Harmful stimuli, including ultraviolet radiation,
ionizing radiation and carcinogens, can result in single-nucleotide
mutations, insertions or deletions, gene fusion, frameshift
mutations, structural mutations or integration and clonal expansion
of the tumor-associated virus genome within the human genome
(1,2). In particular, these genetic
alterations can also cause somatic cell mutagenesis (3–6).
Over the past number of decades, immunotherapy has demonstrated
great potential for the treatment of cancer. This is because tumor
cells produce mutant proteins that can be recognized by the immune
system as antigens, which trigger cellular and humoral immune
responses downstream. Some non-synonymous mutations can give rise
to mutated, non-self peptides that can be presented by human (HLA)
molecules and elicit T-cell responses, which are known as
neoantigens (7). Since leukocyte
antigen neoantigens are not affected by thymus selection or central
tolerance, T cells exhibiting high-avidity likely exist (8). Therefore, immunotherapy of malignant
tumors by targeting these non-synonymous mutant proteins is a
research field that is garnering significant interest (9).
Previous studies have demonstrated that antigens
produced by necrotic tumor cells also have the ability to bind to
MHC-II molecules, which are associated with the functions of
CD4+ T cells (13,14).
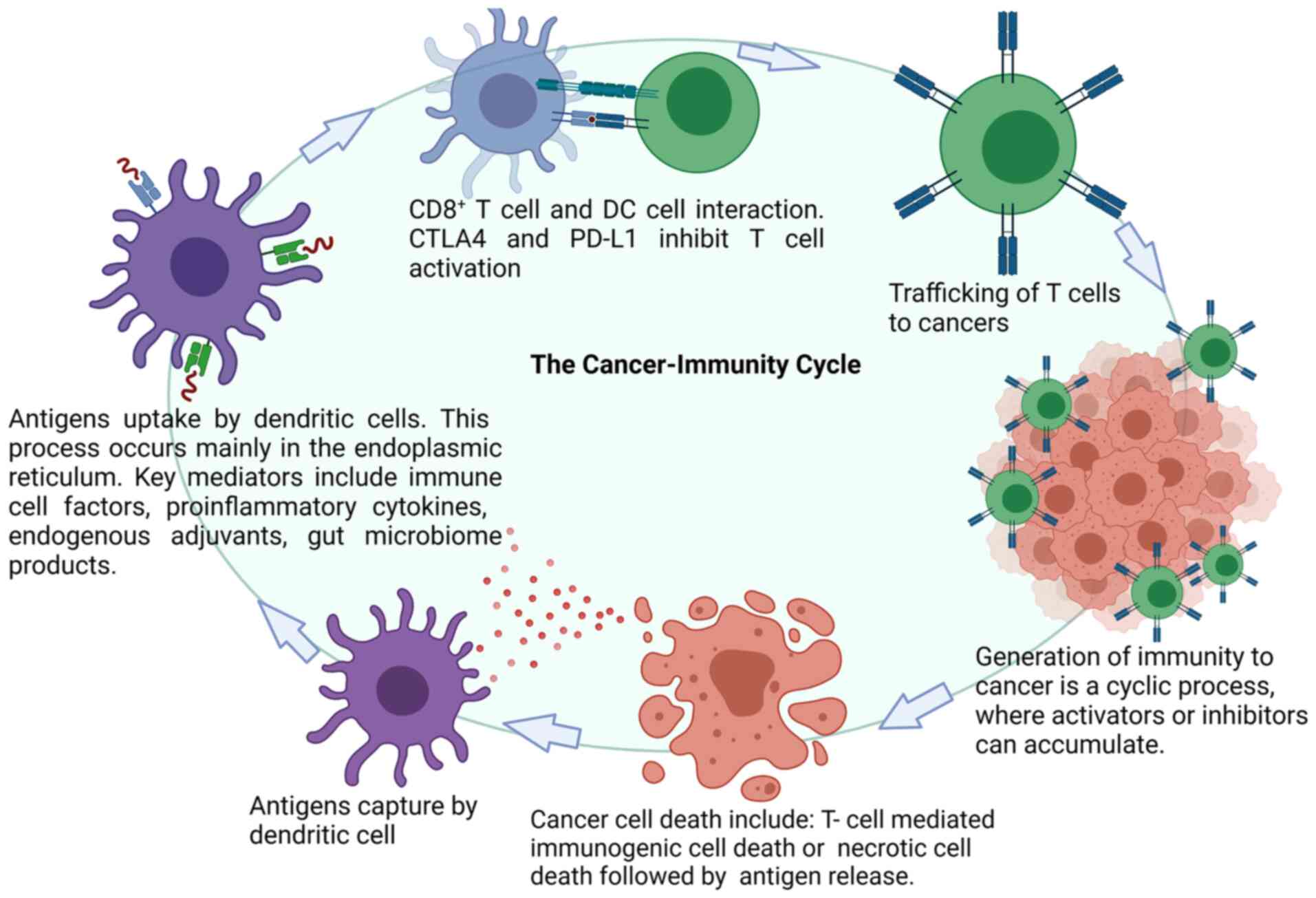
Activated effector T cells recognize and attack cancer cells in the
tumor bed. Additional tumor-associated antigens (TAAs) are released
by dead cancer cells, which increases the breadth and depth of the
cancer-immune cycle (10).
Techniques for tumor immunotherapy have developed
over the past decade, such that notable antitumor activities have
been reported for the treatment of numerous solid tumors, including
melanoma, non-small cell lung cancer (NSCLC), kidney cancer,
prostate cancer and glioblastoma (15–17).
Tumor immunotherapy includes immune enhancement therapy, tumor
vaccines, immune checkpoint blockade therapy and adoptive cellular
therapy (ACT). Immunotherapeutic agents, including immune
checkpoint blockers targeting the programmed cell death
(PD)-1/PD-ligand (L)1 signaling pathway, have been approved by the
US Food and Drug Administration (FDA) for clinical application
(18). In addition, results of a
previous study demonstrated that the neoantigen burden in tumor
tissues is directly and positively associated with the tumor
mutational burden (TMB). TMB is an indicator of the tumor mutation
quantity, which translates into the structures of neoantigens and
is presented to T cells by MHC proteins (19). In particular, melanoma has a high
mutation rate, meaning that PD-1 antibodies are more likely to
mediate beneficial effects. Furthermore, approved immune checkpoint
inhibitors mainly target tumors with high TMB and neoantigen loads,
including melanoma, urothelial cancer and NSCLC (20). For solid tumors with low mutation
loads, it would be more appropriate to apply immunotherapy based on
neoantigens (tumor vaccines and adoptive cell therapies), as
neoantigens have high tumor specificity, without being affected by
thymus selection and the lack of central tolerance (8,21).
Based on previous clinical and tumor immunology
data, tumor cell epitopes can be classified into two categories
(8,22). The first category is TAAs, which
are formed by nonmutant proteins and are not unique to tumor cells.
This type of antigen also exists in non-cancerous cells but is
instead aberrantly expressed during carcinogenesis. The second
category includes peptides that exist only in tumor cells or a
specific tumor cell type, known as tumor-specific antigens (TSAs)
or neoantigens (8).
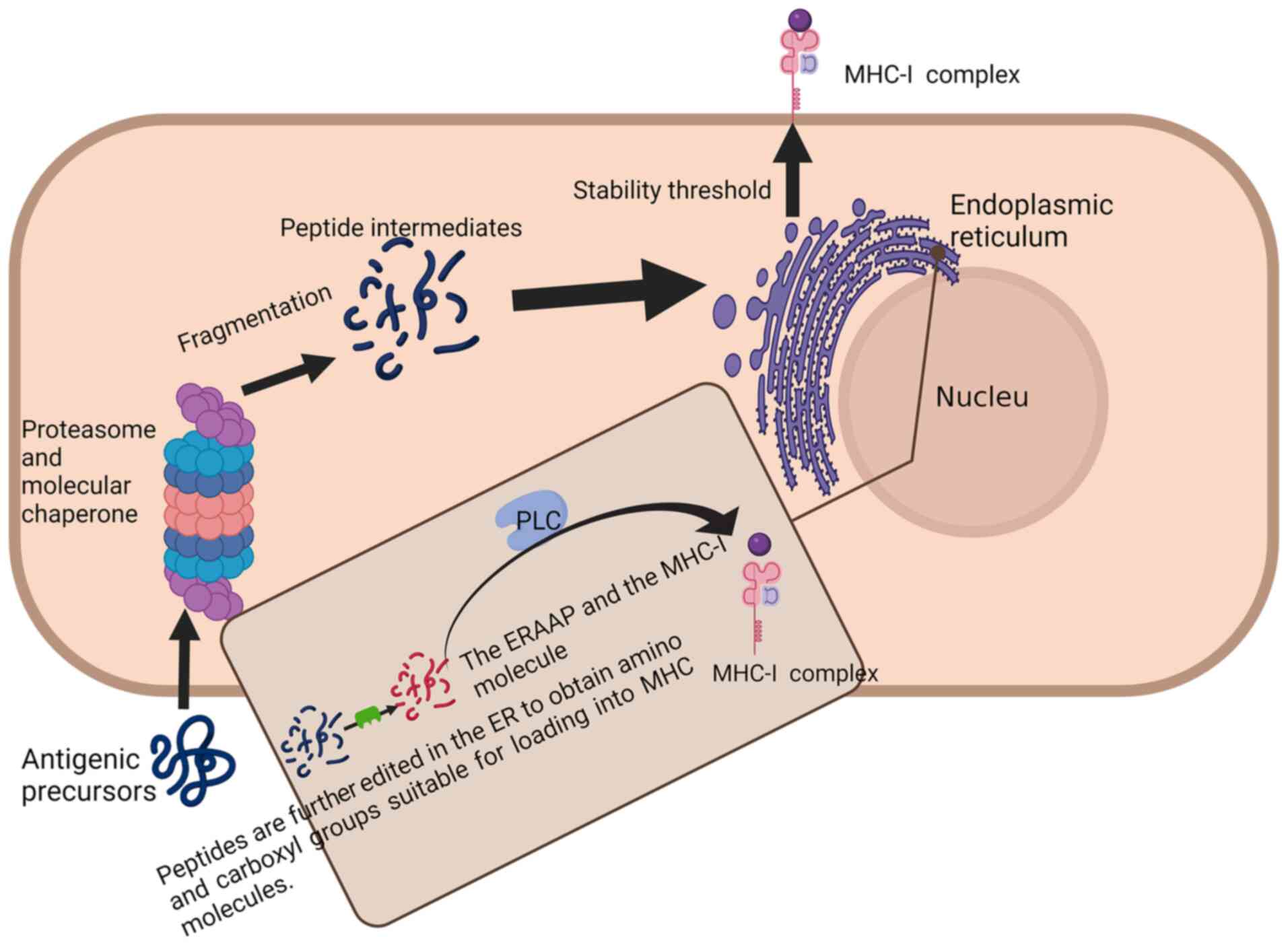
Neoantigens are antigens arising from somatic
mutations that generate these mutant peptides, which are processed
and presented by MHC on the cell surface. Neoantigens exhibiting
potent immunogenicity are not normally present in healthy cells or
tissues and can activate the immune system to eliminate tumor cells
(23). Therefore, neoantigens are
attractive targets for designing precision immunotherapeutic
stratgies, such as antibodies, vaccines and cellular therapeutics.
At present, neoantigens are classified into the following two
types: Private and public neoantigens. Private neoantigens are
mutated antigens that are unique to most neoantigens and typically
differ among patients. Therefore, therapeutic strategies based on
private neoantigens are designed to the specification of each
patient and are also named personalized therapy (24). Previous clinical data demonstrated
that tumor-infiltrating lymphocytes (TILs) from patients with
gastrointestinal cancer can recognize neoantigens expressed by
tumor cells due to somatic mutations, where the majority of the
neoantigen determinants were unique and not shared among patients
(25). By contrast, public
neoantigens refer to mutated antigens that are shared and conserved
among patients with cancer. Immunotherapies targeting public
neoantigens are applicable to groups of patients with analogous
genetic alterations (26).
Neoantigens can induce immune responses with high specificity to
cancer cells because of their underlying mutations, whilst exerting
minimal toxicity to non-cancerous cells. Therefore, screening for
novel tumor neoantigens may serve to be a useful strategy in cancer
immunotherapy.
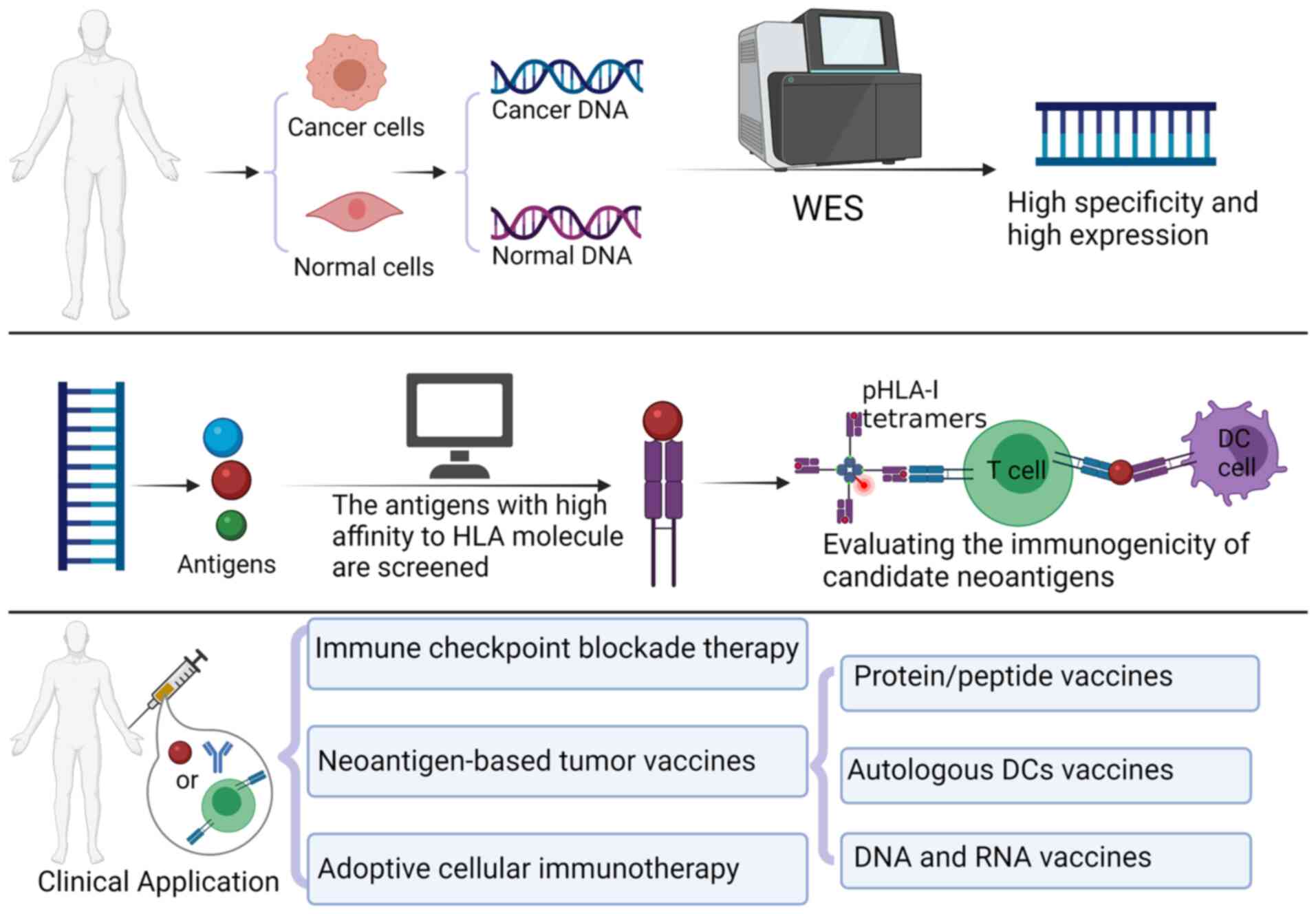
A number of strategies have been devised to screen
for candidate neoantigens, such as whole-exome sequencing (WES),
computer algorithm and immunological effects evaluation (Fig. 3). With the development of
sequencing technology, mutations can be screened using WES
(27). If such mutant proteins are
expressed highly in tumor cells, they exhibit the potential to be
recognized as neoantigens (28).
Subsequently, a computer algorithm can be used to predict the
affinity of neoantigen peptides of interest to HLA-1 molecules.
Peptides with increased predicted levels of immunogenicity may be
selected to be neoantigen candidates. Neoantigen-specific T cells
are thereby isolated from the tumor cell infiltration area or the
peripheral blood samples of patients, expanded to T cells in
vitro and then reinfused back into the body. Subsequently, the
immunological efficacy of the candidate neoantigens can be assessed
(27,28). Previous reports from Chen et
al (29) demonstrated that a
large proportion of the immunogenic neo-epitopes were recognized by
autologous T cells, rendering this a viable pipeline for neoantigen
identification.
However, a number of limitations must be considered.
Algorithms that are currently used for predicting neoantigens are
limited by binding affinity data in vitro and computational
constraints, resulting in a high false discovery rate (29). To circumvent this, Hao et al
(30) proposed a deep
convolutional neural network, named the antigen presentation
prediction model (APPM), for predicting antigen presentation. The
positive predictive value of APPM, combined with the immune epitope
database, can optimize the accuracy further for predicting
neoantigens (28). In addition,
currently applied methods used for screening neoantigens are
relevant to specific HLA alleles. Bulik-Sullivan et al
(31) previously examined a large
HLA peptide and genomic dataset from various human tumors to create
a computational model named EDGE, which increased the positive
predictive value of HLA antigen prediction by ≤ nine-fold.
At present, the most promising method in cancer
immunotherapy is the development of therapeutic tumor vaccine based
on neoantigens. The benefits of this vaccine type are less
tolerance compared with other traditional therapeutic drugs such as
Tarceva, Gleevec and Herceptin, which enables it to activate the
patient's own immune system to induce a sustained antitumor
response (32–34). Despite numerous efforts to develop
cancer vaccines, their conversion into efficacious clinical therapy
have been challenging, with an objective clinical response rate of
only >7% and an overall rate of clinical benefit of only ~20%
(35). To achieve the full
potential of cancer vaccines, personalized neoantigen vaccines have
been introduced (23).
Personalized neoantigen vaccines include DC-, DNA-, RNA- and
synthetic peptide-based vaccines, some of which are currently
undergoing clinical trials (Table
I).
Tumor lytic products are some of the earliest immune
vaccines to be applied for tumor therapy (36). Tumor cells are typically obtained
during surgery and subsequently digested either by irradiation or
tumor cell lysis. Complete tumor lysate contains all potential
antigens (TAAs and TSAs), including neoantigens. Several clinical
trials have begun with using tumor lytic products. Chiang et
al (37) previously used
hypochlorite to oxidize the cleavage products to enhance
antigenicity, which improved treatment efficacy by DCs. Bencherif
et al (38) demonstrated a
cryogen-based whole tumor cell vaccine containing DC-activating
factors, such as granulocyte-macrophage colony-stimulating factor,
which can be used for injection. This vaccine has been demonstrated
to be capable of regressing melanoma in mice (33). However, despite intensive research
efforts into developing autologous tumor cells, a myriad of
problems remain to be solved, including the maintenance of
large-scale tumor cell culture, control of vaccine quality and
standardization of vaccine production.
Protein/peptide vaccines have been extensively
studied in cancer therapy trials due to their safety, cost
effectiveness and ease of storage. Nevertheless, due to the high
variety of unique peptide epitopes, tendency to degrade easily and
low molecular weights, protein/peptide vaccines exhibit two main
limitations: Low immunogenicity and MHC restriction. Previous
studies have demonstrated that the addition of an immune adjuvant
to the peptide vaccine is essential for inducing an effective
immune response (39,40). Traditional adjuvants, such as
Freund's, bacterial and cytokine adjuvants (41), have all been used to activate the
body's immune system and maintain the structure of the antigen. In
addition, advances in nanotechnology have created opportunities for
the development of novel types of adjuvants. For example, 5–100 nm
nanovaccines (IL-2 and a lymphoma-specific antigen into liposomal
particles) were found to be retained in lymphoid tissues with
advanced-stage follicular lymphoma for a prolonged period, so that
they can easily recognized and presented by immune cells in the
lymphatic system (42).
For neoantigens, preparation of a peptide vaccine is
key due to the high levels of immunogenicity (43). It has previously been suggested
that new types of adjuvants coupled with neoantigen peptides,
including charge-modified peptide-toll-like receptor (TLR)-7/8a
conjugates assembled into nanoparticles, can significantly improve
the cytotoxicity of CD8+ T cells (44). Ni et al (45) previously prepared a bi-adjuvant
neoantigen nanovaccine (banNV), containing a peptide neoantigen
[ADP-dependent glucokinase (Adpgk)] along with two other adjuvants,
namely the TLR 7/8 agonist R848 and TLR9 agonist CpG, for
colorectal cancer immunotherapy in mice. Results from this previous
study revealed a highly potent immunogenic effect of this banNV
coupled with reduced acute systemic toxicity, suggesting that
banNVs can serve as a potential therapeutic neoantigen vaccine for
the treatment of cancer (38). A
variety of novel technologies are currently under development with
aims of faciliatating neoantigen-specific T-cell activation
(46–48).
Neoantigens obtained by screening a single peptide
epitope exhibits weak immunogenicity, short half-lives and high HLA
restriction, such that patients typically mount an ineffective
immune response following vaccination. Therefore, research efforts
are currently focusing on the development of a multitude of
personalized vaccines containing a variety of epitopes to enhance
the antitumor response (49).
After obtaining the potential private and public neoantigens,
multiplex vaccines containing 2–5 neoantigens in the form of long
synthetic peptides are developed (50). A personalized long peptide
neoantigen vaccine containing 20 neoepitopes has been previously
synthesized and injected into patients with phase Ib glioblastoma
(41). The results demonstrated
that neoantigen-specific CD8+ and CD4+ T
cells were able to infiltrate into the tumor (51). In addition, Zeng et al
(52) previously reported a case
of personalized neoantigen immunotherapy for renal collecting duct
carcinoma (CDC). According to the patient's specific mutations, 13
neoantigens were screened and identified, following which the
corresponding long peptide neoantigen vaccines were prepared
(42). A total of 3 months later,
biopsy samples collected from the CDC sites exhibited a lower
mutant allele frequency corresponding to 92% of the neoantigens,
suggesting that tumor cells harboring these neoantigens were
effectively eliminated (42).
Nevertheless, vaccines developed based on personalized neoantigens
require a prolonged development period, which may delay the
treatment of cancer (53).
Therefore, vaccines designed based on public neoantigens may be the
novel therapeutic agent with the highest potential for the
treatment of cancer. A synthetic long peptide for isocitrate
dehydrogenase was previously used to design a neoantigen vaccine
using public neoantigens, which yielded promising results regarding
the survival of patients with late-stage melanoma (54).
DC-based tumor vaccines have revealed a high
potential for both preclinical and clinical applications (55,56).
Since they are highly effective antigen-presenting cells (APCs),
DCs serve an important role in the regulation of both innate and
adaptive immune responses, in addition to having a unique ability
to activate effector and memory T cells. DC vaccines loaded with
antigens have been demonstrated to induce more potent immune
responses compared with vaccines composed of only antigens and
adjuvants (57–59). For example, the objective response
rate of patients with metastatic melanoma treated with an
antigen-adjuvanted vaccine was only 2.6%, whilst that of metastatic
melanoma treated with a DC vaccine was 9.5% (35). Therefore, neoantigen-based DC
vaccines hold high potential for cancer therapy. In addition,
Carreno et al (59)
previously reported that DC vaccines loaded with neoantigens can
trigger T-cell-specific responses, which enhanced the immune
response in three patients with melanoma. In particular, two
patients remained stable whereas one patient exhibited no adverse
effects or cancer recurrence (44). In another study, Zhang et al
(57) found that the
neoantigen-pulsed DC vaccine was superior to the neoantigen
peptide-adjuvant immune vaccine in activating the immune response
and inhibiting murine lung carcinoma growth and spread. In
addition, it was previously demonstrated that plasma cell-like DCs
are also potent antitumor inducers. Plasma cell-like DCs expand the
effects of neoantigens and increase the number of specific
CD8+ T cells by presenting neoantigen peptides from
melanoma (60).
Nucleic acid vaccines are anticipated to replace
traditional vaccines in the near future due to their unique
benefits. Specifically, nucleic acid vaccines are non-infectious,
such that RNA vaccines cannot integrate into host cell genome,
eliminating the possibility of insertion mutation (61). Additionally, nucleic acids can be
quickly absorbed and expressed throughout the body with high levels
of efficiency (24,62). Nucleic acid vaccines can also be
used to exploit the strong immunogenicity of neoantigens to reverse
immune tolerance, turning ‘cold’ tumors into ‘hot’ tumors.
Importantly, these types of vaccines can be rapidly developed in a
cost-effective manner (63). At
present, nucleic acid cancer vaccines targeting neoantigens have
been investigated in various clinical trials (64,65).
However, further investigation into the coding regions of the
nucleic acids in the vaccines is required to improve the levels of
immunogenicity. Tondini et al (66) previously designed a circular DNA
vaccine that used a plasmid to express the three neoantigenic
determinants (dolichyl-phosphate
N-acetylglucosaminephosphotransferase 1, RalBP1-associated Eps
domain-containing 1 and Adpgk) before evaluating its efficacy in
mice. The results obtained revealed that this polymer DNA vaccine
induced prophylactic protection against the B16 melanoma expressing
ovalbumin (49). Furthermore, Li
et al (67) identified a
novel CpG oligodeoxynucleotide for promoting the immune response to
inhibit melanoma tumor growth effectively. Specifically, CpG
combined with mRNA cancer vaccines exhibited improved antitumor
efficacy (50). Overall, these
aforementioned findings provide a novel theoretical basis for the
development of DNA or mRNA vaccines to further emphasize the
importance of immunotherapy strategy development (66,68).
Numerous types of regulatory signals that can
negatively regulate the tumor-killing ability of T cells are named
immune checkpoints. The therapeutic field designed to suppress
these associated signaling pathways leading to T-cell exhaustion is
named immune checkpoint blockade therapy. Immune checkpoint
inhibitors can continuously enhance the immune function of T cells
in cancer (69). Previous studies
have reported that PD-1 is a key signaling molecule in tumor immune
evasion, which exerts immunosuppressive effects by binding to PD-L1
to inhibit T-cell proliferation and activation (69,70).
In addition, cytotoxic T lymphocyte protein 4 (CTLA-4) has been
shown to block T-cell activation by binding to CD80 or CD86 on APCs
(71). CTLA-4 and PD-1/PD-L1
mono-antibodies are the most extensively used immune checkpoint
blockers for cancer immunotherapy. Therefore, monoclonal antibodies
have been designed to target these types of immune checkpoint
molecules (CTLA-4 and PD-1/PD-L1) to eliminate immunosuppression,
thereby restoring the antitumor immune response (72–76).
At present, FDA-approved immune checkpoint
inhibitors include the following antibodies (18,77):
i) In total, three anti-PD-1 antibodies, including pembrolizumab
(Keytruda), nivolumab (Opdivo) and cemiplimab (Libtayo); ii) three
anti-PD-L1 antibodies, including atezolizumab (Tecentriq),
durvalumab (Imfinzi) and avelumab (Bavencio); and iii) an
anti-CTLA-4 antibody, namely ipilimumab (Bristol-Myers Squibb).
Antibodies targeting T-cell immune checkpoint receptors PD-1/PD-L1
have demonstrated notable efficacy against melanoma, NSCLC and
glioblastoma (78–80). However, the sole use of immune
checkpoint inhibitors confers limited effects on improving immune
system functions and is exceptionally susceptible to drug
resistance (20). Therefore, an
effective strategy may be the combination of immune checkpoint
blockers with immunotherapy based on neoantigens. The combination
of neoantigen vaccines and immune checkpoint blockade therapy may
enhance the ability of the immune system to recognize
low-immunogenic molecules and shared TAAs by mimicking antigen
epitope transmission and blocking the immune escape-associated
pathway. The specific peptides produced by cancer cells bind to HLA
molecules with high efficiency and are presented to CD8+
and CD4+ T cells by APCs, thereby inhibiting
autoimmunity and maximizing the therapeutic effect of neoantigens
(81). Liu et al (82) previously demonstrated that the
efficacy of the combination of anti-PD-L1 antibody and a neoantigen
vaccine was superior to that of anti-PD-L1 alone in an aggressive
orthotopic murine glioblastoma model. Similarly, Duraiswamy et
al (83) revealed that the
efficacy of PD-1 and CTLA-4 dual-blockade combined with the
neoantigen vaccine in suppressing CT26 colon carcinoma and ID8-VEGF
ovarian carcinoma was mediated by restoring T-cell functions. These
studies suggest that the combined therapy of neoantigen vaccines
and immune checkpoint inhibitors holds great potential for the
treatment of cancer.
ACT was previously used to isolate immune cells,
such as DCs, lymphokine-activated killer cells, TILs and
cytokine-induced killer cells from patients for subsequent
amplification in vitro prior to re-infusion (84). TCR is a T-cell-specific receptor
that participates in antigen recognition by naturally-occurring T
cells. Due to its unique structure and function, TCR only
recognizes peptides bound to major MHC molecules (85). Follow-up immunotherapy following
the in vitro amplification of TILs is a widely practiced
treatment method (86). Tumor
antigen-specific T cells can recognize antigenic epitopes on the
surface of tumor cells and kill them. This has been frequently
exploited for treating patients who did not respond well to immune
checkpoint inhibitor therapy or surgery (21). ACT with TILs has been demonstrated
to confer high levels of therapeutic efficacy in metastatic
melanoma (74). In 10 patients
with melanoma who were not previously treated with TIL infusion,
they exhibited an overall response rate of 50% (87). In addition, neoantigen-specific T
cells were detected in the tumor-infiltrating T cells of three
patients. In total, six of the nine detected neoantigens were found
to increase the response of specific T lymphocytes in the
peripheral blood after the infusion of TILs (88). TIL-based adoptive T-cell therapies
targeting neoantigens have demonstrated potential in patients with
metastatic breast cancer (89).
This highlight a basis for the development of novel personalized
ACT against cancer.
Specific T lymphocytes have been screened in the
tumor-infiltrating area for amplification and reinfusion. Tran
et al (90) identified a
GTPase KRAS G12D-targeting mutation (KRAS treatment gene, codon 12
mutation) in metastatic colorectal cancer. Neoantigen (KRAS
G12D)-specific cell therapy resulted in the significant regression
of the cancer. Sun et al (91) created an RNA mutanome vaccine based
on neoantigens, which activated neoantigen-reactive T (NRT) cells.
Following the adoptive transfer of these NRT cells, they exerted a
significant antitumor effect in mouse lung cancer (78). These results suggest that adoptive
NRT cell therapy is a feasible and effective therapeutic approach
for lung cancer.
It should be emphasized that the amplification of T
cells from bodily fluids or tissues requires a complex procedure.
Notably, it is difficult to obtain high-affinity TCR+ T
cells, where T cells amplified in vitro cannot survive in
the recipient for a prolonged period of time following infusion. In
addition, different types of antigens exhibit individual
variations, even in tumors within the same tissue type (92). Therefore, it is difficult to share
neoantigens among patients.
Cancer immunotherapy has emerged as a novel strategy
for treating malignant tumors. Specifically, immune responses
targeting designed neoantigens has attracted considerable
attraction according to findings from numerous clinical trials.
Therefore, screening for novel neoantigens has become a key focus
in the field of immunotherapy. With the rapid and continuous
development of sequencing technology and bioinformatics algorithms,
tumor mutation sites have been efficiently and accurately examined
to accelerate this process. These neoantigens identified have been
used as vaccines to stimulate the immune system and generate an
antitumor response in patients with cancer.
However, a significant number of limitations remain
that must be addressed prior to the broader application of
neoantigen-targeting immunotherapies. During the development and
progression of tumors, numerous neoantigens with high levels of
diversity are produced, which limits the option for developing a
standardized model. Furthermore, previous studies have reported
that only a small fraction of non-synonymous mutations identified
by tumor WES are immunogenic (93,94).
Therefore, screening for specific neoantigens associated with
specific tumors is critical. Cancers treated using personalized
immunotherapies, such as ACT or vaccinations, may also generate a
potently immunosuppressive local environment to prevent the
activation of neoantigen-specific T cells (95). Rational strategies are therefore
required to identify candidate neoantigens and evaluate their
immunogenicity. Further limitations include the loss of neoantigens
with heterogeneous expression profiles inside the treated tumor,
which may result in the selection of subclones devoid of the target
neoantigen (76).
In conclusion, the emergence of novel therapies,
including neoantigen vaccines and ACT based on neoantigens, is
expected to revolutionize the treatment of cancer based on
precision medicine. The use of neoantigen vaccines have
demonstrated encouraging outcomes and are more ideally suited for
combination therapies, including those with checkpoint inhibitors,
surgery, radiation therapy and chemotherapy. In addition,
neoantigen-based therapeutic strategies hold potential for the
treatment of cancer, such that an increase in the spectra of human
malignancies that can respond to cancer immunotherapy will be
developed.
Not applicable.
The present review was supported by the National Natural Science
Foundation of China (grant no. 81870237), the Foundation of Health
Commission of Weifang (grant no. wfwsjk_2019_025), the Research
Project of Shandong Provincial Education Department (grant no.
J14LK12) and the Weifang Medical University Doctoral Startup
Fund.
Not applicable.
XF, ZG and JL wrote the manuscript. JW drafted the
figure and table and revised the manuscript. XG and HL reviewed and
edited the manuscript. ZG and YL contributed to the conception and
design of the article and acquired funding. Data authentication is
not applicable. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Tomasetti C and Vogelstein B: Cancer
etiology. Variation in cancer risk among tissues can be explained
by the number of stem cell divisions. Science. 347:78–81. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu S, Powers S, Zhu W and Hannun YA:
Substantial contribution of extrinsic risk factors to cancer
development. Nature. 529:43–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Compagni A and Christofori G: Recent
advances in research on multistage tumorigenesis. Br J Cancer.
83:1–5. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paul S and Régulier E: Molecular basis of
oncogenesis. Ann Biol Clin (Paris). 59:393–402. 2001.(In French).
PubMed/NCBI
|
|
5
|
Spandidos DA: Oncogenes and tumor
suppressor genes as paradigms in oncogenesis. J BUON. 12 (Suppl
1):S9–S12. 2007.PubMed/NCBI
|
|
6
|
Shen L, Shi Q and Wang W: Double agents:
Genes with both oncogenic and tumor-suppressor functions.
Oncogenesis. 7:252018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Plaen E, Lurquin C, Van Pel A, Mariamé
B, Szikora JP, Wölfel T, Sibille C, Chomez P and Boon T:
Immunogenic (tum-) variants of mouse tumor P815: Cloning of the
gene of tum-antigen P91A and identification of the tum-mutation.
Proc Natl Acad USA. 85:2274–2278. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wirth TC and Kühnel F: Neoantigen
targeting-dawn of a new era in cancer immunotherapy? Front Immunol.
8:18482017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Finn OJ: Human tumor antigens yesterday,
today, and tomorrow. Cancer Immunol Res. 5:347–354. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen DS and Mellman I: Oncology meets
immunology: The cancer-immunity cycle. Immunity. 39:1–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jongsma MLM, Guarda G and Spaapen RM: The
regulatory network behind MHC class I expression. Mol Immunol.
113:16–21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shastri N, Nagarajan N, Lind KC and
Kanaseki T: Monitoring peptide processing for MHC class I molecules
in the endoplasmic reticulum. Curr Opin Immunol. 26:123–127. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren Y, Cherukuri Y, Wickland DP, Sarangi
V, Tian S, Carter JM, Mansfield AS, Block MS, Sherman ME, Knutson
KL, et al: HLA class-I and II restricted neoantigen loads predict
overall survival in breast cancer. Oncoimmunology. 9:17449472020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Axelrod ML, Cook RS, Johnson DB and Balko
JM: Biological consequences of MHC-II expression by tumor cells in
cancer. Clin Cancer Res. 25:2392–2402. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dantoing E, Piton N, Salaün M, Thiberville
L and Guisier F: Anti-PD1/PD-L1 immunotherapy for non-small cell
lung cancer with actionable oncogenic driver mutations. Int J Mol
Sci. 22:62882021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kok VC: Current understanding of the
mechanisms underlying immune evasion from PD-1/PD-L1 immune
checkpoint blockade in head and neck cancer. Front Oncol.
10:2682020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raphael I, Kumar R, McCarl LH, Shoger K,
Wang L, Sandlesh P, Sneiderman CT, Allen J, Zhai S, Campagna ML, et
al: TIGIT and PD-1 immune checkpoint pathways are associated with
patient outcome and anti-tumor immunity in glioblastoma. Front
Immunol. 12:6371462021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Twomey JD and Zhang B: Cancer
immunotherapy update: FDA-approved checkpoint inhibitors and
companion diagnostics. AAPS J. 23:392021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miller A, Asmann Y, Cattaneo L, Braggio E,
Keats J, Auclair D, Lonial S; MMRF CoMMpass Network, ; Russell SJ
and Stewart AK: High somatic mutation and neoantigen burden are
correlated with decreased progression-free survival in multiple
myeloma. Blood Cancer J. 7:e6122017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yi M, Qin S, Zhao W, Yu S, Chu Q and Wu K:
The role of neoantigen in immune checkpoint blockade therapy. Exp
Hematol Oncol. 7:282018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van den Bulk J, Verdegaal EME, Ruano D,
Ijsselsteijn ME, Visser M, van der Breggen R, Duhen T, van der
Ploeg M, de Vries NL, Oosting J, et al: Neoantigen-specific
immunity in low mutation burden colorectal cancers of the consensus
molecular subtype 4. Genome Med. 11:872019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yarchoan M, Johnson BA III, Lutz ER,
Laheru DA and Jaffee EM: Targeting neoantigens to augment
antitumour immunity. Nat Rev Cancer. 17:5692017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blass E and Ott PA: Advances in the
development of personalized neoantigen-based therapeutic cancer
vaccines. Nat Rev Clin Oncol. 18:215–229. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pardi N, Hogan MJ, Porter FW and Weissman
D: mRNA vaccines-a new era in vaccinology. Nat Rev Drug Discov.
17:261–279. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Parkhurst MR, Robbins PF, Tran E, Prickett
TD, Gartner JJ, Jia L, Ivey G, Li YF, El-Gamil M, Lalani A, et al:
Unique neoantigens arise from somatic mutations in patients with
gastrointestinal cancers. Cancer Discov. 9:1022–1035. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klebanoff CA and Wolchok JD: Shared cancer
neoantigens: Making private matters public. J Exp Med. 215:5–7.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garcia-Garijo A, Fajardo CA and Gros A:
Determinants for neoantigen identification. Front Immunol.
10:13922019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hutchison S and Pritchard AL: Identifying
neoantigens for use in immunotherapy. Mamm Genome. 29:714–730.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen F, Zou Z, Du J, Su S, Shao J, Meng F,
Yang J, Xu Q, Ding N, Yang Y, et al: Neoantigen identification
strategies enable personalized immunotherapy in refractory solid
tumors. J Clin Invest. 129:2056–2070. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hao Q, Wei P, Shu Y, Zhang YG, Xu H and
Zhao JN: Improvement of neoantigen identification through
convolution neural network. Front Immunol. 12:6821032021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bulik-Sullivan B, Busby J, Palmer CD,
Davis MJ, Murphy T, Clark A, Busby M, Duke F, Yang A, Young L, et
al: Deep learning using tumor HLA peptide mass spectrometry
datasets improves neoantigen identification. Nat Biotechnol. Dec
17–2018.(Epub ahead of print). PubMed/NCBI
|
|
32
|
Guo C, Manjili MH, Subjeck JR, Sarkar D,
Fisher PB and Wang XY: Therapeutic cancer vaccines: Past, present,
and future. Adv Cancer Res. 119:421–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schindler T, Bornmann W, Pellicena P,
Miller WT, Clarkson B and Kuriyan J: Structural mechanism for
STI-571 inhibition of abelson tyrosine kinase. Science.
289:1938–1942. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fuster LM and Sandler AB: Select clinical
trials of erlotinib (OSI-774) in non-small-cell lung cancer with
emphasis on phase III outcomes. Clin Lung Cancer. 6 (Suppl
1):S24–S29. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rosenberg SA, Yang JC and Restifo NP:
Cancer immunotherapy: Moving beyond current vaccines. Nat Med.
10:909–915. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
de Gruijl TD, van den Eertwegh AJ, Pinedo
HM and Scheper RJ: Whole-cell cancer vaccination: From autologous
to allogeneic tumor- and dendritic cell-based vaccines. Cancer
Immunol Immunother. 57:1569–1577. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chiang CL, Hagemann AR, Leskowitz R, Mick
R, Garrabrant T, Czerniecki BJ, Kandalaft LE, Powell DJ Jr and
Coukos G: Day-4 myeloid dendritic cells pulsed with whole tumor
lysate are highly immunogenic and elicit potent anti-tumor
responses. PLoS One. 6:e287322011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bencherif SA, Warren Sands R, Ali OA, Li
WA, Lewin SA, Braschler TM, Shih TY, Verbeke CS, Bhatta D, Dranoff
G and Mooney DJ: Injectable cryogel-based whole-cell cancer
vaccines. Nat Commun. 6:75562015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lim YT: Vaccine adjuvant materials for
cancer immunotherapy and control of infectious disease. Clin Exp
Vaccine Res. 4:54–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu G, Zhang F, Ni Q, Niu G and Chen X:
Efficient nanovaccine delivery in cancer immunotherapy. ACS Nano.
11:2387–2392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Obeid J, Hu Y and Slingluff CL Jr:
Vaccines, adjuvants, and dendritic cell activators-current status
and future challenges. Semin Oncol. 42:549–561. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luo M, Samandi LZ, Wang Z, Chen ZJ and Gao
J: Synthetic nanovaccines for immunotherapy. J Control Release.
263:200–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Izumoto S: Peptide vaccine. Adv Exp Med
Biol. 746:166–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lynn GM, Sedlik C, Baharom F, Zhu Y,
Ramirez-Valdez RA, Coble VL, Tobin K, Nichols SR, Itzkowitz Y,
Zaidi N, et al: Peptide-TLR-7/8a conjugate vaccines chemically
programmed for nanoparticle self-assembly enhance CD8 T-cell
immunity to tumor antigens. Nat Biotechnol. 38:320–332. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ni Q, Zhang F, Liu Y, Wang Z, Yu G, Liang
B, Niu G, Su T, Zhu G, Lu G, et al: A bi-adjuvant nanovaccine that
potentiates immunogenicity of neoantigen for combination
immunotherapy of colorectal cancer. Sci Adv. 6:eaaw60712020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Esposito A, Criscitiello C and Curigliano
G: Immune checkpoint inhibitors with radiotherapy and locoregional
treatment: Synergism and potential clinical implications. Curr Opin
Oncol. 27:445–451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ott PA, Hu-Lieskovan S, Chmielowski B,
Govindan R, Naing A, Bhardwaj N, Margolin K, Awad MM, Hellmann MD,
Lin JJ, et al: A Phase Ib trial of personalized neoantigen therapy
plus anti-PD-1 in patients with advanced melanoma, non-small cell
lung cancer, or bladder cancer. Cell. 183:347–362.e24. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shaw SM, Middleton J, Wigglesworth K,
Charlemagne A, Schulz O, Glossop MS, Whalen GF, Old R, Westby M,
Pickford C, et al: AGI-134: A fully synthetic α-Gal glycolipid that
converts tumors into in situ autologous vaccines, induces
anti-tumor immunity and is synergistic with an anti-PD-1 antibody
in mouse melanoma models. Cancer Cell Int. 19:3462019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fennemann FL, de Vries IJM, Figdor CG and
Verdoes M: Attacking tumors from all sides: Personalized multiplex
vaccines to tackle intratumor heterogeneity. Front Immunol.
10:8242019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J,
Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, et al: An
immunogenic personal neoantigen vaccine for patients with melanoma.
Nature. 547:217–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Keskin DB, Anandappa AJ, Sun J, Tirosh I,
Mathewson ND, Li S, Oliveira G, Giobbie-Hurder A, Felt K, Gjini E,
et al: Neoantigen vaccine generates intratumoral T cell responses
in phase Ib glioblastoma trial. Nature. 565:234–239. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zeng Y, Zhang W, Li Z, Zheng Y, Wang Y,
Chen G, Qiu L, Ke K, Su X, Cai Z, et al: Personalized
neoantigen-based immunotherapy for advanced collecting duct
carcinoma: Case report. J Immunother Cancer. 8:e0002172020.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Caron E, Aebersold R, Banaei-Esfahani A,
Chong C and Bassani-Sternberg M: A case for a human
immuno-peptidome project consortium. Immunity. 47:203–208. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hellmann MD and Snyder A: Making it
personal: Neoantigen vaccines in metastatic melanoma. Immunity.
47:221–223. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fu C, Zhou L, Mi QS and Jiang A: DC-based
vaccines for cancer immunotherapy. Vaccines (Basel). 8:7062020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wculek SK, Cueto FJ, Mujal AM, Melero I,
Krummel MF and Sancho D: Dendritic cells in cancer immunology and
immunotherapy. Nat Rev Immunol. 20:7–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang R, Yuan F, Shu Y, Tian Y, Zhou B, Yi
L, Zhang X, Ding Z, Xu H and Yang L: Personalized neoantigen-pulsed
dendritic cell vaccines show superior immunogenicity to
neoantigen-adjuvant vaccines in mouse tumor models. Cancer Immunol
Immunother. 69:135–145. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tang L, Zhang R, Zhang X and Yang L:
Personalized neoantigen-Pulsed DC vaccines: Advances in clinical
applications. Front Oncol. 11:7017772021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Carreno BM, Magrini V, Becker-Hapak M,
Kaabinejadian S, Hundal J, Petti AA, Ly A, Lie WR, Hildebrand WH,
Mardis ER and Linette GP: Cancer immunotherapy. A dendritic cell
vaccine increases the breadth and diversity of melanoma
neoantigen-specific T cells. Science. 348:803–808. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Charles J, Chaperot L, Hannani D, Bruder
Costa J, Templier I, Trabelsi S, Gil H, Moisan A, Persoons V,
Hegelhofer H, et al: An innovative plasmacytoid dendritic cell
line-based cancer vaccine primes and expands antitumor T-cells in
melanoma patients in a first-in-human trial. Oncoimmunology.
9:17388122020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pastor F, Berraondo P, Etxeberria I,
Frederick J, Sahin U, Gilboa E and Melero I: An RNA toolbox for
cancer immunotherapy. Nat Rev Drug Discov. 17:751–767. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lopes A, Vandermeulen G and Préat V:
Cancer DNA vaccines: Current preclinical and clinical developments
and future perspectives. J Exp Clin Cancer Res. 38:1462019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sahin U, Derhovanessian E, Miller M, Kloke
BP, Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B,
et al: Personalized RNA mutanome vaccines mobilize poly-specific
therapeutic immunity against cancer. Nature. 547:222–226. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cafri G, Gartner JJ, Zaks T, Hopson K,
Levin N, Paria BC, Parkhurst MR, Yossef R, Lowery FJ, Jafferji MS,
et al: mRNA vaccine-induced neoantigen-specific T cell immunity in
patients with gastrointestinal cancer. J Clin Invest.
130:5976–5988. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Platten M, Bunse L, Wick A, Bunse T, Le
Cornet L, Harting I, Sahm F, Sanghvi K, Tan CL, Poschke I, et al: A
vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature.
592:463–468. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tondini E, Arakelian T, Oosterhuis K,
Camps M, van Duikeren S, Han W, Arens R, Zondag G, van Bergen J and
Ossendorp F: A poly-neoantigen DNA vaccine synergizes with PD-1
blockade to induce T cell-mediated tumor control. Oncoimmunology.
8:16525392019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li Q, Ren J, Liu W, Jiang G and Hu R: CpG
oligodeoxynucleotide developed to activate primate immune responses
promotes antitumoral effects in combination with a neoantigen-based
mRNA cancer vaccine. Drug Des Devel Ther. 15:3953–3963. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Duperret EK, Perales-Puchalt A, Stoltz R,
G HH, Mandloi N, Barlow J, Chaudhuri A, Sardesai NY and Weiner DB:
A synthetic DNA, multi-neoantigen vaccine drives predominately MHC
class I CD8+ T-cell responses, impacting tumor
challenge. Cancer Immunol Res. 7:174–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang Z and Wu X: Study and analysis of
antitumor resistance mechanism of PD1/PD-L1 immune checkpoint
blocker. Cancer Med. 9:8086–8121. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tan CL, Kuchroo JR, Sage PT, Liang D,
Francisco LM, Buck J, Thaker YR, Zhang Q, McArdel SL, Juneja VR, et
al: PD-1 restraint of regulatory T cell suppressive activity is
critical for immune tolerance. J Exp Med. 218:e201822322021.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Buchbinder EI and Desai A: CTLA-4 and PD-1
pathways: Similarities, differences, and implications of their
inhibition. Am J Clin Oncol. 39:98–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wisdom AJ, Mowery YM, Riedel RF and Kirsch
DG: Rationale and emerging strategies for immune checkpoint
blockade in soft tissue sarcoma. Cancer. 124:3819–3829. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Xu F, Jin T, Zhu Y and Dai C: Immune
checkpoint therapy in liver cancer. J Exp Clin Cancer Res.
37:1102018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Pianko MJ, Liu Y, Bagchi S and Lesokhin
AM: Immune checkpoint blockade for hematologic malignancies: A
review. Stem Cell Investig. 4:322017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kabacaoglu D, Ciecielski KJ, Ruess DA and
Algül H: Immune checkpoint inhibition for pancreatic ductal
adenocarcinoma: Current limitations and future options. Front
Immunol. 9:18782018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Anagnostou V, Smith KN, Forde PM, Niknafs
N, Bhattacharya R, White J, Zhang T, Adleff V, Phallen J, Wali N,
et al: Evolution of neoantigen landscape during immune checkpoint
blockade in non-small cell lung cancer. Cancer Discov. 7:264–276.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Isaacsson Velho P and Antonarakis ES:
PD-1/PD-L1 pathway inhibitors in advanced prostate cancer. Expert
Rev Clin Pharmacol. 11:475–486. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang X, Guo G, Guan H, Yu Y, Lu J and Yu
J: Challenges and potential of PD-1/PD-L1 checkpoint blockade
immunotherapy for glioblastoma. J Exp Clin Cancer Res. 38:872019.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Jiang Y, Chen M, Nie H and Yuan Y: PD-1
and PD-L1 in cancer immunotherapy: Clinical implications and future
considerations. Hum Vaccin Immunother. 15:1111–1122. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Peng M, Mo Y, Wang Y, Wu P, Zhang Y, Xiong
F, Guo C, Wu X, Li Y, Li X, et al: Neoantigen vaccine: An emerging
tumor immunotherapy. Mol Cancer. 18:1282019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liu CJ, Schaettler M, Blaha DT,
Bowman-Kirigin JA, Kobayashi DK, Livingstone AJ, Bender D, Miller
CA, Kranz DM, Johanns TM and Dunn GP: Treatment of an aggressive
orthotopic murine glioblastoma model with combination checkpoint
blockade and a multivalent neoantigen vaccine. Neuro Oncol.
22:1276–1288. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Duraiswamy J, Kaluza KM, Freeman GJ and
Coukos G: Dual blockade of PD-1 and CTLA-4 combined with tumor
vaccine effectively restores T-cell rejection function in tumors.
Cancer Res. 73:3591–3603. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Rohaan MW, Wilgenhof S and Haanen JBAG:
Adoptive cellular therapies: The current landscape. Virchows Arch.
474:449–461. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Rath JA and Arber C: Engineering
strategies to enhance TCR-based adoptive T cell therapy. Cells.
9:14852020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Weber J, Atkins M, Hwu P, Radvanyi L,
Sznol M and Yee C; Immunotherapy Task Force of the NCI
Investigational Drug Steering Committee, : White paper on adoptive
cell therapy for cancer with tumor-infiltrating lymphocytes: A
report of the CTEP subcommittee on adoptive cell therapy. Clin
Cancer Res. 17:1664–1673. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Rohaan MW, van den Berg JH, Kvistborg P
and Haanen JBAG: Adoptive transfer of tumor-infiltrating
lymphocytes in melanoma: A viable treatment option. J Immunother
Cancer. 6:1022018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
van den Berg JH, Heemskerk B, van Rooij N,
Gomez-Eerland R, Michels S, van Zon M, de Boer R, Bakker NAM,
Jorritsma-Smit A, van Buuren MM, et al: Tumor infiltrating
lymphocytes (TIL) therapy in metastatic melanoma: Boosting of
neoantigen-specific T cell reactivity and long-term follow-up. J
Immunother Cancer. 8:e0008482020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zacharakis N, Chinnasamy H, Black M, Xu H,
Lu YC, Zheng Z, Pasetto A, Langhan M, Shelton T, Prickett T, et al:
Immune recognition of somatic mutations leading to complete durable
regression in metastatic breast cancer. Nat Med. 24:724–730. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tran E, Robbins PF, Lu YC, Prickett TD,
Gartner JJ, Jia L, Pasetto A, Zheng Z, Ray S, Groh EM, et al:
T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J
Med. 375:2255–2262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Sun J, Zhang J, Hu H, Qin H, Liao X, Wang
F, Zhang W, Yin Q, Su X, He Y, et al: Anti-tumour effect of
neo-antigen-reactive T cells induced by RNA mutanome vaccine in
mouse lung cancer. J Cancer Res Clin Oncol. 147:3255–3268. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Rosenberg SA and Restifo NP: Adoptive cell
transfer as personalized immunotherapy for human cancer. Science.
348:62–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lu YC, Yao X, Crystal JS, Li YF, El-Gamil
M, Gross C, Davis L, Dudley ME, Yang JC, Samuels Y, et al:
Efficient identification of mutated cancer antigens recognized by T
cells associated with durable tumor regressions. Clin Cancer Res.
20:3401–3410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Gros A, Parkhurst MR, Tran E, Pasetto A,
Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts
IM, et al: Prospective identification of neoantigen-specific
lymphocytes in the peripheral blood of melanoma patients. Nat Med.
22:433–438. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Bailey P, Chang DK, Forget MA, Lucas FA,
Alvarez HA, Haymaker C, Chattopadhyay C, Kim SH, Ekmekcioglu S,
Grimm EA, et al: Exploiting the neoantigen landscape for
immunotherapy of pancreatic ductal adenocarcinoma. Sci Rep.
6:358482016. View Article : Google Scholar : PubMed/NCBI
|