Introduction
Gastrointestinal stromal tumor (GIST) is the most
common mesenchymal tumor of the human gastrointestinal (GI) tract
(1). GISTs occur throughout the GI
tract, but most of them arise in the stomach (60–70%) or small
intestine (20–30%) (2). Activating
mutations in the c-kit gene and PDGFRA gene, encoding
KIT tyrosine kinase and platelet-derived growth factor receptor
tyrosine kinase, respectively, are considered the main oncogenic
drivers of GIST (3). The minority
of GISTs harboring neither c-kit gene nor PDGFRA gene
may have mutations in the NF1, BRAF, or SDH complex
genes (4–6).
GISTs are neoplasms with malignant potential varying
from virtual indolence to rapid progression. Up to 20% of GIST
patients have overt metastases at diagnosis, and the metastases
typically occur in the abdominal cavity or the liver. Small
intestinal GISTs are considered to have a worse prognosis than
gastric GISTs because of their higher risk of metastasis and
tumor-related death (7). Recent
research has shown that small intestinal GISTs exhibit more
aggressive features such as high pathological grade and large size
than gastric GISTs (8). Distinct
transcription profiles related to the anatomical location of GISTs
have also been reported previously (9). The results of hierarchical clustering
analysis of the transcripts showing that GISTs, roughly divided
into two groups such as gastric GISTs and small intestinal GISTs,
may partially account for the more aggressive behavior of small
intestinal GISTs than gastric GISTs of similar size and mitotic
rate. Thus, the widely used risk classifications for GIST
metastasis such as the Armed Forces Institute of Pathology (AFIP)
classification and modified Fletcher classification include tumor
location as a risk factor.
We have recently reported that cell adhesion
molecule 1 (CADM1) is expressed specifically in most small
intestinal GISTs but not in most gastric GISTs (10). CADM1 is a member of the
immunoglobulin superfamily that was initially known as
spermatogenic immunoglobulin superfamily (SgIGSF) and synaptic cell
adhesion molecule (SynCAM) (11).
CADM1 is also a tumor suppressor of lung cancer (TSLC1)
(12). Loss of CADM1
expression probably due to methylation of the CADM1 gene
promoter is frequently found in various types of epithelial cancer,
such as gastric cancer, breast cancer, and esophageal squamous cell
carcinoma (13–15). Therefore, CADM1 in those cancers is
considered to act as a tumor suppressor. In contrast, CADM1 appears
to be a tumor promoter in adult T cell leukemia/lymphoma (ATLL)
(16) and acute myelocytic
leukemia (17,18), which show high CADM1
expression with enhancement of tumor growth, tissue invasion by the
tumor cells, and tumor cell adhesion to the vascular endothelium
(18). Recent research also
reported that CADM1 is highly expressed in ~80% of small
cell lung cancers (SCLCs) and promotes malignant features of them
(19,20).
In the present study, we examined whether CADM1
affects proliferation, migration, invasion, adhesion to endothelial
cells, and transendothelial migration of GIST cells. GIST-T1 cells
with high CADM-1 expression induced by CADM1 cDNA
transfection, when compared to the original GIST-T1 cells with very
low CADM1 expression, had decreased ability to grow,
migrate, and invade but increased ability to adhere to endothelial
cells and emigrate by transendothelial migration. CADM1 might play
a role in tumor metastasis by facilitating adhesion between
vascular endothelial cells and GIST cells and subsequent
transendothelial migration of GIST cells with high CADM1
expression. There is a possibility that the higher expression of
CADM1 in small intestinal GISTs than in gastric GISTs could
contribute to the higher metastatic rate of small intestinal GISTs.
CADM1 might provide a new strategy for inhibiting metastasis of
small intestinal GISTs.
Materials and methods
Tumor tissue samples
Fresh tissue samples of a representative gastric
GIST and a representative small intestinal GIST were collected
intraoperatively, frozen, and stored at −80°C until use. RNA and
protein were extracted from the samples. The RNA and protein in the
present experiments have been used in previous experiments
(10).
Cell lines
A human GIST cell line, GIST-T1, which is derived
from a metastatic pleural tumor from gastric GIST in a Japanese
woman, was purchased from Cosmo Bio. It harbors a heterozygous
c-kit gene mutation at exon 11 (an in-frame deletion of 19
amino acids from Val560 to Tyr578). The GIST-T1 cell line was
maintained in Dulbecco's modified Eagle's medium (Sigma-Aldrich;
Merck KGaA) supplemented with 10% fetal bovine serum (FBS)
(BioWest), 100 U/ml of penicillin G and 100 µg/ml of streptomycin
(Invitrogen; Thermo Fisher Scientific) at 37°C in 5%
CO2. Human umbilical vein endothelial cells (HUVECs)
(Takara) were grown in Endothelial Cell Growth Medium 2
(Takara).
CADM1 cDNA transfection into GIST-T1
cells
Full length of CADM1 cDNA was amplified by
the reverse transcriptase polymerase chain reaction (PCR) using the
primers listed in Table SI, Ampli
Taq Gold (Thermo Fisher Scientific), and mRNA extracted from a
small intestinal GIST highly expressing CADM1 mRNA.
Amplified DNA fragment was electrophoresed and collected. After
cutting with the EcoRI and XhoI enzymes, it was subcloned into
EcoRI and XhoI sites of the pcDNA™ 3.1/Zeo (+) mammalian expression
vector (Thermo Fisher Scientific). To verify product authenticity,
the obtained vector with CADM1 cDNA was sequenced using ABI
BigDye Terminator ver. 3.1 (Applied Biosystems; Thermo Fisher
Scientific) and an ABI Prism 3100-Avant Genetic Analyzer (Applied
Biosystems; Thermo Fisher Scientific). GIST-T1 cells
(1×106) were suspended with 3 µg of the vector with
full-length CADM1 cDNA in 100 µl of Cell Line Nucleofector
kit V solution (Lonza) and electroporated using the Amaxa
Nucleofector II machine (program T-030) (Lonza) according to the
manufacturer's protocol. GIST-T1 cells stably expressing
CADM1 (GIST-T1-CAD) were selected in 250 µg/ml of Zeocin
(Thermo Fisher Scientific), and a monoclonal cell population was
isolated by limiting dilution. All experiments using recombinant
DNA were approved by the Committee for Recombinant DNA Experiments
of Hyogo College of Medicine (no. 24015).
Western blotting
GIST-T1 cells, GIST-T1-CAD cells, a representative
gastric GIST tissue, and a representative small intestinal GIST
tissue were lysed in CelLytic M Cell Lysis Reagent (Sigma-Aldrich;
Merck KGaA) containing 5 mM NAF, 1 mM Na3VO4,
and proteinase inhibitor cocktail (Roche). As described previously
(10), almost all gastric GISTs
express a very low level of CADM1 protein and almost all small
intestinal GISTs apparently express CADM1 protein. Western blot
analysis was performed as previously reported (10). Briefly, anti-CADM1 chicken
monoclonal antibody (clone. 3E1, MBL International), anti-KIT
rabbit polyclonal antibody (A4502, Dako) or anti-β-actin mouse
monoclonal antibody (ab8226, Abcam) were used for primary
antibodies after electrophoresis and membrane transfer. Then,
membranes were incubated either with horse radish peroxidase
(HRP)-conjugated donkey anti-chicken IgY antibody (EMD Millipore,
Sigma-Aldrich; Merck KGaA), HRP-conjugated goat anti-rabbit IgG
antibody, or HRP-conjugated goat anti-mouse IgG antibody (Dako)
after the electrophoresis and membrane transfer. Proteins of
interest were then visualized by incubation with enhanced
chemiluminescence (ECL) reagent (Promega).
Real Time (RT)-quantitative (q)
PCR
Total RNA was extracted from GIST-T1 cells,
GIST-T1-CAD cells, a representative gastric GIST tissue, and a
representative small intestinal GIST tissue using RNeasy mini kit
(Qiagen, Inc.), and 10 µg of total RNA was applied for RT-qPCR
templates. As described previously (10), almost all gastric GISTs express a
very low level of CADM1 mRNA and almost all small intestinal
GISTs apparently express CADM1 mRNA. TaqMan RT-qPCR analysis
was performed using the Applied Biosystems STEP ONE™ standard
real-time PCR system (Applied Biosystems; Thermo Fisher Scientific)
and sets of primers and probes of Human CADM1
(Hs00204937_m1) and Human GAPDH (Hs99999905_m1) in TaqMan™
Gene Expression Assay (4331182, Applied Biosystems; Thermo Fisher
Scientific) according to the manufacturer's instructions.
Cell proliferation assay
GIST-T1 and GIST-T1-CAD cells were plated in 24-well
plates (Corning Incorporated) at 2×104 cells per well in
growth medium. After incubation for 1, 3, 5, and 7 days, cells were
trypsinized, resuspended in Accumax (Innovative Cell Technologies),
and counted by hemocytometer (Z1, Beckman Coulter). Six wells were
used for each cell type in each experiment. The cell proliferation
assay was repeated three times.
Migration assay
Migration ability was assessed by the wound-healing
assay. GIST-T1 and GIST-T1-CAD cells were seeded in 6-well plates
at 3×105 cells and allowed to grow to 90% confluence.
The cell monolayer was scratched with a sterile micropipette tip,
and then serum-free medium was added into plates after washing the
cells thrice with PBS. Photographs of images captured at ×200
magnification were taken at the same six selected locations for
each well under a phase contrast microscope (All-in-One Microscope;
Keyence). The area that remained clear after 0, 1, 2, 3, and 4 days
was quantified with ImageJ (National Institutes of Health) and the
covered area was calculated by comparing to the area of the wound
at day 0. This assay was repeated three times.
Migration and invasion assays
The migration assay was performed using Falcon cell
culture inserts (Corning Incorporated) without Matrigel and the
invasion assay was performed using 24-well BD Bio-Coat Matrigel
Invasion Chambers (BD Biosciences) according to the manufacturer's
protocol. GIST-T1 and GIST-T1-CAD cells were resuspended at a
density of 5×105 cells/l in 0.5 ml of the serum-free
medium and added into the upper chamber of the insert. DMEM
supplemented with 10% FBS (0.75 ml) was added to the lower
chambers. After incubation for 2 days, non-migrated or non-invaded
cells were removed from the upper surface of the membranes using a
cotton tipped swab. The cells adhering to the bottom surface of the
membrane were fixed and permeabilized in 10% neutral formalin and
100% methanol, respectively. Migrated or invaded cells were stained
by Giemsa staining and counted in nine selected microscope fields
per membrane. The experiments were conducted three times.
Tumor-endothelial cell adhesion
assay
Static adhesion assay using fluorescence-labeled
tumor cells was performed. HUVECs (2.5×105 cells/well)
pretreated with or without 10 ng/ml TNF-α (Invitrogen; Thermo
Fisher Scientific) were cultured in 96-well plates overnight. TNF-α
has the potential to stimulate endothelial cell adhesion. GIST-T1
and GIST-T1-CAD cells were labeled with 2 µg/ml Calcein-AM (Dōjindo
Laboratories) at 37°C for 30 min, washed thrice with PBS, and
resuspended at 2.5×106 cells/ml with serum-free DMEM,
and followed by pipetting onto confluent HUVECs monolayers. After
coculturing for 2 h, medium and unbounded tumor cells were removed
and discarded. Adherent tumor cells and endothelial cells were
washed three times with PBS. Then the amount of Calcein-AM
fluorescence was measured using a fluorescence microplate reader
(2030 ARVO X4, PerkinElmer Life and Analytical Sciences), at an
excitation wavelength of 485 nm and emission wavelength of 530
nm.
Transendothelial migration assay
HUVECs (2×105) pretreated with 10 ng/ml
TNF-α were seeded onto 24-well Transwell Inserts and cultured
overnight. After formation of a confluent HUVEC monolayer, tumor
cells labeled with Calcien-AM were added to the upper chamber, and
cells were cocultured for 48 h. After incubation, the non-migrated
cells which were present on the upper side of the membrane were
removed with a cotton tipped swab, and the transmigrated cells on
the bottom side of the membrane were fixed with 10% neutral
formalin. Transmigrated cells were visualized using a fluorescence
microscope and counted from 10 random fields under ×200
magnification. Experiments were performed in triplicate and
repeated three times.
Statistical analysis
Statistical analysis of proliferation assay,
wound-healing assay was performed by two-way mixed ANOVA followed
by Bonferroni's multiple comparison test. The significance of cell
migration and Matrigel invasion in transwell assay, adhesion assay
and transendothelial migration assay was analyzed by unpaired
Student t-test. P<0.01 was considered statistically
significant.
Results
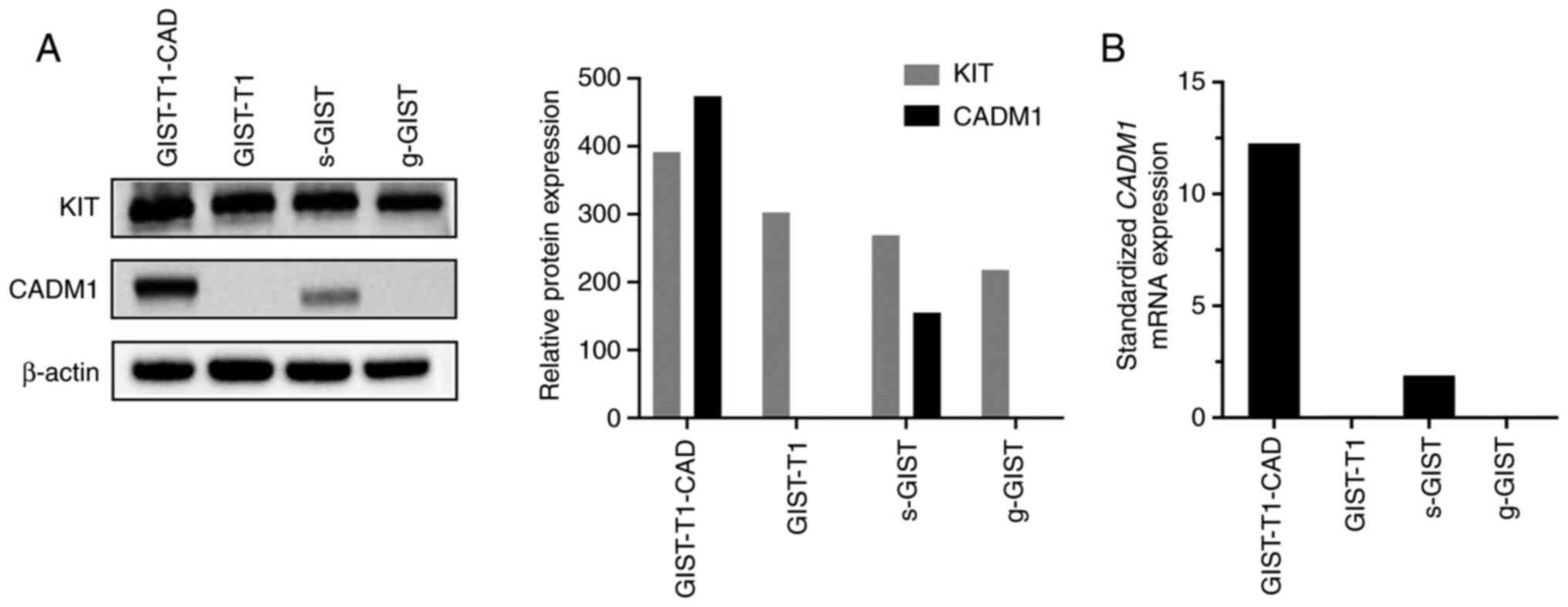
GIST-T1-CAD cells show high expression
of CADM1
GIST-T1 cells are a cell line of GIST cells
originally derived from the stomach. Consistent with our previous
report (10) showing that
CADM1 expression was much weaker in GISTs of gastric origin
than in GISTs of small intestinal origin, no CADM1 protein and
CADM1 mRNA were detected in original GIST-T1 cells by
western blotting and RT-qPCR, respectively (Fig. 1A and B). Using transfection of full
length of CADM1 cDNA into GIST-T1 cells, we tried to
establish GIST-T1 cells stably expressing CADM1 (GIST-T1-CAD
cells). Western blotting and RT-qPCR, respectively, revealed high
expression of CADM1 protein and CADM1 mRNA in the obtained
GIST-T1-CAD cells (Fig. 1A and
B).
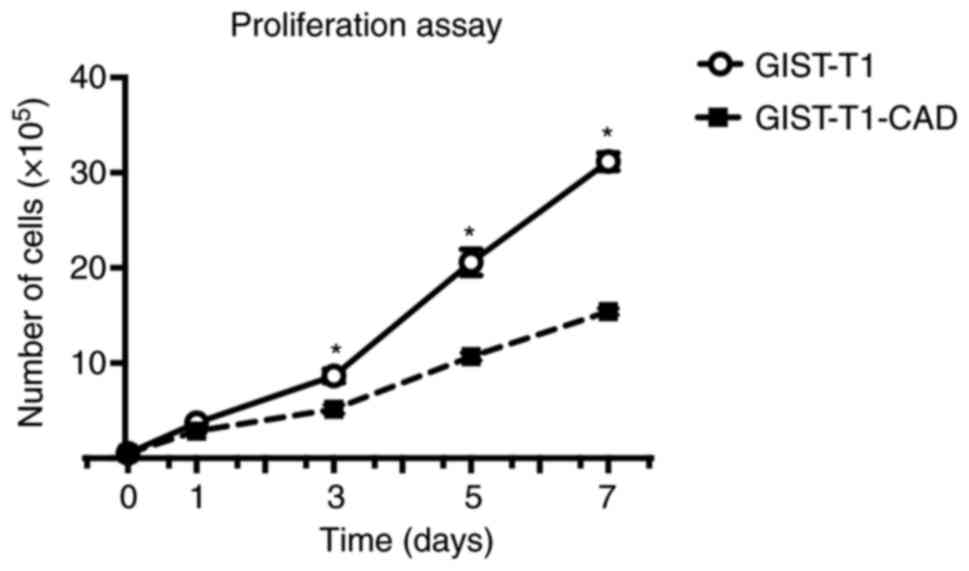
High CADM1 expression suppresses the
proliferation, migration, and invasion of cultured GIST cells
To investigate whether CADM1 is involved in the
proliferation of GIST cells, we compared proliferative ability
between GIST-T1 cells and GIST-T1-CAD cells. Cell number was
counted at days 0, 1, 3, 5, and 7 after seeding with
2×104 of both cells. After day 3, the number of
GIST-T1-CAD cells was significantly smaller than the number of
GIST-T1 cells (Fig. 2)
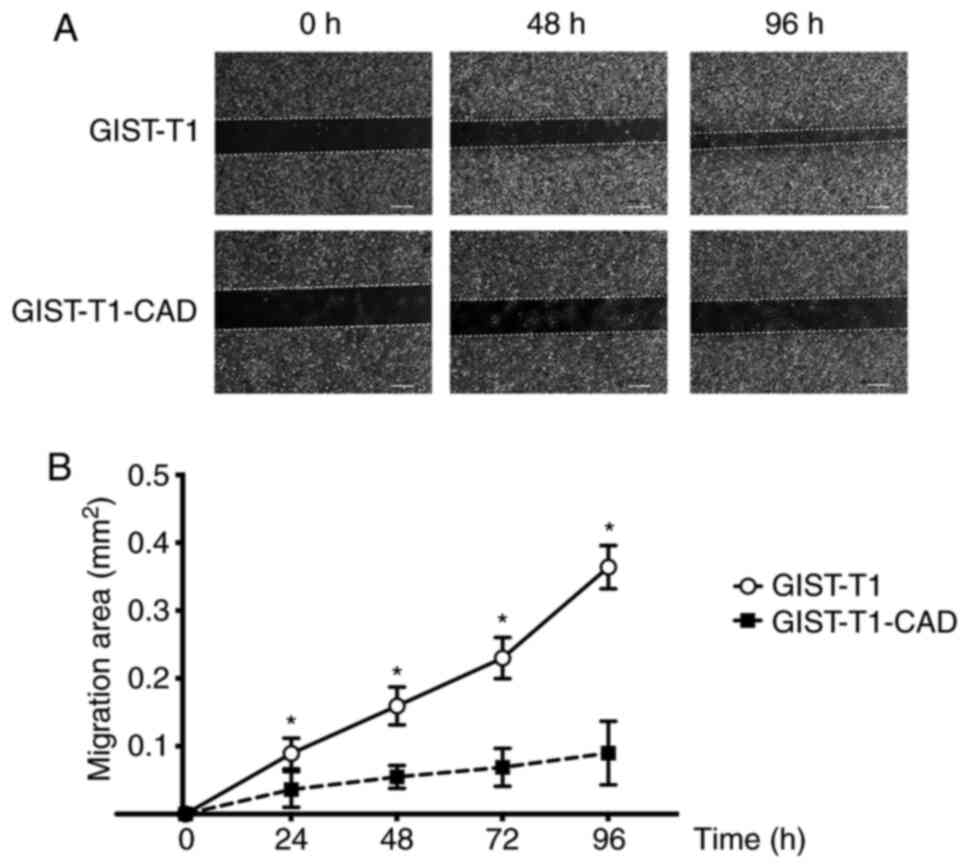
(P<0.001). To evaluate the effect of CADM1 on the ability of
GIST cells to migrate, we performed a wound healing assay. Closure
of scratches in GIST-T1 and GIST-T1-CAD cell monolayers was
measured at 0, 1, 2, 3, and 4 days. Closure of scratches in
GIST-T1-CAD cell monolayers was significantly slower than closure
of scratches in GIST-T1 cell monolayers at 1, 2, 3, and 4 days
(Fig. 3A and B) (P<0.001). We
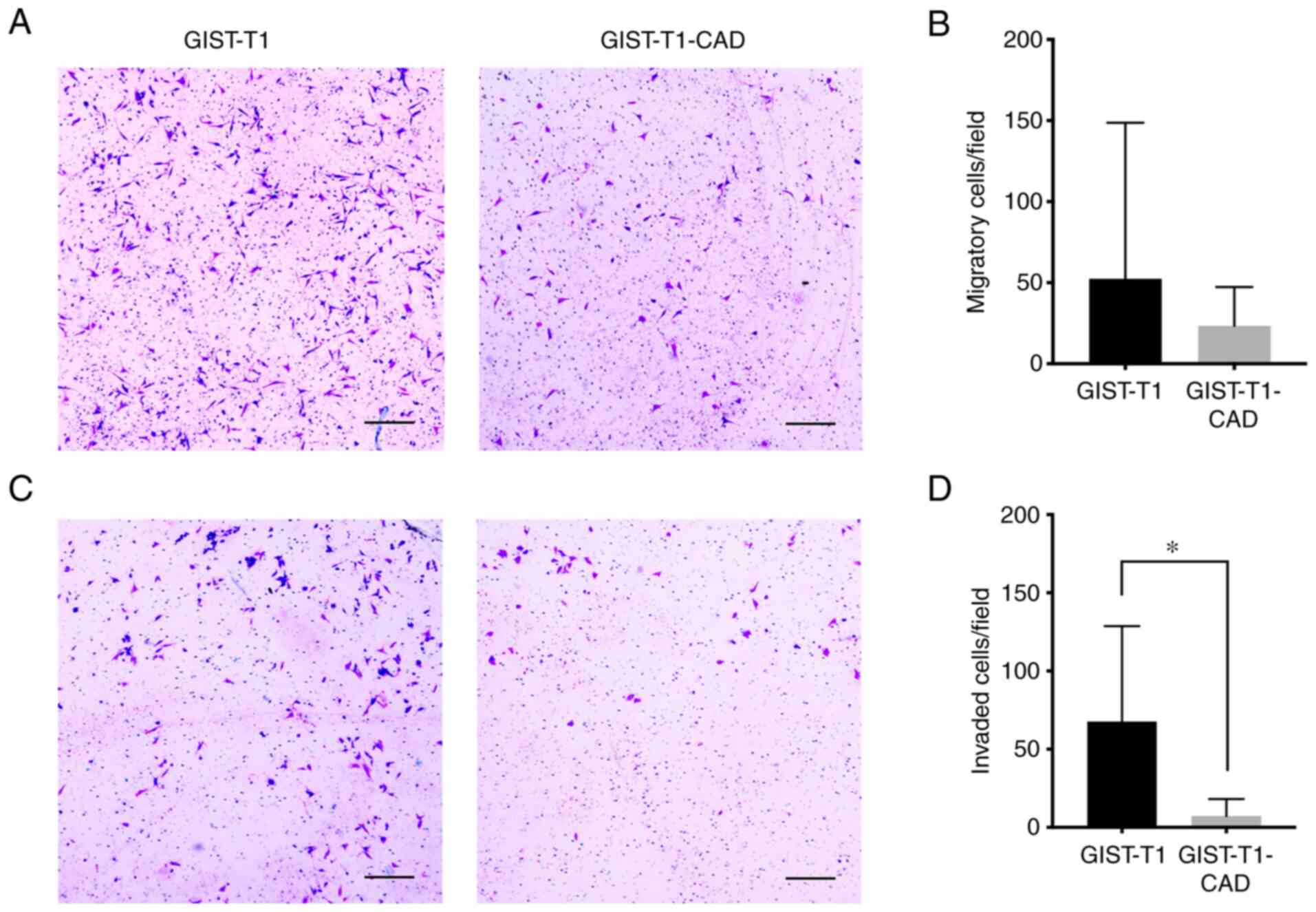
also examined the effect of CADM1 on migration of GIST cells
through transwell membranes without Matrigel and invasion of GIST
cells through transwell membranes coated with Matrigel. Fewer
GIST-T1-CAD cells than GIST-T1 cells appeared to migrate through
the transwell membrane (Fig. 4A and
B), but the difference was not statistically significant (P=
0.1416). On the other hand, statistically significantly fewer
GIST-T1-CAD cells than GIST-T1 cells invaded the Matrigel-coated
transwell membrane (Fig. 4C and D)
(P<0.001).
High CADM1 expression augments
adherence to human endothelial cells and transmigration of cultured
GIST cells through human endothelium
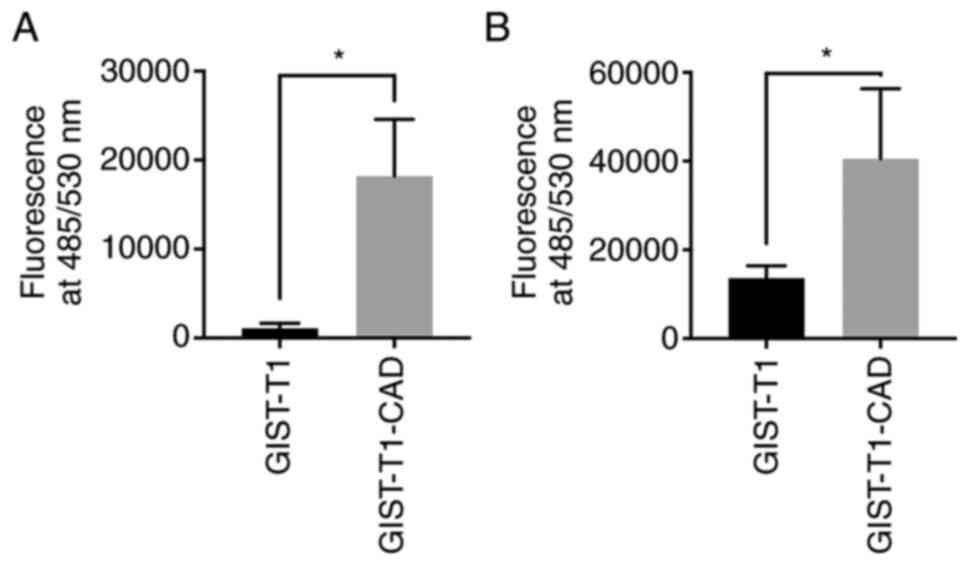
To explore the effect of CADM1 on GIST cell-vascular
endothelial cell adhesion, we performed a static adhesion assay
based on the binding of fluorescence-labeled GIST-T1 cells and
fluorescence-labeled GIST-T1-CAD cells to HUVECs monolayers. We
firstly examined the ability of tumor cells to adhere to
TNF-α-unstimulated endothelium. GIST-T1-CAD cell-HUVEC adherence
was 16.6 times greater than GIST-T1 cell-HUVEC adherence (Fig. 5A) (P<0.001). After overnight
stimulation of HUVECs by TNF-α (10 ng/ml for 12 h), the number of
GIST-T1-CAD cells adhering to HUVECs and GIST-T1 cells adhering to
HUVECs was augmented. Even after stimulation of HUVECs by TNF-α,
the number of GIST-T1-CAD cells adhering to HUVECs was 3 times
greater than that of GIST-T1 cells adhering to HUVECs (Fig. 5B) (P<0.0001). To determine the
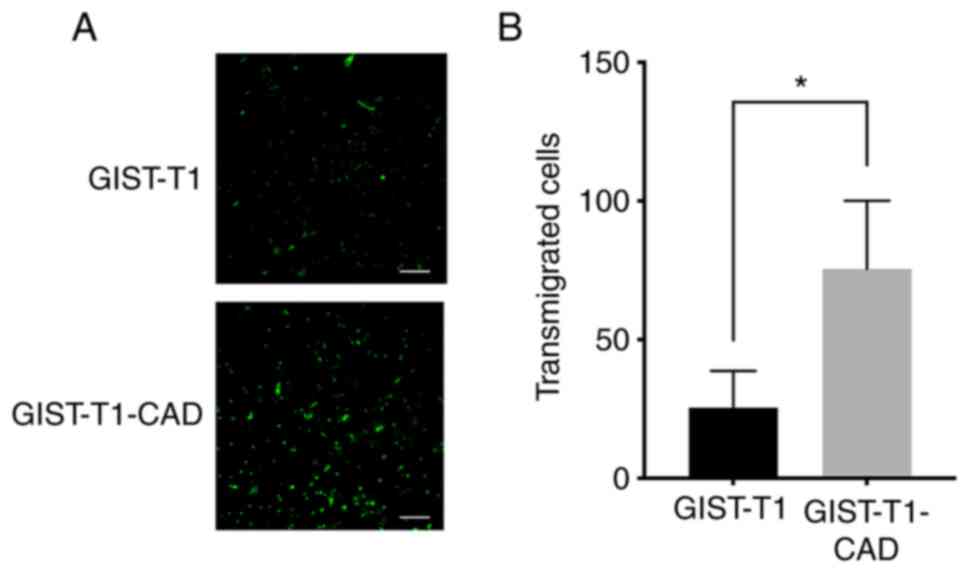
effect of CADM1 on GIST cell transmigration across endothelium, the
transendothelial migration assay was performed using
TNF-α-stimulated HUVECs, GIST-T1 cells, and GIST-T1-CAD cells.
Number of migrated Calcein-AM-labeled GIST-T1-CAD cells was
significantly larger than the number of migrated Calcein-AM-labeled
GIST-T1 cells (Fig. 6A and B)
(P<0.001).
Discussion
We have recently reported that CADM1 is
expressed specifically in most small intestinal GISTs but not in
most gastric GISTs (10). Patients
with small intestinal GISTs are considered to have a worse
prognosis than patients with gastric GISTs because of their higher
risk of metastases and tumor-related death (7). Therefore, we tried to clarify whether
high CADM1 expression in small intestinal GISTs affects the
biological behavior of GISTs. In the present study, proliferation,
migration, invasion, adhesion to endothelial cells, and
transendothelial migration were compared between original GIST-T1
cells with very low CADM1 expression and GIST-T1 cells with
high CADM1 expression induced by CADM1 cDNA
transfection (GIST-T1-CAD cells). GIST-T1-CAD cells showed lower
ability to grow, migrate, and invade, but higher ability to adhere
to endothelium and transmigrate across endothelium than the
original GIST-T1 cells. These results suggested that CADM1 might
facilitate the metastasis of GISTs by increasing tumor cell
adherence to vascular endothelial cells and subsequent passage
through the vascular endothelium but not by increasing tumor cell
growth and motility.
CADM1 expression is frequently lost in
numerous types of epithelial neoplasms (13–15),
and CADM1 is considered to be a tumor suppressor in epithelial
neoplasms. In contrast, upregulated CADM1 appears to promote
ATLL (16) and AML (17) progression through enhancement of
tumor cell growth, tissue invasion, and adhesion to the vascular
endothelium (18). There is also
recent research that high CADM1 expression in SCLCs might
promote the malignant features of the cancer (19,20).
In the present study, we showed that high CADM1 expression
decreased tumor cell growth and motility but increased tumor cell
adherence to vascular endothelial cells and subsequent
transmigration across vascular endothelium. Thus, high CADM1
expression in GISTs appears to have two roles, as a tumor
suppressor and a tumor promoter. Poorer prognosis in small
intestinal GISTs might indicate that the metastasis promoter role
through strong adherence to endothelial cells exceeds the tumor
suppressor role through reduced tumor growth and motility.
Expression of CADM1 is extremely low in most
gastric GISTs (10). The loss of
CADM1 expression frequently found in a variety of cancers is
considered to be due to aberrant hypermethylation of the
CADM1 promoter. In our preliminary research, there was no
methylation of CADM1 promoter in not only small intestinal
GISTs but also gastric GISTs (unpublished data), and methylation is
not considered to be the cause of low CADM1 expression in
gastric GISTs. Therefore, we should clarify the cause of low
CADM1 expression in gastric GISTs.
In the present study, expression of CADM1 in
cultured GIST cells increased their ability to adhere to
endothelium and transmigrate across endothelium. This is similar to
previous reports showing CADM1 promotes ATLL cell infiltration of
organs (18). CADM1 promotes an
invasive phenotype of ATLL cells by activating the Rac
pathway through PDZ-BM interaction with TIAM1 (21). A recent study reported that CADM1
recruits 4.1R to the cell-cell contact site and can enhance the
malignant features of SCLC (20).
In addition, 4.1R modulates the localization of several G-protein
coupled receptors including Duffy/ACKR1 (22). Besides, it had been reported that
CADM1 activates PI3K signaling by forming a tripartite protein
complex with the p85 subunit of PI3K through the
membrane-associated guanylate kinases (MAGuKs), membrane
palmitoylated protein 3 (MPP3) and Drosophila tumor suppressor
discs large (Dlg) (23), which
play roles in the extension of epithelial cells. Further
examination of CADM1 involvement in the mechanism of GIST
cell adhesion to endothelium and transmigration of GIST cells
across endothelium is required.
CADM1 is ubiquitously expressed in vascular
endothelial cells (24). In our
study, compared to the original GIST-T1 cells, GIST-T1-CAD cells
showed a much higher ability to adhere to TNF-α unstimulated
HUVECs, suggesting that CADM1-mediated homotypic contacts between
GIST-T1-CAD cells and HUVECs is extremely important for their
adhesion. The adhesion of the original GIST-T1 cells to HUVECs was
significantly enhanced by pre-stimulation of the HUVECs with TNF-α.
TNF-α can upregulate expression of the intercellular adhesion
molecule type 1 (ICAM-1), E-selectin, and vascular cell
adhesion molecule type 1 (VCAM-1) (25,26)
in vascular endothelial cells. Promotion of original GIST-T1 cell
adhesion to TNF-α-stimulated HUVECs might be significantly induced
by increased expression of those adhesion molecules on TNF-α
stimulated HUVECs. On the other hand, improved GIST-T1-CAD cell
adhesion to HUVECs after TNF-α-stimulation might derive from
increased expression of not only those adhesion molecules but also
CADM1 on TNF-α-stimulated HUVECs. Detailed mechanisms underlying
the change in the ability of GIST-T1-CAD cells and original GIST-T1
cells to adhere to HUVECs before and after TNF-α stimulation should
be clarified.
Recently, anti-CADM1 antibodies were developed as a
promising candidate agent for reducing ATLL cell invasion via
blocking cell adhesion. The antibodies appear to show a minimal
cytotoxic effect on the growth of the ATLL cell line (27). Such antibodies are also expected to
inhibit GIST cell adhesion to the endothelium and show inhibition
of GIST metastasis. Moreover, anti-CADM1 antibodies could cause
cell damage via antibody dependent-cellular cytotoxicity or
complement-dependent cytotoxicity. The anti-tumor effect of
antibody-drug conjugates using CADM1 antibodies might also be
stronger. Anti-CADM1 antibodies could become a new treatment
strategy for small intestinal GISTs.
There are some limitations in our study. First, we
did not examine expression of surface adhesion molecules other than
CADM1 that may affect both migration/invasion of GIST-T1
cells and adhesion between GIST-T1 cells and HUVECs. CADM1
cDNA transfection to GIST-T1 cells may also change the expression
of such surface adhesion molecules. We are planning to examine the
expression levels of those molecules in HUVECs and GIST-T1 cells
before and after CADM1 cDNA transfection. Second, we did not
examine that CADM1 expression in HUVECs was really augmented
after TNF-α stimulation. We will examine whether expression levels
of not only CADM1 but also other surface adhesion molecules
increase in HUVECs after TNF-α stimulation in the near future.
Third, we only carried out in vitro experiments concerning
migration, invasion and adhesion of GIST-T1 and GIST-T1-CAD cells,
but CADM1 contribution to metastatic activity in GIST should be
evaluated by in vivo experiments. In vivo studies
using mouse models of metastasis and GIST cells are being
planned.
In summary, CADM1 in GISTs might act as a suppressor
of tumor growth, migration, and matrix invasion, but stronger GIST
cell-endothelial cell interaction induced by high CADM1
expression could serve as a potential target for the treatment of
small intestinal GISTs, especially for inhibiting GIST
metastasis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JY, TK, NK, TY, MY, KI, AI and SH participated in
data collection and discussion of the findings. JY, TK, NK, TY and
MY carried out the experiments. JY and TK performed the statistical
analysis. JY and SH wrote the manuscript. JY and SH confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Experiments using recombinant DNA were approved by
the Committee for Recombinant DNA Experiments of Hyogo College of
Medicine (approval no. 24015; Nishinomiya, Japan). The use of fresh
human gastrointestinal stromal tumor (GIST) tissue samples for GIST
assays, including gene analysis, was approved by the Ethical
Committee of Hyogo College of Medicine (approval no. 28), and the
patients/participants provided their written informed consent to
participate in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hirota S, Isozaki K, Moriyama Y, Hashimoto
K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M,
et al: Gain-of-function mutations of c-kit in human
gastrointestinal stromal tumors. Science. 279:577–580. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors-definition, clinical, histological,
immunohistochemical, and molecular genetic features and
differential diagnosis. Virchows Arch. 438:1–12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heinrich MC, Corless CL, Duensing A,
McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A,
Town A, et al: PDGFRA activating mutations in gastrointestinal
stromal tumors. Science. 299:708–710. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kinoshita K, Hirota S, Isozaki K, Ohashi
A, Nishida T, Kitamura Y, Shinomura Y and Matsuzawa Y: Absence of
c-kit gene mutations in gastrointestinal stromal tumours from
neurofibromatosis type 1 patients. J Pathol. 202:80–85. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Agaram NP, Wong GC, Guo T, Maki RG, Singer
S, Dematteo RP, Besmer P and Antonescu CR: Novel V600E BRAF
mutations in imatinib-naive and imatinib-resistant gastrointestinal
stromal tumors. Genes Chromosomes Cancer. 47:853–859. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pantaleo MA, Astolfi A, Urbini M, Nannini
M, Paterini P, Indio V, Saponara M, Formica S, Ceccarelli C,
Casadio R, et al: Analysis of all subunits, SDHA, SDHB, SDHC, SDHD,
of the succinate dehydrogenase complex in KIT/PDGFRA wild-type
GIST. Eur J Hum Genet. 22:32–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Emory TS, Sobin LH, Lukes L, Lee DH and
O'Leary TJ: Prognosis of gastrointestinal smooth-muscle (stromal)
tumors: Dependence on anatomic site. Am J Surg Pathol. 23:82–87.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang Z, Wang F, Liu S and Guan W:
Comparative clinical features and short-term outcomes of gastric
and small intestinal gastrointestinal stromal tumours: A
retrospective study. Sci Rep. 9:100332019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Antonescu CR, Viale A, Sarran L,
Tschernyavsky SJ, Gonen M, Segal NH, Maki RG, Socci ND, DeMatteo RP
and Besmer P: Gene expression in gastrointestinal stromal tumors is
distinguished by KIT genotype and anatomic site. Clin Cancer Res.
10:3282–3290. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan J, Kihara T, Kimura N, Hashikura Y,
Ohkouchi M, Isozaki K, Takahashi T, Nishida T, Ito A and Hirota S:
Differential expression of CADM1 in gastrointestinal stromal tumors
of different sites and with different gene abnormalities. Pathol
Oncol Res. 27:6020082021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Biederer T, Sara Y, Mozhayeva M, Atasoy D,
Liu X, Kavalali ET and Sudhof TC: SynCAM, a synaptic adhesion
molecule that drives synapse assembly. Science. 297:1525–1531.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuramochi M, Fukuhara H, Nobukuni T, Kanbe
T, Maruyama T, Ghosh HP, Pletcher M, Isomura M, Onizuka M, Kitamura
T, et al: TSLC1 is a tumor-suppressor gene in human non-small-cell
lung cancer. Nat Genet. 27:427–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allinen M, Peri L, Kujala S,
Lahti-Domenici J, Outila K, Karppinen SM, Launonen V and Winqvist
R: Analysis of 11q21-24 loss of heterozygosity candidate target
genes in breast cancer: Indications of TSLC1 promoter
hypermethylation. Genes Chromosomes Cancer. 34:384–389. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Honda T, Tamura G, Waki T, Jin Z, Sato K,
Motoyama T, Kawata S, Kimura W, Nishizuka S and Murakami Y:
Hypermethylation of the TSLC1 gene promoter in primary gastric
cancers and gastric cancer cell lines. Jpn J Cancer Res.
93:857–860. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng D, Wu X, Zheng J, Zhuang Y, Chen J,
Hong C, Zhang F, Wu M and Lin D: Loss of CADM1/TSLC1 expression is
associated with poor clinical outcome in patients with esophageal
squamous cell carcinoma. Gastroenterol Res Pract. 2016:69476232016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sasaki H, Nishikata I, Shiraga T, Akamatsu
E, Fukami T, Hidaka T, Kubuki Y, Okayama A, Hamada K, Okabe H, et
al: Overexpression of a cell adhesion molecule, TSLC1, as a
possible molecular marker for acute-type adult T-cell leukemia.
Blood. 105:1204–1213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fisser MC, Rommer A, Steinleitner K,
Heller G, Herbst F, Wiese M, Glimm H, Sill H and Wieser R:
Induction of the proapoptotic tumor suppressor gene cell adhesion
molecule 1 by chemotherapeutic agents is repressed in therapy
resistant acute myeloid leukemia. Mol Carcinog. 54:1815–1819. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dewan MZ, Takamatsu N, Hidaka T,
Hatakeyama K, Nakahata S, Fujisawa J, Katano H, Yamamoto N and
Morishita K: Critical role for TSLC1 expression in the growth and
organ infiltration of adult T-cell leukemia cells in vivo. J Virol.
82:11958–11963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kikuchi S, Iwai M, Sakurai-Yageta M,
Tsuboi Y, Ito T, Maruyama T, Tsuda H, Kanai Y, Onizuka M, Sato Y
and Murakami Y: Expression of a splicing variant of the CADM1
specific to small cell lung cancer. Cancer Sci. 103:1051–1057.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Funaki T, Ito T, Tanei ZI, Goto A, Niki T,
Matsubara D and Murakami Y: CADM1 promotes malignant features of
small-cell lung cancer by recruiting 4.1R to the plasma membrane.
Biochem Biophys Res Commun. 534:172–178. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Masuda M, Maruyama T, Ohta T, Ito A,
Hayashi T, Tsukasaki K, Kamihira S, Yamaoka S, Hoshino H, Yoshida
T, et al: CADM1 interacts with Tiam1 and promotes invasive
phenotype of human T-cell leukemia virus type I-transformed cells
and adult T-cell leukemia cells. J Biol Chem. 285:15511–15522.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baines AJ, Lu HC and Bennett PM: The
Protein 4.1 family: Hub proteins in animals for organizing membrane
proteins. Biochim Biophys Acta. 1838:605–619. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murakami S, Sakurai-Yageta M, Maruyama T
and Murakami Y: Trans-homophilic interaction of CADM1 activates
PI3K by forming a complex with MAGuK-family proteins MPP3 and Dlg.
PLoS One. 9:e828942014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tatsumi K, Taatjes DJ, Wadsworth MP,
Bouchard BA and Bovill EG: Cell adhesion molecule 1 (CADM1) is
ubiquitously present in the endothelium and smooth muscle cells of
the human macro- and micro-vasculature. Histochem Cell Biol.
138:815–820. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ross EA, Douglas MR, Wong SH, Ross EJ,
Curnow SJ, Nash GB, Rainger E, Scheel-Toellner D, Lord JM, Salmon M
and Buckley CD: Interaction between integrin alpha9beta1 and
vascular cell adhesion molecule-1 (VCAM-1) inhibits neutrophil
apoptosis. Blood. 107:1178–1183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sana TR, Janatpour MJ, Sathe M, McEvoy LM
and McClanahan TK: Microarray analysis of primary endothelial cells
challenged with different inflammatory and immune cytokines.
Cytokine. 29:256–269. 2005.PubMed/NCBI
|
|
27
|
Chilmi S, Nakahata S, Fauzi YR, Ichikawa
T, Tani C, Suwanruengsri M, Yamaguchi R, Matsuura T, Kurosawa G and
Morishita K: Development of anti-human CADM1 monoclonal antibodies
as a potential therapy for adult T-cell leukemia/lymphoma. Int J
Hematol. 112:496–503. 2020. View Article : Google Scholar : PubMed/NCBI
|