Introduction
Cervical cancer (CC) is the second most common type
of cancer in women aged 20–39 years worldwide, and represents a
serious threat to public health (1).
Each year, 265,700 individuals die from the disease globally, and
thus, efforts have been made to improve the timely diagnosis and
effective treatment of CC (2,3).
Currently, hysterectomy has become increasingly popular and is
widely used as a treatment for early-stage CC (4). Targeted therapies have also become an
effective strategy for CC treatment (5).
Circular RNAs (circRNAs/circ), a novel class of
non-coding RNAs (ncRNAs), are characterized by a covalently closed
loop without a 5 cap and 3 polyadenylated tail, and are generated
through abnormal transcription and splicing (6). Accumulating evidence has demonstrated
that circRNAs exert effects on multiple biological and pathological
processes, suggesting that they may be involved in the occurrence
and development of numerous diseases, including cancer (7,8). For
example, circ-acetyl-CoA carboxylase α (ACACA) has been previously
reported to promote the proliferation of non-small cell lung cancer
(NSCLC) cells by modulating proliferation, migration and glycolysis
via microRNA (miRNA/miR)-1183 and the PI3K/AKT signaling pathway
(9). However, to the best of our
knowledge, the role of circ-ACACA in CC remains to be
determined.
miRNAs are small ncRNA molecules that can sponge
target genes and regulate their expression (3). A previous study demonstrated that
miR-582-5p inhibits the proliferation and invasion of NSCLC cells
by downregulating Notch1 expression (10). In addition, bone metastasis of
prostate cancer is inhibited by the combined effects of miR-582-3p
and miR-582-5p via modulation of TGF-β signaling transduction
(11). Long ncRNA urothelial cancer
associated 1 targets miR-582-5p and promotes the progression and
drug resistance of bladder cancer cells through the inhibition of
autophagy related 7-mediated autophagy (12). The expression levels of miR-582-5p
have also been reported to be downregulated in endometrial and
gastric cancer, and miR-582-5p overexpression inhibits cell
proliferation and promotes apoptosis by targeting AKT3 (13). Furthermore, the inhibitory effect of
miR-582-5p on the proliferation of colorectal cancer and
hepatocellular carcinoma cells has been identified to be achieved
by targeting certain genes (14).
However, to the best of our knowledge, the role of miR-582-5p in CC
has not yet been reported.
Endoplasmic reticulum oxidoreductase 1α (ERO1α;
ERO1A) is an oxidase located in the endoplasmic reticulum, which
promotes the formation of disulphide bonds in granulocyte-colony
stimulating factor (15).
Accumulating evidence has demonstrated the close association
between upregulated expression levels of ERO1A and poor prognosis
in multiple types of cancer, such as pancreatic cancer,
cholangiocarcinoma and breast cancer (16–19). In
a previous study, knockdown of ERO1A expression reduced the
proliferation, migration and tumorigenesis of CC cells by
downregulating H2O2-associated
epithelial-to-mesenchymal transition (20). Additionally, ERO1A has been
demonstrated to affect the functions of pancreatic cancer cells by
activating the Wnt/β-catenin signaling pathway to enhance the
progression of pancreatic cancer (17). A previous study also demonstrated
that upregulated expression levels of ERO1A are associated with the
poor prognosis of gastric cancer (21). Furthermore, endoplasmic reticulum
stress-dependent ERO1A expression has been demonstrated to enhance
aerobic glycolysis in pancreatic cancer (16). Hypoxia-inducible ERO1A also promotes
the development of colorectal cancer by regulating integrin-β1 and
integrin-β1-associated signaling in colorectal cancer cells
(22).
Therefore, the present study aimed to investigate
the potential role of circ-ACACA in CC and its regulatory effects
on the miR-582-5p/ERO1A signaling axis.
Materials and methods
Cell lines and culture
Cervical squamous cell carcinoma cell lines (Ca Ski
and SiHa), a endocervical adenocarcinoma cell line (HeLa) and the
normal cervical epithelial cell line (End1/E6E7) were obtained from
The Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences. All cells were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 100 mg/l and streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) and 100 U/l penicillin (Gibco; Thermo Fisher
Scientific, Inc.) and maintained in an atmosphere of 37°C with 5%
CO2.
Cell transfection
pIRES vectors containing short hairpin RNA
(shRNA)-circ-ACACA#1, shRNA-circ-ACACA#2, shRNA-negative control
(NC), miR-582-5p mimic (50 nM; sense, 5′-UUACAGUUCAACCAGUUACU-3′
and antisense, 5′-UAACUGGUUGAACAACUGUAAUU-3′), mimic-NC (50 nM;
sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′), miR-582-5p inhibitor (50 nM;
5′-AGUAACUGGUUGAACAACUGUAA-3′), NC inhibitor (50 nM;
5′-CAGUACUUUUGUGUAGUACAAA-3′), pcDNA3.1-ERO1A and pcDNA3.1-NC
(empty plasmid) were purchased from Shanghai GenePharma Co., Ltd.
HeLa cells (1×106 cells per well) were plated and
incubated in six-well plates for 24 h and then transfected for 48 h
with the corresponding plasmids, oligonucleotides or NCs using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C. The transfected cells were used for
subsequent experiments at 48 h after transfection. Untreated cells
were used as the control group.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from CC cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturers protocols. Total RNA was
reverse transcribed into cDNA using the PrimeScript™ Strand cDNA
synthesis kit (Takara Biotechnology Co., Ltd.). The temperature
protocol for this step was as follows: 70°C for 5 min, 37°C for 5
min and 42°C for 1 h. qPCR was subsequently performed using a Power
SYBR® Green PCR Master mix (Invitrogen; Thermo Fisher
Scientific, Inc.) on an ABI 7500 Real-Time PCR Detection system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used: Initial denaturation at 95°C
for 10 min; followed by 40 cycles of denaturation at 95°C for 15
sec and annealing at 60°C for 1 min; and a final extension of 10
min at 72°C. Primers pairs used in this study were as follows:
circ-ACACA forward, 5′-GTGGCTTTGAAGGAGCTGTC-3′ and reverse,
5′-CAGACATGCTGGACCTTGAA-3′; miR-582-5p forward,
5′-GCGGTTACAGTTGTTCAACC-3′ and reverse, 5′-CTCAACTGGTGTCGTGGA-3′;
ERO1A forward, 5′-ATGACATCAGCCAGTGTGGA-3′ and reverse,
5′-CATGCTTGGTCCACTGAAGA-3′; GAPDH forward,
5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse,
5′-GCCATCACGCCACAGTTTC-3′; and U6 forward,
5′-GGAACGATACAGAGAAGATTAGC-3′ and reverse,
5′-TGGAACGCTTCACGAATTTGCG-3′. The miRNA expression level was
normalized to U6 and GAPDH was applied as the internal control of
mRNAs, following quantification using the 2−∆∆Cq method
(23).
RNase R treatment assay
Six units of RNase R (Geneseed Biotech, Inc.) were
added into every 2 µg RNA and incubated at 37°C for 20 min. After
RNase R treatment, the mRNA expression of circ-ACACA and ACACA was
detected by RT-qPCR.
Cell cytoplasm/nucleus fraction
isolation
Cell cytoplasm/nucleus fraction isolation was
performed using the Nuclear/Cytosol Fractionation Kit (Cell
Biolabs, Inc.). Briefly, extracted RNAs from the cytoplasm or
nucleus were determined by RT-qPCR which was performed in the
previous RT-qPCR section. The relative expression levels of
circ-ACACA, ACACA, nuclear control transcript (U6) and cytoplasmic
control transcript (β-actin) were measured. β-actin and U6 were
used as the internal control for cytosolic and nuclear fractions,
respectively.
MTT assay
The viability of transfected HeLa cells was detected
using MTT reagent (Sigma-Aldrich; Merck KGaA). Briefly, cells
(6×103 cells/well) were plated into 96-well plates and
incubated for 24, 48 or 72 h. At each time point, 10 µl MTT was
added to each well. Following incubation, 100 µl DMSO
(Sigma-Aldrich; Merck KGaA) was added to each well to dissolve the
purple formazan crystals. The absorbance of each well was measured
at a wavelength of 490 nm using a microplate reader (Bio-Rad
Laboratories, Inc.).
Colony formation assay
A total of 2×103 HeLa cells/well were
seeded into a six-well plate and maintained in a humidified
incubator with 5% CO2 at 37°C. Following 48 h of
incubation, the plates were washed with PBS twice, and then
colonies were fixed with 10% paraformaldehyde for 10 min at room
temperature and stained with 0.1% crystal violet for 15 min at room
temperature. After the plates were washed with phosphate buffer
saline (PBS) at room temperature for 1 min and dried, the number of
colonies was counted under an inverted light microscope.
Cell invasion assay
To detect the ability of cell invasion, Transwell
plates with a 8-µm pore insert precoated with Matrigel (BD
Biosciences) overnight at 37°C were obtained. A total of
2×104 transfected HeLa cells were trypsinized and plated
into the upper chamber of transwell plates with serum-free DMEM.
The lower chambers were filled with 800 µl DMEM supplemented with
10% FBS. Following 48 h of incubation at 37°C, the invasive cells
were fixed with 95% ethanol for 20 min at 37°C and stained with
0.1% crystal violet for 10 min at 37°C. The number of cells in five
randomly selected fields of view was counted under a light
microscope.
Wound healing assay
HeLa cells were plated into 96-well plates at a
density of 4×103 cells/well and cultured until they
reached 80% confluence. The cells were washed with PBS three times
and then cultured in medium supplemented with 1% FBS for 24 h. An
artificial wound was created across the cell monolayer by
scratching the center of all wells with a 10-µl pipette tip.
Migratory cells were visualized using an inverted light microscope.
ImageJ software (version 1.52r; National Institutes of Health) was
used to determine the wound healing rate.
TUNEL assay
Following culture for 24 h, 1×105 HeLa
cells were seeded in a 6-well plate. Cells were fixed with 4%
paraformaldehyde at 4°C for 20 min, permeabilized with 0.1% Triton
X-100 in PBS and subsequently incubated with TUNEL reagents (EMD
Millipore). Cell nuclei was stained with diaminobenzene for 10 min
at room temperature. The apoptosis of TUNEL-positive cells was
observed under a fluorescence microscope (magnification, ×200;
Olympus Corporation) and cells were counted in five randomly
selected microscopic fields.
Glycolysis assay
Glycolysis was determined using a Seahorse XF
Glycolytic Rate assay kit (Agilent Technologies, Inc.) on a
Seahorse XFe96 analyzer (Agilent Technologies, Inc.) according to
the manufacturers protocol. Briefly, 2×104 HeLa cells
per well transfected with shRNA-circ-ACACA, miR-582-5p,
shRNA-circ-ACACA + miR-582-5p inhibitor or respective NCs were
plated into a 96-well plate. After the probes were calibrated, 10
mmol glucose, 10 µmol oligomycin and 50 mmol 2-deoxyglucose were
serially injected to detect the extracellular acidification rate
(ECAR). Data were analyzed using Seahorse XFe24 Wave 2.2 software
(Agilent Technologies, Inc.).
Glucose consumption and lactate
production assays
HeLa cells and cell culture medium were collected
following transfection for 48 h. The concentration of glucose in
the cell culture medium was determined using a Glucose assay kit
(Sigma-Aldrich; Merck KGaA), whereas the lactate concentration was
measured using a Lactic Acid assay kit (Seebio), according to the
manufacturers protocols.
Measurement of intracellular
metabolite generation
13C-labeled intracellular metabolites
were identified as previously described (24). Briefly, 1×107 HeLa cells
were incubated with 2 g/l 13C-labeled glucose for 2 h at
37°C. Metabolites were subsequently extracted and evaluated using a
liquid chromatography system equipped with a TripleTOF®
5600 mass spectrometer (SCIEX; AB SCIEX). Electrospray positive ion
mode was used along with the following parameters: ion voltage,
5,500 V; declustering potential, 80 V; source temperature, 60°C;
curtain gas, 35 psi; and 100–1,000 m/z.
Western blotting
Total protein was extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology). Protein
concentration was determined using a BCA kit (Beyotime Institute of
Biotechnology) and protein samples (40 µg protein/lane) were
separated via 12% SDS-PAGE. The separated proteins were
subsequently transferred onto PVDF membranes (EMD Millipore) and
the membranes were blocked with 5% skimmed milk for 1.5 h at room
temperature. The membranes were then incubated with primary
antibodies overnight at 4°C. Following the primary antibody
incubation, the membranes were rinsed with TBS-0.2% Tween 20 and
incubated with the goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (dilution, 1:2,000; cat.
no. ab205718; Abcam) at room temperature for 1 h. GAPDH was used as
the internal loading control. Protein bands were visualized using
an ECL detection system (Thermo Fisher Scientific, Inc.) and
densitometric analysis was performed using ImageJ software (version
1.52r; National Institutes of Health). The following primary
antibodies were used: anti-hypoxia-inducible factor 1α (HIF-1A;
cat. no. 36169T; 1:1,000; Cell Signaling Technology, Inc.),
anti-lactate dehydrogenase A (LDHA; cat. no. 3582T; 1:1,000; Cell
Signaling Technology, Inc.), anti-glucose transporter type 1
(GLUT1; cat. no. 12939S; 1:1,000; Cell Signaling Technology, Inc.),
anti-hexokinase-2 (HK2; cat. no. 2867S; 1:1,000; Cell Signaling
Technology, Inc.), anti-ERO1A (cat. no. 3264T; 1:1,000; Cell
Signaling Technology, Inc.) and anti-GAPDH (cat. no. 5174T;
1:1,000; Cell Signaling Technology, Inc.). GAPDH was used as an
internal control.
Dual-luciferase reporter assay
The binding site between circ-ACACA and miR-582-5p
as well as miR-582-5p and ERO1A was predicted using the
Encyclopedia of RNA Interactomes (ENCORI) database (http://starbase.sysu.edu.cn/). To validate the binding
relationship between miR-582-5p and circ-ACACA or ERO1A, wild-type
(WT) or mutant (MUT) 3-untranslated region sequences of circ-ACACA
or ERO1A were cloned into a pmiRGLO dual-luciferase miRNA target
expression reporter (Promega Corporation). HeLa cells were
subsequently transfected with miR-582-5p mimic or mimic-NC and WT
or MUT reporters using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). The relative
luciferase activity was measured at 48 h post-transfection using a
Dual Luciferase Reporter assay system (Promega Corporation). And
firefly luciferase activity was normalized to Renilla
luciferase activity.
Statistical analysis
All experiments were repeated independently in
triplicate. Statistical analysis was performed using SPSS v20.0
software (IBM Corp.). Data are presented as the mean ± SD.
Statistical differences between two groups were determined using an
unpaired Students t-test, while one-way ANOVA followed by Tukeys
post hoc test was used for comparisons among ≥3 groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
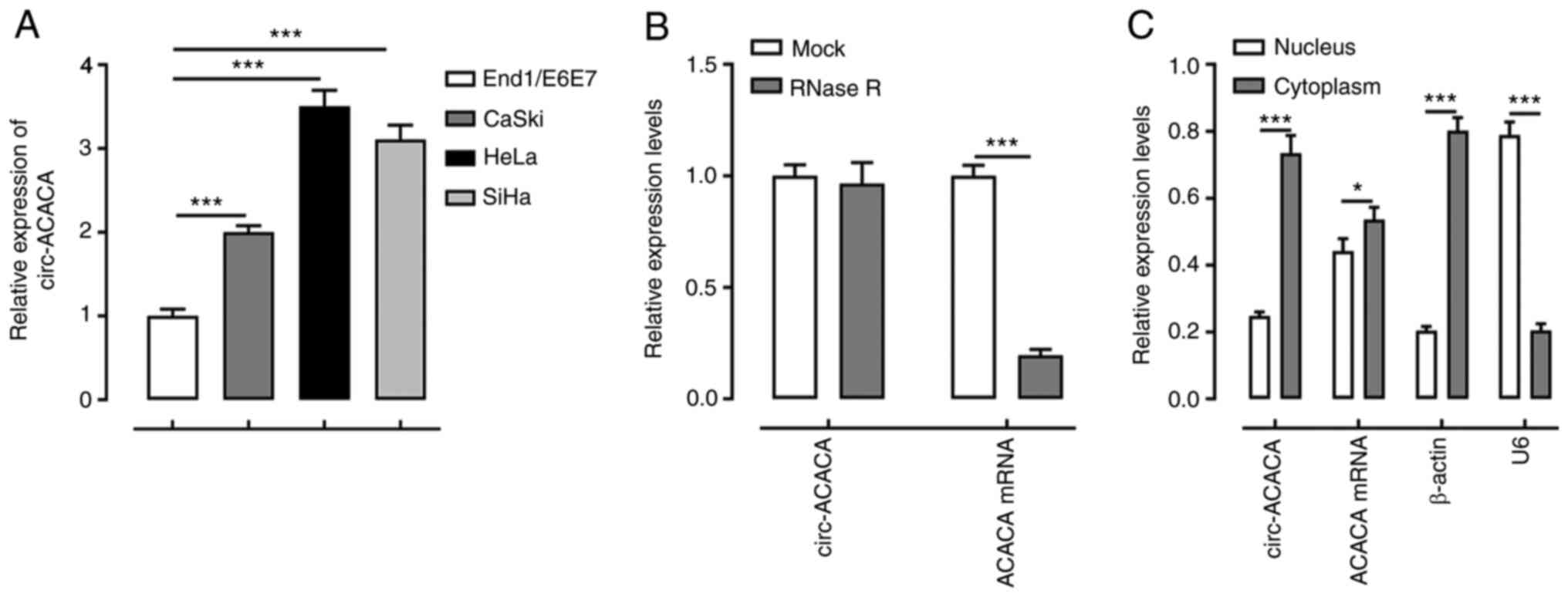
circ-ACACA expression is upregulated
in CC cells
To investigate the role of circ-ACACA in CC, its
expression levels in CC cells were first determined using RT-qPCR.
The results revealed that the mRNA expression levels of circ-ACACA
were upregulated in CC cells compared with End1/E6E7 cells
(Fig. 1A). The expression levels of
circ-ACACA were the highest in HeLa cells among the cell lines
examined. Therefore, HeLa cells were used as the CC cell model in
subsequent experiments. As shown in Fig.
1B, circ-ACACA was not dissolved, while ACACA mRNA was
dissolved by RNase R. Furthermore, circ-ACACA was expressed at a
higher level in the cytoplasm compared with the nucleus (Fig. 1C). These results suggested that
circ-ACACA expression may be upregulated in CC cells.
Silencing of circ-ACACA inhibits the
proliferation, invasion and migration, while promoting the
apoptosis of CC cells
circ-ACACA expression was subsequently knocked down
to determine its effects in CC cells. The expression levels of
circ-ACACA were downregulated following transfection with
shRNA-circ-ACACA#1 and shRNA-circ-ACACA#2 compared with shRNA-NC,
especially in shRNA-circ-ACACA#2. Therefore, shRNA-circ-ACACA#2 was
selected for use in subsequent experiments (Fig. 2A). The effects of transfection of
shRNA-circ-ACACA on CC cells were determined using functional
assays. MTT and colony formation assays demonstrated that cell
viability and proliferation were decreased in HeLa cells
transfected with shRNA-circ-ACACA as compared with the shRNA-NC
group (Fig. 2B and C). Furthermore,
transfection with shRNA-circ-ACACA increased the levels of
apoptosis compared with the shRNA-NC group (Fig. 2D and E). circ-ACACA silencing also
notably inhibited cell invasion and migration compared with the
shRNA-NC group (Fig. 2F-I). These
results suggested that silencing of circ-ACACA may inhibit the
proliferation, invasion and migration, and promote the apoptosis of
CC cells.
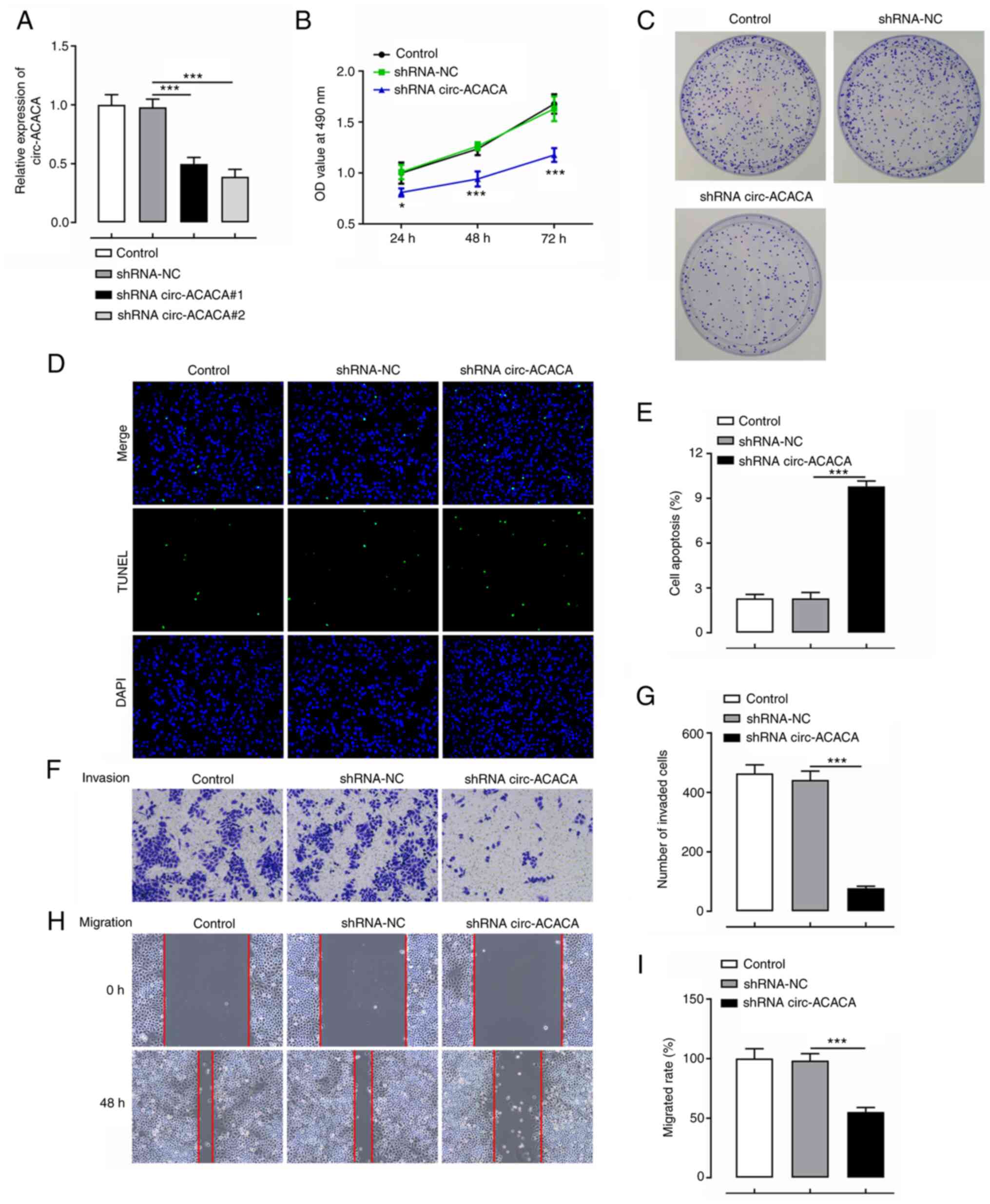 | Figure 2.Knockdown of circ-ACACA inhibits the
proliferation, invasion and migration, and promotes the apoptosis
of cervical cancer cells. (A) Expression levels of circ-ACACA
following the transfection of cells with shRNA-circ-ACACA were
detected using reverse transcription-quantitative PCR. (B) Cell
viability was analyzed using an MTT assay. *P<0.05,
***P<0.001 vs. shRNA-NC. (C) Cell proliferation was determined
using a colony formation assay. (D) Cell apoptosis was analyzed
using a TUNEL assay. Magnification, ×200. (E) The apoptosis rate of
TUNEL assay. (F) HeLa cell invasion was determined using transwell
assay following transfection with shRNA-circ-ACACA. Magnification,
×100. (G) The number of invaded cells. (H) HeLa cell migration was
tested using wound healing assay following transfection with
shRNA-circ-ACACA. Magnification, ×200. (I) The cell migration rate.
*P<0.05, ***P<0.001. circ-ACACA, circular RNA
acetyl-CoA-carboxylase α; NC, negative control; OD, optical
density; shRNA, short hairpin RNA. |
Silencing of circ-ACACA suppresses
glycolysis in CC cells
It was subsequently investigated whether knockdown
of circ-ACACA led to the attenuation of glycolysis. As displayed in
Fig. 3A, ECAR was notably decreased
after transfection with shRNA-circ-ACACA when compared with the
shRNA-NC group. Lactate production and glucose consumption assays
were performed to evaluate the effect of circ-ACACA on glycolysis.
As shown in Fig. 3B and C, the
transfection with shRNA-circ-ACACA markedly decreased the
production of lactate and increased the levels of glucose compared
with the shRNA-NC group. In addition, HeLa cells were incubated
with 13C-labeled glucose and liquid chromatography-mass
spectrometry was performed to gain more in-depth insights into the
metabolic flux of glucose. As shown in Fig. 3D, the ratio of 13C-marked glucose was
notably decreased following circ-ACACA-knockdown relative to the
shRNA-NC group. As expected, the expression levels of
glycolysis-related proteins, including HIF-1A, LDHA, GLUT1 and HK2,
were markedly downregulated following the knockdown of circ-ACACA
compared with those in the shRNA-NC group (Fig. 3E). These results indicated that
silencing of circ-ACACA may inhibit glycolysis in CC cells.
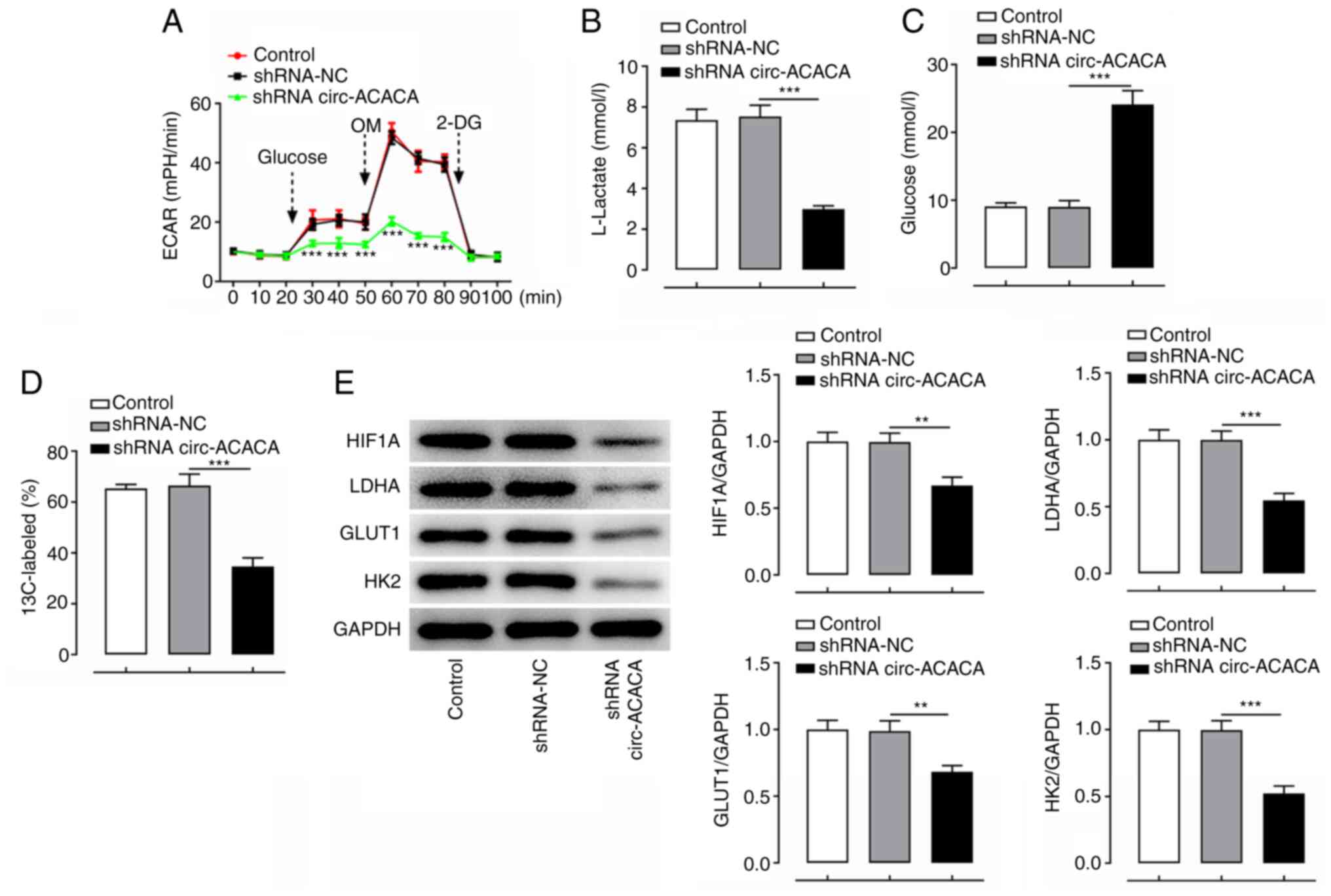 | Figure 3.Silencing of circ-ACACA suppresses
glycolysis in cervical cancer cells. (A) ECAR after HeLa cells were
transfected with shRNA-circ-ACACA. ***P<0.001 vs. shRNA-NC. (B)
Lactate levels and (C) glucose content after HeLa cells were
transfected with shRNA-circ-ACACA. (D) Metabolite analysis and (E)
glycolysis-related protein expression after HeLa cells were
transfected with shRNA-circ-ACACA. **P<0.01, ***P<0.001.
circ-ACACA, circular RNA acetyl-CoA-carboxylase α; ECAR,
extracellular acidification rate; GLUT1, glucose transporter type
1; HIF1A, hypoxia-inducible factor 1α; HK2, hexokinase-2; LDHA,
lactate dehydrogenase A; NC, negative control; shRNA, short hairpin
RNA; OM, oligomycin; 2-DG, 2-deoxy-D-glucose. |
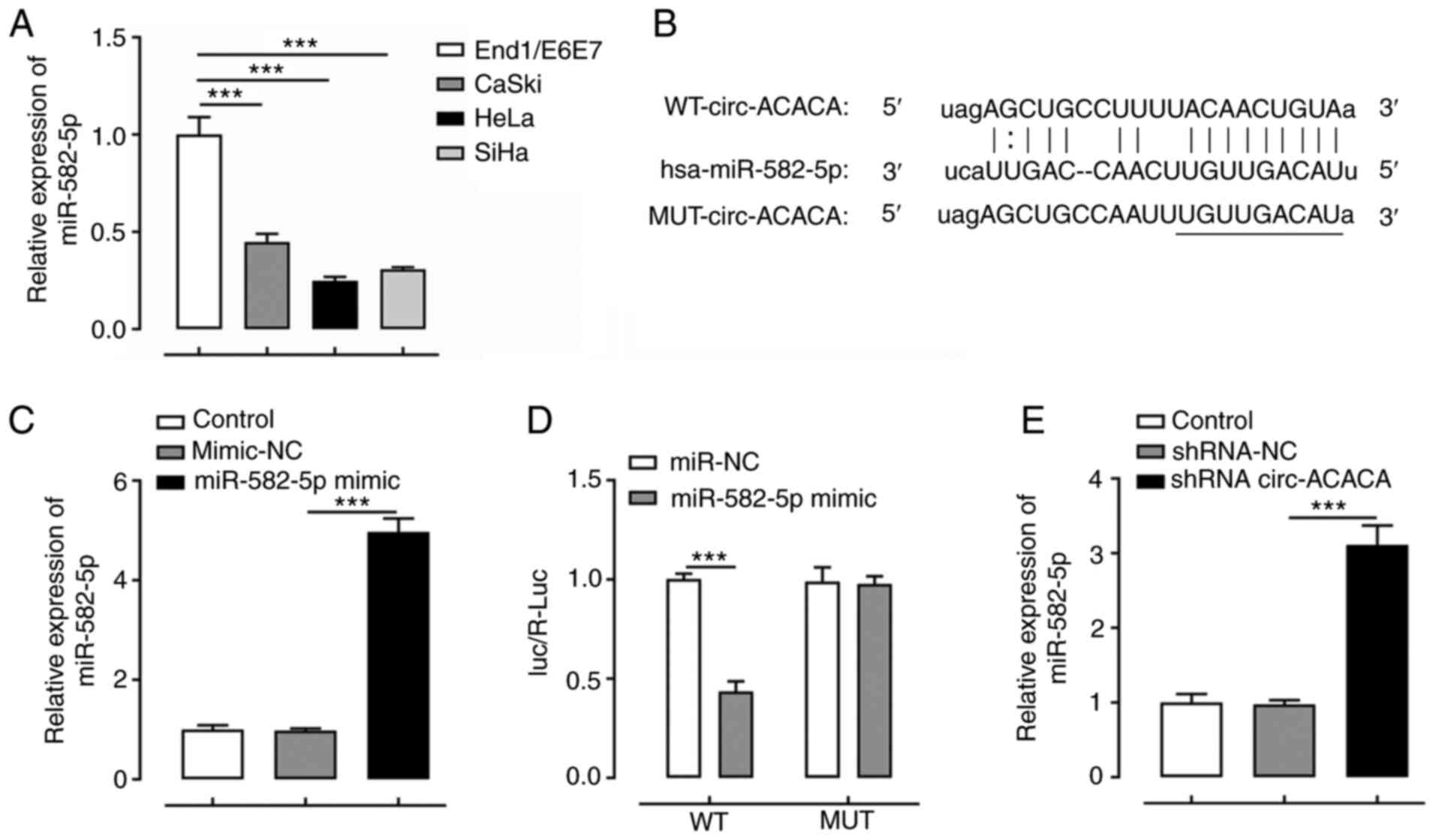
circ-ACACA negatively regulates the
expression levels of miR-582-5p
To determine the regulatory effect of circ-ACACA on
the expression levels of miR-582-5p, the expression levels of
miR-582-5p were first analyzed in various CC cell lines. The
results revealed that miR-582-5p expression was downregulated in CC
cells compared with End1/E6E7 cells (Fig. 4A). Subsequently, a binding site
between circ-ACACA and miR-582-5p was predicted using ENCORI
(Fig. 4B). Following the
overexpression of miR-582-5p by transfection with the miR-582-5p
mimic, a dual-luciferase reporter assay was used to demonstrate the
binding relationship between circ-ACACA and miR-582-5p (Fig. 4C and D). As shown in Fig. 4E, knockdown of circ-ACACA markedly
upregulated the expression levels of miR-582-5p compared with those
in the shRNA-NC group. These data suggested that circ-ACACA may
negatively regulate miR-582-5p expression in HeLa cells.
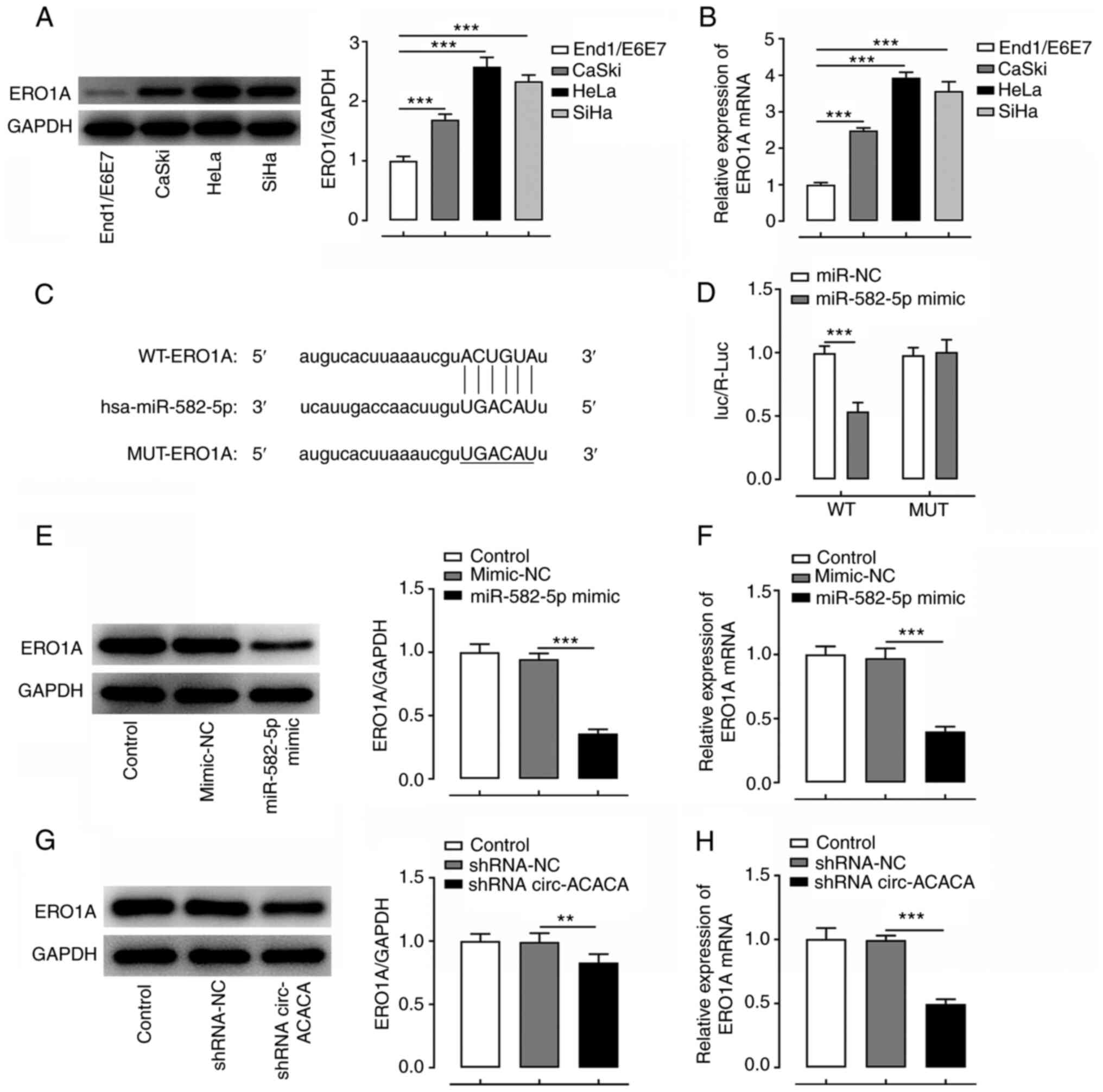
ERO1A is a target gene of
miR-582-5p
ERO1AIt was hypothesized that circ-ACACA may exert
its promoting effects on the progression of CC via the
miR-582-5p/ERO1A signaling axis. To verify this hypothesis, the
expression levels of ERO1A in various CC cell lines were
determined. As illustrated in Fig. 5A
and B, ERO1A expression was notably unregulated in CC cells
compared with End1/E6E7 cells. Since ERO1A was predicted to target
and bind with miR-582-5p using ENCORI database. A binding
association between ERO1A and miR-582-5p was predicted using ENCORI
(Fig. 5C) and verified using a
dual-luciferase reporter assay (Fig.
5D). Subsequently, RT-qPCR and western blotting demonstrated
that transfection with the miR-582-5p mimic markedly reduced the
mRNA and protein expression levels of ERO1A (Fig. 5E and F). Furthermore, transfection
with shRNA-circ-ACACA markedly downregulated the mRNA and protein
expression levels of ERO1A compared with the shRNA-NC group
(Fig. 5G and H). These findings
suggested that ERO1A may be a target of miR-582-5p, and that
circ-ACACA may upregulate the expression levels of ERO1A by
sponging and negatively regulating miR-582-5p expression.
Knockdown of miR-582-5p or
overexpression of ERO1A reverses the effects of circ-ACACA
silencing on CC cell proliferation, viability, invasion, migration
and apoptosis
To investigate whether circ-ACACA could affect the
functions of CC cells in vitro via the miR-582-5p/ERO1A
signaling axis, ERO1A was overexpressed in CC cells. The expression
levels of ERO1A were markedly upregulated in the pcDNA3.1-ERO1A
group compared with the pcDNA3.1-NC group, demonstrating the
successful establishment of ERO1A-overexpressing CC cells (Fig. 6A and B). Additionally, miR-582-5p
expression was notably downregulated after transfection with
miR-582-5p inhibitor compared with that in the NC inhibitor group
(Fig. 6C). Knockdown of circ-ACACA
reduced the viability, proliferation, invasion and migration, and
enhanced the apoptosis of HeLa cells, and these effects were all
partially reversed following the transfection with the miR-582-5p
inhibitor or pcDNA3.1-ERO1A (Fig.
6D-K). In addition, transfection with circ-ACACA reduced the
levels of ECAR, and these effects were subsequently reversed by
transfection with the miR-582-5p inhibitor or pcDNA3.1-ERO1A
(Fig. 7A). Furthermore, the results
of the lactate production and glucose consumption assays revealed
that the decreased lactate production and enhanced glucose levels
induced by transfection with shRNA-circ-ACACA were partially
recovered following the transfection with the miR-582-5p inhibitor
or pcDNA3.1-ERO1A (Fig. 7B and C).
Finally, the shRNA-circ-ACACA-induced decreases in the ratio of
13C-marked glucose and expression levels of
glycolysis-related proteins were partially reversed following
transfection with the miR-582-5p inhibitor or pcDNA3.1-ERO1A
(Fig. 7D and E). Overall, these
results suggested that knockdown of miR-582-5p or overexpression of
ERO1A alleviates the effects of circ-ACACA silencing on CC cell
proliferation, viability, invasion, migration and apoptosis.
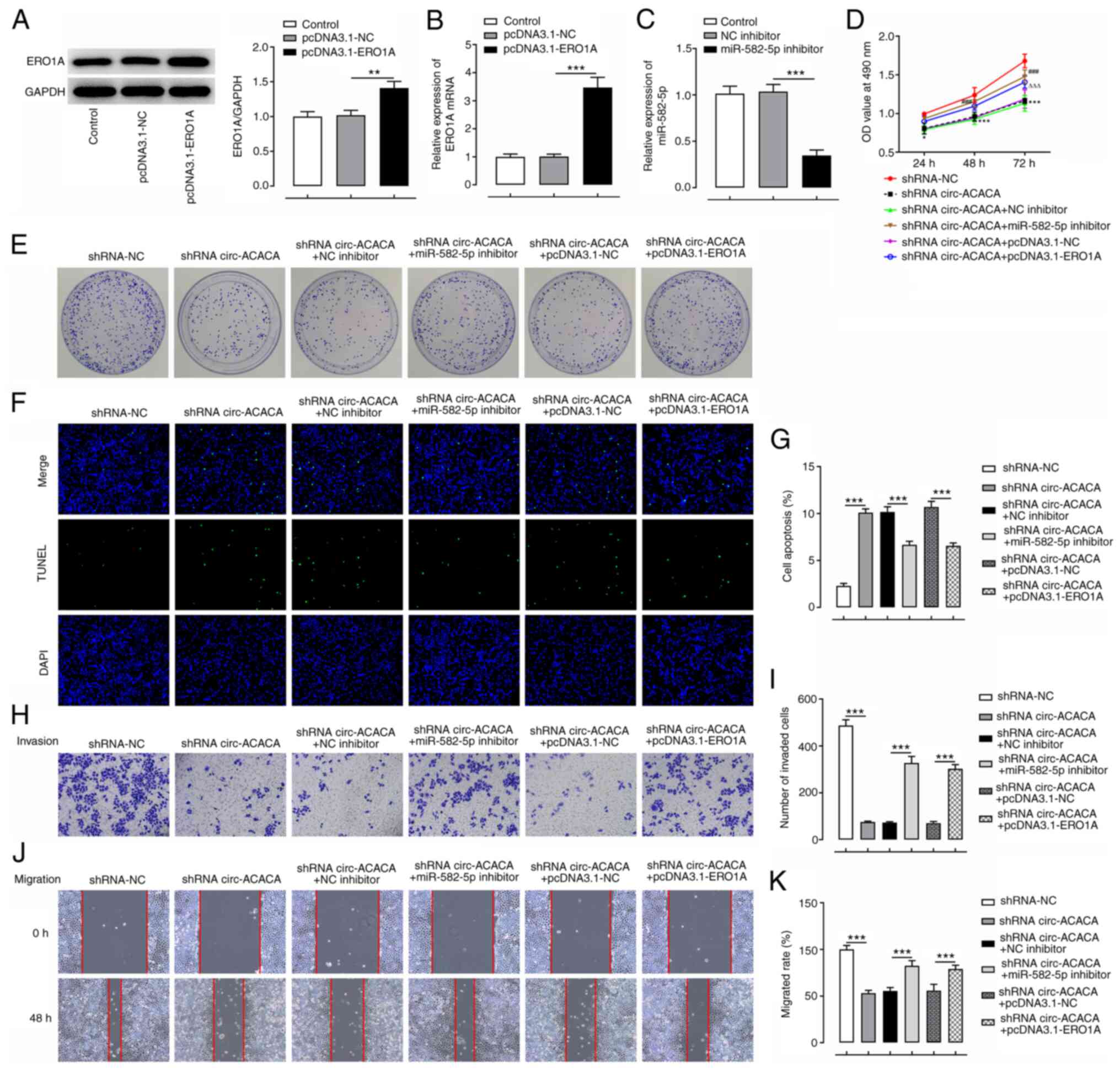 | Figure 6.circ-ACACA/miR-582-5p/ERO1A promotes
the proliferation, invasion and migration, and inhibits the
apoptosis of cervical cancer cells. Expression levels of ERO1A were
examined by (A) western blotting and (B) RT-qPCR after
pcDNA3.1-ERO1A was transfected into HeLa cells. (C) miR-582-5p
expression was evaluated by RT-qPCR after transfection with
miR-582-5p inhibitor. **P<0.01, ***P<0.001. (D) Cell
viability was detected using an MTT assay. *P<0.05,
***P<0.001 vs. shRNA-NC; ###P<0.001 vs. shRNA
circ-ACACA+NC inhibitor; ΔΔΔP<0.001 vs. shRNA
circ-ACACA+pcDNA 3.1-NC. (E) Cell proliferation was determined
using a colony formation assay. (F) Cell apoptosis was evaluated
using TUNEL staining. Magnification, ×200. (G) The apoptosis rate
of TUNEL assay. (H) Cell invasion was evaluated by Transwell assay.
(I) The number of invaded cells. Magnification, ×100. (J) Cell
migration was assessed by wound healing assay. (K) The cell
migration rate. Magnification, ×100. ***P<0.001. circ-ACACA,
circular RNA acetyl-CoA-carboxylase α; ERO1A, endoplasmic reticulum
disulphide oxidase 1α; miR, microRNA; NC, negative control; OD,
optical density; RT-qPCR, reverse transcription-quantitative PCR;
shRNA, short hairpin RNA. |
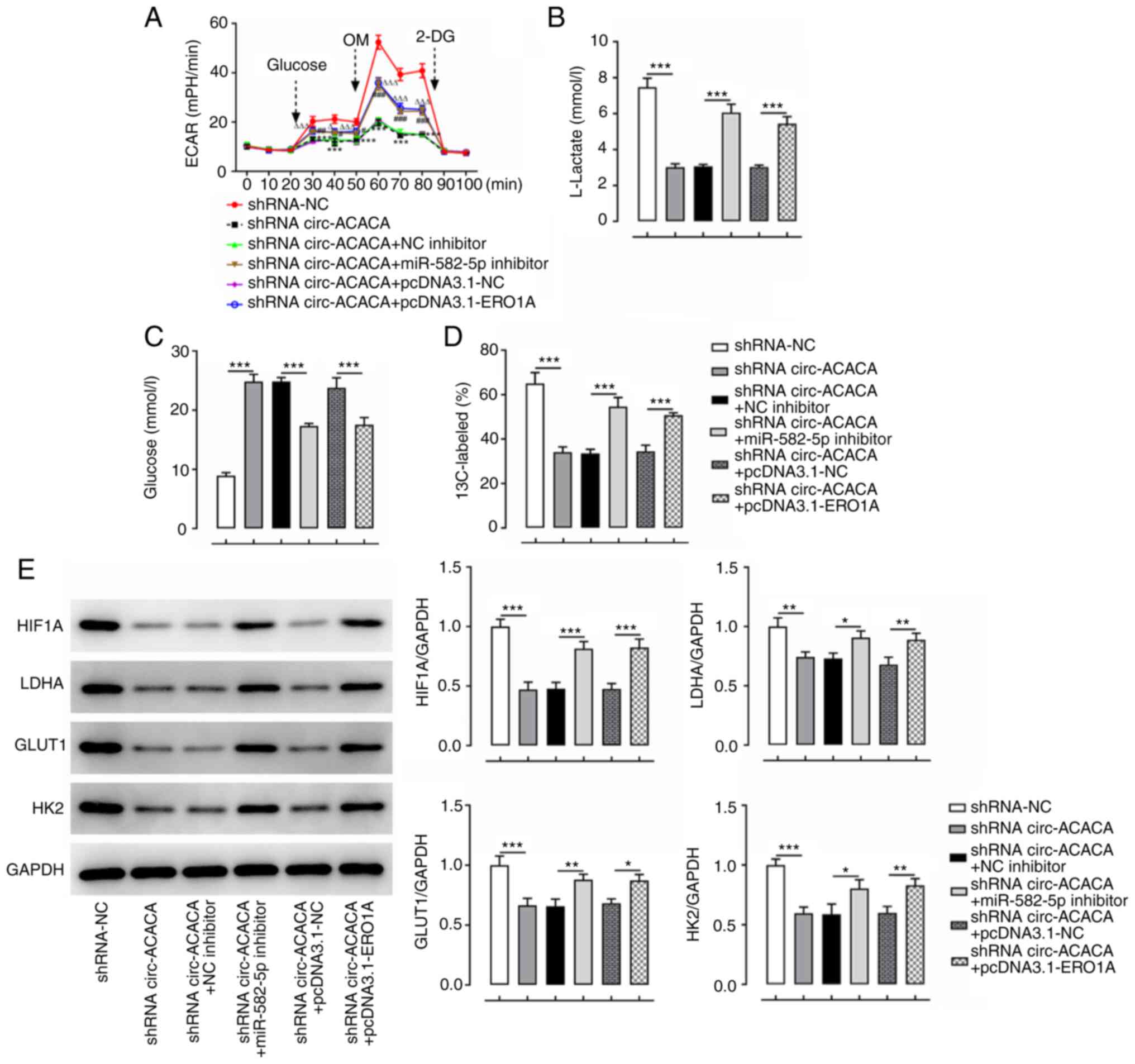 | Figure 7.circ-ACACA/miR-582-5p/ERO1A promotes
the glycolysis of cervical cancer cells. (A) ECAR after HeLa cells
were transfected with shRNA-circ-ACACA and miR-582-5p inhibitor or
pcDNA3.1-ERO1A. *P<0.05, **P<0.01, ***P<0.001 vs.
shRNA-NC; #P<0.05, ##P<0.01,
###P<0.001 vs. shRNA circ-ACACA+NC inhibitor;
ΔP<0.05 and ΔΔΔP<0.001 vs. shRNA
circ-ACACA+pcDNA3.1-NC. (B) Lactate levels and (C) glucose content
were assessed with commercially available kits. (D) Metabolite
analysis and (E) glycolysis-related protein expression after HeLa
cells were transfected with shRNA-circ-ACACA and miR-582-5p
inhibitor or pcDNA3.1-ERO1A. *P<0.05, **P<0.01,
***P<0.001. circ-ACACA, circular RNA acetyl-CoA-carboxylase α;
ECAR, extracellular acidification rate; ERO1A, endoplasmic
reticulum disulphide oxidase 1α; GLUT1, glucose transporter type 1;
HIF1A, hypoxia-inducible factor 1α; HK2, hexokinase-2; LDHA,
lactate dehydrogenase A; miR, microRNA; NC, negative control;
shRNA, short hairpin RNA; OM, oligomycin; 2-DG,
2-deoxy-D-glucose. |
Discussion
ncRNAs have been suggested to serve important roles
in the progression of numerous types of tumor, such as prostate,
non-small cell lung, colorectal and cervical cancer (25–29).
circRNAs, which are single-stranded ncRNAs, have been revealed to
be involved in the development of multiple cancer types (30). Due to the increased stability,
evolutionary conservation and high abundance of circRNAs, they have
been established as essential players in numerous physiological and
pathophysiological processes (31).
Previous research has demonstrated that circ-ACACA expression is
upregulated in NSCLC tissues and cells, and silencing of circ-ACACA
hinders the cellular functions of NSCLC cells, including
proliferation, invasion and migration, via regulation of the
miR-1183 and PI3K/AKT signaling pathway (9). Therefore, it was hypothesized that
circ-ACACA may also serve as an oncogene to promote the
tumorigenesis of CC. The results of the present study revealed that
circ-ACACA expression was significantly upregulated in CC cells,
and circ-ACACA was expressed at a higher level in the cytoplasm
compared with the nucleus. Silencing of circ-ACACA inhibited the
viability, proliferation, invasion and migration, and enhanced the
apoptosis of CC cells.
Alteration of energy metabolism, especially abnormal
activation of the glycolysis pathway has been observed in diverse
human cancer types, including CC (32–36).
Cancer cells are dependent on the glycolytic pathway to meet their
high energy demands; thus, increased glycolysis is a hallmark of
cancer cells (37). Notably,
knockdown of circ-ACACA could hinder proliferation and migration of
NSCLC cells and also reduce the glycolysis rate (9). In the present study, the effect of
circ-ACACA on glycolysis in CC cells was determined. Higher lactate
levels have been previously identified to increase the risk of
recurrence and metastasis in patients with CC, and are associated
with poor overall survival (38).
Furthermore, high lactate content in the tumor microenvironment has
been revealed to induce highly acidic conditions, which hinders the
function of several chemotherapeutic drugs, such as tamoxifen,
cisplatin and adriamycin (39,40). In
the present study, silencing of circ-ACACA reduced the ECAR and
lactate levels. Increased glucose uptake is another characteristic
of cancer cells (37), and the
present study revealed that glucose uptake was decreased in HeLa
cells transfected with shRNA-circ-ACACA. HIF-1A, LDHA, GLUT1 and
HK2 serve important roles in glycolytic metabolism (39). The present study revealed that
transfection with shRNA-circ-ACACA downregulated the expression
levels of these factors, indicating that shRNA-circ-ACACA may
inhibit glycolysis. Therefore, these results indicated the
suppressive effect of silencing of circ-ACACA on glycolysis and the
progression of CC.
The unique cellular stability and ability of
circRNAs to sponge miRNAs and proteins suggests their potential as
novel molecular markers for targeted therapies for cancer (40). miRNAs are known to serve an important
role in the pathogenesis of most types of cancer (41). Efforts have been made to determine
the effect of miR-582 on cancer development (42). A previous study reported that
miR-582-5p is differentially expressed during the development of
multiple types of cancer, indicating that the expression levels of
miR-582-5p depend on the specific type of cancer tissue or cells
(42). The results of the present
study demonstrated that the expression levels of miR-582-5p were
downregulated in CC cells, and dual-luciferase reporter assays
verified the binding between circ-ACACA and miR-582-5p. RT-qPCR
analysis revealed that the silencing of circ-ACACA markedly
upregulated the expression levels of miR-582-5p, demonstrating the
negative association between circ-ACACA and miR-582-5p.
circRNAs have been reported to act as miRNA sponges
in circRNA/miRNA/mRNA signaling axes (43). In the present study, bioinformatics
analysis was used to predict that ERO1A could interact with
miR-582-5p, which prompted further investigations to determine the
role of ERO1A in CC cells. ERO1A, which is a protein involved in
the oxidative protein folding of molecules involved in cancer
progression, has been reported to be induced by hypoxia in HeLa
cells (44). The expression levels
of ERO1A have been reported to be upregulated in numerous types of
cancer cells compared with normal cells, such as breast, pancreatic
and colorectal cancer (19,17,22),
which was consistent with the present findings that CC cells
exhibited upregulated ERO1A expression compared with End1/E6E7
cells. Notably, transfection with the miR-582-5p mimic
downregulated the expression levels of ERO1A in HeLa cells. Whether
circ-ACACA affected the functions of HeLa cells via regulation of
the miR-582-5p/ERO1A signalling axis was subsequently investigated.
The results demonstrated that transfection with shRNA-circ-ACACA
decreased the proliferation, invasion and migration, and promoted
the apoptosis of HeLa cells, while these effects were reversed
following transfection with the miR-582-5p inhibitor or
pcDNA3.1-ERO1A.
In conclusion, to the best of our knowledge, the
present study was the first to investigate the role of circ-ACACA
in CC development and provided evidence to suggest that the
knockdown of circ-ACACA may decrease proliferation, invasion,
migration and glycolysis, and promote apoptosis in HeLa cells via
targeting of the miR-582-5p/ERO1A signaling axis. These results
highlight the potential of circ-ACACA as a novel biomarker for CC.
However, the lack of experiments regarding the clinical value of
circ-ACACA/miR-582-5p/ERO1A in CC tissue samples to determine its
clinical applicability, as well as in vivo antitumor effects
in an animal model are limitations of the present study.
Additionally, based on a previous study in NSCLC (9), the circ-ACACA/miR-1183/PI3K/protein
kinase B signaling pathway and whether there is a direct
interaction between circ-ACACA and miR-1183 should also be explored
in CC in future studies. Therefore, comprehensive analysis is
required in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors contributions
DH and CL interpreted the data and performed
experiments. DH collected the data, searched the literature,
designed the study and wrote the manuscript. CL revised the
manuscript. Both authors read and approval the final manuscript. DH
and CL confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan Y, Sheng W, Meng Y, Cao Y and Li R:
LncRNA PTENP1 inhibits cervical cancer progression by suppressing
miR-106b. Artif Cells Nanomed Biotechnol. 48:393–407. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fader AN: Surgery in cervical cancer. N
Engl J Med. 379:1955–1957. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan Y, Meng Y, Yang S, Wang L, Zhi W,
Lazare C, Cao C and Wu P: Screening of cervical cancer with
self-collected cervical samples and next-generation sequencing. Dis
Markers. 2018:48265472018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilusz JE and Sharp PA: Molecular biology.
A circuitous route to noncoding RNA. Science. 340:440–441. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jia YJ, Liu MJ and Wang SX: CircRNA
hsa_circRNA_0001776 inhibits proliferation and promotes apoptosis
in endometrial cancer via downregulating LRIG2 by sponging miR-182.
Cancer Cell Int. 20:4122020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang W, Liu T, Li TS and Zhao XD:
Hsa_circRNA_102002 facilitates metastasis of papillary thyroid
cancer through regulating miR-488-3p/HAS2 axis. Cancer Gene Ther.
28:279–293. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu W, Xi W, Li H, Yang M and Yao X:
Circular RNA circ-ACACA regulates proliferation, migration and
glycolysis in non-small-cell lung carcinoma via miR-1183 and
PI3K/PKB pathway. Int J Mol Med. 45:1814–1824. 2020.PubMed/NCBI
|
|
10
|
Liu J, Liu S, Deng X, Rao J, Huang K, Xu G
and Wang X: MicroRNA-582-5p suppresses non-small cell lung cancer
cells growth and invasion via downregulating NOTCH1. PLoS One.
14:e02176522019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang S, Zou C, Tang Y, Wa Q, Peng X, Chen
X, Yang C, Ren D, Huang Y, Liao Z, et al: miR-582-3p and miR-582-5p
suppress prostate cancer metastasis to bone by repressing TGF-β
signaling. Mol Ther Nucleic Acids. 16:91–104. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu J, Li W, Ning J, Yu W, Rao T and Cheng
F: Long noncoding RNA UCA1 targets miR-582-5p and contributes to
the progression and drug resistance of bladder cancer cells through
ATG7-mediated autophagy inhibition. Onco Targets Ther. 12:495–508.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L and Ma L: Upregulation of miR-582-5p
regulates cell proliferation and apoptosis by targeting AKT3 in
human endometrial carcinoma. Saudi J Biol Sci. 25:965–970. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Huang W, Ran Y, Xiong Y, Zhong Z,
Fan X, Wang Z and Ye Q: miR-582-5p inhibits proliferation of
hepatocellular carcinoma by targeting CDK1 and AKT3. Tumour Biol.
36:8309–8316. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka T, Kutomi G, Kajiwara T, Kukita K,
Kochin V, Kanaseki T, Tsukahara T, Hirohashi Y, Torigoe T, Okamoto
Y, et al: Cancer-associated oxidoreductase ERO1-α promotes immune
escape through up-regulation of PD-L1 in human breast cancer.
Oncotarget. 8:24706–24718. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Yang J, Lin C, Liu W, Huo Y, Yang
M, Jiang SH, Sun Y and Hua R: Endoplasmic reticulum
stress-dependent expression of ERO1L promotes aerobic glycolysis in
pancreatic cancer. Theranostics. 10:8400–8414. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han F, Xu Q, Zhao J, Xiong P and Liu J:
ERO1L promotes pancreatic cancer cell progression through
activating the Wnt/catenin pathway. J Cell Biochem. 119:8996–9005.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan W, Wang X, Liu T, Chen L, Han L, Xu J,
Jin G, Harada K, Lin Z and Ren X: Expression of endoplasmic
reticulum oxidoreductase 1-α in cholangiocarcinoma tissues and its
effects on the proliferation and migration of cholangiocarcinoma
cells. Cancer Manag Res. 11:6727–6739. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zilli F, Marques Ramos P, Auf der Maur P,
Jehanno C, Sethi A, Coissieux MM, Eichlisberger T, Sauteur L,
Rouchon A, Bonapace L, et al: The NFIB-ERO1A axis promotes breast
cancer metastatic colonization of disseminated tumour cells. EMBO
Mol Med. 13:e131622021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Li T, Zhang L, Shangguan F, Shi
G, Wu X, Cui Y, Wang X, Wang X, Liu Y, et al: Targeting the
functional interplay between endoplasmic reticulum oxidoreductin-1α
and protein disulfide isomerase suppresses the progression of
cervical cancer. EBioMedicine. 41:408–419. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seol SY, Kim C, Lim JY, Yoon SO, Hong SW,
Kim JW, Choi SH and Cho JY: Overexpression of Endoplasmic Reticulum
Oxidoreductin 1-α (ERO1L) is associated with poor prognosis of
gastric cancer. Cancer Res Treat. 48:1196–1209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takei N, Yoneda A, Sakai-Sawada K, Kosaka
M, Minomi K and Tamura Y: Hypoxia-inducible ERO1α promotes cancer
progression through modulation of integrin-β1 modification and
signalling in HCT116 colorectal cancer cells. Sci Rep. 7:93892017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamamoto T, Takano N, Ishiwata K, Ohmura
M, Nagahata Y, Matsuura T, Kamata A, Sakamoto K, Nakanishi T, Kubo
A, et al: Reduced methylation of PFKFB3 in cancer cells shunts
glucose towards the pentose phosphate pathway. Nat Commun.
5:34802014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guarnerio J, Zhang Y, Cheloni G, Panella
R, Mae Katon J, Simpson M, Matsumoto A, Papa A, Loretelli C, Petri
A, et al: Intragenic antagonistic roles of protein and circRNA in
tumorigenesis. Cell Res. 29:628–640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang G, Liu Y, Yang J, Wang H and Xing Z:
Inhibition of circ_0081234 reduces prostate cancer tumor growth and
metastasis via miR-1/MAP3K1 axis. J Gene Med. e33762021.PubMed/NCBI
|
|
27
|
Yang C, Shi J, Wang J, Hao D, An J and
Jiang J: Circ_0006988 promotes the proliferation, metastasis and
angiogenesis of non-small cell lung cancer cells by modulating
miR-491-5p/MAP3K3 axis. Cell Cycle. 20:1334–1346. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Li S, Zhang G and Han H:
Sevoflurane inhibits malignant progression of colorectal cancer via
hsa_circ_0000231-mediated miR-622. J Biol Res (Thessalon).
28:142021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xie J, Chen Q, Zhou P and Fan W: Circular
RNA hsa_circ_0000511 improves epithelial mesenchymal transition of
cervical cancer by regulating hsa-mir-296-5p/HMGA1. J Immunol Res.
2021:99645382021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ding L, Zhao Y, Dang S, Wang Y, Li X, Yu
X, Li Z, Wei J, Liu M and Li G: Circular RNA circ-DONSON
facilitates gastric cancer growth and invasion via NURF complex
dependent activation of transcription factor SOX4. Mol Cancer.
18:452019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Zheng F, Xiao X, Xie F, Tao D, Huang
C, Liu D, Wang M, Wang L, Zeng F and Jiang G: CircHIPK3 sponges
miR-558 to suppress heparanase expression in bladder cancer cells.
EMBO Rep. 18:1646–1659. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pelicano H, Martin DS, Xu RH and Huang P:
Glycolysis inhibition for anticancer treatment. Oncogene.
25:4633–4646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hua Q, Mi BM, Xu F, Wen J, Zhao L, Liu J
and Huang G: Hypoxia-induced lncRNA-AC020978 promotes proliferation
and glycolytic metabolism of non-small cell lung cancer by
regulating PKM2/HIF-1α axis. Theranostics. 10:4762–4778. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun SF, Li WW, Ma XM and Luan H: Long
noncoding RNA LINC00265 promotes glycolysis and lactate production
of colorectal cancer through regulating of miR-216b-5p/TRIM44 axis.
Digestion. 101:391–400. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shang RZ, Wang M, Dai B, Du J, Wang J, Liu
Z, Qu S, Yang X, Liu J, Xia C, et al: Long noncoding RNA SLC2A1-AS1
regulates aerobic glycolysis and progression in hepatocellular
carcinoma via inhibiting the STAT3/FOXM1/GLUT1 pathway. Mol Oncol.
14:1381–1396. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu J, Tan QQ and Lie T: USP22 promotes the
expression of GLUT1 and HK2 to facilitate growth and glycolysis in
cervical cancer cells. Eur J Gynaecol Oncol. 41:790–796. 2020.
View Article : Google Scholar
|
|
37
|
Yeung C, Gibson AE, Issaq SH, Oshima N,
Baumgart JT, Edessa LD, Rai G, Urban DJ, Johnson MS, Benavides GA,
et al: Targeting glycolysis through inhibition of lactate
dehydrogenase impairs tumor growth in preclinical models of ewing
sarcoma. Cancer Res. 79:5060–5073. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Walenta S, Wetterling M, Lehrke M,
Schwickert G, Sundfør K, Rofstad EK and Mueller-Klieser W: High
lactate levels predict likelihood of metastases, tumor recurrence,
and restricted patient survival in human cervical cancers. Cancer
Res. 60:916–921. 2000.PubMed/NCBI
|
|
39
|
Wang M, Wang W, Wang J and Zhang J:
MiR-182 promotes glucose metabolism by upregulating
hypoxia-inducible factor 1α in NSCLC cells. Biochem Biophys Res
Commun. 504:400–405. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kristensen LS, Hansen TB, Veno MT and
Kjems J: Circular RNAs in cancer: Opportunities and challenges in
the field. Oncogene. 37:555–565. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rupaimoole R, Calin GA, Lopez-Berestein G
and Sood AK: miRNA deregulation in cancer cells and the tumor
microenvironment. Cancer Discov. 6:235–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tian Y, Guan Y, Su Y, Luo W, Yang G and
Zhang Y: MiR-582-5p inhibits bladder cancer-genesis by suppressing
TTK Expression. Cancer Manag Res. 12:11933–11944. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tang W, Ji M, He G, Yang L, Niu Z, Jian M,
Wei Y, Ren L and Xu J: Silencing CDR1as inhibits colorectal cancer
progression through regulating microRNA-7. Onco Targets Ther.
10:2045–2056. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Takei N, Yoneda A, Kosaka M, Sakai-Sawada
K and Tamura Y: ERO1α is a novel endogenous marker of hypoxia in
human cancer cell lines. BMC Cancer. 19:5102019. View Article : Google Scholar : PubMed/NCBI
|