Introduction
Chemotherapy may induce both acute toxicities
(mucositis, infection, bleeding) and late toxicities (atrophy,
xerostomia) in patients with cancer (1). Oral mucositis is one of the most
common oral complications resulting from cancer chemotherapy.
According to an article published in 2020, globally 30–40% of
patients with cancer treated with chemotherapy develop oral or
gastro-intestinal mucositis (2).
In patients with head and neck cancer (HNC) treated with both
chemo- and radiotherapy, 90% of the patients developed mucositis
(3). Oral mucositis is often
accompanied with acute oral pain and a compromised nutritional
status, in addition to reduced kidney, liver and salivary gland
function (1,4). To the best of our knowledge, the
mechanism of chemotherapy-induced mucositis is unknown and likely
involves a multistep process, which may be associated with several
factors. Chemotherapy can have adverse effects on any healthy
rapidly multiplying cells including the mucosal lining of the mouth
and gastro-intestinal tract. This may lead to the loss of the
renewal capacity of the epithelium, which ultimately results in
atrophy and ulcers (5–7). Mucositis is not limited to the
epithelium; it might involve all the mucosal tissues. The severity
of mucositis depends not only on anticancer regimens but also on
patients' characteristics. Female patients, elderly patients and
patients with a dihydropyrimidine dehydrogenase deficiency may
develop severe 5-FU-induced mucositis (8). There are very few therapeutic options
available for mucositis prevention or treatment, and unfortunately
their effectiveness is poor or negligible (7,9–14).
Therefore, novel strategies to alleviate mucositis in patients with
cancer are urgently needed.
Elental® (Ajinomoto Co., Inc.) is a
widely used nutritional supplement in Japan for patients suffering
from malnutrition. This elemental diet (ED) has a special formula
consisting of 18 amino acids with high L-glutamine (2,415 mg/100
gm) and L-leucine (1,124 mg/100 gm) content. It also contains eight
minerals, 14 vitamins and dextrin as the major energy source
(Table I) (3). Several reports have demonstrated that
ED with glutamine has beneficial effects against Crohn's disease
and chemotherapy-induced mucositis in patients with cancer
(15–20). Moreover, weight loss is a common
side effect of advanced cancer and leucine can stimulate muscle
protein synthesis (16). In our
recent clinical studies, we successfully used Elental®
to treat malnutrition as well as chemotherapy-induced oral
mucositis and dermatitis in patients with HNC (21,22).
Tanaka et al (3) reported
Elental® can reduce adverse events in patients with
esophageal cancer receiving docetaxel/cisplatin/5-fluorouracil in
multicenter study of a phase III randomized controlled clinical
trial. These observations led to the hypothesis that
Elental® might be useful against 5-fluorouracil
(5-FU)-induced atrophic changes in salivary glands.
 | Table I.Composition of Elental®
(one package=80 g). |
Table I.
Composition of Elental®
(one package=80 g).
| Composition | Amount |
|---|
| Energy (kcal) | 300 |
| Carbohydrate
(g) |
|
|
Dextrin | 63.41 |
| Fat (g) |
|
| Soy
bean oil | 0.51 |
| Amino
acid total (g) | 14.1 |
| Amino acid
(mg) |
|
|
L-Isoleucine | 642 |
|
L-Leucine | 899 |
| Lysine
hydrochloride | 888 |
|
L-Methionine | 648 |
|
L-Phenylalanine | 871 |
|
L-Threonine | 523 |
|
L-Tryptophan | 151 |
|
L-Valine | 701 |
|
L-Histidine hydrochloride
monohydrate | 501 |
|
L-Arginine hydrochloride | 1,125 |
|
L-Alanine | 899 |
|
L-Aspartic acid magnesium
potassium | 1,036 |
|
L-Aspartic acid sodium
monohydrate | 867 |
|
L-Glutamine | 1,932 |
|
Aminoacetic acid | 505 |
|
L-Proline | 630 |
|
L-Serine | 1,159 |
|
L-Tyrosine | 110 |
| Branched-chain
amino acids or BCAA (mg) | 2,242 |
Cytochrome c (Cytc) and Cytc oxidase (COX) catalyze
the terminal reaction of themitochondrial electron transport chain
(ETC), which is important in the production of cellular energy
(23). Among numerous
mitochondrial marker genes, COX subunit 4 (COX IV) has a pivotal
role in the regulation of cellular energy metabolism, mitochondrial
function and oxidative phosphorylation (24–26).
Malfunction or reduced activity of COX is caused by mitochondrial
defects, which might be associated with a number of human diseases
and disorders, including stroke, heart or liver diseases, and
cancer (23,26). Under these stressful conditions,
the ETC produces reactive oxygen species, which may trigger cell
death processes and tissue damage (23,26).
Moreover, downregulation of COX IV may result in mitochondrial
dysfunction and oxidative stress, which may inhibit epithelial
repair processes and wound healing (25,27,28).
In patients with cancer undergoing chemotherapy and
radiotherapy, a high incidence of mucositis is often correlated
with decreased saliva secretion (29,30).
Salivary gland hypofunction may induce xerostomia, which negatively
affects oral health as well as the nutritional status of patients
(30). Saliva, a mixture of water,
ions and proteins, is mainly produced by three pairs of major
salivary glands: The parotid, submandibular and sublingual glands.
Saliva is rich in immunoglobulin A (IgA) and epidermal growth
factor (EGF) (31). IgA plays an
important role in mucosal immunity and EGF is required for tissue
repair in salivary glands (24,32–34).
It has been suggested that reduction in both saliva and EGF may be
associated with the severity of oral mucositis (32,35,36).
It is also generally accepted that salivary EGF may promote wound
healing and may be an effective treatment for gut ulcers (30).
In the present study, an animal model was used to
evaluate the efficacy of the ED, Elental®, for treating
oral mucositis and salivary gland atrophy associated with cancer
chemotherapy. The present examined the effect of
Elental® treatment on the body weight and salivary gland
weight of 5-FU-treated mice, as well as its beneficial effects
against 5-FU-induced oral mucositis. Furthermore, the underlying
mechanisms of the healing effects of Elental® were
investigated.
Materials and methods
Animals
A total of 6 ICR female mice at 10 weeks of age
(average, weight, 31.5; range, 30–33 g) were purchased from CLEA
Japan Inc. They were housed in a temperature-controlled (20–25°C)
and pathogen free environment with ~69.31% humidity and a 12 h
light/dark cycle. The mice were provided with a commercial diet
(CRF-1; Oriental Yeast Co., Ltd.) and sterilized water ad
libitum. Surgical procedures and animal treatments were
conducted in accordance with the Guidelines for Animal
Experimentation of Yamaguchi University (Ube, Japan). All in
vivo experiments were approved by the Institutional Animal Care
and Use Committee of Yamaguchi University (approval no.
55-017).
Oral mucositis induction and ED
treatment in mice
After a 7 day habituation period, mice were divided
into two groups: Control group (n=3) and ED group (n=3) with
similar mean body weights. In order to induce atrophic changes in
the salivary glands, all mice were injected intraperitoneally with
20 mg/kg/day of 5-FU (Kyowa Kirin Co., Ltd.) twice per week for 3
weeks. Simultaneously, the ED group received 1.6 kcal/0.8 ml/day of
Elental® (Ajinomoto Co., Inc.) orally (twice/day; 5
times/week for 3 weeks) and the control group received the same
amount of saline administered orally (twice/day, 5 times/week for 3
weeks). The duration of the experiment was 21 days. The body weight
and health of the mice were checked every other day until the end
of the experiment (day 21). The humane end point of the experiment
was decided as ≥40% loss of body weight, severe diarrhea or loss of
appetite. On day 21, the experiment was terminated, and all six
mice were sacrificed via cervical dislocation. Mortality was
confirmed by checking the loss of respiratory movement and
discoloration of the ocular bulb. The salivary glands (the
submandibular gland and sublingual gland) were collected and
weighed. Next, half of each samples were kept at −80°C in RNA-later
(Thermo Fisher Scientific, Inc.) for whole transcriptome analysis,
and the other half of the samples were fixed with 10% neutral
buffered formalin (Mildform® 10N; FUJIFILM Wako Pure
Chemical Corporation) at room temperature overnight and were
paraffin-embedded. The tissue sections were then subjected to
hematoxylin and eosin (HE) staining, immunohistochemical (IHC)
staining, and terminal deoxynucleotidyl transferase (TdT)-mediated
nick end labeling (TUNEL) assays as described below.
HE staining and observation
Salivary glands were fixed in 10% neutral buffered
formalin at 4°C for 24 h, and then were embedded in paraffin. The 4
µm-thick tissue sections were subjected to HE staining. The entire
procedure of HE staining was carried out at room temperature. The
tissue sections were immersed in xylene followed by rehydration in
graded ethanol series (100–70%). After washing with tap water, the
tissue sections were immersed in hematoxylin for 10 min, 1% acid
ethanol for 30 sec and 1% ammonia water for 30 sec. The sections
were washed with running tap water again and then treated with
eosin for 1 min, followed by graded ethanol (70–100%) and xylene.
After clearing the section with Histo-clear (National Diagnostics),
the slides were mounted with glass coverslips using DPX mounting
medium (Sigma-Aldrich; Merck KGaA). The stained sections were
observed at ×200 magnification using a BX51 fluorescence microscope
(Olympus Corporation).
Whole transcriptome analysis
Total RNA was isolated from salivary gland samples
using the RNeasy Mini kit (Qiagen GmbH). The quality of RNA was
examined with an Agilent 2100 Bioanalyzer (Agilent Technologies,
Inc.) using RNA 6000 Nano kit (Agilent Technologies, Inc.) after
the concentrations were determined by Qubit (Thermo Fisher
Scientific, Inc.). The RNA Integrity Number values were >7 in
all samples, indicating that the samples contained high-quality
RNA. The expression libraries were produced using the NEBNext ultra
II RNA library prep kit (cat. no. E7770L; New England BioLabs,
Inc.) and NEBNext Multiplex Oligos for Illumina (cat. no. E7335S;
New England BioLabs, Inc.). According to the manufacturer's
protocol, total RNA (500 ng) extracted from each sample was
reverse-transcribed into cDNA with the NEBNext Ultra II RNA First
Strand Synthesis Module (cat. no. E7771; New England BioLabs,
Inc.), and then the index sequences were inserted during PCR
amplification. Following initial denaturation at 98°C for 30 sec,
amplification was performed for 9 cycles of a denaturation at 98°C
for 10 sec, annealing at 65°C for 75 sec and extension at 65°C for
75 sec with a final extension at 65°C for 5 min. Primers were as
follows: NEBNext Index 6 Primer for Illumina,
5′-CAAGCAGAAGACGGCATACGAGATATTGGCGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT-3′;
NEBNext Index 7,
5′-CAAGCAGAAGACGGCATACGAGATGATCTGGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT-3′;
NEBNext Index 8,
5′-CAAGCAGAAGACGGCATACGAGATTCAAGTGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT-3′;
NEBNext Index 9,
5′-CAAGCAGAAGACGGCATACGAGATCTGATCGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT-3′;
NEBNext Index 10,
5′-CAAGCAGAAGACGGCATACGAGATAAGCTAGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT-3′;
NEBNext Index 11,
5′-CAAGCAGAAGACGGCATACGAGATGTAGCCGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT-3′
and NEBNext Universal PCR Primer,
5′-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCT-3′.
The quality of the library was examined with a Bioanalyzer (Agilent
Technologies, Inc.) after purification using AMPure XP beads (cat.
no. A63882; Beckman Coulter, Inc.). The libraries were sequenced on
an Illumina Next-seq sequencer (Illumina, Inc.) with an Illumina
NextSeq High Output 150 bp pair-end cycle sequencing kit (cat. no.
20024907; Illumina, Inc.). More than 30 million reads in each
sample were detected in the reaction and were trimmed and mapped
with the mouse reference genome GRCm38 release-92 using CLC
Genomics Workbench software (ver.8.01; Qiagen GmbH); and the
mapping ratio was 98%.
Principal component analysis
(PCA)
PCA is a type of data dimension reduction algorithm
which was used to verify the quality of the gene expression data.
The analyses of differential gene expression profiles were
conducted using JMP Pro software (ver.15.0.0; SAS Institute, Inc.)
and then dimension reduction analysis was performed. The principal
component 1 (PC1) was plotted on the x-axis and the principal
component 2 (PC2) was plotted on the y-axis of the scatter diagram.
Each data point represents a sample. The differential gene
expression pattern based on the control (C) and ED (E) groups were
analyzed using PCA.
Ingenuity network analysis
Co-relation analysis was conducted with Prism
(ver.9.0.3; GraphPad Software, Inc.). The gene sets that
demonstrated significantly increased or decreased expression in the
co-relation analysis were then examined by network analysis using
the IPA software (version 8.6, Qiagen GmbH). The IPA software
revealed the molecular and cellular functions of the dataset
members as well as the canonical pathways represented in the
dataset. The IPA software is derived from a vast amount of
molecular interactions reported in the literature and the software
is updated weekly (37). The IPA
uses a Fisher's exact test to determine whether the differentially
expressed genes are significantly related to pathways compared with
the whole ingenuity knowledge base.
Volcano plot
A volcano plot was constructed to identify the
differentially expressed gene between the ED group and the control
group. The horizontal and vertical coordinates denoted the average
expression value of each gene between these two groups. The
screening criteria cutoff comprised a log2 fold change of >3 or
<-3 with P<0.01 using JMP Pro software (ver.15.0.0; SAS
Institute, Inc.). The significantly upregulated genes are shown in
red, and the significantly downregulated genes are shown in
green.
IHC staining
The expression profiles of COX IV and EGF in mice
salivary glands were detected using IHC analyses. Paraffin-embedded
4 µm-thick tissue sections were immersed in xylene at room
temperature and then rehyraded in graded ethanol (100–70%). Next,
the sections were washed with phosphate buffered saline (PBS) at
room temperature, immersed in a Target Retrieval Solution (pH 9;
Agilent Technologies, Inc.) and heated in a microwave for 10 min.
Endogenous peroxidase activity was quenched with a 0.3% hydrogen
peroxide/methanol mixture for 20 min. Next, the sections were
rinsed in PBS and incubated with Dako REAL™
peroxidase-blocking solution (Agilent Technologies, Inc.) at room
temperature for 30 min, followed by an overnight incubation at 4°C
with anti-COX IV rabbit polyclonal antibody (1:200; cat. no.
11242-1-AP; ProteinTech Group, Inc.) and anti-EGF rabbit polyclonal
antibody (1:200; cat. no. AP12878c; Abcepta). After rinsing in PBS
for 10 min, the tissue sections were incubated with a secondary
antibody (undiluted; Dako REAL™ EnVision™
Detection system, HRP conjugated; cat. no. K5007; Agilent
Technologies, Inc.) at room temperature for 30 min, and the
expression of COX IV and EGF were detected using the Dako
REAL™ EnVision™ detection system (Agilent
Technologies, Inc.) according to the manufacturer's instructions.
Lastly, the tissues were rinsed in tap water, and then
counterstained with hematoxylin for 1–2 min at room temperature.
They were subsequently dehydrated in graded ethanol (70–100%) and
xylene, cleared with Histo-clear (National Diagnostics) and mounted
with glass coverslips using DPX mounting medium (Sigma-Aldrich;
Merck KGaA). The tissue sections were then observed at ×200
magnification using a BX51 fluorescence microscope (Olympus
Corporation).
TUNEL assay
In order to detect apoptotic cells in control and
ED-treated salivary glands, a TUNEL assay was performed with 4
µm-thick paraffin sections from biopsy tissues using the
DeadEnd™ Colorimetric TUNEL System (Promega Corporation)
according to the manufacturer's instructions. Briefly, the tissue
sections were deparaffinized by xylene and rehydrated in graded
ethanol (100–50%) at room temperature. The tissues were then
incubated in 0.85% NaCl for 5 min, fixed in 4% paraformaldehyde
solution for 15 min at room temperature and washed with PBS. Next,
they were treated with 20 µg/ml proteinase K at room temperature
for 15 min and were immersed in 4% paraformaldehyde solution again
at room temperature for 5 min followed by a PBS wash. The tissues
were incubated in a 3% hydrogen peroxide solution and then in an
equilibration buffer (0.05 M phosphate buffer containing 0.145 M
NaCl, pH 7.4). Next, the rTdT reaction mix (rTdT enzyme and
biotinylated nucleotide mix dissolved in equilibration buffer) was
added to the samples and they were incubated in a humidified
chamber at 37°C for 60 min. The enzymatic reaction was stopped by
immersing the tissues into a stop wash buffer for 10 min at room
temperature. After a PBS wash, the tissue sections were incubated
with anti-digoxigenin-peroxidase conjugate for 30 min at room
temperature, and then with diaminobenzidine for 5 min at room
temperature. Hematoxylin was used as a counterstain at room
temperature for 1 min. In three random fields of each tissue
section, at least 100 cells were counted under ×100 magnification
using a BX51 fluorescence microscope (Olympus Corporation). The
number of apoptotic cells (TUNEL-positive cells) was calculated by
dividing the number of TUNEL-positive cells by the total number of
counted cells, and the result was expressed as a percentage
(TUNEL-positive score).
Statistical analysis
All data are indicated as the mean ± standard
deviation. The mice body weight data and the TUNEL data from the ED
group were compared with the control group using Mann-Whitney U
tests. P<0.05 was considered to indicate a statistically
significant difference. StatView software (version 5.0J; SAS
Institute, Inc.) was used for statistical calculations.
Results
Body weights and health of the control
and ED group members
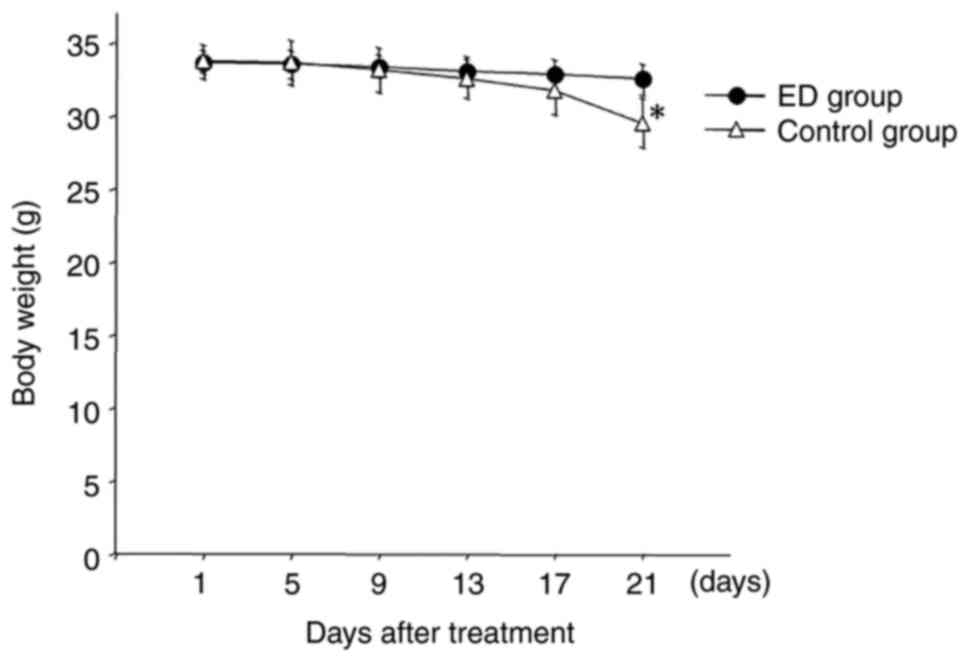
Fig. 1 presents the
body weight changes in mice during the treatment period. The body
weight of mice in the ED group remained stable even after 5-FU
administration throughout the treatment period. However, the body
weight gradually decreased in the control group after day 9. At the
end of the experiment (day 21), a significant difference in body
weight was observed between the ED group and the control group.
During the experiment, all mice were healthy until they were
sacrificed on day 21. These data demonstrated that
Elental® helped to maintain the body weight of
5-FU-treated mice.
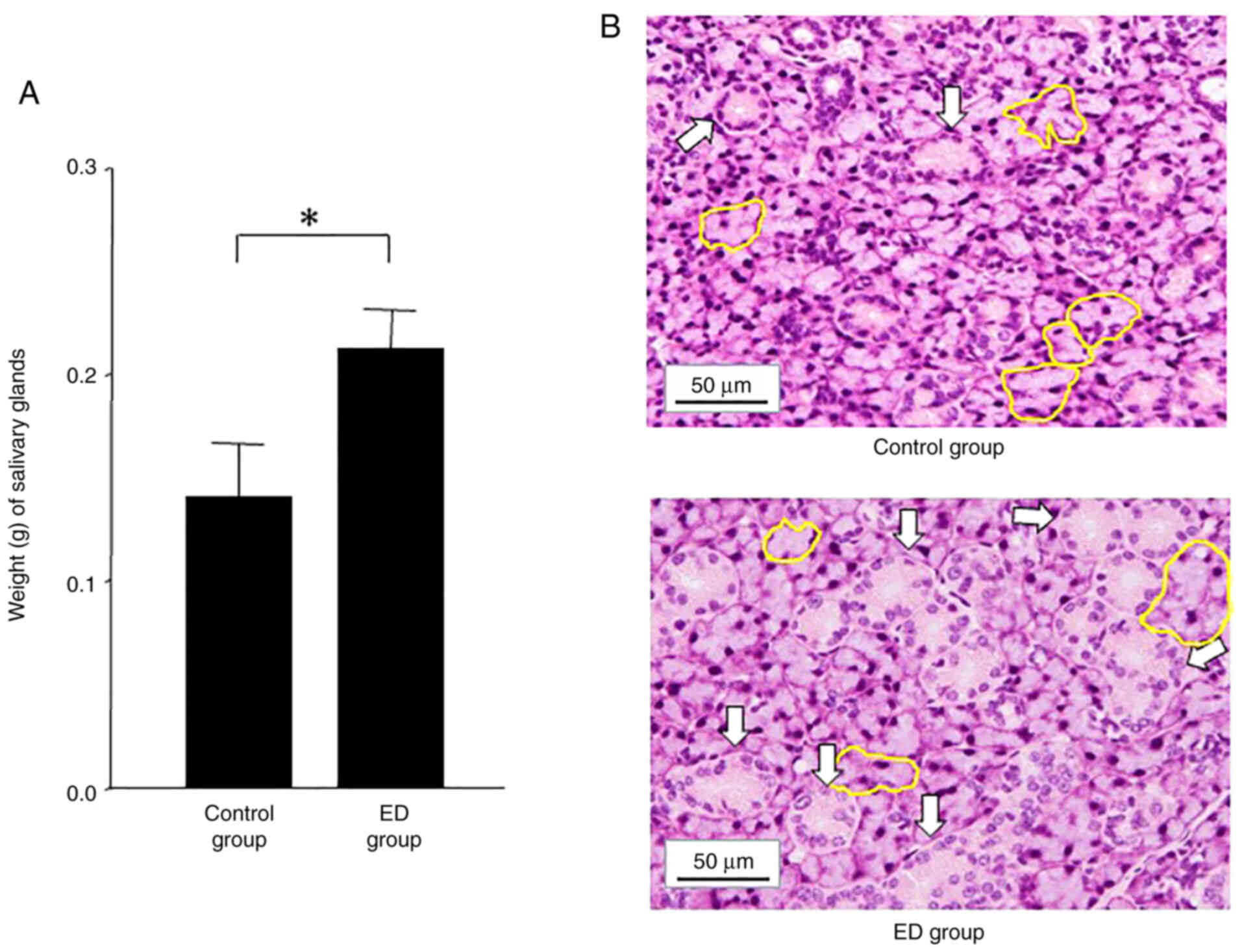
Salivary gland weight and
histology
The weight and histology of murine salivary glands
were compared between the control and ED groups. The salivary gland
weight was higher in the ED group (0.212±0.190 g) compared with the
control group (0.140±0.027 g) (Fig.
2). The histology of the salivary glands is presented in
Fig. 2. A higher level of atrophy
was observed in the salivary acini cells from control group mice
compared with ED group mice. Moreover, histological changes in the
granular duct area of the salivary glands were more prominent in
the ED group mice compared with the control group mice (Fig. 2). In addition, the granular duct
cells in the ED group appeared larger compared with the control
group. In brief, ED treatment could heal 5-FU-damaged salivary
glands in nude mice.
ED mechanism of action in the salivary
gland
The ED mechanism of action was investigated by whole
transcriptome analysis, which identified differentially expressed
genes between the salivary glands from the ED group and control
group of mice. These differentially expressed genes were subjected
to IPA in order to assign functions to the genes as well as
identify any pathways represented in the differentially expressed
gene dataset. PCA was used to verify the data quality (Fig. 3A). The PCA data demonstrated that
the distance between the samples in the control group (C) was
small, indicating that the gene expression patterns were similar.
However, the distance between samples from the ED group (E) was
relatively large; therefore, the gene expression patterns of E were
different compared with that of C (Fig. 3A). A volcano map was created which
represents the average value of each expressed gene between the ED
group and control group. Significantly upregulated genes are
presented in red, and significantly downregulated genes are
presented in green (Fig. 3B). IPA
analysis of these upregulated genes (>1.5-fold) revealed that
the ‘oxidative phosphorylation’ pathway was the top canonical
pathway related to these differentially expressed genes. The second
pathway among the top canonical pathways was the ‘mitochondrial
dysfunction’-related pathway (Fig.
3B). The data obtained from IPA were also used to understand
the relationships and functional interaction network among these
differentially expressed genes. Fig.
3C presents the oxidative phosphorylation pathway-related
network and Fig. 3D indicates the
mitochondrial function-related network associated with these
differentially expressed genes (Control vs. ED). These data
demonstrated that a number of the upregulated genes in the salivary
glands of the ED group were associated wih mitochondrial functions.
This suggested that the healing function of Elental®
might be associated with the recovery of mitochondrial function in
5-FU-damaged cells.
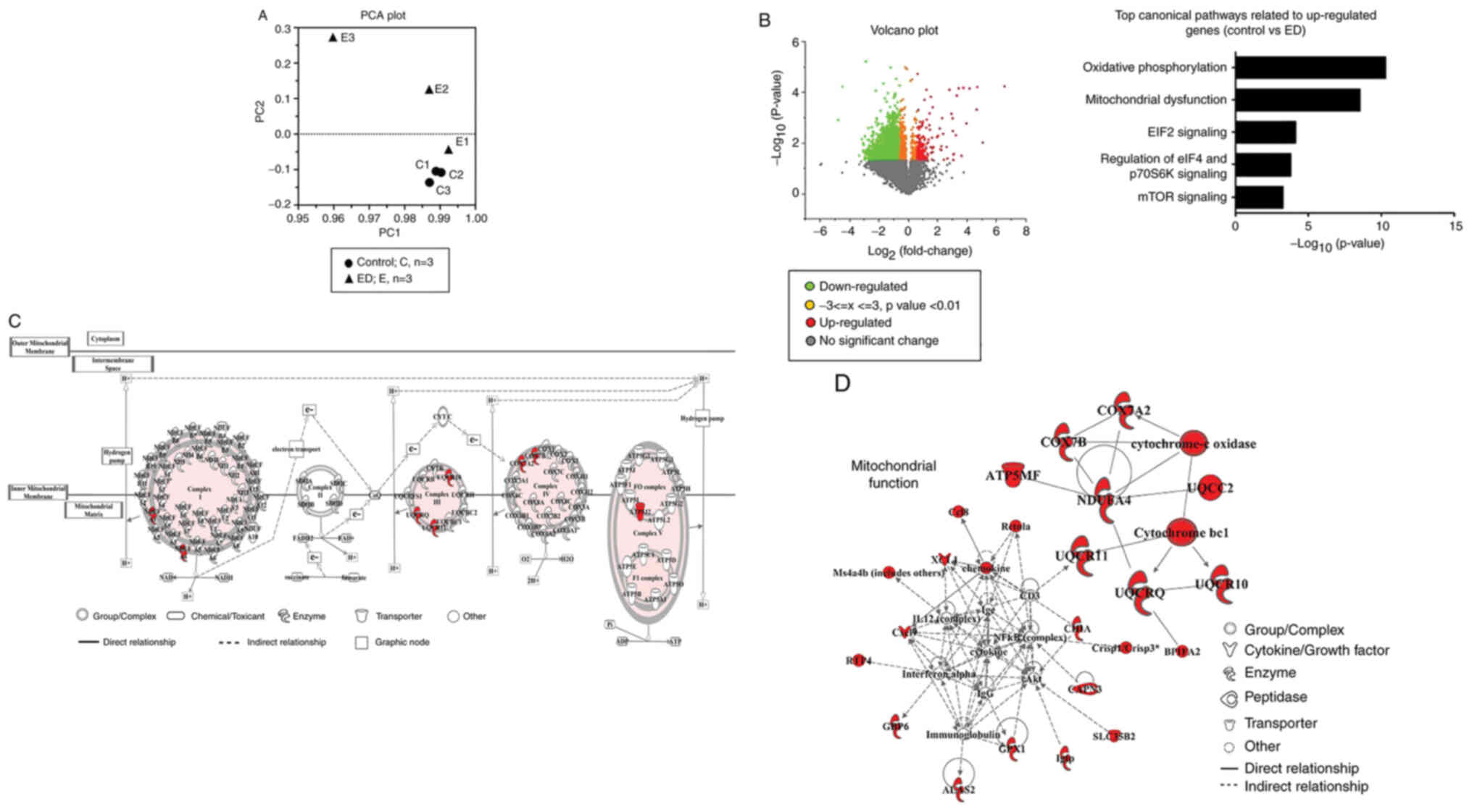 | Figure 3.Data obtained from whole
transcriptome analysis, PCA and IPA of 5-FU-injected ED) mice and
saline-treated (control) mice. On day 21 of treatment, the mice
were sacrificed and the salivary glands were collected for whole
transcriptome analysis to identify the differentially expressed
genes in the salivary glands from the ED group compared with the
control group. (A) PCA was used to verify the data quality. PC1 and
PC2 were plotted on the x-axis and y-axis, respectively, to draw
the scatter diagram, where each point represents a sample. E1, E2
and E3 represent the ED group; and C1, C2 and C3 represent the
control group. (B) Left panel: A volcano plot showing each
differentially expressed gene in the ED group compared with the
control group. The significantly upregulated genes are shown in
red, and the significantly downregulated genes are shown in green.
Right panel: IPA analysis of the upregulated (>1.5-fold) genes
identified with the volcano plot indicated that these genes were
mostly associated with oxidative phosphorylation and mitochondrial
dysfunction pathways. (C) IPA analysis of the differentially
expressed genes in the salivary glands from the ED group compared
with the control group, which were associated with the oxidative
phosphorylation pathway-related network. (D) Mitochondrial
function-related network constructed from the IPA data of the
differentially expressed genes in the salivary glands from the ED
group compared with the control group (large block letters refer to
the upregulated genes). PCA, principal component analysis; IPA,
Ingenuity Pathways Analysis; ED, elemental diet; 5-FU,
5-fluorouracil; PC, principal component; E, ED group; C, control
group. |
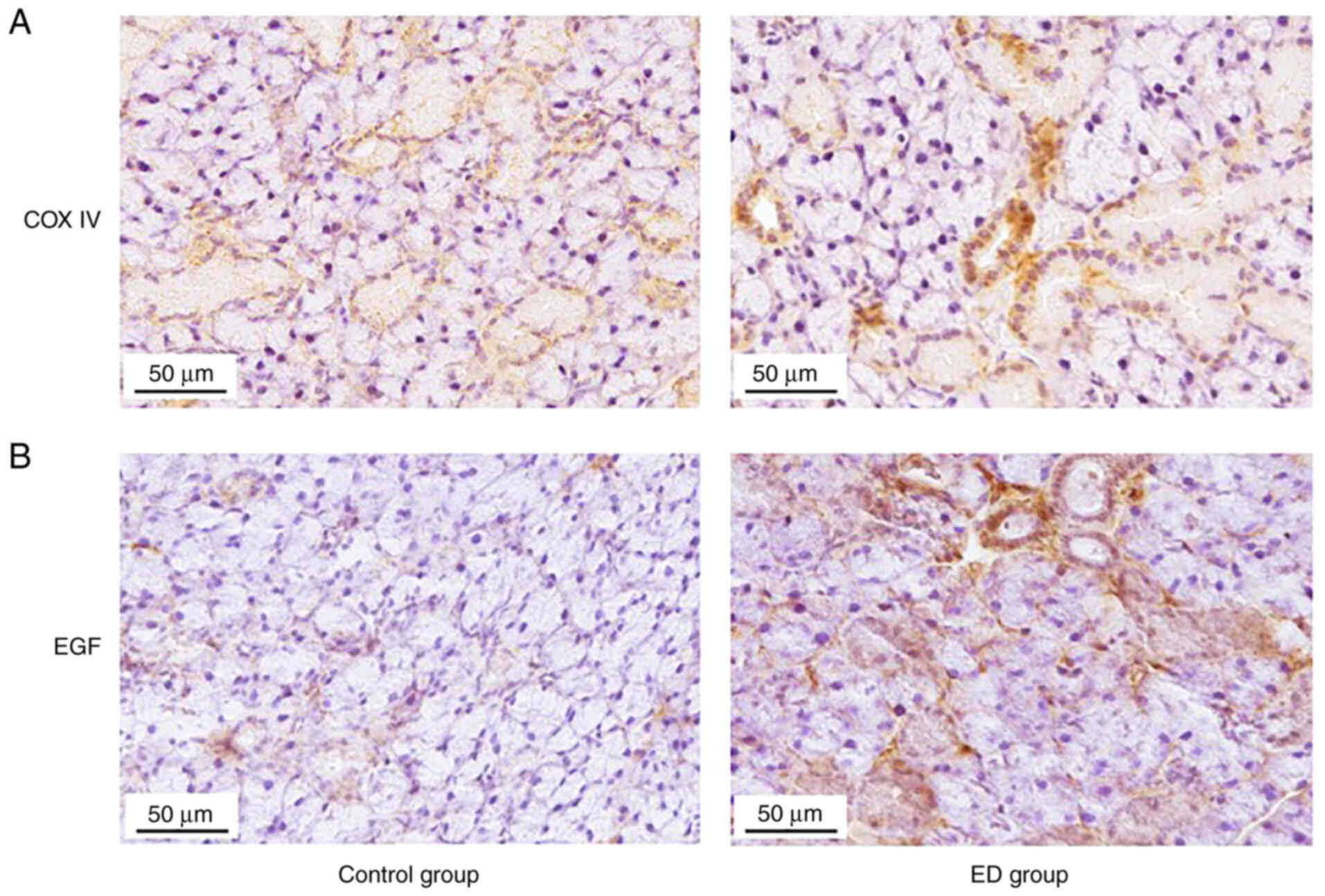
Expression of COX IV and EGF in
salivary glands of 5-FU-treated mice
The expression of COX IV in the salivary glands of
control and ED mice were examined by IHC because it is an important
mitochondrial marker. IHC analysis was also used to detect the
expression of EGF in salivary gland tissues. The expression of both
COX IV (Fig. 4A) and EGF (Fig. 4B) in the salivary glands were
higher in the ED group compared with the control group. These data
indicated that ED might heal 5-FU-damaged salivary gland tissues by
upregulating EGF and by recovering mitochondrial functions in
cells.
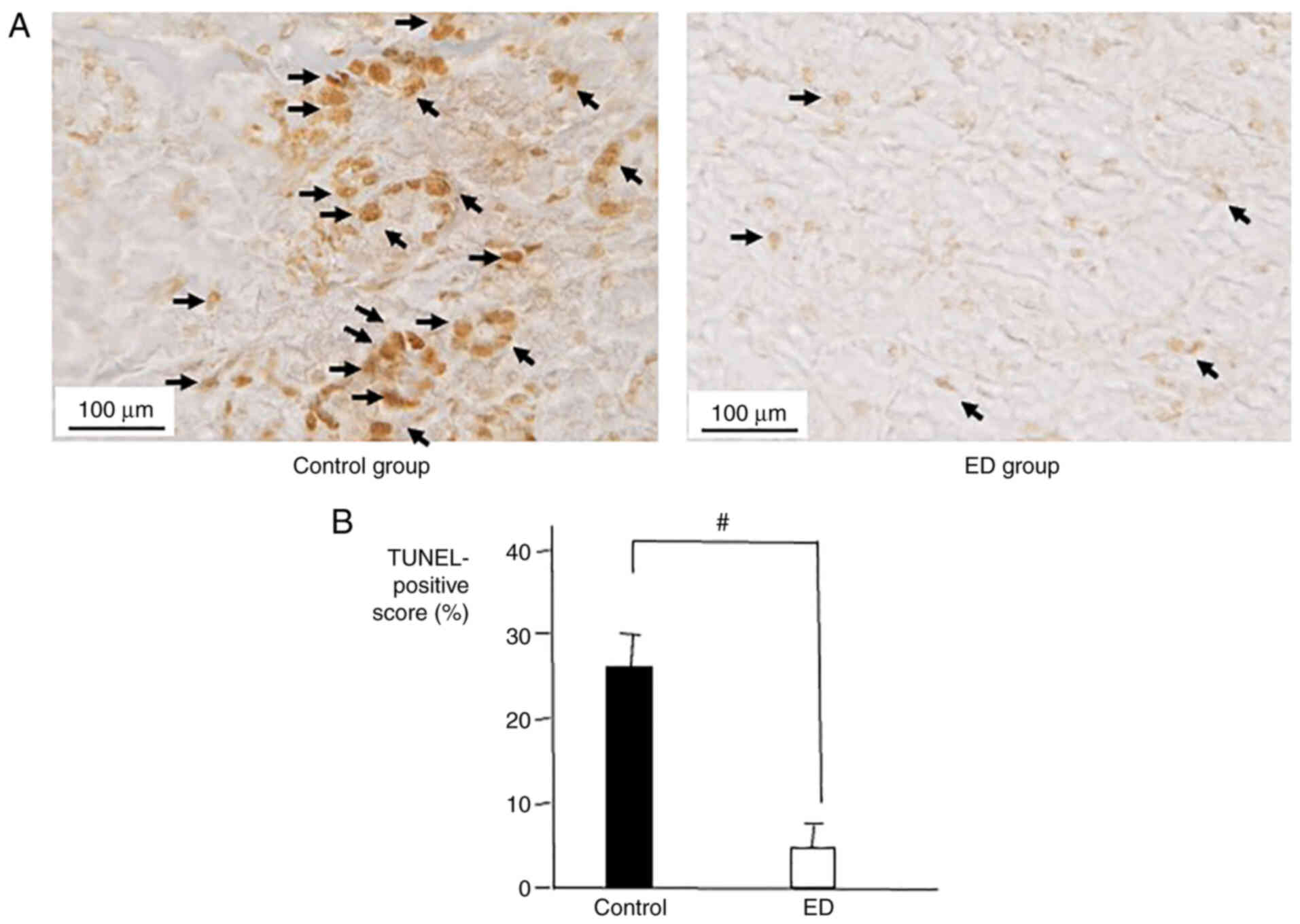
TUNEL-positive cells in salivary
glands of 5-FU-treated mice
The rate of apoptosis in the salivary glands of mice
was analyzed to evaluate the tissue damage caused by 5-FU as well
as the protective and recovery effects of ED against that damage.
The number of TUNEL-positive cells in the salivary glands of the
control group was significantly higher compared with the ED group
(Fig. 5). The TUNEL data
demonstrated that ED could protect salivary glands from tissue
damage caused by 5-FU.
Discussion
Oral mucositis is a life-threatening disease and one
of the most common side effects of chemotherapy and radiotherapy.
Oral mucositis and salivary gland dysfunction caused by 5-FU-based
chemotherapy may result in a decreased quality of life and an
increased treatment cost (1).
Until now, very few agents exhibit protective and healing effects
against mucositis (7,9,11,14,38).
Elental® is an easily digestible nutritional supplement
or ED which is composed of free amino acids and dextrin (3). In Japan, Elental® is
administered to malnourished high-risk patients for the maintenance
and improvement of their nutritional status (3,16,18).
Elental® and other EDs were reported to be effective in
the treatment of inflammatory bowel diseases such as Crohn's
disease and chemotherapy-induced oral mucositis (17–22).
However, the detailed mechanisms contributing to these beneficial
effects of EDs are not clear. In the present study, the efficacy of
an ED, Elental®, against 5-FU-induced salivary gland
atrophy and dysfunction was examined.
Antineoplastic drugs reportedly injure salivary
gland tissues and decrease saliva secretion volume (25,39).
McCarthy et al (35)
reported that the incidence of 5-FU-induced oral mucositis is
higher in patients with lower saliva secretion volumes. Notably,
Kawashima et al (39)
reported that 5-FU treatment (40 mg/kg/day) decreases the volume of
saliva in mice, and then a 7-day treatment of Elental®
recovers the saliva volume and increases mouse body weight. In
addition, this study indicated that Elental® may protect
the submandibular acini against 5-FU-induced atrophic changes
(40). In our previous study, we
used an experimental protocol (a 7-day treatment of
Elental®) similar to the one developed by Kawashima
et al to understand the protective effects of
Elental® on salivary glands of 5-FU-administered mice
(39). Additionally, this study
also investigated which components of Elental® are most
effective against the 5-FU-induced damage of the salivary glands.
The data indicated that among all the components of
Elental®, amino acids exert the highest ameliorating
effects against 5-FU-induced atrophic changes in salivary glands
(41). However, both of these
studies do not focus on the underlying mechanism of action of
Elental® responsible for its protective effects
(40,41). In our present study, a different
treatment protocol was used compared with our previous study. In
the present study, mice were treated with 20 mg/kg/day 5-FU (twice
per week for 3 weeks). Elental® was administered for 21
days or 3 weeks (5 times a week) to understand its long-term
effects. Moreover, this study did not focus on the different
components of Elental® this time. The main focus was to
examine the underlying mechanisms of the healing effects of
Elental® by whole transcriptome analysis and IPA.
Over the past few years, the usefulness of EDs
against the negative side effects of antineoplastic drugs has been
observed in patients with cancer at Yamaguchi University Hospital.
Elental® was effective against chemotherapy- and/or
radiotherapy-induced mucositis and dermatitis in HNC patients, as
well as in vitro and in vivo (21,22,42,43).
Based on these results, it was hypothesized that
Elental® may help to alleviate oral mucositis by
preserving saliva secretion volume and improving the oral
environment.
The current study demonstrated that
Elental® helped to maintain the body weight of
5-FU-treated mice. Moreover, the weight of 5-FU-damaged salivary
glands and the size of granular duct cells in salivary glands were
increased in the ED group compared with the control group. These
findings suggested a possible healing effect of Elental®
against 5-FU-induced salivary gland atrophy and oral mucositis.
In previous clinical trials, oral squamous cell
carcinoma patients with oral mucositis are advised to swish
Elental® suspension in their mouth and swallow it. None
of the patients complained of any irritation, pain or difficulty
during oral administration (22).
Moreover, murine back skin wounds are healed by topical application
of Elental®, which suggests that it may have direct
soothing effects on the wounded areas upon contact (43). Therefore, it was assumed that
Elental® is suitable for patients with severe oral
mucositis who are unable to eat solid foods.
A reduced saliva secretion volume hampers the
salivary clearance ability and decreases the concentrations of
secretory IgA, which possess antibacterial activity (33). In addition, EGF is required during
tissue repair in salivary glands (32,34).
The present study demonstrated that Elental® upregulated
the expression of EGF. Notably, the present whole transcriptome
analysis and IPA data revealed that a number of the upregulated
genes in the salivary glands of the ED group of mice are associated
with mitochondrial functions. This indicated that the healing
function of Elental® may be associated with the
functional recovery of mitochondria in 5-FU-damaged cells.
COX IV is a typical mitochondrial marker and is
essential for the regulation of cellular energy metabolism and
mitochondrial function. It is also a highly regulated enzyme, which
controls the oxidative phosphorylation pathway (24,26).
Decreased expression of COX IV results in impaired COX activity
followed by mitochondrial dysfunction and sensitization of cells to
apoptosis, which may hamper the normal functions of cells and
tissues including damage repair mechanisms (25,26,44).
The present data indicated upregulated expression of COX IV in the
salivary gland tissues from the ED group, which suggested that
Elental® might facilitate the recovery of 5-FU-damaged
mitochondrial function. Moreover, increased EGF expression and
reduced apoptosis were observed in the salivary glands of the ED
group compared with the control group, which also suggested that
Elental® may protect salivary glands from 5-FU-induced
atrophic changes. These data supported the hypothesis that the
Elental®-mediated repair of 5-FU-induced damage of the
salivary glands promotes adequate saliva secretion and adjustment
of oral bacterial flora. Therefore, Elental® may be used
to treat xerostomia and oral mucositis in patients with cancer.
The present in vivo experiments were
performed twice (n=3 per group) and the findings were almost
similar. However, there are some imitations of the present study.
The sample size was small (n=3) per group for each experiment and
no true control group (without 5-FU) was included. Therefore,
future studies will include a true control group and a larger
sample size (n=5-10 mice per group) to generate more reliable
data.
In conclusion, the present study demonstrated that
ED Elental® may be able to alleviate adverse effects of
5-FU-based chemotherapy including oral mucositis and atrophic
changes in the salivary glands. In order to further investigate the
healing mechanisms of EDs, the effects of EDs on genes involved in
oxidative phosphorylation and mitochondrial dysfunction will be
examined further. In addition, further studies are required to
clarify the mechanisms of the healing and protective effects of EDs
in a clinical setting.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by a Grant-in-Aid from
the Japanese Ministry of Education, Science and Culture (grant no.
55-017).
Availability of data and materials
The data generated in the present study are openly
available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE196407
[accession no. GSE196407 (access code: knabcecwrhuhpcr)].
Authors' contributions
KH designed the study. KH, RF and TF performed the
experiments. KH, KW and YM analyzed and interpreted the data. KH,
TF, KW and YM wrote and revised the manuscript. YM and KH confirmed
the authenticity of all the raw data. KM assisted in data
interpretation, revised the manuscript and provided valuable
suggestions during the study. All authors have read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
All in vivo experiments were approved by the
Institutional Animal Care and Use Committee of Yamaguchi University
(approval no. 55-017; Ube, Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Epstein JB, Thariat J, Bensadoun RJ,
Barasch A, Murphy BA, Kolnick L, Popplewell L and Maghami E: Oral
complications of cancer and cancer therapy: From cancer treatment
to survivorship. CA Cancer J Clin. 62:400–422. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pulito C, Cristaudo A, Porta C, Zapperi S,
Blandino G, Morrone A and Stranoet S: Oral mucositis: The hidden
side of cancer therapy. J Exp Clin Cancer Res. 39:2102020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tanaka Y, Takeuchi H, Nakashima Y, Nagano
H, Ueno T, Tomizuka K, Morita S, Emi Y, Hamai Y, Hihara J, et al:
Effects of an elemental diet to reduce adverse events in patients
with esophageal cancer receiving
docetaxel/cisplatin/5-fluorouracil: A phase III randomized
controlled trial-EPOC 2 (JFMC49-1601-C5). ESMO Open. 6:1002772021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reyes-Gibby CC, Melkonian SC, Wang J, Yu
RK, Shelburne SA, Lu C, Gunn GB, Chambers MS, Hanna EY, Yeung SJ
and Shete S: Identifying novel genes and biological processes
relevant to the development of cancer therapy-induced mucositis: An
informative gene network analysis. PLoS One. 12:e01803962017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sayles C, Hickerson SC, Bhat RR, Hall J,
Garey KW and Trivedi MV: Oral glutamine in preventing
treatment-related mucositis in adult patients with cancer: A
systematic review. Nutr Clin Pract. 31:171–179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lalla RV, Bowen J, Barasch A, Elting L,
Epstein J, Keefe DM, McGuire DB, Migliorati C, Nicolatou-Galitis O,
Peterson DE, et al: MASCC/ISOO clinical practice guidelines for the
management of mucositis secondary to cancer therapy. Cancer.
120:1453–1461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abdel Moneim AE, Guerra-Librero A, Florido
J, Shen YQ, Fernández-Gil B, Acuña-Castroviejo D and Escames G:
Oral mucositis: Melatonin gel an effective new treatment. Int J Mol
Sci. 18:10032017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kyllo RL and Anadkat MJ: Dermatologic
adverse events to chemotherapeutic agents, part 1: Cytotoxics,
epidermal growth factor receptors, multikinase inhibitors, and
proteasome inhibitors. Semin Cutan Med Surg. 33:28–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Keefe DM, Schubert MM, Elting LS, Sonis
ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, McGuire DB,
Hutchins RD and Peterson DE; Mucositis Study Section of the
Multinational Association of Supportive Care in Cancer and the
International Society for Oral Oncology, . Updated clinical
practice guidelines for the prevention and treatment of mucositis.
Cancer. 109:820–831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peterson DE, Bensadoun RJ and Roila F;
ESMO Guidelines Working Group, : Management of oral and
gastrointestinal mucositis: ESMO clinical recommendations. Ann
Oncol. 20 (Suppl 4):S174–S177. 2009. View Article : Google Scholar
|
|
11
|
Henke M, Alfonsi M, Foa P, Giralt J,
Bardet E, Cerezo L, Salzwimmer M, Lizambri R, Emmerson L, Chen MG
and Berger D: Palifermin decreases severe oral mucositis of
patients undergoing postoperative radiochemotherapy for head and
neck cancer: A randomized, placebo-controlled trial. J Clin Oncol.
29:2815–2820. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bensinger W, Schubert M, Ang KK, Brizel D,
Brown E, Eilers JG, Elting L, Mittal BB, Schattner MA, Spielberger
R, et al: NCCN task force report. Prevention and management of
mucositis in cancer care. J Natl Compr Canc Netw. 6 (Suppl
1):S1–S24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Svanberg A, Ohrn K and Birgegård G: Oral
cryotherapy reduces mucositis and improves nutrition-a randomised
controlled trial. J Clin Nurs. 19:2146–2151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scully C, Epstein J and Sonis S: Oral
mucositis: A challenging complication of radiotherapy,
chemotherapy, and radiochemotherapy. Part 2: Diagnosis and
management of mucositis. Head Neck. 26:77–84. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamamoto T, Nakahigashi M, Umegae S,
Kitagawa T and Matsumoto K: Impact of elemental diet on mucosal
inflammation in patients with active Crohn's disease: Cytokine
production and endoscopic and histological findings. Inflamm Bowel
Dis. 11:580–588. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishikawa T, Yasuda T, Doi T, Okayama T,
Sakamoto N, Gen Y, Dohi O, Yoshida N, Kamada K, Uchiyama K, et al:
The amino acid-rich elemental diet Elental® preserves
lean body mass during chemo- or chemoradiotherapy for esophageal
cancer. Oncol Rep. 36:1093–1100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamoto T, Nakahigashi M, Saniabadi AR,
Iwata T, Maruyama Y, Umegae S and Matsumoto K: Impacts of long-term
enteral nutrition on clinical and endoscopic disease activities and
mucosal cytokines during remission in patients with Crohn's
disease: A prospective study. Inflamm Bowel Dis. 13:1493–1501.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamamoto T, Nakahigashi M, Umegae S,
Kitagawa T and Matsumoto K: Impact of long-term enteral nutrition
on clinical and endoscopic recurrence after resection for Crohn's
disease: A prospective, non-randomized, parallel, controlled study.
Aliment Pharmacol Ther. 25:67–72. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fukui T, Itoh Y, Orihara M, Yoshizawa K,
Takeda H, Kawada S and Yoshioka T: Elental prevented and reduced
oral mucositis during chemotherapy in patients esophageal cancer.
Gan To Kagaku Ryoho. 38:2597–2601. 2011.(In Japanese). PubMed/NCBI
|
|
20
|
Ogata Y, Takeuchi M, Ishibashi N, Kibe S,
Takahashi K, Uchida S, Murakami N, Yahara T and Shirouzu K:
Efficacy of Elental on prevention for chemotherapy-induced oral
mucositis in colorectal cancer patients. Gan To Kagaku Ryoho.
39:583–587. 2012.(In Japanese). PubMed/NCBI
|
|
21
|
Harada K, Ferdous T, Horinaga D, Uchida K,
Mano T, Mishima K, Park S, Hanazawa H, Takahashi S, Okita A, et al:
Efficacy of elemental diet on prevention for
chemoradiotherapy-induced oral mucositis in patients with oral
squamous cell carcinoma. Support Care Cancer. 24:953–959. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harada K, Minami H, Ferdous T, Kato Y,
Umeda H, Horinaga D, Uchida K, Park SC, Hanazawa H, Takahashi S, et
al: The Elental® elemental diet for
chemoradiotherapy-induced oral mucositis: A prospective study in
patients with oral squamous cell carcinoma. Mol Clin Oncol.
10:159–167. 2019.PubMed/NCBI
|
|
23
|
Hüttemann M, Helling S, Sanderson TH,
Sinkler C, Samavati L, Mahapatra G, Varughese A, Lu G, Liu J,
Ramzan R, et al: Regulation of mitochondrial respiration and
apoptosis through cell signaling: Cytochrome c oxidase and
cytochrome c in ischemia/reperfusion injury and inflammation.
Biochim Biophys Acta. 1817:598–609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bikas A, Jensen K, Patel A, Costello J,
Reynolds SM, Mendonca-Torres MC, Thakur S, Klubo-Gwiezdzinska J,
Ylli D, Wartofsky L, et al: Cytochrome C oxidase subunit 4 (COX4):
A potential therapeutic target for the treatment of medullary
thyroid cancer. Cancers (Basel). 12:25482020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vogt S, Ruppert V, Pankuweit S, Paletta
JPJ, Rhiel A, Weber P, Irqsusi M, Cybulski P and Ramzan R:
Myocardial insufficiency is related to reduced subunit 4 content of
cytochrome c oxidase. J Cardiothorac Surg. 13:952018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Park JS, Deng JH and Bai Y:
Cytochrome c oxidase subunit IV is essential for assembly and
respiratory function of the enzyme complex. J Bioenerg Biomembr.
38:283–291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cano Sanchez M, Lancel S, Boulanger E and
Neviere R: Targeting oxidative stress and mitochondrial dysfunction
in the treatment of impaired wound healing: A systematic review.
Antioxidants (Basel). 7:982018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hoffmann RF, Jonker MR, Brandenburg SM, de
Bruin HG, Ten Hacken NHT, van Oosterhout AJM and Heijink IH:
Mitochondrial dysfunction increases pro-inflammatory cytokine
production and impairs repair and corticosteroid responsiveness in
lung epithelium. Sci Rep. 9:150472019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saegusa Y, Ichikawa T, Iwai T, Goso Y,
Ikezawa T, Nakano M, Shikama N, Saigenji K and Ishihara K: Effects
of acid antisecretory drugs on mucus barrier of the rat against
5-fluorouracil-induced gastrointestinal mucositis. Scand J
Gastroenterol. 43:531–537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stempniewicz A, Ceranowicz P and Warzecha
Z: Potential therapeutic effects of gut hormones, ghrelin and
obestatin in oral mucositis. Int J Mol Sci. 20:15342019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Van Leeuwen SJM, Proctor GB, Laheij AMGA,
Potting CMJ, Smits O, Bronkhorst EM, Hazenberg MD, Haverman TM,
Brennan MT, Von Bültzingslöwen I, et al: Significant salivary
changes in relation to oral mucositis following autologous
hematopoietic stem cell transplantation. Bone Marrow Transplant.
56:1381–1390. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dumbrigue HB, Sandow PL, Nguyen KH and
Humphreys-Beher MG: Salivary epidermal growth factor levels
decrease in patients receiving radiation therapy to the head and
neck. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 89:710–716.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harrison T, Bigler L, Tucci M, Pratt L,
Malamud F, Thigpen JT, Streckfus C and Younger H: Salivary sIgA
concentrations and stimulated whole saliva flow rates among women
undergoing chemotherapy for breast cancer: An exploratory study.
Spec Care Dentist. 18:109–112. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brand HS, Ligtenberg AJ and Veerman EC:
Saliva and wound healing. Monogr Oral Sci. 24:52–60. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McCarthy GM, Awde JD, Ghandi H, Vincent M
and Kocha WI: Risk factors associated with mucositis in cancer
patients receiving 5-fluorouracil. Oral Oncol. 34:484–490. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Epstein JB, Tsang AH, Warkentin D and Ship
JA: The role of salivary function in modulating
chemotherapy-induced oropharyngeal mucositis: A review of the
literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
94:39–44. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
QIAGEN, . Online Ingenuity Knowledge
Base-Articles. https://qiagen.secure.force.com/KnowledgeBase/KnowledgeIPAPage?id=kA41i000000L5sBFebruary
27–2022
|
|
38
|
Wie SM, Wellberg E, Karam SD and Reyland
ME: Tyrosine kinase inhibitors protect the salivary gland from
radiation damage by inhibiting activation of protein kinase C-δ.
Mol Cancer Ther. 16:1989–1998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kawashima R, Fujimaki M, Ikenoue Y, Danjo
K, Koizumi W and Ichikawa T: Influence of an elemental diet on
5-fluorouracil-induced morphological changes in the mouse salivary
gland and colon. Support Care Cancer. 24:1609–1616. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fujiwara R, Harada K, Ferdous T and
Mishima K: Amino acids may have protective effects on salivary
glands of 5-FU-administered mice. In Vivo. 36:198–205. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jensen SB, Pedersen AM, Reibel J and
Nauntofte B: Xerostomia and hypofunction of the salivary glands in
cancer therapy. Support Care Cancer. 11:207–225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Harada K, Ferdous T, Kobayashi H and
Ueyama Y: Elemental diet accelerates the recovery from oral
mucositis and dermatitis induced by 5-fluorouracil through the
induction of fibroblast growth factor 2. Integr Cancer Ther.
17:423–430. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Harada K, Takenawa T, Ferdous T, Mizukami
Y and Mishima K: Elemental diet directly affects
chemotherapy-induced dermatitis and raw wound areas. Mol Clin
Oncol. 13:209–215. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hao YH, Zhang J, Wang H, Wang HY, Dong J,
Xu XP, Yao BW, Wang LF, Zhou HM, Zhao L and Peng RY: HIF-1α
regulates COXIV subunits, a potential mechanism of self-protective
response to microwave induced mitochondrial damages in neurons. Sci
Rep. 8:104032018. View Article : Google Scholar : PubMed/NCBI
|