Introduction
Gastrointestinal cancer is a health issue with
worldwide concern, of which gastric and colorectal cancers are the
most common types (1,2). Despite the declining incidence and
mortality, gastric cancer remains the third leading cause of
cancer-related mortality in the world (1,3).
Nearly one million gastric cancer cases are diagnosed worldwide
yearly, about half of which are found in the Chinese population
(3). The incidence of colorectal
cancer ranks third in the world, with highest morbidity and
mortality in Asian populations (4). This distribution may be related to
particular diet habits, increased level of stress and/or the
Helicobacter pylori (H. pylori) infection prevalence in the
Asian population (5,6). China and South Korea prefer high-salt
foods such as pickles and kimchi. Koreans consume more than twice
the daily salt intake recommended by the World Health Organization
(7,8), and a high-salt diet can lead to a
series of gastrointestinal diseases. Approximately half of the
world's population is infected with H. pylori, while more
than 55% are found in China (9,10).
Some studies have shown the relationship among vegetable
consumption, gastrointestinal tumors and H. pylori (11–14),
confirming that the increased consumption of fibers that are
abundant in fresh fruits and vegetables is correlated with a
reduced risk of gastrointestinal cancer (11).
Historically, garlic consumption has been associated
with medicinal properties in ancient cultures of Indochina, the
Mediterranean and Northern Africa (15). Garlic was shown to be able to
reduce the risk of carcinogenesis in breast cancer, pancreatic
cancer and esophageal cancer models (16–18).
The S-allyl cysteine, diallyl disulfide, and other compounds found
in garlic were suggested to have anticancer effects in cellular
models (15,19,20).
Many potential anticancer mechanisms of these compounds were
proposed, including the inhibition of cell proliferation, changes
in enzyme activity and immune regulation (21,22).
The active ingredients in garlic oil correspond mainly to a family
of organosulfur molecules, which selectively increase redox stress
in cancer cells, leading to apoptosis and death (23).
Previous meta-analyses and reviews exploring the
relationship between garlic consumption and the risk of gastric and
colorectal cancers have come to inconsistent conclusion (4,14,24–30).
While some studies have found that garlic intake could reduce the
risk of gastric and colorectal cancers (14,30),
others have shown that this effect may be overestimated (28). In a recent study by Li et al
(13) with a follow-up of 22.3
years, garlic supplementation was found to be associated with
reduced gastric cancer mortality (OR=0.81, 95% CI=0.57-1.13), with
a delayed effect on gastric cancer mortality. Although this finding
provides a potential opportunity for the prevention of gastric
cancer, further large-scale intervention trials are needed to
confirm the effect. Based on the prospective data from the Nurses'
Health Study involving 121,700 nurses [relative risk (RR)=1.21, 95%
CI=0.94-1.57] and the Health Professionals Follow-up Study
(RR=1.00, 95% CI=0.71-1.42) involving 512,529 male health
professionals, Meng et al (31) found no association between garlic
consumption and the risk of colorectal cancer. However, this study
was excluded in this research due to the lack of OR or RR data.
Different diets in various populations, various levels of garlic
consumption, and diverse patterns of garlic intake may cause
inconsistent results from the different studies. Therefore, the
effect of garlic on gastrointestinal cancer needs to be further
confirmed. We conducted this meta-analysis to update the
epidemiological evidence for the association between garlic and
gastrointestinal cancer.
Materials and methods
Search strategy
This systemic review and meta-analysis is reported
in accordance with the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses (PRISMA) statement. The study was
registered in PROSPERO (CRD42020179464). The authors completed the
data search in September 2021. All relevant studies that related to
garlic intake for gastric and colorectal cancers from 1980 to 2021
were identified by searching in the following databases: Pubmed
(https://pubmed.ncbi.nlm.nih.gov/),
Embase (https://www.embase.com/landing?status=grey) and
Cochrane Library (https://www.cochranelibrary.com/), with key terms
including: ‘garlic’, ‘allium’, ‘stomach’, ‘gastric’, ‘colon’,
‘neoplasms’, ‘cancer’ and ‘tumor’. The detailed searching
strategies in each database are shown in Tables SI-SIII. All studies that met the
requirements were reviewed. In addition, we expanded the search
fields by including the references of the screened articles.
Study selection
During the database compilation, two investigators
(YaW and DL) reviewed the full text of all the screened
publications to determine whether the studies met the selection
criteria. Further refinement of the database was completed by a
third investigator (YuW). Studies were selected according to the
following criteria: i) randomized controlled trials, case-control
trials, or with cohort design; ii) studies that include the
evaluation of the association between garlic intake and gastric or
colorectal cancers over nearly 30 years; iii) studies that provide
odds ratio (OR) or relative risk (RR) and with 95% confidence
interval (CI) or providing sufficient information for OR/RR and 95%
CI calculation; iv) studies published within the last 30 years. The
exclusion criteria included: i) reviews or meta-analyses; ii)
non-English literature; iii) studies that lacked OR or RR data, or
without sufficient data estimation results; iv) studies for which
animal, cell, in vitro, and in vivo experiments were
excluded. Since the majority of related studies were published in
English, we chose not to include non-English studies which were
very few and had lack of representativeness. The studies that were
included were all non-truncated ones.
Data extraction
Data mining was performed by two investigators.
Disagreements were resolved by consultation with a third
investigator. The following information was extracted: author, year
of publication, study period, study type, country, number of
subjects, risk estimates and their 95% CI, description of garlic
intake categories, and adjusted variables.
Risk of bias assessment
For randomized controlled trials (RCTs), we assessed
the risk of bias using the Cochrane Risk of Bias assessment tool
(32). The following
characteristics were evaluated: random sequence generation,
allocation concealment, blinding of participants and personnel,
blinding of outcome assessment, incomplete outcome data, selective
reporting and other biases. According to the recommendations of the
Cochrane Handbook, a judgment to risk of bias was determined as
three categories, including low risk, unclear risk and high risk.
We used the Newcastle-Ottawa Scale (NOS) to assess the risk of bias
in nonrandomized studies and scored the studies in three
categories: selection (four questions), comparability of study
groups (two questions), and ascertainment of exposure or outcome
(three questions). Regarding the comparability, the study groups
were awarded a maximum of two points; all the other questions were
assigned a score of one point (33).
Statistical analysis
We first collected the OR of gastric cancer in
various studies. Since the incidences of gastric cancer and
colorectal cancer are relatively low, the approximate OR was
obtained based on the RR. Then we explored the sources of
heterogeneity and conducted a subgroup analysis by garlic intake
level, geographic area, and the type of study.
The heterogeneity was assessed using the Cochrane's
Q test and I2 statistic. P-values <0.1 and
I2 values >50% suggested the existence of
heterogeneity. If significant heterogeneity existed, a random
effect model was selected; otherwise, the fixed-effects model was
used. Meanwhile, I2 values of <30%, 30–60%, and
>60% were considered to indicate low, moderate, and high
heterogeneity, respectively. Results were assessed using forest
plots. All data analysis was performed by STATA 12.0 (https://www.stata.com/).
Sensitivity analysis and subgroup
analysis
Sensitivity analysis was performed to identify
potential sources of heterogeneity according to garlic consumption
level, research type and geographical area. Subgroup analysis was
conducted to identify the cause of heterogeneity. Random effect
model and fixed effect model were selected according to different
degrees of heterogeneity.
Publication bias
Publication bias was assessed by conducting Begg's
and Egger's funnel plot asymmetry tests, a P-value <0.1
suggested publication bias with statistical significance.
Results
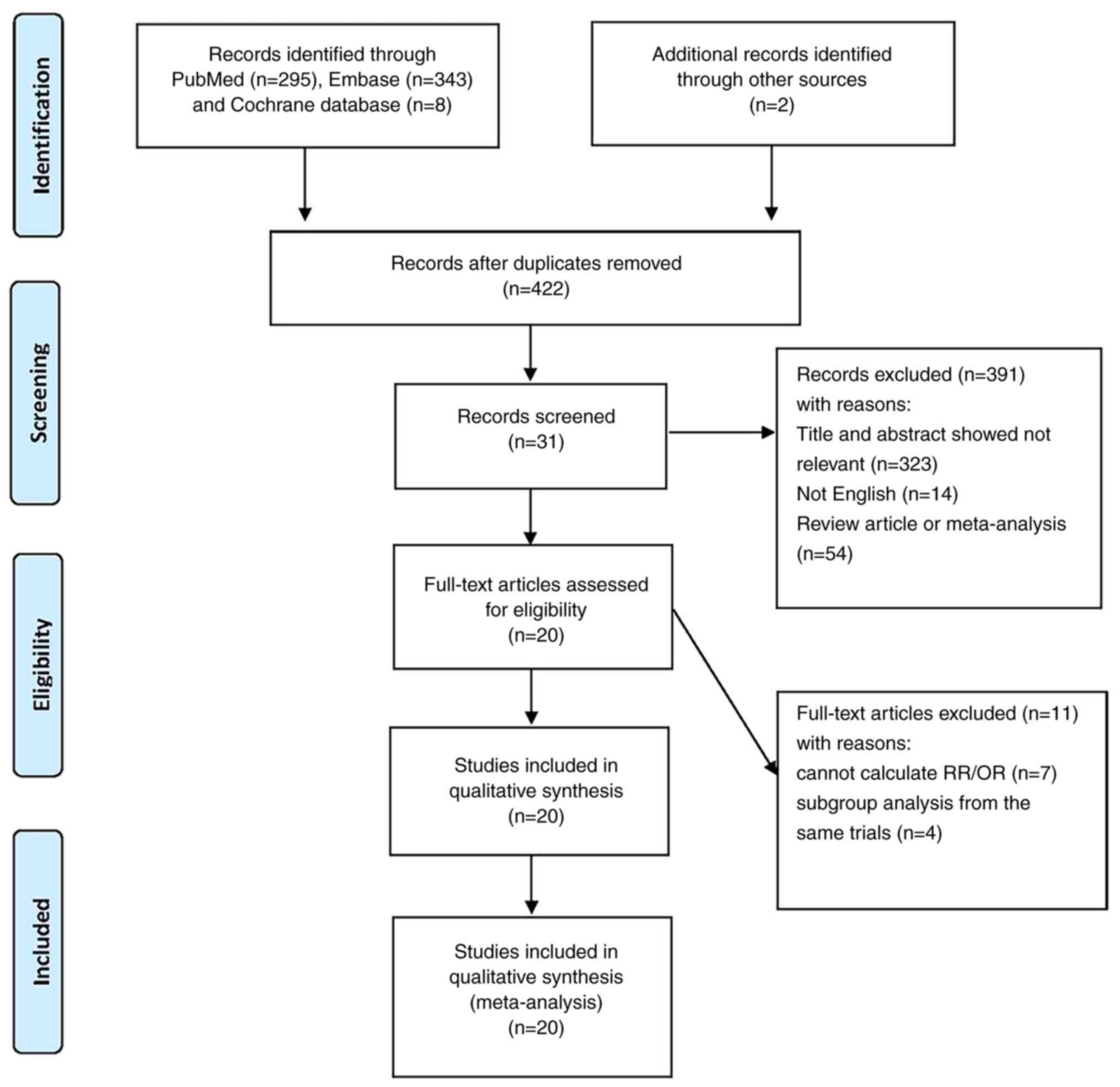
Study selection and
characteristics
A total of 648 articles were initially identified,
of which 226 articles were excluded as duplicate studies. Then we
reviewed the titles and abstracts of each literature study
according to inclusion and exclusion criteria. We excluded
additional articles, among which 323 were irrelevant to this study,
54 were meta-analyses and review, and 14 were non-English
literature. After a careful review of full texts in the remaining
31 articles, we finally included 20 articles after excluding 4
articles from the same study and 7 articles with insufficient data
(12,13,18,34–50).
The flow of the literature search is documented in Fig. 1.
Of the 20 included articles (Table I), 11 were about garlic and gastric
cancer, and 9 were about garlic and colorectal cancer. The 11
studies on garlic and gastric cancer were published between 1989
and 2020, including 3,299 patients with gastric cancer and 133,801
controls from one randomized controlled trial (13), 8 case-control studies (18,34,35,37,38,40,41,47)
and two cohort studies (36,39).
The study by Setiawan et al (35) was a large population-based
case-control study of Shanghai and Qingdao, thus we divided this
study into a and b to represent the results of Shanghai and
Qingdao, respectively. The study by Kim et al (39) was composed of The Nurses' Health
Study (NHS) and The Health Professionals Follow-up Study (HPFS);
therefore, we split the results into two parts. The 9 studies on
garlic and colorectal cancer include 7 case-control studies
(12,43,44,46,48–50)
and two cohort studies (42,45),
published between 1994 and 2018 involving 8,519 colorectal cancer
patients and 52,423 controls. Of the 9 studies, 4 were conducted in
Europe, 2 in Asia, 2 in the US and 1 in Australia. Both Franceschi
et al (46) and Dorant
et al (42) studies
included colon and rectal cancer, thus we believe that it was
reasonable to separate colon and rectal cancers.
 | Table I.Details of all 20 studies included in
this analysis. |
Table I.
Details of all 20 studies included in
this analysis.
| Authors of the
study (year) | Country | Study design | Study period | Cases/Controls | Garlic consumption
types | Consumption | OR/RR (95% CI) | Adjustment | (Refs.) |
|---|
| You et al
(1989) | China | Population-based
case-control study | 1984- 1986 | 564/1,131 | Garlic | 0 (kg/year) 0.1-1.5
(kg/year) >1.5 (kg/year) | 1.00 0.8 (0.5-1.2)
0.7 (0.4-1.0) | Age, sex, family
income, and intake of other allium vegetables | (38) |
| Dorant et al
(1996) | The
Netherlands | Cohort study | 1986-1990 | 152 (male 119,
female 33)/3,340 (male 1,627, female 1,713) | Garlic
supplement | No supplements
Exclusively garlic | 1.00 1.27
(0.61-2.64) | Age, alcohol
intake, vitamin C intake, and b-carotene as continuous variables
and sex, smoking status, education, history of stomach di-orders,
and family history of stomach cancer as categorical variables | (36) |
| Gao et al
(1999) | China | Population-based
case-control study | 1995-1997 | 153 (male 140,
female 53)/234 (male 154, female 80) | Garlic | <1 time/month
1–3 times/month ≥1 time/week | 1.00 0.4
(0.21-0.76) 0.31 (0.22-0.44) | Age, sex, income,
smoking, drinking, tea consumption and intake of leftover gruel,
pickled vegetables, meat, fruit, tomatoes, eggs and snap
beans. | (40) |
| Muñoz et al
(2001) | Venezuela | Population- based
case-control study | 1991-1997 | 292/485 | Garlic | Less than once/week
Several times/week Every day | 1.00 0.7 (0.4-1.0)
0.5 (0.3-0.8) | Age, sex and
SES | (37) |
| Takezaki et
al (2001) | China | Population-based
case-control study | 1996-2000 | 187 (male 137,
female 50)/333 (male 235,) female 98 | Garlic | <1 time/week 1–2
times/week 3–5 times/week Everyday | 1.00 1.00
(0.56-1.81) 0.72 (0.42-1.24) 0.66 (0.37-1.17) | Age, sex, smoking
and drinking habits | (18) |
| De Stefani et
al (2001) | Uruguay | Hospital-based
case-control study | 1997-2000 | 160 (male 114,
female 46)/320 (male 225, female 95) | Garlic |
| 0.67
(0.38-1.18) | Age, sex,
residence, urban/rural status, education, body mass index (BMI),
and total energy intake, and total fruit intake | (34) |
| Setiawan et
al (2005a) | China | Population-based
case-control study | 1991-1993 | 750 (male 478,
female 272)/750 (male 478, female 272) | Garlic | Never Occasional
Often | 1.00 1.11
(0.87-1.41) 0.68 (0.37-1.26) | Matching variables
(age, sex), education, BMI, pack-years of smoking, alcohol
drinking, salt intake, and vegetable and fruit intake | (35) |
| Setiawan et
al (2005b) | China | Population-based
case-control study | 1991-1993 | 201 (male 143,
female 58)/201 (male143, female 58) | Garlic | Never Occasional
Often | 1.00 0.71
(0.27-1.88) 0.45 (0.15-1.30) | Matching variables
(age, sex), education, BMI, pack-years of smoking, alcohol
drinking, salt intake, and vegetable and fruit intake | (35) |
| Pourfarzi et
al (2009) | Iran | Population-based
case-control study | 1999- 2005 | 217 (male 151,
female 66)/394 (male 265, female 129) | Garlic | Never or
infrequently 1–2 times/week >3 times/week | 1.00 0.48
(0.25-0.91) 0.35 (0.13-0.95) | Sex, age group,
education, family history of GC, citrus fruits, garlic, onion, red
meat, fish, dairy products, strength and warmth of tea, preference
for salt intake and H. pylori. | (41) |
| Kim et al
(2018) NHS | USA | Cohort study | 1984-2014 | 138/76,948 | Garlic | Never 0-<1 per
week 1–4 per week 5 per week | 1.00 1.08
(0.67-1.73) 0.81 (0.50-1.31) 1.34 (0.72-2.47) | Age, Caucasian,
BMI, physical activity, smoking status, alcohol consumption,
current multivitamin use, current aspirin use, personal history of
diabetes mellitus, and intakes of total calorie, red/processed
meat, fruits, vegetables, and coffee | (39) |
| Kim et al
(2018) HPFS | USA | Cohort study | 1984-2014 | 154/46,244 | Garlic | Never 0-<1 per
week 1–4 per week 5 per week | 1.00 1.13
(0.75-1.72) 1.15 (0.74-1.77) 1.45 (0.76-2.78) | Age, Caucasian,
BMI, physical activity, smoking status, alcohol consumption,
current multivitamin use, current aspirin use, personal history of
diabetes mellitus, and intakes of total calorie, red/processed
meat, fruits, vegetables, and coffee | (39) |
| Li et al
(2019) | China | Randomized
controlled trial | 1995-2017 | 151/3,241 | Garlic
supplementation | Twice a day | 0.81
(0.57-1.13) | Baseline histology,
age, sex, history of ever using alcohol, and history of ever
smoking | (13) |
| Yuan et al
(2020) | China | Hospital-based
case-control study | 2014-2016 | 180/180 | Garlic |
| 0.35
(0.18-0.67) | Dietary/lifestyle
habits, psychological factors, serum PG I level, serum PG II level,
PG I/II ratio, serum G-17 level and H. pylori infection | (47) |
| Steinmetz et
al (1994) | USA | Prospective cohort
Study | 1986-1990 | 212/35004 | Garlic | L1 (0) L2 (0.5) L3
(1.0) | 1.00 1.07
(0.77-1.50) 0.68 (0.46-1.02) | Age and energy
intake | (45) |
| Witte et al
(1996) | USA | Case control
study | 1991-1993 | 488 (male 325,
female 163)/488 (male 325, female 163) | Garlic | None 0.5 1.0-2.5
>-3.0 | 1.00 0.92
(0.64-1.34) 0.98 (0.61-1.56) 0.66 (0.43-1.01) | Race, BMI, physical
activity, smoking, calories, and saturated fat using conditional
logistic, regression dietary fiber, folate, β-carotene, and vitamin
C | (49) |
| Dorant et al
(1996) (colon) | The
Netherlands | Cohort study | 1986-1989 | 293 (male 150,
female 143)/3,123 (male 1,525, female 1598) | Garlic
supplement | No supplement
Exclusively garlic | 1.00 1.36
(0.79-2.35) | Age, vitamin C and
(J-carotene as continuous variables, and sex, smoking status,
education, family history of intestinal cancer, previous history of
chronic intestinal disease or chole cystectomy as categorical
variables | (42) |
| Dorant et al
(1996) (rectum) | The
Netherlands | Cohort study | 1986-1989 | 150 (male 93,
female 57)/3,123 (male 1,525, | Garlic
supplement | No supplement
Exclusively garlic | 1.00 1.28
(0.63-2.60) | Age, vitamin C and
(J-carotene as continuous variables, and sex, smoking female 1,598)
status, education, family history of intestinal cancer, previous
history of chronic intestinal disease or cholecystectomy as
categorical variables | (42) |
| Franceschi et
al (1997) (colon) | Italy | Case control
study | 1991-1996 | 1,225/5,155 | Cooked garlic |
| 0.9 (0.8-1.0) | Age, sex, center,
year of interview, education, physical activity, alcohol and energy
intake | (46) |
| Franceschi et
al (1997) (rectum) | Italy | Case control
study | 1991-1996 | 728/5,155 | Cooked garlic |
| 0.9 (0.8-1.0) | Age, sex, center,
year of interview, education, physical activity, alcohol and energy
intake | (46) |
| Levi et al
(1999) | Switzerland | Case control
study | 1992-1997 | 223 (male 142,
female 81)/491 (male 211, female 280) | Garlic | Low Medium
High | 1.00 0.51
(0.35-0.74) 0.32 (0.18-0.57) | Age, sex,
education, smoking, alcohol, BMI, physical activity and total
energy intake | (44) |
| Galeone et
al (2006) | Italy | Case control
study | 1991-2004 | 2,280 (male 1,318,
female 962)/4,765 (male 2,403, female 2,362) | Garlic | None or low
Intermediate High | 1 0.88 (0.78, 0.98)
0.74 (0.63,0.86) | Age, sex, study
center, education, BMI, energy intake, alcohol consumption, smoking
habit, and physical activity | (48) |
| Annema et al
(2011) | Australia | Population-based
case-control study | 2005-2007 | 834 (male 514,
female 320)/939 (male 551, female 388) | Garlic | <0.02
(servings/day) 0.02-<0.14 (servings/day) 0.14-<0.28
(servings/day) 0.28+ (servings/day) | 1.00 0.92
(0.67-1.26) 0.84 (0.62-1.15) 0.86 (0.68-1.09) | Adjusted for sex,
age, BMI at age 20 years, energy intake, multivitamin use, alcohol
consumption, physical activity, smoking, diabetes and socioeconomic
status | (50) |
| Wang et al
(2018) | China | Case control
study | 2015-2016 | 317 (male 145,
female 172)/317 (male 146, female 171) | Garlic |
| 0.499
(0.341-0.732) |
| (43) |
| Wu et al
(2019) | China | Hospital-based
matched case-control study | 2009-2011 | 833/833 | Garlic | <0.60 (kg/year)
0.60-2.60 (kg/year) 2.60-3.65 (kg/year) >3.65 (kg/year) | 1.00 0.49
(0.35-0.66) 0.43 (0.30-0.59) 0.56 (0.39-0.79) | BMI, family history
of CRC (first degree), education level, smoking, passive smoking,
alcohol, the consumption of red meat, milk, other vegetables,
fruit, total energy, fiber, calcium, fat, vitamin C, vitamin D,
cholesterol, and folic acid | (12) |
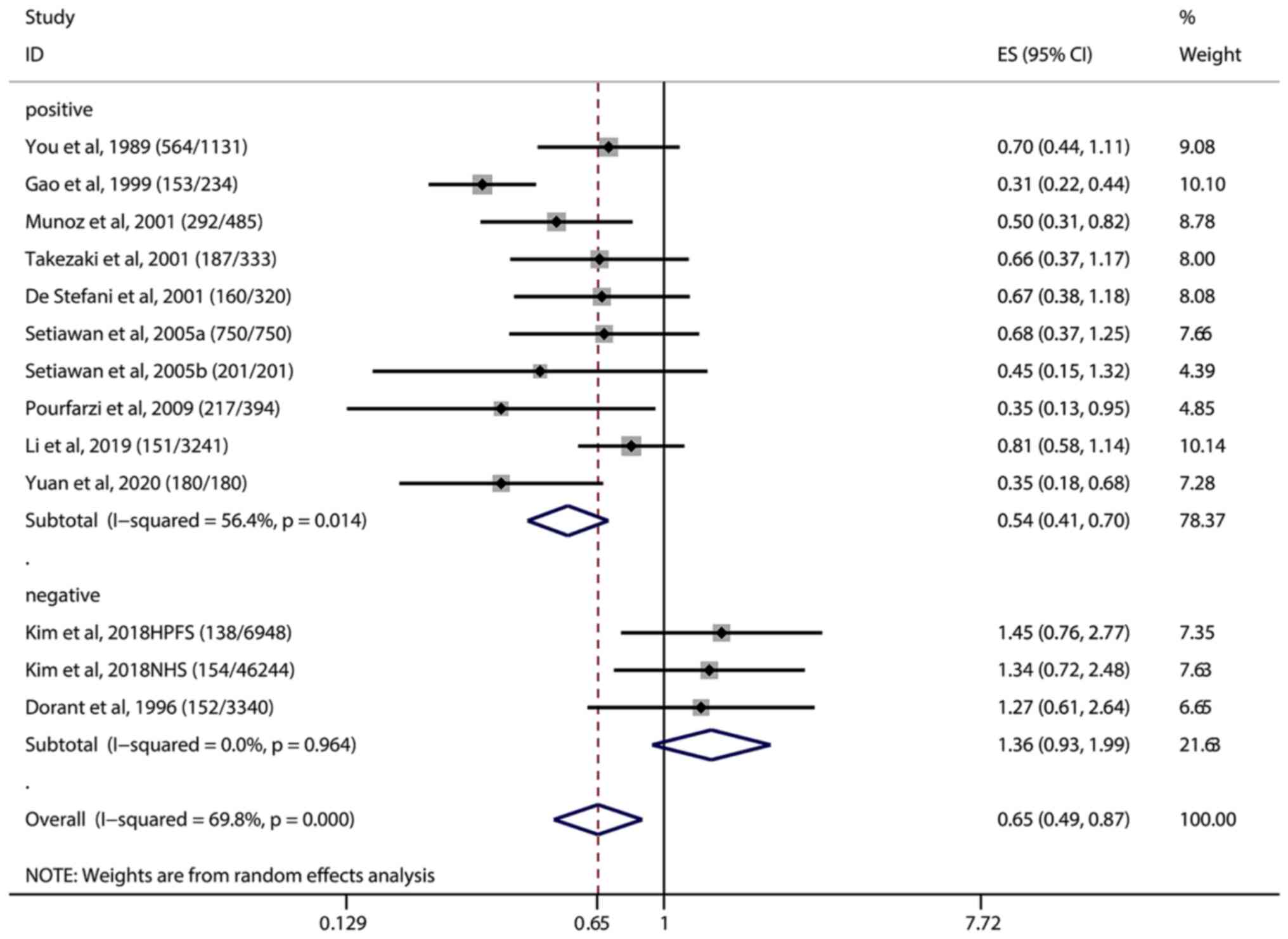
Overall and subgroup analysis of
evidence
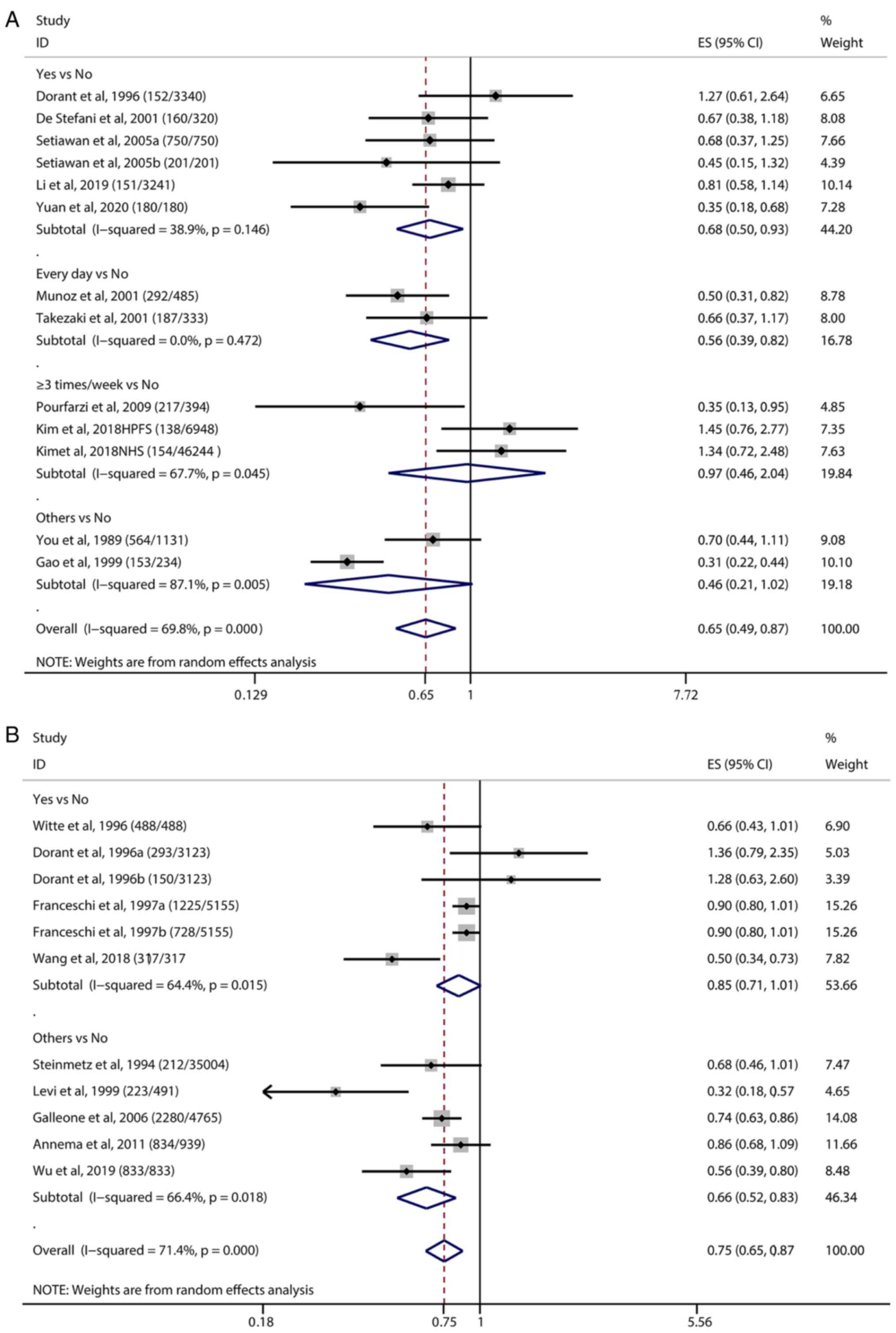
We conducted an overall estimation by categories of
garlic consumption (Fig. 2). The
ORs of all the studies were extracted for the meta-analysis. The OR
obtained by the pooled analysis was 0.65 (95% CI=0.49-0.87,
P<0.001), indicating that garlic intake was associated with a
lower risk of gastric cancer in individuals compared with those
without garlic intake (Fig. 2A).
Participants who consumed garlic every day had a significant lower
risk of gastric cancer than those who did not consume garlic.
In the subgroup analysis by geographic area
(Fig. S1), the estimated OR of
the studies in Asia, Europe and America was 0.53 (95%
CI=0.38-0.73), 1.27 (95% CI=0.61-2.64), 0.87 (95% CI=0.52-1.47,
P<0.05), respectively (Table
SIV). In addition, the comprehensive analysis of prospective
studies showed that garlic intake correlated with a small reduction
in gastric cancer (OR=1.07, 95% CI=0.79-1.47), while the
retrospective studies showed garlic intake had a more significant
effect (OR=0.50, 95% CI=0.39-0.64) (Fig. S2).
We found that among those 11 included studies, 2
studies (36,39) containing 126,976 subjects showed
that garlic intake had no significant association with the
incidence of gastric cancer (OR=1.36, 95% CI=0.93-1.99), while 9
studies (13,18,34,35,37,38,40,41,47)
containing 9,944 subjects showed that garlic intake could
significantly reduce the incidence of gastric cancer (OR=0.54, 95%
CI=0.41-0.70) (P<0.05) (Fig.
3).
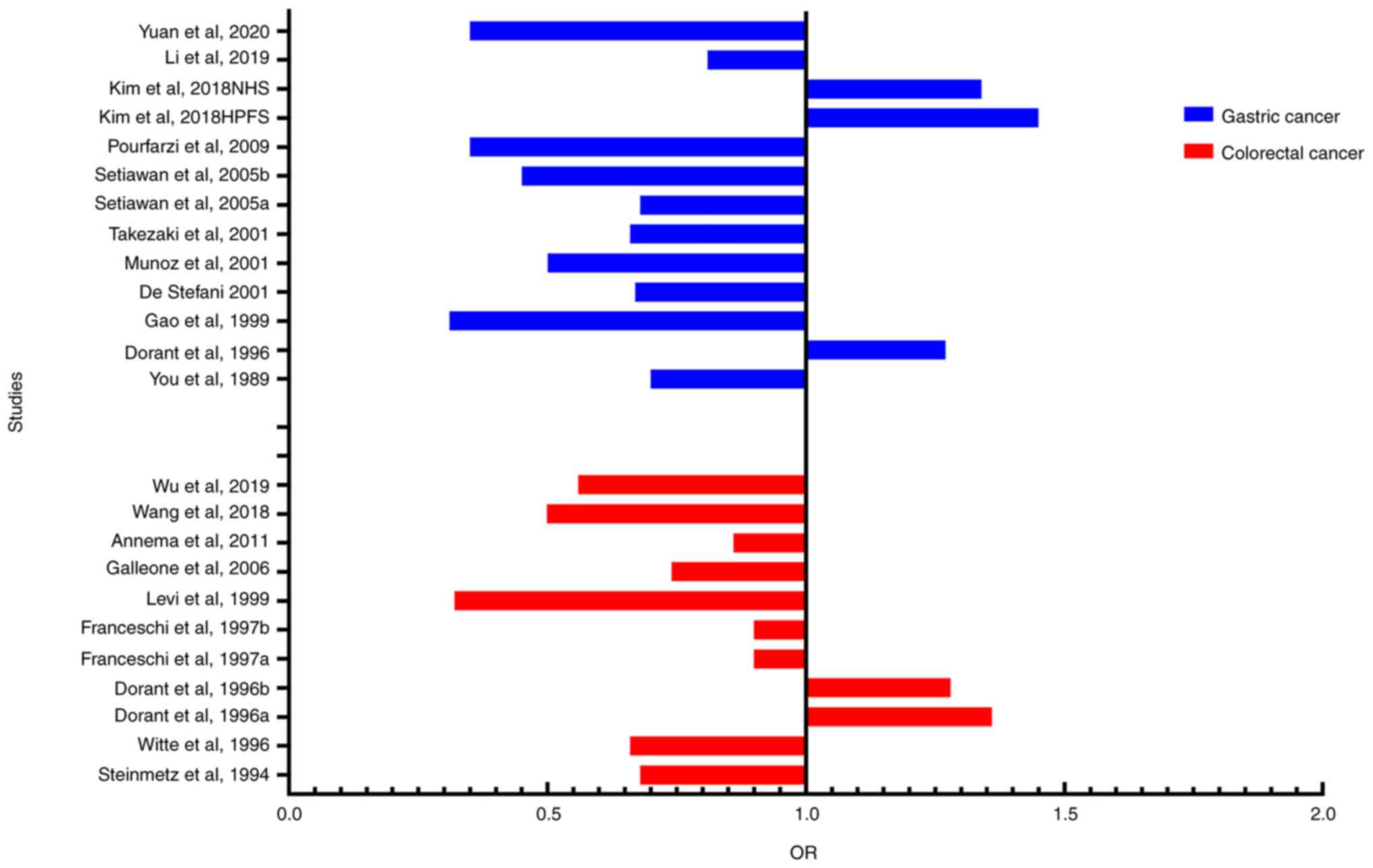
 | Figure 3.Positive and negative association
studies between garlic intake and gastric cancer. The
(number/number) after each study in the figure indicates the
(Cases/Controls), Vertical solid black line: invalid line; red
dashed line: pooled effect size; horizontal black solid line: the
width of the line represents the confidence interval (CI) of each
study, the black diamond in the middle represents the OR of each
study, and the gray square represents the weight of each study.
Among the 11 included studies, 2 studies showed that garlic intake
had no association with the incidence of gastric cancer (OR=1.36,
95% CI=0.93-1.99), including 12,6976 subjects, and 9 studies showed
that garlic intake could reduce the incidence of gastric cancer
(OR=0.54, 95% CI=0.41-0.70), including 9,944 subjects. OR, odds
ratio; ES, effect size. |
A total of 9 studies estimated the association
between garlic intake and the risk of colorectal cancer (Fig. 2B). The meta-analysis using the
random-effects model showed a combined estimated OR of 0.75 (95%
CI=0.65-0.87, P<0.001), suggesting that garlic intake could
significantly reduce the risk of colorectal cancer. Among the 9
included research studies, only Dorant et al (42) and Franceschsi et al
(46) estimated the OR values for
colon cancer and rectal cancer separately, without providing the
total OR value.
Compared to the retrospective studies (OR=0.72, 95%
CI=0.62-0.84, P<0.001), the results of the prospective study
(OR=1.01, 95% CI=0.62-1.65, P<0.1) showed an insignificant
effect of garlic intake on reducing the risk of colorectal cancer
(Table SV; Fig. S3). Subgroup analyses of
geographical regions (Fig. S4)
showed that garlic intake significantly reduced the risk of
colorectal cancer in Asia compare to other regions.
Heterogeneity assessment and
sensitivity analysis
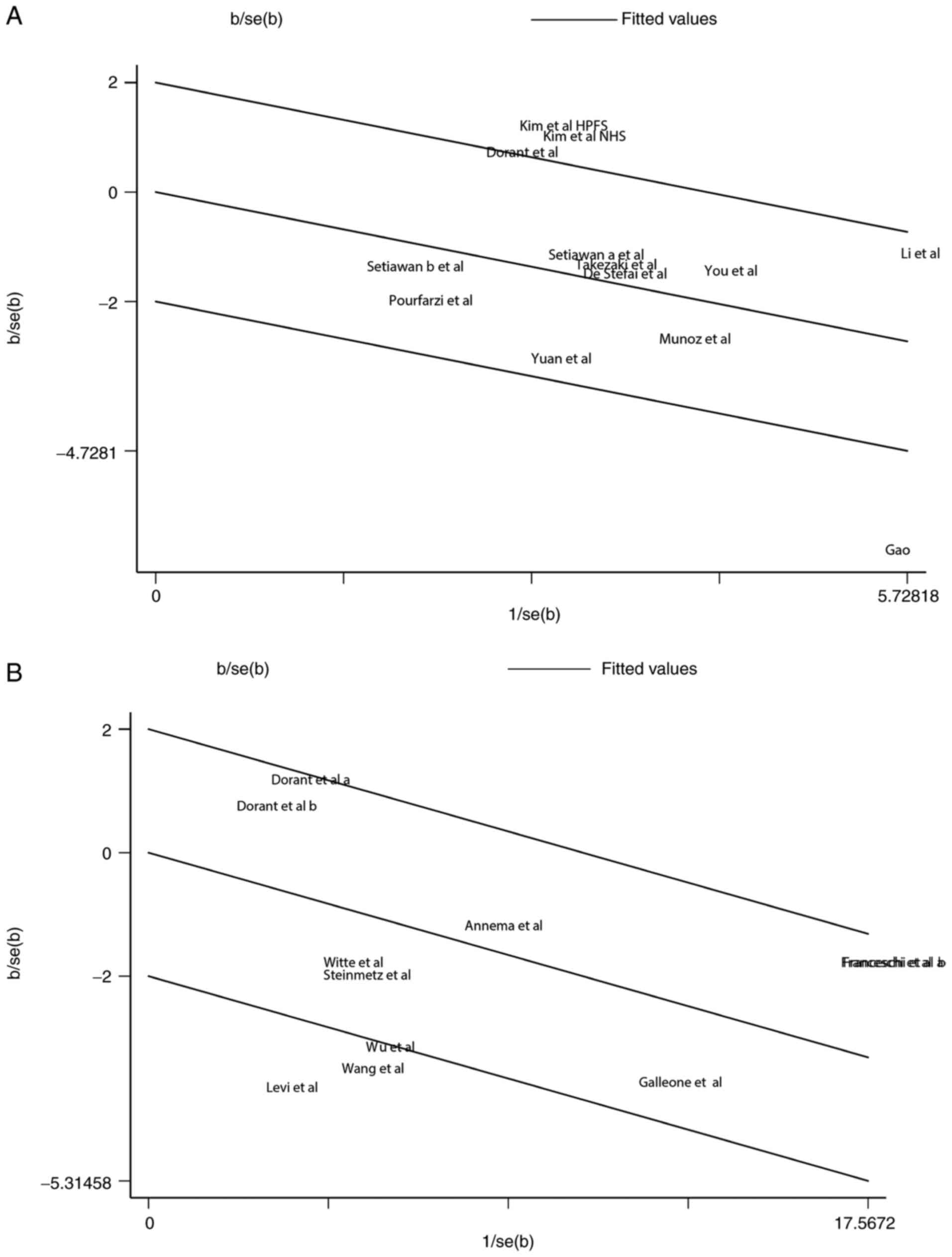
The random effect model suggested a strong
heterogeneity with I2=69.8%, and P<0.1 in the studies
of garlic and gastric cancer which were selected for the
meta-analysis. Therefore, we conducted Galbraith test to further
identify the source of heterogeneity. The result of Galbraith test
showed that the studies of Gao et al (40) and Kim et al (39) were the main sources of
heterogeneity (Fig. 4A). The
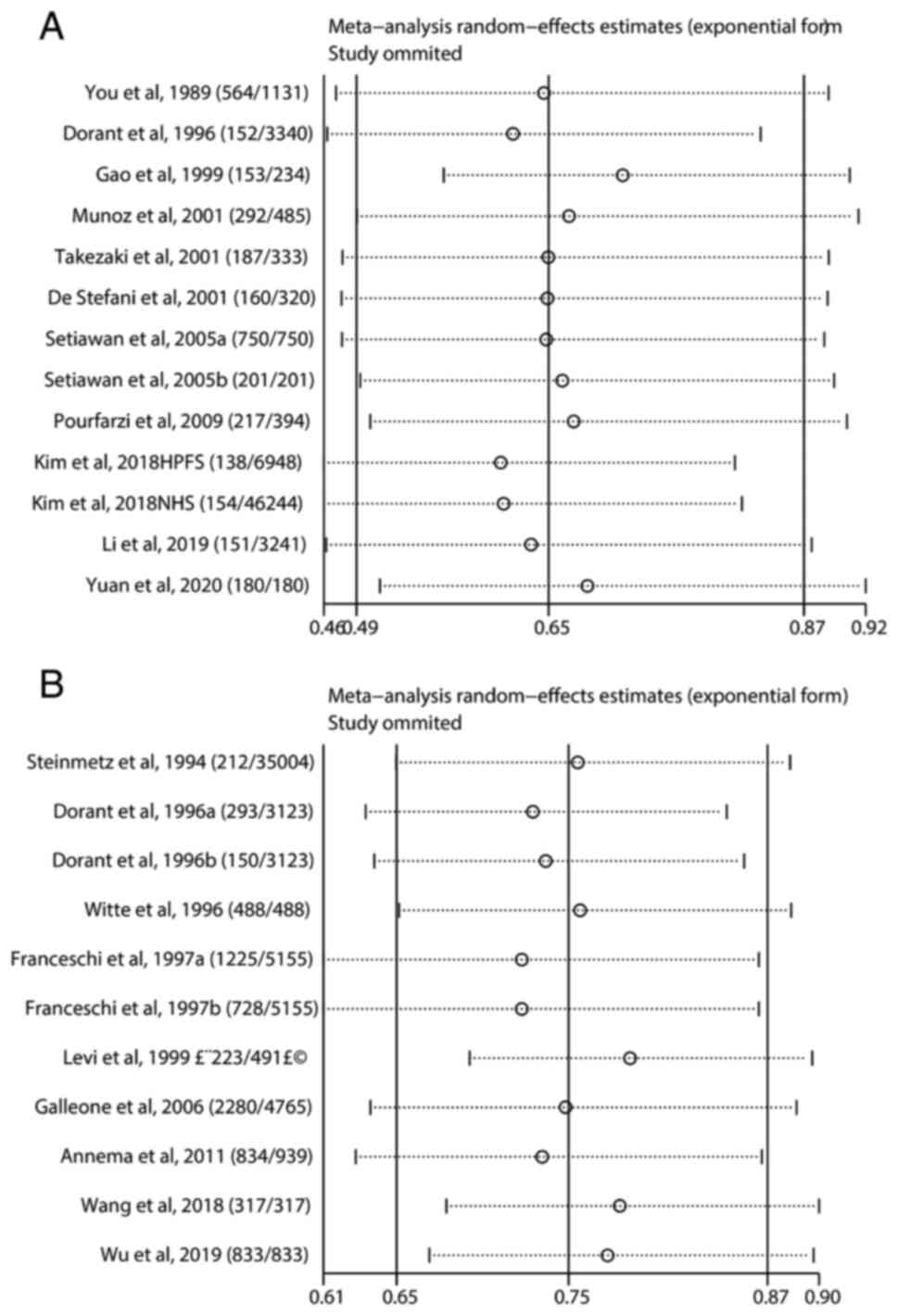
result of sensitivity analysis showed that our results were stable,
and there was no significant difference in the pooled results
(Fig. 5A).
For the 9 studies of garlic and colorectal cancer, a
significant heterogeneity was also suggested (I2=71.4%,
P<0.001). According to the results of Galbraith test, three
studies [Wu et al (12),
Levi et al (44) and Wang
et al (43)] were indicated
as the main sources of heterogeneity (Fig. 4B). Sensitivity analysis was used to
estimate the impact of each study on the overall estimate (Fig. 5B). The results of the sensitivity
analysis showed that no articles exceeded the limits and there were
no significant differences among the studies. Our meta-analysis
suggested that garlic can reduce the risk of gastrointestinal
cancers, and most of the included studies are consistent with this
conclusion (Fig. 6).
Risk of bias assessment
A randomized controlled trial evaluated by the
Cochrane risk assessment tool was rated as low risk, and the
non-randomized controlled trials were scored using a NOS scale, as
shown in Table SVI and Table SVII.
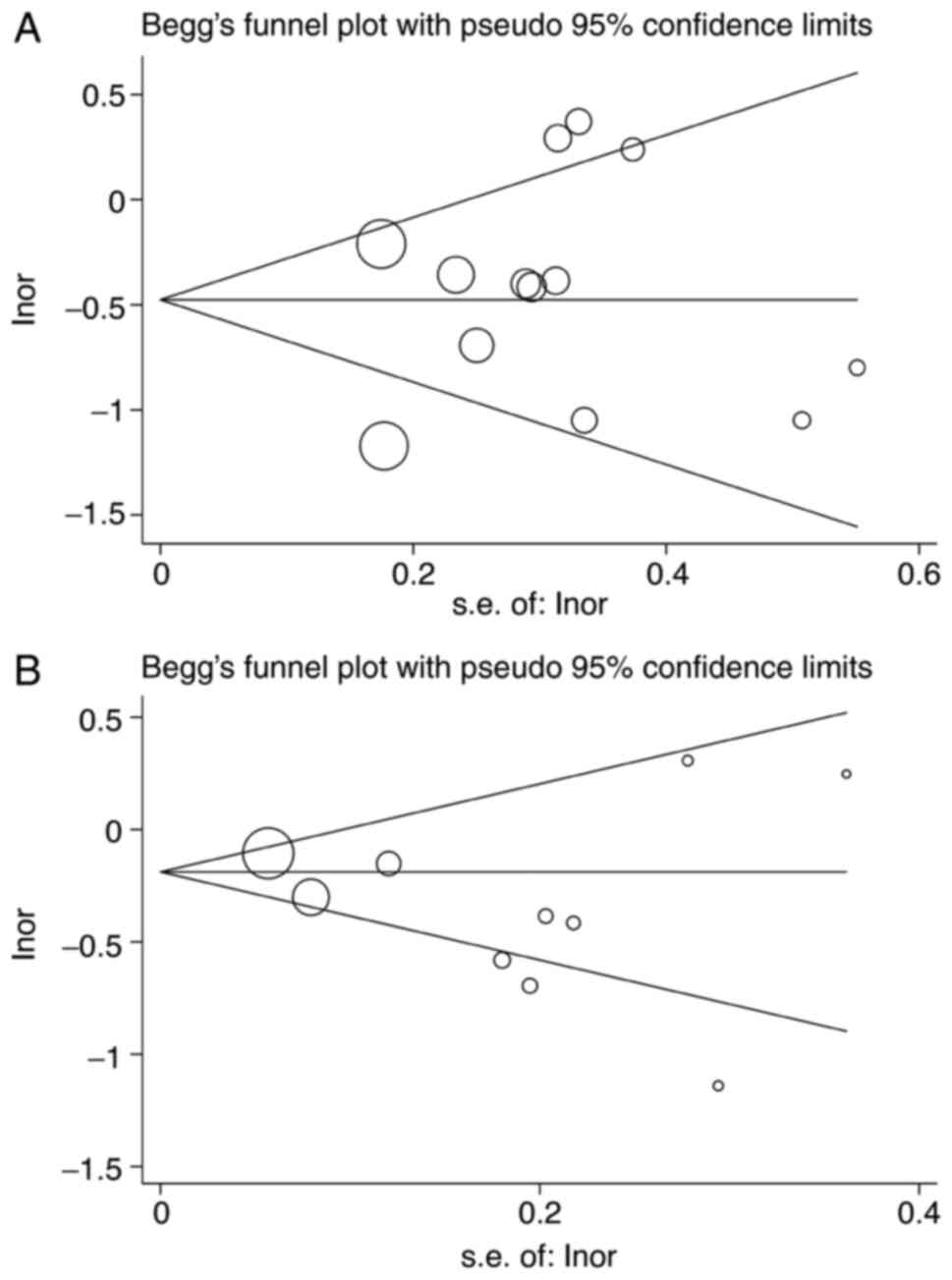
Publication bias
Potential publication bias was assessed using the
Begg (Fig. 7) and Egger tests
(Table SVIII). There was no
significant evidence of publication bias for gastric and colorectal
cancers.
Discussion
This meta-analysis combined the results of 20
studies regarding the association of garlic consumption with
gastric cancer (11 studies) and colorectal cancer (9 studies). Our
results indicated that garlic intake significantly reduces the risk
of gastric cancer (OR=0.65, 95% CI=0.49-0.87, P<0.001) and
colorectal cancer (OR=0.75, 95% CI=0.65-0.87, P<0.001),
consistent with the epidemiological evidence supporting the
correlation between garlic intake and a reduced risk of gastric and
colorectal cancer. The results of the geographical subgroup
analysis showed that a greater risk reduction occurs in the Asian
region compared with other geographical regions. We suspect one of
the possible reasons is that garlic consumption is higher in Asia,
especially in China, where the habit of eating raw garlic leads to
a higher consumption than other countries in the world (51,52).
Some studies have also analyzed the effects of allium and onion on
gastrointestinal tumors. We speculate that the active ingredients
may be the same or similar to garlic, and that these foods may have
a superimposed effect on gastrointestinal cancers (12,18,38,40,41).
In addition, the European population may be under-represented since
there was only one study conducted in Europe. Our meta-analysis
incorporated the results of the latest research by Li et al
(13) and summarized the recent
studies. Although most of the included studies were retrospective
case-control studies, lacking blinding and randomized control
(53), we still found that garlic
intake was associated with a reduced risk of gastric and colorectal
cancer. Compared to the previous meta-analysis on the relationship
between garlic and gastric and colorectal cancer by Fleischauer
et al (28), our
meta-analysis included more studies and conducted a subgroup
analysis with a focus on garlic intake, resulting in more reliable
conclusions. Due to the various dietary patterns in the different
studies, we cannot exclude the effects of other factors, such as
vegetable and fiber intake. Additionally, each study had different
confounding factors, and most studies adjusted them, such as sex,
age, and others. From the results of the subgroup analysis, it was
shown that the intake of garlic can reduce the likelihood of
gastric cancer compared with the non-intake of garlic. Although a
previous meta-analysis by others showed that the protective effect
of garlic on gastric and colorectal cancer may be overestimated
(28), the results of the
comprehensive analysis in this study indicated the preventive
function of garlic in gastrointestinal tumors.
A further review was conducted on the molecular
mechanisms of the anticancer effects of garlic (Fig. 8). Based on previous literature,
garlic contains a variety of organic sulfur compounds, mainly
including S-allylmercaptocysteine (SAMC), diallyl disulfide (DADS),
diallyl trisulfide (DATS) and allicin, which are the main
components which produce potential antitumor effects. We searched
the Pubmed database with key terms including ‘gastrointestinal
tumors’, ‘garlic’, ‘mechanism’, ‘pathways’, and reviewed biological
functions of these four organic sulfur compounds. These organic
sulfur compounds demonstrate potential antitumor activity through
various underlying mechanisms. First, organic sulfur compounds can
regulate the cell cycle. DADS and DATS can activate the P53/P21
pathway, while DADS can inhibit the expression of cyclin B1, cdc2,
and cdc25c proteins, leading to G2/M phase arrest in tumor cells
(54,55). SAMC and DADS can inhibit the
polymerization of tubulin and thus affect the function of the
spindle, resulting in mitotic arrest (56). In addition, allicin induces cell
cycle arrest in the S phase of the cell cycle (57). Second, organic sulfides can induce
cell apoptosis in the following ways. DADS, DATS, SAMC and allicin
promote the release of cytochrome c from mitochondria,
thereby activating caspase family proteins, such as caspase 3 and
caspase 9, and inducing apoptosis (23,56,58–62);
DADS, DATS and SAMC can activate the p53 pathway, resulting in the
decreased expression of Bcl-2 and increased expression of Bax
(54,58,63–65);
SAMC and DATS significantly activate three pathways of the MAPKs
pathway, including ERK, JNK and p38 (63,64);
Moreover, DATS can significantly upregulate the level of glycogen
synthase kinase 3 β (GSK3β) to increase the digestion of β-catenin,
indicating that DATS can inhibit the Wnt/β-catenin pathway, a key
component in the occurrence and development of tumors. DATS can
also increase reactive oxygen species (ROS) production and activate
the AMPK pathway (23,55); Allicin can reduce phosphorylated
signal transducer and activator of transcription 3 (STAT3) to
inhibit the STAT3 pathway, as well as activate Nrf2 and induce its
translocation to the nucleus (66,67).
Third, DADS can inhibit matrix metalloproteinase (MMP)-2, MMP-9,
tissue inhibitor of metalloproteinases-1 (TIMP-1), TIMP-2 and
PI3K/AKT pathways to inhibit cell metastasis (68).
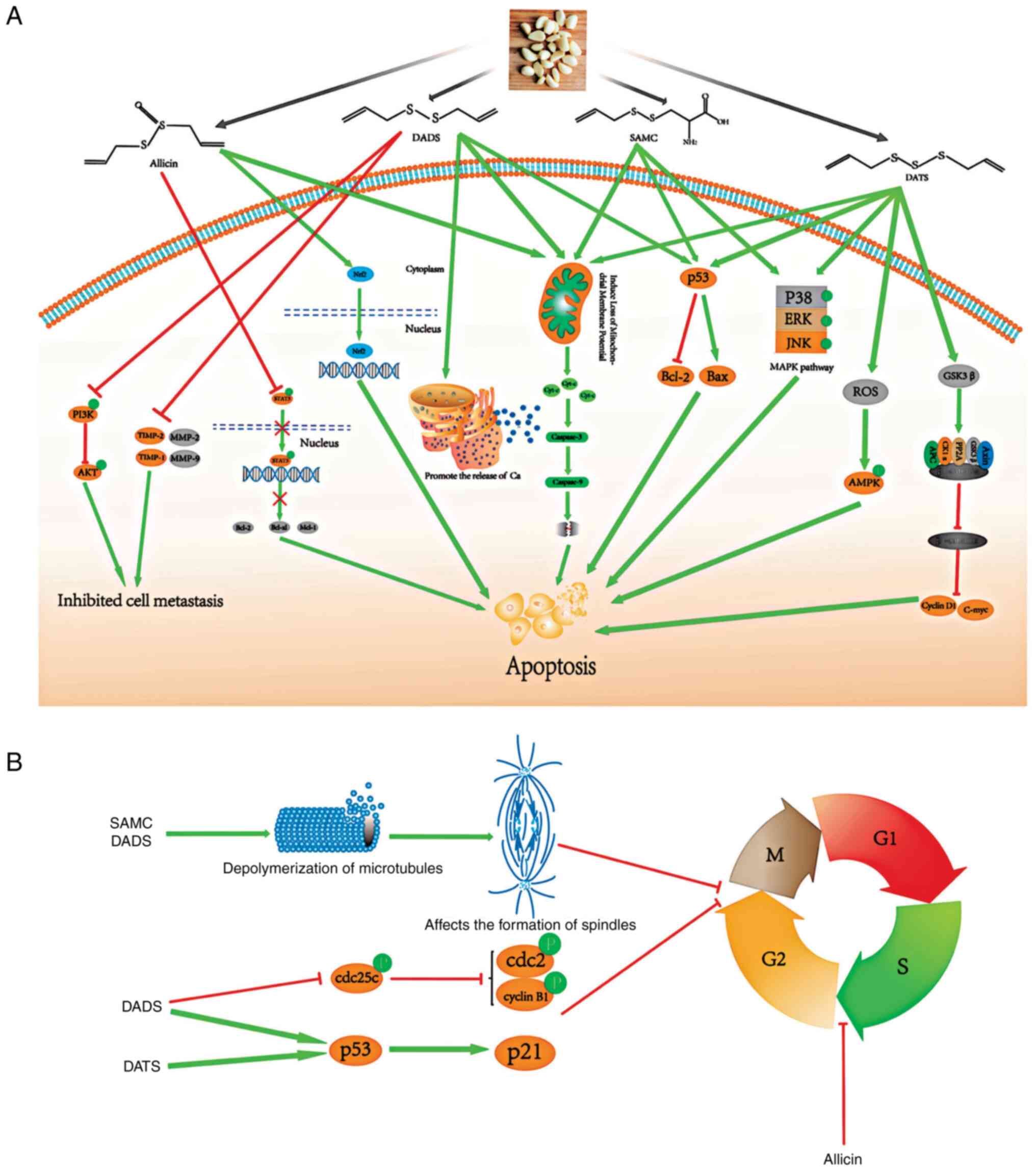 | Figure 8.Potential molecular mechanisms of the
anticancer effects of garlic. (A) DADS, DATS, SAMC and allicin can
all promote the release of cytochrome c from mitochondria,
thereby activating the caspase family proteins, such as caspase 3
and caspase 9, and inducing apoptosis; DADS, DATS and SAMC can
activate the p53 pathway, resulting in decreased expression of
Bcl-2 and increased expression of Bax. SAMC and DATS significantly
activated the three pathways of MAPKs, including ERK, JNK and p38.
In addition, the Wnt/β-catenin pathway plays a key role in the
occurrence and development of tumors, DATS can significantly
upregulate the level of GSK3β, thereby increasing the digestion of
β-catenin, indicating that DATS can inhibit the Wnt/β-catenin
pathway. DATS can also increase ROS production and activate the
AMPK pathway. Allicin can reduce phosphorylated STAT3, thereby
inhibiting the STAT3 pathway, and allicin can also activates Nrf2
and induces its translocation to the nucleus. Moreover, DADS can
inhibit MMP-2 and MMP-9. (B) DADS and DATS can activate the P53/P21
pathway, and DADS can also inhibit the expression of cyclin B1,
cdc2, and cdc25c proteins, leading to G2/M phase arrest of tumor
cells. SAMC and DADS can affect the polymerization of tubulin and
thus affect the function of the spindle, leading to mitotic arrest.
Finally, allicin induces cell cycle arrest in the S phase. DADS,
diallyl disulfide; DATS, diallyl trisulfide; SAMC,
S-allylmercaptocysteine; GSK3β, glycogen synthase kinase 3 β; ROS,
reactive oxygen species; STAT3, signal transducer and activator of
transcription 3; MMP, matrix metalloproteinase. |
To summarize, DADS, DATS, SAMC, and allicin
participate in tumor-related biological process through various
mechanisms, eventually leading to apoptosis, cell cycle arrest, and
migration inhibition in tumor cells. A medical compound containing
active ingredients from garlics may exert potential tumor
preventive or therapeutic effects through the above-mentioned
mechanisms in the human body, representing a novel antitumor
treatment alternative.
This meta-analysis has the following limitations. i)
Only a small set of randomized controlled trials are included in
the date, most of which are case-control and cohort studies.
Compared with randomized controlled trials, case-control and cohort
studies have more unaccounted parameters in blind control and
follow-up, resulting in higher propensity of bias. ii) This
meta-analysis included studies conducted in different countries
since the 1990s. Not all studies were primarily based on onion
vegetables, and there was inconsistent stratification among the
studies. iii) Most of the included studies were conducted in China,
where the incidence of gastric cancer is generally higher than the
rest of the world. Moreover, garlic intake is relatively high in
the diet of Chinese people. iv) Many studies did not control other
diets, and the type of garlic consumption remains unstandardized.
It is difficult to determine the minimum garlic intake for a
tumor-protective effect. The minimum and maximum consumption levels
varied greatly among the different studies.
The quantified I2 test showed that the
included studies had significant heterogeneity, and Galbraith test
suggested that some studies might be the sources. Therefore, we
explored the possible cause for the heterogeneity. First, most of
the included studies were retrospective studies with various
confounding factors, and recall bias may have produced different
results from the prospective studies. Second, most studies had
collected data in the form of questionnaires instead of objective
measurement. Third, studies conducted in Asia, especially in China,
where garlic is a highly consumed food, may lead to certain bias on
the results when pooled together with studies conducted in other
places with much lower garlic consumption.
In summary, our meta-analysis provides strong
evidence that garlic can reduce the risk of gastric and colorectal
cancers. The conclusion was mainly based on case-control studies
with many potential confounders, and further research is warranted
to validate it.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present work was financially supported by grants from the
National Natural Science Foundation of China [32000098, 31671468],
the China Postdoctoral Science Foundation [2020M682167,
2020T130070ZX], the Science and Technology Development Plan of
Jinan Municipal Health Commission [2020-3-09], Jinan 2020 Science
and Technology Innovation Development Plan [202019035], and the
Academic Promotion Programme of Shandong First Medical University
[2019QL024].
Availability of data and materials
The study was registered in PROSPERO
(CRD42020179464).
Authors' contributions
HJL designed the review and meta-analysis. YYW and
HJL conceived and wrote the review. YFW and DRL acquired and
analysed the data. MYJ and PH analyzed and confirmed the integrity
of the data found in the literature. YSW was involved in drafting
the manuscript. All authors contributed to the analysis, reviewed
the results and read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Authors' information
ORCID: Huanjie Li, orcid.org/0000-0002-4997-0927;
Yunshan Wang, orcid.org/0000-0003-3767-6728.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gausman V, Dornblaser D, Anand S, Hayes
RB, O'Connell K, Du M and Liang PS: Risk factors associated with
early-onset colorectal cancer. Clin Gastroenterol Hepatol.
18:2752–2759.e2. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou X, Qian H, Zhang D and Zeng L: Garlic
intake and the risk of colorectal cancer: A meta-analysis. Medicine
(Baltimore). 99:e185752020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Larsson SC, Orsini N and Wolk A: Processed
meat consumption and stomach cancer risk: A meta-analysis. J Natl
Cancer Inst. 98:1078–1087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sugano K: Effect of Helicobacter pylori
eradication on the incidence of gastric cancer: A systematic review
and meta-analysis. Gastric Cancer. 22:435–445. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park HK, Lee Y, Kang BW, Kwon KI, Kim JW,
Kwon OS, Cobb LK, Campbell NRC, Blakeman DE and Kim CI: Progress on
sodium reduction in South Korea. BMJ Glob Health. 5:e0020282020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee HS, Duffey KJ and Popkin BM: Sodium
and potassium intake patterns and trends in South Korea. J Hum
Hypertens. 27:298–303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ford AC, Yuan Y and Moayyedi P:
Helicobacter pylori eradication therapy to prevent gastric cancer:
Systematic review and meta-analysis. Gut. 69:2113–2121. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hooi JKY, Lai WY, Ng WK, Suen MMY,
Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu
JCY, et al: Global prevalence of Helicobacter pylori infection:
Systematic review and meta-Analysis. Gastroenterology. 153:420–429.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hou N, Huo D and Dignam JJ: Prevention of
colorectal cancer and dietary management. Chin Clin Oncol.
2:132013.PubMed/NCBI
|
|
12
|
Wu X, Shi J, Fang WX, Guo XY, Zhang LY,
Liu YP and Li Z: Allium vegetables are associated with reduced risk
of colorectal cancer: A hospital-based matched case-control study
in China. Asia Pac J Clin Oncol. 15:e132–e141. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li WQ, Zhang JY, Ma JL, Li ZX, Zhang L,
Zhang Y, Guo Y, Zhou T, Li JY, Shen L, et al: Effects of
Helicobacter pylori treatment and vitamin and garlic
supplementation on gastric cancer incidence and mortality:
Follow-up of a randomized intervention trial. BMJ. 366:l50162019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Ying X, Shan F and Ji J: The
association of garlic with helicobacter pylori infection and
gastric cancer risk: A systematic review and meta-analysis.
Helicobacter. 23:e125322018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nicastro HL, Ross SA and Milner JA: Garlic
and onions: Their cancer prevention properties. Cancer Prev Res
(Phila). 8:181–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Desai G, Schelske-Santos M, Nazario CM,
Rosario-Rosado RV, Mansilla-Rivera I, Ramírez-Marrero F, Nie J,
Myneni AA, Zhang ZF, Freudenheim JL and Mu L: Onion and garlic
intake and breast cancer, a case-control study in Puerto Rico. Nutr
Cancer. 72:791–800. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chan JM, Wang F and Holly EA: Vegetable
and fruit intake and pancreatic cancer in a population-based
case-control study in the San Francisco bay area. Cancer Epidemiol
Biomarkers Prev. 14:2093–2097. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takezaki T, Gao CM, Wu JZ, Ding JH, Liu
YT, Zhang Y, Li SP, Su P, Liu TK and Tajima K: Dietary protective
and risk factors for esophageal and stomach cancers in a
low-epidemic area for stomach cancer in Jiangsu Province, China:
Comparison with those in a high-epidemic area. Jpn J Cancer Res.
92:1157–1165. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
You WC, Chang YS, Heinrich J, Ma JL, Liu
WD, Zhang L, Brown LM, Yang CS, Gail MH, Fraumeni JF Jr and Xu GW:
An intervention trial to inhibit the progression of precancerous
gastric lesions: Compliance, serum micronutrients and S-allyl
cysteine levels, and toxicity. Eur J Cancer Prev. 10:257–263. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang HR, Pan YM and Zhang L: Mechanism for
allicin to sensitize gastric cancer cells to chemotherapy. World
Chinese J Digestol. 27:1248–1255. 2019. View Article : Google Scholar
|
|
21
|
Wu S and Li Z: Effect of allicin on the
proliferation and apoptosis of gastric cancer cells SGC-7901
through inhibiting autophagy of endoplasmic reticulum. Anti-Tumor
Pharmacy. 9:226–230. 2019.
|
|
22
|
Arreola R, Quintero-Fabián S, López-Roa
RI, Flores-Gutiérrez EO, Reyes-Grajeda JP, Carrera-Quintanar L and
Ortuño-Sahagún D: Immunomodulation and anti-inflammatory effects of
garlic compounds. J Immunol Res. 2015:4016302015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu CS, Huang AC, Lai KC, Huang YP, Lin MW,
Yang JS and Chung JG: Diallyl trisulfide induces apoptosis in human
primary colorectal cancer cells. Oncol Rep. 28:949–954. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu B, Zou L, Qi L, Zhong R and Miao X:
Allium vegetables and garlic supplements do not reduce risk of
colorectal cancer, based on meta-analysis of prospective studies.
Clin Gastroenterol Hepatol. 12:1991.2001.e1-4; quiz e121.
2014.PubMed/NCBI
|
|
25
|
Turati F, Guercio V, Pelucchi C, La
Vecchia C and Galeone C: Colorectal cancer and adenomatous polyps
in relation to allium vegetables intake: A meta-analysis of
observational studies. Mol Nutr Food Res. 58:1907–1914. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kodali RT and Eslick GD: Meta-analysis:
Does garlic intake reduce risk of gastric cancer? Nutr Cancer.
67:1–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu JY, Hu YW, Zhou JJ, Zhang MW, Li D and
Zheng S: Consumption of garlic and risk of colorectal cancer: An
updated meta-analysis of prospective studies. World J
Gastroenterol. 20:15413–15422. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fleischauer AT, Poole C and Arab L: Garlic
consumption and cancer prevention: Meta-analyses of colorectal and
stomach cancers. Am J Clin Nutr. 72:1047–1052. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chiavarini M, Minelli L and Fabiani R:
Garlic consumption and colorectal cancer risk in man: A systematic
review and meta-analysis. Public Health Nutr. 19:308–317. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Turati F, Pelucchi C, Guercio V, La
Vecchia C and Galeone C: Allium vegetable intake and gastric
cancer: A case-control study and meta-analysis. Mol Nutr Food Res.
59:171–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meng S, Zhang X, Giovannucci EL, Ma J,
Fuchs CS and Cho E: No association between garlic intake and risk
of colorectal cancer. Cancer Epidemiol. 37:152–155. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
De Stefani E, Correa P, Boffetta P, Ronco
A, Brennan P, Deneo-Pellegrini H and Mendilaharsu M: Plant foods
and risk of gastric cancer: A case-control study in Uruguay. Eur J
Cancer Prev. 10:357–364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Setiawan VW, Yu GP, Lu QY, Lu ML, Yu SZ,
Mu L, Zhang JG, Kurtz RC, Cai L, Hsieh CC and Zhang ZF: Allium
vegetables and stomach cancer risk in China. Asian Pac J Cancer
Prev. 6:387–395. 2005.PubMed/NCBI
|
|
36
|
Dorant E, van den Brandt PA, Goldbohm RA
and Sturmans F: Consumption of onions and a reduced risk of stomach
carcinoma. Gastroenterology. 110:12–20. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Muñoz N, Plummer M, Vivas J, Moreno V, De
Sanjosé S, Lopez G and Oliver W: A case-control study of gastric
cancer in Venezuela. Int J Cancer. 93:417–423. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
You WC, Blot WJ, Chang YS, Ershow A, Yang
ZT, An A, Henderson BE, Fraumeni JF Jr and Wang TG: Allium
vegetables and reduced risk of stomach cancer. J Natl Cancer Inst.
81:162–164. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim H, Keum N, Giovannucci EL, Fuchs CS
and Bao Y: Garlic intake and gastric cancer risk: Results from two
large prospective US cohort studies. Int J Cancer. 143:1047–1053.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao CM, Takezaki T, Ding JH, Li MS and
Tajima K: Protective effect of allium vegetables against both
esophageal and stomach cancer: A simultaneous case-referent study
of a high-epidemic area in Jiangsu Province, China. Jpn J Cancer
Res. 90:614–621. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pourfarzi F, Whelan A, Kaldor J and
Malekzadeh R: The role of diet and other environmental factors in
the causation of gastric cancer in Iran-A population based study.
Int J Cancer. 125:1953–1960. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dorant E, van den Brandt PA and Goldbohm
RA: A prospective cohort study on the relationship between onion
and leek consumption, garlic supplement use and the risk of
colorectal carcinoma in The Netherlands. Carcinogenesis.
17:477–484. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang W, Dong Z, Zhang X, Li W, Li P and
Chen X: Dietary and the risk of sporadic colorectal cancer in
China: A case-control study. Iran J Public Health. 47:1327–1335.
2018.PubMed/NCBI
|
|
44
|
Levi F, Pasche C, La Vecchia C, Lucchini F
and Franceschi S: Food groups and colorectal cancer risk. Br J
Cancer. 79:1283–1287. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Steinmetz KA, Kushi LH, Bostick RM, Folsom
AR and Potter JD: Vegetables, fruit, and colon cancer in the Iowa
women's health study. Am J Epidemiol. 139:1–15. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Franceschi S, Parpinel M, La Vecchia C,
Favero A, Talamini R and Negri E: Role of different types of
vegetables and fruit in the prevention of cancer of the colon,
rectum, and breast. Epidemiology. 9:338–341. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yuan P, Lin L, Zheng K, Wang W, Wu S,
Huang L, Wu B, Chen T, Li X and Cai L: Risk factors for gastric
cancer and related serological levels in Fujian, China:
hospital-based case-control study. BMJ Open. 10:e0423412020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Galeone C, Pelucchi C, Levi F, Negri E,
Franceschi S, Talamini R, Giacosa A and La Vecchia C: Onion and
garlic use and human cancer. Am J Clin Nutr. 84:1027–1032. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Witte JS, Longnecker MP, Bird CL, Lee ER,
Frankl HD and Haile RW: Relation of vegetable, fruit, and grain
consumption to colorectal adenomatous polyps. Am J Epidemiol.
144:1015–1025. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Annema N, Heyworth JS, McNaughton SA,
Iacopetta B and Fritschi L: Fruit and vegetable consumption and the
risk of proximal colon, distal colon, and rectal cancers in a
case-control study in Western Australia. J Am Diet Assoc.
111:1479–1490. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Myneni AA, Chang SC, Niu R, Liu L, Swanson
MK, Li J, Su J, Giovino GA, Yu S, Zhang ZF and Mu L: Raw garlic
consumption and lung cancer in a Chinese population. Cancer
Epidemiol Biomarkers Prev. 25:624–633. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shi X, Lv Y, Mao C, Yuan J, Yin Z, Gao X
and Zhang Z: Garlic consumption and all-cause mortality among
Chinese oldest-old individuals: A population-based cohort study.
Nutrients. 11:15042019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Powell JT and Sweeting MJ: Retrospective
studies. Eur J Vasc Endovasc Surg. 50:6752015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yin X, Zhang R, Feng C, Zhang J, Liu D, Xu
K, Wang X, Zhang S, Li Z, Liu X and Ma H: Diallyl disulfide induces
G2/M arrest and promotes apoptosis through the p53/p21 and MEK-ERK
pathways in human esophageal squamous cell carcinoma. Oncol Rep.
32:1748–1756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Choi YH: Diallyl trisulfide induces
apoptosis and mitotic arrest in AGS human gastric carcinoma cells
through reactive oxygen species-mediated activation of
AMP-activated protein kinase. Biomed Pharmacother. 94:63–71. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xiao D, Pinto JT, Gundersen GG and
Weinstein IB: Effects of a series of organosulfur compounds on
mitotic arrest and induction of apoptosis in colon cancer cells.
Mol Cancer Ther. 4:1388–1398. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shang A, Cao SY, Xu XY, Gan RY, Tang GY,
Corke H, Mavumengwana V and Li HB: Bioactive compounds and
biological functions of garlic (Allium sativum L.). Foods.
8:2462019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yin X, Zhang J, Li X, Liu D, Feng C, Liang
R, Zhuang K, Cai C, Xue X, Jing F, et al: DADS suppresses human
esophageal xenograft tumors through RAF/MEK/ERK and
mitochondria-dependent pathways. Int J Mol Sci. 15:12422–12441.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang W, Ha M, Gong Y, Xu Y, Dong N and
Yuan Y: Allicin induces apoptosis in gastric cancer cells through
activation of both extrinsic and intrinsic pathways. Oncol Rep.
24:1585–1592. 2010.PubMed/NCBI
|
|
60
|
Zhang X, Zhu Y, Duan W, Feng C and He X:
Allicin induces apoptosis of the MGC-803 human gastric carcinoma
cell line through the p38 mitogen-activated protein
kinase/caspase-3 signaling pathway. Mol Med Rep. 11:2755–2760.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Park SY, Cho SJ, Kwon HC, Lee KR, Rhee DK
and Pyo S: Caspase-independent cell death by allicin in human
epithelial carcinoma cells: Involvement of PKA. Cancer Lett.
224:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Xiao D, Pinto JT, Soh JW, Deguchi A,
Gundersen GG, Palazzo AF, Yoon JT, Shirin H and Weinstein IB:
Induction of apoptosis by the garlic-derived compound
S-allylmercaptocysteine (SAMC) is associated with microtubule
depolymerization and c-Jun NH(2)-terminal kinase 1 activation.
Cancer Res. 63:6825–6837. 2003.PubMed/NCBI
|
|
63
|
Jiang X, Zhu X, Huang W, Xu H, Zhao Z, Li
S, Li S, Cai J and Cao J: Garlic-derived organosulfur compound
exerts antitumor efficacy via activation of MAPK pathway and
modulation of cytokines in SGC-7901 tumor-bearing mice. Int
Immunopharmacol. 48:135–145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhu X, Jiang X, Li A, Sun Y, Liu Y, Sun X,
Feng X, Li S and Zhao Z: S-allylmercaptocysteine suppresses the
growth of human gastric cancer xenografts through induction of
apoptosis and regulation of MAPK and PI3K/Akt signaling pathways.
Biochem Biophys Res Commun. 491:821–826. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yan JY, Tian FM, Hu WN, Zhang JH, Cai HF
and Li N: Apoptosis of human gastric cancer cells line SGC 7901
induced by garlic-derived compound S-allylmercaptocysteine (SAMC).
Eur Rev Med Pharmacol Sci. 17:745–751. 2013.PubMed/NCBI
|
|
66
|
Li X, Ni J, Tang Y, Wang X, Tang H, Li H,
Zhang S and Shen X: Allicin inhibits mouse colorectal tumorigenesis
through suppressing the activation of STAT3 signaling pathway. Nat
Prod Res. 33:2722–2725. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bat-Chen W, Golan T, Peri I, Ludmer Z and
Schwartz B: Allicin purified from fresh garlic cloves induces
apoptosis in colon cancer cells via Nrf2. Nutr Cancer. 62:947–957.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yin X, Feng C, Han L, Ma Y, Jiao Y, Wang
J, Jia L, Jing F, Gao X, Zhang Y and Zhang J: Diallyl disulfide
inhibits the metastasis of type II esophageal-gastric junction
adenocarcinoma cells via NF-κB and PI3K/AKT signaling pathways
in vitro. Oncol Rep. 39:784–794. 2018.PubMed/NCBI
|