Introduction
Lung cancer is one of the most common malignancies,
that threatens the health and life of those affected. Lung cancer
is the leading cause of cancer incidence and mortality in both men
and women; it is estimated that there were 2.1 million new
diagnosed cases and 1.8 million mortalities in 2018 worldwide
(1,2). Lung cancer is classified into two major
pathohistological subtypes, small cell lung cancer (SCLC) and
non-small cell lung cancer (NSCLC) (3). NSCLC accounts >85% of all cases of
lung cancer (4). Despite major
advancements in diagnosis and treatment, the incidence and
mortality rates of lung cancer has remained high in recent years
(5). Thus, it remains critical to
identify and develop potential therapeutic targets in the hope of
decreasing the risk of lung cancer.
A previous study demonstrated that consumption of
legumes decreased the risk of lung cancer by 23% (6). Isoflavones, coumarins and lignin were
reported to be the three main phytochemicals in soybeans (7). Currently, there is a focus on the
potential health benefits of isoflavones, including genistein and
daidzein. The effect of soybeans and their ingredients on tumors
has become a hot topic (8), which
could provide evidence on the preventive and protective effects of
soybeans and their products for lung cancer.
Several long non-coding (lnc) RNAs and their
biological functions have been identified with the use of high
throughput technology (9). For
example, BCYRN1 was found to function as a competing endogenous RNA
to inhibit glioma progression by sponging microRNA-619-5p to
regulate CUEDC2 expression and the PTEN/AKT/p21 pathway (10). Zhu et al (11) identified key lncRNAs in rectal
adenocarcinoma using RNA sequencing and dysregulation of lncRNAs
have been associated with lung cancer initiation and progression.
lncRNAs play a vital role in regulating biological processes
through epigenetic, transcriptional and post-transcriptional levels
(12–15). Abnormal expression of lncRNAs have
been associated with apoptosis, invasion and metastasis of lung
cancer, which is conducive to the progression of lung cancer
(16–18), and it has been demonstrated that
lncRNAs act as effective markers for the diagnosis and prognosis of
patients with lung cancer (19).
Thus, the present study aimed to investigate the potential
functions of daidzein on H1299 lung cancer cells by analyzing
lncRNA and mRNA expression profiles, using microarray analysis. The
results provide novel insight into the molecular mechanism of
daidzein in lung cancer cell lines.
Materials and methods
Cell lines and cell culture
The human H1299 lung cancer cells were purchased
from Wuhan University (Hubei, China). The cells were maintained in
high-glucose medium supplemented with 10% fetal bovine serum
(Haoyang Biological Products Technology Co., Ltd.) and 1%
penicillin/streptomycin (Beijing Solarbio Science & Technology
Co., Ltd.), at 37°C in a humidified incubator with 5%
CO2.
Extraction of RNA from H1299 cells and
RNA quality control
Total RNA was extracted from the H1299 cells using
TRIzol® (Gibco; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. A total of 4 ml medium,
supplemented with dimethyl sulfoxide (Beijing Solarbio Science
& Technology Co., Ltd.) was added to the cells in the control
group for 24 h at 37°C, while 4 ml 10 µM daidzein (Shanxi Huike
Plant Development Co., Ltd.) was added to the cells in the
treatment group for 24 h at 37°C. The RNA was extracted from the
cells in both groups after 24 h. The purity and concentration of
the RNA was determined from optical density 260/280 ratio using a
spectrophotometer (NanoDrop ND-1000; Thermo Fisher Scientific,
Inc.). RNA integrity was determined by 1% formaldehyde denaturing
gel electrophoresis.
RNA labeling and microarray
hybridization
cDNA labeled with fluorescent dyes (Cy5 and
Cy3-dCTP) was produced using they Eberwine's linear RNA
amplification method and subsequent enzymatic reaction, as
previously described (20), which
has also been improved using CapitalBio cRNA Amplification and
Labeling kit (CapitalBio Technology, Inc.) to produce higher yields
of labeled cDNA.
In detail, double-stranded cDNA (containing the T7
RNA polymerase promoter sequence) was synthesized from 1 µg total
RNA using the CbcScript reverse transcriptase according to the
manufacturer's instructions (CapitalBio Technology, Inc.), with T7
Oligo (dT) and T7 Oligo (dN). Following double-stranded cDNA
synthesis using DNA polymerase and RNase H, the products were
purified using a PCR NucleoSpin Extract II kit (Macherey-Nagel,
GmbH and Co., KG) and eluted with 30 µl elution buffer. The eluted
double-stranded cDNA products were then vacuum evaporated to 16 µl
and reverse transcribed at 37°C for 14 h using a T7 Enzyme Mix. The
amplified cRNA was purified using the RNA Clean-up kit (MN). The
Klenow enzyme labeling method was used following RT using CbcScript
II reverse transcriptase. Briefly, 2 µg amplified RNA was mixed
with 4 µg random nanomer, denatured at 65°C for 5 min, then cooled
on ice. Subsequently, 5 µl 4X first-strand buffer, 2 µl 0.1M DTT
and 1.5 µl CbcScript II reverse transcriptase was added, then the
samples were incubated at 25°C for 10 min, and 37°C for 90 min. The
cDNA products were purified using a PCR NucleoSpin Extract II kit
(Macherey-Nagel, GmbH and Co., KG), then vacuum evaporated to 14
µl. The cDNA was mixed with 4 µg random nanomer, heated to 95°C for
3 min, then snap cooled on ice for 5 min. Next, 5 µl Klenow buffer,
dNTP and Cy5-dCTP or Cy3-dCTP (GE Healthcare) was added to a final
concentration of 240 µM each for the dNTPs and 40 µM Cy-dCTP. A
total of 1.2 µl Klenow enzyme was then added and the samples were
incubated at 37°C for 90 min. The labeled cDNA was purified with a
PCR NucleoSpin Extract II kit (Macherey-Nagel, GmbH and Co., KG)
and resuspended in elution buffer. The labeled controls and test
samples labeled with Cy5-dCTP and Cy3-dCTP were dissolved in 80 µl
hybridization solution containing 3XSSC, 0.2% SDS, 5X Denhardt's
solution and 25% formamide. The DNA in the hybridization solution
was denatured at 95°C for 3 min prior to loading onto a microarray.
The arrays were hybridized in an Agilent Hybridization Oven
(Agilent Technologies, Inc.) overnight at a rotation speed of 20
rpm at 42°C then washed with two consecutive solutions (0.2% SDS
and 2X SSC at 42°C for 5 min, then 0.2X SSC for 5 min at room
temperature).
Microarray imaging and data
analysis
The lncRNA + mRNA array data was analyzed for data
summarization, normalization and quality control using the
GeneSpring software v13.0 (Agilent Technologies, Inc.). To select
the differentially expressed lncRNAs and mRNAs fold-change (FC) ≥2
and ≤-2 and P<0.05 (unpaired t-test). The data was
log2 transformed and the median value was determined
using the Adjust Data function in the CLUSTER v3.0 software
(http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm),
then further analyzed using hierarchical clustering with average
linkage (21). Finally, tree
visualization was performed using Java Treeview (Stanford
University School of Medicine). Kyoto Encyclopedia of Genes and
Genomes (KEGG) (https://www.genome.jp/kegg/) and Gene Ontology (GO)
(http://www.geneongoloty.org/) analyses
were performed to determine the function of differentially
expressed mRNAs in biological pathways and GO terms (with a cut-off
of P<0.05). After gene annotation function, STRING (Search Tool
for the Retrieval of Interacting Genes/Proteins; http://string-db.org/) was used to identify the
relationship between protein expression levels, in which a
protein-protein interaction network was created.
Co-expression analysis and
transcription factor prediction
Co-expression analysis is used to perform
correlation analysis on the signal value trend of differential
expressed lncRNA and mRNA in all samples (experimental group and
control group) after comparison. Correlation, >0.99 or <-0.99
was used as the screening criteria, with P<0.05. The lncRNA/mRNA
relationship pair, that met the aforementioned conditions was
considered to have a co expression relationship. Transcription
factor prediction (TFs_predict), which uses the Match-1.0 Public
(http://gene-regulation.com/pub/programs.html%23match)
transcription factor prediction tool, was used to predict the
binding sites of TFs, 2,000 bp upstream and 500 bp downstream of
the start site of each lncRNA based on the co-expression results,
and network diagrams are created using Cytoscape (http://www.cytoscape.org/download.php)
software.
RT-quantitative PCR (RT-qPCR)
Total RNA from the cell cultures was extracted using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.),
reverse transcribed into cDNA using the EasyScript®
One-Step gDNA Removal and cDNA Synthesis SuperMix kit (TransGen
Biotech Co., Ltd.) for 15 min at 42°C, 5 sec at 85°C, then stored
at 4°C until further use. qPCR was performed on a CFX Connect
Real-Time system (Bio-Rad Laboratories, Inc.) with
TransStart® Top Green qPCR SuperMix (TransGen Biotech
Co., Ltd.), according to the manufacturer's protocol. GAPDH served
as an internal control and was included in the same PCR reaction
with differentially expressed lncRNAs and mRNAs for RT-qPCR. The
primer sequences used for the qPCR of the lncRNAs and mRNAs are
listed in Table I. Relative
expression levels were quantified using the 2−ΔΔCq
method (22).
 | Table I.Primer sequences used for lncRNAs and
mRNAs in reverse transcription-quantitative PCR. |
Table I.
Primer sequences used for lncRNAs and
mRNAs in reverse transcription-quantitative PCR.
| Name | Forward primer
sequence (5′-3′) | Reverse primer
sequence (5′-3′) |
|---|
|
ENST00000608897.1 |
GTGCAAATACAGGCCAAGTCAG |
TCCCCAAAAAGATGCCAAGG |
|
ENST00000444196.1 |
TCGTGTGATCTGCCAGTTTC |
TGGAAGGCAGGATTTTGACC |
|
ENST00000608741.1 |
TCTTCCTGGTGCACTCAGATG |
TTGCAGTCCTAACGCGACTC |
| XR_242163.1 |
CATACGAGATGGAGATATCATCC |
CAAATTCTTCTTCTCTAAGATG |
|
ENST00000505196.1 |
AGCCCGGAAATAAGAATGGC |
TTCTGGCCTGTGATGAACTCC |
|
ENST00000498032.1 |
TCAAGAGACCGAGACCATCC |
CTCACTACAAGCTCCGCCT |
| ROPN1L |
TGTGCCTGCCGAAGGAAAAAT |
GTTCAAGGACCCACCAAGCAT |
| FANCC |
CTGCCATATTCCGGGTTGTTG |
AGCACTGCGTAAACACCTGAA |
| FAM149B1 |
ATCTACTGAAGGAAGCTCGGAC |
CACACTCAACTTCTGCTCATACA |
| TRHR |
CCAAACACAGCTTCAGCCAC |
GGCTCACCAGGTAGCAGTTT |
| IGF1 |
GCTCTTCAGTTCGTGTGTGGA |
GCCTCCTTAGATCACAGCTCC |
| TTN |
CCCCATCGCCCATAAGACAC |
CCACGTAGCCCTCTTGCTTC |
| GAPDH |
GGAGTCCACTGGCGTCTTCA |
GTCATGAGTCCTTCCACGATACC |
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (IBM Corp.). The data are presented as the mean ± SD.
Differences between groups were assessed using a two tailed
unpaired t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
In vitro experiments
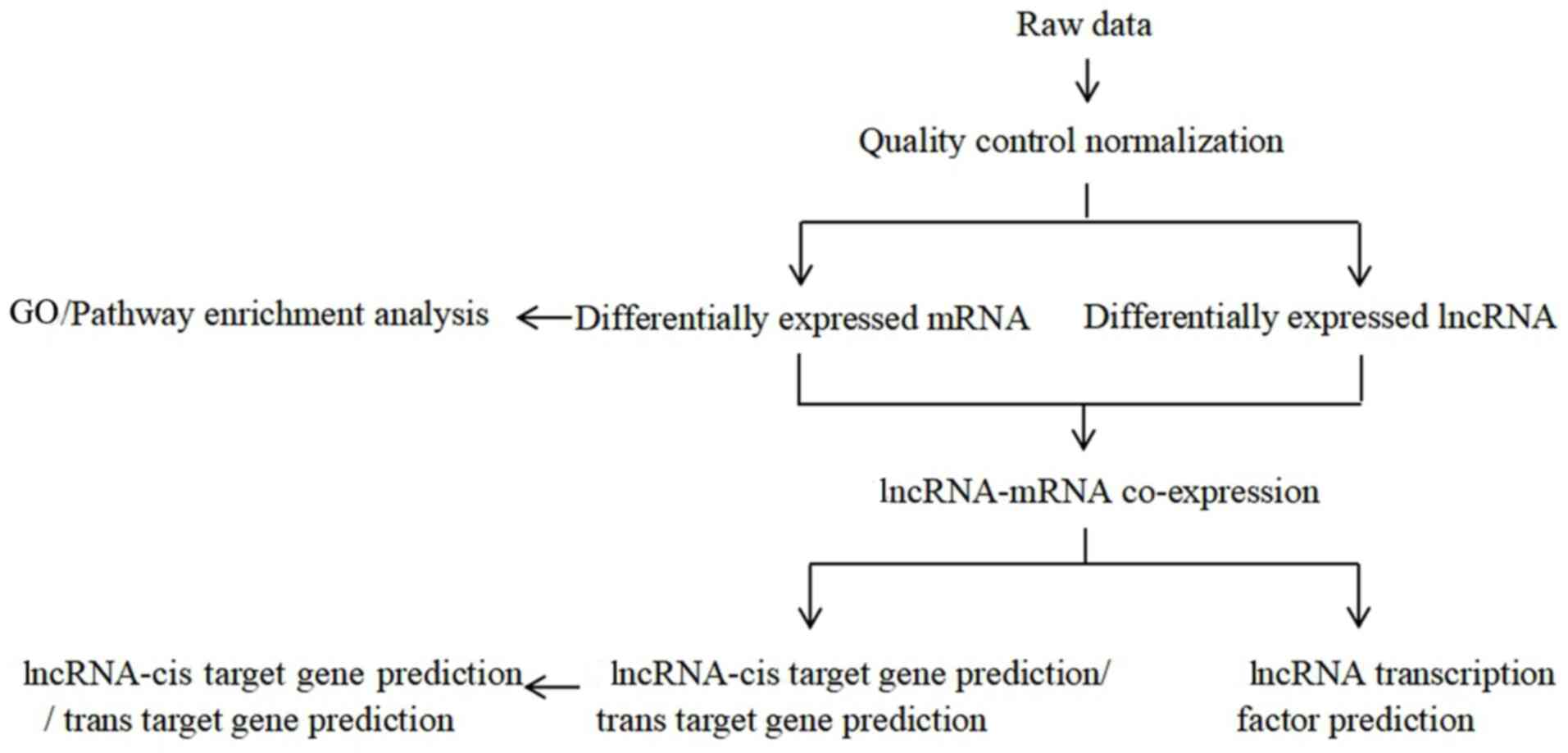
The preliminary in vitro experiments showed
that daidzein had the strongest inhibitory effect on the
proliferation of H1299 cells (data not shown); therefore, these
cells were used for further experiments. The soybean isoflavones
were extracted, which are the active ingredients in legumes, and
daidzein and its derivatives were synthesized and purified. The
experimental flow chart of the present study is presented in
Fig. 1.
Overview of lncRNAs and mRNAs
profiles
The present study aimed to investigate the effect of
daidzein on H1299 cells. Gene cluster analysis of the mRNAs and
lncRNAs was performed following treatment with daidzein. The
clustering map demonstrated similarities between the samples.
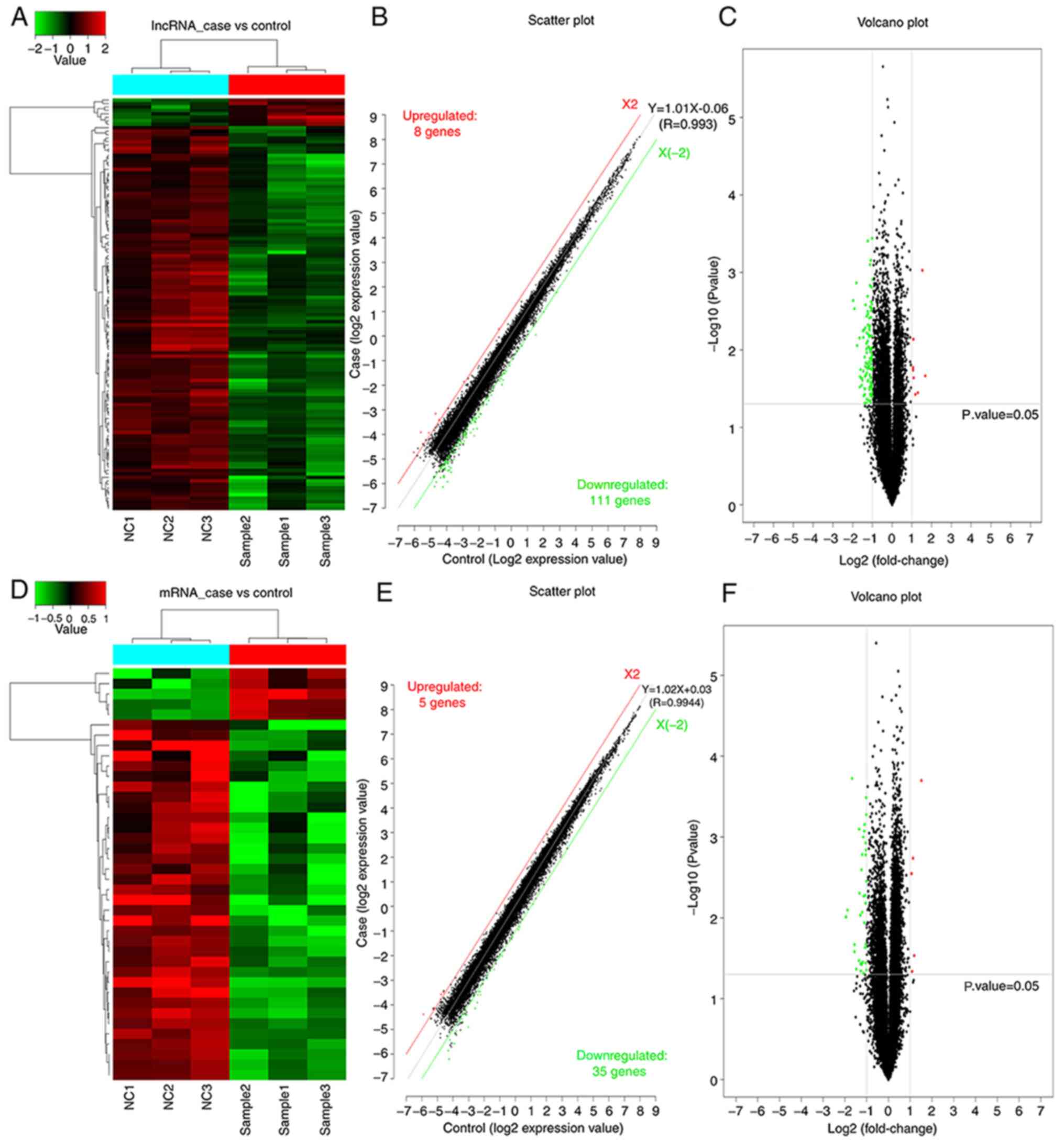
As presented in Fig.
2, a significant difference in both mRNAs and lncRNAs was
observed between the experimental group of cells treated with
daidzein and the control group of untreated with daidzein.
Following the addition of daidzein, the expression level of eight
lncRNAs was upregulated in the H1299 cells, while 111 lncRNAs were
downregulated. In addition, five mRNAs were upregulated and 35
mRNAs were downregulated. In Fig. 2A and
D, the top sample tree represents the similarity clustering
relationship between the different samples. The top color block
represents the expected grouping of the samples manually set prior
to the cluster analysis, and the samples of the same color indicate
that the experiment is expected to be a group. The red line in
Fig. 2B and E, X2, is the threshold
boundary line of the upregulated lncRNA/mRNAs, while the green line
X (−2) is the threshold boundary line of the downregulated
lncRNA/mRNAs, and the middle gray line is the fitted line of the
overall expression amount. The equation in is the fitted line
equation, and R represents the correlation coefficient between the
two sets of samples.
KEGG pathway enrichment and GO
analysis
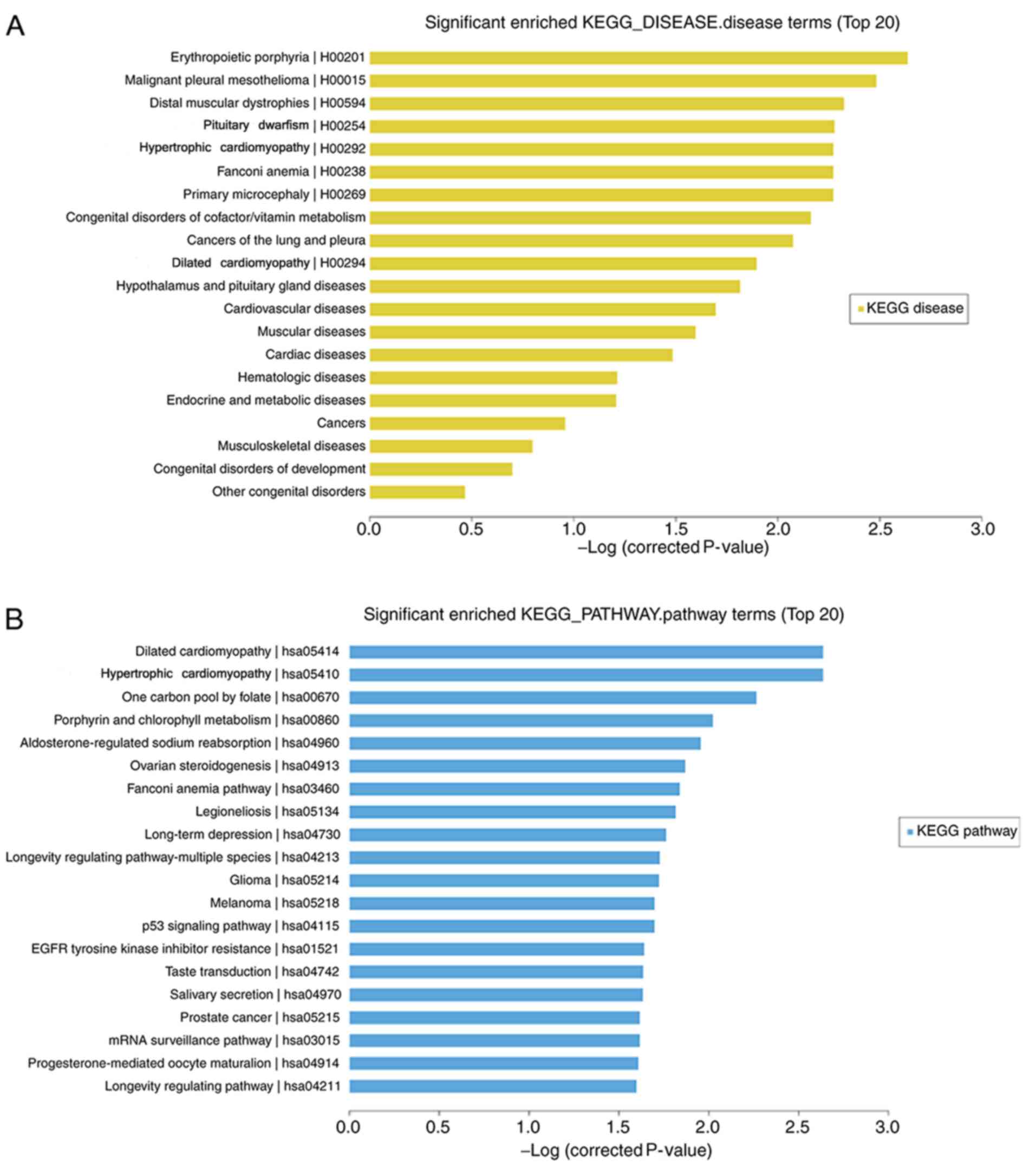
Microarray analysis revealed that 40 mRNAs (5
upregulated and 35 downregulated) were significantly differentially
expressed in the H1299 cell line treated with daidzein compared
with that in the cells treated without daidzein (FC≥2.0;
P<0.05). The top 20 significantly enriched KEGG pathway and
disease terms were selected. The top three terms that were enriched
for gene-affected diseases following daidzein treatment were:
‘Erythropoietic protoporphyrin’, ‘malignant pleural mesothelioma’
and ‘distal myopathy’. The addition of daidzein also affected the
genes involved in diseases, including cancer of the lung and
pleura, the results of which were consistent with the research
model (Fig. 3A). KEGG pathway
analysis revealed that ‘dilated cardiomyopathy’ (hsa05414),
‘hypertrophic cardiomyopathy’ (hsa05410), ‘long-term depression’
(hsa04730), ‘p53 signaling pathway’ (hsa04115) were included in the
top 20 pathways (Fig. 3B).
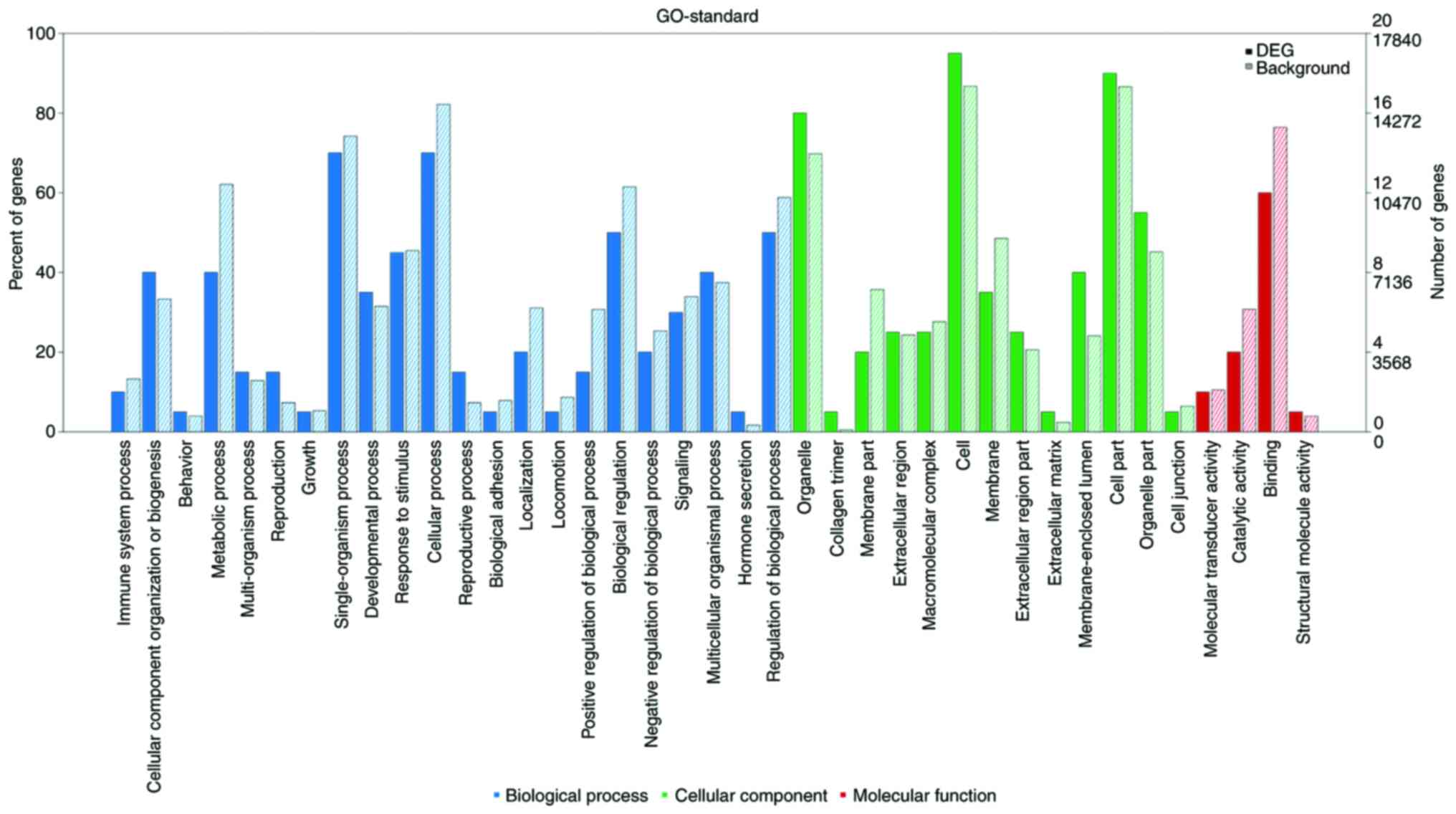
As shown in Fig. 4,
numerous differentially expressed mRNAs, compared with that to the
background mRNAs, were enriched in biological processes, molecular
function and cellular component categories, and the top 20
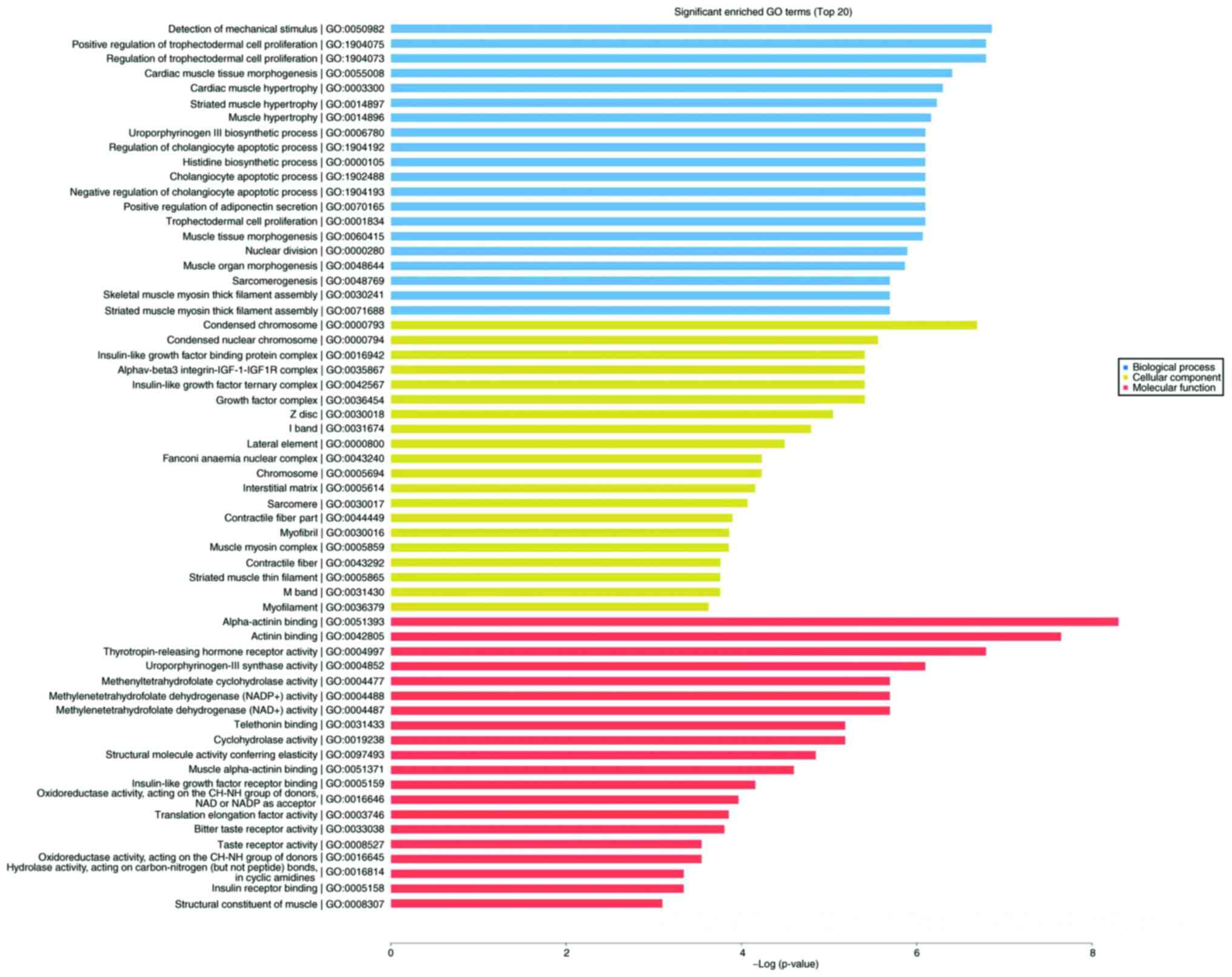
significantly enriched terms were selected from GO analysis. The
results (Fig. 5) indicated that the
most significantly enriched GO terms associated with the
differentially expressed mRNAs were ‘detection of mechanical
stimulus’ (biological processes; GO:0050982; P=0.0010488989),
‘condensed chromosome’ (cellular component; GO:0000793;
P=0.0012408009), ‘α-actinin binding’ (molecular function;
GO:0051393; P=0.0002475636). Several of the differentially
expressed genes were localized in the cytoplasm and altered the
expression of molecular functional genes associated with
‘binding’.
Co-expression analysis
Co-expression analysis was performed to determine
the association between lncRNA and mRNA pairs, with similar
expression profiles, at the genomic level. The association between
differentially expressed mRNAs and lncRNAs following the addition
of daidzein is presented in Fig.
S1A. Following the addition of daidzein, the co-expressed mRNAs
with differentially expressed lncRNAs included: XLOC_I2_013457,
LOC284825, CIQTNF3, FILP1, FANCC, SPATA4, FLJ41455, ANKRD12, SYCP2,
RTKN2 and MTHFD2L.
Transcription factor prediction
The transcription factor was predicted (TFs_predict)
using the Match-1.0 Public transcription factor prediction tool
(Fig. S1B). Based on the
co-expression results, the binding of transcription factors, 2,000
bp upstream and 500 bp downstream of each lncRNA initiation sites
was predicted. The relevant transcription factors of differentially
expressed lncRNAs, following the addition of daidzein to the H1299
cells, included Oct-1, HNF-1, HNF-4, Pax-4, COMP1, Pax-6, FOXD3 and
FOXJ2.
RT-qPCR validation of microarray
data
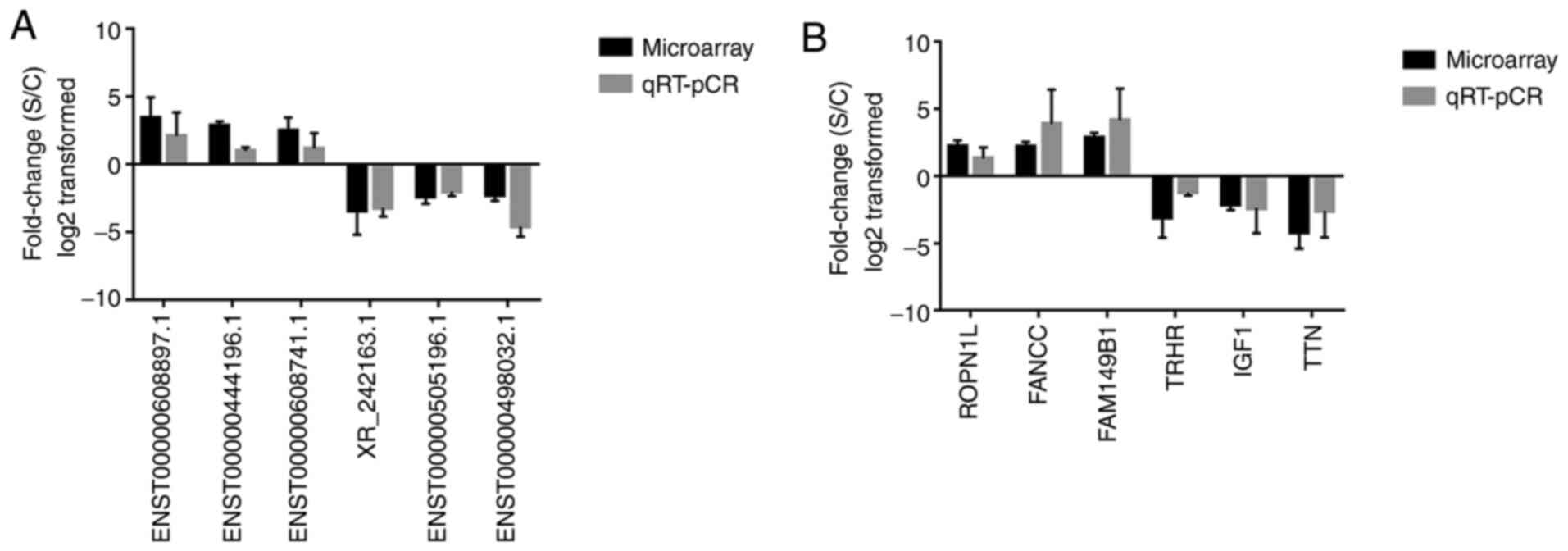
A total of six differentially expressed lncRNAs and
six mRNAs were randomly selected to validate the microarray data.
RT-qPCR analysis was performed to determine the fold changes of
selected lncRNAs and mRNAs, respectively (Fig. 6). The fold-change was positive when
the expression was upregulated and negative when the expression was
downregulated. Thus, the RT-qPCR results were consistent with the
microarray data.
Construction of core gene interaction
protein association
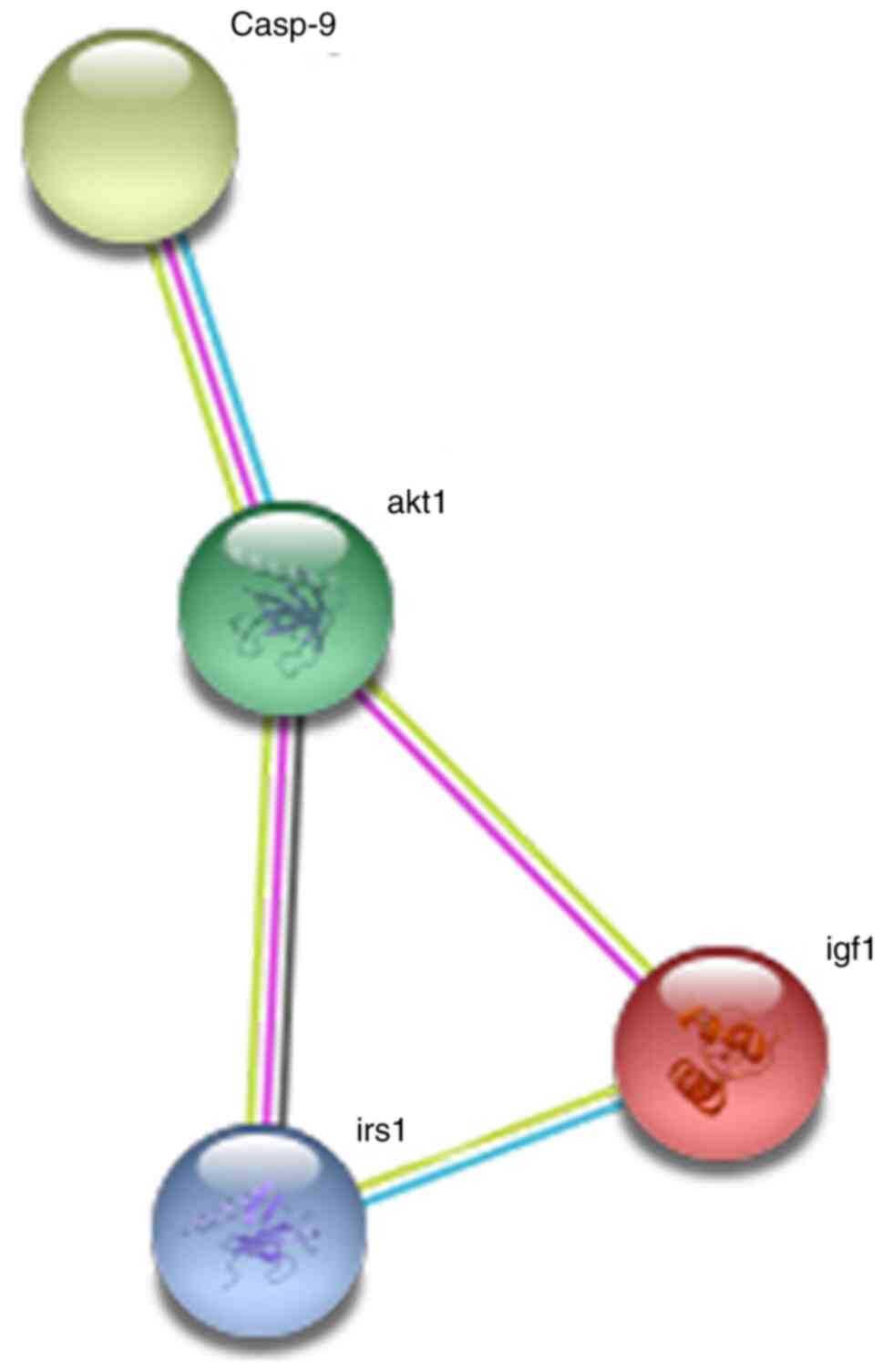
As presented in Fig.
7, the somatostatin C protein encoded by the insulin-like
growth factor-1 (IGF1) gene interacted directly with the IGF1
receptor, IRS, AKT and caspase-9 proteins. Collectively, these
results suggested that the IGF1 gene plays an important role in the
development of tumors.
Discussion
Lung cancer is one of the most common malignant
tumors, that threatens the health and life of those affected
(23). Due to the complexity of its
molecular mechanisms, there has been an increased effort to
identify early and accurate prevention, diagnosis and treatment to
decrease the mortality rate (24).
Previous studies have demonstrated that soy products inhibit tumor
development (25–30). An epidemiological study reported that
soy-related foods can decrease the risk of lung cancer mortality
(31). A previous study demonstrated
that isoflavones were also associated with a decreased risk of lung
cancer, both in vivo and in vitro (32); however, the specific molecular
mechanism remains unclear. In addition, gene chip technology has
enabled the investigation of differential gene expression,
particularly in the identification of tumor differential genes
(33,34).
A previous study reported that lncRNAs were
differentially expressed in normal and cancer cells (35). lncRNAs are non-coding RNA molecules,
~200 nucleotides in length (36).
Aberrant lncRNA expression is a major characteristic of several
types of cancer, which has been demonstrated to play an important
role in promoting the development and progression of human cancer
(37). For example, upregulated
expression of the carcinogenic lncRNA, HOTAIR has been associated
with breast, colorectal and liver cancers (38,39).
Zhang et al (40)
demonstrated that overexpression of lncRNA, UFC1 promoted the
proliferation and migration of gastric cancer cells. The
association between lncRNA and lung cancer has also been
extensively investigated (41–43).
However, there is lack of research into the molecular mechanism of
action of soybean and its products on lung cancer cells, using the
expression profile data of microarray.
The present study analyzed the difference in mRNA
and lncRNA expression levels between daidzein-treated and
-untreated H1299 lung cancer cells, based on the original results
of the gene chip. Bioinformatics (KEGG enrichment and GO analyses)
and RT-qPCR were performed to observe the expression of
differential genes to further investigate the molecular mechanism
of soy isoflavones and their derivatives in NSCLC. Sample
clustering demonstrated that there was a significant difference
between the experimental and the control groups, suggesting that
daidzein could significantly affect the lncRNA and mRNA expression
levels in lung cancer cells. The results of this experiment
demonstrated that following treatment with daidzein, eight and 11
lncRNAs were up- and downregulated in H1299 lung cancer cells,
respectively. In addition, five and 35 mRNAs were up- and
downregulated, respectively. To obtain preliminary insights into
lncRNA target gene function, GO and KEGG enrichment analyses were
performed using the lncRNA target gene pool. KEGG analysis of the
differentially expressed lncRNAs identified the top 20 diseases
associated, which included cancers of the lung and pleura and
malignant pleural mesothelioma diseases. KEGG analysis also
demonstrated that the addition of daidzein affected the pathways
involved in cancer-associated genes, namely the P53 signaling
pathway, suggesting that the addition of daidzein could affect
tumor proliferation. GO analysis revealed that the genes involved
in stress-related biological processes changed following addition
of daidzein to lung cancer cells. Several of the differentially
expressed genes were localized in the cytoplasm and altered the
expression of molecular functional genes associated with ‘binding’.
Collectively, these results suggested that the molecular mechanism
of action of daidzein in the lung cancer cells may be associated
with the alteration of molecular functions associated with
‘binding’ in the cytoplasm. RT-q PCR was performed using six
randomly selected lncRNAs and mRNAs in samples from a lung cancer
cell line to validate the microarray results. A total of six
lncRNAs and mRNAs identified in the microarray analysis were
confirmed to be aberrantly expressed in lung cancer cells via
RT-qPCR analysis.
As lung cancer is a serious threat to human health,
researchers have been investigating the molecular mechanisms that
inhibit lung cancer from all aspects, including the proliferation,
invasion, metastasis, autophagy and apoptosis of lung cancer cells
(44–47). There are several studies
investigating the apoptotic mechanism associated with the p53 gene
(48–50). It is well-known that p53 plays a key
role in inducing apoptosis in numerous types of cancer, such as
lung, gastric and prostate cancers (51–53). In
a previous study, daidzein inhibited the proliferation and NF-κB
signaling pathway in lung cancer cells (54). Therefore, it was hypothesized that
daidzein could promote the apoptosis of cancer cells via the P53
apoptosis pathway, which could inhibit the proliferation of lung
cancer cells. From the microarray analysis, in the present study,
the P53 pathway was included in the top 20 pathways with
differential gene enrichment, which provides a potential mechanism
for future research, to further identify the specific mechanism
involved.
Previous studies have demonstrated that high IGF1
expression level was associated with an increased risk of breast,
prostate, colorectal and lung cancers (55–58). In
addition, the IGF-binding protein, that regulates the IGF1 gene,
has been found to have proapoptotic activities, both dependent and
independent of p53 (59). Zhang
et al (60) reported that the
IGF1 receptor plays a key role in radiation-induced apoptosis of
lung cancer cells. Furthermore, a previous study demonstrated that
the occurrence of lung cancer is associated with the dysregulation
of IGF1, which affects the tumor suppressor gene, p53 (61). Consistent with these previous
findings, the results of the present study demonstrated that IGF1
was differentially expressed and the addition of daidzein to the
lung cancer cell line affected the p53 signaling pathway.
The present study has two major limitations. First,
the study only focused on the H1299 cell line of NSCLC; therefore,
other NSCLC cell lines and those from SCLC will also be
investigated in future research. Second, the present study lacks
clinical data, which will be also be included in future studies to
validate the results in the present study.
It has been hypothesized that IGF1 also plays a key
role in the effects of daidzein in lung cancer. The present study
constructed a protein-protein interaction network of the IGF1 gene
using the STRING online database. The results suggested that IGF1
could mediate apoptosis by interacting with other genes in
cancer-related pathways, thereby inhibiting tumor cell
proliferation. This IGF1 protein forms a signaling pathway with the
IGF1 receptor, IRS, AKT and caspase-9 proteins. Notably, IGF1 or a
protein that interacted with IGF1 was involved in malignant tumors
via the PI3K/pAKT pathway (62,63) AKT,
also known as protein kinase B or Rac, is an oncogenic protein,
that plays an important role in cell survival and apoptosis
(64). Insulin growth factors and
survival factors, including nerve growth factor and peptide trophic
factors could activate the AKT signaling pathway (65). Abnormal expression of AKT and
overactivation of AKT-associated pathways have been found to be
involved in the development of different types of cancer, including
lung, breast, ovarian and pancreatic cancers, and have been
associated with the proliferation and survival of lung cancer cells
and apoptosis (66–69). Taken together, these findings
suggested that the AKT signaling pathway could be used as a
therapeutic target for lung cancer.
In mammalian cells, one of the endogenous pathways
of apoptosis is the activation of caspase-9 (70). Caspases are proteolytic enzymes, that
are involved in cell apoptosis. Caspase-9 is one of the most
critical components of apoptosis in the caspase family (54). Previous studies have provided
valuable insight for the potential of daidzein to induce apoptosis
via different pathways and delay the progression of lung cancer
(71,72). Thus, the IGF1 gene has been
considered as a promising candidate gene, and the gene network
should be the focus of follow-up research on the molecular
mechanism of soybean action in lung cancer. Collectively, the
results of the present study provide a novel biomarker for the
treatment of lung cancer; however, further research is required to
validate the results.
In conclusion, the results of the present study
revealed a set of lncRNAs which were differentially expressed in
lung cancer cells following treatment with daidzein. Taken
together, these findings suggested that daidzein could
significantly affect lncRNA expression in lung cancer cells.
Furthermore, the results also demonstrated that the molecular
mechanism of action of daidzein in lung cancer cells may be
associated with the alteration of molecular functions associated
with ‘binding’ in the cytoplasm. The results presented here provide
novel insight into the molecular mechanism of daidzein in the H1299
lung cancer cell line. In addition, genes were also identified that
were co-expressed with mRNAs, as well as transcription factors
predicted by lncRNAs, and promising core genes and signaling
pathways. Collectively, the present study provided novel insights
into the molecular mechanism of lncRNAs associated with the effect
of daidzein in lung cancer and suggested that daidzein may
effectively delay the progression of lung cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Jiangxi Province (grant no.
2020BAB206067).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request. The high-throughput sequencing data is publicly
available from the GEO database (accession no. GSE181093).
Authors' contributions
LFL, SXH, XW and JL conceived and designed the
present study. LFL drafted the initial manuscript. LFL performed
the experiments, data collection, and wrote the manuscript. XWX and
SXH contributed to the methodology, performed the experiments and
analyzed the data. XBW contributed to data collection and analyzed
the data. All authors have read and approved the final manuscript.
XWX and SXH obtained materials and confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
lncRNA
|
long non-coding RNA
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
NSCLC
|
non-small cell lung cancer
|
|
SCLC
|
small cell lung cancer
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
IGF1
|
insulin-like growth factor 1
|
References
|
1
|
Mattiuzzi C and Lippi G: Current cancer
epidemiology. J Epidemiol Glob Health. 9:217–222. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Sun K, Zheng R, Zeng H, Zhang S,
Xia C, Yang Z, Li H, Zou X and He J: Cancer incidence and mortality
in China, 2014. Chin J Cancer Res. 30:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davidson MR, Gazdar AF and Clarke BE: The
pivotal role of pathology in the management of lung cancer. J
Thorac Dis. 5 (Suppl 5):S463–S478. 2013.PubMed/NCBI
|
|
4
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gridelli C, Rossi A and Maione P:
Treatment of non-small-cell lung cancer: State of the art and
development of new biologic agents. Oncogene. 22:6629–6638. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang G, Shu XO, Chow WH, Zhang X, Li HL,
Ji BT, Cai H, Wu S, Gao YT and Zheng W: Soy food intake and risk of
lung cancer: Evidence from the shanghai women's health study and a
meta-analysis. Am J Epidemiol. 176:846–855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Benassayag C, Perrot-Applanat M and Ferre
F: Phytoestrogens as modulators of steroid action in target cells.
J Chromatogr B Analyt Technol Biomed Life Sci. 777:233–248. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andres S, Abraham K, Appel KE and Lampen
A: Risks and benefits of dietary isoflavones for cancer. Crit Rev
Toxicol. 41:463–506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng F, Wang R, Zhang Y, Zhao Z, Zhou W,
Chang Z, Liang H, Zhao W, Qi L, Guo Z and Gu Y: Differential
expression analysis at the individual level reveals a lncRNA
prognostic signature for lung adenocarcinoma. Mol Cancer.
16:982017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mu M, Niu W, Zhang X, Hu S and Niu C:
LncRNA BCYRN1 inhibits glioma tumorigenesis by competitively
binding with miR-619-5p to regulate CUEDC2 expression and the
PTEN/AKT/p21 pathway. Oncogene. 39:6879–6892. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu X, Wang D, Lin Q, Wu G, Yuan S, Ye F
and Fan Q: Screening key lncRNAs for human rectal adenocarcinoma
based on lncRNA-mRNA functional synergistic network. Cancer Med.
8:3875–3891. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Z, Yang B, Zhang M, Guo W, Wu Z, Wang
Y, Jia L, Li S; Cancer Genome Atlas Research Network, ; Xie W and
Yang D: lncRNA epigenetic landscape analysis identifies EPIC1 as an
oncogenic lncRNA that interacts with MYC and promotes cell-cycle
progression in cancer. Cancer Cell. 33:706–720. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hauptman N and Glavač D: Long non-coding
RNA in cancer. Int J Mol Sci. 14:4655–4669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bao Z, Yang Z, Huang Z, Zhou Y, Cui Q and
Dong D: LncRNADisease 2.0: An updated database of long non-coding
RNA-associated diseases. Nucleic Acids Res. 47:D1034–D1037. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen G, Wang Z, Wang D, Qiu C, Liu M, Chen
X, Zhang Q, Yan G and Cui Q: LncRNADisease: A database for
long-non-coding RNA-associated diseases. Nucleic Acids Res.
41:D983–D986. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tokgun O, Tokgun PE, Inci K and Akca H:
lncRNAs as potential targets in small cell lung cancer:
MYC-dependent regulation. Anticancer Agents Med Chem. 20:2074–2081.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu T, Wang Y, Chen D, Liu J and Jiao W:
Potential clinical application of lncRNAs in non-small cell lung
cancer. Onco Targets Ther. 11:8045–8052. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lai XN, Li J, Tang LB, Chen WT, Zhang L
and Xiong LX: miRNAs and LncRNAs: Dual roles in TGF-β
signaling-regulated metastasis in lung cancer. Int J Mol Sci.
21:11932020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang M, Ma X, Zhu C, Guo L, Li Q, Liu M
and Zhang J: The prognostic value of long non coding RNAs in non
small cell lung cancer: A meta-analysis. Oncotarget. 7:81292–81304.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Patterson TA, Lobenhofer EK,
Fulmer-Smentek SB, Collins PJ, Chu TM, Bao W, Fang H, Kawasaki ES,
Hager J, Tikhonova IR, et al: Performance comparison of one-color
and two-color platforms within the microarray quality control
(MAQC) project. Nat Biotechnol. 24:1140–1150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eisen MB, Spellman PT, Brown PO and
Botstein D: Cluster analysis and display of genome-wide expression
patterns. Proc Natl Acad Sci U S A. 95:14863–14868. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li QW, Ma L, Qiu B, Yang H, Zhu YJ, Qiang
MY, Liu SR, Chen NB, Guo JY, Cai LZ, et al: Differential expression
profiles of long noncoding RNAs in synchronous multiple and
solitary primary esophageal squamous cell carcinomas: A microarray
analysis. J Cell Biochem. Oct 15–2018.(Epub ahead of print).
|
|
23
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gong WJ, Peng JB, Yin JY, Li XP, Zheng W,
Xiao L, Tan LM, Xiao D, Chen YX, Li X, et al: Association between
well-characterized lung cancer lncRNA polymorphisms and
platinum-based chemotherapy toxicity in Chinese patients with lung
cancer. Acta Pharmacol Sin. 38:581–590. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao T, Jin F, Li J, Xu Y, Dong H, Liu Q,
Xing P, Zhu G, Xu H and Miao Z: Dietary isoflavones or
isoflavone-rich food intake and breast cancer risk: A meta-analysis
of prospective cohort studies. Clin Nutr. 38:136–145. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tse G and Eslick GD: Soy and isoflavone
consumption and risk of gastrointestinal cancer: A systematic
review and meta-analysis. Eur J Nutr. 55:63–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wada K, Tsuji M, Tamura T, Konishi K,
Kawachi T, Hori A, Tanabashi S, Matsushita S, Tokimitsu N and
Nagata C: Soy isoflavone intake and stomach cancer risk in Japan:
From the Takayama study. Int J Cancer. 137:885–892. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zaheer K and Humayoun AM: An updated
review of dietary isoflavones: Nutrition, processing,
bioavailability and impacts on human health. Crit Rev Food Sci
Nutr. 57:1280–1293. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Applegate C, Rowles J, Ranard K, Jeon S
and Erdman J: Soy consumption and the risk of prostate cancer: An
updated systematic review and meta-analysis. Nutrients. 10:402018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Woo HD and Kim J: Dietary flavonoid intake
and risk of stomach and colorectal cancer. World J Gastroentero.
19:1011–1019. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nachvak SM, Moradi S, Anjom-Shoae J,
Rahmani J, Nasiri M, Maleki V and Sadeghi O: Soy, soy isoflavones,
and protein intake in relation to mortality from all causes,
cancers, and cardiovascular diseases: A systematic review and
dose-response meta-analysis of prospective cohort studies. J Acad
Nutr Diet. 119:1483–1500. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khan N and Mukhtar H: Dietary agents for
prevention and treatment of lung cancer. Cancer Lett. 359:155–164.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pan W: A comparative review of statistical
methods for discovering differentially expressed genes in
replicated microarray experiments. Bioinformatics. 18:546–554.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu Z, Fan C, Oh DS, Marron JS, He X,
Qaqish BF, Livasy C, Carey LA, Reynolds E, Dressler L, et al: The
molecular portraits of breast tumors are conserved across
microarray platforms. BMC Genomics. 7:962006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fu X, Ravindranath L, Tran N, Petrovics G
and Srivastava S: Regulation of apoptosis by a prostate-specific
and prostate cancer-associated noncoding gene, PCGEM1. DNA Cell
Biol. 25:135–141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iyer MK, Niknafs YS, Malik R, Singhal U,
Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et
al: The landscape of long noncoding RNAs in the human
transcriptome. Nat Genet. 47:199–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai M, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xue X, Yang YA, Zhang A, Fong K, Kim J,
Song B, Li S, Zhao JC and Yu J: LncRNA HOTAIR enhances ER signaling
and confers tamoxifen resistance in breast cancer. Oncogene.
35:2746–2755. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kazemzadeh M, Safaralizadeh R and Orang
AV: LncRNAs: Emerging players in gene regulation and disease
pathogenesis. J Genet. 94:771–784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang X, Liang W, Liu J, Zang X, Gu J, Pan
L, Shi H, Fu M, Huang Z, Zhang Y, et al: Long non-coding RNA UFC1
promotes gastric cancer progression by regulating miR-498/Lin28b. J
Exp Clin Cancer Res. 37:1342018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chi Y, Wang D, Wang J, Yu W and Yang J:
Long non-coding RNA in the pathogenesis of cancers. Cells.
8:10152019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen R, Li WX, Sun Y, Duan Y, Li Q, Zhang
AX, Hu JL, Wang YM and Gao YD: Comprehensive analysis of lncRNA and
mRNA expression profiles in lung cancer. Clin Lab. 63:313–320.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wei MM and Zhou GB: Long non-coding RNAs
and their roles in non-small-cell lung cancer. Genomics Proteomics
Bioinformatics. 14:280–288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Han Q, Lin X, Zhang X, Jiang G, Zhang Y,
Miao Y, Rong X, Zheng X, Han Y, Han X, et al: WWC3 regulates the
Wnt and Hippo pathways via dishevelled proteins and large tumour
suppressor 1, to suppress lung cancer invasion and metastasis. J
Pathol. 242:435–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shao L, Li H, Chen J, Song H, Zhang Y, Wu
F, Wang W, Zhang W, Wang F, Li H and Tang D: Irisin suppresses the
migration, proliferation, and invasion of lung cancer cells via
inhibition of epithelial-to-mesenchymal transition. Biochem Biophys
Res Commun. 485:598–605. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bai X, Meng L, Sun H, Li Z, Zhang X and
Hua S: MicroRNA-196b inhibits cell growth and metastasis of lung
cancer cells by targeting Runx2. Cell Physiol Biochem. 43:757–767.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu G, Pei F, Yang F, Li L, Amin AD, Liu
S, Buchan JR and Cho WC: Role of autophagy and apoptosis in
non-small-cell lung cancer. Int J Mol Sci. 18:3672017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gan PP, Zhou YY, Zhong MZ, Peng Y, Li L
and Li JH: Endoplasmic reticulum stress promotes autophagy and
apoptosis and reduces chemotherapy resistance in mutant p53 lung
cancer cells. Cell Physiol Biochem. 44:133–151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu J, Su C, Zhao F, Tao J, Hu D, Shi A,
Pan J and Zhang Y: Paclitaxel promotes lung cancer cell apoptosis
via MEG3-P53 pathway activation. Biochem Biophys Res Commun.
504:123–128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu YH, Liu GH, Mei JJ and Wang J: The
preventive effects of hyperoside on lung cancer in vitro by
inducing apoptosis and inhibiting proliferation through Caspase-3
and P53 signaling pathway. Biomed Pharmacother. 83:381–391. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Goldar S, Khaniani MS, Derakhshan SM and
Baradaran B: Molecular mechanisms of apoptosis and roles in cancer
development and treatment. Asian Pac J Cancer Prev. 16:2129–2144.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang Y, Li Q, Wei S, Sun J, Zhang X, He
L, Zhang L, Xu Z and Chen D: ZNF143 suppresses cell apoptosis and
promotes proliferation in gastric cancer via ROS/p53 axis. Dis
Markers. 2020:58631782020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gupta A, Behl T, Heer HR, Deshmukh R and
Sharma PL: Mdm2-P53 interaction inhibitor with cisplatin enhances
apoptosis in colon and prostate cancer cells in-vitro. Asian Pac J
Cancer Prev. 20:3341–3351. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ellis HM and Horvitz HR: Genetic control
of programmed cell death in the nematode C. elegans. Cell.
44:817–829. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sarfstein R, Nagaraj K, LeRoith D and
Werner H: Differential effects of insulin and IGF1 receptors on ERK
and AKT subcellular distribution in breast cancer cells. Cells.
8:14992019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ma JB, Bai JY, Zhang HB, Jia J, Shi Q,
Yang C, Wang X, He D and Guo P: KLF5 inhibits STAT3 activity and
tumor metastasis in prostate cancer by suppressing IGF1
transcription cooperatively with HDAC1. Cell Death Dis. 11:4662020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hu J, Liu X, Chi J, Che K, Feng Y, Zhao S,
Wang Z and Wang Y: Expressions of IGF-1, ERK, GLUT4, IRS-1 in
metabolic syndrome complicated with colorectal cancer and their
associations with the clinical characteristics of CRC. Cancer
Biomark. 21:883–891. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang YA, Sun Y, Palmer J, Solomides C,
Huang LC, Shyr Y, Dicker AP and Lu B: IGFBP3 modulates lung
tumorigenesis and cell growth through IGF1 signaling. Mol Cancer
Res. 15:896–904. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Furstenberger G and Senn HJ: Insulin-like
growth factors and cancer. Lancet Oncol. 3:298–302. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang H, Zhang C and Wu D: Activation of
insulin-like growth factor 1 receptor regulates the
radiation-induced lung cancer cell apoptosis. Immunobiology.
220:1136–1140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Brambilla E, Gazzeri S, Gouyer V and
Brambilla C: Mechanisms of lung oncogenesis. Rev Prat. 43:807–814.
1993.(In French). PubMed/NCBI
|
|
62
|
Wang LJ, Li QJ, Le Y, Ouyang HY, He MK, Yu
ZS, Zhang YF and Shi M: Prognostic significance of sodium-potassium
ATPase regulator, FXYD3, in human hepatocellular carcinoma. Oncol
Lett. 15:3024–3030. 2018.PubMed/NCBI
|
|
63
|
Velloso FJ, Bianco AF, Farias JO, Torres
NE, Ferruzo PY, Anschau V, Jesus-Ferreira HC, Chang TH, Sogayar MC,
Zerbini LF and Correa RG: The crossroads of breast cancer
progression: Insights into the modulation of major signaling
pathways. Onco Targets Ther. 10:5491–5524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chuang CH, Cheng TC, Leu YL, Chuang KH,
Tzou SC and Chen CS: Discovery of Akt kinase inhibitors through
structure-based virtual screening and their evaluation as potential
anticancer agents. Int J Mol Sci. 16:3202–3212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: A play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yu X, Yuan Y, Zhi X, Teng B, Chen X, Huang
Q, Chen Y, Guan Z and Zhang Y: Correlation between the protein
expression of A-kinase anchor protein 95, cyclin D3 and AKT and
pathological indicators in lung cancer tissues. Exp Ther Med.
10:1175–1181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kim BM, Kim DH, Park JH, Surh YJ and Na
HK: Ginsenoside Rg3 inhibits constitutive activation of NF-kB
signaling in human breast cancer (MDA-MB-231) cells: ERK and akt as
potential upstream targets. J Cancer Prev. 19:23–30. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bellacosa A, Testa JR, Moore R and Larue
L: A portrait of AKT kinases: Human cancer and animal models depict
a family with strong individualities. Cancer Biol Ther. 3:268–275.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cheng JQ, Lindsley CW, Cheng GZ, Yang H
and Nicosia SV: The Akt/PKB pathway: Molecular target for cancer
drug discovery. Oncogene. 24:7482–7492. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hirsch T, Marzo I and Kroemer G: Role of
the mitochondrial permeability transition pore in apoptosis. Biosci
Rep. 17:67–76. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Koo J, Cabarcas-Petroski S, Petrie JL,
Diette N, White RJ and Schramm L: Induction of proto-oncogene BRF2
in breast cancer cells by the dietary soybean isoflavone daidzein.
BMC Cancer. 15:9052015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Guo S, Wang Y, Li Y, Li Y, Feng C and Li
Z: Daidzein-rich isoflavones aglycone inhibits lung cancer growth
through inhibition of NF-kB signaling pathway. Immunol Lett.
222:67–72. 2020. View Article : Google Scholar : PubMed/NCBI
|