamyari H, Rahmati M,
Mohammadi A, Nahand JS, Rezaei A, Mirzaei H and Hadjati J:
Gene-knocked out chimeric antigen receptor (CAR) T cells: Tuning up
for the next generation cancer immunotherapy. Cancer Lett.
423:95–104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Johnson LA, Scholler J, Ohkuri T, Kosaka
A, Patel PR, McGettigan SE, Nace AK, Dentchev T, Thekkat P, Loew A,
et al: Rational development and characterization of humanized
anti-EGFR variant III chimeric antigen receptor T cells for
glioblastoma. Sci Transl Med. 7:275ra222015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alonso-Camino V, Sánchez-Martín D, Compte
M, Nuñez-Prado N, Diaz RM, Vile R and Alvarez-Vallina L: CARbodies:
Human antibodies against cell surface tumor antigens selected from
repertoires displayed on T cell chimeric antigen receptors. Mol
Ther Nucleic Acids. 2:e932013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao Y, Wang QJ, Yang S, Kochenderfer JN,
Zheng Z, Zhong X, Sadelain M, Eshhar Z, Rosenberg SA and Morgan RA:
A herceptin-based chimeric antigen receptor with modified signaling
domains leads to enhanced survival of transduced T lymphocytes and
antitumor activity. J Immunol. 183:5563–5574. 2009. View Article : Google Scholar : PubMed/NCBI
|