Introduction
According to cancer statistics from the American
Cancer Society, pancreatic cancer is characterized by the lowest
5-year survival rate (10%) among all cancers (1). In addition, early diagnosis is
extremely difficult due to the nonspecific symptoms of pancreatic
cancer (2). Notably, 80–85% of
patients with local invasion and distant metastasis at the
diagnosis of pancreatic cancer receive systemic therapy with
gemcitabine (3). Gemcitabine
(GEM), a deoxycytidine nucleotide analog, is a typical first-line
chemotherapeutic drug for pancreatic cancer treatment.
Unfortunately, GEM resistance frequently occurs in patients with
pancreatic cancer, which results in a high incidence, high
mortality, and substantial disease burden in patients (4,5). In
addition, a 5-year survival rate below 5% is found in patients with
drug-resistant and metastatic pancreatic cancer, suggesting that
drug resistance is a critical problem that urgently needs to be
solved (6). The available evidence
described above indicates that a late diagnosis and high
therapeutic resistance are the major challenges in pancreatic
cancer treatment. Thus, the development of a new strategy designed
to enhance GEM chemosensitivity may be beneficial for pancreatic
cancer treatment.
Taiwanofungus camphoratus (Antrodia
cinnamomea), often referred to as ‘Niu-chang-chih’, is a
medicinal fungus that only grows on the native tree Cinnamomum
kanehirai Hayata in Taiwan (7). Several bioactive compounds are
present in Taiwanofungus camphoratus, including
polysaccharides, triterpenes, diterpenes, succinic acid
derivatives, and ubiquinone (7).
4-Acetylantroquinonol B (4-AAQB) is the ubiquinone derivative of
Taiwanofungus camphoratus, which is identified as the major
antiproliferative compound for hepatoma cells (8). In addition, 4-AAQB is known to
participate in modulating many physiological and pathological
processes, such as anti-inflammatory and antioxidant ability
(9), amelioration of nonalcoholic
steatohepatitis (9) and inhibition
of osteoclast formation (10),
breast cancer (11), glioblastoma
(12), and hepatic cancer stem
cell tumorigenicity (13).
High mobility group box 1 (HMGB1) and the receptor
for advanced glycation end products (RAGE) are crucial for tumor
progression, drug resistance, and metastasis in pancreatic
adenocarcinoma (14,15). Accumulating evidence suggests that
RAGE expression is elevated in pancreatic cancer and pancreatic
cancer cell lines but not in adjacent normal epithelial tissue
(16,17). Moreover, our previous study found
that RAGE/HMGB1 upregulates the expression of MDR1 in GEM-resistant
MiaPaCa-2 (MiaPaCa-2GEMR) cells (18–20).
This indicates that RAGE/HMGB1 plays a crucial role in pancreatic
cancer progression and chemoresistance. However, the roles of
4-AAQB in suppressing pancreatic cancer and enhancing GEM
chemosensitivity remain unclear. The aim of this study was to
investigate the underlying mechanisms of cytotoxicity and enhanced
chemosensitivity mediated by 4-AAQB treatment in human pancreatic
cancer MiaPaCa-2 and MiaPaCa-2GEMR cells.
Materials and methods
Chemicals
4-Acetylantroquinonol B (4-AAQB) was kindly provided
by Grape King Bio Ltd. Sodium bicarbonate (NaHCO3),
2′,7′-dichlorofluorescein diacetate (DCFH-DA),
penicillin-streptomycin solution (PS), MTT, DMSO, TEMED, SDS,
glycine, Tris, isopropanol, Tween-20, bovine serum albumin (BSA),
Triton X-100, sodium chloride (NaCl), and GEM were purchased from
MilliporeSigma. Propidium iodide (PI) and Annexin V staining kit
(cat. no. 559763) was purchased from BD Biosciences. BCA protein
assay kit (BC03-500) was purchased from Energenesis Biomedical.
β-actin (cat. no. NB600-501), Beclin-1 (cat. no. NB110-87318),
vascular endothelial growth factor-A (cat. no. VEGFA, NB100-664),
LC3 II (cat. no. NB100-2220), and Atg5 (cat. no. NB110-53818)
antibodies were purchased from Novus Biologicals. Bax (cat. no.
5023), Bcl-xL (cat. no. 2762), PI3K (cat. no. 4292), phospho-PI3K
(cat. no. 4228), Akt (cat. no. 9272), phospho-Akt (cat. no. 9271),
HMGB1, PI3K catalytic subunit type III (Vps34; cat. no. 4263), and
MDR1 (cat. no. 6893) antibodies were obtained from Cell Signaling
Technology, Inc. The anti-RAGE (cat. no. PA5-24787) antibody was
purchased from Invitrogen (Thermo Fisher Scientific, Inc.).
Dulbecco's modified eagle medium (DMEM) high glucose medium, fetal
bovine serum (FBS), and horse serum were purchased from Gibco
(Thermo Fisher Scientific, Inc.).
Cell culture
The Homo sapiens pancreatic cancer cell line
MiaPaCa-2 was purchased from the Bioresource Collection and
Research Center (Hsinchu, Taiwan). The GEM-resistant
MiaPaCa-2GEMR cell line was established by gradually
increasing GEM concentrations to 0.5 µM GEM to induce tolerance as
described in a previous report (21). Cells were cultured in DMEM (high
glucose) medium supplemented with 10% fetal bovine serum (FBS) plus
2.5% horse serum and 1% PS antibiotic solution (100 U/ml penicillin
and 100 µg/ml streptomycin). The two cell lines were maintained in
a 37°C incubator with 5% CO2.
Cell viability
MiaPaCa-2 and MiaPaCa-2GEMR cells
(8×103 cells/well) were cultured in a 96-well plate
overnight. The cells were incubated with various concentrations
(0.1–5 µM) of 4-AAQB at 37°C for 48 h. The medium was discarded,
and the cells were washed in PBS three times. Cells were incubated
with MTT solution (0.5 mg/ml) for 1 h at 37°C. The MTT solution was
removed and the formazan crystals were dissolved in 200 µl DMSO.
Cell viability was determined using a microplate reader (FLUOstar
Omega) at 570 nm and calculated as: Cell viability (%) = Absorbance
of the sample/Absorbance of the control) ×100.
Reactive oxygen species (ROS)
measurement
MiaPaCa-2 and MiaPaCa-2GEMR cells
(6×104 cells/well) were cultured in a 24-well plate
overnight. Cells were treated with 4-AAQB (2 and 5 µM) with or
without GEM at 37°C for 48 h. The cells were then incubated with
DCFH-DA at 37°C for 30 min. The cellular ROS level was measured
using a Fluostar Galaxy reader (BMG Labtechnologies Ltd.) with
maximum excitation and emission spectra of 485 and 520 nm,
respectively. The level of ROS was calculated as the rate of change
in contrast to the level in untreated cells.
Cell cycle analysis
MiaPaCa-2 and MiaPaCa-2GEMR cells
(6×104 cells/ml) were cultured in a 6-well plate
overnight. Cells were incubated with various concentrations (2 and
5 µM) of 4-AAQB at 37°C for 48 h. The medium was discarded and
cells were washed with PBS three times. Cells were harvested by
trypsinization and fixed with 70% ethanol at −20°C for 2 h. Cells
(1×105) were stained with 0.5 ml PI/RNase staining
buffer (cat. no. 550825, BD Biosciences) at room temperature for 30
min. The cell cycle was evaluated using a BD Accuri C6 Plus flow
cytometer (BD Biosciences). The cell cycle phase distribution was
analyzed by ModFit LT 3.1 software (Verity Software House).
Annexin V and PI staining
MiaPaCa-2 and MiaPaCa-2GEMR cells
(6×104 cells/ml) were cultured in a 6-well plate
overnight. Cells were incubated with 4-AAQB (2 and 5 µM) at 37°C
for 48 h, double-stained with Annexin V and PI at room temperature
for 15 min and analyzed using a BD Accuri C6 Plus flow cytometer
(BD Biosciences). Quadrant 1 (negative for Annexin V and PI)
indicates living cells, quadrant 2 (Annexin V-positive,
PI-negative) indicates early apoptotic cells, quadrant 3 (positive
for Annexin V and PI) indicates the late apoptotic cells, and
quadrant 4 (Annexin V-negative, PI-positive) indicates necrotic
cells. The data was analyzed by ModFit LT 3.1 software (Verity
Software House).
Western blot analysis
MiaPaCa-2 and MiaPaCa-2GEMR cells
(1×106) were incubated with 4-AAQB (2 and 5 µM) at 37°C
for 48 h. The expression of proteins related to apoptosis (Bcl-xL
and Bax), autophagy (Atg5, Beclin-1, and LC3 II), and GEM
resistance-associated signaling (PI3K, phospho-PI3K, Akt,
phospho-Akt, Vps34, RAGE, HMGB1, and MDR1) was determined using
western blotting as described in a previous report (21). Briefly, cells were lysed by
ice-cold RIPA lysis buffer (Thermo Fisher Scientific, Inc.)
containing a cocktail of 1% protease inhibitors (P&C Biotech,
Inc.) and 1% phosphatase inhibitors (P&C Biotech, Inc.), and
the protein concentration was determined using a BCA protein assay
kit (Energenesis Biomedical Co., Ltd.) according to the user guide.
A total of 50 µg protein per lane was separated using 10–12%
SDS-PAGE, then transferred to PVDF membranes and blocked with 5%
non-fat milk in TBS-0.1% Tween-20 (TBST) at room temperature for 1
h. PVDF membranes were incubated primary antibodies (1:1,000)
overnight at 4°C. After washing three times with TBST at room
temperature for 10 min, the membranes were incubated HRP-conjugated
secondary antibodies (1:10,000) at room temperature for 2 h. After
antibody incubation, the protein was visualized using the ECL
detecting reagent (PerkinElmer, Inc.), and the density of the
target protein bands was quantified with the corresponding internal
reference proteins by the Biospectrum 810 UVP VisionWorks LS Image
Acquisition and Analysis Software (UVP).
Chemosensitivity analysis
MiaPaCa-2 and MiaPaCa-2GEMR cells
(4×104 cells/ml) were cultured in a 96-well plate
overnight. The increase in chemosensitivity was evaluated through
both cotreatment with 4-AAQB and GEM and 4-AAQB pretreatment
methods. For cotreatment, cells were incubated with 4-AAQB (2 and 5
µM) plus GEM at 37°C for 48 h. For pretreatment, cells were
pretreated with 4-AAQB (2 and 5 µM) at 37°C for 48 h and then
incubated with GEM at 37°C for another 48 h. In the pretreatment
method, cells unexposed to 4-AAQB or GEM were incubated in complete
medium until the end of the experiment. After treatments,
chemosensitivity was determined using the MTT analysis.
Cell migration assay
Cell migration was evaluated using a gap closure
assay. The Ibidi® culture inserts were placed in a
24-well plate, and then MiaPaCa-2 and MiaPaCa-2GEMR
cells (5×105 cells/ml) were seeded into the inserts and
cultured with complete medium overnight. After cell attachment, the
culture insert was removed and then the migration distance was
recorded after exposure to 4-AAQB (2 and 5 µM) with or without GEM
(0.5 µM) at 37°C for 48 h in serum free condition. The cell
migration rate was calculated using ImageJ software (version 1.34s;
National Institutes of Health). The migration area of the various
treatment groups was calculated as a percentage of the untreated
group using the following formula: Mean migration area of the
experimental group/mean migration area of the control group
×100%.
Cell invasion assay
Cell invasion was measured using a
Transwell® system with an 8-µm pore size (Corning, Inc.)
as described previously (22).
Briefly, 0.5 mg/ml Matrigel (Thermo Fisher Scientific, Inc.) was
coated on the upper chambers of the inserts at 37°C for 1 h.
MiaPaCa-2 and MiaPaCa-2GEMR cells (5×104
cells/well) were cultured with 4-AAQB (2 and 5 µM) under serum-free
conditions at 37°C for 48 h on the upper chambers and medium
containing 10% FBS served as a chemoattractant in the lower
chambers. The invading cells were subsequently fixed in 100%
methanol for 10 min at room temperature, stained with 0.5% crystal
violet for 10 min at room temperature. The total invasive number of
cells was calculated using the Power IX71 Inverted Fluorescence
Microscope (Olympus Corporation).
Statistical analysis
All experiments were repeated independently three
times. The data were analyzed using one-way ANOVA followed by
Tukey's post hoc test using the SPSS 20 software (IBM, Corp.) and
are shown as the mean ± SD. P<0.05 was considered to indicate a
statistically significant difference.
Results
4-AAQB induces cytotoxicity and cell
cycle arrest in pancreatic cancer cells
MiaPaCa-2GEMR cells were generated to
explore the molecular mechanisms of GEM resistance in pancreatic
cancer cells (21). The structure
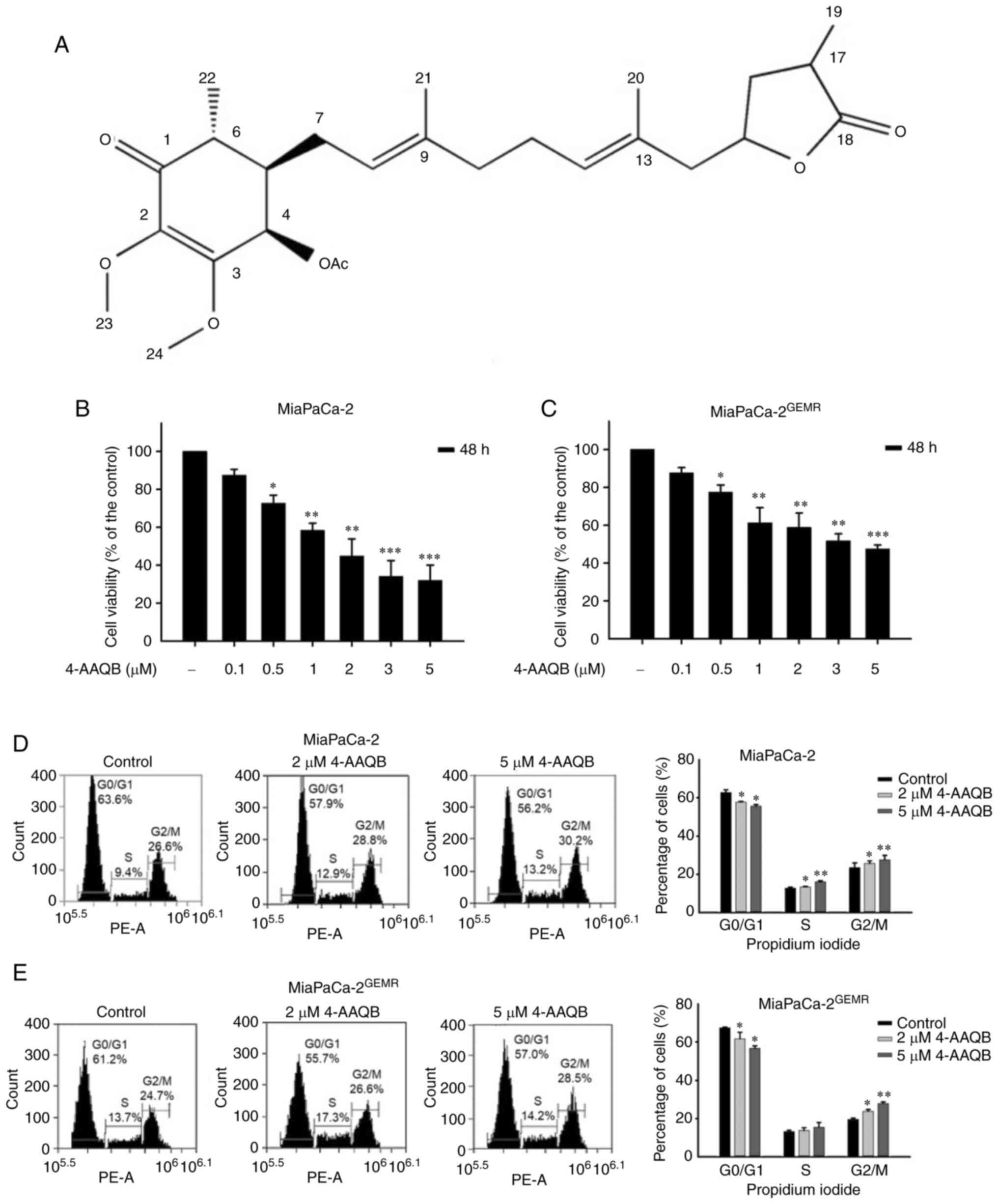
of 4-AAQB is shown in Fig. 1A. The
effect of 4-AAQB on cell viability was evaluated in both MiaPaCa-2
and MiaPaCa-2GEMR cells. Cytotoxicity was significantly
increased by 4-AAQB treatment in a dose-dependent manner (0.1–5 µM)
for 48 h (Fig. 1B and C). The
IC50 values of 4-AAQB in MiaPaCa-2 and
MiaPaCa-2GEMR cells were 1.61 and 3.8 µM, respectively
(Fig. 1B and C). Doses of 2 and 5
µM were used in subsequent experiments.
Cell viability is directly affected by cell cycle
arrest, apoptosis, and autophagy in cancer cells (18). S-phase cell cycle arrest was
observed in MiaPaCa-2 cells subjected to 4-AAQB treatment, whereas
the percentage of G0/G1 phase cells was significantly reduced in
4-AAQB-treated MiaPaCa-2 cells compared with untreated MiaPaCa-2
cells (Fig. 1D). In addition, this
coordinated change in G2/M cell cycle arrest was observed in
4-AAQB-treated MIAPaCa-2GEMR cells (Fig. 1E). Previous studies have found that
cell cycle regulators not only influence cell division but also
induce programmed cell death (23). In the present study, treatment with
4-AAQB led to dose-dependent cell cycle arrest, suggesting that
4-AAQB treatment may inhibit cell cycle progression in pancreatic
cancer cells.
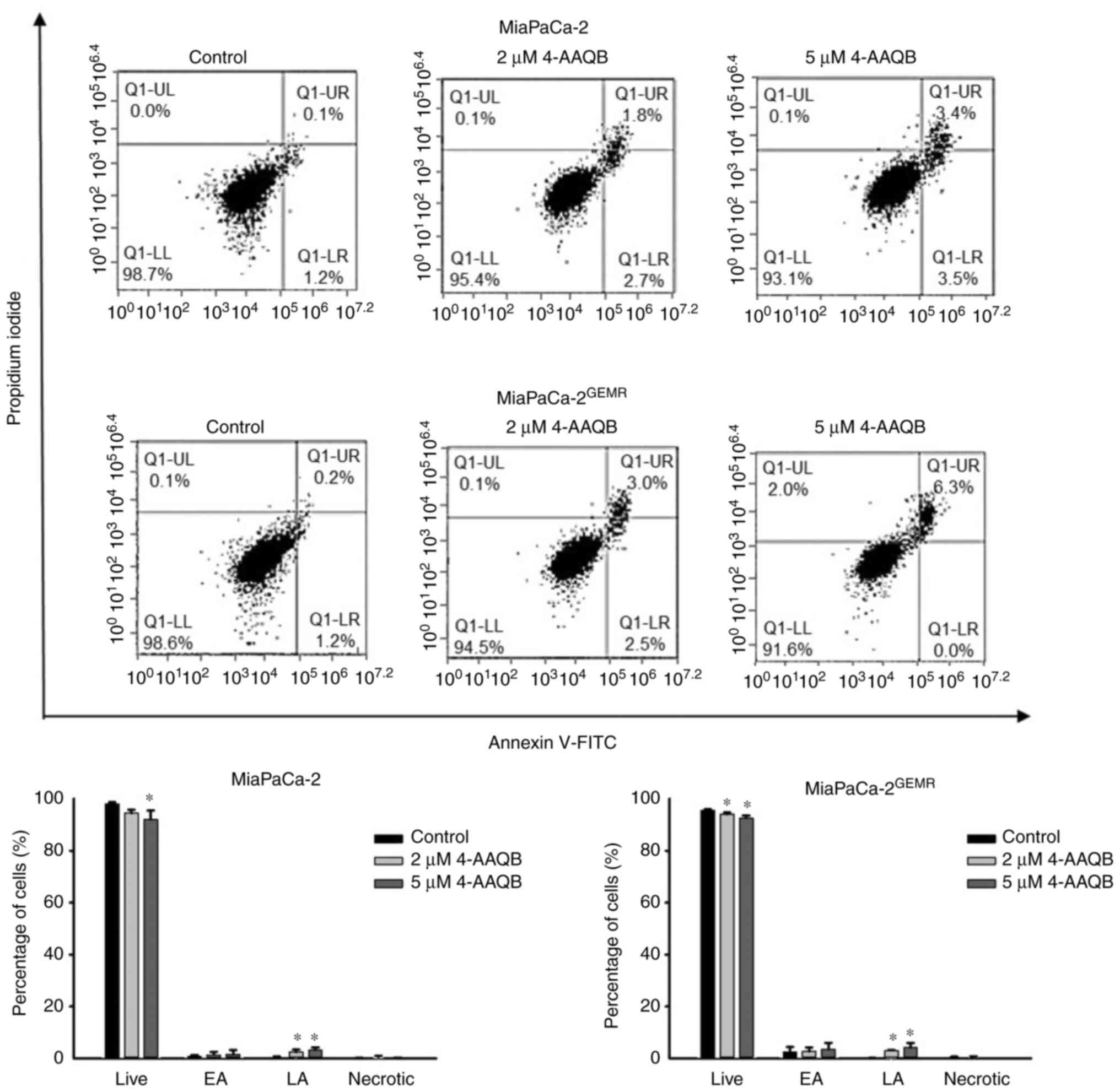
4-AAQB treatment promotes apoptosis
and inhibits autophagy
Emerging evidence suggests that to increase
apoptosis and decrease autophagy are powerful therapeutic
strategies for pancreatic cancer progression (24). In the present study, the percentage
of late apoptotic cells was significantly induced in a
dose-dependent manner following 4-AAQB treatment in both cell lines
(Fig. 2). Western blot analysis
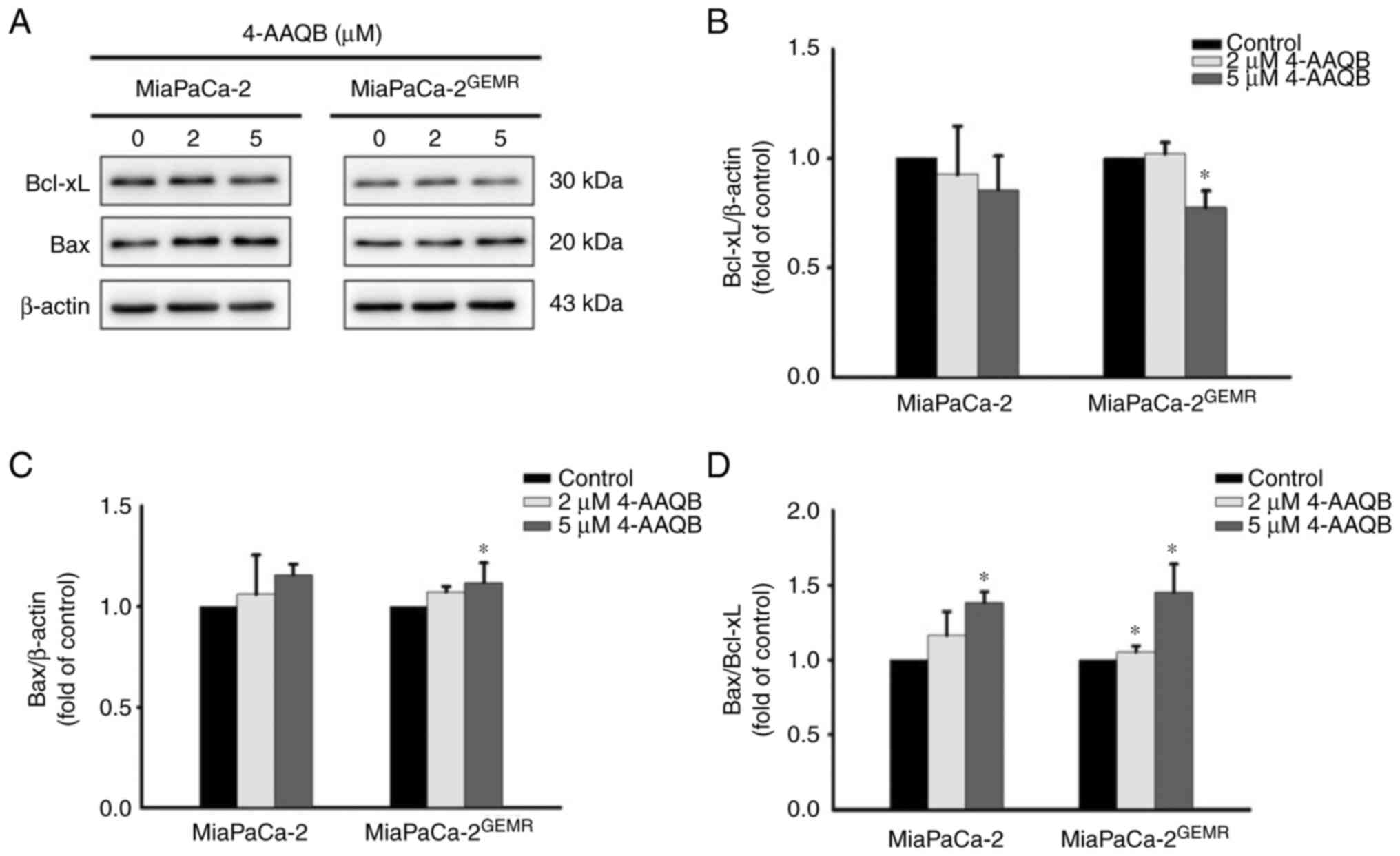
was used to examine the expression of apoptosis-associated
proteins. Consistent with published evidence (24), although the levels of Bcl-xL were
not significantly altered by 2 µM 4-AAQB treatment, however, it was
significantly reduced following 5 µM 4-AAQB treatment in
MiaPaCa-2GEMR cells compared with the untreated cells
(Fig. 3A and B). The levels of Bax
significantly increased in MiaPaCa-2GEMR cells treated
with 4-AAQB (Fig. 3A and C).
Moreover, the Bax/Bcl-xL ratio significantly increased in 5 µM
4-AAQB-treated MiaPaCa-2 and MiaPaCa-2GEMR cells
(Fig. 3D).
The pro-apoptotic protein Bax directly inhibits
autophagy (25). Furthermore,
clinical evidence has revealed that autophagy is involved in
resistance to gemcitabine/nab-paclitaxel chemotherapy and its
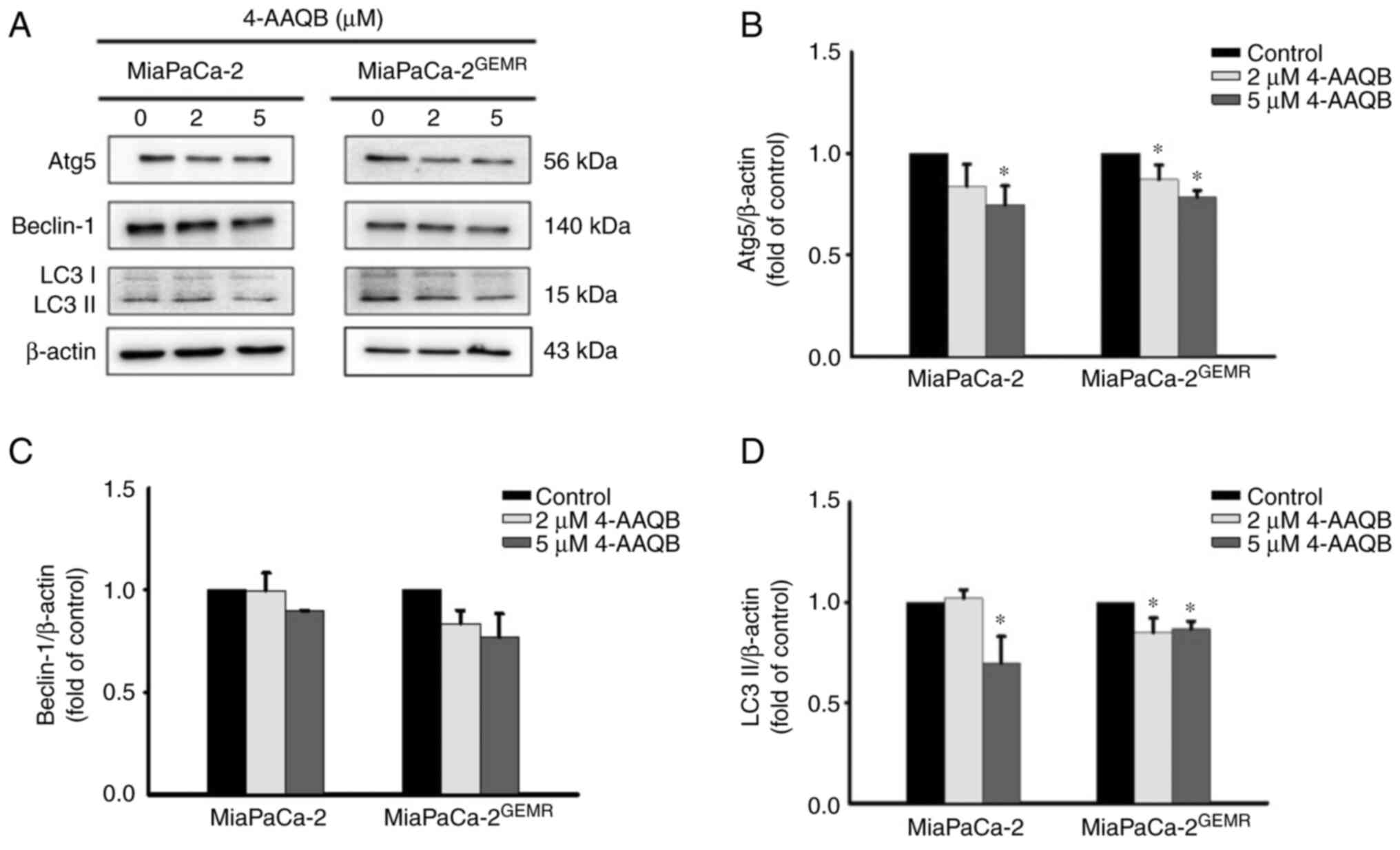
cytotoxic response in patients with pancreatic cancer (26). Thus, the expression levels of
autophagy-associated proteins (Atg5, Beclin-1 and LC3 II) were
assessed using western blotting. The protein expression levels of
Atg5 and LC3 II were significantly reduced after 4-AAQB treatment
in both cell lines compared with the untreated cells (Fig. 4).
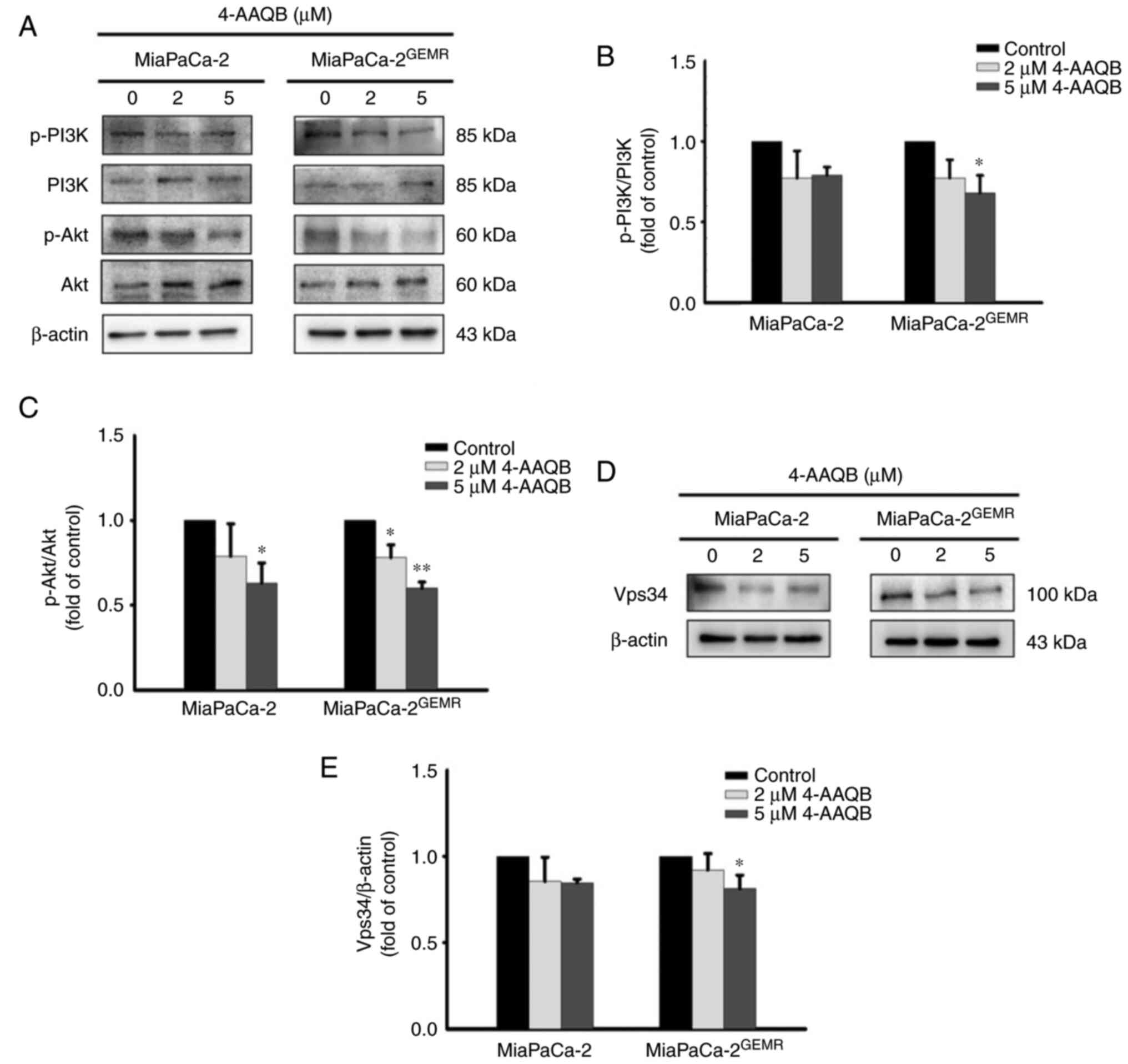
The PI3K/Akt signaling pathway is critical for tumor
survival and autophagy initiation (27,28).
The phosphorylation of PI3K and Akt significantly decreased in
4-AAQB-treated MiaPaCa-2 and MiaPaCa-2GEMR cells
compared with untreated cells (Fig.
5A-C). Vps34 binds to beclin-1 and subsequently activates
autophagy (29). Additionally, a
previous study indicated that VPS34 was essential for the autophagy
process (30). In the present
study, the expression of the Vps34 protein was significantly
reduced following the 4-AAQB incubation in both cell lines
(Fig. 5D and E).
Chemosensitivity and ROS levels are
enhanced through the suppression of the RAGE-HMGB1-initiated
PI3K/Akt/MDR1 signaling axis in both cell lines treated with
4-AAQB
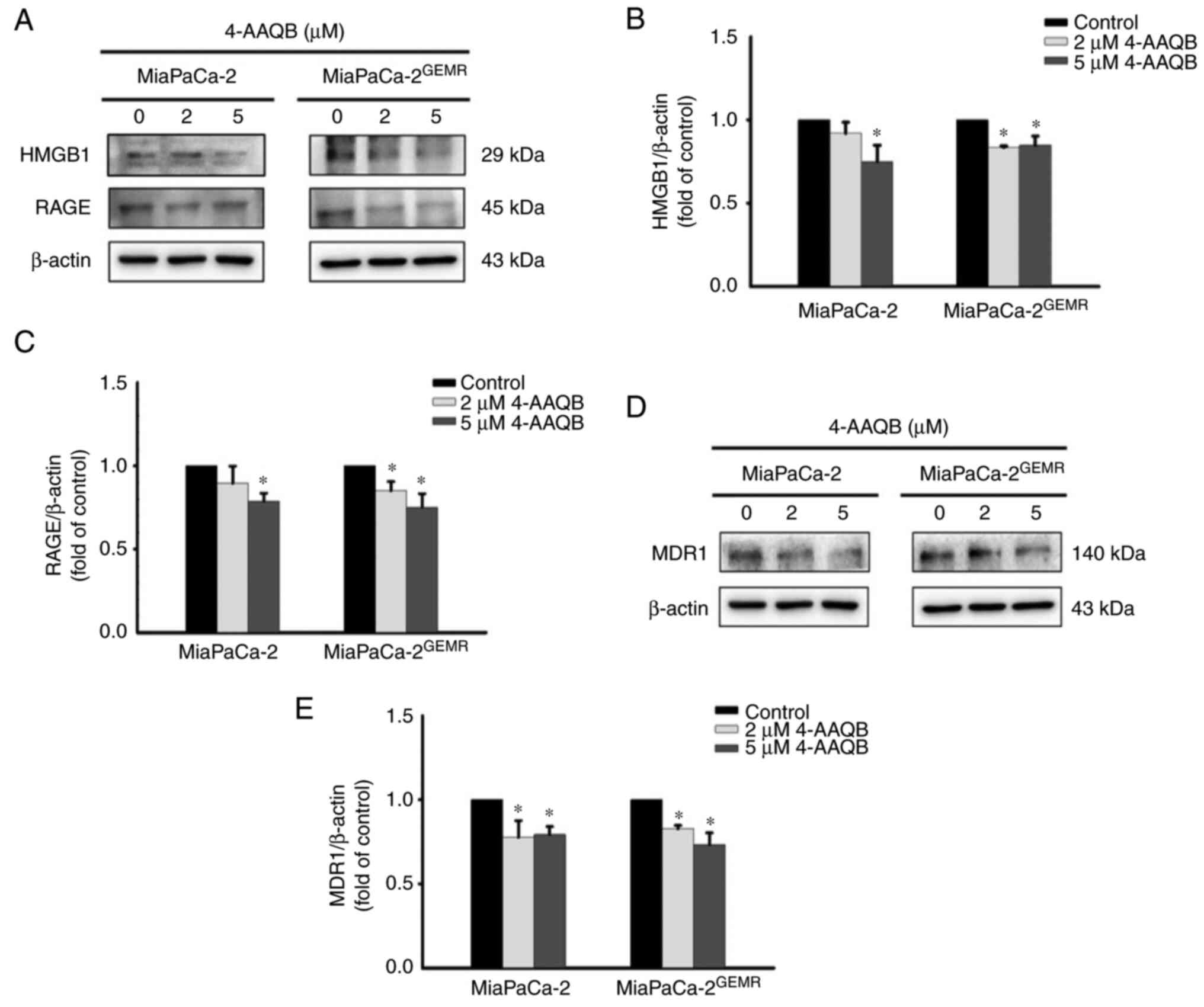
As shown in our previous study, the PI3K/Akt/MDR1
axis is triggered by HMGB1 and RAGE engagement during GEM
chemoresistance (19). Thus, the
protein expression levels of HMGB1, RAGE, and MDR1 were examined.
The levels of HMGB1 and RAGE were significantly inhibited after 5
µM 4-AAQB treatment in MiaPaCa-2 cells (Fig. 6A-C). Moreover, treatment with both
2 and 5 µM 4-AAQB effectively inhibited HMGB1 and RAGE expression
in MiaPaCa-2GEMR cells (Fig. 6A-C). Accordingly, the level of the
MDR1 protein was reduced by 4-AAQB treatment in a dose-dependent
manner in both cell lines (Fig. 6D and
E).
A previous study showed that suppression of the
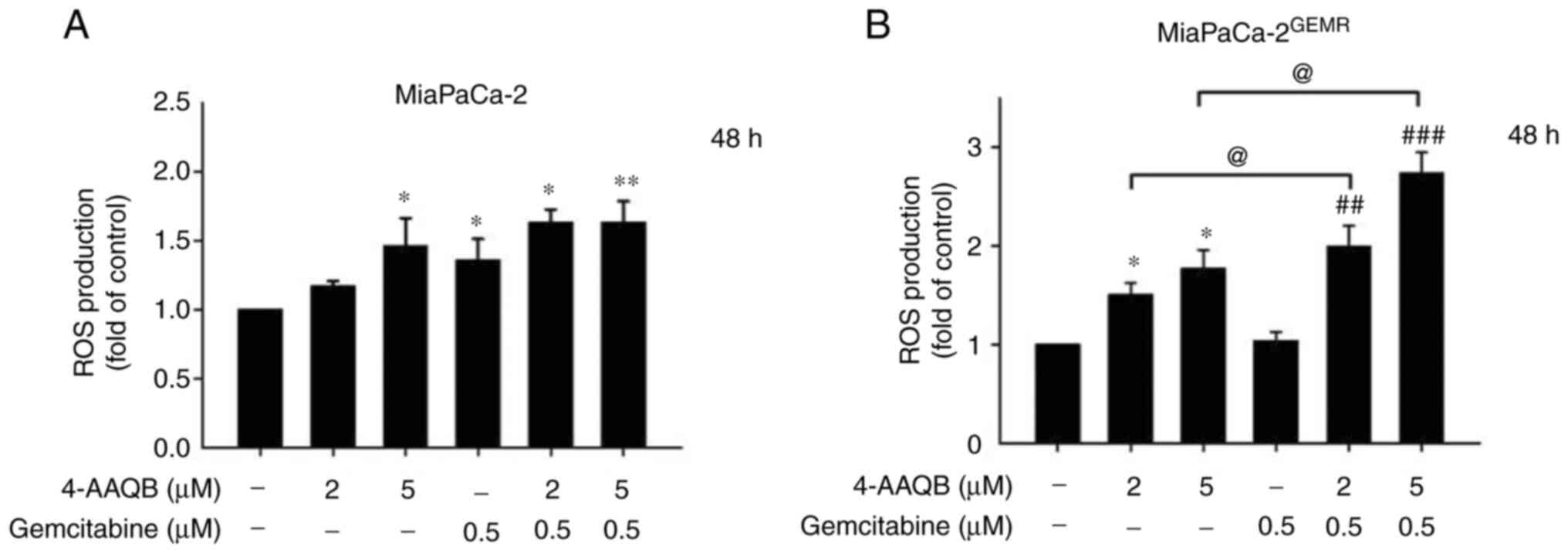
PI3K/AKT pathway by inducing ROS generation may represent a
strategy for cancer treatment (31). Additionally, GEM induces ROS,
resulting in an increase in pancreatic cancer cell death, although
the opposite effect is observed in GEM-resistant cells (32). In the present study, ROS levels
were measured using DCFH-DA staining. The results showed that ROS
levels significantly increased in a dose-dependent manner following
4-AAQB treatment in both cell lines (Fig. 7A and B). Notably, ROS accumulation
was dramatically increased by 4-AAQB combination with GEM treatment
in MiaPaCa-2GEMR cells compared with 4-AAQB-treated
alone or GEM-treated alone condition (Fig. 7B).
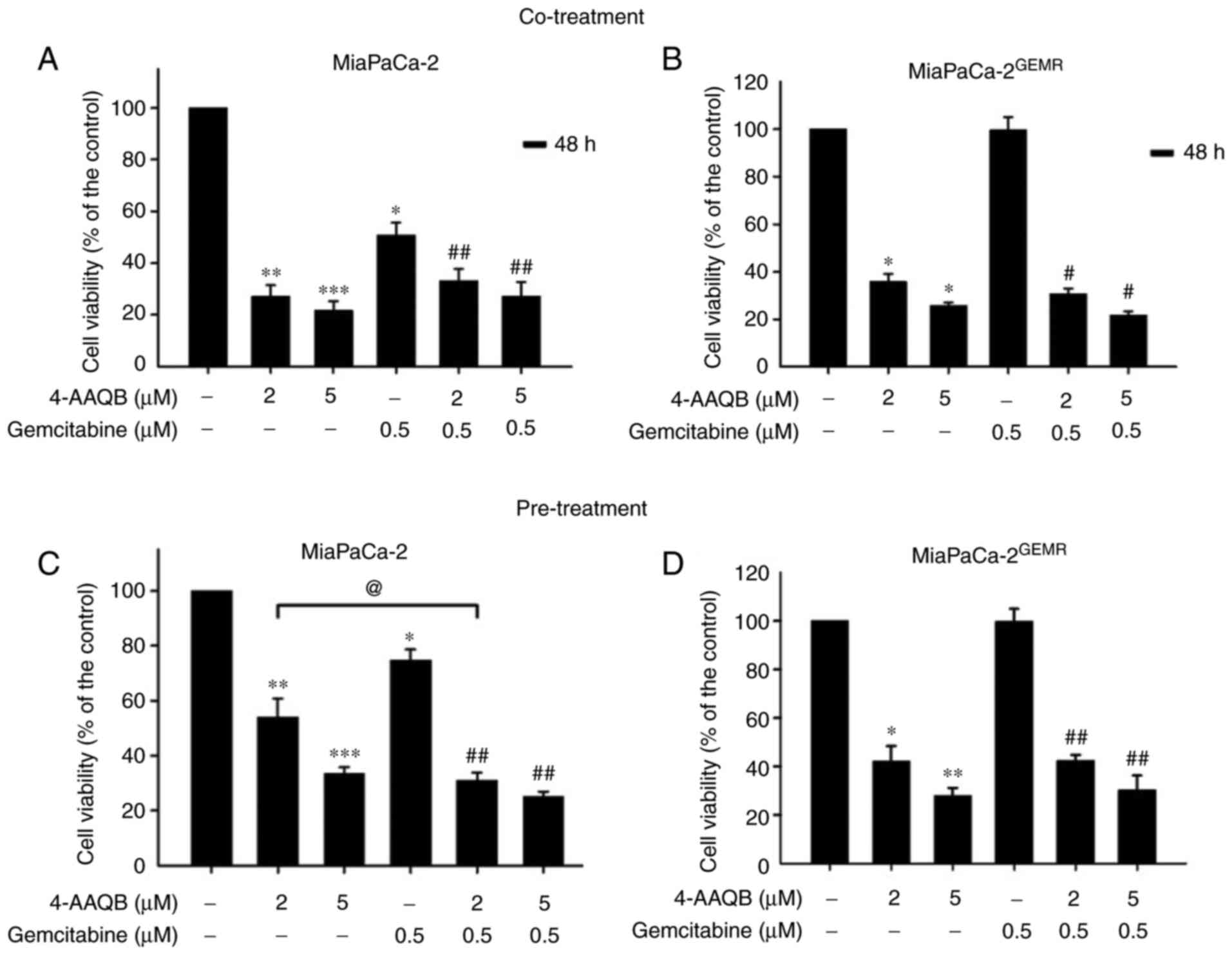
Pancreatic cancer is highly malignant with great
metastatic capacity (33). An
increase in chemosensitivity is important for preventing the local
invasion and long distant metastasis of pancreatic cancer (34). The ability of 4-AAQB to increase
chemosensitivity was evaluated using 4-AAQB cotreatment with GEM
and 4-AAQB pretreatment methods in both cell lines to select an
effective drug treatment pattern. Consistent with the results
presented in Fig. 1B, cell
viability was significantly reduced by GEM and 4-AAQB (2 and 5 µM)
treatment in MiaPaCa-2 cells compared with untreated cells
(Fig. 8A). Compared with GEM
treatment, chemosensitivity was effectively enhanced following
4-AAQB cotreatment with GEM in MiaPaCa-2 cells (Fig. 8A). Cell viability was not altered
following 0.5 µM GEM treatment in MiaPaCa-2GEMR cells
(Fig. 8B), indicating that the
GEM-resistant pancreatic cancer cell lines was successfully
established. Cell viability was significantly reduced both by
4-AAQB treatment alone and by 4-AAQB/GEM co-treatment in cells
(Fig. 8B). In addition, the effect
of the 4-AAQB pre-treatment on enhancing chemosensitivity was also
evaluated. Cells were pretreated with 4-AAQB for 48 h, then
incubated with GEM for another 48 h. In MiaPaCa-2 cells, the GEM
and 4-AAQB (2 and 5 µM) treatment significantly reduced cell
viability (Fig. 8C). Additionally,
chemosensitivity was significantly increased following 4-AAQB
pre-treatment in MiaPaCa-2 cells compared with GEM-treated cells
(Fig. 8C). In addition, cell
viability was significantly inhibited following 4-AAQB (2 and 5 µM)
pre-treatment with or without GEM incubation in
MiaPaCa-2GEMR cells (Fig.
8D).
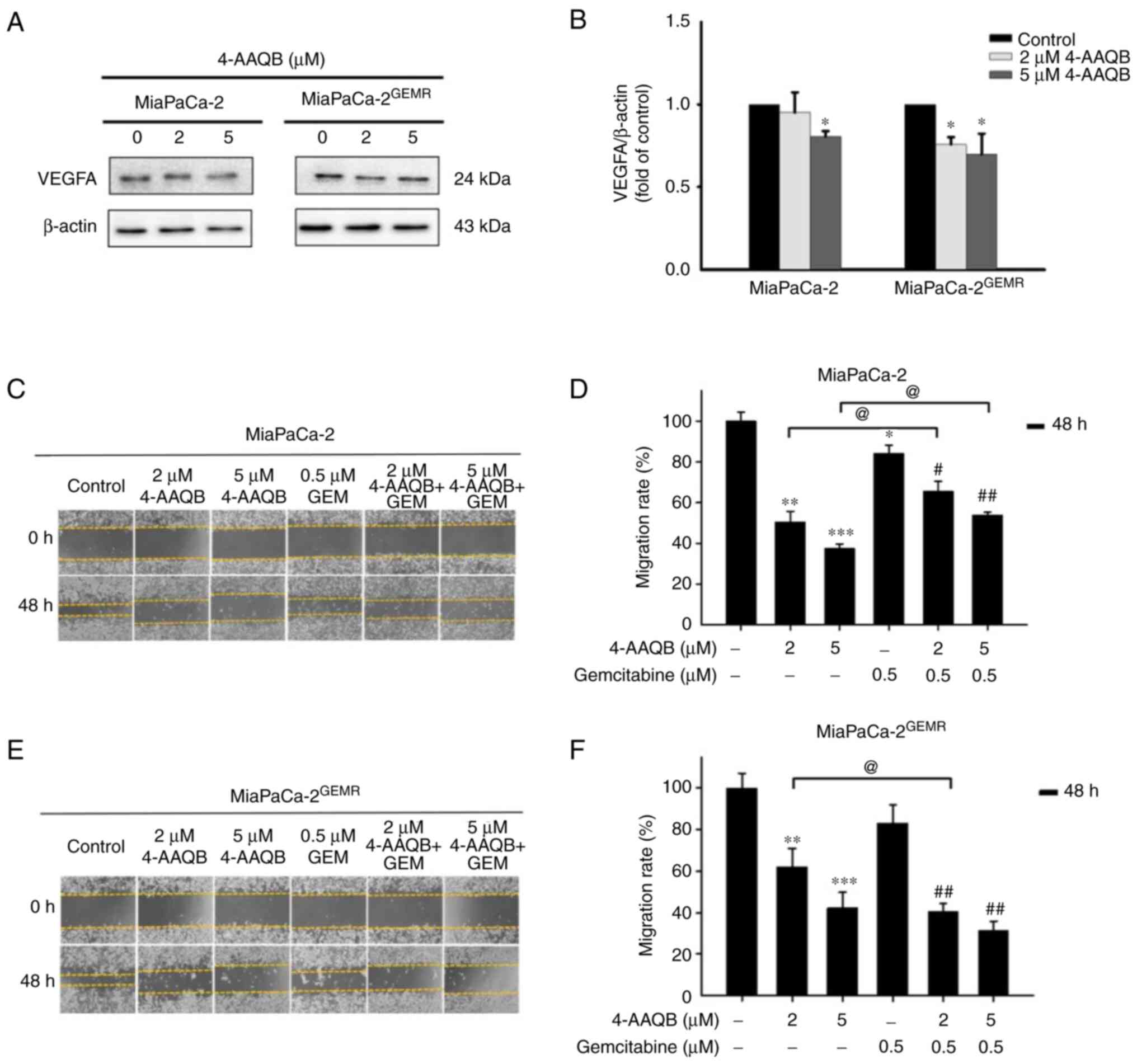
4-AAQB treatment effectively repressed
VEGFA-mediated cell migration and invasion. VEGFA plays important
roles in cell proliferation, angiogenesis, migration, invasion, and
cancer metastasis (35). According
to a previous study, the upregulation of VEGFA in patients with
pancreatic cancer is associated with metastatic disease and shorter
overall survival (36). The next
experiments were carried out to assess whether 4-AAQB treatment was
effective at inhibiting VEGFA production and subsequently
preventing cell migration and invasion. Interestingly, VEGFA
protein levels significantly decreased in both cell lines subjected
to 4-AAQB treatment (Fig. 9A and
B). Cell migration was inhibited following 4-AAQB, GEM, and
4-AAQB/GEM co-treatment in MiaPaCa-2 cells compared with untreated
cells (Fig. 9C and D).
Unexpectedly, the cell migration distance was not substantially
reduced by 4-AAQB/GEM co-treatment compared to 4-AAQB treatment
alone in MiaPaCa-2 cells (Fig. 9C and
D). In addition, 4-AAQB (2 and 5 µM) treatment effectively
decreased MiaPaCa-2GEMR cell migration compared to
untreated cells (Fig. 9E and F).
Notably, 2 µM 4-AAQB/GEM co-treatment significantly decreased cell
migration compared to that of 4-AAQB-treated
MiaPaCa-2GEMR cells (Fig.
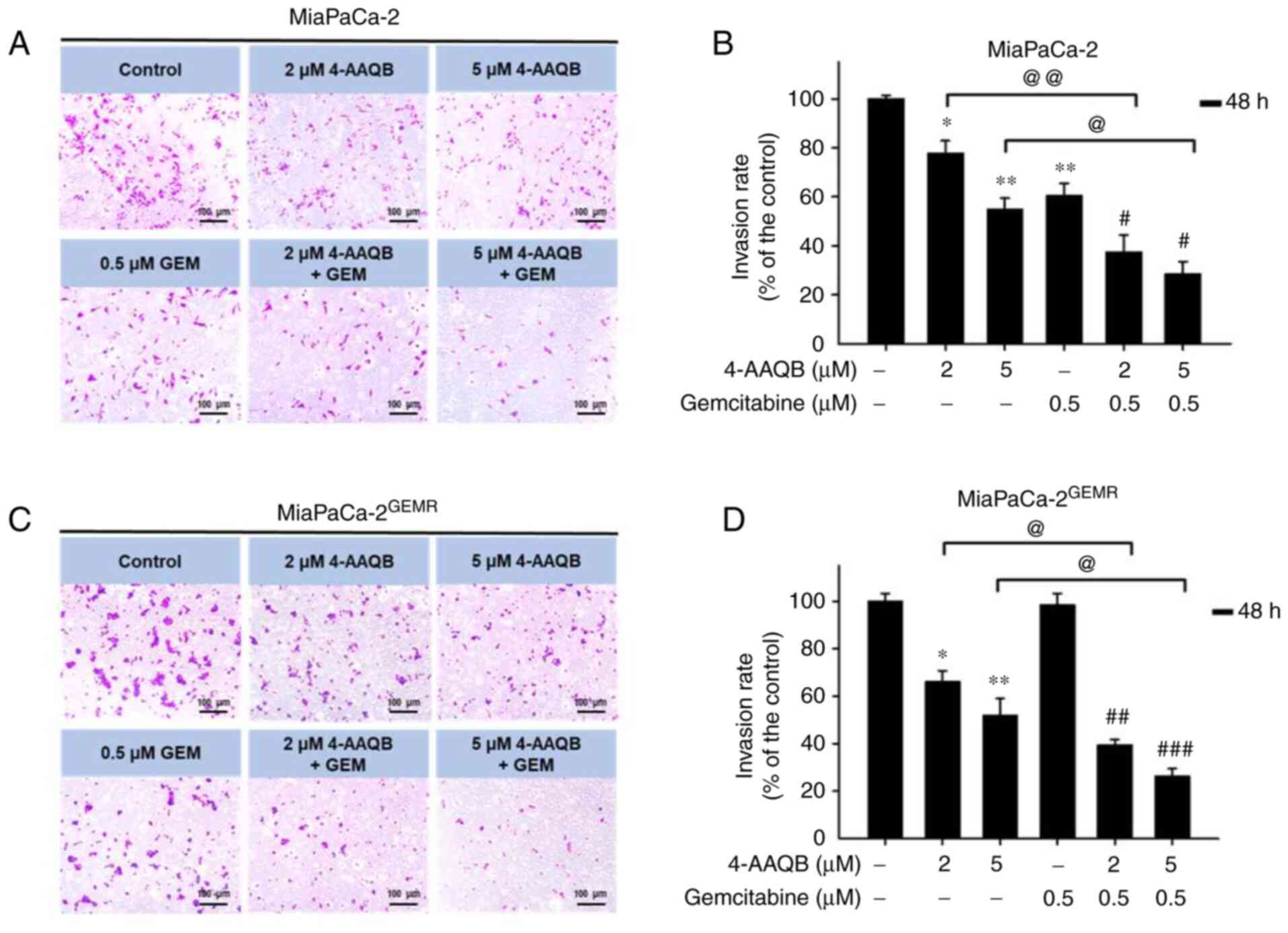
9E and F). In addition, the inhibitory effect of 4-AAQB on cell
invasion was also evaluated. The cell invasion abilities of
MiaPaCa-2 and MiaPaCa-2GEMR cells were significantly
reduced by 4-AAQB (2 and 5 µM) treatment (Fig. 10). Notably, 4-AAQB combined with
GEM significantly enhanced the inhibition of cell invasion compared
with 4-AAQB-treated MiaPaCa-2 cells l and MiaPaCa-2GEMR
cells (Fig. 10B and D).
Discussion
GEM is known to induce ROS accumulation, cell cycle
arrest, and apoptosis (37,38).
However, GEM resistance inhibits ROS production and consequently
inhibits cell death in pancreatic ductal adenocarcinoma (32). 4-AAQB triggers cell cycle arrest,
apoptosis, and DNA damage by suppressing CDK2/CDK4 expression in
human breast cancer and hepatocellular carcinoma cells (11,39).
The present study also found that 4-AAQB upregulated cell cycle
arrest and apoptosis. This suggested that 4-AAQB may play a vital
role in regulating cyclin-dependent kinases (CDKs) in pancreatic
cancer cells and the cell cycle-related markers such as CDK1, CDK2,
CDK4 or CDK5 were not further evaluated; however, this should be
further analyzed in future in vitro studies. Moreover, RAGE
downregulation in pancreatic tumor cells is sensitive to
H2O2-induced oxidative injury, indicating
that RAGE plays a protective role against oxidative injury
(40,41). In the present study, 4-AAQB
treatment effectively induced ROS accumulation by inhibiting
RAGE/HMG1 levels and promoting cell cycle arrest and apoptosis in
both MiaPaCa-2 and GEM-resistant cells. The cumulative evidence
suggests 4-AAQB plays an important role the regulation of cancer
cell death.
4-AAQB is a novel bioactive compound, and its effect
on GEM chemosensitivity remains unclear. A previous study has
reported that 4-AAQB plays a critical role in inhibiting tumor
growth by attenuating cisplatin chemoresistance and inhibiting
Atg5-dependent autophagy in ovarian cancer (42). Increased autophagy in pancreatic
cancer is strongly associated with early metastasis and
chemotherapy resistance, indicating that autophagy may serve as a
therapeutic target for pancreatic cancer (43). Additionally, a clinical trial found
that the administration of an autophagy inhibitor
(hydroxychloroquine) in combination with GEM therapy effectively
increases the survival rate through the activation of immune
responses in patients with resectable pancreatic cancer (26). The findings of the present study
revealed that autophagy-associated protein (LC3 II and Atg5) levels
and GEM resistance were significantly reduced in 4-AAQB-treated
cells.
Based on accumulating evidence, apoptosis and
autophagy may compete with each other; for example, tioconazole
represses autophagy-related 4A cysteine peptidase (ATG4A) and ATG4B
expression, which subsequently enhances chemotherapy-induced
apoptosis in glioma, colorectal, and breast cancer cells (44). Additionally, spautin-1 (an
autophagy inhibitor) has been shown to increase apoptosis by
inactivating the PI3K/Akt pathway in chronic myeloid leukemia cells
(45). Moreover, a previous report
found that cell cycle arrest and apoptosis were induced in
4-AAQB-treated MDA-MB-231 and Hs578T human breast cancer cells
(11). The findings from the
present study corroborate the aforementioned results and indicate
that 4-AAQB induces cell cycle arrest, apoptosis, and GEM
chemosensitivity by inhibiting autophagy and the PI3K/Akt signaling
axis.
Our previous studies demonstrated that the
RAGE/HMGB1-initiated PI3K/Akt/MDR1 biochemical cascade was strongly
related to GEM chemoresistance in human pancreatic cancer cells and
xenograft mouse models (18–21).
Moreover, 4-AAQB has been shown to suppress autophagy and enhance
cisplatin sensitivity by suppressing PI3K/Akt/mTOR/p70S6K signaling
in ovarian cancer cells (46). In
addition, PI3K/Akt signaling is the dominant regulator of the
epithelial-mesenchymal transition (EMT), which contributes to tumor
migration, invasion, and metastasis (47). 4-AAQB (5–10 µM) treatment
significantly downregulated VEGF and reduced cell migration and
invasion in a dose-dependent manner in DLD-1, HT-29, and HCT-116
colorectal cancer cells (48,49).
In addition, previous evidence showed that cell migration studies
had shown the importance of cell moving ability; however, a 2D
substrate (Matrigel matrix) was used in the evaluation of cell
invasion that may deliver inconsistent results due to cell adhesion
with a different substrate such as polystyrene cell culture plate
or Matrigel matrix-coated (50). A
previous study compared the motility-associated protein profiles
between human bladder cancer T24T cells (higher invasion but lower
migration abilities) and its parental non-metastatic T24 cells
(51). The results has
demonstrated that high cell migratory activity does not necessarily
mean high invasive activity in highly metastatic human bladder
cancer T24T cells via SOD2 and MMP-2 regulated metastatic behaviors
(51). Although the inhibitory
effect of 4-AAQB/GEM co-treatment on invasion was greater than that
on migration of MiaPaCa-2 cells, cell migration and invasion were
reduced by 4-AAQB treatment in both MiaPaCa-2 and GEM-resistant
cells, suggesting that 4-AAQB may play a critical role in the
inhibition of tumor metastasis.
In conclusion, the present study described the
mechanism through which 4-AAQB downregulates autophagy and enhances
cytotoxicity, ROS accumulation, cell cycle arrest, apoptosis, and
GEM sensitivity through the suppression of the RAGE/HMGB1-initiated
PI3K/Akt/MDR1 signaling pathway in pancreatic cancer cells.
Accordingly, this 4-AAQB/GEM combination strategy might represent a
novel approach for the treatment of GEM-resistant pancreatic
cancer.
Acknowledgements
Not applicable.
Funding
This research work was supported by a grant from Grape King Bio
Ltd., Taiwan (grant no. BR-MM003).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YYC and GCY conceived and designed the experiments.
YYC, TJL and SYC performed the experiments. YYC, TWL, SYC and CCC
analyzed and interpreted the data. SYC, and GCY wrote the
manuscript. YYC, SYC, and GCY confirmed the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors TJL, TWL, CCC are employed by Grape King
Bio Ltd. All the authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
4-AAQB
|
4-acetylantroquinonol B
|
|
ATG5
|
autophagy-related protein 5
|
|
HMGB1
|
high mobility group box 1 protein
|
|
MDR1
|
multidrug resistance protein 1
|
|
RAGE
|
receptor for advanced glycation
end-products
|
|
VEGFA
|
vascular endothelial growth factor
A
|
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Traub B, Link KH and Kornmann M: Curing
pancreatic cancer. Semin Cancer Biol. 76:232–246. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mizrahi JD, Surana R, Valle JW and Shroff
RT: Pancreatic cancer. Lancet. 395:2008–2020. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang J, Lok V, Ngai CH, Zhang L, Yuan J,
Lao XQ, Ng K, Chong C, Zheng ZJ and Wong MCS: Worldwide burden of,
risk factors for, and trends in pancreatic cancer.
Gastroenterology. 160:744–754. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amrutkar M and Gladhaug IP: Pancreatic
cancer chemoresistance to gemcitabine. Cancers (Basel). 9:1572017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Binenbaum Y, Na'ara S and Gil Z:
Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug
Resist Updat. 23:55–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuang Y, Li B, Wang Z, Qiao X and Ye M:
Terpenoids from the medicinal mushroom Antrodia camphorata:
Chemistry and medicinal potential. Nat Prod Rep. 38:83–102. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin YW, Pan JH, Liu RH, Kuo YH, Sheen LY
and Chiang BH: The 4-acetylantroquinonol B isolated from mycelium
of Antrodia cinnamomea inhibits proliferation of hepatoma
cells. J Sci Food Agric. 90:1739–1744. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yen IC, Tu QW, Chang TC, Lin PH, Li YF and
Lee SY: 4-Acetylantroquinonol B ameliorates nonalcoholic
steatohepatitis by suppression of ER stress and NLRP3 inflammasome
activation. Biomed Pharmacother. 138:1115042021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu CH, Ou CH, Yen IC and Lee SY:
4-Acetylantroquinonol B inhibits osteoclastogenesis by inhibiting
the autophagy pathway in a simulated microgravity model. Int J Mol
Sci. 21:69712020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Satriyo PB, Su CM, Ong JR, Huang WC, Fong
IH, Lin CC, Aryandono T, Haryana SM, Deng L, Huang CC, et al:
4-Acetylantroquinonol B induced DNA damage response signaling and
apoptosis via suppressing CDK2/CDK4 expression in triple negative
breast cancer cells. Toxicol Appl Pharmacol. 422:1154932021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu HW, Su YK, Bamodu OA, Hueng DY, Lee
WH, Huang CC, Deng L, Hsiao M, Chien MH, Yeh CT and Lin CM: The
disruption of the β-catenin/TCF-1/STAT3 signaling axis by
4-acetylantroquinonol B inhibits the tumorigenesis and cancer
stem-cell-like properties of glioblastoma cells, in vitro and in
vivo. Cancers (Basel). 10:4912018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li TY and Chiang BH: 4-Acetylantroquinonol
B from Antrodia cinnamomea enhances immune function of
dendritic cells against liver cancer stem cells. Biomed
Pharmacother. 109:2262–2269. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang D, Loze MT, Zeh HJ and Kang R: The
redox protein HMGB1 regulates cell death and survival in cancer
treatment. Autophagy. 6:1181–1183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arumugam T, Ramachandran V, Gomez SB,
Schmidt AM and Logsdon CD: S100P-derived RAGE antagonistic peptide
reduces tumor growth and metastasis. Clin Cancer Res. 18:4356–4364.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sparvero LJ, Asafu-Adjei D, Kang R, Tang
D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ and Lotze
MT: RAGE (receptor for advanced glycation endproducts), RAGE
ligands, and their role in cancer and inflammation. J Transl Med.
7:172009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang R, Tang D, Schapiro NE, Loux T,
Livesey KM, Billiar TR, Wang H, Van Houten BV, Lotze MT and Zeh HJ:
The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor
growth by regulating mitochondrial bioenergetics. Oncogene.
33:567–577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin JH, Chen SY, Lu CC, Lin JA and Yen GC:
Ursolic acid promotes apoptosis, autophagy, and chemosensitivity in
gemcitabine-resistant human pancreatic cancer cells. Phytother Res.
34:2053–2066. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lan CY, Chen SY, Kuo CW, Lu CC and Yen GC:
Quercetin facilitates cell death and chemosensitivity through
RAGE/PI3K/AKT/mTOR axis in human pancreatic cancer cells. J Food
Drug Anal. 27:887–896. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lia ZY, Chen SY, Weng MH and Yen GC:
Ursolic acid restores sensitivity to gemcitabine through the
RAGE/NF-κB/MDR1 axis in pancreatic cancer cells and in a mouse
xenograft model. J Food Drug Anal. 29:262–274. 2021.
|
|
21
|
Hsu YH, Chen SY, Wang SY, Lin JA and Yen
GC: Pterostilbene enhances cytotoxicity and chemosensitivity in
human pancreatic cancer cells. Biomolecules. 10:7092020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang HY, Chen SY, Wu CH, Lu CC and Yen
GC: Glycyrrhizin attenuates the process of
epithelial-to-mesenchymal transition by modulating HMGB1 initiated
novel signaling pathway in prostate cancer cells. J Agric Food
Chem. 67:3323–3332. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Connolly P, Garcia-Carpio I and Villunger
A: Cell-cycle cross talk with caspases and their substrates. Cold
Spring Harb Perspect Biol. 12:a0364752020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bedoui S, Herold MJ and Strasser A:
Emerging connectivity of programmed cell death pathways and its
physiological implications. Nat Rev Mol Cell Biol. 21:678–695.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gil J, Ramsey D, Szmida E, Leszczynski P,
Pawlowski P, Bebenek M and Sasiadek MM: The BAX gene as a candidate
for negative autophagy-related genes regulator on mRNA levels in
colorectal cancer. Med Oncol. 34:162017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zeh HJ, Bahary N, Boone BA, Singhi AD,
Miller-Ocuin JL, Normolle DP, Zureikat AH, Hogg ME, Bartlett DL,
Lee KK, et al: A randomized phase II preoperative study of
autophagy inhibition with high-dose hydroxychloroquine and
gemcitabine/nab-paclitaxel in pancreatic cancer patients. Clin
Cancer Res. 26:3126–3134. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu L, Wei J and Liu P: Attacking the
PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment
in human cancer. Semin Cancer Biol. S1044-579X(21)00188-7. 2021.
View Article : Google Scholar
|
|
28
|
Bernard M, Cardin GB, Cahuzac M, Ayad T,
Bissada E, Guertin L, Bahig H, Nguyen-Tan PF, Filion E, Ballivy O,
et al: Dual inhibition of autophagy and PI3K/AKT/mTOR pathway as a
therapeutic strategy in head and neck squamous cell carcinoma.
Cancers (Basel). 12:23712020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Su H, Yang F, Wang Q, Shen Q, Huang J,
Peng C, Zhang Y, Wan W, Wong CCL, Sun Q, et al: VPS34 acetylation
controls its lipid kinase activity and the initiation of canonical
and non-canonical autophagy. Mol Cell. 67:907–921.e7. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jaber N, Dou Z, Chen JS, Catanzaro J,
Jiang YP, Ballou LM, Selinger E, Ouyang X, Lin RZ, Zhang J and Zong
WX: Class III PI3K Vps34 plays an essential role in autophagy and
in heart and liver function. Proc Natl Acad Sci USA. 109:2003–2008.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wen C, Wang H, Wu X, He L, Zhou Q, Wang F,
Chen S, Huang L, Chen J, Wang H, et al: ROS-mediated inactivation
of the PI3K/AKT pathway is involved in the antigastric cancer
effects of thioredoxin reductase-1 inhibitor chaetocin. Cell Death
Dis. 10:8092019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ju HQ, Gocho T, Aguilar M, Wu M, Zhuang
ZN, Fu J, Yanaga K, Huang P and Chiao PJ: Mechanisms of overcoming
intrinsic resistance to gemcitabine in pancreatic ductal
adenocarcinoma through the redox modulation. Mol Cancer Ther.
14:788–798. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aiello NM, Brabletz T, Kang Y, Nieto MA,
Weinberg RA and Stanger BZ: Upholding a role for EMT in pancreatic
cancer metastasis. Nature. 547:E7–E8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng X, Carstens JL, Kim J, Scheible M,
Kaye J, Sugimoto H, Wu CC, LeBleu VS and Kalluri R:
Epithelial-to-mesenchymal transition is dispensable for metastasis
but induces chemoresistance in pancreatic cancer. Nature.
527:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Parveen A, Subedi L, Kim HW, Khan Z, Zahra
Z, Farooqi MQ and Kim SY: Phytochemicals targeting VEGF and
VEGF-related multifactors as anticancer therapy. J Clin Med.
8:3502019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hosein AN, Brekken RA and Maitra A:
Pancreatic cancer stroma: An update on therapeutic targeting
strategies. Nat Rev Gastroenterol Hepatol. 17:487–505. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao H, Wu S, Li H, Duan Q, Zhang Z, Shen
Q, Wang C and Yin T: ROS/KRAS/AMPK signaling contributes to
gemcitabine-induced stem-like cell properties in pancreatic cancer.
Mol Ther Oncolytics. 14:299–312. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu W, Liu Q, Pan J and Sui Z: miR-373-3p
enhances the chemosensitivity of gemcitabine through cell cycle
pathway by targeting CCND2 in pancreatic carcinoma cells. Biomed
Pharmacother. 105:887–898. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin YW and Chiang BH:
4-acetylantroquinonol B isolated from Antrodia cinnamomea
arrests proliferation of human hepatocellular carcinoma HepG2 cell
by affecting p53, p21 and p27 levels. J Agric Food Chem.
59:8625–8631. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kang R, Tang D, Lotze MT and Zeh HJ III:
RAGE regulates autophagy and apoptosis following oxidative injury.
Autophagy. 7:442–444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kang R, Tang D, Livesey KM, Schapiro NE,
Lotze MT and Zeh HJ III: The receptor for advanced glycation
end-products (RAGE) protects pancreatic tumor cells against
oxidative injury. Antioxid Redox Signal. 15:2175–2184. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu M, Bamodu OA, Huang WC, Zucha MA, Lin
YK, Wu ATH, Huang CC, Lee WH, Yuan CC, Hsiao M, et al:
4-Acetylantroquinonol B suppresses autophagic flux and improves
cisplatin sensitivity in highly aggressive epithelial cancer
through the PI3K/Akt/mTOR/p70S6K signaling pathway. Toxicol Appl
Pharmacol. 325:48–60. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Piffoux M, Eriau E and Cassier PA:
Autophagy as a therapeutic target in pancreatic cancer. Br J
Cancer. 124:333–344. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu PF, Tsai KL, Hsu CJ, Tsai WL, Cheng
JS, Chang HW, Shiau CW, Goan YG, Tseng HH, Wu CH, et al: Drug
repurposing screening identifies tioconazole as an ATG4 inhibitor
that suppresses autophagy and sensitizes cancer cells to
chemotherapy. Theranostics. 8:830–845. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shao S, Li S, Qin Y, Wang X, Yang Y, Bai
H, Zhou L, Zhao C and Wang C: Spautin-1, a novel autophagy
inhibitor, enhances imatinib-induced apoptosis in chronic myeloid
leukemia. Int J Oncol. 44:1661–1668. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tam C, Rao S, Waye MMY, Ng TB and Wang CC:
Autophagy signals orchestrate chemoresistance of gynecological
cancers. Biochim Biophys Acta Rev Cancer. 1875:1885252021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chang TC, Yeh CT, Adebayo BO, Lin YC, Deng
L, Rao YK, Huang CC, Lee WH, Wu AT, Hsiao M, et al:
4-Acetylantroquinonol B inhibits colorectal cancer tumorigenesis
and suppresses cancer stem-like phenotype. Toxicol Appl Pharmacol.
288:258–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bamodu OA, Yang CK, Cheng WH, Tzeng DTW,
Kuo KT, Huang CC, Deng L, Hsiao M, Lee WH and Yeh CT:
4-Acetyl-antroquinonol B suppresses SOD2-enhanced cancer stem
cell-like phenotypes and chemoresistance of colorectal cancer cells
by inducing hsa-miR-324 re-expression. Cancers (Basel). 10:2692018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jin H, Yu Y, Hu Y, Lu C, Li J, Gu J, Zhang
L, Huang H, Zhang D, Wu XR, et al: Divergent behaviors and
underlying mechanisms of cell migration and invasion in
non-metastatic T24 and its metastatic derivative T24T bladder
cancer cell lines. Oncotarget. 6:522–536. 2015. View Article : Google Scholar : PubMed/NCBI
|