Introduction
Breast cancer is one of the most common malignant
tumors in women worldwide, and triple-negative breast cancer
accounts for 15-20% of all breast cancer cases (1,2). At
present, various clinical trials have been conducted to treat and
delay the progression of breast cancer, but mortality and morbidity
among patients remain high (3).
Several carcinogens and signaling pathways are known to be involved
in the progression of breast cancer. Among them, signal transducer
and activator of transcription 3 (STAT3) has been reported to play
a pivotal role in breast cancer development (4). Unlike normal cells, in which the
activity of STAT3 is strictly regulated, STAT3 activity in breast
cancer contributes to tumorigenesis in a multifaceted manner
(5). Interleukin-6 (IL-6),
G-protein-coupled receptors (GPCRs), and Toll-like receptors (TLRs)
are known to be involved in STAT3 activation in various types of
cancer, including colon cancer (6,7).
Once STAT3 signaling is activated, it induces carcinogenesis
through the expression of various genes associated with apoptosis
(survivin, Bcl-xL, and Bcl-2), proliferation (cyclin D1), and
angiogenesis (VEGF) (8,9). Additionally, several protein tyrosine
phosphatases (PTPs) promote the death of cancer cells by STAT3
inactivation (10,11). SH2 domain containing phosphatase
1/2 (SHP-1/2) and protein tyrosine phosphatase 1B (PTP-1B) are
known to be associated with STAT3 inactivation. SHP-1 is highly
expressed in normal lymphocytes, whereas its expression is
decreased in most cancer cells (12). In addition, SHP-1 expression in
cancer cells effectively suppresses target genes such as VEGF-1,
cyclin D1, and survivin (13).
Thus, dephosphorylation of STAT3 by increasing the expression of
SHP-1 may be an efficient strategy for the treatment of various
cancer types.
In previous decades, many researchers have suggested
that natural compounds may act as potent anti-cancer drugs with
high efficacy and low side effects. Various candidates have been
studied and examined for their anti-cancer properties and
underlying mechanisms. Minecoside (MIN), an active compound
extracted from Veronica peregrina L., belongs to the family
Scrophulariaceae (14). The entire
plant has been used as a traditional drug for the treatment of
menstrual irregularities, fractures, and traumatic injuries
(14). Moreover, several compounds
from Veronica peregrina L. have been reported to exhibit
antioxidant activity (14). A
recent study revealed that MIN suppressed the invasive capability
of cancer cells by inhibiting CXCR4 expression via blocking NF-κB
(15). However, the mechanism
underlying the regulation of STAT3 activation by MIN has not been
completely understood. Therefore, we investigated whether MIN could
modulate the apoptosis of breast cancer cells by regulating the
STAT3 signaling pathway.
Materials and methods
Purity analysis of minecoside
Minecoside was isolated from the Catalpa ovata
according to a previously protocol (16). Purity analysis of isolated
minecoside was carried on Shiseido CapCell PAK C18 column
(Sigma-Aldrich) particle size 5 µm (150×4.6 mm) using a Waters 2695
system (Waters Corporation). The mobile phase consisted of water
with 0.1% formic acid (solvent A), acetonitrile with 0.1% formic
acid (solvent B), which were applied in the following gradient
elution: 5% B (0–5 min), 5-95% B (5–30 min). The injection volume
was 10 µl, and the flow rate was 0.6 ml/min. The UV chromatogram of
minecoside was acquired at 330 nm and integrated. Purity of
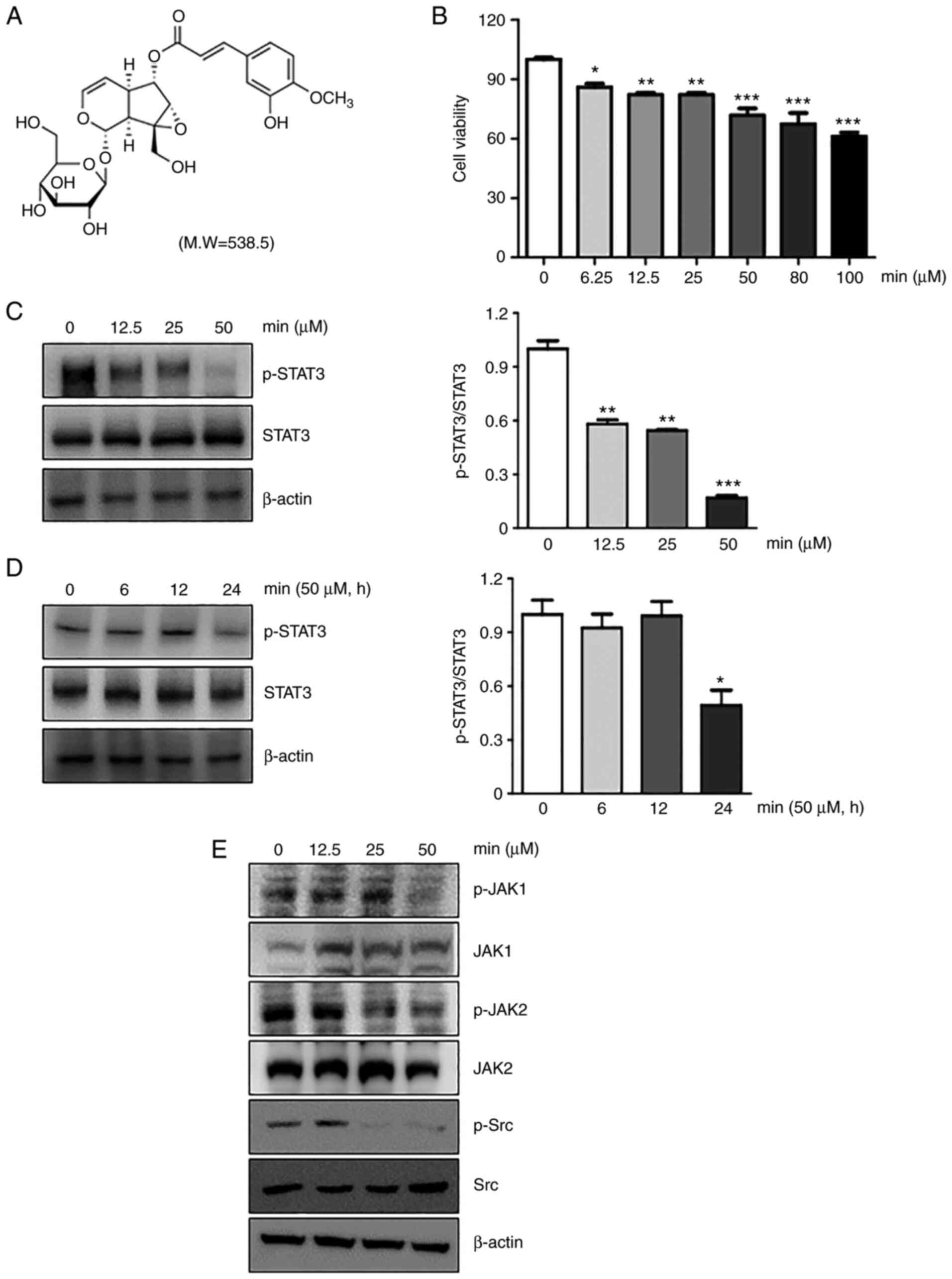
minecoside was 90.4±0.4%. The chemical structure of MIN is shown in
Fig. 1A. As preliminary
experiments with minecoside (MIN) had been performed by the
authors, identical conditions were adhered to throughout the study
(15).
Materials
Antibodies against phospho-STAT3 (1:1,000; rabbit,
monoclonal; cat. no. 9145), STAT3 (1:1,000; rabbit, monoclonal;
cat. no. 12640), p-JAK1 (1:1,000; rabbit, polyclonal; cat. no.
3331), p-JAK2 (1:1,000; rabbit, monoclonal; cat. no. 8082), p-Src
(1:1,000; rabbit, polyclonal; cat. no. 2101), Src (1:1,000; rabbit,
polyclonal; cat. no. 2108), SHP-1 (1:1,000; rabbit, monoclonal;
cat. no. 3759), cleaved PARP (1:1,000; rabbit, monoclonal; cat. no.
5625), cleaved caspase-9 (1:1,000; rabbit, monoclonal; cat. no.
7237), cleaved caspase-3 (1:1,000; rabbit, polyclonal; cat. no.
9661), Bcl-2 (1:1,000; rabbit, monoclonal; cat. no. 3498), β-actin
(1:1,000; rabbit, monoclonal; cat. no. 4970) and anti-rabbit IgG
(1:5,000; rabbit, polyclonal; cat. no. 14708) were obtained from
Cell Signaling Technology, Inc. CXCR4 antibody (1:10,000; rabbit,
polyclonal; cat. no. ab227767) was obtained from Abcam. Antibodies
to VEGF (1:1,000; rabbit, polyclonal; cat. no. sc-152), JAK1
(1:1,000; rabbit, polyclonal; cat. no. sc-277), JAK2 (1:1,000;
rabbit, polyclonal; cat. no. sc-278), Bcl-xL (1:1,000; mouse,
monoclonal; cat. no. sc-8392), Cyclin D1 (1:1,000; rabbit,
polyclonal; cat. no. sc-718) and goat anti-mouse IgG (1:5,000;
mouse, monoclonal; cat. no. 2355) were purchased from Santa Cruz
Biotechnology, Inc. RIPA buffers were purchased from Cell Signaling
Technology, Inc. DMEM, fetal bovine serum (FBS), and
antibiotic-antimycotic mixture were purchased from Gibco BRL;
Thermo Fisher Scientific, Inc. The DIG gel shift kit for EMSA was
purchased from Roche Diagnostics.
Cell culture
MDA-MB-231 cells were obtained from the American
Type Culture Collection and cultured in DMEM supplemented with 10%
FBS and 1% antibiotics at 37°C in a humidified incubator with 5%
CO2. For the western blot assay, MDA-MB-231 cells were
treated with the indicated concentrations of MIN (0, 12.5, 25, and
50 µM) for 24 h or at the indicated times (0, 6, 12, and 24 h) at
50 µM concentration.
Cell viability assay by Cell Counting
Kit-8 (CCK-8)
To determine the optimal concentration of MIN
capable of inhibiting STAT3 activity, MDA-MB-231 cells
(5×104 cells/well) were seeded into 96-well plates. MIN
was added at various concentrations (0–100 µM) at 37°C for 24 h.
Subsequently, 10 µl CCK-8 solution was added to each well, and the
cells were incubated at 37°C for 2 h. The cell viability was
determined by measuring the absorbance at 490 nm using a
GloMax® Explorer Multimode Microplate Reader (Promega
Corporation).
Western blot assay
As described in a previous study (17), whole-cell extracts were lysed with
RIPA buffer and then the extracted proteins were separated by 10%
SDS-PAGE and electrotransferred to PVDF membranes. The membranes
were immunoblotted with the aforementioned primary and secondary
antibodies.
Electrophoretic mobility shift assay
(EMSA)
As previously described (17), binding activity of STAT3 to
consensus oligonucleotides was measured with extracting nuclear
proteins from MIN-treated MDA-MB-231 cells using non-radioactive
EMSA assay (DIG Gel Shift Kit; Roche Diagnostics). Oligonucleotide
probes labeled with DIG containing consensus binding sites for
STAT3 (5-CTTCATTTCCCGTAAATCCCTAAAGCT −3 and
5-AGCTTTAGGGATTTACGGGAAATG A-3) were used.
Immunofluorescence assay
As described in a previous study (15), immunofluorescence assay was
performed to check STAT3 nuclear translocation. The cells were
blocked with 5% BSA for 1 h and then incubated with the anti-STAT3
antibody at room temperature for 1 h. After being washed with PBS,
the slides were incubated with the secondary antibody Alexa Flour
488 for 30 min and counterstained for nuclei with Hoechst-33342 at
37°C for 10 min. The fluorescence image was measured under ×200
magnification using a fluorescence microscope (Nikon ECLIPSE Ti-U;
Nikon Corporation).
TUNEL assay
Detection of DNA fragments in situ using
terminal deoxyribonucleotidyl transferase-mediated dUTP-digoxigenin
nick end labeling (TUNEL) assay kits (Roche Diagnostics) was
applied to investigate active cell death. Briefly, cells were
treated with 50 µM MIN for 24 h, then washed with PBS. The cells
were fixed with freshly prepared 4% paraformaldehyde for 1 h at
room temperature and treated with 0.2% Triton X-100 solution for 2
min on ice. Intracellular DNA fragments were then labeled by
exposing the cells to TUNEL reaction mixture for 1 h at 37°C in a
humidified atmosphere, with protection from light. The cells were
washed with PBS twice, then transferred to slides and analyzed
under a fluorescence microscope (Nikon ECLIPSE Ti-U; Nikon
Corporation).
Statistical analysis
Experimental data are presented as the mean ± SEM
obtained from three independent experiments. Statistical
significance was assessed by one-way analysis of variance (ANOVA)
followed by Tukey's honest significant difference test or Students'
t-test using graph pad prism 6 software package (GraphPad Software
Inc.) and ImageJ (imagej.nih.gov/ij). The statistics of the cell
viability assay and western blot were determined by triplicate
experiments using one-way ANOVA with Tukey's post hoc test. The
statistical analysis of STAT3 translocation was determined by
Students' t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
MIN downregulates constitutive
activation of STAT3 in MDA-MB-231 cells
Since constitutive activation of STAT3 is found in
numerous cancer types, including >40% of primary breast tumors
(4), and a recent study has
reported anti-cancer effects of MIN in breast cancer (15), whether MIN affects STAT3 activation
was examined in the present study. To determine the optimal
concentration of MIN, the cell viability at the indicated
concentrations of MIN (0, 6.25, 12.5, 25, 50, 80 and 100 µM) were
evaluated. Based on the results, 50 µM of MIN was selected as an
optimal concentration and used throughout the study (Fig. 1B). As shown in Fig. 1C and D, MIN reduced the
phosphorylation of STAT3 in a dose- and time-dependent manner in
MDA-MB-231 cells. The reduction ratio of phosphorylated STAT3 to
STAT3 was from 15 to 55%.
MIN downregulates upstream signaling
pathway responsible for STAT3 activation
Janus kinases 1/2 (JAK1/2) and Src kinase were
reported to contribute to STAT3 activation and are involved in
cancer cell growth (18). Thus,
whether MIN could inhibit the upstream signaling molecules that
activate STAT3 was examined. The results showed that MIN suppressed
the constitutive activation of JAK1, JAK2, and Src kinase at 50 µM
in MDA-MB-231 cells (Fig. 1E).
MIN suppresses DNA binding of STAT3 in
MDA-MB-231 cells
Based on the fact that the phosphorylation of STAT3
regulates gene transcription through dimerization, nuclear
translocation, and DNA binding (19), whether MIN can regulate the DNA
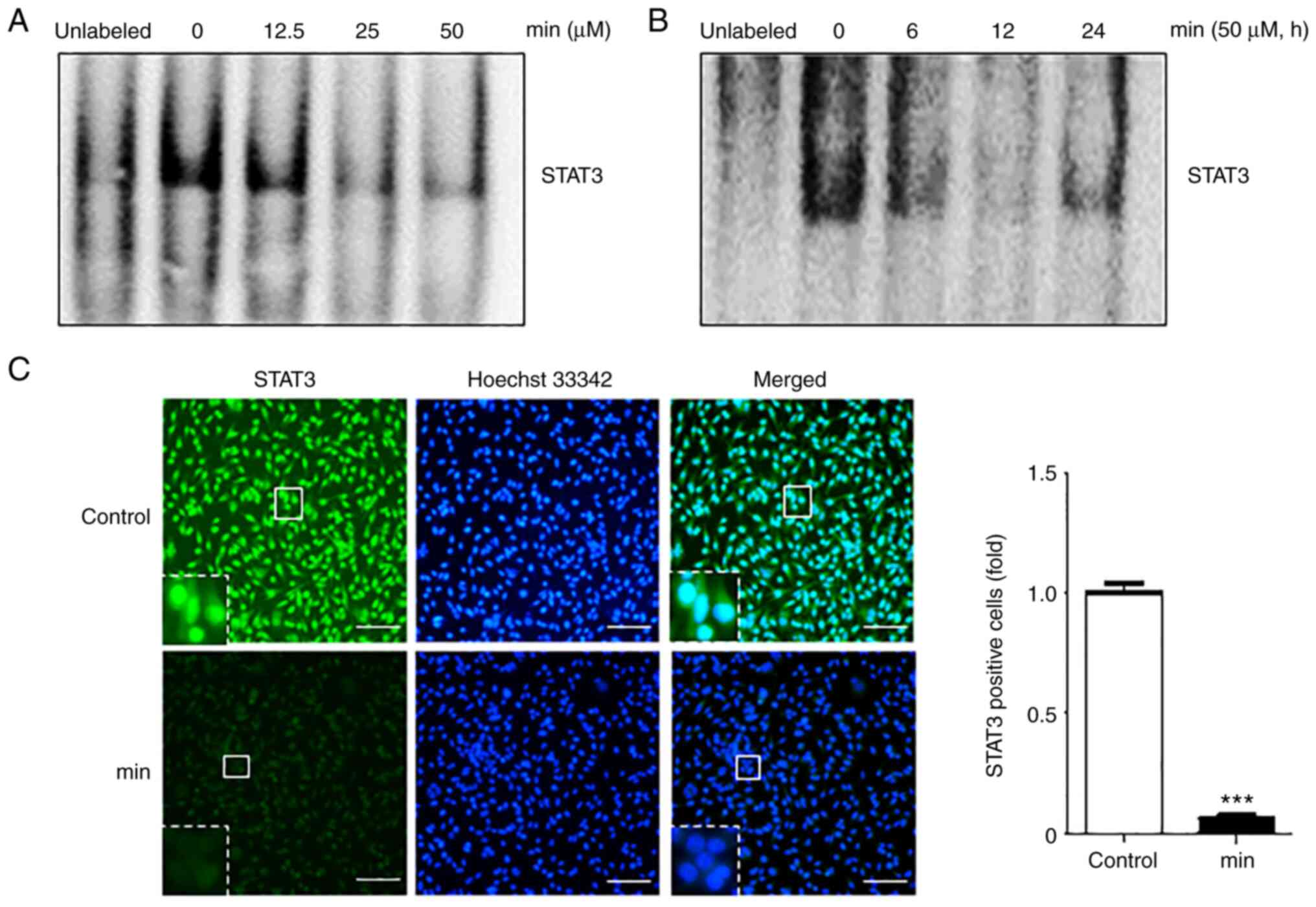
binding activity of STAT3 was investigated. After cells were
treated with the indicated concentrations of MIN for 24 h or with
50 µM of MIN for different time periods, EMSA was performed to
examine the DNA binding activity of STAT3. The results showed that
MIN suppressed STAT3-DNA binding in a dose- and time-dependent
manner (Fig. 2A and B).
To check whether MIN inhibits STAT3-DNA binding by
blocking STAT3 nuclear translocation, an immunofluorescence assay
was performed. As shown in Fig.
2C, 50 µM MIN suppressed the translocation of STAT3 into the
nucleus as compared to the control groups.
MIN induces the expression of
SHP-1
Previous findings showed that, several PTPs can
modulate the STAT3 signaling pathway in various cancer cells
(20). Therefore, whether the
regulation of STAT3 activity by MIN was due to PTP induction was
examined. Several studies reported that loss of SHP-1 in cancer
cells improves STAT3 signaling (12,21).
In addition, SHP-1 was proposed as a promising drug target in the
development of STAT3 inhibitors because it can dephosphorylate JAKs
(22) as well as STAT3 (11). Thus, whether MIN modulated SHP-1
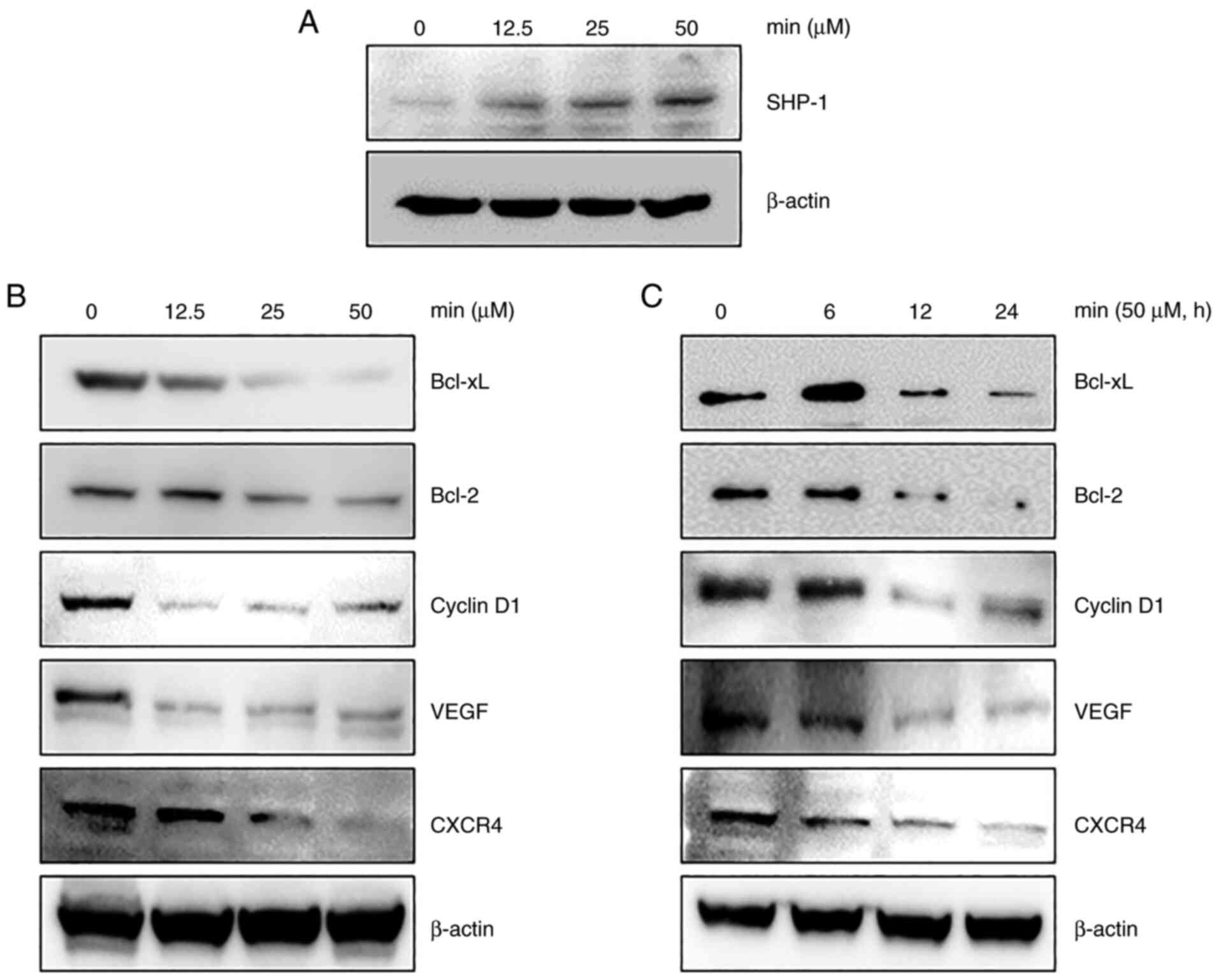
expression in MDA-MB-231 cells was examined. As shown in Fig. 3A, MIN treatment increased SHP-1
expression in a concentration-dependent manner.
MIN inhibits the expression of various
genes involved in growth, angiogenesis, invasion, and
metastasis
STAT3 activation induces the expression of various
genes involved in cancer cell survival and proliferation (9). The anti-apoptotic proteins Bcl-2
(23), Bcl-xL (24), invasive protein CXCR4 (25), angiogenic protein VEGF (26), and cell cycle control protein
cyclin D1 (27) are known to be
induced upon STAT3 activation. Thus, whether MIN affects the
expression of these genes by regulating STAT3 activity was
investigated. As shown in Fig. 3B and
C, MIN downregulated the expression of Bcl-xL, Bcl-2, cyclin
D1, VEGF, and CXCR4 in a dose- and time-dependent manner.
MIN induces caspase-dependent
apoptosis in MDA-MB-231 cells
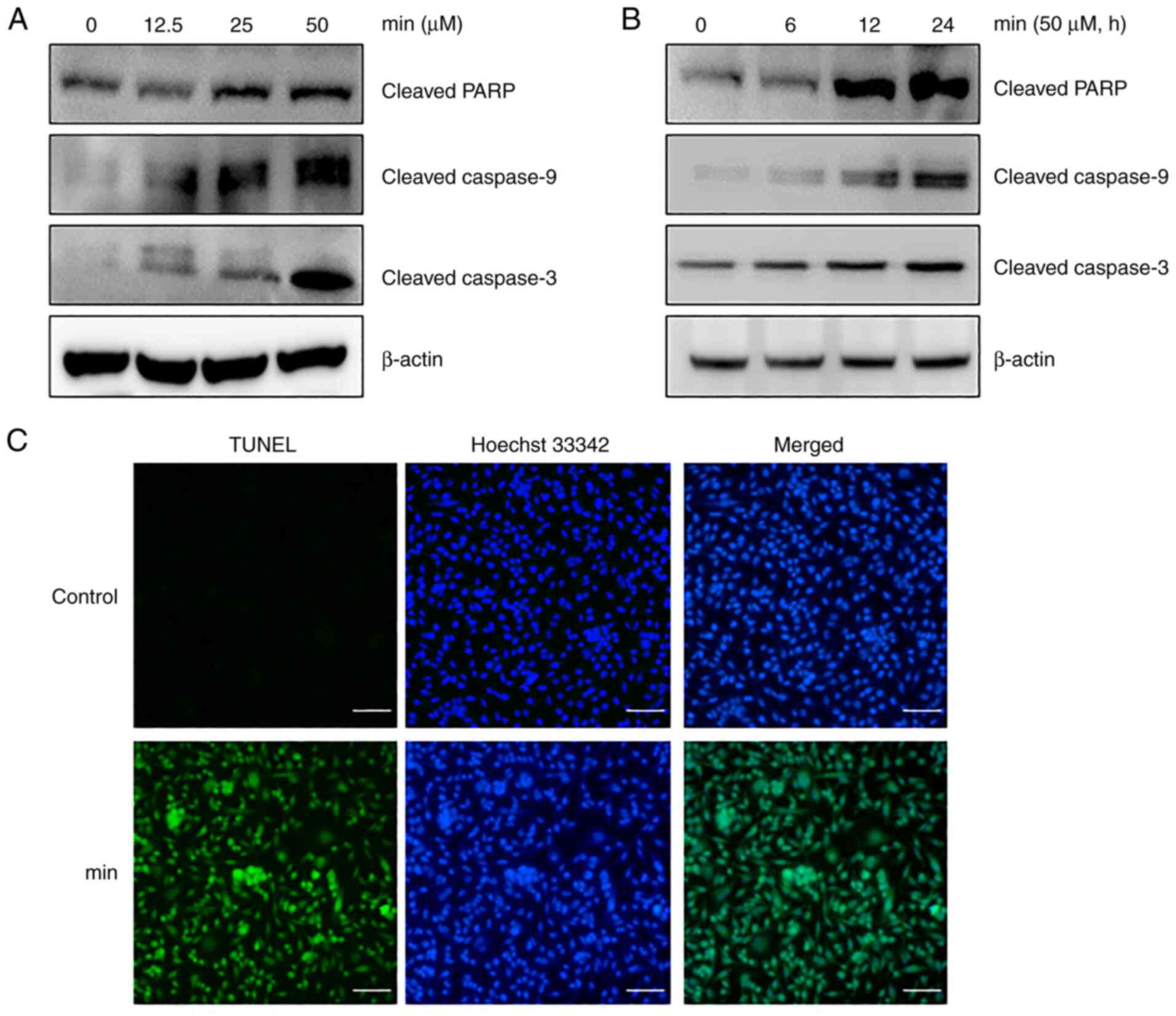
To further examine the apoptotic progression induced
by MIN, major proteins involved in the caspase pathway were
examined using western blot analysis. The results showed that MIN
upregulated the expression of cleaved forms of caspase-9,
caspase-3, and PARP in a dose- and time-dependent manner (Fig. 4A and B). To confirm MIN-induced
apoptosis, a TUNEL assay, which is a commonly used method for
identifying apoptosis, was performed. The TUNEL analysis revealed
that MIN increased the number of apoptotic cells (Fig. 4C). Taken together, these results
strongly suggested that MIN induced caspase-dependent apoptosis in
MDA-MB-231 cells.
Discussion
Although the specific role of STAT3 in the onset and
progression of breast cancer is not fully defined, the activation
of IL-6/JAK2/STAT3 pathways is well known to promote carcinogenesis
(4,28). In particular, breast cancer is
associated with inflammatory conditions with high levels of growth
factors and cytokines (6). For
example, inflammatory cytokines, such as IL-6, are known to promote
cancer cell growth, invasion, migration, metastasis, angiogenesis,
and drug resistance through the JAK/STAT3 pathway (23,26,29).
Based on the aforementioned studies, we investigated whether MIN
promoted apoptosis by inhibiting constitutive STAT3 activation in
breast cancer cells.
The results showed that, MIN suppressed constitutive
STAT3 activation in MDA-MB-231 cells. The phosphorylation of STAT3
at Tyr705, an important step in STAT3 activation, was inhibited by
MIN in a dose- and time-dependent manner. STAT3 phosphorylation is
mediated through the activation of non-receptor protein tyrosine
kinases such as JAK1 and JAK2. Src kinase is also known to play an
important role in the phosphorylation of STAT3 (30,31).
Indeed, although upstream kinases are important in tumorigenesis,
STAT3, which is considered a key transcription factor for gene
expression involved in malignancy, was the primary focus of the
present study. The results demonstrated that MIN inhibited JAK1,
JAK2, and Src kinase activation, suggesting that MIN suppresses
STAT3 phosphorylation via the inactivation of upstream kinases.
Since the phosphorylation of STAT3 regulates gene transcription
through dimerization, nuclear translocation, and DNA binding
(19), whether MIN can affect
STAT3 translocation and DNA binding activity was investigated. The
results revealed that MIN suppressed STAT3-DNA binding as well as
the nuclear translocation of STAT3.
Several PTPs modulate the STAT3 signaling pathway in
various cancer cells (20). In
particular, the SH2 domain-containing phosphatase-1 (SHP-1)
possesses a tumor-suppressive potential by virtue of negatively
regulating STAT3 signaling (12);
it is considered an antagonist of tyrosine kinases related to tumor
growth and anti-apoptosis (32).
SHP-1 expression is known to be reduced or abolished in estrogen
receptor-negative breast cancer cell lines. In the present study,
whether the inhibitory effect of MIN on STAT3 activation was
related to SHP-1 expression was examined. The results showed that,
MIN treatment increased SHP-1 expression in a
concentration-dependent manner. The increase in SHP-1 expression by
MIN was confirmed via the association with the inhibition of
constitutive STAT3 activation in subsequent experiments. Besides
SHP-1, the involvement of other PTPs in the regulation of STAT3 by
MIN should be investigated in future studies.
STAT3 activation is observed in various malignant
tumors, including lung, breast, liver, colon, prostate, stomach,
pancreas, kidney, and brain cancers (33). This is because STAT3 activation
upregulates a variety of cellular signaling required for cell
survival (Bcl-xL, Bcl-2, c-myc, Mcl-1, and survivin), proliferation
(cyclin D1), invasion (MMP-9, CXCR4, Rho, and Rac), and
angiogenesis (VEGF) (23,25,28,33).
Findings of the present study showed that MIN also downregulated
STAT3-mediated protein expression such as cyclin D1, Bcl-2, Bcl-xL,
CXCR4, and VEGF. In a previous study, the inhibitory effect of MIN
on CXCR4 expression was found to be mediated via the blockade of
transcription factor NF-κB (15).
A gene that contains only NF-κB binding sites may be responsive to
NF-κB, but not STAT3. However, a gene that contains both NF-κB and
STAT3 binding sites may be regulated by both in a cooperative
manner (34). Thus, investigation
of the key role of MIN on crosstalk between STAT3 and NF-κB is
imperative.
Since MIN inhibited the expression of proteins
related to proliferation (cyclin D1), and cell survival (Bcl-2,
Bcl-xL), whether MIN affected apoptotic progression was examined.
The results showed that MIN upregulated caspase-dependent apoptosis
in MDA-MB-231 cells. A major limitation of the present study was
that the inhibitory effect of MIN was not examined in other cancer
cells or in an animal model.
In conclusion, the results of the present study
provide evidence that MIN exerts anticancer activity via inhibition
of the JAK/STAT3 signaling pathway, especially in breast cancer
cells. Further studies using animal models are required to
determine the potential of this molecule as an anticancer drug.
Acknowledgements
Not applicable.
Funding
This research was supported by a grant from the Korea Health
Technology R&D Project through the Korea Health Industry
Development Institute (KHIDI), funded by the Ministry of Health
& Welfare, Republic of Korea (HF20C0038).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
BP and BK conceived the study. BK developed the
methodology, and obtained and validated the data. BK performed the
experiments. BP and KL analyzed and interpreted the data. BK and BP
prepared the original draft. BP and KL revised the draft. BK and BP
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis C, Siegel R, Bandi P and Jemal A:
Breast cancer statistics, 2011. CA Cancer J Clin. 61:409–418. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Banerjee K and Resat H: Constitutive
activation of STAT3 in breast cancer cells: A review. Int J Cancer.
138:2570–2578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Laudisi F, Cherubini F, Monteleone G and
Stolfi C: STAT3 interactors as potential therapeutic targets for
cancer treatment. Int J Mol Sci. 19:17872018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garbers C, Aparicio-Siegmund S and
Rose-John S: The IL-6/gp130/STAT3 signaling axis: Recent advances
towards specific inhibition. Curr Opin Immunol. 34:75–82. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Becker C, Fantini MC, Schramm C, Lehr HA,
Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, et al:
TGF-beta suppresses tumor progression in colon cancer by inhibition
of IL-6 trans-signaling. Immunity. 21:491–501. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kundu J, Choi BY, Jeong CH, Kundu JK and
Chun KS: Thymoquinone induces apoptosis in human colon cancer
HCT116 cells through inactivation of STAT3 by blocking JAK2- and
Src-mediated phosphorylation of EGF receptor tyrosine kinase. Oncol
Rep. 32:821–828. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Crowe PJ, Goldstein D and Yang JL:
STAT3 inhibition, a novel approach to enhancing targeted therapy in
human cancers (Review). Int J Oncol. 41:1181–1191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gu F, Dubé N, Kim JW, Cheng A,
Ibarra-Sanchez Mde J, Tremblay ML and Boisclair YR: Protein
tyrosine phosphatase 1B attenuates growth hormone-mediated
JAK2-STAT signaling. Mol Cell Biol. 23:3753–3762. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu CY, Tseng LM, Su JC, Chang KC, Chu PY,
Tai WT, Shiau CW and Chen KF: Novel sorafenib analogues induce
apoptosis through SHP-1 dependent STAT3 inactivation in human
breast cancer cells. Breast Cancer Res. 15:R632013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu C, Sun M, Liu L and Zhou GW: The
function of the protein tyrosine phosphatase SHP-1 in cancer. Gene.
306:1–12. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joo MK, Park JJ, Yoo HS, Lee BJ, Chun HJ,
Lee SW and Bak YT: Epigenetic regulation and anti-tumorigenic
effects of SH2-containing protein tyrosine phosphatase 1 (SHP1) in
human gastric cancer cells. Tumour Biol. 37:4603–4612. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kwak JH, Kim HJ, Lee KH, Kang SC and Zee
OP: Antioxidative iridoid glycosides and phenolic compounds from
Veronica peregrina. Arch Pharm Res. 32:207–213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim B, Min YH and Park B: Minecoside
modulates cell invasion via regulation of CXCR4 expression in
breast and colon cancer cells. Planta Med. 86:331–337. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park S, Shin H, Park Y, Choi I, Park B and
Lee KY: Characterization of inhibitory constituents of NO
production from Catalpa ovata using LC-MS coupled with a cell-based
assay. Bioorg Chem. 80:57–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim B, Lee KY and Park B: Crocin
suppresses constitutively active STAT3 through induction of protein
tyrosine phosphatase SHP-1. J Cell Biochem. 118:3290–3298. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Masciocchi D, Gelain A, Villa S,
Meneghetti F and Barlocco D: Signal transducer and activator of
transcription 3 (STAT3): A promising target for anticancer therapy.
Future Med Chem. 3:567–597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim M, Morales LD, Jang IS, Cho YY and Kim
DJ: Protein tyrosine phosphatases as potential regulators of STAT3
signaling. Int J Mol Sci. 19:27082018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han Y, Amin HM, Franko B, Frantz C, Shi X
and Lai R: Loss of SHP1 enhances JAK3/STAT3 signaling and decreases
proteosome degradation of JAK3 and NPM-ALK in ALK+
anaplastic large-cell lymphoma. Blood. 108:2796–2803. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiao H, Berrada K, Yang W, Tabrizi M,
Platanias LC and Yi T: Direct association with and
dephosphorylation of Jak2 kinase by the SH2-domain-containing
protein tyrosine phosphatase SHP-1. Mol Cell Biol. 16:6985–6992.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Real PJ, Sierra A, De Juan A, Segovia JC,
Lopez-Vega JM and Fernandez-Luna JL: Resistance to chemotherapy via
Stat3-dependent overexpression of Bcl-2 in metastatic breast cancer
cells. Oncogene. 21:7611–7618. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Xu J and Xing G: Lycorine inhibits
the growth and metastasis of breast cancer through the blockage of
STAT3 signaling pathway. Acta Biochim Biophys Sin (Shanghai).
49:771–779. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Xiao Q, Bai X, Yu Z, Sun M, Zhao H,
Mi X, Wang E, Yao W, Jin F, et al: Activation of STAT3 is involved
in malignancy mediated by CXCL12-CXCR4 signaling in human breast
cancer. Oncol Rep. 32:2760–2768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Niu G, Wright KL, Huang M, Song L, Haura
E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al:
Constitutive Stat3 activity up-regulates VEGF expression and tumor
angiogenesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Johnston PA and Grandis JR: STAT3
signaling: Anticancer strategies and challenges. Mol Interv.
11:18–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burke WM, Jin X, Lin HJ, Huang M, Liu R,
Reynolds RK and Lin J: Inhibition of constitutively active Stat3
suppresses growth of human ovarian and breast cancer cells.
Oncogene. 20:7925–7934. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang T, Niu G, Kortylewski M, Burdelya L,
Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola
D, et al: Regulation of the innate and adaptive immune responses by
Stat-3 signaling in tumor cells. Nat Med. 10:48–54. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ihle JN: STATs: Signal transducers and
activators of transcription. Cell. 84:331–334. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schreiner SJ, Schiavone AP and Smithgall
TE: Activation of STAT3 by the Src family kinase Hck requires a
functional SH3 domain. J Biol Chem. 277:45680–45687. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang TT, Su JC, Liu CY, Shiau CW and Chen
KF: Alteration of SHP-1/p-STAT3 signaling: A potential target for
anticancer therapy. Int J Mol Sci. 18:12342017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Santoni M, Massari F, Del Re M, Ciccarese
C, Piva F, Principato G, Montironi R, Santini D, Danesi R, Tortora
G and Cascinu S: Investigational therapies targeting signal
transducer and activator of transcription 3 for the treatment of
cancer. Expert Opin Investig Drugs. 24:809–824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grivennikov SI and Karin M: Dangerous
liaisons: STAT3 and NF-kappaB collaboration and crosstalk in
cancer. Cytokine Growth Factor Rev. 21:11–19. 2010. View Article : Google Scholar : PubMed/NCBI
|