Introduction
Prostate cancer is a malignancy that arises from the
lateral posterior lobes or glands of the prostate, which seriously
threatens the health of those affected (1). Over the past decades, the therapeutic
strategies for prostate cancer have made significant progression,
particularly androgen-deprivation therapy (2). However, the acquired resistance to
medicines targeting androgen receptor has proved a major challenge
in effectively treating prostate cancer. Thus, it is important to
identify novel therapeutic targets for prostate cancer (2).
Ferroptosis is a newly established non-apoptotic
form of regulated cell death caused by failed regulation of
glutathione-dependent lipid-peroxide-scavenging (3). Ferroptosis is biochemically
characterized by generation of intracellular ferrous iron
(Fe2+), elevated lipid peroxidation and accumulated
lethal reactive oxygen species (ROS) (3). Ferroptosis plays an important role in
regulating different types of cancer, including triple-negative
breast cancer, renal cancer and gastric cancer (3). For example, cysteine dioxygenase 1
suppresses gastric cancer progression by inducing ferroptosis of
gastric cancer cells (4). In
addition, tumor suppressor BRCA1-associated protein 1 inhibits the
development of renal cancer by inducing ferroptosis via
upregulation of Solute carrier family 7 member 11 (5). However, the role and regulatory
mechanisms of ferroptosis in prostate cancer remain unclear.
Several enzymes play critical roles during
ferroptosis, of which glutathione peroxidase 4 (GPX4) has been well
studied (6–10). GPX4 is an antioxidant enzyme that
downregulates lipid hydroperoxides and protects cells from damage
of accumulated ROS, and it is considered a suppressor of
ferroptosis (7). Increasing
evidence suggest that GPX4 plays an important role in tumors
(6,8). A recent study revealed that RSL3
induces cell death in colorectal cancer cells by triggering
ferroptosis via the inactivation of GPX4 (9). In addition, GPX4 contributes to the
vulnerability of ferroptosis in clear-cell carcinomas (CCCs), and
thus is a therapeutic target in CCCs (10). However, the regulatory mechanisms
of GPX4 in prostate cancer remain unknown.
Various genes are involved in tumorigenesis. For
example, mutations of acetyl-CoA carboxylase alpha and DEP domain
containing MTOR interacting protein are associated with lung cancer
(11). MicroRNAs (miRNAs/miRs) are
a principal member of non-coding RNAs, defined as highly conserved
short RNAs, 21–25 nucleotides in length (12). In the past decades, the functions
of miRNAs have been widely studied in various diseases and
pathological processes, particularly in cancers (13–15).
However, only a few miRNAs, including miR-9, miR-137 and miR-522
have been identified to be associated with ferroptosis (16–19).
miR-15a is a widely studied miRNA in cancers, including prostate
cancer (20,21). A bioinformatics study revealed that
miR-15a expression was notably downregulated in blood samples of
patients with prostate cancer compared with healthy subjects,
suggesting that circulating miR-15a may be a potential diagnostic
biomarker for prostate cancer (22). Jin et al (23) and Bonci et al (24) reported that miR-15a functions as a
tumor suppressor to inhibit the proliferation and invasion of
prostate cancer cells by downregulating Wnt/β-catenin and TGF-β
signaling.
The present study aimed to investigate the function
of miR-15a on ferroptotic cell death of prostate cancer cells via
regulation of GPX4 to determine whether miR-15a and GPX4 may be
used as potential therapeutic targets for prostate cancer.
Materials and methods
Cell culture
The human prostate cancer cell line, LNCAP was
purchased from the American Type Culture Collection and maintained
in DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100
U/ml penicillin and 100 µg/ml streptomycin, at 37°C with 5%
CO2.
Target prediction
Targetscan (http://www.targetscan.org) was used to predict the
binding sites between miRNA-15a and the 3′-untranslated region
(UTR) of GPX4 mRNA.
Cell transfection
miR-15a mimics, miR-15a inhibitor (anti-miR-15a) and
negative control (NC), small interfering (si)-GPX4 and si-NC were
designed and purchased from Guangzhou RiboBio Co., Ltd. The
following sequences were used: MiR-15a mimics,
5′-UAGCAGCACAUAAUGGUUUGUG-3′; miR-15a inhibitor,
5′-CACAAACCAUUAUGUGCUGCUA-3′; mimic NC,
5′-UUUGUACUACACAAAAGUACUG-3′; inhibitor NC,
5′-UCUACUCUUUCUAGGAGGUUGUGA-3′; si-GPX4 forward,
5′-GUGGAUGAAGAUCCAACCCdTdT-3′ and reverse,
3′-GGGUUGGAUCUUCAUCCACdTdT-5′; si-NC forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
3′-ACGUGACACGUUCGGAGAATT-5′. LNCAP cells were seeded into 6-well
plates at a density of 1×105 cells/well and incubated
overnight to achieve monolayer confluence. Following starvation
with serum-free medium for 12 h, cells were transfected with
miR-15a mimics or si-GPX4 at 50 nmol/l using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions and
cultured at 37°C for 48 h, after which subsequent experiments were
performed.
Reverse transcription-quantitative
(RT-qPCR)
Total RNA was extracted from LNCAP cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. Total RNA was
reverse transcribed into cDNA using the cDNA synthesis kit (Takara
Bio, Inc.). The reverse transcribed reaction was performed as 42°C
for 60 min and 85°C for 5 min. qPCR was subsequently performed
using the SYBR Green Super Mix kit (BD Biosciences) and the
thermocycling conditions were as follows: 40 Cycles of 95°C for 30
sec, 95°C for 15 sec and 60°C for 15 sec. The following primer
sequences were used for qPCR: U6 forward, 5′-CTCGCTTCGGCAGCACA-3′
and reverse, 5′-AACGCTTCACGAATTTGCGT-3′; miR-15a forward,
5′-ACACTCCAGCTGGGTAGCAGCACATAATGGTTTG-3′ and reverse,
5′-CTCAACTGGTGTCGTGGA-3′; GAPDH forward, 5′-AAGAAGGTGGTGAAGCAGGC-3′
and reverse, 5′-TCCACCACCCAGTTGCTGTA-3′; and GXP4 forward,
5′-GCCGGGACCATGTGCGCGTC-3′ and reverse,
5′-CAGGATCCGCAAACCACACTC-3′. Small endogenous nucleolar U6 snRNA
and GAPDH were used as internal controls for normalization of
miR-15a and GXP4 mRNA expression levels, respectively. Relative
expression levels were calculated using the 2−ΔΔCq
method (17).
Western blotting
Following transfection, LNCAP cells were lysed in 1×
ice-cold RIPA lysis buffer (Beyotime Institute of Biotechnology),
centrifuged at 13,000 × g at 4°C for 10 min, and the deposit was
discarded. Protein concentration was determined using the BCA kit
(Takara Biotechnology, Inc.). A total of 35 ug protein was
determined via 10% SDS-PAGE, transferred onto PVDF membranes and
blocked with 5% non-fat milk dissolved in 1×TBST for 1 h at room
temperature. The membranes were incubated with primary antibodies
against GPX4 (1:1,000; cat. no. ab252833; Abcam) and GAPDH
(1:1,000; cat. no. ab8245; Abcam) overnight at 4°C. Following the
primary incubation, membranes were incubated with corresponding
secondary antibodies including goat anti-rabbit IgG H&L (HRP;
1:20,000; cat. no. ab6721; Abcam) and rabbit anti-mouse IgG H&L
(HRP; 1:20,000; cat. no. ab6728; Abcam) for 2 h at room
temperature. Protein bands were visualized using the ECL kit
(Invitrogen; Thermo Fisher Scientific, Inc.) and captured using the
Gel imaging system (BD Biosciences). All antibodies were purchased
from Abcam and diluted according to the manufacturer's
instructions.
Biotin RNA pull-down assay
Biotin-labelled wild-type (WT) miR-15a
(Bio-miR-15a-WT), biotin-labelled mutated (MUT) miR-15a
(Bio-miR-15a-MUT) and biotin-labelled NC (Bio-NC) were purchased
from Guangzhou RiboBio Co., Ltd. The sequences were as follows:
Bio-miR-15a-WT, 5′-Bio-UAGCAGCACAUAAUGGUUUGUG-3′; Bio-miR-15a-MUT,
5′-Bio-UGAUGAUGCAUAAUGGUUUGUG-3′; and Bio-NC,
5′-Bio-UUUGUACUACACAAAAGUACUG-3′. Next, RNA pull-down was performed
by Pierce™ Magnetic RNA-Protein Pull-Down kit (Thermo Fisher
Scientific, Inc.) according to manufacturer's instructions.
Briefly, LNCAP cells were transfected with Bio-miR-15a-WT,
Bio-miR-15a-MUT or the NC. Following transfection for 48 h at 37°C,
cells were lysed and whole cell extractions were collected by
centrifuging at 13,000 × g at 4°C for 10 min. Subsequently, 100 µl
lysates were incubated with Streptavidin Magnetic Beads
(MedChemExpress) overnight at 4°C according to manufacturer's
protocol. The biotinylated nucleic acids coated beads were
subsequently diluted using magnetic to separate the co-precipitated
RNA. The samples were purified, reverse transcribed into cDNA and
detected via RT-qPCR analysis.
Dual-luciferase reporter assay
The sequence of GPX4 mRNA (NM_002085.5) was obtained
from GenBank of The National Center for Biotechnology Information.
The WT complete sequence and MUT binding sites of miR-15a complete
sequence of GPX4 3′-UTR were subcloned into the psiCHECK™−2 vector
(Promega Corporation) and named as GPX4-UTR WT and GPX4-UTR MUT,
respectively. LNCAP cells were transfected with GPX4-UTR WT or
GPX4-UTR MUT and miR-15a mimics, anti-miR-15a or mi-NC using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Cells were lysed and the luciferase activities were measured using
the dual-luciferase reporter assay kit (Promega Corporation) 48 h
post-transfection, which was normalized by comparing with
Renilla luciferase activity.
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay (Abcam) was performed to assess the
proliferation of LNCAP cells. Cells were digested and seeded into
96-well plates at a density of 1×103 cells/well and
transfected with mi-NC, miR-15a mimics, si-NC, siGPX4 or treated
with normal DMEM (Gibco; Thermo Fisher Scientific, Inc.) as the
control, respectively. CCK-8 solution (10 µl) was added to each
well and further incubated for 2 h. Absorbance was measured at a
wavelength of 450 nm using an absorbance reader (Multiscan MK3;
Thermo Fisher Scientific, Inc.). The inhibitory ratio of each group
was calculated as follows: (1-average absorbance value ÷ average
absorbance value of control group) ×100%.
Lactate dehydrogenase (LDH)
detection
LDH is considered a cell damage biomarker (4). First, 1×105 cells were
seeded per well of the 6-well plates and transfected with mi-NC,
miR-15a mimics, si-NC, siGPX4 and siGPX4. The levels of LDH were
detected using the L-Lactate Dehydrogenase (Crude Enzyme) kit (cat.
no. P3532-100 ml) purchased from Beyotime Institute of
Biotechnology according to the manufacturer's instructions.
Iron assay
LNCAP cells were transfected with mi-NC, miR-15a
mimics, si-NC and siGPX4 for 48 h at 37°C and then the
intracellular ferrous iron (Fe2+) level was determined
using the Iron Assay kit (Abcam), according to the manufacturer's
instructions. Cells were collected and washed twice in PBS, and
subsequently homogenized with buffer in the kit. The supernatant
was collected and incubated with iron reducer at room temperature
for 30 min. Next, the supernatant was mixed with iron probe and
incubated at room temperature for 1 h. Absorbance was measured at a
wavelength of 593 nm using a Multiscan MK3 absorbance reader
(Thermo Fisher Scientific, Inc.).
ROS activity assay
Cells were seeded into 6-well plates and transfected
with mi-NC, miR-15a mimics, si-NC and siGPX4. Following the culture
at 37°C for 48 h, cells were stained with fresh medium containing
C11-BODIPY dye (Beyotime Institute of Biotechnology) at 37°C for 20
min in culture incubator. Next, cells were collected, washed and
suspended in PBS. The cell suspension was detected using a FACSC
Calibur (BD Biosciences).
Evaluation of the mitochondrial
membrane potential (MMP)
LNCAP cells were transfected with mi-NC, miR-15a
mimics, si-NC and siGPX4 at 37°C for 48 h, and their MMP was
subsequently analyzed via staining using the JC-1 Assay kit
(Beyotime Institute of Biotechnology). Results were detected using
the FACSC Calibur (BD Biosciences), according to the manufacturer's
instructions.
Lipid peroxidation detection
LNCAP cells were lysed with 1X ice-cold RIPA lysis
buffer (Beyotime Institute of Biotechnology), centrifuged at 13,000
× g at 4°C for 10 min and the deposit was discarded. The
supernatant was used to detect lipid peroxidation using the Lipid
Peroxidation MDA Assay kit (Beyotime Institute of Biotechnology),
according to the manufacturer's instructions.
Statistical analysis
Data are presented as the mean ± SD. Analysis of
statistical differences were performed using SPSS 20 software (IBM
Corp.) Unpaired Student's t-test was used to compare differences
between two groups, while one-way ANOVA followed by Tukey's post
hoc test was used to compare differences between multiple groups.
P<0.05 was considered to indicate a statistically significant
difference. Each experiment was performed three times.
Results
miR-15a affects GPX4 expression in
prostate cancer cells
GPX4 plays a critical role in ferroptosis, whereby
its suppression is considered a characteristic of ferroptosis
(4–7). To determine the role of miR-15a in
prostate cancer, Targetscan was used to predict the potential
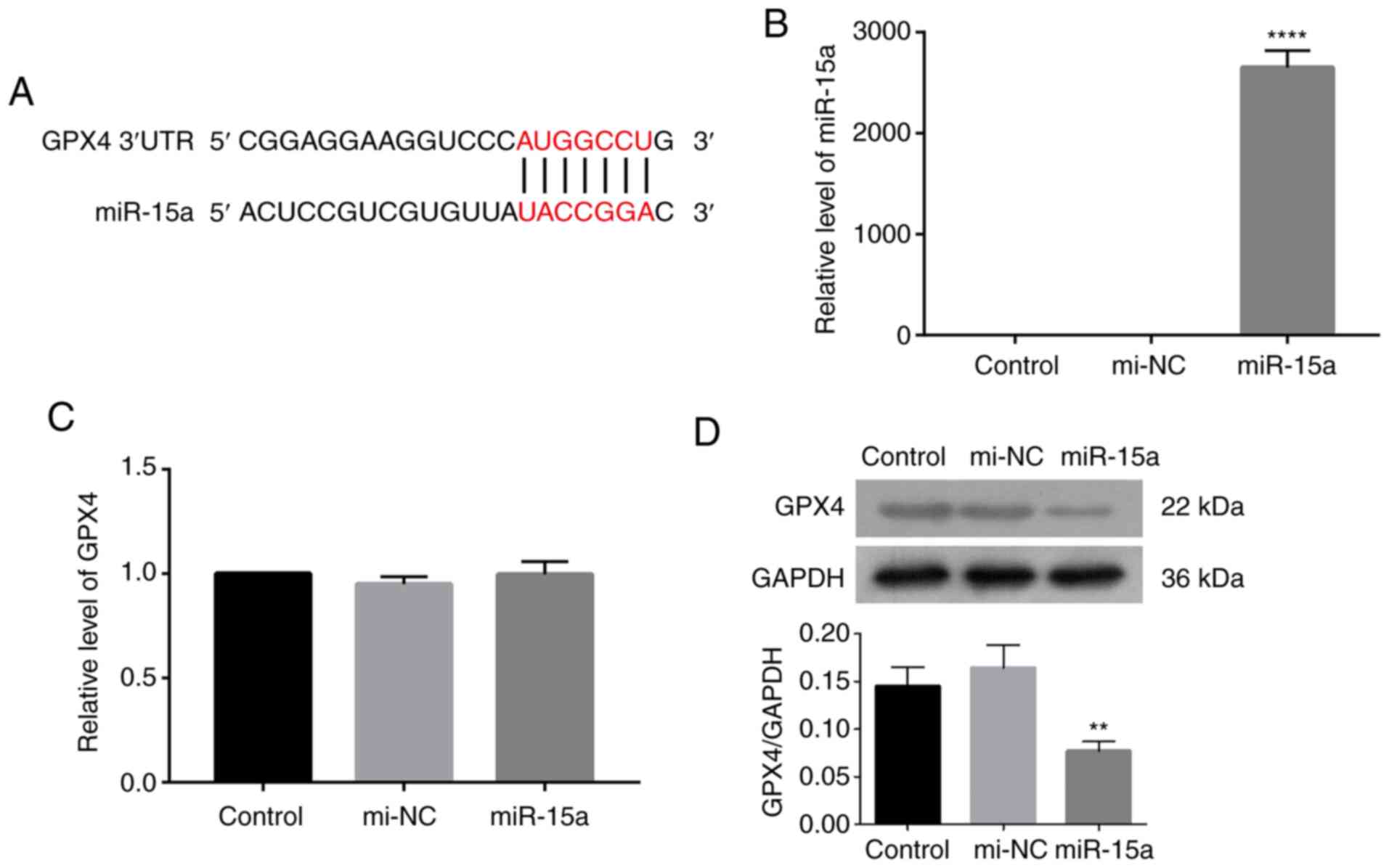
targets of miR-15a. The results revealed a potential binding
between miR-15a and GPX4 (Fig.
1A). The present study aimed to investigate the direct
regulation of miR-15a to GPX4 by transfecting LNCAP cells with
miR-15a mimics. Transfection efficiency of miR-15a mimics was
assessed via RT-qPCR analysis. Results indicated that transfection
of miR-15a mimics significantly upregulated miR-15a level in LNCAP
cells compared with that of LNCAP cells transfected with mimic
controls (Fig. 1B). As presented
in Fig. 1C and D, transfection
with miR-15a mimics decreased GPX4 protein expression, while little
change was observed at the mRNA level. Taken together, these
results suggest that miR-15a may regulate GPX4 expression at a
translational level.
Validation of direct interaction
between miR-15a and GPX4
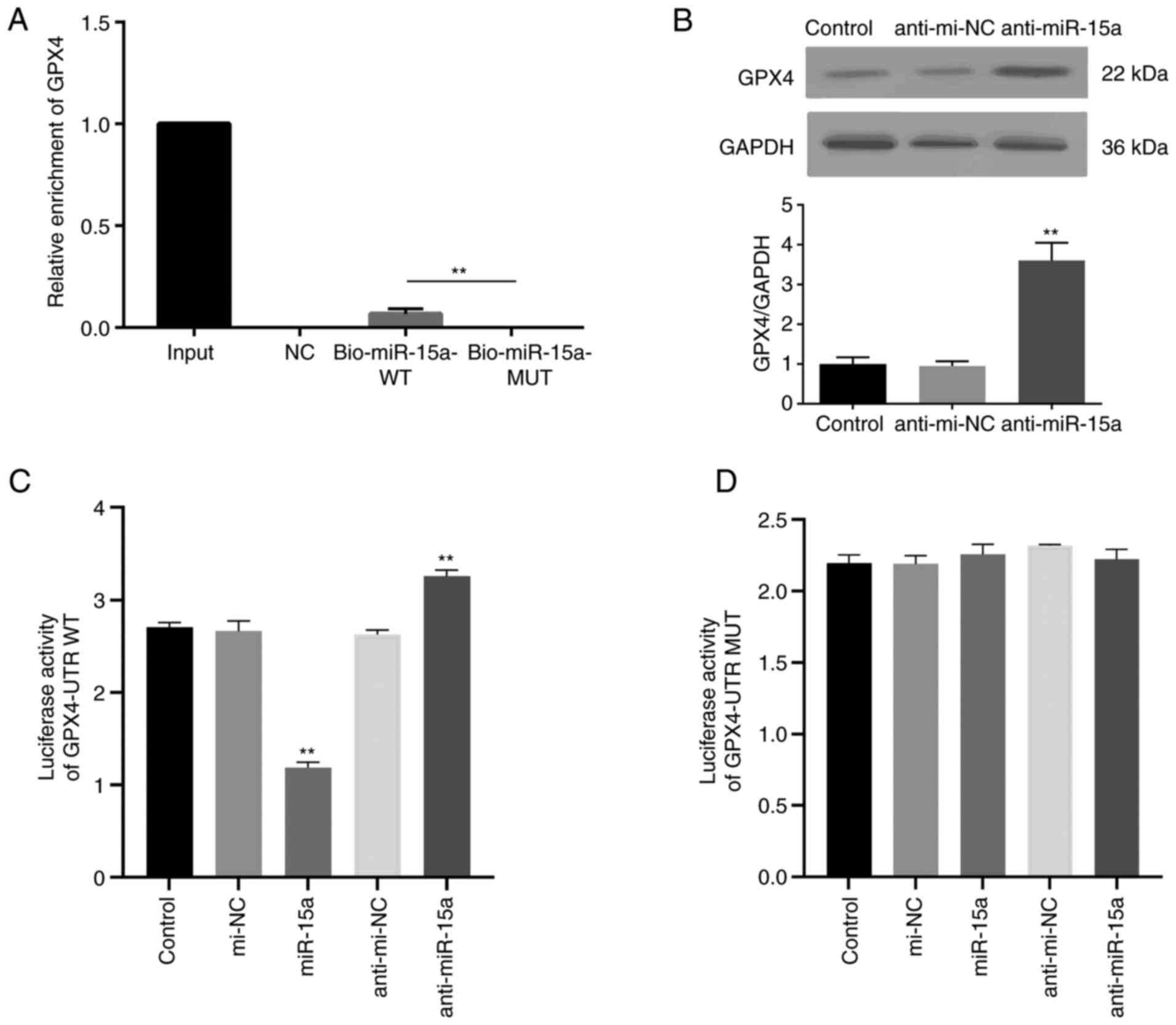
The direct interaction between miR-15a and GPX4 was
assessed. Bio-miR-15a-WT, Bio-miR-15a-MUT and Bio-NC were
transfected into LNCAP cells, and the enrichment of GPPX4 was
assessed. As presented in Fig. 2A,
GPX4 mRNA was detected in the WT miR-15a group but not the MUT
group. To further identify the regulatory effect of miR-15a on
GPX4, miR-15a inhibitor (anti-miR-15a) were used. Usually, miRNA
inhibitors do not decrease miRNA expression (18,20).
The results of the present study demonstrated that transfection
with miR-15a inhibitor did not decrease miR-15a expression
(Fig. S1). MiRNA inhibitors can
competitively bind with miRNA to suppress the association between
miRNA and target mRNA, and reverse the inhibitory effect of miRNA
on the translation of the target mRNA (18,20).
As presented in Fig. 2B,
transfection with miR-15a inhibitor notably increased GPX4 protein
expression. The dual-luciferase reporter assay was performed with
transfection of plasmids containing GPX4-UTR WT or GPX4-UTR MUT
miR-15a binding sequences of GPX4 mRNA 3′-UTR. The luciferase
activity of the GPX4-UTR WT group decreased following
co-transfection with miR-15a mimics, the effects of which were
reversed following transfection with anti-miR-15a (Fig. 2C). However, the luciferase activity
of the GPX4-UTR MUT group did not change following transfection
(Fig. 2D). Collectively, these
results highlight the direct regulatory role of miR-15a on
GPX4.
miR-15a inhibits prostate cancer cell
proliferation and causes cell death via GPX4
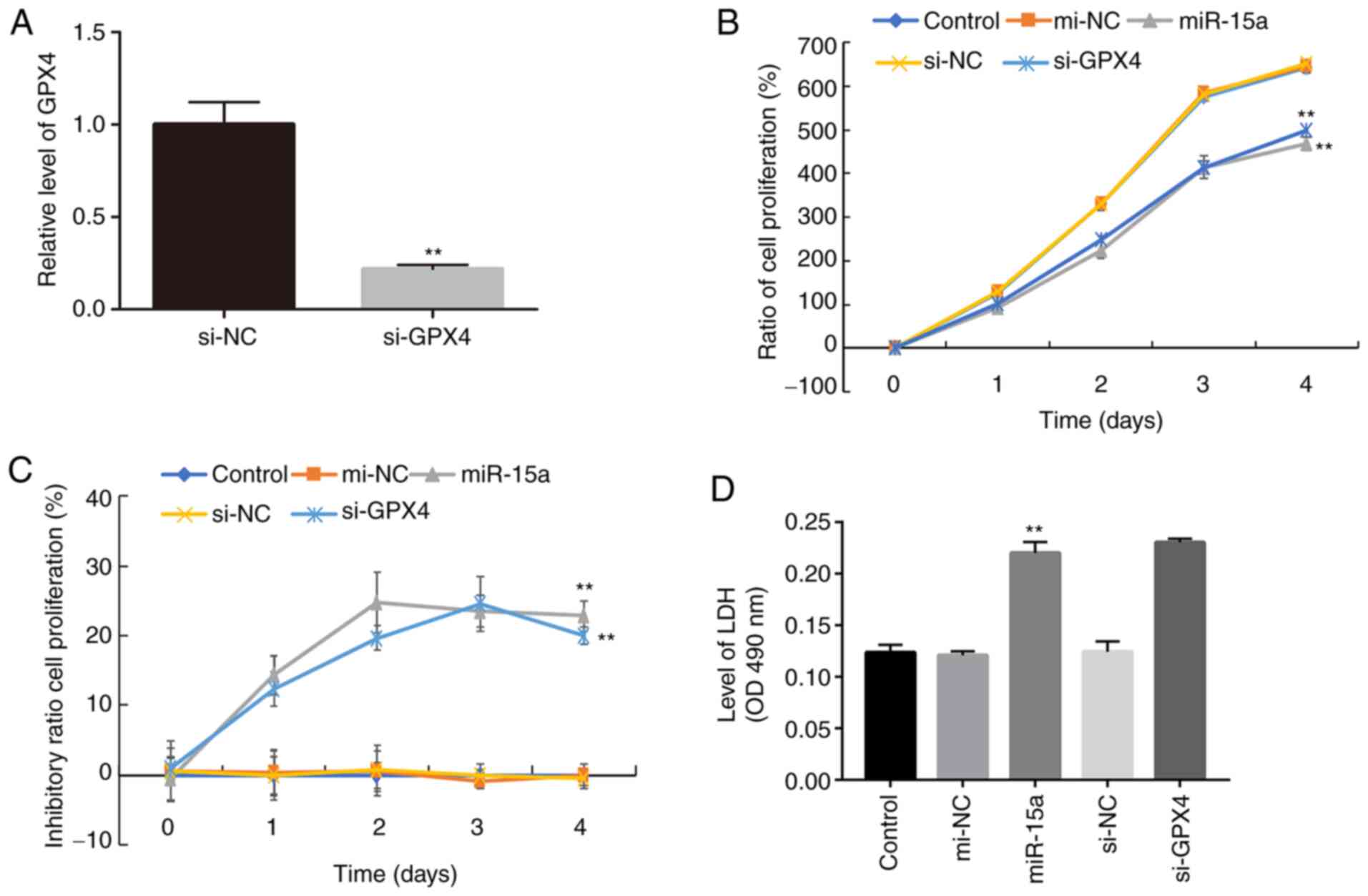
To further elucidate the role of miR-15a and GPX4 in
prostate cancer cells, cell proliferation was assessed following
transfection with miR-15a mimics or si-GPX4. The results
demonstrated that transfection with si-GPX4 significantly decreased
GPX4 mRNA expression in LNCAP cells (Fig. 3A). In addition, the results of the
CCK-8 assay demonstrated a significant decline in the proliferation
of cells transfected with miR-15a mimics or si-GPX4 compared with
the control groups (Fig. 3B).
Similar inhibitory effects were observed in the miR-15 mimics and
si-GPX4 groups (Fig. 3C), further
confirming that miR-15a functions by regulating GPX4.
Ferroptosis is a novel type of necrosis,
characterized by destruction of the cell membrane structure and the
release of LDH (4). Thus, the
present study detected LDH levels in the culture medium. As
presented in Fig. 3D, transfection
with miR-15a mimics and si-GPX4 significantly increased LDH levels,
which is associated with elevated cell death (4).
miR-15a induces prostate cancer cell
ferroptosis via GPX4
Ferroptosis is characterized by elevated lipid
peroxidation, disrupted mitochondrial membrane density and
accumulation of intracellular iron (3,4,9).
Thus, the present study assessed the accumulation of redox active
metal Fe2+, ROS, integrity of mitochondrial membrane and
lipid peroxidation. The levels of intracellular Fe+ and
lipid ROS accumulation notably increased in LNCAP cells transfected
with miR-15a mimics or si-GPX4 (Fig.
4A and B). In addition, the present study detected the
integrity of mitochondrial membrane via flow cytometric analysis
using JC-1 staining. The decreased membrane potential following
transfection with miR-15a mimics and si-GPX4 indicated disrupted
membrane structure (Fig. 4C).
Furthermore, transfection with both miR-15a mimics and si-GPX4
significantly increased the levels of MDA (Fig. D), which is a
marker of lipid peroxidation (9).
Taken together, these results suggest that miR-15a triggers
ferroptosis via GPX4 in prostate cancer cells.
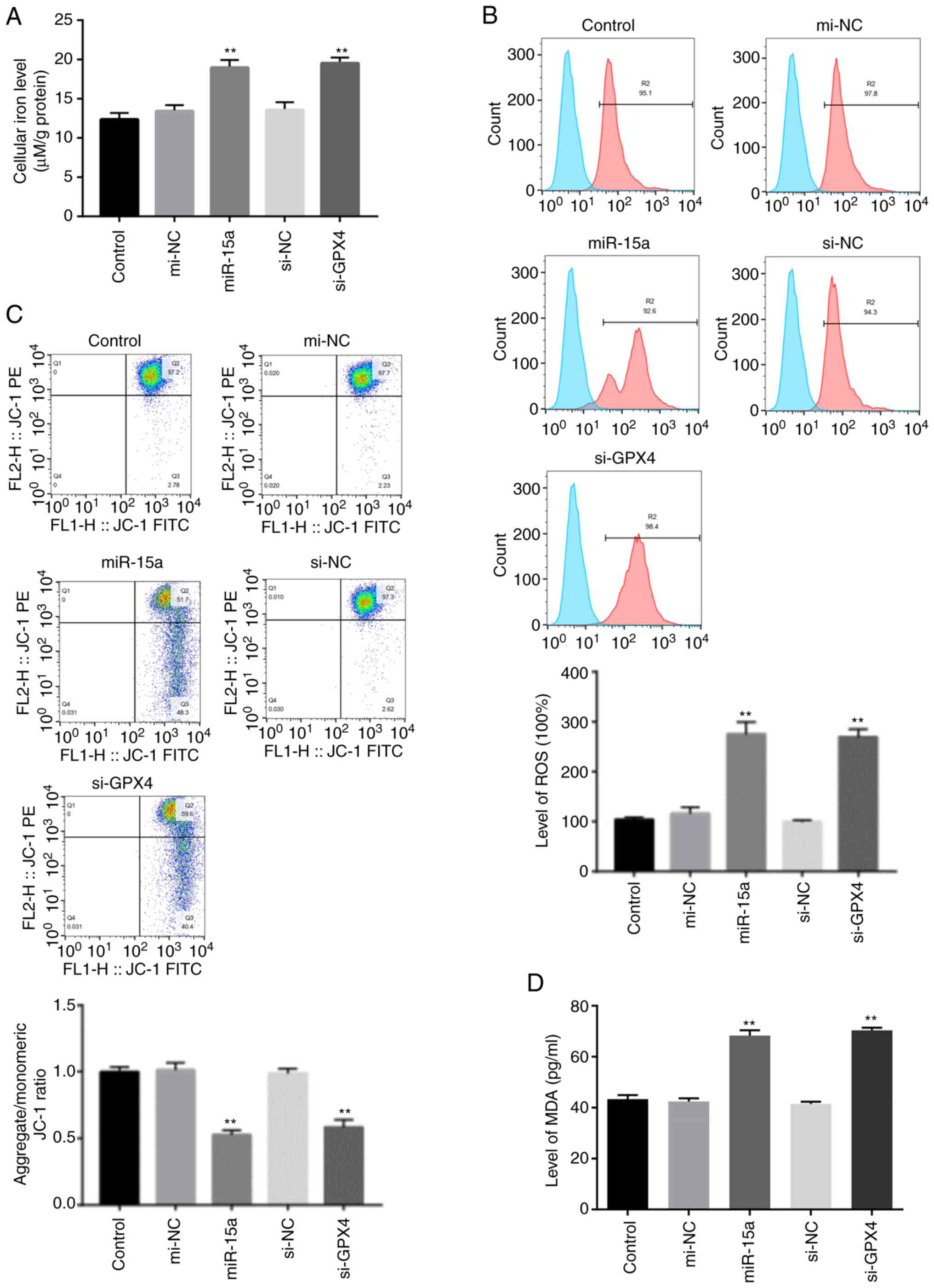 | Figure 4.miR-15a induces prostate cancer cell
ferroptosis via GPX4. (A) Intracellular ferrous iron levels were
determined via the iron assay in LNCAP cells transfected with
miR-15a mimics, si-GPX4 or NCs. (B) Lipid ROS levels were measured
via flow cytometric analysis using C11-BODIPY in LNCAP cells
transfected with miR-15a mimics, si-GPX4 or NCs. (C) MMP was
detected via JC-1 staining and flow cytometric analysis in LNCAP
cells transfected with miR-15a mimics, si-GPX4 or NCs. (D) MDA
levels were measured in LNCAP cells transfected with miR-15a
mimics, si-GPX4 or NCs. **P<0.01 vs. control group. miR,
microRNA; GPX4, glutathione peroxidase 4; si, small interfering;
NC, negative control; ROS, reactive oxygen species; MMP,
mitochondrial membrane potential; MDA, malondialdehyde. |
Discussion
Recent studies have reported several novel
strategies for diagnosis and therapies of cancers, among which,
serum miRNAs are promising targets for cancer research and
treatment (25–27). miRNAs manly function by interacting
with the 3′-UTR of target mRNAs to induce degradation of mRNAs or
suppress their translation, leading to the alteration of regulatory
factors in cellular physiological processes, including cell
proliferation, differentiation, autophagy and apoptosis (28). Several studies have revealed the
function of miRNAs in prostate cancer (29,30).
For example, one analysis of miRNAs in prostate lesions identified
global loss of miRNA expression during progression of prostate
cancer (31). In addition,
downregulated miR-15a expression has been observed in patients with
prostate cancer compared with healthy subjects (32). Several studies have implicated that
miR-15a may inhibit the proliferation and metastasis of prostate
cancer cells (23,33).
In the present study, bioinformatics analysis
revealed the potential interaction between miR-15a and the 3′-UTR
of GPX4 mRNA, suggesting that miR-15a may regulate GPX4 expression.
In addition, transfection with miR-15a mimics altered GPX4 protein
expression, but not GPX4 mRNA expression, suggesting that miR-15a
regulates the translation of GPX4. miRNA pull-down and the
dual-luciferase reporter assays were also performed. The enrichment
of GPX4 and the enhanced luciferase activity demonstrated the
direct binding of miR-15a to the 3-™UTR of GPX4 mRNA.
A previous study reported that apoptosis of prostate
cancer cells is involved in the development of prostate cancer
(34). However, the role of
ferroptosis in prostate cancer progression remains unclear.
Ferroptosis is a newly discovered form of ferrous iron-associated
cell death (3,4), first described in 2012 by Dixon et
al (35). Within the past
decade, several studies have revealed the regulatory function of
ferroptosis in different types of cancer (36,37).
As the key suppressor for ferroptosis, GPX4 interacts with
glutathione to decrease peroxidase reaction within biological
membranes, thereby suppressing the accumulation of lipid ROS and
activation of ferroptosis (38).
In the present study, the suppressed cell proliferation and
elevated levels of released cell death biomarker LDH (4) confirmed the induced cell death
following transfection with miR-15a mimics and si-GPX4. The unique
biochemical characteristics of ferroptosis consist of accumulated
ferrous iron and lipid ROS (10,36).
The destructed cell membrane structure induced by ferroptosis can
results in extracellular release of LDH (4). Here, to confirm that the suppressed
cell proliferation was indeed caused by ferroptosis, lipid ROS
accumulation was measured via flow cytometric analysis using
C11-BIODIPY probes, the MMP via JC-1 staining, as well as the
cellular iron level. In accordance with the aforementioned features
of ferroptosis, transfection with miR-15a mimics and si-GPX4
simultaneously increased ROS and Fe2+ generation, and
disrupted the structure of the mitochondrial membrane.
The present study is not without limitations. First,
only one prostate cancer cell line was assessed. Thus, prospective
studies will focus on assessing androgen receptor negative prostate
cancer cell lines. In addition, it would be useful to assess normal
cell lines to determine whether the protocol is selective.
In conclusion, the results of the present study
demonstrated that miR-15a induced ferroptosis of prostate cancer
cells by targeting GPX4. These findings may provide novel insights
and targets for the treatment of prostate cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81772631 and 81974362), the
Shenzhen Science and Technology Innovation Committee (grant nos.
JCYJ20190814121001751, JCYJ201908143000229 and
JCYJ20190814110203636), the Shenzhen Key Medical Discipline
Construction Fund (grant no. SZXK013) and the Natural Science
Foundation of Guangdong Province (grant No. 2018A030313530).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XP and PX initiated the project, designed the
experiments and analyzed the data. PX made the figures and drafted
the initial manuscript. YW performed the experiments and revised
the manuscript. ZD and ZT performed the experiments. XP and PX
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
miRNA
|
microRNA
|
|
GPX4
|
glutathione peroxidase 4
|
|
siRNA
|
small interfering RNA
|
|
ROS
|
reactive oxygen species
|
|
MMP
|
mitochondrial membrane potential
|
|
LDH
|
lactate dehydrogenase
|
References
|
1
|
Omar MI, Roobol MJ, Ribal MJ, Abbott T,
Agapow PM, Araujo S, Asiimwe A, Auffray C, Balaur I, Beyer K, et
al: Introducing PIONEER: A project to harness big data in prostate
cancer research. Nat Rev Urol. 17:351–362. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu C, Xia H, Bai S, Zhao J, Edwards H, Li
X, Yang Y, Lyu J, Wang G, Zhan Y, et al: CUDC-907, a novel dual
PI3K and HDAC inhibitor, in prostate cancer: Antitumour activity
and molecular mechanism of action. J Cell Mol Med. 24:7239–7253.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hassannia B, Vandenabeele P and Vanden
Berghe T: Targeting ferroptosis to iron out cancer. Cancer Cell.
35:830–849. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hao S, Yu J, He W, Huang Q, Zhao Y, Liang
B, Zhang S, Wen Z, Dong S, Rao J, et al: Cysteine dioxygenase 1
mediates erastin-induced ferroptosis in human gastric cancer cells.
Neoplasia. 19:1022–1032. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Shi J, Liu X, Feng L, Gong Z,
Koppula P, Sirohi K, Li X, Wei Y, Lee H, et al: BAP1 links
metabolic regulation of ferroptosis to tumour suppression. Nat Cell
Biol. 20:1181–1192. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seibt TM, Proneth B and Conrad M: Role of
GPX4 in ferroptosis and its pharmacological implication. Free Radic
Biol Med. 133:144–152. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Louandre C, Marcq I, Bouhlal H, Lachaier
E, Godin C, Saidak Z, François C, Chatelain D, Debuysscher V,
Barbare JC, et al: The retinoblastoma (Rb) protein regulates
ferroptosis induced by sorafenib in human hepatocellular carcinoma
cells. Cancer Lett. 356:971–977. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sui X, Zhang R, Liu S, Duan T, Zhai L,
Zhang M, Han X, Xiang Y, Huang X, Lin H and Xie T: RSL3 drives
ferroptosis through GPX4 Inactivation and ROS production in
colorectal cancer. Front Pharmacol. 9:13712018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou Y, Palte MJ, Deik AA, Li H, Eaton JK,
Wang W, Tseng YY, Deasy R, Kost-Alimova M, Dančík V, et al: A
GPX4-dependent cancer cell state underlies the clear-cell
morphology and confers sensitivity to ferroptosis. Nat Commun.
10:16172019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baldassarri M, Fallerini C, Cetta F,
Ghisalberti M, Bellan C, Furini S, Spiga O, Crispino S, Gotti G,
Ariani F, et al: Omic approach in non-smoker female with lung
squamous cell carcinoma pinpoints to germline susceptibility and
personalized medicine. Cancer Res Treat. 50:356–365. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karbasforooshan H, Roohbakhsh A and Karimi
G: SIRT1 and microRNAs: The role in breast, lung and prostate
cancers. Exp Cell Res. 367:1–6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang
G, Song J, Li Z, Zhang Z and Yuan W: Effect of exosomal miRNA on
cancer biology and clinical applications. Mol Cancer. 17:1472018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guelfi G, Cochetti G, Stefanetti V,
Zampini D, Diverio S, Boni A and Mearini E: Next Generation
Sequencing of urine exfoliated cells: An approach of prostate
cancer microRNAs research. Sci Rep. 8:71112018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang K, Wu L, Zhang P, Luo M, Du J, Gao
T, O'Connell D, Wang G, Wang H and Yang Y: miR-9 regulates
ferroptosis by targeting glutamic-oxaloacetic transaminase GOT1 in
melanoma. Mol Carcinog. 57:1566–1576. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo M, Wu L, Zhang K, Wang H, Zhang T,
Gutierrez L, O'Connell D, Zhang P, Li Y, Gao T, et al: miR-137
regulates ferroptosis by targeting glutamine transporter SLC1A5 in
melanoma. Cell Death Differ. 25:1457–1472. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tomita K, Fukumoto M, Itoh K, Kuwahara Y,
Igarashi K, Nagasawa T, Suzuki M, Kurimasa A and Sato T: MiR-7-5p
is a key factor that controls radioresistance via intracellular
Fe2+ content in clinically relevant radioresistant
cells. Biochem Biophys Res Commun. 518:712–718. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang H, Deng T, Liu R, Ning T, Yang H,
Liu D, Zhang Q, Lin D, Ge S, Bai M, et al: CAF secreted miR-522
suppresses ferroptosis and promotes acquired chemo-resistance in
gastric cancer. Mol Cancer. 19:432020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cui Y, Yang Y, Ren L, Yang J, Wang B, Xing
T, Chen H and Chen M: miR-15a-3p suppresses prostate cancer cell
proliferation and invasion by targeting SLC39A7 via downregulating
Wnt/β-catenin signaling pathway. Cancer Biother Radiopharm.
34:472–479. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jia X, Liu H, Xu C, Han S, Shen Y, Miao X,
Hu X, Lin Z, Qian L, Wang Z and Gong W: MiR-15a/16-1 deficiency
induces IL-10-producing CD19+ TIM-1+ cells in
tumor microenvironment. J Cell Mol Med. 23:1343–1353. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Al-Kafaji G, Said HM, Alam MA and Al Naieb
ZT: Blood-based microRNAs as diagnostic biomarkers to discriminate
localized prostate cancer from benign prostatic hyperplasia and
allow cancer-risk stratification. Oncol Lett. 16:1357–1365.
2018.PubMed/NCBI
|
|
23
|
Jin W, Chen F, Wang K, Song Y, Fei X and
Wu B: miR-15a/miR-16 cluster inhibits invasion of prostate cancer
cells by suppressing TGF-β signaling pathway. Biomed Pharmacother.
104:637–644. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bonci D, Coppola V, Musumeci M, Addario A,
Giuffrida R, Memeo L, D'Urso L, Pagliuca A, Biffoni M, Labbaye C,
et al: The miR-15a-miR-16-1 cluster controls prostate cancer by
targeting multiple oncogenic activities. Nat Med. 14:1271–1277.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimomura A, Shiino S, Kawauchi J,
Takizawa S, Sakamoto H, Matsuzaki J, Ono M, Takeshita F, Niida S,
Shimizu C, et al: Novel combination of serum microRNA for detecting
breast cancer in the early stage. Cancer Sci. 107:326–334. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qadir MI and Faheem A: miRNA: A diagnostic
and therapeutic tool for pancreatic cancer. Crit Rev Eukaryot Gene
Expr. 27:197–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yokoi A, Matsuzaki J, Yamamoto Y, Yoneoka
Y, Takahashi K, Shimizu H, Uehara T, Ishikawa M, Ikeda SI, Sonoda
T, et al: Integrated extracellular microRNA profiling for ovarian
cancer screening. Nat Commun. 9:43192018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Omidkhoda N, Wallace Hayes A, Reiter RJ
and Karimi G: The role of MicroRNAs on endoplasmic reticulum stress
in myocardial ischemia and cardiac hypertrophy. Pharmacol Res.
150:1045162019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang J, Jia P, Zhao Z and Shen B: Key
regulators in prostate cancer identified by co-expression module
analysis. BMC Genomics. 15:10152014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hart M, Nolte E, Wach S, Szczyrba J,
Taubert H, Rau TT, Hartmann A, Grässer FA and Wullich B:
Comparative microRNA profiling of prostate carcinomas with
increasing tumor stage by deep sequencing. Mol Cancer Res.
12:250–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leite KR, Tomiyama A, Reis ST,
Sousa-Canavez JM, Sañudo A, Camara-Lopes LH and Srougi M: MicroRNA
expression profiles in the progression of prostate cancer-from
high-grade prostate intraepithelial neoplasia to metastasis. Urol
Oncol. 31:796–801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zidan HE, Abdul-Maksoud RS, Elsayed WSH
and Desoky EAM: Diagnostic and prognostic value of serum miR-15a
and miR-16-1 expression among egyptian patients with prostate
cancer. IUBMB Life. 70:437–444. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aqeilan RI, Calin GA and Croce CM: miR-15a
and miR-16-1 in cancer: Discovery, function and future
perspectives. Cell Death Differ. 17:215–220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Antognelli C, Mezzasoma L, Fettucciari K,
Mearini E and Talesa VN: Role of glyoxalase I in the proliferation
and apoptosis control of human LNCaP and PC3 prostate cancer cells.
Prostate. 73:121–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu M, Gai C, Li Z, Ding D, Zheng J, Zhang
W, Lv S and Li W: Targeted exosome-encapsulated erastin induced
ferroptosis in triple negative breast cancer cells. Cancer Sci.
110:3173–3182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shin D, Kim EH, Lee J and Roh JL: Nrf2
inhibition reverses resistance to GPX4 inhibitor-induced
ferroptosis in head and neck cancer. Free Radic Biol Med.
129:454–462. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bebber CM, Müller F, Prieto Clemente L,
Weber J and von Karstedt S: Ferroptosis in cancer cell biology.
Cancers (Basel). 12:1642020. View Article : Google Scholar : PubMed/NCBI
|