Over the past decade, tumor treatment has been
revolutionized by moving away from chemotherapy and radiation and
toward tumor immunotherapy and targeted therapy. Tumor
immunotherapy, which modulates immune responses against tumors, has
shown appreciable efficacy in multiple cancer types and is
considered to be a novel and promising therapy for tumors. However,
the efficacy of tumor immunotherapy has been found to be poor in
the majority of patients, despite its notable efficacy in in an
appreciable proportion of patients with cancer (1–3). It
has been reported that the hyporesponsiveness or unresponsiveness
of patients to tumor immunotherapy may be due to the heterogeneity
of the tumor immune microenvironment (TIME) (4,5). The
TIME and its components may vary widely during neoplastic
progression and among different patients, and these variations, as
well as the heterogeneity of the TIME and its components, have a
profound effect on the outcome of tumor immunotherapy (4,5). As
a result, it is crucial to understand the roles of the TIME and its
components during neoplastic progression and in different patients
in order to improve the efficacy of tumor immunotherapy. With the
deepening of research and the development of technology, our
understanding of the complexity and heterogeneity of the TIME and
its components and their effects on the response of patients to
tumor immunotherapy has also improved. Deeper analysis of the
complexity and heterogeneity of the TIME and its components is
likely to uncover advanced biomarkers that may prove useful in
identifying patient populations responsive to current tumor
immunotherapy, and will benefit the search for novel targets for
therapeutic modulation. The aim of the present review was to
provide a summary of the current knowledge centered around the
TIME, focusing on its components and their association with tumor
immunotherapy, in order to improve the ability to study, predict
and guide immunotherapeutic responsiveness and uncover novel
therapeutic targets.
Tumor cells, the dominant cellular components of the
TIME, play an important role in the TIME, and they can directly
inhibit the function of immune cells via secreting tumor antigens
or creating a microenvironment that is not conducive to the
metabolism of immune cells, thereby causing inactivation and
inhibition of immune cell function (6). The tumor cells can also inhibit the
function of immune cells through secreting inhibitory cytokines,
capturing chemokines, secreting VEGF, which can suppress dendritic
cell (DC) maturation and activate regulatory T cells (Tregs)
directly, and activating immune checkpoints (6,7).
Immune cells, also a dominant cellular component of
the TIME, serve an important role in the TIME, principally consist
of T cells, B cells, monocytes-macrophages, natural killer (NK)
cells, DCs and their subsets.
T cells, the main immune cells in the TIME, induce
an antitumor immune response by recognizing antigens on tumor
cells. The proportion and subsets of T cells in the TIME are the
major factors affecting tumor progression (8).
Exhaustive T cells, a special subset of T cells, are
characterized by dysmetabolic disorder, poor self-renewal ability,
piecemeal loss of function, as well as sustained high expression of
inhibitory immune checkpoints like cytotoxic lymphocyte antigen-4
(CTLA-4), programmed cell death protein-1 (PD-1), T-cell
immunoglobulin and mucin-domain containing-3 (TIM-3), lymphocyte
activation gene-3 (LAG-3) and T-cell immunoglobulin and ITIM domain
(TIGIT), among others (8,9). T-cell exhaustion can be classified
into ‘pre-exhaustion’ and ‘terminal exhaustion’ stages.
‘Pre-exhausted’ T cells retaining their T-cell function persist
in vivo for 30–40 days and eventually differentiate into
‘terminally exhausted’ T cells (10,11).
Studies show that ‘pre-exhausted’ T cells express PD-1,
T-cell-specific transcription factor-1 as well as the chemokine
receptor C-X-C chemokine receptor type 5 (12,13).
PD-1 blockers primarily act on ‘pre-exhausted’ rather than
‘terminally exhausted’ T cells (10). Patients with melanoma with more
‘pre-exhausted’ T cells respond better to immune checkpoint
blockade therapy for longer periods of time (10), indicating that increasing the
numbers of ‘pre-exhausted’ T cells may contribute to better
response to immune checkpoint blockers (10). Therefore, various approaches have
been employed in an attempt to convert ‘terminally exhausted’ T
cells into ‘pre-exhausted’ T cells or younger memory T cells
(9,14).
Abundant B cells may be found in tumors and
tumor-draining lymph nodes, and they are common immune cells of the
TIME (21). B cells, serving as
APCs or recruiting DCs, participate in antigen presentation and
consequently adjust T-cell differentiation and activation (22). The extent of infiltration by B
cells, particularly memory B cells and plasma cells, was shown to
be associated with the progression and prognosis of gastric cancer
(23). Antibody-induced
circulating immune complexes can inhibit antitumor immune response,
leading to poor prognosis in patients with pancreatic ductal
adenocarcinoma and bone marrow tumors (24,25).
Lymphotoxin secreted by B cells can accelerate tumor angiogenesis
via activating STAT3 signaling, which in turn promotes cell
proliferation in prostate cancer, melanoma and lung cancer
(26). B cells can also promote
bladder cancer metastasis by increasing the expression of
extracellular matrix and remodeling-related genes (27). B cells, by secreting TGF-β, promote
the production of reactive oxygen species and nitric oxide in
myeloid cells, as well as the transformation of CD4+ T
cells into Tregs, thereby suppressing the function of
CD4+ T, CD8+ T and NK cells, and accelerating
tumor growth and metastasis (26,28).
CD20 monoclonal antibody was found to restrain the function of
CD4+ and CD8+ T cells in melanoma (26).
In addition, B cells can directly destroy tumor
cells and participate in antitumor immunity; they also express
TNF-related apoptosis-inducing ligand to induce the lysis of
melanoma cells and granulosin B to trigger the lysis of breast
cancer cells, serving a protective role in patients with breast
cancer and melanoma (26,29). Furthermore, activated B cells
enhance T-cell-mediated antitumor responses in patients with
cervical cancer (30). These
findings suggest that B cells in the TIME have a dual function, as
they may promote as well as inhibit tumor growth.
NK cells, the predominant members of the innate
lymphocyte family, are a class of natural immune cells that exhibit
strong cytolytic activity against tumors (31). NK cells may be divided into the
immature subset of CD56+ CD16− cells that can
secrete a large quantity of cytokines, and the mature subset of
CD56− CD16+ cells that are strongly cytotoxic
(32,33). NK cells recognize and destroy
target cells via surface receptors, such as TIGIT, LAG-3 and PD-1.
Allogeneic NK cells can discern and kill acute myeloid leukemia
(AML) cells in hematopoietic stem cell transplantation (32), which is of significant therapeutic
value in AML (34,35). NK cell activity is modulated by
blocking NK cell immune checkpoints, as NK cells express multiple
immune checkpoint receptors, such as killer cell Ig-like receptor
and CD94/NKG2A, and express multiple immune checkpoints, including
TIM-3, TIGIT, CD96 and LAG-3, which can interact with their cognate
ligands on tumor cells or on other immune cells. Moreover, NK cells
are innate lymphoid cells that efficiently kill tumor cells without
MHC specificity (36). A novel
strategy often employed in tumor immunotherapy is through applying
NK cell immune checkpoint inhibitors (37). The IgG4 anti-NKG2A antibody
monalizumab was used to treat various solid tumors, and was shown
to be generally well-tolerated (37,38).
The combination of monalizumab and the PD-1/PD-L1 disrupting agent
durvalumab was used to treat colorectal cancer, which was also
well-tolerated. The disease in 11 patients was stable and the
disease control rate was 24% at 16 weeks in the expansion cohort.
In addition, the anti-EGFR antibody cetuximab is an established
therapeutic approach to squamous cell carcinoma of the head and
neck, acting through induction of antibody-dependent cytotoxicity
through the CD16 (FcγRIII) receptor expressed on NK cells (39). The rationale for this approach
relies on evidence that squamous cell carcinomas of the head and
neck are strongly positive for HLA-E and are infiltrated by NK
cells (40). This regimen was also
well-tolerated, characterized mostly by grade 1–2 adverse events,
with an overall response rate of 31% and disease stabilization rate
of 54% (38). Furthermore, it is
not necessary for NK cells to go through the process of antigen
recognition, which indicates that NK cells can eliminate tumor
cells without sensitization, preferentially eliminating tumor stem
cells (41). It must be pointed
out that the decrease in the number of NK cells may be associated
with cancer risk (42). Compared
with T cells, NK cells have a shorter persistence and may also
represent a safer and more effective adoptive immunotherapy for
solid tumors and hematological malignancies (43). Therefore, regulation of NK cell
function and enhancement of NK cell toxicity are the dominant means
of NK cell-based tumor immunotherapy (44,45).
DCs mobilize naive T cells differentiate into
effector cells and, thus, exert antitumor immunomodulatory effects
by recognizing foreign antigens. Lysosomal-associated membrane
glycoprotein 3-positive DCs, a mature subset of DCs, can express a
variety of immune-related ligands and regulate the functions of a
variety of lymphocytes and their subsets (46). Reducing the recruitment and the
number of CD103+ DC leads to poor infiltration and
dysfunction of CD8+ T cells in the TIME (8). Compared with the untreated control,
DC vaccines, alone or in combination with PD-1 inhibitors, have
shown better tumor control and milder toxicity compared with the
untreated control (47,48). The aforementioned findings indicate
that DCs can affect the function of other immune cells in TIME.
Tumor-associated macrophages (TAMs), the most
abundant population of tumor-infiltrating immune cells, refers to
the macrophages located in or near the tumor (49). In response to tumor antigen
stimulation, macrophages can differentiate into two subtypes: The
M1 subtype, which promotes antitumor immunity, and the M2 subtype,
which plays a role in tumor progression (49). TAMs, which tend to differentiate
into the M2 subtype, are involved in tumorigenesis and tumor
progression (50). It has been
reported that an increase in TAMs is associated with poor prognosis
in patients with cancer (51).
Nanocomposites can promote the transformation of M2 to M1
macrophages, suppress tumor angiogenesis, reshape the TIME, present
antigens to T cells, stimulate T cells to release cytokines,
stimulate NK cells to infiltrate to tumor cells, and activate
antitumor immune response to kill tumor cells (52,53).
Therefore, TAMs in the TIME may also affect the outcome of tumor
immunotherapy.
Fibroblasts, an important type of mesenchymal cells,
maintain organ structure and homeostasis by secreting cytokines,
chemokines, growth factors and extracellular matrix.
Carcinoma-associated fibroblasts (CAFs) are among the most
important immune cells in TIME, which attract and mobilize
immunocytes with inhibitory function through the release of
cytokines, such as IL-6 and TGF-β, as well as chemokines, such as
C-X-C motif chemokine ligand (CXCL)1, CXCL12 and C-C motif
chemokine ligand 2 (54). In
addition, CAFs attract macrophages, T cells and NK cells to the
tumor stroma (49) and they also
induce resident macrophages and neutrophils to differentiate into
M2 macrophages and N2 neutrophils, thereby serving an antitumor
immunosuppressive role (55). It
has been found that tumorigenic signals in melanoma interfere with
T-cell-mediated antitumor responses by regulating the phenotype of
CAFs (8). Paracrine signaling
between tumor cells and fibroblasts can lead to chemoresistance,
thereby negatively affecting chemotherapeutic efficacy in patients
with breast cancer (56). CAFs
induce phosphorylation of heat shock transcription factor-1 at
S326, as well as proliferation, epithelial-to-mesenchymal
transition and cancer stem cell-like transition of gallbladder
cancer (GBC) cells by secreting thrombospondin-4 and binding to
integrin α2, a transmembrane receptor on GBC cells (57). CAF exon LINC00659 accelerates the
proliferation, invasion and migration of colorectal cancer cells
via the microRNA-342-3p/annexin 2 axis (58). Activated CAFs promote the invasion
and migration of ovarian cancer cells via the TGF-β/collagen type
VI alpha 1 chain signaling pathway (59). These findings indicate that CAFs in
the TIME can affect the functions of other immune cells and
cytokines, the efficacy of tumor immunotherapy and tumor
growth.
Vascular endothelial cells, another non-immune cell
type in the TIME, highly express PD-L1, which suppress
CD8+ T-cell infiltration and facilitate
Foxp3+ T-cell aggregation, thus forming a type of
‘immunosuppressive barrier’ (60).
There are reports that anlotinib can downregulate the expression of
PD-L1 in vascular endothelial cells to suppress tumor growth
(60). These findings indicate
that the vascular endothelial cells in the TIME also affect the
functions of other immune cells and immune checkpoints to affect
tumor immunotherapy.
IL-2, which activates and promotes the proliferation
of T cells and NK cells, is the most promising cytokine in tumor
immunotherapy (49). IL-2
activates aromatic hydrocarbon receptors to regulate
CD8+ T-cell failure (61). Second-generation IL-2 based on
CD122 can induce the production of NARA1 interleukin. NARA1 with
longer half-life in vivo can completely avoid binding to
CD25 and stimulate proliferation and activation of CD8+
T cells and NK cells more effectively (62).
The application of immune checkpoint inhibitors has
been a novel approach to and research hotspot in tumor
immunotherapy in recent years, and has also shown considerable
efficacy in the treatment of several tumors (66–68).
First-generation immune checkpoint blockade tumor immunotherapy
based on antibodies acts by blocking the interaction between
receptors and/or ligand molecules, such as CTLA-4 and PD-1, that
are involved in T-cell activation or reduced function (8).
PD-1, a member of the CD28 family, has two ligands
with different expression patterns, PD-L1 (B7-H1) and PD-L2
(49), which can be used not only
as an index of predicting tumor occurrence, but also as tumor
prognostic index (69,70). The combination of PD-1 with PD-L1,
through the PI3K-AKT signaling pathway, releases immunosuppressive
signals to inhibit the activation and proliferation of T cells, as
well as to induce T-cell tolerance and exhaustion (49,71).
It can also directly affect the proliferation of cytotoxic T cells
through the SH2 containing protein tyrosine phosphatase-2/Ras/MAPK
signaling pathway (72). PD-L1
alone or in combination with LAG-3 blocker and CXCL13 can
contribute to delayed tumor growth and is associated with survival
benefits (73,74). It has been pointed out that the
monovalent bispecific antibody MEDI5752 can suppress PD-1 and
CTLA-4, thus enhancing the blocking effect on activated
PD-1+ T cells (75).
Tumor immunotherapy with CTLA-4 and PD-1 monoclonal antibodies to
block immune checkpoints has also achieved notable efficacy in a
number of tumors (6,20). Pembrolizumab and durvalumab, as
PD-1 monoclonal antibodies, have also shown marked efficacy in
patients with esophageal cancer and are available as second-line
treatment in patients with esophageal squamous cell carcinoma
(ESCC) (76), which has been
approved for clinical use for ESCC in Japan (46).
LAG-3 is an immune checkpoint expressed on the
surface of various lymphocytes, including activated T cells, Tregs,
B cells, NK cells and plasmacytoid DCs (49). LAG-3, which has a similar structure
to CD4 but higher affinity to APCs, can compete with MHC II complex
antigens, thereby inhibiting T-cell activation (49). Anti-LAG-3 monoclonal antibody, as
well as bispecific antibodies targeting LAG-3 and PD-L1, can block
the immunosuppression mediated by LAG-3 and PD-L1, thus enhancing
the activity of T cells, in order to suppress cell proliferation
and tumor growth, which may prove beneficial for numerous patients
with cancer (77,78).
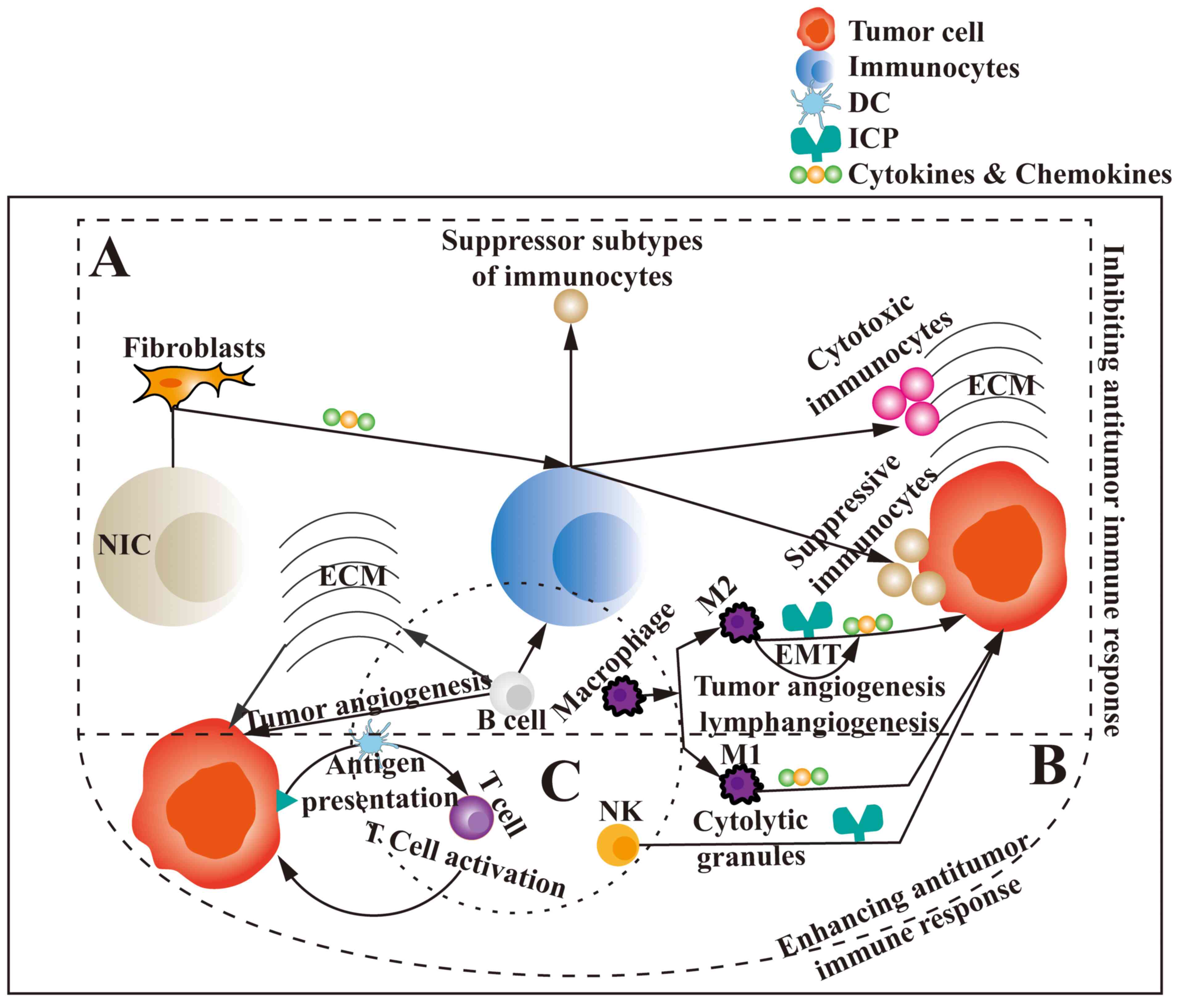
In conclusion, the TIME is complex and has numerous
components that may serve as tumor immunotherapy targets;
furthermore, these components can interact and affect one another,
which greatly affects the efficiency of tumor immunotherapy
(Fig. 1). Moreover, the TIME
differs among different patients and at different time points.
Therefore, fully elucidating the changes occurring in the TIME and
its components during tumor development in specific patients may be
the key to administering effective tumor immunotherapy. Further
research must be conducted in follow-up studies on tumor
immunotherapy, in order to improve the specificity and
effectiveness of this treatment modality in cancer management.
Not applicable.
The present study was supported by grants from Natural Science
Foundation of Guizhou Province (CN) (grant no. Qian ke he cheng guo
[2019] 4444), Beijing Medical and health public welfare foundation
(grant no. YWJKJJHKYJJ-B184054) and Key Project of the Science and
Technology Ministry of China (grant no.
2017ZXl0203206-005-002).
Not applicable.
CZ contributed to literature review and search, as
well as to the writing of the manuscript. QL and YX were involved
in the design, acquisition and analysis of data and drafting the
manuscript.. XG and WL contributed to the design of the study,
interpretation of data and revised the manuscript critically for
important intellectual content. All authors have read and approved
the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Hao M, Hou S, Li W, Li K, Xue L, Hu Q, Zhu
L, Chen Y, Sun H, Ju C and Zhang C: Combination of metabolic
intervention and T cell therapy enhances solid tumor immunotherapy.
Sci Transl Med. 12:eaaz66672020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perets R, Bar J, Rasco DW, Ahn MJ, Yoh K,
Kim DW, Nagrial A, Satouchi M, Lee DH, Spigel DR, et al: Safety and
efficacy of quavonlimab, a novel anti-CTLA-4 antibody (MK-1308), in
combination with pembrolizumab in first-line advanced
non-small-cell lung cancer. Ann Oncol. 32:395–403. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keung EZ, Lazar AJ, Torres KE, Wang WL,
Cormier JN, Ashleigh Guadagnolo B, Bishop AJ, Lin H, Hunt KK, Bird
J, et al: Phase II study of neoadjuvant checkpoint blockade in
patients with surgically resectable undifferentiated pleomorphic
sarcoma and dedifferentiated liposarcoma. BMC Cancer. 18:9132018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thorsson V, Gibbs DL, Brown SD, Wolf D,
Bortone DS, Ou Yang TH, Porta-Pardo E, Gao GF, Plaisier CL, Eddy
JA, et al: The immune landscape of cancer. Immunity.
48:812–830.e14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eckstein M and Gupta S: New insights in
predictive determinants of the tumor immune microenvironment for
immune checkpoint inhibition: A never ending story? Ann Transl Med.
7 (Suppl 3):S1352019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beckermann KE, Dudzinski SO and Rathmell
JC: Dysfunctional T cell metabolism in the tumor microenvironment.
Cytokine Growth Factor Rev. 35:7–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fukumura D, Kloepper J, Amoozgar Z, Duda
DG and Jain RK: Enhancing cancer immunotherapy using
antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol.
15:325–340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Binnewies M, Roberts EW, Kersten K, Chan
V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI,
Ostrand-Rosenberg S, Hedrick CC, et al: Understanding the tumor
immune microenvironment (TIME) for effective therapy. Nat Med.
24:541–550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ando M, Ito M, Srirat T, Kondo T and
Yoshimura A: Memory T cell, exhaustion, and tumor immunity. Immunol
Med. 43:1–9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miller BC, Sen DR, Al Abosy R, Bi K,
Virkud YV, LaFleur MW, Yates KB, Lako A, Felt K, Naik GS, et al:
Author correction: Subsets of exhausted CD8+ T cells
differentially mediate tumor control and respond to checkpoint
blockade. Nat Immunol. 20:15562019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miller BC, Sen DR, Al Abosy R, Bi K,
Virkud YV, LaFleur MW, Yates KB, Lako A, Felt K, Naik GS, et al:
Subsets of exhausted CD8+ T cells differentially mediate
tumor control and respond to checkpoint blockade. Nat Immunol.
20:326–336. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He R, Hou S, Liu C, Zhang A, Bai Q, Han M,
Yang Y, Wei G, Shen T, Yang X, et al: Follicular CXCR5- expressing
CD8(+) T cells curtail chronic viral infection. Nature.
537:412–428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Im SJ, Hashimoto M, Gerner MY, Lee J,
Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al:
Defining CD8+ T cells that provide the proliferative
burst after PD-1 therapy. Nature. 537:417–421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kagoya Y, Tanaka S, Guo T, Anczurowski M,
Wang CH, Saso K, Butler MO, Minden MD and Hirano N: A novel
chimeric antigen receptor containing a JAK-STAT signaling domain
mediates superior antitumor effects. Nat Med. 24:352–359. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho MK, Park JG, Iwata H and Kim EY:
2,3,7,8-Tetrachlorodibenzo-p-dioxin prompted differentiation to
CD4+CD8−CD25+ and
CD4+CD8+CD25+ Tregs and altered
expression of immune-related genes in the thymus of chicken
embryos. Ecotoxicol Environ Saf. 211:1119472021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pompura SL, Wagner A, Kitz A, LaPerche J,
Yosef N, Dominguez-Villar M and Hafler DA: Oleic acid restores
suppressive defects in tissue-resident FOXP3 Tregs from patients
with multiple sclerosis. J Clin Invest. 131:e1385192021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shevyrev D and Tereshchenko V: Treg
heterogeneity, function, and homeostasis. Front Immunol.
10:31002020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wherry EJ and Kurachi M: Molecular and
cellular insights into T cell exhaustion. Nat Rev Immunol.
15:486–499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kurtulus S, Sakuishi K, Ngiow SF, Joller
N, Tan DJ, Teng MW, Smyth MJ, Kuchroo VK and Anderson AC: TIGIT
predominantly regulates the immune response via regulatory T cells.
J Clin Invest. 125:4053–4062. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zarour HM: Reversing T-cell dysfunction
and exhaustion in cancer. Clin Cancer Res. 22:1856–1864. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuen GJ, Demissie E and Pillai S: B
lymphocytes and cancer: A love-hate relationship. Trends Cancer.
2:747–757. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van de Veen W, Globinska A, Jansen K,
Straumann A, Kubo T, Verschoor D, Wirz OF, Castro-Giner F, Tan G,
Rückert B, et al: A novel proangiogenic B cell subset is increased
in cancer and chronic inflammation. Sci Adv. 6:eaaz35592020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ni Z, Xing D, Zhang T, Ding N, Xiang D,
Zhao Z, Qu J, Hu C, Shen X, Xue X and Zhou J: Tumor-infiltrating B
cell is associated with the control of progression of gastric
cancer. Immunol Res. 69:43–52. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Satoh M, Takano S, Sogawa K, Noda K,
Yoshitomi H, Ishibashi M, Mogushi K, Takizawa H, Otsuka M, Shimizu
H, et al: Immune-complex level of cofilin-1 in sera is associated
with cancer progression and poor prognosis in pancreatic cancer.
Cancer Sci. 108:795–803. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakamura K and Smyth MJ: Myeloid
immunosuppression and immune checkpoints in the tumor
microenvironment. Cell Mol Immunol. 17:1–12. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rubio AJ, Porter T and Zhong X: Duality of
B Cell-CXCL13 axis in tumor immunology. Front Immunol.
11:5211102020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ou Z, Wang Y, Liu L, Li L, Yeh S, Qi L and
Chang C: Tumor microenvironment B cells increase bladder cancer
metastasis via modulation of the IL-8/androgen receptor (AR)/MMPs
signals. Oncotarget. 6:26065–26078. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bodogai M, Moritoh K, Lee-Chang C,
Hollander CM, Sherman-Baust CA, Wersto RP, Araki Y, Miyoshi I, Yang
L, Trinchieri G and Biragyn A: Immunosuppressive and prometastatic
functions of myeloid-derived suppressive cells rely upon education
from tumor-associated B cells. Cancer Res. 75:3456–3465. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tabuchi Y, Shimoda M, Kagara N, Naoi Y,
Tanei T, Shimomura A, Shimazu K, Kim SJ and Noguchi S: Protective
effect of naturally occurring anti-HER2 autoantibodies on breast
cancer. Breast Cancer Res Treat. 157:55–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rossetti RAM, Lorenzi NPC, Yokochi K, Rosa
MBSF, Benevides L, Margarido PFR, Baracat EC, Carvalho JP, Villa LL
and Lepique AP: B lymphocytes can be activated to act as antigen
presenting cells to promote anti-tumor responses. PLoS One.
13:e01990342018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bald T, Krummel MF, Smyth MJ and Barry KC:
The NK cell-cancer cycle: Advances and new challenges in NK
cell-based immunotherapies. Nat Immunol. 21:835–847. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tarazona R, Lopez-Sejas N, Guerrero B,
Hassouneh F, Valhondo I, Pera A, Sanchez-Correa B, Pastor N, Duran
E, Alonso C and Solana R: Current progress in NK cell biology and
NK cell-based cancer immunotherapy. Cancer Immunol Immunother.
69:879–899. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Del Zotto G, Marcenaro E, Vacca P, Sivori
S, Pende D, Della Chiesa M, Moretta F, Ingegnere T, Mingari MC,
Moretta A and Moretta L: Markers and function of human NK cells in
normal and pathological conditions. Cytometry B Clin Cytom.
92:100–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Handgretinger R, Lang P and André MC:
Exploitation of natural killer cells for the treatment of acute
leukemia. Blood. 127:3341–3349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Myers JA and Miller JS: Exploring the NK
cell platform for cancer immunotherapy. Nat Rev Clin Oncol.
18:85–100. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim N and Kim HS: Targeting checkpoint
receptors and molecules for therapeutic modulation of natural
killer cells. Front Immunol. 9:20412018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Minetto P, Guolo F, Pesce S, Greppi M,
Obino V, Ferretti E, Sivori S, Genova C, Lemoli RM and Marcenaro E:
Harnessing NK cells for cancer treatment. Front Immunol.
10:28362019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tinker AV, Hirte HW, Provencher D, Butler
M, Ritter H, Tu D, Azim HA Jr, Paralejas P, Grenier N, Hahn SA, et
al: Dose-ranging and cohort-expansion study of monalizumab
(IPH2201) in patients with advanced gynecologic malignancies: A
Trial of the Canadian cancer trials group (CCTG): IND221. Clin
Cancer Res. 25:6052–6060. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Metes D, Galatiuc C, Moldovan I, Morel PA,
Chambers WH, DeLeo AB, Rabinowich H, Schall R, Whiteside TL, Sulica
A, et al: Expression and function of Fc gamma RII on human natural
killer cells. Nat Immun. 13:289–300. 1994.PubMed/NCBI
|
|
40
|
Braud VM, Allan DS, O'Callaghan CA,
Söderström K, D'Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI,
Phillips JH, et al: HLA-E binds to natural killer cell receptors
CD94/NKG2A, B and C. Nature. 391:795–799. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Grossenbacher SK, Canter RJ and Murphy WJ:
Natural killer cell immunotherapy to target stem-like tumor cells.
J Immunother Cancer. 4:192016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Imai K, Matsuyama S, Miyake S, Suga K and
Nakachi K: Natural cytotoxic activity of peripheral-blood
lymphocytes and cancer incidence: An 11-year follow-up study of a
general population. Lancet. 356:1795–1799. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lupo KB and Matosevic S: Natural killer
cells as allogeneic effectors in adoptive cancer immunotherapy.
Cancers (Basel). 11:7692019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Al-Attar A, Presnell SR, Clasey JL, Long
DE, Walton RG, Sexton M, Starr ME, Kern PA, Peterson CA and Lutz
CT: human body composition and immunity: Visceral adipose tissue
produces IL-15 and muscle strength inversely correlates with NK
Cell function in elderly humans. Front Immunol. 9:4402018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang LX, Tong X, Li C, Giddens JP and Li
T: Glycoengineering of antibodies for modulating functions. Annu
Rev Biochem. 88:433–459. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Baba Y, Nomoto D, Okadome K, Ishimoto T,
Iwatsuki M, Miyamoto Y, Yoshida N and Baba H: Tumor immune
microenvironment and immune checkpoint inhibitors in esophageal
squamous cell carcinoma. Cancer Sci. 111:3132–3141. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ding Z, Li Q, Zhang R, Xie L, Shu Y, Gao
S, Wang P, Su X, Qin Y, Wang Y, et al: Personalized neoantigen
pulsed dendritic cell vaccine for advanced lung cancer. Signal
Transduct Target Ther. 6:262021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Teng CF, Wang T, Shih FY, Shyu WC and Jeng
LB: Therapeutic efficacy of dendritic cell vaccine combined with
programmed death 1 inhibitor for hepatocellular carcinoma. J
Gastroenterol Hepatol. 36:1988–1996. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jia Y, Liu L and Shan B: Future of immune
checkpoint inhibitors: Focus on tumor immune microenvironment. Ann
Transl Med. 8:10952020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen Y, Song Y, Du W, Gong L, Chang H and
Zou Z: Tumor-associated macrophages: An accomplice in solid tumor
progression. J Biomed Sci. 26:782019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hwang I, Kim JW, Ylaya K, Chung EJ, Kitano
H, Perry C, Hanaoka J, Fukuoka J, Chung JY and Hewitt SM:
Tumor-associated macrophage, angiogenesis and lymphangiogenesis
markers predict prognosis of non-small cell lung cancer patients. J
Transl Med. 18:4432020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Han S, Wang W, Wang S, Yang T, Zhang G,
Wang D, Ju R, Lu Y, Wang H and Wang L: Tumor microenvironment
remodeling and tumor therapy based on M2-like tumor associated
macrophage-targeting nano-complexes. Theranostics. 11:2892–2916.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shan H, Dou W, Zhang Y and Qi M: Targeted
ferritin nanoparticle encapsulating CpG oligodeoxynucleotides
induces tumor-associated macrophage M2 phenotype polarization into
M1 phenotype and inhibits tumor growth. Nanoscale. 12:22268–22280.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Costa A, Kieffer Y, Scholer-Dahirel A,
Pelon F, Bourachot B, Cardon M, Sirven P, Magagna I, Fuhrmann L,
Bernard C, et al: Fibroblast heterogeneity and immunosuppressive
environment in human breast cancer. Cancer Cell. 33:463–479.e10.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
De Jaeghere EA, Denys HG and De Wever O:
Fibroblasts fuel immune escape in the tumor microenvironment.
Trends Cancer. 5:704–723. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Maia A, Gu Z, Koch A, Berdiel-Acer M, Will
R, Schlesner M and Wiemann S: IFNβ1 secreted by breast cancer cells
undergoing chemotherapy reprograms stromal fibroblasts to support
tumour growth after treatment. Mol Oncol. 15:1308–1329. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shi Y, Sun L, Zhang R, Hu Y, Wu Y, Dong X,
Dong D, Chen C, Geng Z, Li E and Fan Y: Thrombospondin 4/integrin
α2/HSF1 axis promotes proliferation and cancer stem-like traits of
gallbladder cancer by enhancing reciprocal crosstalk between
cancer-associated fibroblasts and tumor cells. J Exp Clin Cancer
Res. 40:142021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhou L, Li J, Tang Y and Yang M: Exosomal
LncRNA LINC00659 transferred from cancer-associated fibroblasts
promotes colorectal cancer cell progression via miR-342-3p/ANXA2
axis. J Transl Med. 19:82021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang Y, Liu Z, Yang X, Lu W, Chen Y, Lin
Y, Wang J, Lin S and Yun JP: H3K27 acetylation activated-COL6A1
promotes osteosarcoma lung metastasis by repressing STAT1 and
activating pulmonary cancer-associated fibroblasts. Theranostics.
11:1473–1492. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu S, Qin T, Liu Z, Wang J, Jia Y, Feng
Y, Gao Y and Li K: Anlotinib alters tumor immune microenvironment
by downregulating PD-L1 expression on vascular endothelial cells.
Cell Death Dis. 11:3092020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu Y, Zhou N, Zhou L, Wang J, Zhou Y,
Zhang T, Fang Y, Deng J, Gao Y, Liang X, et al: IL-2 regulates
tumor-reactive CD8+ T cell exhaustion by activating the
aryl hydrocarbon receptor. Nat Immunol. 22:358–369. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sahin D, Arenas-Ramirez N, Rath M, Karakus
U, Hümbelin M, van Gogh M, Borsig L and Boyman O: An IL-2-grafted
antibody immunotherapy with potent efficacy against metastatic
cancer. Nat Commun. 11:64402020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Renavikar PS, Sinha S, Brate AA,
Borcherding N, Crawford MP, Steward-Tharp SM and Karandikar NJ:
IL-12-induced immune suppressive deficit during CD8+
T-cell differentiation. Front Immunol. 11:5686302020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tucker CG, Mitchell JS, Martinov T,
Burbach BJ, Beura LK, Wilson JC, Dwyer AJ, Singh LM, Mescher MF and
Fife BT: Adoptive T Cell Therapy with IL-12-preconditioned
low-avidity T cells prevents exhaustion and results in enhanced T
cell activation, enhanced tumor clearance, and decreased risk for
autoimmunity. J Immunol. 205:1449–1460. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Agliardi G, Liuzzi AR, Hotblack A, De Feo
D, Núñez N, Stowe CL, Friebel E, Nannini F, Rindlisbacher L,
Roberts TA, et al: Intratumoral IL-12 delivery empowers CAR-T cell
immunotherapy in a pre-clinical model of glioblastoma. Nat Commun.
12:4442021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jiang X, Ren L, Tebon P, Wang C, Zhou X,
Qu M, Zhu J, Ling H, Zhang S, Xue Y, et al: Cancer-on-a-chip for
modeling immune checkpoint inhibitor and tumor interactions. Small.
17:e20042822021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Duchemann B, Pluvy J, Crestani B, Zalcman
G and Nunes H: Immune checkpoint blockade for patients with lung
cancer and idiopathic pulmonary fibrosis. Eur J Cancer.
145:179–182. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ceresoli GL and Pasello G: Immune
checkpoint inhibitors in mesothelioma: A turning point. Lancet.
397:348–349. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wakita A, Motoyama S, Nanjo H, Sato Y,
Yoshino K, Sasaki T, Kawakita Y, Liu J, Imai K, Saito H and
Minamiya Y: PD-L1 expression is a prognostic factor in patients
with thoracic esophageal cancer treated without adjuvant
chemotherapy. Anticancer Res. 37:1433–1441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yoshida H, Nomizo T, Ozasa H, Tsuji T,
Funazo T, Yasuda Y, Ajimizu H, Yamazoe M, Kuninaga K, Ogimoto T, et
al: PD-L1 polymorphisms predict survival outcomes in advanced
non-small-cell lung cancer patients treated with PD-1 blockade. Eur
J Cancer. 144:317–325. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tan CL, Kuchroo JR, Sage PT, Liang D,
Francisco LM, Buck J, Thaker YR, Zhang Q, McArdel SL, Juneja VR, et
al: PD-1 restraint of regulatory T cell suppressive activity is
critical for immune tolerance. J Exp Med. 218:e201822322021.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Liotti F, Kumar N, Prevete N, Marotta M,
Sorriento D, Ieranò C, Ronchi A, Marino FZ, Moretti S, Colella R,
et al: PD-1 blockade delays tumor growth by inhibiting an intrinsic
SHP2/Ras/MAPK signalling in thyroid cancer cells. J Exp Clin Cancer
Res. 40:222021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Marcq E, Van Audenaerde JRM, De Waele J,
Merlin C, Pauwels P, van Meerbeeck JP, Fisher SA and Smits ELJ: The
search for an interesting partner to combine with PD-L1 Blockade in
Mesothelioma: Focus on TIM-3 and LAG-3. Cancers (Basel).
13:2822021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yang M, Lu J, Zhang G, Wang Y, He M, Xu Q,
Xu C and Liu H: CXCL13 shapes immunoactive tumor microenvironment
and enhances the efficacy of PD-1 checkpoint blockade in high-grade
serous ovarian cancer. J Immunother Cancer. 9:e0011362021.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Dovedi SJ, Elder MJ, Yang C, Sitnikova SI,
Irving L, Hansen A, Hair J, Jones DC, Hasani S, Wang B, et al:
Design and efficacy of a monovalent bispecific PD-1/CTLA-4 antibody
that enhances CTLA-4 blockade on PD-1+ activated T
cells. Cancer Discov. 11:1100–1117. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kojima T, Shah MA, Muro K, Francois E,
Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH, et al:
Randomized phase III KEYNOTE-181 study of pembrolizumab versus
chemotherapy in advanced esophageal cancer. J Clin Oncol.
38:4138–4148. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lecocq Q, Keyaerts M, Devoogdt N and
Breckpot K: The next-generation immune checkpoint LAG-3 and its
therapeutic potential in oncology: Third time's a charm. Int J Mol
Sci. 22:752020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Atkinson V, Khattak A, Haydon A, Eastgate
M, Roy A, Prithviraj P, Mueller C, Brignone C and Triebel F:
Eftilagimod alpha, a soluble lymphocyte activation gene-3 (LAG-3)
protein plus pembrolizumab in patients with metastatic melanoma. J
Immunother Cancer. 8:e0016812020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Harjunpää H and Guillerey C: TIGIT as an
emerging immune checkpoint. Clin Exp Immunol. 200:108–119. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Judge SJ, Darrow MA, Thorpe SW, Gingrich
AA, O'Donnell EF, Bellini AR, Sturgill IR, Vick LV, Dunai C,
Stoffel KM, et al: Analysis of tumor-infiltrating NK and T cells
highlights IL-15 stimulation and TIGIT blockade as a combination
immunotherapy strategy for soft tissue sarcomas. J Immunother
Cancer. 8:e0013552020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Han HS, Jeong S, Kim H, Kim HD, Kim AR,
Kwon M, Park SH, Woo CG, Kim HK, Lee KH, et al: TOX-expressing
terminally exhausted tumor-infiltrating CD8+ T cells are
reinvigorated by co-blockade of PD-1 and TIGIT in bladder cancer.
Cancer Lett. 499:137–147. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li W, Deng C, Yang H, Lu X, Li S, Liu X,
Chen F, Chen L, Shu X, Zhang L, et al: Expansion of circulating
peripheral TIGIT+CD226+ CD4 T cells with
enhanced effector functions in dermatomyositis. Arthritis Res Ther.
23:152021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Han JH, Cai M, Grein J, Perera S, Wang H,
Bigler M, Ueda R, Rosahl TW, Pinheiro E, LaFace D, et al: Effective
Anti-tumor response by TIGIT blockade associated with FcγR
Engagement and myeloid cell activation. Front Immunol.
11:5734052020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Simon S, Voillet V, Vignard V, Wu Z,
Dabrowski C, Jouand N, Beauvais T, Khammari A, Braudeau C, Josien
R, et al: PD-1 and TIGIT coexpression identifies a circulating CD8
T cell subset predictive of response to anti-PD-1 therapy. J
Immunother Cancer. 8:e0016312020. View Article : Google Scholar : PubMed/NCBI
|