Introduction
Bone sarcomas (BSs) and soft-tissue sarcomas (STSs)
are a heterogeneous group of mesenchymal malignancies with >50
histological subtypes. They are rare conditions, with estimated
incidences of STS and BS averaging 4–5 and 1 per 100,000 cases per
year, respectively, accounting for ~1% of all malignancies
(1). The rarity and diversity of
sarcomas are critical hurdles in a better understanding of sarcomas
and improving their therapeutic outcomes. The median overall
survival for advanced leiomyosarcoma is ~2 years, but for most
other advanced STS, it is shorter than 1 year and only ~10% of
patients survive for 5 years (2).
Most STSs do not respond well to cytotoxic chemotherapy and their
treatment options are limited and generally palliative, while the
expected benefits are tempered by significant side effects.
Therefore, novel therapeutic options are necessary to improve the
clinical outcomes of sarcomas (3).
Cancer immunotherapy is an attractive therapeutic approach, as it
exerts anti-tumor effects through mechanisms different from those
of conventional antitumor treatments. Thus, checkpoint inhibitors
and adoptive T-cell therapies have been investigated as novel
therapeutic options for sarcomas (3–6).
WT1 was initially isolated as a tumor suppressor
gene responsible for Wilms' tumor, a pediatric renal neoplasm
(7). However, WT1 is overexpressed
in various cancer types, such as leukemia (8,9), as
well as lung (10), colorectal
(11), bone sarcoma and STS
(12), and has been indicated to
have oncogenic roles in these cancer types (13). Due to the tumor-specific expression
and high immunogenicity of WT1, WT1-targeted immunotherapy has been
considered a promising novel therapeutic strategy for various
cancers. The WT1 protein is a ubiquitous tumor-associated antigen
(TAA) and ranks as the top protein in terms of clinical
immunotherapy usefulness among 75 TAAs (14). Consequently, our and other groups
have demonstrated the clinical utility of WT1-targeted
immunotherapies in multiple forms as a WT1 peptide cancer vaccine
(15–24), WT1 peptide-pulsed or WT1
mRNA-electroporated dendritic cell vaccine (25,26)
and WT1-specific T-cell receptor-transduced T-cell therapy
(27,28).
The induction of an immune response against the
target antigen is essential for clinical efficacy in WT1-targeted
cancer immunotherapy. In previous studies by our group, the
delayed-type hypersensitivity skin reaction and IgG antibody
production against a WT1 peptide were analyzed to evaluate the
induction of WT1-specific immune responses after administering the
WT1 peptide vaccine (15,16,18–23).
The WT1 peptide IgG antibody has been examined as an
immuno-monitoring marker indicating the activation of WT1-specific
T helper cell (Th) responses by the WT1 peptide vaccine.
Considering the essential roles of Th cells in the induction and
maintenance of anti-tumor immune responses, the WT1-235 peptide IgG
correlates with longer survival and may be a predictive marker in
patients with recurrent glioblastoma treated with the WT1-235
peptide vaccine (29).
Furthermore, B cells have been reported to correlate with a good
prognosis of patients with sarcoma receiving immunotherapy.
Petitprez et al (30)
reported that the presence of B cells in the tumor microenvironment
is the strongest prognostic factor for STS. These results indicate
the essential roles of B cells and humoral immune responses in
immunotherapy for sarcoma. However, the importance of humoral
immunity, including B-cell functions, in the efficacy of the WT1
peptide vaccine remains undetermined. IgM is the first antibody
isotype expressed during B-cell development and the first humoral
antibody response; furthermore, its production does not require Th
cells. Therefore, epitope-specific IgM antibodies may also be
helpful monitoring tools for humoral immune responses to tumor
antigens.
In the present study, the serum levels of IgG and
IgM against WT1 epitopes were examined in WT1-235 peptide
vaccine-treated sarcoma patients, focusing on the humoral immune
responses prior to WT1 peptide vaccine treatment. The WT1 epitopes
examined included WT1-235, the target epitope of the vaccine, and
two non-target WT1 epitopes, WT1-332 and WT1-271. The association
between WT1 epitope-specific antibodies and WT1-235-specific
cellular immune responses in the first month of vaccine therapy, as
well as the clinical outcomes of vaccine therapy, were also
analyzed.
Materials and methods
WT1-235 peptide cancer vaccine
WT1-235 peptide vaccine immunotherapy was
administered with approval from the Ethical Review Board of the
Osaka University Faculty of Medicine (Osaka, Japan). The
eligibility criteria included the following: Histologically
confirmed sarcoma not amenable to potentially curative therapies,
WT1 protein expression in tumor cells, human leukocyte antigen
(HLA)-A*24:02 positivity, age range of 16–85 years and good organ
function. The Good Manufacturing Practice-grade, 9-mer modified
WT1-derived peptide (mWT1-235; 235–243 a.a., CYTWNQMNL; Peptide
Institute and Multiple Peptide Systems) was used for immunization.
Patients were intradermally administered 3 mg of mWT1-235 peptide
emulsified with Montanide ISA51 adjuvant (Seppic) once per week for
12 consecutive weeks. After the 3-month treatment protocol, the WT1
peptide vaccine immunotherapy was continued until disease
progression or intolerable adverse events were observed. Trials
were registered at the University hospital Medical Information
Network (UMIN) Clinical Trials Registry (https://www.umin.ac.jp/ctr) as UMIN000002001
(registered on May 24, 2009) and then UMIN000015997 (registered on
December 20, 2014). The present study was approved by the ethical
review board of the Faculty of Medicine, Osaka University (Osaka,
Japan). Written informed consent was obtained from the participants
at Osaka University Hospital (Osaka, Japan). These clinical trials
are one-armed and therefore do not adhere to the Consolidated
Standards Of Reporting Trials statement (31). The present study was performed in
accordance with the Japanese Ethical Guidelines for Medical and
Biological Research Involving Human Subjects.
Patient assessment
Antitumor effects were assessed by determining the
response of target lesions on CT scans according to the Response
Evaluation Criteria in Solid Tumors v1.0 (32). When tumor progression was slowing
or inhibited during the treatment protocol period, WT1 peptide
vaccination was continued after the 3-month protocol, mostly at
intervals of 2–4 weeks. Safety was assessed by monitoring and
recording adverse events, vital signs, clinical chemistry,
hematology and urinalysis. Adverse events were graded according to
the Common Terminology Criteria for Adverse Events v3.0 (33).
Serum samples
Sera were obtained with written informed consent
from the patients at Osaka University Hospital (Osaka, Japan) at
the indicated time-points. Serum samples were stored at −20°C until
use.
Peripheral blood mononuclear cell
(PBMC) samples
Blood samples were obtained with written informed
consent from the patients. PBMCs were isolated from heparinized
whole blood using the standard Ficoll-Paque separation method and
were cryopreserved in liquid nitrogen until use.
ELISA
WT1 epitope-specific IgG and IgM antibodies in serum
were measured by ELISA as described previously (29). The amino acid sequences of the
capture antigen peptides for WT1-235, WT1-325 and WT1-271
antibodies were CMTWNQMNLPKK, CAYPGCNKRYFKLSHLQM and
YESDNHTTPILCGAQYRI, respectively. The peptides were synthesized by
PH Japan. Using a peptide coating kit (cat. no. MK100; Takara Bio,
Inc.), WT1 peptides or citrate (negative control) were immobilized
on the bottom surface of each well as capture antigens, according
to the manufacturer's instructions. After blocking with 1X Blocking
One (Nacalai Tesque), patient sera diluted 1:100 with the blocking
buffer from the peptide coating kit were added to each well and
incubated at 4°C overnight. All serum samples were measured in
duplicate. After washing with Tris-buffered saline containing
Tween-20 (TBST), horseradish peroxidase (HRP)-conjugated rabbit
anti-human IgG (cat. no. 309-035-003; Jackson ImmunoResearch
Europe, Ltd.) or HRP-conjugated goat anti-human IgM antibody (cat.
no. A80-100P; Bethyl Laboratories, Inc.), diluted at 1:2,000 in
TBST, was added to each well and incubated at room temperature for
2 h. After washing with TBST, corresponding third antibody,
HRP-conjugated goat anti-rabbit IgG (cat. no. ab6721; Abcam) or
HRP-conjugated rabbit anti-goat IgG antibody (cat. no. 546; MBL
International Co.), diluted 1:5,000 in TBST, was added to each well
and incubated at room temperature for 2 h. Bound WT1
epitope-specific IgG or IgM antibodies were colorimetrically
detected using 3,3′,5,5′-tetramethylbenzidine substrate (KPL,
Inc.). The absorbance was measured at 450 nm using a microplate
reader (MULTISKAN FC; Thermo Fisher Scientific, Inc.). The antibody
titer for each serum sample was determined as the average
absorbance value of the duplicate wells after subtracting the
absorbance value of the negative control well. The cutoff levels
for positivity for WT1-235 IgM, WT1-235 IgG, WT1-271 IgM, WT1-271
IgG, WT1-332 IgM and WT1-332 IgG were set at 0.10, 0.15, 0.13,
0.06, 0.06 and 0.15, respectively, based on the absorbance values
of the mean + 3× standard deviation from five independent assays in
negative control serum samples.
Enzyme-linked immunospot (ELISPOT)
assay
After hydrophilization, the bottom membrane of each
well in a 96-well filtration plate (Merck KGaA) was incubated with
capture antibodies, including anti-human IFN-γ monoclonal antibody
(anti-human IFN-γ mAb 1-D1K; final concentration, 15 µg/ml in PBS;
cat. no. 3420-3-250; Mabtech AB) and anti-human IL-10 monoclonal
antibody (anti-human IL-10 mAb; final concentration, 15 µg/ml in
PBS; cat. no. 3430-3-250; Mabtech AB), at 4°C overnight. After
blocking the membranes with 1X Blocking One (cat. no. 03953-95;
Nacalai Tesque, Inc.) at 37°C for 2 h, thawed PBMCs suspended in
FBS-free RPMI1640 medium (5×104 cells per 100 µl;
Nacalai Tesque, Inc.) were seeded in each well in triplicate and
the antigen peptide was added to the medium at a final
concentration of 10 µg/ml. The cells were incubated for antigenic
stimulation at 37°C for 48 h. After removing the cell suspension,
each membrane was incubated with the corresponding detection
antibodies: A biotinylated anti-human IFN-γ monoclonal antibody
(final concentration, 1.3 µg/ml; cat. no. 3420-6-250; Mabtech AB)
and a biotinylated anti-human IL-10 monoclonal antibody (final
concentration, 1.3 µg/ml; cat. no. 3430-6-250; Mabtech AB) in PBS
containing 1% bovine serum albumin (Nacalai Tesque, Inc.) and 0.05%
Tween-20 at 4°C overnight. After washing with PBS, each membrane
was incubated with alkaline phosphatase-conjugated streptavidin
(diluted 1:500 with PBS containing 0.05% Tween-20; cat. no. 3310-8;
Mabtech AB) at room temperature for 1 h. After washing both sides
of the membranes, the spots were colored using
5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium
solution (Nacalai Tesque, Inc.), followed by washing with deionized
water. After drying at 4°C overnight, the membranes were punched
out using an acrylic device ‘ELI 8’ (Create Ltd.) onto a scotch
tape. The membranes were then sandwiched with another strip of
scotch tape and scanned with a scanner (Canoscan LiDE200; Canon,
Inc.) at a resolution of 1,200 dpi. The generated digital images
were analyzed for spot counting with the assistance of particle
analysis using Image J software (version 1.50i; National Institutes
of Health). WT1 antigen-specific IFN-γ or IL-10
production/secretion by PBMCs was described as the antigen-specific
immune response (IR) index as follows: (Number of spot-forming
cells under antigen-stimulated test conditions)/(number of
spot-forming cells under antigen-free control conditions) (34). The cutoff level for positive
detection of antigen-specific cytokine production/secretion was 1.0
in the IR index.
Statistical analysis
Differences in the WT1-235 IgM, WT1-235 IgG, WT1-271
IgM, and WT1-271 IgG antibody titers were analyzed using Welch's
t-test. The antibody titers were expressed as the mean of
duplicated assay results in dot plots. The association between WT1
antibody production and clinical outcomes was analyzed using
Fisher's exact probability test and Cramer's V-test. P<0.05 was
considered to indicate a statistically significant difference in
Welch's t-test and Fisher's exact probability test. A P-value of
0.20-0.29 was considered to indicate moderate association in
Cramer's V-test. The statistical analysis was performed using
Statcel4 EXCEL Addin software (OMS Publishing Ltd.).
Results
Patient characteristics
In the present study, WT1 epitope-specific humoral
immune responses were analyzed in 31 patients (21 male and 10
female patients; age range, 16–79 years; median age, 36 years) with
advanced sarcoma out of 33 patients who participated in the
clinical trial between April 2004 and November 2012, which was due
to sample availability. These included various types of sarcomas,
such as osteosarcoma, chondrosarcoma, rhabdomyosarcoma, clear cell
sarcoma and Ewing's sarcoma. Of these 31 patients, 15 completed the
3-month treatment protocol, whereas the treatment of the remaining
16 was discontinued owing to disease progression; however, no
adverse events were encountered (Table
I). The treatment period was 1-1,387 days (median, 85 days). No
severe WT1-235 peptide-related adverse events were observed.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Patient ID | Age, years | Sex | Disease | Tumor control | Vaccine period
(days) |
|---|
| 1 | 68 | M | UPS | PD | 141 |
| 2 | 21 | M | PNET | PD | 39 |
| 3 | 27 | M | Fibrosarcoma | PD | 29 |
| 4 | 18 | M | PNET | PD | 15 |
| 5 | 63 | M | Chondrosarcoma | PD | 1 |
| 6 | 16 | F | Mesenchymal
chondrosarcoma | SD | 1,050 |
| 7 | 51 | M | Clear cell
sarcoma | PD | 78 |
| 8 | 26 | M | DSRCT | PD | 43 |
| 9 | 18 | M | DSRCT | SD | 253 |
| 10 | 39 | M |
Rhabdomyosarcoma | PD | 22 |
| 11 | 33 | M | Chondrosarcoma | PD | 99 |
| 12 | 36 | F | Malignant
schwannoma | SD | 869 |
| 13 | 59 | F | Liposarcoma | PD | 49 |
| 14 | 31 | M | Osteosarcoma | NE | 121 |
| 15 | 57 | M | UPS | PD | 71 |
| 16 | 41 | F |
Rhabdomyosarcoma | NE | 379 |
| 17 | 20 | M | Ewing's
sarcoma | NE | 29 |
| 18 | 19 | F | Ewing's
sarcoma | NE | 35 |
| 19 | 55 | M | Undifferentiated
sarcoma | PD | 545 |
| 20 | 60 | M | Chondrosarcoma | PD | 433 |
| 21 | 35 | M | Osteosarcoma | PD | 85 |
| 22 | 59 | F | UPS | PD | 99 |
| 23 | 33 | F | Clear cell
sarcoma | PD | 85 |
| 24 | 34 | M | Osteosarcoma | PD | 43 |
| 25 | 42 | F | UPS | PD | 36 |
| 26 | 21 | M |
Rhabdomyosarcoma | NE | 1,387+ |
| 27 | 37 | M | Osteosarcoma | PD | 92 |
| 28 | 79 | M | Leiomyosarcoma | SD | 1,072+ |
| 29 | 24 | M | Osteosarcoma | PD | 15 |
| 30 | 74 | F | Leiomyosarcoma | PD | 106 |
| 31 | 59 | F | Chordoma | PD | 183 |
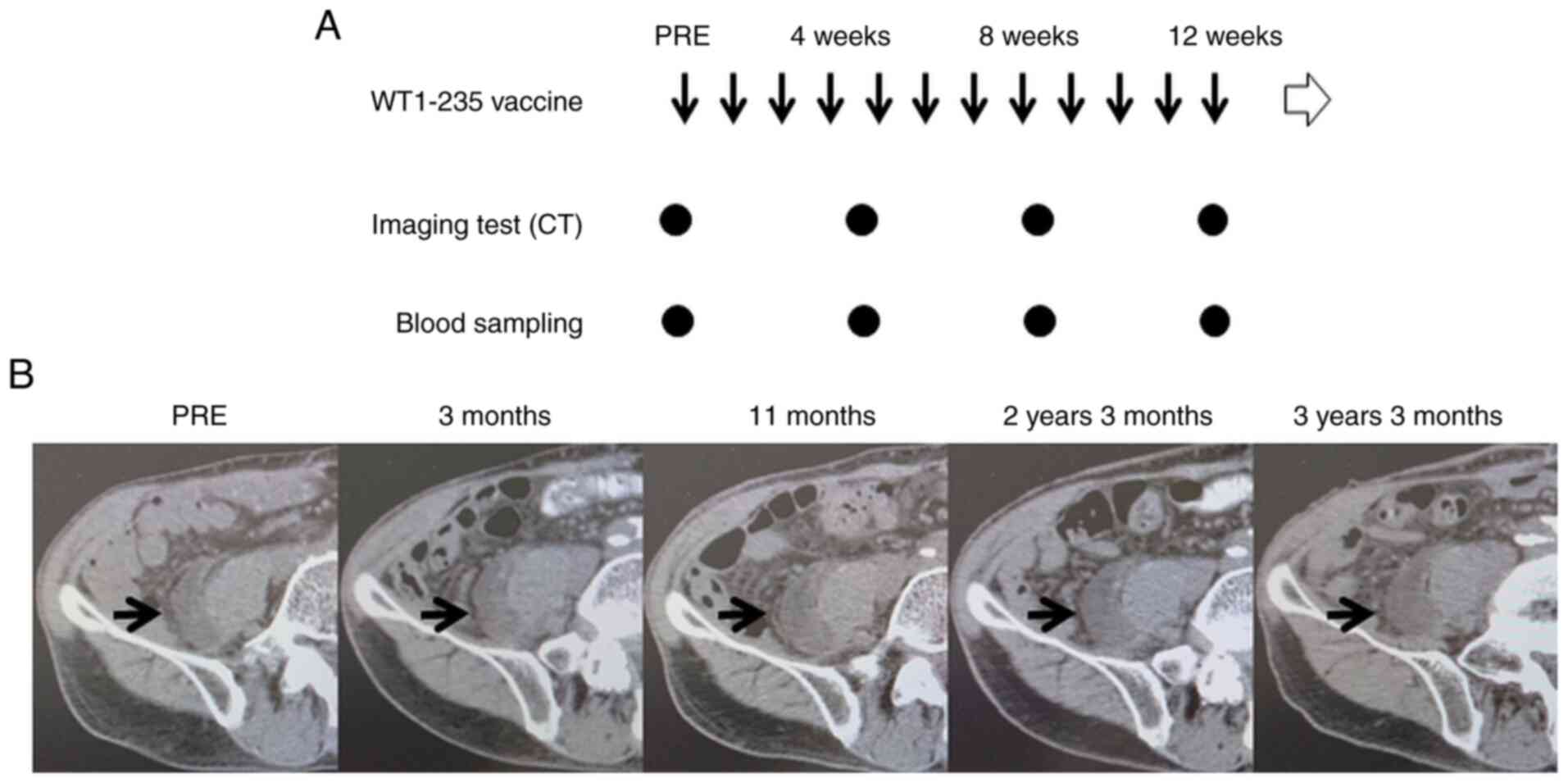
Representative case
Details of a representative case are provided in
Fig. 1. A 79-year-old male patient
with leiomyosarcoma had previously undergone two surgical
resections but was diagnosed with a second recurrence (patient ID,
28). The tumor lesion included a tumor adjacent to the right
iliopsoas muscle. The patient refused further surgical resection
with amputation of the right lower extremity and visited our
hospital to receive the WT1-235 peptide cancer vaccine. As he met
the criteria for the trial, the WT1-235 peptide cancer vaccine was
started as a monotherapy. The tumor remained stable during the
3-month treatment period and the WT1-235 peptide vaccine was
continued for >3 years. A slight enlargement of the tumor was
observed during this period (Fig.
1).
Production of WT1-235 CTL
epitope-specific IgG and IgM antibodies
To analyze the humoral immune responses to the
WT1-235 CTL epitope, which is the target antigen of the WT1 peptide
cancer vaccine, serum IgG and IgM antibodies against the WT1-235
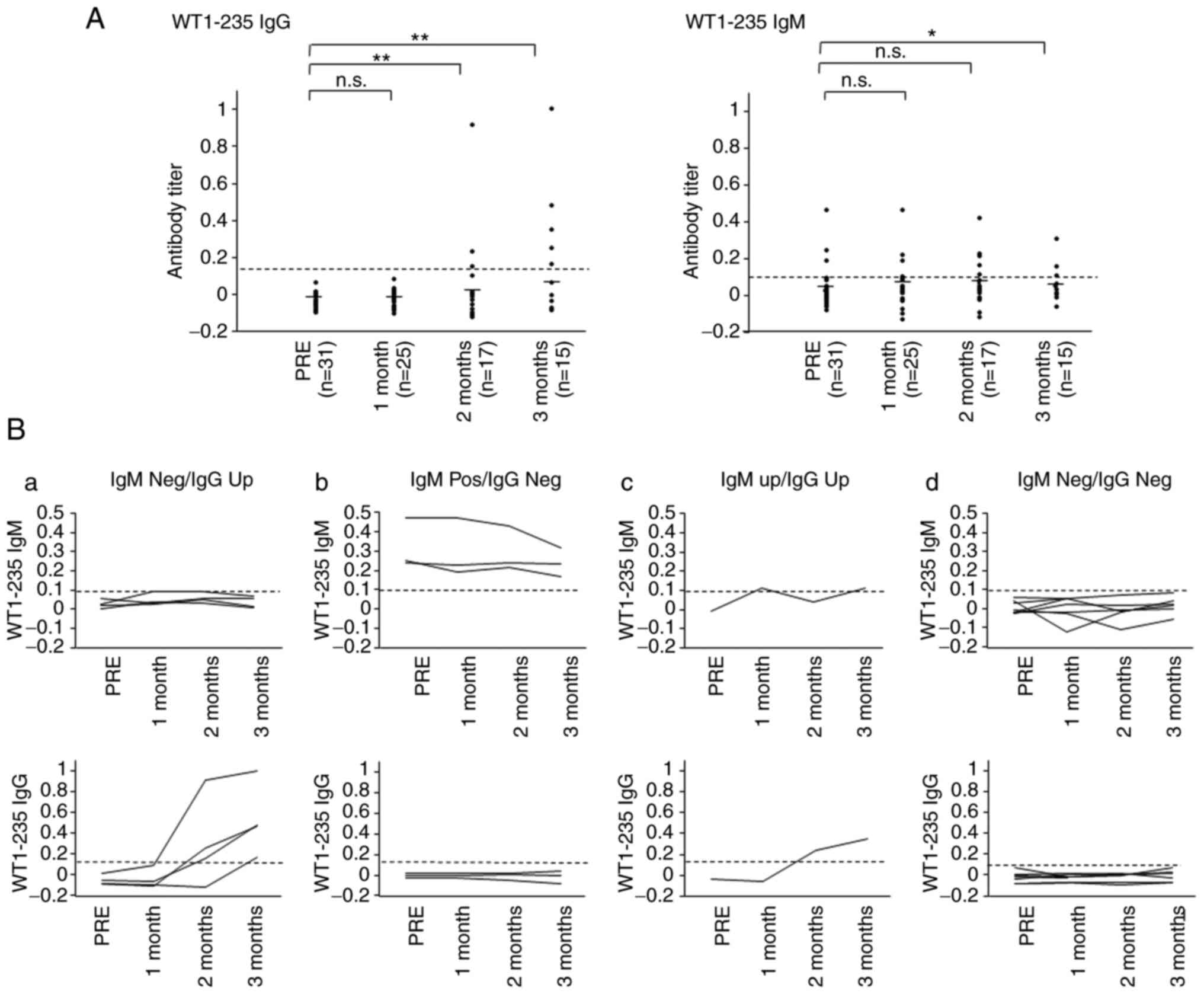
peptide were examined. The WT1-235 IgG antibody was undetectable in
all cases examined prior to vaccine treatment. The WT1-235 IgG
antibody levels were significantly elevated and became positive in
five patients at 3 months after the start of treatment. By
contrast, WT1-235 IgM antibody levels were positive at the start
and remained stable during the 3-month treatment protocol in three
(9.6%) of the 31 patients. Furthermore, one patient exhibited a
transient increase in serum WT1-235 IgM antibody levels. The
remaining 27 patients were negative for the WT1-235 IgM antibody
during the 3-month protocol treatment. After 3 months of
vaccination, the WT1-235 IgM antibody levels were significantly
lower than before vaccine initiation (Fig. 2A). The 15 patients who completed
the protocol treatment were divided into four groups based on the
production of WT1-235 IgG and IgM antibodies as follows: i) WT1-235
IgM was positive prior to vaccination and WT1 IgG was negative
after vaccination (WT1-235 IgM Pos/IgG Neg; n=3); ii) WT1-235 IgM
was negative prior to and after vaccination, but WT1 IgG became
positive during the treatment protocol (WT1-235 IgM Neg/IgG Up;
n=4); iii) both WT1-235 IgM and IgG became positive after
vaccination (WT1-235 IgM Up/IgG Up; n=1); and iv) both WT1-235 IgM
and IgG were negative prior to and after vaccination (WT1-235 IgM
Neg/IgG Neg; n=7). The levels of WT1-235 IgG and IgM in each
patient from the different groups prior to and during the first
three months of vaccine treatment are presented in Fig. 2B.
Immune recognition of WT1 antigens
prior to vaccination with the WT1 peptide
As described above, four patients exhibited WT1-235
IgG production without the preceding WT1-235 IgM elevation during
the first 3 months of vaccination, suggesting class switching to
WT1-235 IgG prior to vaccination. To understand the humoral immune
responses to WT1 antigens prior to the vaccine treatment, the
production of IgM and IgG antibodies against two non-target WT1
epitopes, WT1-332 and WT1-271, was examined. The WT1-332 epitope is
an HLA class II-binding helper T lymphocyte (HTL) epitope (35). The WT1-271 epitope is a newly
identified immunogenic WT1 epitope with elevated serum WT1-271 IgG
antibody levels in patients with intestinal cancers (Ito et
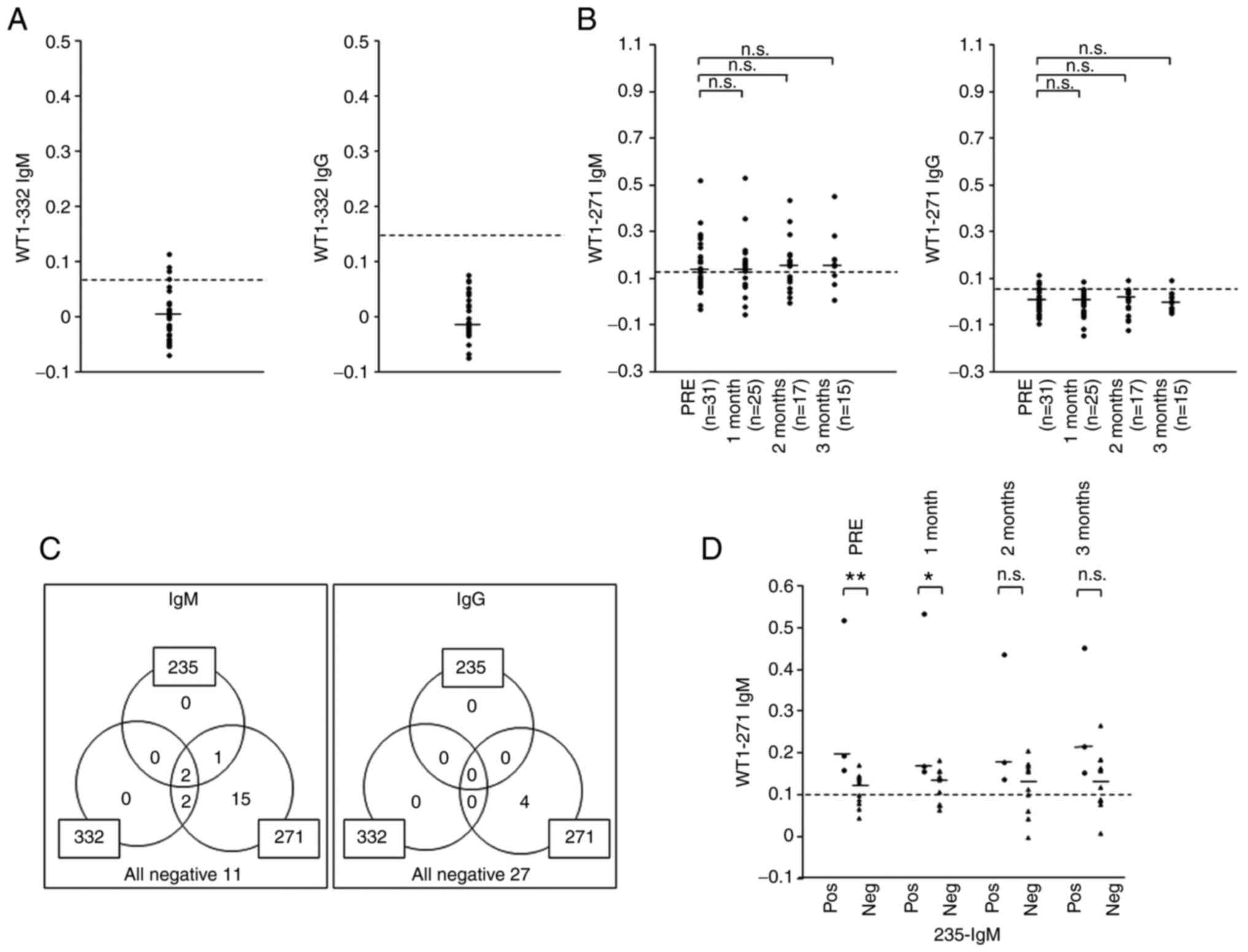
al, unpublished data). The WT1-332 IgM antibody was detected in
the serum of four (12.9%) of the 31 patients, whereas the WT1-332
IgG antibody was undetectable in all patients prior to vaccination
(Fig. 3A). Serum WT1-271 IgM
antibody was detected in 20 (64.5%) patients prior to vaccination.
The WT1-271 IgG antibody was also positive in the serum of four
(12.9%) of the 31 patients prior to vaccine treatment (Fig. 3B). These results indicated that the
immune system recognized the WT1 antigen prior to administration of
the WT1-235 peptide vaccine in most patients. Due to the high
positivity rate for WT1-271 IgM prior to vaccination, WT1-271 IgM
and IgG production was analyzed after administering the WT1-235
vaccine. The WT1-271 IgM antibody remained positive during the
3-month protocol treatment period in most patients at levels
similar to those prior to vaccine treatment. The WT1-271 IgG
antibody remained detectable but exhibited no significant elevation
during the 3-month treatment protocol.
Furthermore, the production of IgM and IgG
antibodies against the three WT1 antigens prior to vaccine
treatment was compared in each patient (Fig. 3C). WT1-271 IgM was detected in all
patients with WT1-235 or WT1-332 IgM production prior to
vaccination. In 15 patients, only WT1-271 IgM was detected. These
results indicated that the immune system had already recognized and
responded to the WT1 antigen in these patients, even if they were
negative for WT1-235 IgM or WT1-332 IgM prior to the vaccine
treatment.
Next, it was examined whether WT1-271 IgM antibody
production was associated with WT1-235 epitope-specific IgM
production prior to vaccine treatment. Serum WT1-271 IgM levels
were significantly higher in the WT1-235 IgM-positive group than in
the WT1-235 IgM-negative group at the start of and at 1 month after
initiating vaccine treatment (Fig.
3D). These results indicated that WT1-235 IgM production was
associated with WT1-271 IgM production in these tumor-bearing
patients.
Association between WT1-235 antibody
production and WT1-235-specific cellular immune responses
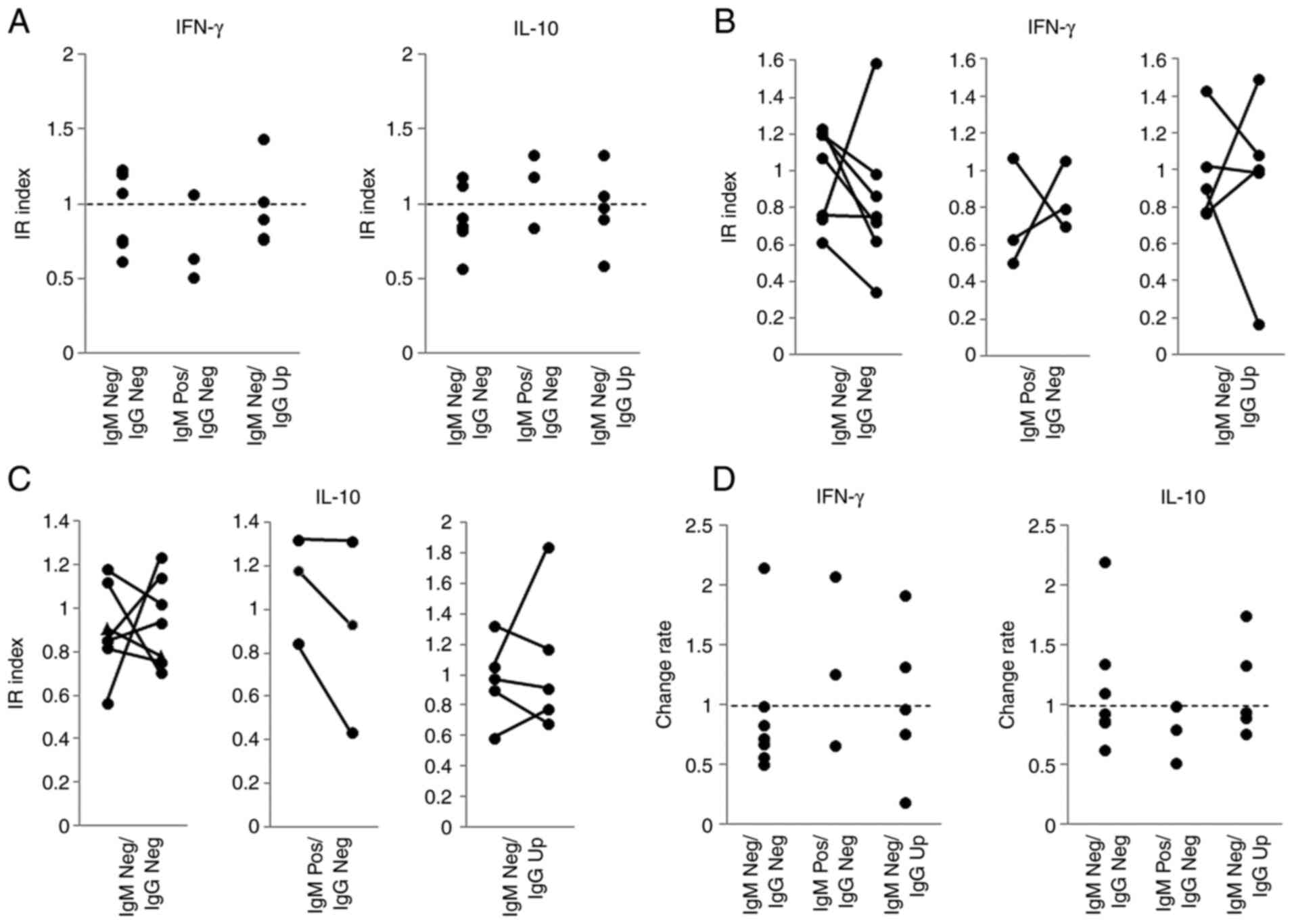
To characterize the immune responses in patients
with positive WT1-235 IgM antibodies prior to vaccine treatment,
WT1-235 epitope-specific IFN-γ and IL-10 production/secretion by
PBMCs from seven 235-IgM Neg/IgG Neg patients (patient IDs: 6, 10,
18, 25, 30, 32 and 33), three 235-IgM Pos/IgG Neg patients (patient
IDs: 1, 13 and 22) and five 235-IgM Neg/IgG Up patients (patient
IDs: 7, 14, 21, 28 and 29) were examined. No significant difference
in WT1-235-specific IFN-γ and IL-10 production/secretion was
obtained with the ELISPOT assay prior to WT1-235 vaccine
administration among the three patient groups (Fig. 4A). Next, the changes in IFN-γ and
IL-10 production/secretion were analyzed (Fig. 4B) and their rates of change were
calculated by dividing their IR index at 1 month by the
corresponding IR index prior to vaccine treatment. Of note, six of
the seven 235-IgM Neg/IgG Neg patients exhibited a decrease in
IFN-γ production and secretion. All three patients with WT1-235 IgM
antibody prior to vaccine treatment exhibited a decrease in IL-10
production and secretion in the first month (Fig. 4C).
Association between WT1
epitope-specific antibody production and clinical outcomes
The association between the production of WT1-235
and WT1-271 IgM antibodies prior to vaccine treatment and tumor
progression during the treatment protocol was also analyzed. No
significant association was observed between the production of
these WT1 antibodies and clinical outcomes (Table II). However, a combination of
WT1-235 and −271 IgM antibody positivity prior to treatment was
significantly associated with unfavorable tumor control at 3 months
after vaccine administration (Cramer's V=0.256; Table II).
 | Table II.Association between WT1 IgM antibody
and clinical outcomes. |
Table II.
Association between WT1 IgM antibody
and clinical outcomes.
|
Antibody/status | Stable disease,
n | Progressive
disease, n | Statistical
parameter |
|---|
| WT1-235 IgM |
|
| P=0.432 |
| Positive | 0 | 3 |
|
| Negative | 4 | 19 |
|
| WT1-271 IgM |
|
| P=0.432 |
| Positive | 2 | 17 |
|
| Negative | 2 | 5 |
|
| WT1-235 IgM-WT1-271
IgM |
|
| Cramer's
V=0.256 |
|
Positive-positive | 0 | 3 |
|
|
Positive-negative | 2 | 14 |
|
|
Negative-negative | 2 | 5 |
|
Discussion
Sarcomas are a group of rare malignancies that have
constrained the development of relevant therapeutic agents. WT1 is
overexpressed in various types of sarcomas and may be considered a
common target antigen for immunotherapy in these rare malignancies
(36–38). In the present clinical trial for
sarcomas, it was observed that administration of the WT1-235
peptide vaccine resulted in long-term stable disease in two
patients with advanced STS. No standard treatment has been
established for the majority of patients with advanced sarcomas.
Furthermore, the effectiveness of immune checkpoint inhibitors for
treating STS and bone sarcoma is limited (39,40).
Considering the currently limited therapeutic options, the WT1
peptide cancer vaccine may be considered a novel treatment option
for sarcoma.
WT1-235 IgG antibody levels were elevated in five
(33.3%) of 15 patients during the 3-month treatment protocol. A
previous study by our group reported that the WT1-235 antibody
levels were elevated in approximately half of the patients treated
with the WT1-235 peptide cancer vaccine (29). These results suggested that only a
small population of patients with sarcoma has elevated WT1-235 IgG
antibodies. This low rate of WT1-235 IgG antibody elevation may
indicate insufficient activity of WT1-specific helper T cells,
which are required for class switching from IgM to IgG. These
suppressed immune responses may result from the advanced tumor
stage and chemotherapies administered previously. In the present
study, the production of antibodies against the WT1-332 helper
epitope was analyzed and serum WT1-332 IgM antibodies were detected
in four (12.9%) of 31 patients; however, the WT1-332 IgG antibody
was undetectable in all patients prior to vaccination. These
results may indicate a lack of robust WT1-332-specific Th functions
in these patients. A previous study by our group demonstrated that
stimulation with the WT1-332 helper peptide enhanced the induction
of WT1-235-specific cytotoxic T lymphocytes (CTLs) in vitro
(35). Furthermore, a clinical
trial on patients with recurrent glioblastoma multiforme indicated
that the WT1-332 HTL peptide may be safely combined with the
WT1-235 peptide (41). However,
future clinical studies are required to examine whether a novel
type of WT1 peptide vaccine including both WT1-235 CTL and WT1-332
HTL peptides may induce more robust WT1-specific antitumor immune
responses and achieve improved clinical effects in patients with
sarcoma.
The present study provided several noteworthy
findings on WT1-235-specific humoral immune responses. Although IgM
generally precedes IgG antibody production in infectious diseases,
prior elevation of WT1-235 IgM was observed in only one patient but
not in the remaining four of five patients who exhibited an
increase in WT1-235 IgG antibodies during the 3 months of
treatment. These results indicated that a significant number of
patients had already experienced class switching of
WT1-235-specific B cells in patients with undetectable WT1-235 IgG
prior to vaccination.
Furthermore, the WT1-235 IgM antibody was detected
prior to vaccination in three patients. WT1-235 IgM antibody levels
were detected at similar levels and WT1-235 IgG remained
undetectable during the 3-month treatment period in all three
patients. This lack of class switching to WT1-235 IgG indicated
insufficient WT1-specific Th-cell assistance, suggesting that the
presence of WT1-235 IgM antibody prior to vaccine treatment may be
linked to unfavorable clinical outcomes, as the Th response has an
essential role in the induction and maintenance of anti-tumor CTL
responses. This is comparable with the results of the present study
indicating that WT1-235 IgM was undetectable prior to vaccine
administration in two patients who achieved long-term stable
disease. Furthermore, the combination of WT1-235 and WT1-271 IgM
antibody positivity was moderately associated with unfavorable
tumor control. In addition, patients with WT1-235 IgM antibody
prior to vaccine treatment tended to exhibit a decline in IL-10
production and secretion in the first month of vaccine treatment.
This may explain the unfavorable clinical outcomes in patients with
WT1-235 IgM antibodies, as IL-10 has crucial roles in the
development and function of CD8 T-cell memory (42) and the stimulation of natural killer
cells (43–46). Due to the small number of patients
and the heterogeneity of diseases in the present study, it is
necessary to examine whether IgM antibody production against the
target antigen prior to vaccination may indicate a lack of
robustness in Th responses and correlate with poor prognosis in the
future. In recent years, neoantigens have been identified in
different patients as targets for personalized medicine and a
therapeutic peptide vaccine targeting these antigens has been
considered to be a promising therapeutic option (47). In such settings, the measurement of
IgM antibodies against target antigens may help evaluate
antigen-specific Th responses in each patient and contribute to the
selection of antigen peptides with high clinical potential.
Cancer therapeutic vaccines induce anti-tumor immune
responses through the activation of cancer antigen-specific
cytotoxic progenitor cells. Therefore, predicting whether the
patient immune system is able to recognize and respond to the
target antigen prior to immunotherapy is essential for
individualized therapy. The present study indicated that the
patient population with negative WT1-235 IgM and IgG prior to
WT1-235 vaccine treatment included both patients with class WT1-235
IgG and those with undetectable WT1-235 IgM and IgG during the
3-month treatment. These results indicated that antibodies against
the WT1-235 epitope alone cannot be used to assess the response of
the patient's immune system to the WT1 antigen. The present study
suggested that WT1-271 IgM antibody levels were elevated in 64.5%
of patients, which was much higher than the frequency of WT1-235
IgM (9.6%) and WT1-332 IgM (12.9%). All patients with positive
WT1-235 IgM were also positive for WT1-271 IgM prior to vaccine
treatment. These results indicate that the WT1-271 IgM antibody may
be a marker for WT1-experienced B-cell immunity. Furthermore, a
combination of WT1-235 and WT1-271 IgM antibody positivity was
associated with unfavorable tumor control. Taken together, the
WT1-271 IgM antibody may be helpful in the assessment of antitumor
immunity in patients with cancer, as the presence of WT1-directed
immunity indicates the potential of inducing antitumor immune
responses against WT1.
Recently, multiple studies have reported the
double-faceted roles of B lymphocytes, in addition to antibody
production, in pro- and anti-tumor immunity (48). B cells may have pro-tumorigenic
roles through the production and secretion of immunosuppressive
cytokines, such as IL-10 (49,50),
TGF-β (51) and IL-35 (52), and the induction of apoptosis in
CD4+ T cells through the Fas-FasL system (50). In addition, B cells may have
anti-tumorigenic roles through IFN-γ secretion to enhance tumor
killing by NK cells and CTLs (50,53)
and even kill tumor cells directly via the Fas-FasL system
(51). WT1-235 target
epitope-specific IgG production was thus analyzed in patients with
recurrent glioblastoma treated with the WT1 peptide vaccine to
monitor WT1-specific Th functions. The results indicated that
WT1-235 IgG antibody production during vaccine treatment was
associated with favorable clinical outcomes (29). In the present study, the findings
suggested an association between WT1-235 IgM antibody production
and WT1-235-specific cellular immune responses in the first month
of WT1-235 vaccine treatment. As IgM production does not require
Th-mediated class switching, WT1-235 IgM, rather than WT1-235 IgG,
may be more directly associated with B-cell functions in cellular
immune responses. Future studies are thus required on WT1-specific
B-cell functions, such as immunomodulation through Th1 or Th2
cytokines.
WT1-235 IgG antibodies may contribute to
WT1-235-specific cellular immune responses as an opsonin.
Antibody-mediated opsonization of the cancer antigen MAGE-A3
reportedly promotes its uptake by dendritic cells. Furthermore, the
stimulation of native T cells with antibody-opsonized MAGE-A3
protein induces a CD8 T-cell response rather than a CD4 T-cell
response, indicating the existence of an uptake route-dependent
mechanism by which subsequent immune responses are modulated
(54). Similarly, WT1-235 IgG
antibodies may bind to the WT1-235 peptide administered for
vaccination, acting as an opsonin to promote subsequent
WT1-specific cellular immune responses. This potential effect of
WT1-235 IgG antibodies may explain the association between WT1-235
IgG production and the favorable clinical outcomes in patients with
recurrent glioblastoma treated with the WT1-235 peptide vaccine
that were previously reported by our group (29).
Approximately 10% of the patients with sarcoma that
were assessed produced WT1-235 IgM and the majority produced
WT1-271 IgM antibodies prior to the initiation of vaccination.
These WT1 antibodies may exert direct anti-tumor effects on tumor
cells expressing WT1 antigens on their cell surface. However, the
WT1 protein localizes predominantly to the cytoplasm and is
undetectable on tumor cell surfaces. Furthermore, the direct
binding of WT1-235 and WT1-271 IgM antibodies to tumor cells may
result in tumor destruction through activation of complement
cascades existing in the tumor microenvironment (55). The association between WT1-235 and
WT1-271 IgM antibodies and unfavorable clinical outcomes in the
present study indicates that the direct action of these WT1
antibodies against tumor cells is unlikely in these patients.
Another possible effect of WT1 IgM antibodies is the
modulation of B-cell functions through the IgM receptor, FcµR,
abundantly expressed in B cells (56,57).
FcµR promotes B-cell activation by interacting with the B-cell
receptor (BCR) to enhance BCR signaling (58,59).
A common feature of FcµR deletion in mice is the production of
autoreactive antibodies (60,61),
suggesting the involvement of FcµR in regulating B-cell tolerance.
WT1-specific IgM antibodies are persistently produced in patients
with WT1-expressing tumors. Further studies are required to examine
whether these WT1-specific IgM antibodies modulate B-cell functions
by binding to FcµR in patients treated with the WT1 peptide
vaccine. Overall, the present results indicated that WT1
epitope-specific IgG and IgM antibodies may be helpful as
immune-monitoring markers for WT1 peptide cancer vaccine
immunotherapy.
Acknowledgements
The authors would like to thank Dr Seiji Hayashi
(Kinki National Hospital, Sakai, Japan) and Dr Hiroshi Matsuoka
(Division of Medical Oncology/Hematology, Department of Medicine,
Kobe University Graduate School of Medicine, Kobe, Japan) for their
support as Effect Safety Evaluation Committee members for the
clinical study. The authors would also like to thank Ms. Tomoe
Umeda and Ms. Chiaki Ohta (Osaka University Graduate School of
Medicine, Department of Cancer Immunotherapy, Suita, Japan) for
their contribution to patient enrolment and technical support in
this study.
Funding
This study was supported by a grant from the Ministry of
Education, Culture, Sports, Science and Technology, Japan (grant
no. 20K08732).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
SA designed this study, performed the experiments
and data analysis and prepared the manuscript. NN, KH, NH, HO, EM,
JN, SN, AT, YOk, HS and YOj designed and performed the clinical
trial. HN contributed to the clinical trial through quality control
and preparation of WT1 peptide vaccine, and acquisition and
interpretation of clinical data. MK, MA, RI and MI performed the
sample preparation, ELISPOT assay experiments and data analysis.
FF, SM and SN performed data analysis. YOj designed this study,
supervised the experiments, performed data analysis and prepared
the manuscript. SA and YOk confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent prior
to participation in the study. The study protocol was approved by
the Ethical Committee of Osaka University Hospital (nos. 07094 and
13386 for the clinical trials and no. 11293 for clinical sample
analysis). Trials were registered at the UMIN Clinical Trials
Registry as Umin000002001 and then UMIN000015997.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mazanet R and Antman KH: Sarcomas of soft
tissue and bone. Cancer. 68:463–473. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tawbi HA, Burgess M, Bolejack V, Van Tine
BA, Schuetze SM, Hu J, D'Angelo S, Attia S, Riedel RF, Priebat DA,
et al: Pembrolizumab in advanced soft-tissue sarcoma and bone
sarcoma (SARC028): A multicentre, two-cohort, single-arm,
open-label, phase 2 trial. Lancet Oncol. 18:1493–1501. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shoushtari AN, Van Tine BA and Schwartz
GK: Novel treatment targets in sarcoma: More than just the GIST. Am
Soc Clin Oncol Educ Book. e488–e495. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saerens M, Brusselaers N, Rottey S,
Decruyenaere A, Creytens D and Lapeire L: Immune checkpoint
inhibitors in treatment of soft-tissue sarcoma: A systematic review
and meta-analysis. Eur J Cancer. 152:165–182. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Keung EZ, Lazar AJ, Torres KE, Wang WL,
Cormier JN, Ashleigh Guadagnolo B, Bishop AJ, Lin H, Hunt KK, Bird
J, et al: Phase II study of neoadjuvant checkpoint blockade in
patients with surgically resectable undifferentiated pleomorphic
sarcoma and dedifferentiated liposarcoma. BMC Cancer. 18:9132018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramachandran I, Lowther DE, Dryer-Minnerly
R, Wang R, Fayngerts S, Nunez D, Betts G, Bath N, Tipping AJ,
Melchiori L, et al: Systemic and local immunity following adoptive
transfer of NY-ESO-1 SPEAR T cells in synovial sarcoma. J
Immunother Cancer. 7:2762019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inoue K, Tamaki H, Ogawa H, Oka Y, Soma T,
Tatekawa T, Oji Y, Tsuboi A, Kim EH, Kawakami M, et al: Wilms'
tumor gene (WT1) competes with differentiation-inducing signal in
hematopoietic progenitor cells. Blood. 91:2969–2976. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sugiyama H: Cancer immunotherapy targeting
WT1 protein. Int J Hematol. 76:127–132. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oka Y, Tsuboi A, Kawakami M, Elisseeva OA,
Nakajima H, Udaka K, Kawase I, Oji Y and Sugiyama H: Development of
WT1 peptide cancer vaccine against hematopoietic malignancies and
solid cancers. Curr Med Chem. 13:2345–2352. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oji Y, Kitamura Y, Kamino E, Kitano A,
Sawabata N, Inoue M, Mori M, Nakatsuka S, Sakaguchi N, Miyazaki K,
et al: WT1 IgG antibody for early detection of nonsmall cell lung
cancer and as its prognostic factor. Int J Cancer. 125:381–387.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oji Y, Yamamoto H, Nomura M, Nakano Y,
Ikeba A, Nakatsuka S, Abeno S, Kiyotoh E, Jomgeow T, Sekimoto M, et
al: Overexpression of the Wilms' tumor gene WT1 in colorectal
adenocarcinoma. Cancer Sci. 94:712–717. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakatsuka S, Oji Y, Horiuchi T, Kanda T,
Kitagawa M, Takeuchi T, Kawano K, Kuwae Y, Yamauchi A, Okumura M,
et al: Immunohistochemical detection of WT1 protein in a variety of
cancer cells. Mod Pathol. 19:804–814. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sugiyama H: Wilms' tumor gene WT1: Its
oncogenic function and clinical application. Int J Hematol.
73:177–187. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheever MA, Allison JP, Ferris AS, Finn
OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL,
Weiner LM and Matrisian LM: The prioritization of cancer antigens:
A national cancer institute pilot project for the acceleration of
translational research. Clin Cancer Res. 15:5323–5337. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oka Y, Tsuboi A, Taguchi T, Osaki T, Kyo
T, Nakajima H, Elisseeva OA, Oji Y, Kawakami M, Ikegame K, et al:
Induction of WT1 (Wilms' tumor gene)-specific cytotoxic T
lymphocytes by WT1 peptide vaccine and the resultant cancer
regression. Proc Natl Acad Sci USA. 101:13885–13890. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Izumoto S, Tsuboi A, Oka Y, Suzuki T,
Hashiba T, Kagawa N, Hashimoto N, Maruno M, Elisseeva OA, Shirakata
T, et al: Phase II clinical trial of Wilms tumor 1 peptide
vaccination for patients with recurrent glioblastoma multiforme. J
Neurosurg. 108:963–971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Keilholz U, Letsch A, Busse A, Asemissen
AM, Bauer S, Blau IW, Hofmann WK, Uharek L, Thiel E and
Scheibenbogen C: A clinical and immunologic phase 2 trial of Wilms
tumor gene product 1 (WT1) peptide vaccination in patients with AML
and MDS. Blood. 113:6541–6548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oji Y, Oka Y, Nishida S, Tsuboi A,
Kawakami M, Shirakata T, Takahashi K, Murao A, Nakajima H, Narita
M, et al: WT1 peptide vaccine induces reduction in minimal residual
disease in an imatinib-treated CML patient. Eur J Haematol.
85:358–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hashii Y, Sato-Miyashita E, Matsumura R,
Kusuki S, Yoshida H, Ohta H, Hosen N, Tsuboi A, Oji Y, Oka Y, et
al: WT1 peptide vaccination following allogeneic stem cell
transplantation in pediatric leukemic patients with high risk for
relapse: Successful maintenance of durable remission. Leukemia.
26:530–532. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maeda T, Hosen N, Fukushima K, Tsuboi A,
Morimoto S, Matsui T, Sata H, Fujita J, Hasegawa K, Nishida S, et
al: Maintenance of complete remission after allogeneic stem cell
transplantation in leukemia patients treated with Wilms tumor 1
peptide vaccine. Blood Cancer J. 3:e1302013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miyatake T, Ueda Y, Morimoto A, Enomoto T,
Nishida S, Shirakata T, Oka Y, Tsuboi A, Oji Y, Hosen N, et al: WT1
peptide immunotherapy for gynecologic malignancies resistant to
conventional therapies: A phase II trial. J Cancer Res Clin Oncol.
139:457–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oji Y, Inoue M, Takeda Y, Hosen N,
Shintani Y, Kawakami M, Harada T, Murakami Y, Iwai M, Fukuda M, et
al: WT1 peptide-based immunotherapy for advanced thymic epithelial
malignancies. Int J Cancer. 142:2375–2382. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nishida S, Ishikawa T, Egawa S, Koido S,
Yanagimoto H, Ishii J, Kanno Y, Kokura S, Yasuda H, Oba MS, et al:
Combination gemcitabine and WT1 peptide vaccination improves
progression-free survival in advanced pancreatic ductal
adenocarcinoma: A phase II randomized study. Cancer Immunol Res.
6:320–331. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maslak PG, Dao T, Bernal Y, Chanel SM,
Zhang R, Frattini M, Rosenblat T, Jurcic JG, Brentjens RJ, Arcila
ME, et al: Phase 2 trial of a multivalent WT1 peptide vaccine
(galinpepimut-S) in acute myeloid leukemia. Blood Adv. 2:224–234.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yanagisawa R, Koizumi T, Koya T, Sano K,
Koido S, Nagai K, Kobayashi M, Okamoto M, Sugiyama H and Shimodaira
S: WT1-pulsed dendritic cell vaccine combined with chemotherapy for
resected pancreatic cancer in a phase I study. Anticancer Res.
38:2217–2225. 2018.PubMed/NCBI
|
|
26
|
Anguille S, Van de Velde AL, Smits EL, Van
Tendeloo VF, Juliusson G, Cools N, Nijs G, Stein B, Lion E, Van
Driessche A, et al: Dendritic cell vaccination as postremission
treatment to prevent or delay relapse in acute myeloid leukemia.
Blood. 130:1713–1721. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ochi T, Fujiwara H, Okamoto S, An J, Nagai
K, Shirakata T, Mineno J, Kuzushima K, Shiku H and Yasukawa M:
Novel adoptive T-cell immunotherapy using a WT1-specific TCR vector
encoding silencers for endogenous TCRs shows marked antileukemia
reactivity and safety. Blood. 118:1495–1503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chapuis AG, Egan DN, Bar M, Schmitt TM,
McAfee MS, Paulson KG, Voillet V, Gottardo R, Ragnarsson GB,
Bleakley M, et al: T cell receptor gene therapy targeting WT1
prevents acute myeloid leukemia relapse post-transplant. Nat Med.
25:1064–1072. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oji Y, Hashimoto N, Tsuboi A, Murakami Y,
Iwai M, Kagawa N, Chiba Y, Izumoto S, Elisseeva O, Ichinohasama R,
et al: Association of WT1 IgG antibody against WT1 peptide with
prolonged survival in glioblastoma multiforme patients vaccinated
with WT1 peptide. Int J Cancer. 139:1391–1401. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Petitprez F, de Reyniès A, Keung EZ, Chen
TW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougoüin A,
et al: B cells are associated with survival and immunotherapy
response in sarcoma. Nature. 577:556–560. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schulz KF, Altman DG and Moher D; CONSORT
Group, : CONSORT 2010 Statement: Updated guidelines for reporting
parallel group randomised trials. BMJ. 340:c3322010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hayashi S, Imanishi R, Adachi M, Ikejima
S, Nakata J, Morimoto S, Fujiki F, Nishida S, Tsuboi A, Hosen N, et
al: Reader-free ELISPOT assay for immuno-monitoring in
peptide-based cancer vaccine immunotherapy. Biomed Rep. 12:244–250.
2020.PubMed/NCBI
|
|
35
|
Fujiki F, Oka Y, Tsuboi A, Kawakami M,
Kawakatsu M, Nakajima H, Elisseeva OA, Harada Y, Ito K, Li Z, et
al: Identification and characterization of a WT1 (Wilms Tumor Gene)
protein-derived HLA-DRB1*0405-restricted 16-mer helper peptide that
promotes the induction and activation of WT1-specific cytotoxic T
lymphocytes. J Immunother. 30:282–293. 2007.PubMed/NCBI
|
|
36
|
Ueda T, Oji Y, Naka N, Nakano Y, Takahashi
E, Koga S, Asada M, Ikeba A, Nakatsuka S, Abeno S, et al:
Overexpression of the Wilms' tumor gene WT1 in human bone and
soft-tissue sarcomas. Cancer Sci. 94:271–276. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sotobori T, Ueda T, Oji Y, Naka N, Araki
N, Myoui A, Sugiyama H and Yoshikawa H: Prognostic significance of
Wilms tumor gene (WT1) mRNA expression in soft tissue sarcoma.
Cancer. 106:2233–2240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oue T, Uehara S, Yamanaka H, Takama Y, Oji
Y and Fukuzawa M: Expression of Wilms tumor 1 gene in a variety of
pediatric tumors. J Pediatr Surg. 46:2233–2238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Merchant MS, Wright M, Baird K, Wexler LH,
Rodriguez-Galindo C, Bernstein D, Delbrook C, Lodish M, Bishop R,
Wolchok JD, et al: Phase I clinical trial of ipilimumab in
pediatric patients with advanced solid tumors. Clin Cancer Res.
22:1364–1370. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thanindratarn P, Dean DC, Nelson SD,
Hornicek FJ and Duan Z: Advances in immune checkpoint inhibitors
for bone sarcoma therapy. J Bone Oncol. 15:1002212019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tsuboi A, Hashimoto N, Fujiki F, Morimoto
S, Kagawa N, Nakajima H, Hosen N, Nishida S, Nakata J, Morita S, et
al: A phase I clinical study of a cocktail vaccine of Wilms' tumor
1 (WT1) HLA class I and II peptides for recurrent malignant glioma.
Cancer Immunol Immunother. 68:331–340. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Foulds KE, Rotte MJ and Seder RA: IL-10 is
required for optimal CD8 T cell memory following Listeria
monocytogenes infection. J Immunol. 177:2565–2574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Giovarelli M, Musiani P, Modesti A,
Dellabona P, Casorati G, Allione A, Consalvo M, Cavallo F, di
Pierro F, De Giovanni C, et al: Local release of IL-10 by
transfected mouse mammary adenocarcinoma cells does not suppress
but enhances antitumor reaction and elicits a strong cytotoxic
lymphocyte and antibody-dependent immune memory. J Immunol.
155:3112–3123. 1995.PubMed/NCBI
|
|
44
|
Petersson M, Charo J, Salazar-Onfray F,
Noffz G, Mohaupt M, Qin Z, Klein G, Blankenstein T and Kiessling R:
Constitutive IL-10 production accounts for the high NK sensitivity,
low MHC class I expression, and poor transporter associated with
antigen processing (TAP)-1/2 function in the prototype NK target
YAC-1. J Immunol. 161:2099–2105. 1998.PubMed/NCBI
|
|
45
|
Fujiki F, Oka Y, Kawakatsu M, Tsuboi A,
Tanaka-Harada Y, Hosen N, Nishida S, Shirakata T, Nakajima H,
Tatsumi N, et al: A clear correlation between WT1-specific Th
response and clinical response in WT1 CTL epitope vaccination.
Anticancer Res. 30:2247–2254. 2010.PubMed/NCBI
|
|
46
|
McKay K, Moore PC, Smoller BR and Hiatt
KM: Association between natural killer cells and regression in
melanocytic lesions. Hum Pathol. 42:1960–1964. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Blass E and Ott PA: Advances in the
development of personalized neoantigen-based therapeutic cancer
vaccines. Nat Rev Clin Oncol. 18:215–229. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Largeot A, Pagano G, Gonder S, Moussay E
and Paggetti J: The B-side of cancer immunity: The underrated tune.
Cells. 8:4492019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sarvaria A, Madrigal JA and Saudemont A: B
cell regulation in cancer and anti-tumor immunity. Cell Mol
Immunol. 14:662–674. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tao H, Lu L, Xia Y, Dai F, Wang Y, Bao Y,
Lundy SK, Ito F, Pan Q, Zhang X, et al: Antitumor effector B cells
directly kill tumor cells via the Fas/FasL pathway and are
regulated by IL-10. Eur J Immunol. 45:999–1009. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Olkhanud PB, Damdinsuren B, Bodogai M,
Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP and Biragyn A:
Tumor-evoked regulatory B cells promote breast cancer metastasis by
converting resting CD4+ T cells to T-regulatory cells.
Cancer Res. 71:3505–3515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pylayeva-Gupta Y, Das S, Handler JS, Hajdu
CH, Coffre M, Koralov SB and Bar-Sagi D: IL35-producing B cells
promote the development of pancreatic neoplasia. Cancer Discov.
6:247–255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Schwartz M, Zhang Y and Rosenblatt JD: B
cell regulation of the anti-tumor response and role in
carcinogenesis. J Immunother Cancer. 4:402016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Moeller I, Spagnoli GC, Finke J, Veelken H
and Houet L: Uptake routes of tumor-antigen MAGE-A3 by dendritic
cells determine priming of naive T-cell subtypes. Cancer Immunol
Immunother. 61:2079–2090. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Roumenina LT, Daugan MV, Petitprez F,
Sautes-Fridman C and Fridman WH: Context-dependent roles of
complement in cancer. Nat Rev Cancer. 19:698–715. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu J, Wang Y, Xiong E, Hong R, Lu Q, Ohno
H and Wang JY: Role of the IgM Fc receptor in immunity and
tolerance. Front Immunol. 10:5292019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kubagawa H, Oka S, Kubagawa Y, Torii I,
Takayama E, Kang DW, Gartland GL, Bertoli LF, Mori H, Takatsu H, et
al: Identity of the elusive IgM Fc receptor (FcmuR) in humans. J
Exp Med. 206:2779–2793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ouchida R, Mori H, Hase K, Takatsu H,
Kurosaki T, Tokuhisa T, Ohno H and Wang JY: Critical role of the
IgM Fc receptor in IgM homeostasis, B-cell survival, and humoral
immune responses. Proc Natl Acad Sci USA. 109:E2699–E2706. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ouchida R, Lu Q, Liu J, Li Y, Chu Y,
Tsubata T and Wang JY: FcµR interacts and cooperates with the B
cell receptor To promote B cell survival. J Immunol. 194:3096–3101.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Honjo K, Kubagawa Y, Suzuki Y, Takagi M,
Ohno H, Bucy RP, Izui S and Kubagawa H: Enhanced auto-antibody
production and Mott cell formation in FcµR-deficient autoimmune
mice. Int Immunol. 26:659–672. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yu J, Duong VHH, Westphal K, Westphal A,
Suwandi A, Grassl GA, Brand K, Chan AC, Föger N and Lee KH: Surface
receptor Toso controls B cell-mediated regulation of T cell
immunity. J Clin Invest. 128:1820–1836. 2018. View Article : Google Scholar : PubMed/NCBI
|