Introduction
Cancer is a common cause of death worldwide; its two
essential characteristics are the loss of cell growth control,
which leads to tumor formation, and the invasion of other tissues
through metastasis. In addition, dissemination through blood or
lymphatic vessels leads to secondary tumors. It has been estimated
that ~18.1 million new cases and 9.6 million deaths were related to
this disease in 2018. In women, the second most common type of
cancer is breast cancer (BC), with 11.6% of cases, and in men,
prostate cancer (PC) is the second most common type of cancer, with
7.1% of cases (1).
Hanahan and Weinberg described six major hallmarks
of cancer progression. These are sustaining proliferative
signaling, evading growth suppressors, activating invasion and
metastasis, enabling replicative immortality, inducing angiogenesis
and resisting cell death (2). In
recent years, most of the knowledge attained has been in
elucidating cancer biology. Generally speaking, a single gene
mutation seldom causes cancer. However, cancer can occur when a
mutation falls in a key gene. These key genes can be categorized
into three main groups: i) Proto-oncogenes; ii) tumor suppressor
genes; and iii) DNA repair genes (3). Advances in cancer biology have
identified possible molecular targets in BC and PC.
BC is a highly heterogeneous disease with complex
classification subtypes such as Luminal A, Luminal B, HER2-positive
and triple-negative. Most BC cases are invasive ductal carcinoma;
however, inflammatory BC is also of concern due to its
aggressiveness and occurrence in different patient populations
(4). Similarly, PC is classified
into three categories regarding its biology: i) Endocrine-driven;
ii) microenvironment-dependent; and iii) tumor cell-autonomous
(5). Several PC studies have
demonstrated the role of the androgen receptor (AR) in its
development and progression (6–10).
The AR is located in the cytoplasm and, when bound with its ligand,
translocates into the cell nucleus recognizing hormone response
elements in regulatory genomic regions (11).
Standard cancer therapies vs. alternative
treatments
The objective of cancer therapy is to keep the
patient free of disease or in remission with a partial or complete
decrease of symptoms. The most common cancer therapies are surgery,
radiotherapy and chemotherapy. In addition to being local
treatments, they possess several disadvantages and limitations. For
example, surgery requires diagnosis at an early stage of the
disease, when metastasis development is less frequent; however,
overdiagnosis has increased the number of mastectomies and
prostatectomies performed each year (12).
Radiotherapy is applied to 30–40% of solid tumors,
alone or combined with other treatments. However, it causes genetic
damage to tumor cells and surrounding healthy tissues, with severe
side effects. Radiation exposure also causes inflammation and,
consequently, oxidative damage. High doses cause chronic damage
that interferes with the healing ability of the tissue (12,13).
Chemotherapy generally lacks specificity, leading to cytotoxic
damage throughout the body and the development of resistance
(14,15). Chemotherapy and radiotherapy have
been shown to cause DNA double-strand breaks, increasing the
production of reactive oxygen species (ROS) and a general stress
response. This damage leads to several possible outcomes besides
apoptosis, such as cell cycle arrest, senescence, mitotic
catastrophe, inflammatory response and fibrosis at the tissue level
(16).
Targeted therapies are directed at a biochemical
pathway or target molecule required for tumor survival (15). Given the limitations of
conventional treatments, the term precision medicine has emerged,
in which therapy is directed according to the characteristics of
the cancer to be treated. This therapy includes antibodies, drugs,
nucleic acids and other therapeutic molecules. However, targeted
therapies could be improved using nanosystems (NSs) as carriers
whose surface can be modified to include the therapeutic principle
and other components to protect the NS and facilitate target cell
recognition and entry.
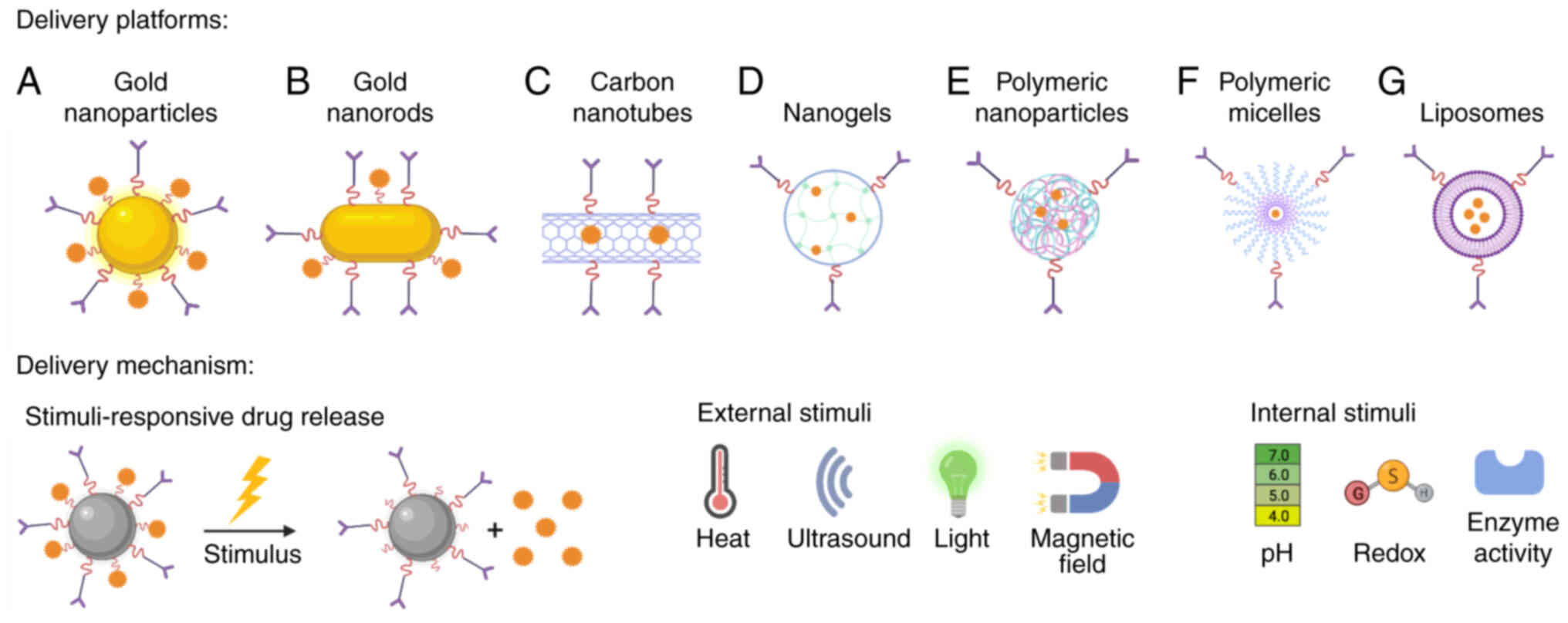
Nanomedicine development involves several types of
nanoplatforms such as metallic and polymeric nanoparticles (NPs),
nanoliposomes and nanomicelles, among others (17). Additionally, stimuli-responsive
drug release systems increase cytotoxicity against cancer cells
(18–20). Some of these nanoplatforms, along
with the most common stimuli-responsive drug release systems, are
shown in Fig. 1.
Strategies for site-directed cancer therapy
using NSs
One highly advantageous characteristic of NSs is
their ability to be functionalized in several ways for specific
guidance to cancer cells and tumors. Several guidance tools are
used, such as antibodies and aptamers. Some of these strategies can
be seen in Fig. 2.
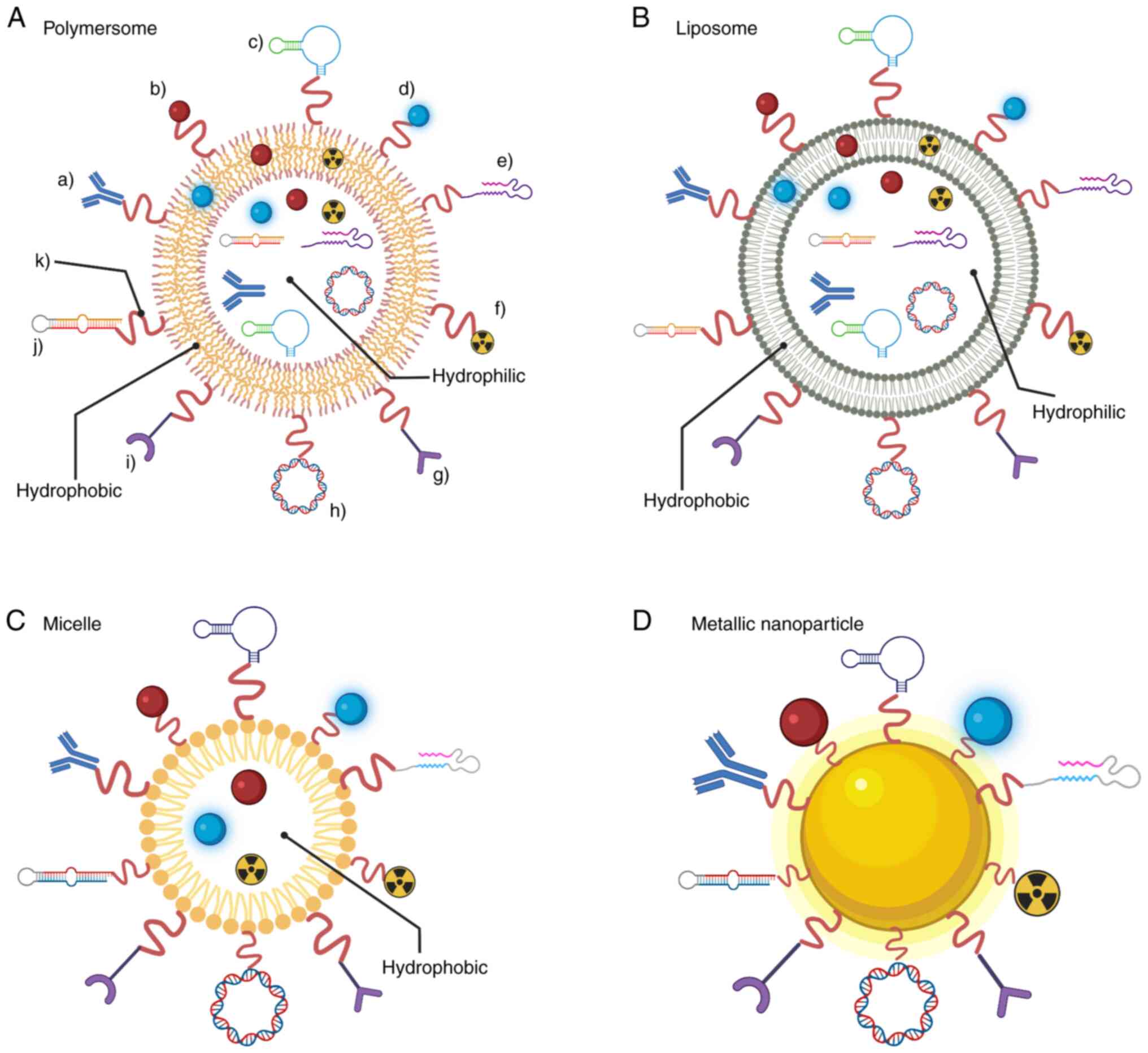 | Figure 2.Functionalization strategies for
specific site-direction of NPs. Antibodies, targeting ligand and
aptamer guidance have been used for highly specific delivery into a
particular cell type. Fluorescent dyes and radioligands have been
used for diagnostic purposes. Drug, DNA vector and pre-miRNA
coupling have been studied for therapeutic purposes. CPPs and
enzymes have been shown to increase cell uptake. A linker chain is
required for coupling to the NP surface. The nature of (A)
polymersomes, (B) liposomes and (C) micelles allows them to be used
as a vehicle for delivering hydrophobic and/or hydrophilic
anticancer agents, among others. The nature of (D) metallic
nanoparticles allows them to be used as theranostics for both drug
delivery and imaging applications. Figure created with
BioRender.com. Key: a), antibodies; b), drug; c), targeting ligand;
d), fluorescent dye; e), CPP; f), radioligand; g), aptamer; h), DNA
vector; i), enzyme; j), pre-miRNA; k), linker chain. NP,
nanoparticle; CPP, cell-penetrating peptide; miRNA, microRNA. |
Other properties rely on the nature of the NP.
Polymersomes, also called polymeric vesicles, have specific
features varying by polymer composition. Generally, they have high
stability, adaptable physicochemical properties, an easily-modified
surface and a highly adjustable permeability membrane (21,22).
Nanomicelles are commonly self-assembled; they can carry
hydrophilic drugs, protecting them from unwanted interactions
(23,24).
Similarly, nanoliposomes possess a specific
characteristic of carrying both hydrophobic and hydrophilic drugs
due to their double lipid membrane (25,26).
Metallic NPs can serve as theranostics by carrying anticancer drugs
and taking advantage of surface plasmon resonance for photothermal
therapy and imaging (27,28). On another approach, nanoliposomes
were used to develop a synthetic vaccine particle carryng rapamycin
(SVP-rapamycin), with the objective of inducing a tolerogenic
immune profile to NPs (29).
The present review discusses one of the most common
guidance tools used, known as cell-penetrating peptides (CPPs).
CPPs are small peptides, usually 5 to 30 amino acids in length,
that are designed from other protein sequences, have a short life
and can penetrate cell membranes in a non-selective way in most
cases (30). However, as will be
discussed further, some cell-specific CPPs have been studied and
developed. Furthermore, the definition of CPPs has been changing
throughout the years since their discovery >20 years ago. One
definition that globally encompasses all descriptions known to date
is ‘any peptide that can, to a measurable degree, enter the
interior of living cells in cell culture, or deliver a
membrane-impermeant cargo’ (31).
This assumption will be considered for the present review.
The first reports of CPPs were from studies related
to human immunodeficiency virus (HIV) and its cellular uptake
(32,33). The TAT peptide, corresponding to
the basic domain of HIV-1 TAT protein, was one of the first
reported. Afterward, a homeodomain of the transcription factor
antennapedia from Drosophila melanogaster also showed
penetrating activity. This finding led to discovery of the CPP,
penetratin, which corresponds to the third helix of the
antennapedia homeodomain (34).
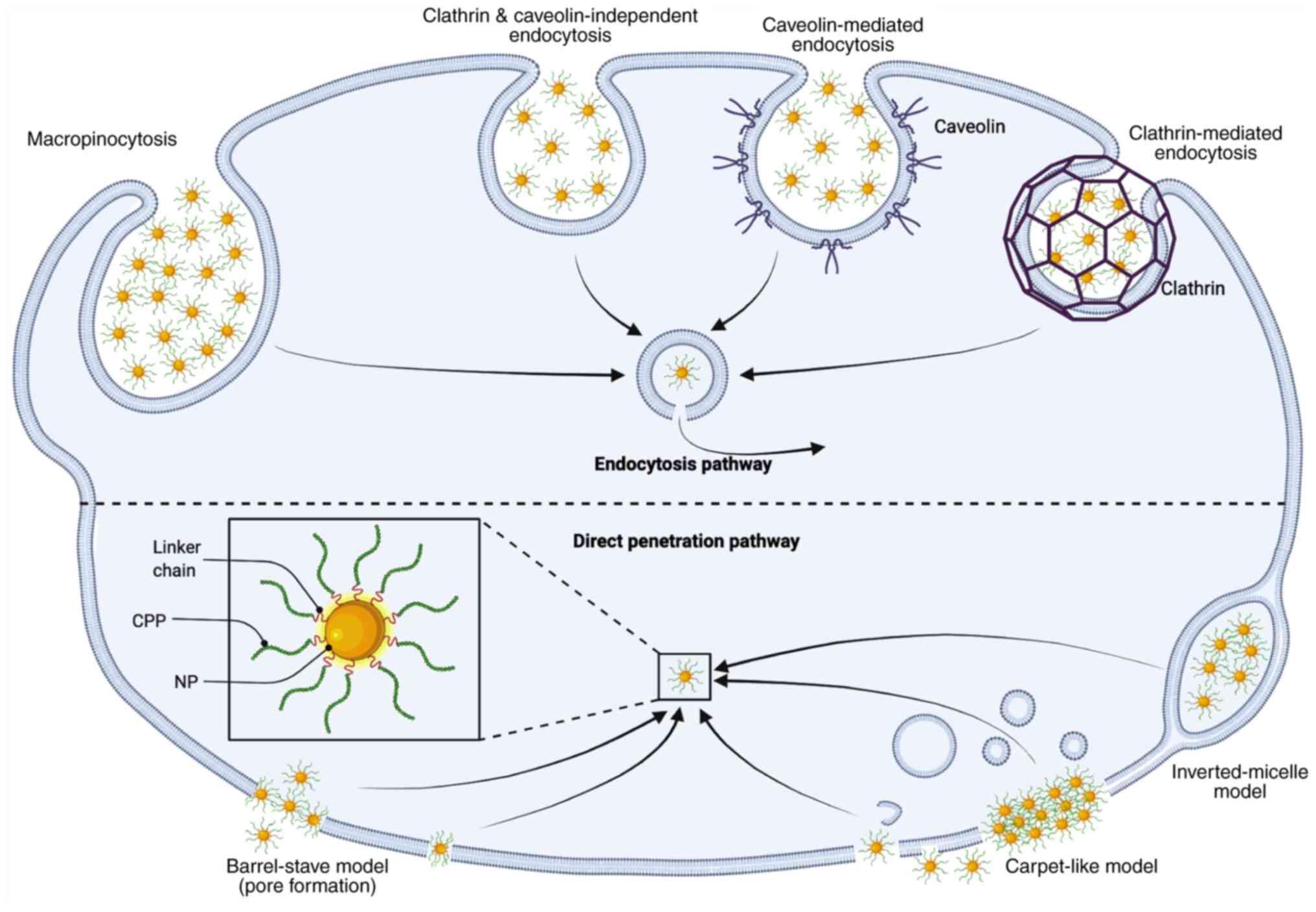
CPPs are added to molecules to improve their
capacity for cell internalization (30). CPPs can deliver cargoes inside the
cell through two primary mechanisms: Direct penetration and
endocytosis, depending on several physicochemical properties
(35). However, other reports
mention some CPPs with a pore formation mechanism (31,36–39).
Four major penetration models have been proposed through these
mechanisms: Reverse vesicle endocytosis, direct translocation,
adaptive translocation and pore formation (34). A general schematic of these models
applied to NSs is presented in Fig.
3. The variety of mechanisms related to the heterogenicity of
sequences of the CPPs impact their biochemical properties. Some of
these properties, which must be considered when developing an NS,
are shown in Table I, along with
the basic classification used for most available CPPs (35). Most of the studies discussed in the
present review involve cationic peptides, which are generally
attributed to a direct penetration mechanism by being adsorbed onto
cell surfaces due to anionic moieties (35,40).
Each CPP sequence discussed in the present review is included when
available.
 | Table I.Examples of CPPs, their
classification and other biochemical characteristics. |
Table I.
Examples of CPPs, their
classification and other biochemical characteristics.
| Classification | CPP name | Sequence length,
aa | Molecular weight,
kDa | Isoelectric
point |
|---|
| Amphipathic | p28 | 28 | 2.91 | 3.49 |
|
| VT5 | 26 | 2.60 | 6.17 |
|
| Pep-1 | 21 | 2.84 | 10.48 |
|
| BPrPr | 28 | 3.19 | 10.65 |
|
| Transportan | 27 | 2.84 | 10.75 |
|
| MAP | 18 | 1.87 | 11.27 |
|
| MPG | 27 | 2.80 | 11.74 |
|
| ARF | 22 | 2.65 | 12.49 |
|
| pVEC | 18 | 2.21 | 12.59 |
|
| Bac7 | 24 | 2.93 | 13.00 |
| Hydrophobic | Pep-7 | 15 | 1.80 | 3.28 |
|
| PFVYLI | 6 | 0.75 | 5.54 |
|
| C105Y | 17 | 1.99 | 8.05 |
| Cationic | DPV1047 | 19 | 2.31 | 12.29 |
|
| Penetratin,
pAntp | 16 | 2.24 | 12.44 |
|
| HIV-1 TAT
protein | 13 | 1.80 | 3.28 |
|
| HIV-1 TAT protein 9
aa | 9 | 1.34 | 12.80 |
|
| Polyarginines
(R7-R9) | Variable, 7-9 | Variable,
1.10-1.40 | Variable,
12.78-12.90 |
The chemical reaction for CPP binding (also known as
conjugation) to the NP depends most on the NP used. Lipid-based NPs
rely on terminal amino and carboxyl groups from the peptide and
lipids. The conjugation reaction is performed under constant
stirring in a solution that contains both the lipids and the CPP
(41,42). CPP conjugation to metallic NPs can
be achieved by a thiol (SH)-ether bond between Cys from the CPP and
a maleimide group in polyethylene glycol (PEG). Different sources
of the required functional groups exist; one example is a
functionalized SH-PEG and a maleimide-PEG (43).
Improving NS properties
One of the challenges in the construction of NSs is
to improve stability, biocompatibility and cell penetration. For
instance, one strategy for inducing apoptosis and inhibiting tumor
cell proliferation involves using small interfering RNAs (siRNAs)
(44–46). The main limitation of this
technology is that when administered in the systemic circulation,
the siRNA faces two main problems. The first is its limited
cell-penetrating properties, and the second, its rapid degradation
by circulating ribonucleases. Incorporating polymers such as PEG
and hyaluronic acid (HA) contributes stealthiness against the
immune system of the host, and improves biocompatibility and
biodegradability (47–50). In addition, cationic polymers such
as chitosan are implemented when the therapeutic agent is RNA,
protecting it against degradation and further cell internalization
(51).
Guided delivery of NSs by CPPs
The most common uses of CPPs include improving
cancer treatment with drugs and gene therapies, among others. In
vitro and in vivo experiments have been conducted to
improve cell penetration, stability, viability and drug
cytotoxicity, and to reduce tumor size. However, a valid concern
raised by several researchers is the lack of specificity of some
CPPs, which could lead to drug delivery to cells in healthy
tissues. Some modifications, such as antibody or antibody fragments
and aptamers, could be incorporated into the NS for specific
targeting. Other researchers have developed chimeric CPPs that
specifically target cancer cells (52–54).
Next, the present review discusses some of the NSs coupled to CPP
for BC and PC.
CPPs coupled to NSs for BC
NSs coupled to CPP to guide drug release
in BC
Doxorubicin (DOX)
DOX is commonly used to treat various types of
cancer, including BC. However, despite wide use, it has well known
undesirable side effects, such as cardiotoxicity (55). Various types of CPP have been
coupled to a variety of NSs, including liposomes, gold,
dendrigraft, iron oxides and polymeric structures. These NSs
carrying DOX and/or other anticancer drugs have been tested in
vivo and in vitro to improve delivery and reduce side
effects. Below, the present review briefly describes some of the
NSs that have been coupled to CPPs.
CPP PVF (PFVYLI)
Hydrophobic CPP PVF coupled to a 100-nm liposome was
evaluated in MCF-7, MDA-MB-435S, MCF-7/Adr (Adriamycin-resistant
human mammary adenocarcinoma) and 4T1 murine BC carcinoma cell
lines. Even when the cell survival rate was higher in cells treated
with the NS in vivo, the NS decreased the tumor weight up to
0.4 g compared with the control (0.9 g) and free DOX (0.7 g), while
keeping the mice at a healthier weight compared with the free
DOX-treated mice (41).
HIV-derived TAT CPP
(YGRKKRRQRRRTAT)
With the ability to permeate the blood-brain
barrier, the HIV-derived TAT CPP was evaluated in MDA-MB-231 cell
lines and metastatic in vivo tumors using gold NPs coupled
with PEG as the carrying vehicle for DOX. In vivo
cell uptake increased 4.8-fold compared with that of the control,
while DOX half maximal inhibitory concentration (IC50)
decreased 80% compared with that of free DOX. Furthermore, no
significant adverse effects were found when the DOX dose of the NS
ranged from 1 to 5 µg. However, at 10 and 15 µg DOX, the mice
exhibited significant weight loss, which a common side effect of
this drug. Also, the development of ascites and peripheral edema
was found in the mice, which a relatively common adverse effect of
DOX metabolism. These results suggest further targeting with this
NS should be achieved before moving into clinical trials (43).
TAT peptide fragment (RKKRRRQRC)
A gold nanostar coupled with mesoporous silica (MS),
TAT fragment and the photosensitizer drug protoporphyrin IX was
used as a theranostic system. Also, Raman detection for
diagnostics, photodynamic therapy (PDT) and cytotoxicity were
evaluated in the BT-549 cell line. Through Raman imaging, it was
determined that TAT CPP activity allowed cell penetration while
maintaining cell viability, although once PDT was applied, the cell
viability was decreased (56). A
similar study used an NS containing TAT and gold NPs along with
anti-HER2 antibody for increased specificity and surface-enhanced
Raman spectroscopy, which was assessed for DOX release rate into
SK-BR-3 cells. After 24 h, in vitro cell viability was
decreased by 39.48% compared with that of the control (57). Another study designed a synthetic
peptide containing TAT, L-lysine residues, fusogenic GALA peptide
and cell targeting peptide (DMPGTVLP) with stearic acid to improve
condensation and stability to a dioleoylphosphatidylethanolamine
(DOPE)-based nanoliposome. This NS was evaluated against MCF-7
cells delivering an siRNA targeting BCL2 mRNA. The chimeric peptide
used in this NS increased cell uptake, and BCL2 silencing was
decreased by ~80%. However, cell viability was decreased by only
20% (58).
Angiopep-2 CPP
(TFFYGGSRGKRNNFKTEEY)
The size of the NS directly impacts tumor
accumulation and diffusion abilities. Keeping this in mind,
researchers developed an NS that could be decreased in size by the
action of metalloproteinase-2, while keeping high specificity for
triple-negative BC (TNBC). Gelatin and dendrigraft polylysine were
used as a carrying vehicle and stabilization agent, while DOX was
used as the anticancer agent in 4T1 cells. As expected, in
vitro cell uptake was increased from 15 to 40%, which
translated into a higher inhibition rate. In vivo,
there was an ~3-fold decrease in tumor volume and a 4-fold decrease
in tumor weight compared with that for free DOX treatment.
Furthermore, no difference in mouse bodyweight was reported,
suggesting the relative safety of this NS (59).
Arginine-rich amphiphile CPP
(lauryl-PPPPRRRR)
A nanoliposome-based system carrying the
arginine-rich amphiphile CPP and DOX or paclitaxel (PTX) was tested
in MCF-7 cells. After 3 h, cell uptake had increased 5-fold with
rhodamine B (a hydrophilic dye) and 30% with Nile red (a
hydrophobic dye) to show this dual amphiphile activity. The drug
uptake and effectiveness of the liposome improved with the NS
compared with the use of free drugs. In vitro, cancer cell
viability was decreased by 50 and 25% in the CPP-DOX-NS and NS-PTX
groups, respectively (60). These
results show a significant difference between NSs with CPPs and
those that do not have them coupled.
QLPVM CPP peptide
The QLPVM CPP was attached to a
1,2-distearoyl-sn-glycero-3-phosphorylethanolamine (DSPE)-PEG-based
nanoliposome carrying tamoxifen (TAM) and DOX, evaluated in MCF-7
cell tumor-bearing mouse models. In vitro cell viability was
dose-dependent for the NS. However, cell viability was lower than
that in the NS-free TAM and NS-free DOX groups. In vivo
tumor inhibition with the combined NS showed the best results. In a
synergistic effect, tumor volume decreased 7-fold compared with
that of the control, with similar results in tumor weight and no
noticeable bodyweight loss or cardiac toxicity (61).
Chimeric R8 CPP
(AVPIR8)
A disadvantage of drug therapy is the existence of
drug-resistant tumors. Thus, the chimeric R8 CPP was fused with an
apoptotic peptide to create a co-delivery nanocomplex in MCF7-DOX
resistant cells. The nanocomplex consisted of binding several
wild-type p53 proteins, chimeric CPPs and p53 DNA. Assembly was
enabled by preparing a mixture of these components and incubating
them at 37°C for 30 min to allow the formation of the nanocomplex.
There was a 36-fold in vitro increase in apoptosis in the
synergistic NS compared with that in free DOX, leading to a 97.9%
decrease in cell viability. In vivo, there was a significant
tumor volume and weight decrease when the mice were treated with
the NS. Tumor volume decreased ~10-fold, while tumor weight was
9-fold lower at 21 days post-injection (62).
R7 CPP (RRRRRRR)
The R7 CPP was coupled in a
polylactic-co-glycolic acid-PEG polymer with folic acid and the
cell-cycle anticancer agent, vincristine sulfate (VCR). Cell uptake
mediated by R7 was not significantly different from cell
uptake mediated by folic acid and its receptors. However, cell
viability was decreased to 30% when 5 nM VCR was added, compared
with 60% cell viability without VCR. These results were further
confirmed by determining an increase in MCF-7 cell percentage in
the G2/M arrest phase from 12.28±1.74% to >33% (63).
R9 CPP (RRRRRRRRR)
A DSPE-PEG-based nanoliposome coupled with
R9 was developed to deliver cabazitaxel in the 4T1 cell
line and murine models. Cell uptake was increased 1.78-fold after 8
h of exposure compared with the control. In vitro
cytotoxicity was enhanced 223-fold to 0.03 µg/ml due to NS
activity. In vivo, the NS localization in the lymph nodes
was reported to increase 1.73-fold at 24 h post-injection. This
finding demonstrates a penetrating activity with long retention
times. Also, primary tumor growth decreased 75.3%, while tumor
inhibition in the lymph nodes was reported at 89.1%, showing that
this NS can inhibit metastasis. The size of this NS was 13 nm; NSs
with sizes <30 nm have been reported to have better access to
lymph nodes (64–66).
iRGD peptide (CRGDK/RGPD/EC)
Vesicles extracted from red blood cell membranes and
coupled to iRGD peptide were used as biological NSs carrying PTX.
In vitro cell uptake in 4T1 cells was higher in the NS
compared with that in the control. However, in vitro cell
viability was similar compared with that of free PTX. In
vivo, the NS decreased tumor growth to 8.5%, 5.6-fold less
compared to the control (67). In
a similar study, the iRGD CPP coupled to iron oxide nanoworms was
used as an NS to inhibit or lower BC metastasis to the brain,
targeting MDA-MB-231 and 4T1 ×enografts in 231Br and 4T1-BR5 mouse
models. Brain imaging showed that 30 days post-injection, the
number of tumors was decreased by 60%, and their size had decreased
5-fold compared with the controls. However, researchers found a
tight window of 12 days for this metastasis decrease (68).
Pentapeptide CALNN CPP (CALNN)
The pentapeptide CALNN CPP coupled with gold NPs was
used to deliver linalool (a monoterpenoid derived from plants) and
evaluate its anticancer activity in MCF-7 cells. In vitro
cytotoxicity was reported at >80% after 48 h of treatment, while
clonogenicity was inhibited. In vivo, the NS conferred no
statistical change in mouse health, suggesting the safety of the NS
for further study. Unfortunately, the study did not include in
vivo anticancer activity assays (69).
CF CPP
(CREKA-CN2-CKDEPQRRSARLSAKPAPPKPEPKPKKAPAKK-NH2)
CREKA, a linear pentapeptide designed to bind to
fibrous protein in the tumor microenvironment, was fused to the CF
peptide (a CPP for nucleus translocation) using a polylactic
acid-based NS as the vehicle. This NS was designed for targeted
delivery of the anticancer drug erlotinib into MDA-MB-231 cell
lines to combat drug resistance in TNBC. Cell uptake was increased
2.3-fold when the pH of the assay was similar to that of the tumor
microenvironment. In vitro cell viability decreased <20%
in the pH simulating the tumor microenvironment, compared with that
in the physiological pH, which maintained 50% viability. In
vivo, a 2.8-fold tumor decrease was observed in mice treated
with the NS, while their body weight was maintained, suggesting an
effective method for TNBC tumor treatment that lacked adverse
effects (70).
tLyP-1 CPP (CGNKRTRG)
The tLyP-1 CPP, which specifically targets tumors,
was used to develop an NS targeting MDA-MD-231 cells. The polymeric
NPs consisted of HA and D-α tocopheryl succinate as a vehicle. The
NS carried the anticancer drug docetaxel. Cell uptake compared with
that of the control increased 2.36-fold, while in vitro,
cell cytotoxicity was higher in the NS with tLyP-1. In vivo
tumor weight decreased 3.5-fold compared to the control (71).
PEGA-pVEC CPP
(CPGPEGAGC-LLIILRRRIRKQAHAHSK)
The PEGA-pVEC CPP that targets BC cells and tumors
was coupled to an NS of colloidal MS as a vehicle for the
anticancer agent epigallocatechin-3-gallate (EGCG) in MCF-7 cells.
In vitro cell inhibition reached 100% at a 100 µg/ml
concentration. In vivo tumor inhibition of up to 89.66% was
reported compared with 69.9% in the control group in murine models
(72). In a similar study,
PEGA-pVEC was used to develop an MS and HA NS as a carrying vehicle
for DOX and an siRNA directed against the BC overexpressed
connective tissue growth factor gene. The NS was tested in
drug-resistant MDA-MB-231 cells. In vitro inhibition
increased from 59 to 80% compared with the control. In vivo,
the tumor inhibition increased up to 60% compared with the control
(73). A similar study developed a
self-aggregating nano-gel based on protamine aggregates carrying
EGCG as an anticancer agent to MDA-MB-231 cells and xenografts.
In vitro, there was a 15-fold increase in cytotoxicity
compared with the control, while in vivo tumor inhibition
was 80% compared with the control (74).
Chimeric arginine-glycine-aspartate
CPP (RGD and RGERPPR)
The chimeric arginine-glycine-aspartate CPP was
evaluated in MDA-MB-231 cells using gambogic acid (GA) as an
anticancer agent and nanostructured lipid nanocarriers. When cells
were treated with a 2 µg/ml concentration, in vitro cell
viability was decreased to <20% compared with that of the
control. However, the best inhibition rate and tumor size decrease
were achieved with the NS carrying RGERPPR alone, suggesting that
only one CPP can benefit a single NS (75).
CPPs coupled to NSs for gene and
recombinant therapy in BC gH625 CPP (HGLASTLTRWAHYNALIRAF)
An NS, delivering a novel, non-disclosed siRNA
consisting of superparamagnetic iron oxide NPs (SPIONs)
functionalized with PEG, and cationic polymers, such as chitosan
and L-arginine with CPP gH625, was designed and evaluated against
the MDA-MB-231 cancer cell line. After 4 h of exposure, the NS
penetrated the cells aided by the gH625 peptide, and the MDA-MB-231
cancer cell line genes were downregulated due to the interference
mechanism of the siRNA. At 72 h, there was cell growth inhibition
of nearly 80% (51). gH625 in an
NS SPION couple was also tested in MDA-MB-231 cells as a
theranostic system. Cell uptake increased 3-fold in the NS compared
with that in the control assay (76).
R9 CPP (RRRRRRRRR)
The Twist gene is a transcription factor for an
epithelial-mesenchymal transition that is heavily involved in the
metastatic activity of tumors. Therefore, a nano-corona NS
consisting of a DOPE NP carrying an siRNA against the Twist gene,
the R9 for tumor penetration, and coated with human
serum albumin for immune system camouflage, was designed and
evaluated in 4T1 cells. The nano-coronas were labeled with IR-780
dye for combination therapy: Twist gene silencing and tumor growth
inhibition using photothermal therapy for thermal ablation. Cell
uptake indicated a higher NS translocation in the cytoplasm after
24 h of incubation; the cell migration rate decreased up to 66%.
The cell viability index was decreased by 90% when treated by
photothermal therapy. In vivo experiments showed the ability
of the NS to penetrate tumors; tumor inhibition progression was
83.6% and metastasis inhibition was 92.2% after 13 days of
combination therapy (77).
TAT protein basic domain-derived CPP
(RKKRRQRRR-Cys)
This CPP was used to develop a lipid-based
nanobubble system carrying an siRNA against an epidermal growth
factor. The main objective was to evaluate the NS when applying
ultrasound irradiation inside tumor cells to enhance the siRNA
anticancer activity targeting MDA-MB-231 cells. Cell inhibition was
increased from 48 to 72 h, while in vivo tumor growth was
decreased by 42.08% (78).
Chimeric Tat-Mu CPP
(YGRKKRRQRRRMRRAHHRRRRASHRRMRGG)
The fusion of a HER2 antibody mimetic-affibody and
CPP Tat-Mu was used to deliver an anti-tissue factor using an
shRNA. The NS was a cationic N,N-di-n-hexadecyl-N,N-dihydroxy ethyl
ammonium chloride-based nanoliposome used as a vehicle to
MDA-MB-231 cells and xenografts. In vitro, cell uptake
increased 7-fold compared with that of the control, while there was
a significant tumor size decrease, to <10% of the original size,
in vivo (79).
Lin TT1 CPP (AKRGARSTA)
The TT1 CPP has specificity against p32, a
mitochondrial essential tumor regulator protein. Iron oxide NPs
were the vehicle for the TT1 CPP fused with the proapoptotic
peptide [D(KLAKLAK)2] (inducing apoptosis through the activation of
caspase-3) and tested in MCF10CA1a and 4T1 cell lines. In
vitro cell uptake increased 4-fold, while a tumor size decrease
of 50% was observed in vivo (80). Another study consisted of a
cholesterol-based nano-micelle carrying TT1 plus two anticancer
components, siRNA targeting PDL-1 and indoleamine 2,3-dioxygenase
inhibitor (1-methyl tryptophan), that led to the activation of
cytotoxic T lymphocytes against the 4T1 cell line. After 4 h, cell
uptake was 16.3-fold higher than that of the control assay
(81).
Penetratin-derived CPP
(CKRRMKWKK)
Ephrin type-A receptor 2 (EphA2) is a transmembrane
protein whose overexpression has been linked to carcinogenesis,
metastasis and a poor clinical prognosis. The YSA peptide, an
ephrin mimetic bound with EphA2, was fused with a
penetratin-derived CPP. DSPE-PEG nanobubbles were used as the
vehicle for an anti-Myc siRNA acting as an anticancer agent. The
in vitro cell uptake was higher with this NS treatment.
In vivo tumor growth inhibition after 24 days of treatment
was decreased to 31.2% compared to the control, while no
significant body weight loss was found in the mice. The NPs with
YSA peptides improve therapeutic effects in vivo (82).
uCendR CPP (RPARSGRSAGGSVA)
A urokinase activatable CPP was developed to ensure
tumor specificity for in vivo targeting of 4T1 cell tumors
in murine models. In addition, silver NPs were coated with
PEGylated neutravidin as a vehicle and for immune system
stealthiness. However, in vivo distribution was determined
only in tumor tissue, without fluorescence in healthy tissue
(83).
Reversibly activatable CPP
(RACPP)
The difference in pH between the tumor
microenvironment and the healthy extracellular matrix has been
exploited along with CPPs to improve the specificity of the NS.
Nanopolymer micelles masked with PEG-lactic acid were used as a
vehicle for the RACPP, containing a pH-sensitive sequence, and PTX
as an anticancer drug targeting 4T1 cells and 4T1-BALB/c tumor
xenografts models. Cell uptake was increased 3-fold, while in
vitro IC50 was decreased from 1.595 to 1.035 µg/ml. In
vivo, tumor size decreased by ~50% at 16 days post-injection,
while the mouse survival rate increased 1.5-fold (20).
MAP CPP (KLALKLALKALKAALKLAY)
In another case, the pH-sensitive MAP CPP was used
with the highly pH-sensitive histidine-glutamate (HE) oligopeptide
(HEHEHEHEHEHEHEHEHEHE) coupled to glutathione-S-transferase acting
as a cargo protein. The delivery activity was measured in
MDA-MB-231 cells and xenografts through fluorescence imaging. At an
acidic pH simulating the tumor microenvironment, there was a 3-fold
increase of fluorescence in vitro. In vivo results
showed that at 6 h post-injection in mice, the system was localized
mainly around the xenograft tumor. These results further show the
advantage of using the pH of the tumor microenvironment for
intelligent drug delivery systems (84).
NGR chimeric CPP (NGR-CKRRMKWKK)
Using a different approach, a thermosensitive NS was
developed using a heat-activatable CPP after mild thermal stimulus
at temperatures ranging from 37 to 42°C, which facilitates drug
delivery. The NS consisted of a nanoliposome that immediately
encapsulated drugs in heating tissue or organs. These were coupled
with a penetratin-derived CPP, the NGR peptide and DOX as the
anticancer agent against MCF-7. In vitro cellular uptake was
increased 5-fold compared with the control, while in vitro
cytotoxicity of the preheated NS showed an increase of 1.5-fold
compared with the NS without preheating treatment. However, both
cases showed increased cytotoxicity compared with that of free DOX.
Similarly, in vivo tumor inhibition was increased in the
preheated NS. Also, there was an ~9-fold tumor volume decrease with
the preheated NS compared with the control, with no apparent body
weight decrease (42).
CPPs coupled to NSs for PC
Considerably less research and NS development have
been conducted for PC, providing an important opportunity in this
area. Certain CPPs have been tested on PC with some success,
similar to BC, due to an observed increase in cell specificity or
uptake. The CPP-NSs described below were designed to improve drug
therapy, for gene therapy or to couple with other therapies such as
electromagnetic field and laser radiation.
Polyarginine-cholesterol CPP
(Chol-R9)
Cancer-associated fibroblasts (CAFs) are involved in
microenvironment remodeling in PC. Therefore, a novel approach was
constructed using a CPP-based amphiphilic peptide,
Chol-R9, an siRNA targeting chemokine ligand 12, and
anti-human FAP-α for their involvement in cancer metastasis. As a
result, there was an in vitro 7-fold increase in CAF uptake
compared with that found using free siRNA, while in vivo,
tumor weight decreased 53.4% (85).
Polyarginine, PSA-selective peptide
and polyanionic shielding peptides (DGGDGGDGGDGGHSSKYQ
G-R8)
A DSPE-PEG-based nanoliposome was developed with
high PC specificity carrying peptides. This NS included
polyarginine CPP and the PSA-selective peptide (HSSKYQ), which
possess a cleavable moiety by PSA to enhance further specificity
towards PC cells. The anticancer agent was an siRNA targeting
polo-like kinase 1. Two cell lines were evaluated, 22Rv1 and PC-3.
The latter was used as the control since it is PSA negative. In
vivo results showed a 54% increase in apoptosis and a 5-fold
tumor volume decrease (86).
Poly arginine (R11)
Among the CPPs used in PC, the proline-rich sequence
R11 has shown a high-efficiency uptake in PC cell lines
coupled to therapeutic systems (87), making it a promising strategy. The
R11 CPP and the anticancer agent tumor suppressor MIR145
were coupled in a polyethyleneimine polymeric NS to increase the
blood circulating time by protecting against the host immune
system. In vitro cell uptake was increased from 5.2 to
87.5%, while in vivo NS accumulation in the tumor increased
3.5-fold compared in both cases with free miRNA. Tumor size was
decreased 7.5-fold, increasing the mouse lifespan up to 18 weeks
(88).
CendR motif peptide (CRGDK)
Another NS with a PC-specific CPP was developed with
the CRGDK peptide targeting neuropilin-1 (Nrp-1) in
glutathione-functionalized gold NPs, along with platinum IV as an
anticancer agent. The NS was tested in PC-3 (Nrp-1 positive) and
DU145 (Nrp-1 negative) cell lines. Cell uptake and in vitro
cytotoxicity were increased 4- and 28.13-fold, respectively
(89).
Polyarginine (R11)
An NS of iron oxide NPs, poly lactic-co-glycolic
acid (R11-Mn-PGLA), polyarginine CPP and the
radio-sensitizer
8-dibenzothiophen-4-yl-2-morpholin-4-yl-chromen-4-one was tested in
PC-3 and PZ-HPV-7 cells. Results showed the uptake of the NS by the
PC3 cells in a dose-dependent manner. However, the R11
CPP increased cell uptake when an electromagnetic field was applied
(90). Using a similar approach,
R11 CPP was used to develop a NS consisting of iron
oxide NP coated with
poly(N-isopropylacrylamide-acrylamide-allylamine) (PMNPs). The
R11-PMNPs were biocompatible with normal cells up to 500
µg/ml. In vitro cell uptake for PC3 and LNCaP cell lines was
higher when PMNPs carried R11. In vivo, tumor
R11-PMNPs accumulation was higher than that of normal
tissue (91).
Chimeric peptide
(Ste-R6L2)
Tripterine is a bioactive compound from Tripterygium
wilfordii that is used in traditional Chinese medicine.
Nanostructured lipid carriers coated with
Ste-R6L2 CPP and loaded with tripterine were
designed for targeting PC-3 cell lines. The IC50
reported was decreased from 0.88±0.08 to 0.55±0.07 µg/ml, while
in vitro apoptosis increased from 0.71 to 14.15%. In
vivo, tumor volume was decreased by 57.2%, while the tumor
inhibition rate increased 1.96-fold to 72.68±6.7% (92).
Chimeric TAT-bombesin peptide
(99mTc-N2S2-Tat(49–57)-Lys3-bombesin)
The gastrin-releasing peptide receptor (GRP-r) is
overexpressed in PC. Bombesin, a 14-amino acid peptide, strongly
binds to GRP-r. For this reason, an NS consisting of bombesin and
TAT CPP was coupled with gold NPs for PC-specific targeting. The NS
also carried radiopharmaceuticals 99mTc and
177Lu. Laser thermal ablation was performed as an
anticancer treatment. In vitro cell uptake was increased
52.5% compared with that of the control in the PC-3 cell line. Once
the cells were laser-irradiated, cell inhibition was 98.64±0.4%
(93).
Ypep CPP (YTFGLKTSFNVQ)
Bacteriophages have also been studied as NSs for the
treatment of PC. The M13 bacteriophage was engineered to express
the Ypep CPP targeting PC-3 cells. As a result, in vitro,
cell uptake increased ~1.5-fold, with nearly 100% cytotoxicity.
Unfortunately, no in vivo assays were performed, which could
increase the therapeutic potential of this approach. However, the
size of the phage could be an important limiting factor, since it
had a width of 6 nm and a length of 930 nm (94).
Conclusions and outlook
The present review described NSs coupled to CPPs as
guidance tools towards BC and PC cells and tumors. These NSs
increased their specificity against cancer cells and tumors,
leading to less cytotoxicity against healthy tissue. A summary of
the components of the NSs discussed in this article and the effect
they have on cancer cells and/or tumors can be found in Table II. One of the most relevant
achievements of several of these NSs is a decrease in the
anticancer drug used. This decrease, coupled with increased
specificity, could lower the risk of adverse effects in patients.
Several advantages and disadvantages should be considered to
increase the specificity for the NS in order to reach clinical
application consistently.
 | Table II.Summary of discussed CPPs in NSs
targeting breast and prostate cancer. |
Table II.
Summary of discussed CPPs in NSs
targeting breast and prostate cancer.
| CPP | Sequence | NP type | Target cells | NS size, nm | Anticancer
agent | Effect of the
NS |
|---|
| PVF | PFVYLI | Nanoliposome | MCF-7
MDA-MB-453S | 100 | DOX | 2.25-fold tumor
weight decrease |
| HIV-derived | YGRKKRRQRRRTAT | Metallic | MDA-MB-231 | 23.4 | DOX | 4.8-fold increase
in vivo cell uptake |
| TAT |
|
|
|
|
|
|
| TAT peptide
fragment | RKKRRRQRC | Metallic | BT-549 | 123 | Protoporphyrin IX
(PpIX) | Cell viability
decrease |
| Angiopep-2 | TFFYGGSRGK
RNNFKTEEY | Polymeric | 4T1 | Reduction 185.7 to
55.6 (intelligent NS designed to reduce its size by action of the
MMP-2) | DOX | 3-fold tumor volume
and 4-fold tumor weight decrease |
| Arginine-rich
amphiphile |
lauryl-PPPPRRRR | Nanoliposome | MCF-7 | 95.26 | DOX and PTX | 50% in vitro
cell viability decrease |
| Not named | QLPVM | Nanoliposome | MCF-7 | 96.93 | DOX and TAM | 7-fold tumor volume
decrease |
| Chimeric
polyarginine |
AVPIR8 | Polymeric | MCF-7
Dox-resistant | <50 | DOX | 97.9% decrease
in vitro cell viability |
| R7
Polyarginine | RRRRRRR | Polymeric | MCF-7 | 235.8 | Vincristine
sulfate | Cell viability
decrease to 30.65% |
| R9
polyarginine | RRRRRRRRR | Nanoliposome | 4T1 | 13 | Cabazitaxel | 1.73-fold increase
in localization at tumor |
| Chimeric iRGD | CRGDK/RGPD/EC | Nanoliposome | 4T1 | 150 | PTX | 5.6-fold decrease
in tumor growth rate |
| Pentapeptide
CALNN | CALNN | Metallic | MCF-7 | <10 | Linalool | 80% increased in
vitro cytotoxicity |
| Chimeric
peptide | CREKA-CN2-CKDEP
QRRSARLSAKPAPPK PEPKPKKAPAKK-NH2 | Polymeric | MDA-MB-231 | N/A | Erlotinib | 2.8-fold tumor size
decrease |
| tLyP-1 | CGNKRTRG | Polymeric | MDA-MB-231 | 110 | DTX | 3.5-fold tumor size
decrease |
| PEGA-pVEC | CPGPEGAGC-LLIIL
RRRIRKQAHAHSK | Polymeric | MCF-7 | 180 |
Epigallocatechin-3 | 89.66% tumor
inhibition |
| Chimeric
arginine-glycine-aspartate | RGD and
RGERPPR | Nanoliposome | MDA-MB-231 | 25.81 | Gambogic acid | 20% higher tumor
size decrease |
| gH625 | HGLASTLTRW
AHYNALIRAF | Metallic | MDA-MB-231 | 79 | Non-disclosed
siRNA | 3-fold increased
cellular uptake |
| Not named | RKKRRQRRR-Cys | Nanoliposome | MDA-MB-231 | 582 | siRNA targeting
epidermal growth factor | Tumor growth
decrease by 42.08% |
| Not named | RKKRRQRRR-Cys | Nanoliposome | MDA-MB-231 | 582 | siRNA targeting
epidermal growth factor | Tumor growth
decrease by 42.08% |
| Chimeric |
YGRKKRRQRRRMRRA | Nanoliposome | MDA-MB-231 | N/A | shRNA anti-tissue
factor | 7-fold tumor size
decrease |
| Tat-Mu |
HHRRRRASHRRMRGG |
|
|
|
|
|
| Lin TT1 | AKRGARSTA | Metallic | 4T1 | 175 | Lin TT1 CPP targets
p32 |
|
|
|
|
|
|
| tumor regulation
protein | 50% tumor size
decrease |
|
Penetratin-derived | CKRRMKWKK | Nanoliposome | MCF-7 | 203 | Anti-Myc siRNA | Tumor growth
inhibition by 31.2% |
| uCendR | RPARSGRSAGGSVA | Metallic | 4T1 | 50 | N/A | In vivo
distribution only in tumor tissue |
| Not named | RACPPKLALKLAL | Polymeric | 4T1 | 25.3 | PTX | Tumor size decrease
by 50% |
| MAP | KALKAALKLAY | Polymeric | MDA-MB-231 | N/A | N/A | 3-fold increased
accumulation in xenograft tumor |
| Chimeric | NGR-CKRRMKWKK | Nanoliposomes | MCF-7 | 89.23 | DOX | 9-fold increased
tumor |
| NGR |
|
|
|
|
| volume
decrease |
|
Polyarginine-cholesterol |
Chol-R9 | Polymeric | Cancer-associated
fibroblasts | 100 | siRNA targeting
CXCL12 | 53.4% tumor weight
decrease |
| Chimeric | DGGDGGDGGD | Nanoliposome | PC-3 and 22v1 | 200 | siRNA targeting
PLK1 | 5-fold tumor volume
decrease |
| polyarginine | GGHSS
KYQG-R8 |
|
|
|
|
|
| CendR Motif | CRGDK | Metallic | PC-3 and DU145 | 5.2 | Platinum IV | 4 and 28.13-fold
increase in vitro cell cytotoxicity in PC-3 and DU145,
respectively |
| Polyarginine | RRRRRRRRRRR | Metallic | PC-3 and LNCaP | 100 | N/A | 5-fold increase
in vivo tumor accumulation |
| Not named |
Ste-R6L2 | Nanoliposome | PC-3 | 126.7 | Tripterine | Tumor volume
decrease by 57.2% |
| TAT-Chimeric
bombesin |
99mTc-N2S2-Tat
(49–57)-Lys3-bombesin | Metallic | PC-3 | 8 | Laser thermal
ablation | 98% in vitro
cell inhibition |
| Ypep | YTFGLKTSFNVQ | Bacteriophage |
| 930 | N/A | 1.5-fold in
vitro cell uptake |
Assays evaluating the efficiency in cell uptake and
drug delivery of each type of CPP could provide knowledge and
insight on this topic. In addition, the proposed assays could be
particularly useful for upcoming scientists developing different
NSs that target BC or PC to increase reproducibility and as a
baseline to compare their results.
Besides specificity and safety, NS design should
consider production costs, development time and processing time. A
cost-benefit analysis should be established to compare the
specificity needs while keeping complexity at manageable levels.
CPPs significantly improved the delivery of anticancer agents
towards cancer cells while showing little effect on healthy tissue
both on in vitro and in vivo assays. Cell uptake is
one of the major contributions to the success of NSs in anticancer
therapy. Still, several concerns exist with nanomedicine, with the
main issue being the clearance of the NPs from the system once
their therapeutic potential has been achieved. Further studies and
clinical trials should provide a better understanding of the
mechanisms involved in developing NS therapy for both PC and
BC.
Acknowledgements
The authors would like to thank Dr. Sergio Lozano
(Office of the Vice Dean of Research, ‘Dr. Jose Eleuterio Gonzalez’
University Hospital (Monterrey, Nuevo León, Mexico) for language
editing and correcting the original manuscript.
Funding
The present study was funded by the Consejo Nacional de Ciencia
y Tecnología, Call for Basic Scientific Research 2017-2018 (grant
no. A1-S-9859).
Availability of data and materials
Not applicable.
Authors' contributions
SLG, CNSD and HLGB designed the theme of the
review. SLG searched and retrieved the relevant literature and
wrote the first draft. CNSD and HLGB reviewed and suggested
corrections. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parsa Y, Mirmalek SA, Kani FE, Aidun A,
Salimi-Tabatabaee SA, Yadollah-Damavandi S, Jangholi E, Parsa T and
Shahverdi E: A review of the clinical implications of breast cancer
biology. Electron Physician. 8:2416–2424. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu D, Si M, Xue HY and Wong HL:
Nanomedicine applications in the treatment of breast cancer:
Current state of the art. Int J Nanomedicine. 12:5879–5892. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Logothetis CJ, Gallick GE, Maity SN, Kim
J, Aparicio A, Efstathiou E and Lin SH: Molecular classification of
prostate cancer progression: Foundation for marker-driven treatment
of prostate cancer. Cancer Discov. 3:849–861. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang A, Zhang J, Plymate S and Mostaghel
EA: Classical and Non-classical roles for pre-receptor control of
DHT metabolism in prostate cancer progression. Horm Cancer.
7:104–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu LL, Xie N, Sun S, Plymate S, Mostaghel
E and Dong X: Mechanisms of the androgen receptor splicing in
prostate cancer cells. Oncogene. 33:3140–3150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Culig Z and Santer FR: Androgen receptor
signaling in prostate cancer. Cancer Metastasis Rev. 33:413–427.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rebello RJ, Pearson RB, Hannan RD and
Furic L: Therapeutic approaches targeting MYC-Driven prostate
cancer. Genes (Basel). 8:712017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blee AM, He Y, Yang Y, Ye Z, Yan Y, Pan Y,
Dugdale J, Kuehn E, Kohli M, Jimenez R, et al: TMPRSS2-ERG controls
luminal epithelial lineage and antiandrogen sensitivity in PTEN and
TP53-mutated prostate cancer. Clin Cancer Res. 24:4551–4565. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nadal M, Prekovic S, Gallastegui N, Helsen
C, Abella M, Zielinska K, Gay M, Vilaseca M, Taulès M, Houtsmuller
AB, et al: Structure of the homodimeric androgen receptor
ligand-binding domain. Nat Commun. 8:143882017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Damyanov CA, Maslev IK and Pavlov VS:
Conventional treatment of cancer realities and problems. Ann
Complement Altern Med. 1:1–9. 2018.
|
|
13
|
Schaue D and Mcbride WH: Opportunities and
challenges of radiotherapy for treating cancer. Nat Rev Clin Oncol.
12:527–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Estanqueiro M, Amaral MH, Conceição J and
Sousa Lobo JM: Nanotechnological carriers for cancer chemotherapy:
The state of the art. Colloids Surfaces B Biointerfaces.
126:631–648. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vanneman M and Dranoff G: Combining
immunotherapy and targeted therapies in cancer treatment. Nat Rev
Cancer. 12:237–251. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao QX, Zhou GX, Lin SJ, Paus R and Yue
ZC: How chemotherapy and radiotherapy damage the tissue:
Comparative biology lessons from feather and hair models. Exp
Dermatol. 28:413–418. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi J, Kantoff PW, Wooster R and Farokhzad
OC: Cancer nanomedicine: Progress, challenges and opportunities.
Nat Rev Cancer. 17:20–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hayashi K, Ono K, Suzuki H, Sawada M,
Moriya M, Sakamoto W and Yogo T: High-frequency,
magnetic-field-responsive drug release from magnetic
nanoparticle/organic hybrid based on hyperthermic effect. ACS Appl
Mater Interfaces. 2:1903–1911. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kong SD, Zhang W, Lee JH, Brammer K, Lal
R, Karin M and Jin S: Magnetically vectored nanocapsules for tumor
penetration and remotely switchable on-demand drug release. Nano
Lett. 10:5088–5092. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang B, Zaro JL, Shen Y, Chen Q, Yu Y, Sun
P, Wang Y, Shen WC, Tu J and Sun C: Acid-sensitive hybrid polymeric
micelles containing a reversibly activatable cell-penetrating
peptide for tumor-specific cytoplasm targeting. J Control Release.
279:147–156. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Q, Song L, Chen S, Gao J, Zhao P and
Du J: A superparamagnetic polymersome with extremely high T2
relaxivity for MRI and cancer-targeted drug delivery. Biomaterials.
114:23–33. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ke W, Li J, Mohammed F, Wang Y, Tou K, Liu
X, Wen P, Kinoh H, Anraku Y, Chen H, et al: Therapeutic polymersome
nanoreactors with tumor-specific activable cascade reactions for
cooperative cancer therapy. ACS Nano. 13:2357–2369. 2019.PubMed/NCBI
|
|
23
|
Gao X, Wang S, Wang BL, Deng S, Liu X,
Zhang XN, Luo LL, Fan RR, Xiang ML, You C, et al: Improving the
anti-ovarian cancer activity of docetaxel with biodegradable
self-assembly micelles through various evaluations. Biomaterials.
53:646–658. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mao HL, Qian F, Li S, Shen JW, Ye CK, Hua
L, Zhang LZ, Wu DM, Lu J, Yu RT, et al: Delivery of doxorubicin
from hyaluronic acid-modified glutathione-responsive ferrocene
micelles for combination cancer therapy. Mol Pharm. 16:987–994.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Z, Tan S, Li S, Shen Q and Wang K:
Cancer drug delivery in the nano era: An overview and perspectives
(Review). Oncol Rep. 38:611–624. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zariwala MG, Bendre H, Markiv A, Farnaud
S, Renshaw D, Taylor KM and Somavarapu S: Hydrophobically modified
chitosan nanoliposomes for intestinal drug delivery. Int J
Nanomedicine. 13:5837–5848. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu Y, Liu X, Cai Z, Zhang H, Gao H, He W,
Wu P, Cai C, Zhu JJ and Yan Z: Enhancing the plasmon resonance
absorption of multibranched gold nanoparticles in the near-infrared
region for photothermal cancer therapy: Theoretical predictions and
experimental verification. Chem Mater. 31:471–482. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bellassai N, D'Agata R, Jungbluth V and
Spoto G: Surface plasmon resonance for biomarker detection:
Advances in Non-invasive cancer diagnosis. Front Chem. 7:5702019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kishimoto TK, Ferrari JD, Lamothe RA,
Kolte PN, Griset AP, O'Neil C, Chan V, Browning E, Chalishazar A,
Kuhlman W, et al: Improving the efficacy and safety of biologic
drugs with tolerogenic nanoparticles. Nat Nanotechnol. 11:890–899.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Böhmová E, Machová D, Pechar M, Pola R,
Venclíková K, Janoušková O and Etrych T: Cell-penetrating peptides:
A useful tool for the delivery of various cargoes into cells.
Physiol Res. 67 (Suppl 2):S267–S279. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kauffman WB, Fuselier T, He J and Wimley
WC: Mechanism matters: A taxonomy of cell penetrating peptides.
Trends Biochem Sci. 40:749–764. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Frankel AD and Pabo CO: Cellular uptake of
the tat protein from human immunodeficiency virus. Cell.
55:1189–1193. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Green M and Loewenstein PM: Autonomous
functional domains of chemically synthesized human immunodeficiency
virus tat trans-activator protein. Cell. 55:1179–1188. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bechara C and Sagan S: Cell-penetrating
peptides: 20 years later, where do we stand? FEBS Lett.
587:1693–1702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guidotti G, Brambilla L and Rossi D:
Cell-penetrating peptides: From basic research to clinics. Trends
Pharmacol Sci. 38:406–424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang K and García AE: Free energy of
translocating an arginine-rich cell-penetrating peptide across a
lipid bilayer suggests pore formation. Biophys J. 104:412–420.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Herce HD, Garcia AE, Litt J, Kane RS,
Martin P, Enrique N, Rebolledo A and Milesi V: Arginine-rich
peptides destabilize the plasma membrane, consistent with a pore
formation translocation mechanism of cell-penetrating peptides.
Biophys J. 97:1917–1925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Islam MZ, Ariyama H, Alam JM and Yamazaki
M: Entry of cell-penetrating peptide transportan 10 into a single
vesicle by translocating across lipid membrane and its induced
pores. Biochemistry. 53:386–396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sharmin S, Islam MZ, Karal MAS, Alam
Shibly SU, Dohra H and Yamazaki M: Effects of lipid composition on
the entry of cell-penetrating peptide oligoarginine into single
vesicles. Biochemistry. 55:4154–4165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lindgren M and Langel U: Classes and
prediction of cell-penetrating peptides. Methods in molec8ular
biology (Clifton N.J.). 683:3–19. 2011.PubMed/NCBI
|
|
41
|
Cai D, Gao W, He B, Dai W, Zhang H, Wang
X, Wang J, Zhang X and Zhang Q: Hydrophobic penetrating peptide
PFVYLI-modified stealth liposomes for doxorubicin delivery in
breast cancer therapy. Biomaterials. 35:2283–2294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang Y, Yang Y, Xie X, Cai X, Zhang H,
Gong W, Wang Z and Mei X: PEGylated liposomes with NGR ligand and
heat-activable cell-penetrating peptide-doxorubicin conjugate for
tumor-specific therapy. Biomaterials. 35:4368–4381. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Morshed RA, Muroski ME, Dai Q, Wegscheid
ML, Auffinger B, Yu D, Han Y, Zhang L, Wu M, Cheng Y and Lesniak
MS: Cell-penetrating peptide-modified gold nanoparticles for the
delivery of doxorubicin to brain metastatic breast cancer. Mol
Pharm. 13:1843–1854. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu X, Xu Y, Solis LM, Tao W, Wang L,
Behrens C, Xu X, Zhao L, Liu D, Wu J, et al: Long-circulating siRNA
nanoparticles for validating Prohibitin1-targeted non-small cell
lung cancer treatment. Proc Natl Acad Sci. 112:7779–7784. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Parvani JG, Gujrati MD, Mack MA, Schiemann
WP and Lu ZR: Silencing β3 integrin by targeted ECO/siRNA
nanoparticles inhibits EMT and metastasis of triple-negative breast
cancer. Cancer Res. 75:2316–2325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vaidya AM, Sun Z, Ayat N, Schilb A, Liu X,
Jiang H, Sun D, Scheidt J, Qian V, He S, et al: Systemic delivery
of tumor-targeting siRNA Nanoparticles against an oncogenic LncRNA
facilitates effective triple-negative breast cancer therapy.
Bioconjug Chem. 30:907–919. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pasut G, Paolino D, Celia C, Mero A,
Joseph AS, Wolfram J, Cosco D, Schiavon O, Shen H and Fresta M:
Polyethylene glycol (PEG)-dendron phospholipids as innovative
constructs for the preparation of super stealth liposomes for
anticancer therapy. J Control Release. 199:106–113. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nag M, Gajbhiye V, Kesharwani P and Jain
NK: Transferrin functionalized chitosan-PEG nanoparticles for
targeted delivery of paclitaxel to cancer cells. Colloids Surfaces
B Biointerfaces. 148:363–370. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ebbesen MF, Olesen MTJ, Gjelstrup MC,
Pakula MM, Larsen EKU, Hansen IM, Hansen PL, Mollenhauer J, Malle
BM and Howard KA: Tunable CD44-specific cellular retargeting with
hyaluronic acid nanoshells. Pharm Res. 32:1462–1474. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhong L, Xu L, Liu Y, Li Q, Zhao D, Li Z,
Zhang H, Zhang H, Kan Q, Wang Y, et al: Transformative hyaluronic
acid-based active targeting supramolecular nanoplatform improves
long circulation and enhances cellular uptake in cancer therapy.
Acta Pharm Sin B. 9:397–409. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ben Djemaa S, David S, Hervé-Aubert K,
Falanga A, Galdiero S, Allard-Vannier E, Chourpa I and Munnier E:
Formulation and in vitro evaluation of a siRNA delivery nanosystem
decorated with gH625 peptide for triple negative breast cancer
theranosis. Eur J Pharm Biopharm. 131:99–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mäe M, Myrberg H, El-Andaloussi S and
Langel Ü: Design of a tumor homing cell-penetrating peptide for
drug delivery. Int J Pept Res Ther. 15:11–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lim KJ, Sung BH, Shin JR, Lee YW, Kim DJ,
Yang KS and Kim SC: A cancer specific cell-penetrating peptide,
BR2, for the efficient delivery of an scFv into cancer cells. PLoS
One. 8:e660842013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fang SL, Fan TC, Fu HW, Chen CJ, Hwang CS,
Hung TJ, Lin LY and Chang MD: A novel cell-penetrating peptide
derived from human eosinophil cationic protein. PLoS One.
8:e573182013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kalyanaraman B: Teaching the basics of the
mechanism of doxorubicin-induced cardiotoxicity: Have we been
barking up the wrong tree? Redox Biol. 29:1013942020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fales AM, Yuan H and Vo-Dinh T:
Cell-penetrating peptide enhanced intracellular Raman imaging and
photodynamic therapy. Mol Pharm. 10:2291–2298. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hossain MK, Cho HY, Kim KJ and Choi JW: In
situ monitoring of doxorubicin release from biohybrid nanoparticles
modified with antibody and cell-penetrating peptides in breast
cancer cells using surface-enhanced Raman spectroscopy. Biosens
Bioelectron. 71:300–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wan Y, Dai W, Nevagi RJ, Toth I and Moyle
PM: Multifunctional peptide-lipid nanocomplexes for efficient
targeted delivery of DNA and siRNA into breast cancer cells. Acta
Biomater. 59:257–268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hu G, Chun X, Wang Y, He Q and Gao H:
Peptide mediated active targeting and intelligent particle size
reduction-mediated enhanced penetrating of fabricated nanoparticles
for triple-negative breast cancer treatment. Oncotarget.
6:41258–41274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sardan M, Kilinc M, Genc R, Tekinay AB and
Guler MO: Cell penetrating peptide amphiphile integrated liposomal
systems for enhanced delivery of anticancer drugs to tumor cells.
Faraday Discuss. 166:269–283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang X, Chen X, Yang X, Gao W, He B, Dai
W, Zhang H, Wang X, Wang J, Zhang X, et al: A nanomedicine based
combination therapy based on QLPVM peptide functionalized liposomal
tamoxifen and doxorubicin against Luminal A breast cancer.
Nanomedicine. 12:387–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang H, Wang H, Liang J, Jiang Y, Guo Q,
Peng H, Xu Q and Huang Y: Cell-penetrating apoptotic peptide/p53
DNA nanocomplex as adjuvant therapy for drug-resistant breast
cancer. Mol Pharm. 11:3352–3360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen J, Li S and Shen Q: Folic acid and
cell-penetrating peptide conjugated PLGA-PEG bifunctional
nanoparticles for vincristine sulfate delivery. Eur J Pharm Sci.
47:430–443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hu H, Wang J, Wang H, Tan T, Li J, Wang Z,
Sun K, Li Y and Zhang Z: Cell-penetrating peptide-based
nanovehicles potentiate lymph metastasis targeting and deep
penetration for anti-metastasis therapy. Theranostics. 8:3597–3610.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cabral H, Makino J, Matsumoto Y, Mi P, Wu
H, Nomoto T, Toh K, Yamada N, Higuchi Y, Konishi S, et al: Systemic
targeting of lymph node metastasis through the blood vascular
system by using size-controlled nanocarriers. ACS Nano.
9:4957–4967. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kang S, Ahn S, Lee J, Kim JY, Choi M,
Gujrati V, Kim H, Kim J, Shin EC and Jon S: Effects of gold
nanoparticle-based vaccine size on lymph node delivery and
cytotoxic T-lymphocyte responses. J Control Release. 256:56–67.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Su J, Sun H, Meng Q, Yin Q, Tang S, Zhang
P, Chen Y, Zhang Z, Yu H and Li Y: Long circulation
red-blood-cell-mimetic nanoparticles with peptide-enhanced tumor
penetration for simultaneously inhibiting growth and lung
metastasis of breast cancer. Adv Funct Mater. 26:1243–1252. 2016.
View Article : Google Scholar
|
|
68
|
Hamilton AM, Aidoudi-Ahmed S, Sharma S,
Kotamraju VR, Foster PJ, Sugahara KN, Ruoslahti E and Rutt BK:
Nanoparticles coated with the tumor-penetrating peptide iRGD reduce
experimental breast cancer metastasis in the brain. J Mol Med.
93:991–1001. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jabir MS, Taha AA, Sahib UI, Taqi ZJ,
Al-Shammari AM and Salman AS: Novel of nano delivery system for
Linalool loaded on gold nanoparticles conjugated with CALNN peptide
for application in drug uptake and induction of cell death on
breast cancer cell line. Mater Sci Eng C Mater Biol Appl.
94:949–964. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wan X, Liu C, Lin Y, Fu J, Lu G and Lu Z:
pH sensitive peptide functionalized nanoparticles for co-delivery
of erlotinib and DAPT to restrict the progress of triple negative
breast cancer. Drug Deliv. 26:470–480. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liang DS, Su HT, Liu YJ, Wang AT and Qi
XR: Tumor-specific penetrating peptides-functionalized hyaluronic
acid-d-α-tocopheryl succinate based nanoparticles for multi-task
delivery to invasive cancers. Biomaterials. 71:11–23. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ding J, Yao J, Xue J, Li R, Bao B, Jiang
L, Zhu JJ and He Z: Tumor-homing Cell-penetrating peptide linked to
colloidal mesoporous silica Encapsulated
(-)-Epigallocatechin-3-gallate as drug delivery system for breast
cancer therapy in vivo. ACS Appl Mater Interfaces. 7:18145–18155.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ding J, Liang T, Zhou Y, He Z, Min Q,
Jiang L and Zhu J: Hyaluronidase-triggered anticancer drug and
siRNA delivery from cascaded targeting nanoparticles for
drug-resistant breast cancer therapy. Nano Res. 10:690–703. 2016.
View Article : Google Scholar
|
|
74
|
Ding J, Liang T, Min Q, Jiang L and Zhu
JJ: ‘Stealth and Fully-Laden’ Drug carriers: Self-assembled
nanogels encapsulated with epigallocatechin gallate and siRNA for
drug-resistant breast cancer therapy. ACS Appl Mater Interfaces.
10:9938–9948. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Huang R, Li J, Kebebe D, Wu Y, Zhang B and
Liu Z: Cell penetrating peptides functionalized gambogic
acid-nanostructured lipid carrier for cancer treatment. Drug Deliv.
25:757–765. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Perillo E, Hervé-Aubert K, Allard-Vannier
E, Falanga A, Galdiero S and Chourpa I: Synthesis and in vitro
evaluation of fluorescent and magnetic nanoparticles functionalized
with a cell penetrating peptide for cancer theranosis. J Colloid
Interface Sci. 499:209–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cao H, Zou L, He B, Zeng L, Huang Y, Yu H,
Zhang P, Yin Q, Zhang Z and Li Y: Albumin biomimetic nanocorona
improves tumor targeting and penetration for synergistic therapy of
metastatic breast cancer. Adv Funct Mater. 27:16056792017.
View Article : Google Scholar
|
|
78
|
Jing H, Cheng W, Li S, Wu B, Leng X, Xu S
and Tian J: Novel cell-penetrating peptide-loaded nanobubbles
synergized with ultrasound irradiation enhance EGFR siRNA delivery
for triple negative Breast cancer therapy. Colloids Surfaces B
Biointerfaces. 146:387–395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Govindarajan S, Sivakumar J, Garimidi P,
Rangaraj N, Kumar JM, Rao NM and Gopal V: Targeting human epidermal
growth factor receptor 2 by a cell-penetrating peptide-affibody
bioconjugate. Biomaterials. 33:2570–2582. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sharma S, Kotamraju VR, Mölder T, Tobi A,
Teesalu T and Ruoslahti E: Tumor-penetrating nanosystem strongly
suppresses breast tumor growth. Nano Lett. 17:1356–1364. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Li G, Gao Y, Gong C, Han Z, Qiang L, Tai
Z, Tian J and Gao S: Dual-blockade immune checkpoint for breast
cancer treatment based on a tumor-penetrating peptide assembling
nanoparticle. ACS Appl Mater Interfaces. 11:39513–39524. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Xie X, Yang Y, Lin W, Liu H, Liu H, Yang
Y, Chen Y, Fu X and Deng J: Cell-penetrating peptide-siRNA
conjugate loaded YSA-modified nanobubbles for ultrasound triggered
siRNA delivery. Colloids Surfaces B Biointerfaces. 136:641–650.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Braun GB, Sugahara KN, Yu OM, Kotamraju
VR, Mölder T, Lowy AM, Ruoslahti E and Teesalu T:
Urokinase-controlled tumor penetrating peptide. J Control Release.
232:188–195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Fei L, Yap LP, Conti PS, Shen WC and Zaro
JL: Tumor targeting of a cell penetrating peptide by fusing with a
pH-sensitive histidine-glutamate co-oligopeptide. Biomaterials.
35:4082–4087. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lang J, Zhao X, Qi Y, Zhang Y, Han X, Ding
Y, Guan J, Ji T, Zhao Y and Nie G: Reshaping prostate tumor
microenvironment to suppress metastasis via cancer-associated
fibroblast inactivation with peptide-assembly-based nanosystem. ACS
Nano. 13:12357–12371. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Xiang B, Dong DW, Shi NQ, Gao W, Yang ZZ,
Cui Y, Cao DY and Qi XR: PSA-responsive and PSMA-mediated
multifunctional liposomes for targeted therapy of prostate cancer.
Biomaterials. 34:6976–6991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhou J, Fan J and Hsieh JT: Inhibition of
mitogen-elicited signal transduction and growth in prostate cancer
with a small peptide derived from the functional domain of
DOC-2/DAB2 delivered by a unique vehicle. Cancer Res. 66:8954–8958.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhang T, Xue X, He D and Hsieh JT: A
prostate cancer-targeted polyarginine-disulfide linked PEI
nanocarrier for delivery of microRNA. Cancer Lett. 365:156–165.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kumar A, Huo S, Zhang X, Liu J, Tan A, Li
S, Jin S, Xue X, Zhao Y, Ji T, et al: Neuropilin-1-targeted gold
nanoparticles enhance therapeutic efficacy of Platinum(IV) drug for
prostate cancer treatment. ACS Nano. 8:4205–4220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Menon JU, Tumati V, Hsieh JT, Nguyen KT
and Saha D: Polymeric nanoparticles for targeted radiosensitization
of prostate cancer cells. J Biomed Mater Res A. 103:1632–1639.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wadajkar AS, Menon JU, Tsai YS, Gore C,
Dobin T, Gandee L, Kangasniemi K, Takahashi M, Manandhar B, Ahn JM,
et al: Prostate cancer-specific thermo-responsive polymer-coated
iron oxide nanoparticles. Biomaterials. 34:3618–3625. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yuan L, Liu CY, Chen Y, Zhang ZH, Zhou L
and Qu D: Antitumor activity of tripterine via cell-penetrating
peptide-coated nanostructured lipid carriers in a prostate cancer
model. Int J Nanomedicine. 8:4339–4350. 2013.PubMed/NCBI
|
|
93
|
Jiménez-Mancilla N, Ferro-Flores G,
Santos-Cuevas C, Ocampo-García B, Luna-Gutiérrez M, Azorín-Vega E,
Isaac-Olivé K, Camacho-López M and Torres-García E: Multifunctional
targeted therapy system based on (99m) Tc/(177) Lu-labeled gold
nanoparticles-Tat(49–57)-Lys(3)-bombesin internalized in nuclei of
prostate cancer cells. J Label Compd Radiopharm. 56:663–671. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
DePorter SM and McNaughton BR: Engineered
M13 bacteriophage nanocarriers for intracellular delivery of
exogenous proteins to human prostate cancer cells. Bioconjug Chem.
25:1620–1625. 2014. View Article : Google Scholar : PubMed/NCBI
|