Introduction
Definitive radiotherapy combined with cisplatin is
widely used for locally advanced squamous cell cancer of the head
and neck (LA-SCCHN) based on Phase III trials showing better
survival compared with radiotherapy alone (1–5).
However, cisplatin is highly nephrotoxic and difficult to
administer in patients with impaired renal function (6). The standard, primary curative
treatment for LA-SCCHN is radiotherapy plus cisplatin, which must
be administered at high doses (100 mg/m2 at 3-week
intervals, a total dosage ≥200 mg/m2) (7,8).
However, in patients with reduced renal function, alternative drugs
may be required. In a randomized Phase III trial evaluating primary
treatment of LA-SCCHN (9),
locoregional control and overall survival were better in patients
who received the combination of radiotherapy and cetuximab compared
with radiotherapy alone. Also, a subsequent analysis found that
quality of life scores were not significantly lower in the group
that received cetuximab plus radiotherapy compared with the group
that received radiotherapy alone (10). Another study found that the 5-year
survival rate was approximately 9% higher in patients with LA-SCCHN
who received radiotherapy plus cetuximab than in those who received
radiotherapy alone (45.6% vs. 36.4%) (11).
Cetuximab is an immunoglobulin-based monoclonal
antibody that is not metabolized or excreted via the kidney. There
are two routes via which antibody preparations can be eliminated
from the body. In the first, antibodies are taken up by
reticuloendothelial cells in the liver and spleen, where some of
the preparation is degraded and the rest becomes bound to the
neonatal Fc receptor and recycled into plasma. In the second,
antibodies can be taken up by cells expressing the target molecule
and phagocytosed by immune cells (12). These elimination routes do not
affect renal function, meaning that dose adjustment according to
renal function is unnecessary. According to some case reports, the
pharmacokinetics of cetuximab do not differ between patients with
renal dysfunction and those with normal renal function. Similar
results were reported from population pharmacokinetics analyses
(13–15). In phase III clinical trials for
LA-SCCHN, the only treatment other than platinum-based chemotherapy
(3,16,17)
to be compared with radiation alone was a cetuximab-based regimen
(9). Thus, cetuximab may be
considered in patients with LA-SCCHN who have renal dysfunction and
cannot tolerate administration of cisplatin. However, there is
still no detailed information on the efficacy and safety of
cetuximab when used in combination with radiotherapy in these
patients.
Cetuximab is classified as a biologic agent and has
a radiosensitizing effect (18–20).
It is approved for the treatment of squamous cell carcinoma of the
head and neck and has a toxicity profile different from that of
cytotoxic agents (21–25). Therefore, if radiotherapy and
cisplatin cannot be administered because of renal dysfunction, we
may choose to use radiotherapy plus cetuximab. However, there are
reports indicating that radiotherapy plus cetuximab is not as
effective as radiotherapy plus cisplatin in LA-SCCHN (21,23–29).
Furthermore, a Phase II clinical study found that acute toxicity
was increased and adherence was reduced in patients who received
radiotherapy plus cetuximab (22).
The toxicity of cetuximab is generally manageable (30–32),
but severe toxicity has been observed (33,34).
Therefore, guidelines recommend that the use of cetuximab instead
of cisplatin must be carefully considered (35).
The randomized Phase III trials to date have
excluded patients with renal dysfunction and have not confirmed the
efficacy or safety of radiotherapy plus cetuximab in these patients
(9). Unlike the populations
typically enrolled in clinical trials, patients with LA-SCCHN
encountered in actual clinical practice often have multiple
comorbidities and adverse prognostic factors. Accordingly, in this
study, we investigated the effects of renal dysfunction on
locoregional control and overall survival in patients with LA-SCCHN
who were treated with the combination of radiotherapy and
cetuximab. Our aim was to determine whether this combination is
safe and effective in these patients.
Patients and methods
Patients
Patients eligible for inclusion in this
single-center retrospective study were those with stage III–IVB
LA-SCCHN with primary sites in the hypopharynx, larynx, and
oropharynx who received radiotherapy plus cetuximab at primary
treatment between July 2013 and October 2018. Patients with cancer
in the nasal cavity, oral cancer, and salivary gland cancer were
excluded. Stage I–II and metastatic or recurrent cases were also
excluded. Patients were also ineligible if they had undergone
surgery or had previously received radiotherapy for head and neck
cancer. The maximum follow-up period was 5 years. Data on the
following patient characteristics were collected: age, sex,
performance status, comorbidities, primary site, human
papillomavirus (HPV) status in cases of oropharynx carcinoma,
clinical stage, tumor stage, lymph node stage, laboratory data,
creatinine clearance (CrCl, calculated by the Cockcroft-Gault
formula), treatment after disease progression, smoking history
(Brinkman index), and history of alcohol consumption. Comorbidities
were quantified using the Charlson Comorbidity Index (CCI)
(36). Details of the cetuximab
and radiation doses used were collected from medical records.
Definition of moderate to severe renal
dysfunction
We compared the prognosis after radiotherapy plus
cetuximab between patients with moderate to severe renal
dysfunction (CrCl <45 ml/min), in whom cisplatin is generally
avoided or a significant dose reduction (50% reduction) is required
(37), and those with normal renal
function.
Treatment plan
All patients received concurrent radiotherapy plus
cetuximab. Cetuximab was administered at a loading dose of 400
mg/m2 followed by 250 mg/m2 starting the
following week. The planned total radiation dose was 70 Gy (2
Gy/day, 5 days/week). Radiotherapy was delivered using
intensity-modulated radiotherapy or three-dimensional conformal
radiotherapy. If chemoradiation enabled removal of residual
disease, salvage surgery was performed.
Assessment of safety and efficacy
We collected data on the reasons for postponing or
discontinuing radiotherapy and/or cetuximab as an indicator of
safety. We also compared the degree of change in clinical
laboratory values before and during treatment. Toxicities during
treatment as confirmed by laboratory data were defined according to
the Common Terminology Criteria for Adverse Events, version 5.0.
Locoregional control and overall survival were compared between
patients with CrCl <45 ml/min (moderate to severe renal
dysfunction) and those with CrCl ≥45 ml/min to evaluate
effectiveness. The maximum observation period was 5 years. Patients
for whom treatment details could not be obtained because of
transfer to another hospital and those who continued treatment
after the end of the observation period were censored. Locoregional
control was defined as no progression of local disease during the
follow-up period. Overall survival was calculated from the first
day of treatment to death or to censoring for any reason. Overall
survival time and locoregional control were compared between
patients with and without renal dysfunction using the Kaplan-Meier
method and the log-rank test. Cox regression models were used to
estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for
background factors contributing to overall survival identified in
univariate and multivariate analyses. Subgroup analyses were
performed in patients aged ≥70 years. Data were also collected on
the response rate to treatment as evaluated by imaging studies as
well as on recurrence or metastasis after treatment and subsequent
treatment after disease progression. Treatment response was
evaluated according to the Response Evaluation Criteria in Solid
Tumors (RECIST) guidelines (38).
Categorical data were compared between groups using Fisher's exact
test. Pre-treatment laboratory values and maximum values during
treatment were compared using the paired t-test or the
Wilcoxon rank-sum test. The degree of variation in laboratory
values among patients with CrCl <45 ml/min and those with CrCl
≥45 ml/min was compared using the two-sample t-test or the
Mann-Whitney U test. All statistical analyses were performed
using IBM SPSS Statistics version 25 (IBM Corp, Armonk, NY).
Two-sided P-values of less than 0.05 were considered statistically
significant.
This study was approved by the Ethical Committee of
the Graduate School of Medicine, Chiba University (accession number
3419) and conducted in accordance with the ethical guidelines for
medical research in humans in Japan. All patients provided written
informed consent to receive radiotherapy plus cetuximab.
Results
Patient characteristics
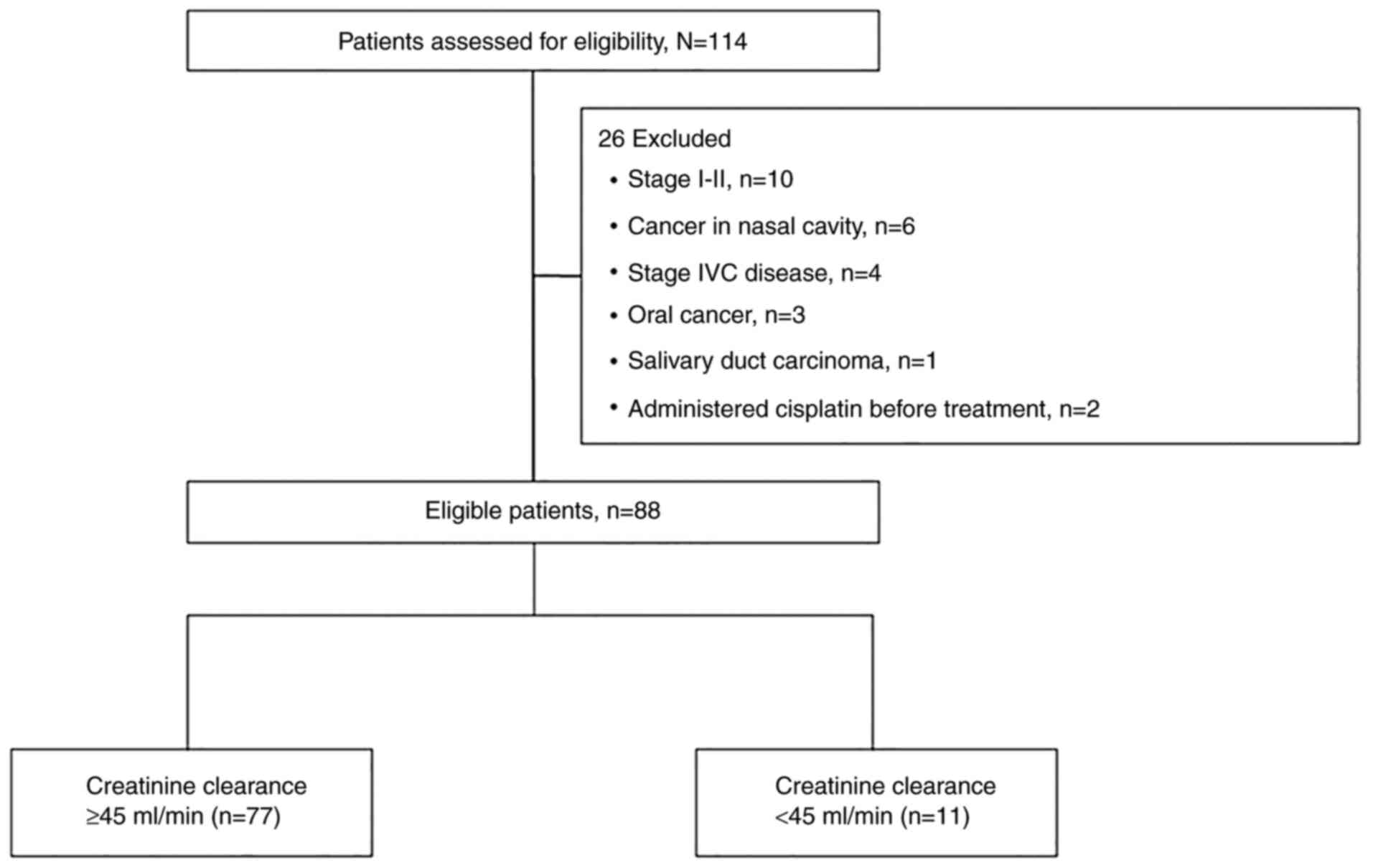
The flow diagram of the study is shown in Fig. 1. The characteristics of the 88
patients included in the study are shown in Table I. Median age was 69 (range 48–84)
years. CrCl was <45 ml/min in 11 patients. The group with CrCl
<45 ml/min was significantly older than the group with CrCl ≥45
ml/min (median age 75 [range 71–84] years vs. 69 [range 48–81]
years; P<0.0001). The proportion of patients with performance
status of ≥2 tended to be higher in the group with CrCl <45
ml/min (18% vs. 4%, P=0.116). Mean CCI (± standard deviation) also
tended to be higher in patients with CrCl <45 ml/min (1.82±1.54
vs. 1.08±1.19; P=0.066). There was no significant difference in
patient background characteristics between the two groups or in
primary site, clinical stage, T classification, N classification,
Brinkman index, or alcohol use, which are generally regarded as
prognostic factors for head and neck cancer.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
|
| CrCl |
|---|
|
|
|
|
|---|
| Variables | Total (n=88) | <45 ml/min
(n=11) | ≥45 ml/min
(n=77) |
|---|
| Median age, years
(range) | 69 (48–84) | 75 (71–84) | 69 (48–81) |
| Median CrCl, ml/min
(range)a | 72.4
(15.1-134.7) | 38.8
(15.1-44.2) | 75.9
(45.4-134.7) |
| Sex, n (%) |
|
|
|
|
Male | 78 (89) | 11
(100) | 67 (87) |
|
Female | 10 (11) | 0 (0) | 10 (13) |
| Performance status,
n (%) |
|
|
|
|
0-1 | 84 (96) | 9
(82) | 75 (96) |
| ≥2 | 4 (4) | 2
(18) | 2 (4) |
| CCI, n (%) |
|
|
|
| 0 | 38 (43) | 3
(27) | 35 (45) |
| ≥1 | 50 (57) | 8
(73) | 42 (55) |
| Primary site, n
(%) |
|
|
|
|
Oropharynx | 37 (42) | 5
(45) | 32 (42) |
|
HPV-positive | 10 | 2 | 8 |
|
HPV-negative | 11 | 2 | 9 |
|
Unknown | 16 | 1 | 15 |
| Larynx
or hypopharynx | 51 (58) | 6
(55) | 45 (58) |
| T classification, n
(%) |
|
|
|
| T1 | 3 (3) | 0 (0) | 3 (4) |
| T2 | 32 (36) | 3
(27) | 29 (38) |
| T3 | 26 (30) | 1 (9) | 25 (32) |
| T4 | 27 (31) | 7
(64) | 20 (26) |
| N classification, n
(%) |
|
|
|
| N0 | 20 (23) | 3
(27) | 17 (22) |
| N1 | 6 (7) | 1 (9) | 5 (6) |
| N2 | 59 (67) | 7
(64) | 52 (68) |
| N3 | 3 (3) | 0 (0) | 3 (4) |
| Clinical stage, n
(%) |
|
|
|
|
III | 17 (19) | 0 (0) | 17 (22) |
|
IVA-B | 71 (81) | 11
(100) | 60 (78) |
| Treatment after
disease progression, n (%) |
|
|
|
|
Yes | 23 (26) | 1 (9) | 22 (29) |
| No | 65 (74) | 10 (91) | 55 (71) |
| Brinkman index, n
(%) |
|
|
|
|
<400 | 31 (35) | 3
(27) | 28 (36) |
|
≥400 | 56 (64) | 8
(73) | 48 (62) |
|
Unknown | 1 (1) | 0 (0) | 1 (1) |
| Alcohol use, n
(%) |
|
|
|
|
Yes | 72 (82) | 10 (91) | 62 (80.5) |
| No | 16 (18) | 1 (9) | 15 (19.5) |
| Median number of
cetuximab cycles (range) | 7
(1–9) | 5
(1–8) | 7
(1–9) |
| Median radiation
dose, Gy (range) | 70 (16–70) | 70 (42–70) | 70 (16–70) |
Safety
The median number of cetuximab doses administered
was significantly lower in the group with CrCl <45 ml/min
(P=0.010). The reasons for discontinuing cetuximab in this group
were deterioration of general condition (n=2), infection (n=2),
fever (n=1), and infusion reaction (n=1). The median radiation dose
was 70 Gy in both groups (Table
I).
Reasons for postponing or discontinuing radiotherapy
and/or cetuximab are shown in Table
II. The incidence of fever tended to be higher in patients with
CrCl ≥45 ml/min (P=0.259). Patients with CrCl <45 ml/min tended
to be more likely to discontinue treatment because of worsening
general condition (P=0.116). The incidence of oral mucositis was
similar between the two groups (P=0.681). There was no significant
difference between the groups in any of the items.
 | Table II.Reasons for postponing or
discontinuing radiation therapy or cetuximab. |
Table II.
Reasons for postponing or
discontinuing radiation therapy or cetuximab.
|
|
| CrCla |
|---|
|
|
|
|
|---|
|
Characteristics | Total, n (%)
(n=88) | <45 ml/min, n
(%) (n=11) | ≥45 ml/min, n (%)
(n=77) |
|---|
| Fever | 19 (21.6) | 1 (9.1) | 18 (23.4) |
| Oral mucositis | 9 (10.2) | 1 (9.1) | 8 (10.4) |
| Pneumonia | 6 (6.8) | 0 (0.0.) | 6 (8.8) |
|
Aspiration pneumonia | 4 (4.6) | 0 (0.0) | 4 (5.2) |
|
Interstitial or aspiration
pneumonia | 1 (1.1) | 0 (0.0) | 1 (1.3) |
|
Drug-induced neutrophil
pneumonia | 1 (1.1) | 0 (0.0) | 1 (1.3) |
| Deterioration of
general condition | 5 (5.7) | 2 (18.2) | 3 (3.9) |
| Salvage
surgery | 4 (4.6) | 0 (0.0) | 4 (5.2) |
| Dermatitis | 3 (3.4) | 1 (9.1) | 2 (2.6) |
| Infusion
reaction | 2 (2.3) | 1 (9.1) | 1 (1.3) |
| Ileus or
sub-ileus | 2 (2.3) | 2 (18.2) | 0 (0.0) |
| Cardiac and
vascular disorder | 2 (2.3) | 0 (0.0) | 2 (2.6) |
| Neutropenia | 1 (1.1) | 0 (0.0) | 1 (1.3) |
| Heart failure
triggered by infection | 1 (1.1) | 1 (9.1) | 0 (0.0) |
| Elevated
hepatobiliary system enzymes | 1 (1.1) | 1 (9.1) | 0 (0.0) |
| Urinary tract
infection | 1 (1.1) | 0 (0.0) | 1 (1.3) |
| Gastrointestinal
bleeding | 1 (1.1) | 0 (0.0) | 1 (1.3) |
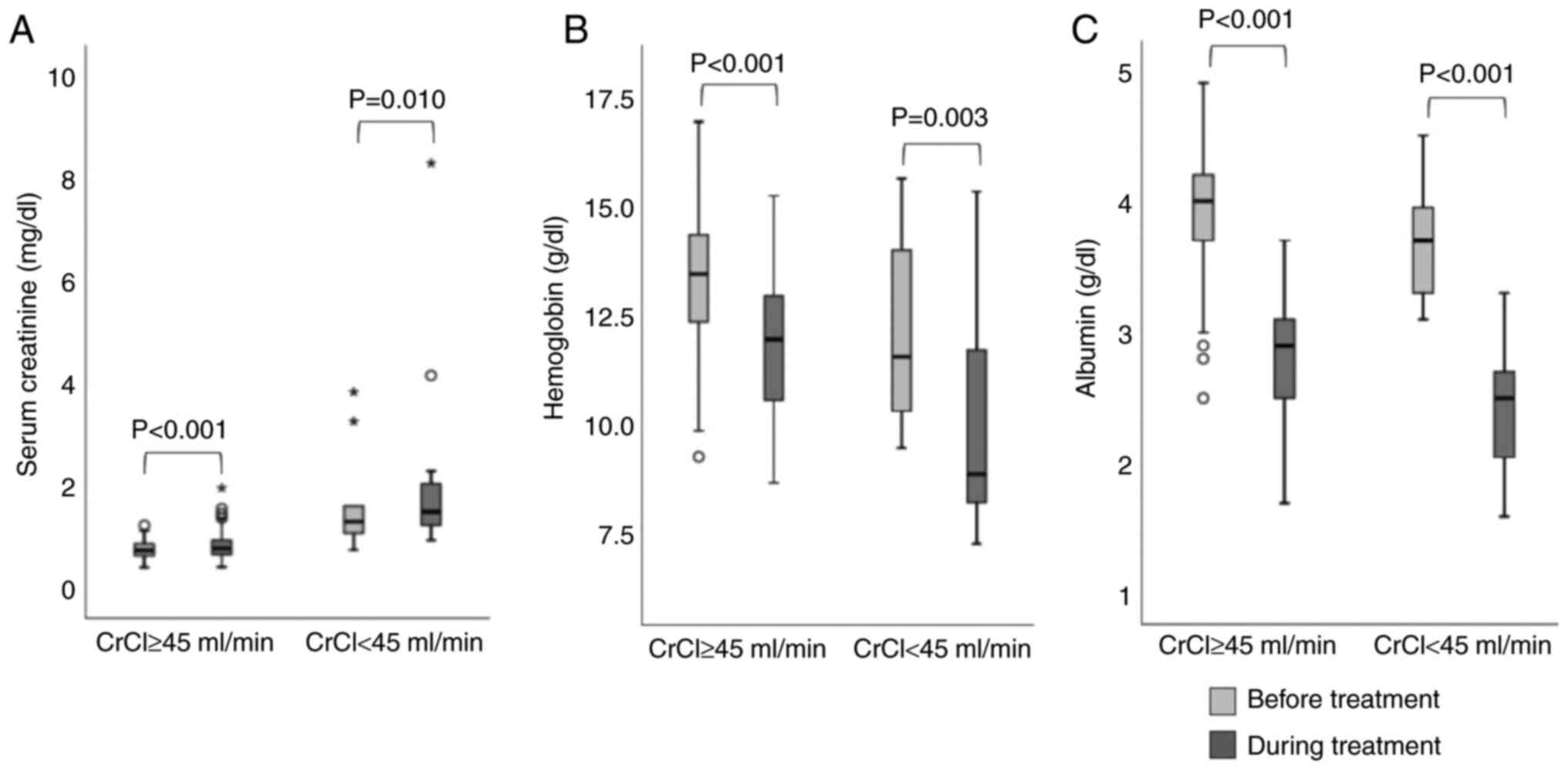
Fig. 2 shows a
box-and-whisker plot of the changes in laboratory values (serum
creatinine, hemoglobin, and albumin) from baseline. The degree of
change in hemoglobin and albumin tended to be greater in patients
with CrCl <45 ml/min, but the difference was not significant.
However, the degree of change in serum creatinine was significantly
greater in patients with CrCl <45 ml/min (median 0.19 [IQR
0.06-0.48] vs. 0.04 [−0.03-0.12]; P=0.006).
Association of response to treatment,
locoregional control, and overall survival with CrCl
Assessment of post-treatment responses based on
imaging studies evaluated according to the RECIST guidelines, there
was no difference in the disease control rate between CrCl <45
ml/min and CrCl ≥45 ml/min (72% vs. 91%; P=0.107). There was no
difference in the proportion of patients with metastasis or
recurrence after treatment between the two groups (46% vs. 43%;
P=0.406).
The data cutoff date for the final analysis of
overall survival was December 10, 2019. The median follow-up period
was 35.9 months. As of the cutoff date, 18.2% of patients with CrCl
<45 ml/min and 20.8% of those with CrCl ≥45 ml/min were still
under observation.
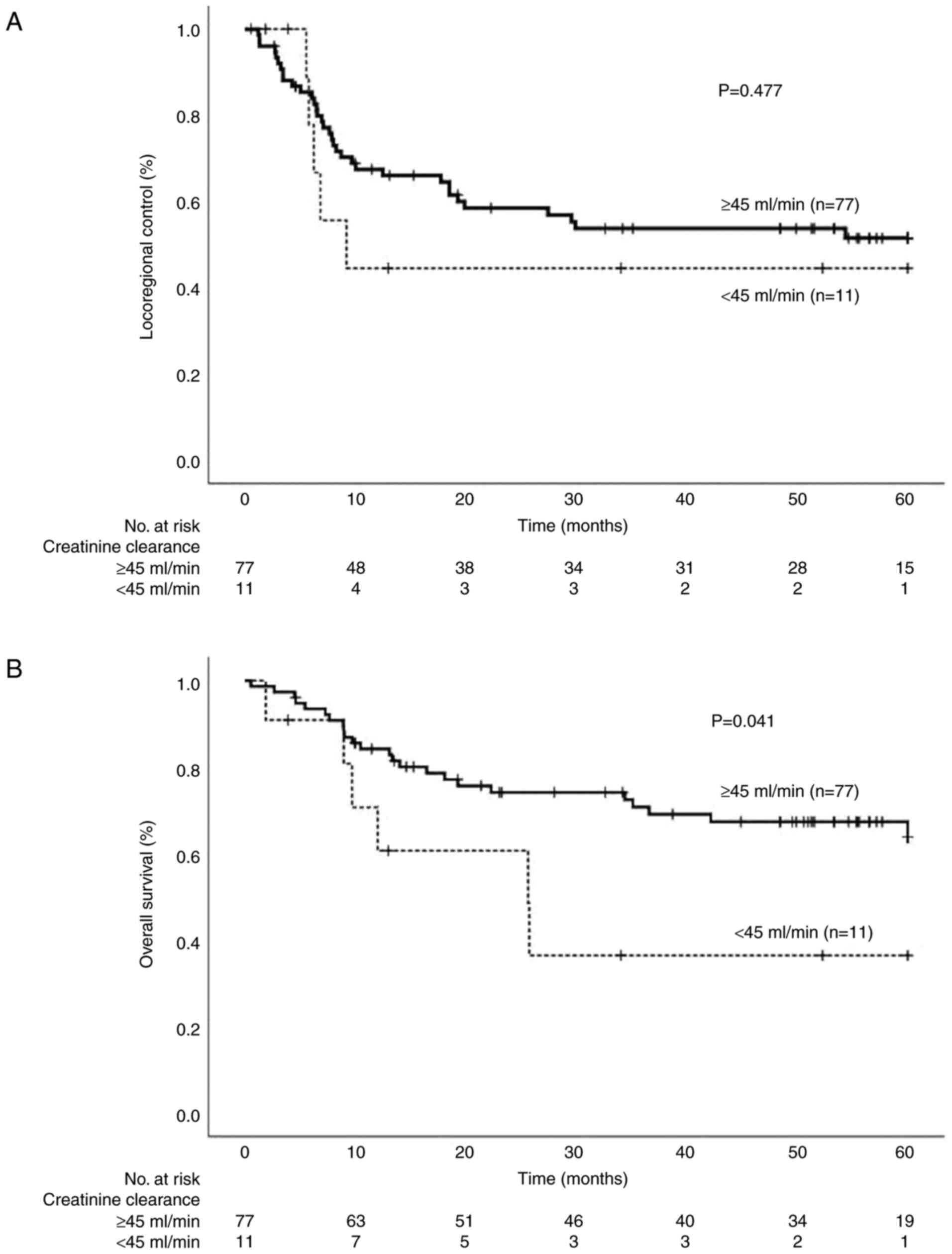
There was no difference in the locoregional control
rate between patients with CrCl <45 ml/min and those with CrCl
≥45 ml/min (Fig. 3A). However,
overall survival was significantly shorter in the group with CrCl
<45 ml/min (Fig. 3B). Patients
with moderate to severe renal dysfunction had median survival of
25.6 months when treated with radiotherapy plus cetuximab. Table III shows the HR for each patient
characteristic identified as contributing to survival by univariate
and multivariate analyses using a Cox proportional hazards model in
all patients. Univariate analysis confirmed that CrCl <45 ml/min
was a significant adverse prognostic factor (HR 2.48, 95% CI
1.01-6.12; P=0.048), as was CCI of ≥1 (HR 2.25, 95% CI 1.00-5.06;
P=0.050). There was no statistically significant difference in
prognosis between the two groups of patients over 70 years of age
and under 70 years of age (HR 1.407, 95% CI 0.686-2.886; P=0.351).
Multivariate analysis was performed by incorporating up to three
variables into the Cox regression model based on the number of
mortality events. Given that calculation of CrCl includes age, we
performed the multivariate analysis without incorporating age and
CrCl into one model. CCI includes items for moderate to severe
renal dysfunction, but the definition is strict, with serum
creatinine set as >3 mg/dl. Only two patients with CrCl <45
ml/min had a serum creatinine level of >3 mg/dl. Furthermore,
there was no correlation between CCI and whether CrCl was <45
ml/min. We added the primary site to the covariates because it is
known to affect prognosis. For these reasons, we used the following
three covariates in the multivariate analysis: whether the CCI
score was ≥1, the primary site, and whether the CrCl was <45
ml/min. In the multivariate analysis, the significant association
remained between shorter overall survival and CrCl <45 ml/min
(adjusted HR 2.52, 95% CI 1.01-6.30; P=0.048).
 | Table III.Univariate and multivariate analyses
of prognostic factors using a Cox proportional hazards regression
model for overall survival in all patients. |
Table III.
Univariate and multivariate analyses
of prognostic factors using a Cox proportional hazards regression
model for overall survival in all patients.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | P-value | HR | P-value | HR |
|---|
| Age (years) | 0.351 |
|
|
|
|
<70 |
| 1 |
|
|
|
≥70 |
| 1.41 |
|
|
| Sex | 0.401 |
|
|
|
|
Male |
| 1 |
|
|
|
Female |
| 0.54 |
|
|
| Performance
status | 0.300 |
|
|
|
|
0-1 |
| 1 |
|
|
| ≥2 |
| 2.17 |
|
|
| CCI | 0.050a |
| 0.120 |
|
| 0 |
| 1 |
| 1 |
| ≥1 |
| 2.25 |
| 1.93 |
| Primary site | 0.083 |
| 0.115 |
|
|
Oropharynx |
| 1 |
| 1 |
| Larynx
or hypopharynx |
| 2.05 |
| 1.94 |
| Clinical stage | 0.389 |
|
|
|
|
III |
| 1 |
|
|
|
IVA-B |
| 1.53 |
|
|
| CrCl | 0.048a |
| 0.048a |
|
| ≥45
ml/min |
| 1 |
| 1 |
| <45
ml/min |
| 2.48 |
| 2.52 |
| Treatment after
disease progression | 0.123 |
|
|
|
| No |
| 1 |
|
|
|
Yes |
| 2.48 |
|
|
| Brinkman index | 0.602 |
|
|
|
|
<400 |
| 1 |
|
|
|
≥400 |
| 1.23 |
|
|
| Alcohol use | 0.984 |
|
|
|
| No |
| 1 |
|
|
|
Yes |
| 1.01 |
|
|
Subgroup analysis in patients aged 70
years or older
As shown in Table
III, age tended to affect prognosis, so we could not rule out
the possibility that age was a confounding factor in the poorer
prognosis seen in patients with CrCl <45 ml/min. Therefore, a
subgroup analysis was performed in patients aged ≥70 years
(Table IV). This cutoff was set
because it was the median age in the full sample, meaning that
about half the patients could be included in the subgroup analysis.
In univariate analysis, the primary site was a significant
prognostic factor (HR 9.37, 95% CI 1.24-71.03; P=0.03). CrCl <45
ml/min tended to be a marginally significant adverse prognostic
factor (HR 2.45, 95% CI 0.88-6.83; P=0.086). In multivariate
analysis, the primary site remained a significant prognostic factor
(adjusted HR 13.56, 95% CI 1.73-106.67; P=0.013). After adjustment
for primary site, CrCl <45 ml/min was a significant adverse
prognostic factor (adjusted HR 4.16, 95% CI 1.39-12.46; P=0.011).
There was no significant difference in mean CCI between patients
with CrCl ≥45 ml/min and those with CrCl <45 ml/min (1.57±1.31
vs. 1.82±1.54; P=0.51).
 | Table IV.Univariate and multivariate analyses
of prognostic factors using a Cox proportional hazards regression
model for overall survival in a subgroup of patients aged 70 years
or older. |
Table IV.
Univariate and multivariate analyses
of prognostic factors using a Cox proportional hazards regression
model for overall survival in a subgroup of patients aged 70 years
or older.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variables | Total, n (%)
(n=41) | P-value | HR | P-value | HR |
|---|
| Sex |
| 0.625 |
|
|
|
|
Male | 40 (98) |
| 1 |
|
|
|
Female | 1 (2) |
| 0.05 |
|
|
| Performance
status |
| 0.631 |
|
|
|
|
0-1 | 38 (93) |
| 1 |
|
|
| ≥2 | 3 (7) |
| 1.67 |
|
|
| CCI |
| 0.184 |
|
|
|
| 0 | 11 (27) |
| 1 |
|
|
| ≥1 | 30 (73) |
| 2.74 |
|
|
| Primary site |
| 0.030a |
| 0.013a |
|
|
Oropharynx | 14 (34) |
| 1 |
| 1 |
| Larynx
or hypopharynx | 27 (66) |
| 9.37 |
| 13.67 |
| Clinical stage |
| 0.532 |
|
|
|
|
III | 9
(22) |
| 1 |
|
|
|
IVA-B | 32 (78) |
| 1.50 |
|
|
| CrCl |
| 0.086 |
| 0.011a |
|
| ≥45
ml/min | 30 (73) |
| 1 |
| 1 |
| <45
ml/min | 11 (27) |
| 2.45 |
| 4.16 |
| Treatment after
disease progression |
| 0.558 |
|
|
|
| No | 32 (78) |
| 1 |
|
|
|
Yes | 9
(22) |
| 1.41 |
|
|
| Brinkman index |
| 0.287 |
|
|
|
|
<400 | 14 (34) |
| 1 |
|
|
|
≥400 | 27 (66) |
| 1.85 |
|
|
| Alcohol use |
| 0.418 |
|
|
|
| No | 5
(12) |
| 1 |
|
|
|
Yes | 36 (88) |
| 2.32 |
|
|
Discussion
In this study, renal dysfunction did not affect
locoregional control but had a significant effect on overall
survival in patients with LA-SCCHN treated with radiotherapy plus
cetuximab. This finding may be a statistical artifact but could
also reflect the impact of differences in patient characteristics.
Patients with CrCl <45 ml/min tended to discontinue or postpone
cetuximab or radiotherapy because of worsening general condition.
These patients may have had background factors that affected their
ability to tolerate this treatment. In general, this treatment may
lead to feeding problems due to damage to the oral mucosa and
dehydration due to the inability to drink water. Patients with
impaired kidney function are more susceptible to the effects of
dehydration. In our study, the magnitude of the change in serum
creatinine during treatment in these patients was severe and
treatment was poorly tolerated in most patients. Deterioration of
renal function and general condition due to dehydration may lessen
the feasibility of additional treatments if the disease progresses
after treatment. In our study, only 9% of patients with CrCl <45
ml/min received therapy after disease progression. In contrast, 29%
of patients with CrCl ≥45 ml/min received additional treatment.
Furthermore, the fact that cisplatin can be used to treat disease
progression in patients with CrCl ≥45 ml/min may have had a
positive impact on prognosis. The combination of poor prognostic
factors in the original renal dysfunction (39) and the abovementioned negative
influencing factors may have had a significant negative impact on
overall survival in patients with CrCl <45 ml/min. There may not
be a single factor; rather, the additive involvement of multiple
factors may be responsible for the significantly shorter overall
survival in patients with CrCl <45 ml/min, even though the
locoregional control rate and disease control rate were
comparable.
A subgroup analysis of patients aged ≥70 years was
performed to investigate the possibility of age as a confounding
factor and also showed that decreased renal function was an adverse
prognostic factor for overall survival. These findings suggest that
prognosis is poor in patients with comorbidities that reduce CrCl
even when they are treated with radiotherapy plus cetuximab.
However, in this study, 82% of these patients had a performance
status of 0–1 and were deemed suitable for this treatment. Given
the subjective nature of clinicians' judgment when evaluating
performance status, objective indicators such as CrCl may be more
useful when considering the treatment options.
Patients with head and neck squamous cell carcinoma,
especially those with involvement of the oropharynx, have better
prognosis if their tumors are HPV-positive than if they are
HPV-negative (40,41). In our study, about 40% of patients
in both groups had oropharyngeal carcinoma. However, HPV status was
unknown in many cases because the study included patients who were
treated at a time when it was not yet common practice to routinely
test for HPV, even in patients with oropharyngeal carcinoma.
Therefore, we did not include HPV status in our analysis. However,
despite these missing data, about 50% of patients with oropharynx
carcinoma in both groups were positive for HPV. We addressed this
problem by adjusting the prognostic risk using the difference in
the primary site as a covariate in multivariate analysis because
about 40% of patients in both groups had oropharyngeal cancer.
The results of a randomized Phase III trial by
Bonner et al (9) led to the
approval of cetuximab for LA-SCCHN by the US Food and Drug
Administration in 2006. Thereafter, there was an increase in use of
cetuximab instead of cisplatin for LA-SCCHN (9). However, a later report suggested that
the therapeutic effect of radiotherapy plus cetuximab in LA-SCCHN
was not as good as that of chemoradiotherapy combined with
cisplatin (21,23,26–29).
RTOG 1016, a non-inferiority trial of radiotherapy plus cetuximab
vs. radiotherapy plus cisplatin in patients with HPV-positive
oropharyngeal cancer conducted by NRG Oncology, found that
prognosis was significantly worse after radiotherapy plus cetuximab
(24). The results of the
De-ESCALaTE HPV trial in low-risk HPV-positive oropharyngeal cancer
were similar (25). In these
studies, there was a difference in the profile of moderate to
severe acute and late toxicities between cetuximab and cisplatin
but the proportion of patients experiencing at least one such event
was similar. However, despite these problems, cetuximab remains an
alternative for patients with renal dysfunction who cannot tolerate
cisplatin because of its toxicity profile. Because adverse events
associated with cetuximab are mainly reversible side effects such
as dermatitis, it is less likely than cisplatin to cause
irreversible renal dysfunction (24,25).
In our study, serum creatinine was transiently elevated but
reversible even in patients with CrCl <45 ml/min, except in
those whose general condition deteriorated during treatment with
radiation plus cetuximab. Disease control was also achieved in
patients with CrCl <45 ml/min as effectively as in those with
CrCl ≥45 ml/min. The results suggest that it may be possible to
reduce symptoms caused by head and neck cancer (e.g., pain,
dysphagia) even in situations where treatment options are limited
by renal dysfunction. These data can be shown as an advantage of
using radiotherapy plus cetuximab in patients with renal
dysfunction. However, serum creatinine was greatly elevated in
patients with CrCl <45 ml/min, likely as a result of dehydration
caused by poor feeding and drinking due to stomatitis. Although
cetuximab does not directly damage the kidneys, dehydration-which
can also occur with radiation plus cetuximab-does affect kidney
function and this should be considered when choosing a treatment
for patients with impaired kidney function. This worsening of the
patient's general condition will further limit the treatment
options for patients with renal dysfunction in the event of disease
progression or recurrence.
The MACH-NC (Meta-Analyses of Chemotherapy in Head
and Neck Cancer) Study Group showed a modest but significant
survival benefit from addition of chemotherapy to radiotherapy in
patients with head and neck squamous cell carcinoma, except in
those aged ≥70 years (42). A
subgroup analysis of the 5-year follow-up study by Bonner et
al (11) showed that the
combination of radiotherapy and cetuximab was less beneficial in
patients aged ≥65 years. However, the eligibility criteria in the
above-mentioned studies only referred to normal renal function. If
judged based solely on the serum creatinine level, renal function
might have been overestimated in the elderly patients in those
studies, which could have affected prognosis. Another report
published before cetuximab was commonly used to treat head and neck
cancer suggested that comorbidities are a poor prognostic factor in
older patients with head and neck cancer (43). The authors of that report concluded
that comorbidity status, but not age, was an independent prognostic
factor. Our findings support this view. In general, elderly
patients with head and neck cancer are a group with many
comorbidities, and the same was true in our study. The CCI value
could not predict the prognosis because comorbidities are so common
in the population over 70 years of age. However, CrCl could predict
the prognosis, suggesting that a decrease in overall organ function
affects renal function, which in turn affects the prognosis.
Honma et al (44) found that renal function was a
prognostic factor in oropharyngeal squamous cell carcinoma. They
speculated that renal dysfunction could have attenuated the
outcomes of chemoradiotherapy by making it more difficult to
administer cisplatin. They also suggested that cetuximab could be
an alternative treatment in the presence of renal dysfunction,
pointing out that their data were collected before cetuximab was
available. However, in our study, which also included patients with
non-oropharyngeal cancers, prognosis was poor even with the use of
cetuximab. Therefore, based solely on pharmacokinetic
considerations, cetuximab should not be used in place of cisplatin
in patients with renal dysfunction. Consideration should be given
to the patient's general condition and the tolerability of
treatment.
We defined a cutoff value of CrCl <45 ml/min as
moderate to severe renal dysfunction. We had originally intended
this to be CrCl <30 ml/min, which is the level at which
cisplatin should be avoided (37),
but this would have limited the number of cases for analysis. Even
for patients with 30 ml/min ≤ CrCl <45 ml/min, a reduction of
cisplatin to 50% is recommended (37). The standard, primary curative
treatment for LA-SCCHN is radiotherapy plus cisplatin, which must
be administered at high doses (100 mg/m2 at 3-week
intervals, total dosage ≥200 mg/m2) (7,8).
When clinicians consider reducing the dose of cisplatin from 100
mg/m2 to 50 mg/m2, they will likely be
concerned about a decrease in therapeutic effect. In such cases,
many clinicians may use cetuximab as an alternative with the hope
of maintaining the therapeutic effect. Considering this, we believe
that a cutoff value of CrCl <45 ml/min is not an obstacle to
resolving clinical questions in actual practice.
The regimens used for primary curative treatment of
LA-SCCHN are platinum-based regimens and cetuximab. The
recommendation for radiotherapy plus cetuximab has been lowered
because of recent clinical studies showing that it is less
effective compared with cisplatin (21,23–29).
A cisplatin-based regimen requires high doses (100 mg/m2
per dose) (7,8). For postoperative chemotherapy,
cisplatin administered in weekly fractions at a single dose of 40
mg/m2 was non-inferior to the high-dose regimen
(45). However, this is not
evidence for the primary curative treatment of LA-SCCHN and thus
cannot be applied as such. Guidelines on primary curative treatment
for LA-SCCHN suggest that high-dose cisplatin may be more effective
than weekly cisplatin, although there has been no direct comparison
(35). A carboplatin-based regimen
is also recommended as primary curative treatment for LA-SCCHN
(17). However, it is difficult to
use in patients with renal dysfunction because the dosage is not
based on renal function calculated using Calvert's formula. To
determine the optimal primary curative treatment for LA-SCCHN,
further studies are needed in special populations such as patients
with renal dysfunction.
Although we found that radiotherapy plus cetuximab
has a poor prognosis in patients with CrCl <45 ml/min, this does
not mean the treatment itself is ineffective. Radiotherapy plus
cetuximab may still achieve a better prognosis than radiation alone
even if CrCl is <45 ml/min. However, the prognosis is worse
overall, so patients should be selected for treatment very
carefully. Particular caution is advised in elderly patients with
renal dysfunction who are ineligible for treatment with cisplatin
and have multiple comorbidities, even if there are no
pharmacokinetic problems. However, the opposite line of thinking
may be possible; that is, radiotherapy plus cetuximab may improve
prognosis in elderly patients without comorbidities that cause
renal dysfunction. However, cisplatin would still be considered the
best option for these patients.
Our analysis of data from actual clinical practice
found that patients with LA-SCCHN who had renal dysfunction due to
aging or complications had poor prognosis when treated with
radiotherapy plus cetuximab. This information was not available
from clinical trials and should be useful when treating elderly
patients with LA-SCCHN in routine clinical practice.
The main limitations of this study are that it had a
single-center retrospective design and included a small number of
cases, which meant that it was not possible to plan the statistical
analysis in advance. Therefore, our finding of a significant
reduction in overall survival despite no difference in locoregional
control may have been a statistical artifact. In addition, we did
not compare local control and overall survival between radiotherapy
plus cetuximab and radiotherapy alone in a population with renal
dysfunction, so we cannot use the results of our study to recommend
radiotherapy alone for patients with renal dysfunction. The results
only showed that patients with renal dysfunction who received
radiotherapy plus cetuximab had a worse prognosis than patients
without renal dysfunction. Therefore, the data may only indicate
that patients with renal dysfunction have a poor prognosis.
In conclusion, this study found that patients with
LA-SCCHN and renal dysfunction who were treated with radiotherapy
plus cetuximab had a poor prognosis. The possibility that the
prognosis may be poor even if this treatment is administered should
be borne in mind when considering the treatment strategy for these
patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CI conceived and designed the study. CI wrote the
paper. CI, YT, TS and II analyzed the data and critically revised
the manuscript. CI and TS confirm the authenticity of all the raw
data. CI, HS, KY, AI, MA, AT, TS, YT, TH and II were involved in
the interpretation of the data and preparation of the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This retrospective study was approved by the Ethical
Committee of the Graduate School of Medicine, Chiba University
(approval number 3419; Chiba, Japan) and conducted in accordance
with the ethical guidelines for medical research in humans in
Japan. All patients provided written informed consent to receive
radiotherapy plus cetuximab.
Patient consent for publication
Not applicable.
Competing interests
TY reports grants and personal fees from Merck
Chemicals, during the study period; and grants and personal fees
from Eli Lilly, grants and personal fees from Ono Pharmaceutical
Co., grants and personal fees from Chugai Pharmaceutical Co.,
grants and personal fees from MSD, grants and personal fees from
Takeda, grants from Daiichi Sankyo, personal fees from Novartis,
grants from Kyowa-Hakko Kirin, personal fees from Boehringer
Ingelheim, personal fees from AstraZeneca, and grants and personal
fees from Taiho Pharmaceutical Co. outside the submitted work. The
other authors declare that they have no competing interests.
References
|
1
|
Brizel DM, Albers ME, Fisher SR, Scher RL,
Richtsmeier WJ, Hars V, George SL, Huang AT and Prosnitz LR:
Hyperfractionated irradiation with or without concurrent
chemotherapy for locally advanced head and neck cancer. N Engl J
Med. 338:1798–1804. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forastiere AA, Goepfert H, Maor M, Pajak
TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et
al: Concurrent chemotherapy and radiotherapy for organ preservation
in advanced laryngeal cancer. N Engl J Med. 349:2091–2098. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adelstein DJ, Li Y, Adams GL, Wagner H Jr,
Kish JA, Ensley JF, Schuller DE and Forastiere AA: An intergroup
phase III comparison of standard radiation therapy and two
schedules of concurrent chemoradiotherapy in patients with
unresectable squamous cell head and neck cancer. J Clin Oncol.
21:92–98. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Denis F, Garaud P, Bardet E, Alfonsi M,
Sire C, Germain T, Bergerot P, Rhein B, Tortochaux J and Calais G:
Final results of the 94–01 French head and neck oncology and
radiotherapy group randomized trial comparing radiotherapy alone
with concomitant radiochemotherapy in advanced-stage oropharynx
carcinoma. J Clin Oncol. 22:69–76. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Corvo R: Evidence-based radiation oncology
in head and neck squamous cell carcinoma. Radiother Oncol.
85:156–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bernier J and Schneider D: Cetuximab
combined with radiotherapy: An alternative to chemoradiotherapy for
patients with locally advanced squamous cell carcinomas of the head
and neck? Eur J Cancer. 43:35–45. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spreafico A, Huang SH, Xu W, Granata R,
Liu CS, Waldron JN, Chen E, Ringash J, Bayley A, Chan KK, et al:
Impact of cisplatin dose intensity on human papillomavirus-related
and -unrelated locally advanced head and neck squamous cell
carcinoma. Eur J Cancer. 67:174–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Szturz P, Wouters K, Kiyota N, Tahara M,
Prabhash K, Noronha V, Adelstein D, Van Gestel D and Vermorken JB:
Low-Dose vs. High-dose cisplatin: Lessons learned from 59
chemoradiotherapy trials in head and neck cancer. Front Oncol.
9:862019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Curran D, Giralt J, Harari PM, Ang KK,
Cohen RB, Kies MS, Jassem J, Baselga J, Rowinsky EK, Amellal N, et
al: Quality of life in head and neck cancer patients after
treatment with high-dose radiotherapy alone or in combination with
cetuximab. J Clin Oncol. 25:2191–2197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bonner JA, Harari PM, Giralt J, Cohen RB,
Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J, et al:
Radiotherapy plus cetuximab for locoregionally advanced head and
neck cancer: 5-year survival data from a phase 3 randomised trial,
and relation between cetuximab-induced rash and survival. Lancet
Oncol. 11:21–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu L: Pharmacokinetics of monoclonal
antibodies and Fc-fusion proteins. Protein Cell. 9:15–32. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dirks NL, Nolting A, Kovar A and Meibohm
B: Population pharmacokinetics of cetuximab in patients with
squamous cell carcinoma of the head and neck. J Clin Pharmacol.
48:267–278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aldoss IT, Plumb T, Zhen WK, Lydiatt DD
and Ganti AK: Cetuximab in hemodialysis: A case report. Head Neck.
31:1647–1650. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krens LL, Baas JM, Verboom MC, Paintaud G,
Desvignes C, Guchelaar HJ and Gelderblom H: Pharmacokinetics and
safety of cetuximab in a patient with renal dysfunction. Cancer
Chemother Pharmacol. 73:1303–1306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Forastiere AA, Zhang Q, Weber RS, Maor MH,
Goepfert H, Pajak TF, Morrison W, Glisson B, Trotti A, Ridge JA, et
al: Long-term results of RTOG 91–11: A comparison of three
nonsurgical treatment strategies to preserve the larynx in patients
with locally advanced larynx cancer. J Clin Oncol. 31:845–852.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bourhis J, Sire C, Graff P, Grégoire V,
Maingon P, Calais G, Gery B, Martin L, Alfonsi M, Desprez P, et al:
Concomitant chemoradiotherapy versus acceleration of radiotherapy
with or without concomitant chemotherapy in locally advanced head
and neck carcinoma (GORTEC 99–02): An open-label phase 3 randomised
trial. Lancet Oncol. 13:145–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang SM and Harari PM: Modulation of
radiation response after epidermal growth factor receptor blockade
in squamous cell carcinomas: Inhibition of damage repair, cell
cycle kinetics, and tumor angiogenesis. Clin Cancer Res.
6:2166–2174. 2000.PubMed/NCBI
|
|
19
|
Milas L, Mason K, Hunter N, Petersen S,
Yamakawa M, Ang K, Mendelsohn J and Fan Z: In vivo enhancement of
tumor radioresponse by C225 antiepidermal growth factor receptor
antibody. Clin Cancer Res. 6:701–708. 2000.PubMed/NCBI
|
|
20
|
Harari PM and Huang SM: Head and neck
cancer as a clinical model for molecular targeting of therapy:
Combining EGFR blockade with radiation. Int J Radiat Oncol Biol
Phys. 49:427–433. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koutcher L, Sherman E, Fury M, Wolden S,
Zhang Z, Mo Q, Stewart L, Schupak K, Gelblum D, Wong R, et al:
Concurrent cisplatin and radiation versus cetuximab and radiation
for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol
Phys. 81:915–922. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Magrini SM, Buglione M, Corvo R, Pirtoli
L, Paiar F, Ponticelli P, Petrucci A, Bacigalupo A, Crociani M,
Lastrucci L, et al: Cetuximab and radiotherapy versus cisplatin and
radiotherapy for locally advanced head and neck cancer: A
randomized phase II trial. J Clin Oncol. 34:427–435. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bauml JM, Vinnakota R, Anna Park YH, Bates
SE, Fojo T, Aggarwal C, Di Stefano J, Knepley C, Limaye S, Mamtani
R, et al: Cisplatin versus cetuximab with definitive concurrent
radiotherapy for head and neck squamous cell carcinoma: An analysis
of Veterans Health Affairs data. Cancer. 125:406–415. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gillison ML, Trotti AM, Harris J, Eisbruch
A, Harari PM, Adelstein DJ, Jordan RCK, Zhao W, Sturgis EM,
Burtness B, et al: Radiotherapy plus cetuximab or cisplatin in
human papillomavirus-positive oropharyngeal cancer (NRG Oncology
RTOG 1016): A randomised, multicentre, non-inferiority trial.
Lancet. 393:40–50. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mehanna H, Robinson M, Hartley A, Kong A,
Foran B, Fulton-Lieuw T, Dalby M, Mistry P, Sen M, O'Toole L, et
al: Radiotherapy plus cisplatin or cetuximab in low-risk human
papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An
open-label randomised controlled phase 3 trial. Lancet. 393:51–60.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Petrelli F, Coinu A, Riboldi V, Borgonovo
K, Ghilardi M, Cabiddu M, Lonati V, Sarti E and Barni S:
Concomitant platinum-based chemotherapy or cetuximab with
radiotherapy for locally advanced head and neck cancer: A
systematic review and meta-analysis of published studies. Oral
Oncol. 50:1041–1048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang C, Chan C, Jiang W, Murphy JD, von
Eyben R, Colevas AD, Pinto H, Lee-Enriquez N, Kong C and Le QT:
Concurrent cetuximab versus platinum-based chemoradiation for the
definitive treatment of locoregionally advanced head and neck
cancer. Head Neck. 37:386–392. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Riaz N, Sherman E, Koutcher L, Shapiro L,
Katabi N, Zhang Z, Shi W, Fury M, Wong R, Wolden S, et al:
Concurrent chemoradiotherapy with cisplatin versus cetuximab for
squamous cell carcinoma of the head and neck. Am J Clin Oncol.
39:27–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiang M, Holsinger FC, Colevas AD, Chen
MM, Le QT and Beadle BM: Survival of patients with head and neck
cancer treated with definitive radiotherapy and concurrent
cisplatin or concurrent cetuximab: A Surveillance, Epidemiology,
and End Results-Medicare analysis. Cancer. 124:4486–4494. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Frampton JE: Spotlight on cetuximab in
squamous cell carcinoma of the head and necky. BioDrugs.
25:129–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Villavicencio M, Granados-García M,
Vilajosana E and Domínguez-Cherit J: Management of radiodermatitis
associated with cetuximab in squamous cell carcinomas of the head
and neck. Int J Dermatol. 56:602–609. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lugtenberg RT, Boers-Doets CB, Witteveen
PO, van Herpen CML, Wymenga ANM, de Groot JWB, Hoeben A, Del Grande
C, van Doorn B, Koldenhof JJ, et al: Prospective practice survey of
management of cetuximab-related skin reactions. Support Care
Cancer. 29:3497–3506. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bonomo P, Loi M, Desideri I, Olmetto E,
Delli Paoli C, Terziani F, Greto D, Mangoni M, Scoccianti S,
Simontacchi G, et al: Incidence of skin toxicity in squamous cell
carcinoma of the head and neck treated with radiotherapy and
cetuximab: A systematic review. Crit Rev Oncol Hematol. 120:98–110.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mayfield JD, Mercado CE, Kaye FJ and
Mendenhall WM: Cetuximab-associated pulmonary toxicity in
concurrent chemoradiation for the treatment of a squamous cell
carcinoma of the head and neck. Head Neck. 41:E55–E58. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Machiels JP, Rene Leemans C, Golusinski W,
Grau C, Licitra L and Gregoire V; EHNS Executive Board. Electronic
address, : simplesecretariat@ehns.org;
ESMO Guidelines Committee. Electronic address: simpleclinicalguidelines@esmo.org;
ESTRO Executive Board. Electronic address: simpleinfo@estro.org: Squamous
cell carcinoma of the oral cavity, larynx, oropharynx and
hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 31:1462–1475. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: Development and validation. J Chronic Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kintzel PE and Dorr RT: Anticancer drug
renal toxicity and elimination: Dosing guidelines for altered renal
function. Cancer Treat Rev. 21:33–64. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Torres da Costa E Silva V, Costalonga EC,
Coelho FO, Caires RA and Burdmann EA: Assessment of kidney function
in patients with cancer. Adv Chronic Kidney Dis. 25:49–56. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ragin CC and Taioli E: Survival of
squamous cell carcinoma of the head and neck in relation to human
papillomavirus infection: Review and meta-analysis. Int J Cancer.
121:1813–1820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ang KK, Harris J, Wheeler R, Weber R,
Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C,
et al: Human papillomavirus and survival of patients with
oropharyngeal cancer. N Engl J Med. 363:24–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pignon JP, le Maitre A, Maillard E and
Bourhis J; MACH-NC Collaborative Group, : Meta-analysis of
chemotherapy in head and neck cancer (MACH-NC): An update on 93
randomised trials and 17,346 patients. Radiother Oncol. 92:4–14.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sanabria A, Carvalho AL, Vartanian JG,
Magrin J, Ikeda MK and Kowalski LP: Comorbidity is a prognostic
factor in elderly patients with head and neck cancer. Ann Surg
Oncol. 14:1449–1457. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Homma A, Hayashi R, Kawabata K, Fujii T,
Iwae S, Hasegawa Y, Nibu K, Kato T, Shiga K, Matsuura K, et al:
Association of impaired renal function and poor prognosis in
oropharyngeal squamous cell carcinoma. Head Neck. 38:1495–1500.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kiyota N, Tahara M, Fujii H, Yamazaki T,
Mitani H, Iwae S, Fujimoto Y, Onozawa Y, Hanai N, Ogawa T, et al:
Phase II/III trial of post-operative chemoradiotherapy comparing
3-weekly cisplatin with weekly cisplatin in high-risk patients with
squamous cell carcinoma of head and neck (JCOG1008). J Clin Oncol.
38 (15_suppl):S65022020. View Article : Google Scholar
|