Gastrointestinal stromal tumor (GIST) is an uncommon
gastrointestinal cancer, accounting for less than 3% of overall
gastrointestinal neoplasms but 80% of those of mesenchymal origin
(1) and approximately half of the
cases are malignant (2). Although
tumors may develop in any part of the gastrointestinal tract, they
occur most frequently in the stomach (60%) and small intestine
(between 20 and 30%) (3–5). The worldwide annual incidence is 7-15
per million (6,7), with geographical variations. For
instance, in Europe and North America, the incidence is 10-15
annual cases per million of population but is higher in Asia at
16-20 per million (6). To evaluate
the prognosis of GIST, a consensus risk assessment of recurrence
was developed by the National Institutes of Health (NIH) in 2008
(Table I), subsequently revised to
the modified NIH risk scale (8).
According to the scale, the principal evaluation indices are the
tumor size and mitosis count, and are divided into four grades: i)
very low, ii) low, iii) intermediate and iv) high risk. A
relationship between GIST risk and prognosis has been well
documented (9). However, there is
considerable variation in both the clinical behavior and prognosis
of GIST, particularly in high-risk populations. Thus, a
comprehensive and objective assessment of GIST biology and
malignant progression, particularly in terms of histological and
clinical features, is important.
PubMed, Cochrane Library and EMBASE databases were
searched for relevant articles using the terms ‘GIST’ and ‘Ki67’ by
JL and ARW. Differences were resolved through discussion with a
third researcher SQL. The search took place on October 14, 2021. In
situations where patients were described in multiple publications,
the most complete or recent articles were selected. As the analysis
was based on published studies, neither ethical approval nor
patient consent was required.
The criteria for inclusion were as follows: i)
Patients must be assessed for Ki67 expression by
immunohistochemistry; ii) The prognostic risk of GIST was assessed
by the NIH Risk System; iii) The full text or original data could
be retrieved during October 2021.
Articles that did not include information on Ki67 in
relation to NIH risk assessment were excluded, as were case reports
and articles describing studies in animals or cell lines.
The required information from the publications was
independently recorded by JL and ARW. Specifically, this
information included the first author, publication date,
classification method, number of NIH risk categories, demographic
parameters (such as age and sex), the sample size and Ki67
measurement. Any disagreements between the two researchers were
resolved through discussion with the third researcher (SQL).
The Newcastle-Ottawa Scale (NOS) was used to verify
the quality of the evidence. Data were analyzed with Review Manager
Version 5.3 (Cochrane Collaboration), with P<0.05 representing
statistical significance. Inter-study heterogeneity was evaluated
using the I2 statistic and Cochran's Q test. When there was no
significant heterogeneity (Q test: P≥0.1), the fixed-effects
(Mantel-Haenszel) model was used to combine odds ratio (OR) values;
otherwise, the random-effect (DerSimonian and Laird) model was
used. The significance of combined ORs was evaluated using the
z-test. Examination of the effects of changes in inclusion criteria
on the final results was conducted by sensitivity analysis. The
combined OR and 95% confidence interval (CI) of dichotomous
variables were calculated. Funnel plots were used to assess
possible publication bias, with bias indicated by plot asymmetry.
Egger's test was applied to evaluate asymmetry in funnel plots, and
unpaired t-tests were used to measure intercept significance
(P<0.05).
The titles and abstracts of the publications were
reviewed, resulting in the exclusion of a number of studies due to
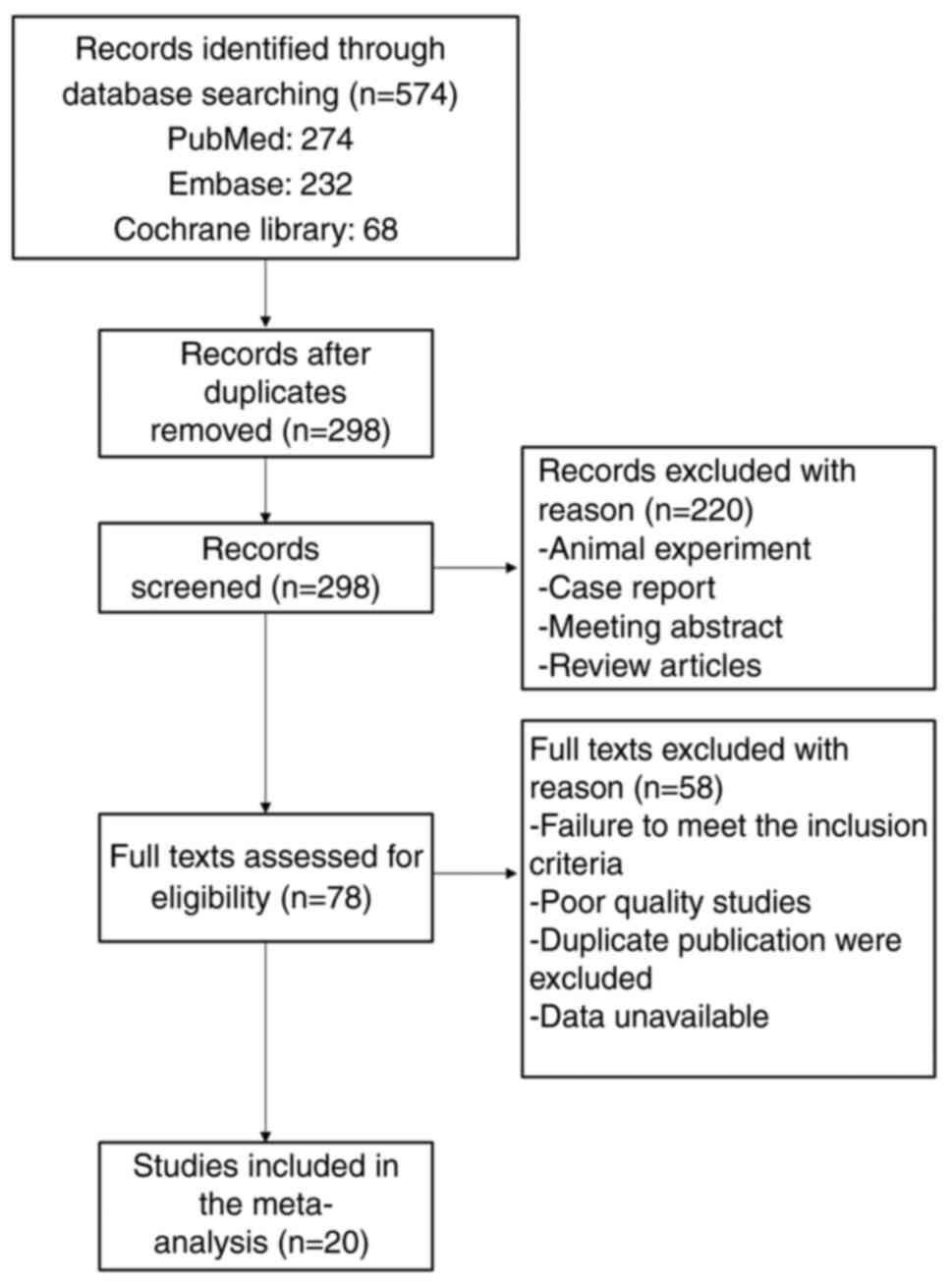
insufficient information for calculating the OR (Fig. 1). A total of 20 studies that
fulfilled the inclusion criteria were finally included in the
analysis (15,22–40).
The NOS was used to verify the quality of the evidence. Table II summarizes the principal
characteristics of these studies. In all, 1682 patient cases were
included. A flow chart of the screening process is shown in
Fig. 1.
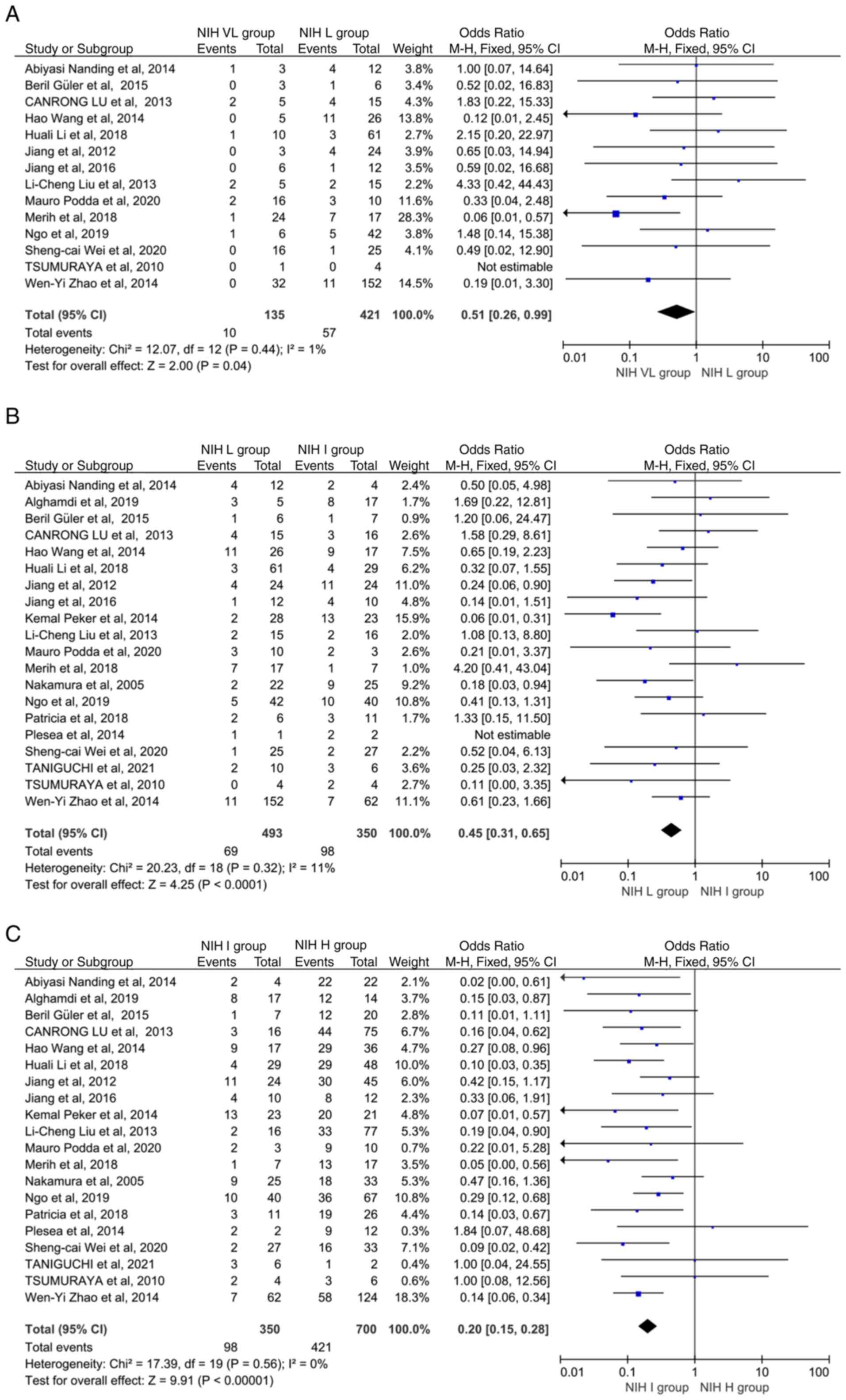
It was found that Ki67 levels were significantly
higher in the NIH L group compared with the NIH VL group (OR: 0.51,
95% CI: 0.26-0.99; P=0.04; P heterogeneity=0.44) (Fig. 2A). There was also greater Ki67
overexpression in the NIH I group compared with the NIH L group
(OR: 0.45, 95% CI: 0.31-0.65; P<0.0001; P heterogeneity=0.32)
(Fig. 2B), while Ki67 levels were
greater in the NIH H group than in the NIH I group (OR: 0.20, 95%
CI: 0.15-0.28; P<0.00001, P heterogeneity=0.56) (Fig. 2C). Due to the small heterogeneity,
the fixed-effects (Mantel-Haenszel) model was used. Heterogeneity
analysis of the 20 studies revealed no heterogeneity (P>0.05),
and sensitivity analysis indicated that no individual study
influenced the pooled OR (data not shown).
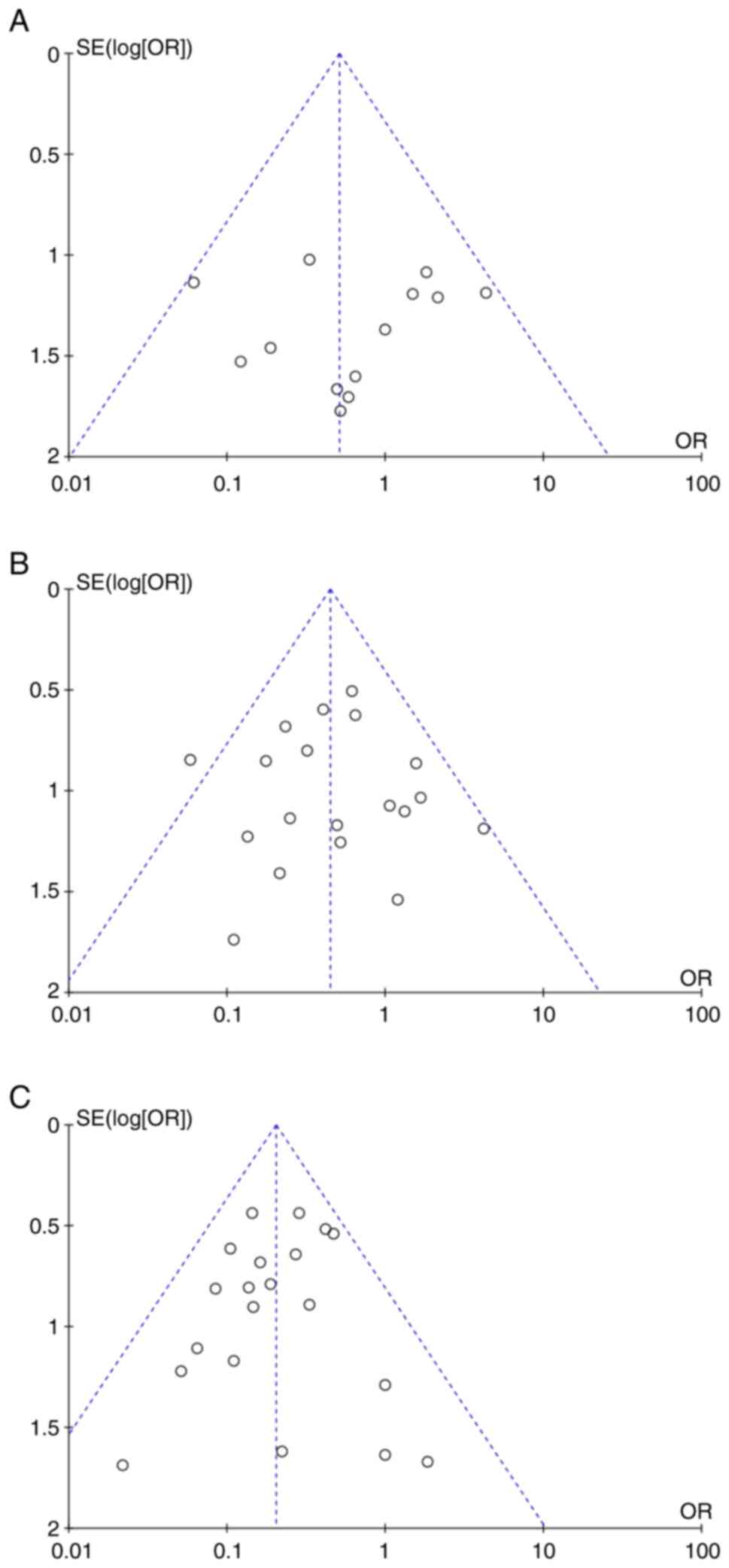
No asymmetry was visible in the funnel plots,
indicating an absence of publication bias (Fig. 3A-C).
GIST develops from the gastrointestinal mesenchyme
and is a relatively common sarcoma of soft tissue (41). The outcome usually depends on the
size, site, and mitotic index of the tumor with tumors <5 cm in
diameter originating in the stomach, with mitotic indices below
5/50 high-power field linked to more favorable prognoses (42,43).
The NIH used these parameters to develop prediction tools for GIST
progression and outcome, assessing the risk of poor outcome as very
low, low, intermediate, or high as outcome prediction tools using
these (8). In addition to this,
numerous studies have been undertaken to investigate the
possibility of basing prediction on molecular, as well as clinical,
factors. A meta-analysis of Asian, European, and North American
patients found that mutations in KIT exon 11 were associated with
superior treatment responses and survival compared with exon 9
polymorphisms (44,45). A previous study showed that
deletions in exon 11 (codons 557 and/or 558) of KIT were linked to
significantly lower rates of disease-free survival in European
patients with GIST (46).
Mutations in exon 18 of PDGFRA have also been associated with
significantly reduced GIST progression and improved outcomes
(47,48). In the present study, a
meta-analysis was conducted at the molecular level to determine
whether KI67 can determine the prognosis of GIST.
To clarify these conflicting reports, the
relationship between Ki67 levels and GIST prognosis was
investigated through meta-analysis. In this meta-analysis, Ki67
levels were found to be higher in the NIH L group than in the NIH
VL group, while those in the NIH I group were significantly
increased in comparison with the NIH L group. Ki67 was also
overexpressed in the NIH H group compared with the NIH I group. In
the present study, different results were obtained compared with
Zhou et al (21). The
results revealed that the higher the risk, the higher the
overexpression rate of Ki67, suggesting that Ki67 expression may be
a useful addition to the NIH assessment system for GIST risk
prediction. Although the mitotic index has been considered to be
only an indication of the M mitotic phase (57), Ki67 is expressed throughout the
cell cycle apart from the G0 phase and is an important predictor of
poor prognosis in GIST (P<0.0003). It was found that Ki67 had
higher observer reliability than the mitotic count in the
evaluation of mitotic activity (32), and the Ki67 index may thus be used
as a replacement index for the mitotic count in the future.
Nevertheless, the present meta-analysis has several
limitations. First, it is difficult to reach a precise conclusion
due to the limited sample size, differences in antibody clones and
possible heterogeneity. Second, the clinicopathological information
of patients was derived from case reports, and differences in the
practices and diagnostic criteria of different pathologists may
also lead to bias. Therefore, since adjuvant imatinib is standard
for high risk GIST, it is considered that a large-scale,
multi-center prospective study is necessary in the future, taking
the low-risk group not receiving imatinib as the control group, and
the high-risk group receiving treatment as the experimental group,
to compare the long-term survival of the results of the two groups,
and use multivariate regression analysis to clarify whether the
Ki67 index, gene mutation site, medication compliance and blood
drug concentration were related to survival outcomes. Despite these
limitations, the present findings contributed to the further
discovery of new predictors of adverse outcomes and to the
improvement of existing classification criteria.
Not applicable.
Funding: No funding was received.
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
JL and SQL contributed to the conception, design and
modification of the study. ARW, XDC and HP extracted the data and
organized the database search. JL and ARW performed the statistical
analysis. SQL, XDC and HP drafted the manuscript. JL and SQL
confirm the authenticity of all the raw data. All authors
contributed to manuscript revision, read, and approved the
submitted version. All authors agreed to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved. All authors read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
El-Menyar A, Mekkodathil A and Al-Thani H:
Diagnosis and management of gastrointestinal stromal tumors: An
up-to-date literature review. J Cancer Res Ther. 13:889–900.
2017.PubMed/NCBI
|
|
2
|
Schaefer IM, Mariño-Enríquez A and
Fletcher JA: What is new in gastrointestinal stromal tumor? Adv
Anat Pathol. 24:259–267. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nishida T, Blay JY, Hirota S, Kitagawa Y
and Kang YK: The standard diagnosis, treatment, and follow-up of
gastrointestinal stromal tumors based on guidelines. Gastric
Cancer. 19:3–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Demetri GD, von Mehren M, Antonescu CR,
DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF,
Schuetze S, et al: NCCN task force report: Update on the management
of patients with gastrointestinal stromal tumors. J Natl Compr Canc
Netw. 8 (Suppl 2):S1–S44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miettinen M and Lasota J: Histopathology
of gastrointestinal stromal tumor. J Surg Oncol. 104:865–873. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van der Graaf WTA, Tielen R, Bonenkamp JJ,
Lemmens V, Verhoeven RHA and de Wilt JHW: Nationwide trends in the
incidence and outcome of patients with gastrointestinal stromal
tumour in the imatinib era. Br J Surg. 105:1020–1027. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma GL, Murphy JD, Martinez ME and Sicklick
JK: Epidemiology of gastrointestinal stromal tumors in the era of
histology codes: Results of a population-based study. Cancer
Epidemiol Biomarkers Prev. 24:298–302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Joensuu H: Risk stratification of patients
diagnosed with gastrointestinal stromal tumor. Hum Pathol.
39:1411–1419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kitamura Y: Gastrointestinal stromal
tumors: Past, present, and future. J Gastroenterol. 43:499–508.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scholzen T and Gerdes J: The Ki-67
protein: From the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Isola J, Helin H and Kallioniemi OP:
Immunoelectron-microscopic localization of a
proliferation-associated antigen Ki-67 in MCF-7 cells. Histochem J.
22:498–506. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
du Manoir S, Guillaud P, Camus E,
Seigneurin D and Brugal G: Ki-67 labeling in postmitotic cells
defines different Ki-67 pathways within the 2c compartment.
Cytometry. 12:455–463. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hutchins JR, Toyoda Y, Hegemann B, Poser
I, Hériché JK, Sykora MM, Augsburg M, Hudecz O, Buschhorn BA,
Bulkescher J, et al: Systematic analysis of human protein complexes
identifies chromosome segregation proteins. Science. 328:593–599.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Visapää H, Bui M, Huang Y, Seligson D,
Tsai H, Pantuck A, Figlin R, Rao JY, Belldegrun A, Horvath S and
Palotie A: Correlation of Ki-67 and gelsolin expression to clinical
outcome in renal clear cell carcinoma. Urology. 61:845–850. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Ren G, Cai R, Chen J, Wu X and Zhao
J: A correlation research of Ki67 index, CT features, and risk
stratification in gastrointestinal stromal tumor. Cancer Med.
7:4467–4474. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H and Liu Q: Prognostic indicators
for gastrointestinal stromal tumors: A review. Transl Oncol.
13:1008122020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang JP, Liu L, Li ZA, Wang Q, Wang XY and
Lin J: Ki-67 labelling index is related to the risk classification
and prognosis of gastrointestinal stromal tumours: A retrospective
study. Gastroenterol Hepatol. 44:103–114. 2021.(In English,
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Qiu H, Zhang P, Feng X, Chen T, Li
Y, Tao K, Li G, Sun X and Zhou Z; China Gastrointestinal Stromal
Tumor Study Group (CN-GIST), : Ki-67 labeling index may be a
promising indicator to identify ‘very high-risk’ gastrointestinal
stromal tumor: A multicenter retrospective study of 1022 patients.
Hum Pathol. 74:17–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sahin S, Ekinci O, Seckin S and Dursun A:
Proliferation markers RacGAP1 and Ki-67 in gastrointestinal stromal
tumors by immunohistochemistry with respect to clinicopathological
features and different risk stratification systems. Int J Clin Exp
Pathol. 10:11723–11736. 2017.PubMed/NCBI
|
|
20
|
Demir L, Ekinci N, Erten C, Kucukzeybek Y,
Alacacioglu A, Somali I, Can A, Dirican A, Bayoglu V, Akyol M, et
al: Does immunohistochemistry provide additional prognostic data in
gastrointestinal stromal tumors? Asian Pac J Cancer Prev.
14:4751–4758. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou Y, Hu W, Chen P, Abe M, Shi L, Tan
SY, Li Y and Zong L: Ki67 is a biological marker of malignant risk
of gastrointestinal stromal tumors: A systematic review and
meta-analysis. Medicine (Baltimore). 96:e79112017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakamura N, Yamamoto H, Yao T, Oda Y,
Nishiyama K, Imamura M, Yamada T, Nawata H and Tsuneyoshi M:
Prognostic significance of expressions of cell-cycle regulatory
proteins in gastrointestinal stromal tumor and the relevance of the
risk grade. Hum Pathol. 36:828–837. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pleşea IE, Chiuţu L, Bordu SI, Georgescu
I, Georgescu EF, Ciobanu D, Mărgăritescu ND, Comănescu V and Nemeş
R: Gastrointestinal stromal tumors-a clinical-morphological study
on 15 cases. Rom J Morphol Embryol. 55 (Suppl 2):S513–S523.
2014.PubMed/NCBI
|
|
24
|
Peker K, Sayar I, Gelincik I, Bulut G,
Kökenek Ünal TD, Şenol S, Gökçe A and Isik A: The diagnostic
importance of matrix metalloproteinase-7 and nestin in
gastrointestinal stromal tumors. Med Sci Monit. 20:674–680. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsumuraya M, Kato H, Miyachi K, Sasaki K,
Tsubaki M, Akimoto K and Sunagawa M: Comprehensive analysis of
genes involved in the malignancy of gastrointestinal stromal
tumors. Anticancer Res. 30:2705–2715. 2010.PubMed/NCBI
|
|
26
|
Jiang L, Cao M, Hu J and Chen J:
Expression of PIN1 in gastrointestinal stromal tumours and its
clinical significance. Anticancer Res. 36:1275–1280.
2016.PubMed/NCBI
|
|
27
|
Güler B, Özyılmaz F, Tokuç B, Can N and
Taştekin E: Histopathological features of gastrointestinal stromal
tumors and the contribution of DOG1 expression to the diagnosis.
Balkan Med J. 32:388–396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Chen P, Liu XX, Zhao W, Shi L, Gu
XW, Zhu CR, Zhu HH and Zong L: Prognostic impact of
gastrointestinal bleeding and expression of PTEN and Ki-67 on
primary gastrointestinal stromal tumors. World J Surg Oncol.
12:892014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao WY, Xu J, Wang M, Zhang ZZ, Tu L,
Wang CJ, Lin TL, Shen YY, Liu Q and Cao H: Prognostic value of Ki67
index in gastrointestinal stromal tumors. Int J Clin Exp Pathol.
7:2298–2304. 2014.PubMed/NCBI
|
|
30
|
Nanding A, Tang L, Cai L, Chen H, Geng J,
Liu X, Ning X, Li X and Zhang Q: Low ING4 protein expression
detected by paraffin-section immunohistochemistry is associated
with poor prognosis in untreated patients with gastrointestinal
stromal tumors. Gastric Cancer. 17:87–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu C, Liu L, Wu X and Xu W: CD133 and
Ki-67 expression is associated with gastrointestinal stromal tumor
prognosis. Oncol Lett. 6:1289–1294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Segales-Rojas P, Lino-Silva LS,
Aguilar-Cruz E and Salcedo-Hernández RA: Association of ki67 index
with recurrence in gastrointestinal stromal tumors. J Gastrointest
Cancer. 49:543–547. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang J and Cao XY, Suo J, Wang YP, He L
and Cao XY: Evaluation of malignancy using Ki-67, p53, EGFR and
COX-2 expressions in gastrointestinal stromal tumors. World J
Gastroenterol. 18:2569–2575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu LC, Xu WT, Wu X, Zhao P, Lv YL and
Chen L: Overexpression of carbonic anhydrase II and Ki-67 proteins
in prognosis of gastrointestinal stromal tumors. World J
Gastroenterol. 19:2473–2480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alghamdi HM, Amr SS, Shawarby MA, Sheikh
SS, Alsayyah AA, Alamri AM, Ismail MH, Almarhabi A, Alrefaee MA and
Ahmed MI: Gastrointestinal stromal tumors. A clinicopathological
study. Saudi Med J. 40:126–130. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ngo QD, Pham QT, Phan DAT, Hoang AV, Hua
TNH and Nguyen ST: Molecular and clinicopathological features of
gastrointestinal stromal tumors in vietnamese patients. J Pathol
Transl Med. 53:361–368. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Podda M, Ferraro G, Di Saverio S, Cois A,
Nardello O, Poillucci G, Marino MV and Pisanu A: Association
between gastrointestinal stromal tumors and other malignancies: It
is only a matter of time ? A case series and an overview of
systematic reviews. J Gastrointest Cancer. 51:914–924. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tepeoğlu M, Özgün G, Tunca MZ, Tezcaner T
and Özdemir BH: Gastrointestinal stromal tumors: A
clinicopathological and immunohistochemical study of 65 cases. Turk
Patoloji Derg. 34:207–214. 2018.PubMed/NCBI
|
|
39
|
Wei SC, Xu L, Li WH, Li Y, Guo SF, Sun XR
and Li WW: Risk stratification in GIST: Shape quantification with
CT is a predictive factor. Eur Radiol. 30:1856–1865. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Taniguchi K, Suzuki A, Serizawa A, Kotake
S, Ito S, Suzuki K, Yamada T, Noguchi T, Amano K, Ota M, et al:
Rapid flow cytometry of gastrointestinal stromal tumours closely
matches the modified fletcher classification. Anticancer Res.
41:131–136. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Joensuu H, Hohenberger P and Corless CL:
Gastrointestinal stromal tumour. Lancet. 382:973–983. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
McCarter MD, Antonescu CR, Ballman KV,
Maki RG, Pisters PW, Demetri GD, Blanke CD, von Mehren M, Brennan
MF, McCall L, et al: Microscopically positive margins for primary
gastrointestinal stromal tumors: Analysis of risk factors and tumor
recurrence. J Am Coll Surg. 215:53–60. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gold JS, Gönen M, Gutiérrez A, Broto JM,
García-del-Muro X, Smyrk TC, Maki RG, Singer S, Brennan MF,
Antonescu CR, et al: Development and validation of a prognostic
nomogram for recurrence-free survival after complete surgical
resection of localised primary gastrointestinal stromal tumour: A
retrospective analysis. Lancet Oncol. 10:1045–1052. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhi X, Zhou X, Wang W and Xu Z: Practical
role of mutation analysis for imatinib treatment in patients with
advanced gastrointestinal stromal tumors: A meta-analysis. PLoS
One. 8:e792752013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yan L, Zou L, Zhao W, Wang Y, Liu B, Yao H
and Yu H: Clinicopathological significance of c-KIT mutation in
gastrointestinal stromal tumors: A systematic review and
meta-analysis. Sci Rep. 5:137182015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wozniak A, Rutkowski P, Schöffski P,
Ray-Coquard I, Hostein I, Schildhaus HU, Le Cesne A, Bylina E,
Limon J, Blay JY, et al: Tumor genotype is an independent
prognostic factor in primary gastrointestinal stromal tumors of
gastric origin: A european multicenter analysis based on
ConticaGIST. Clin Cancer Res. 20:6105–6116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rubió-Casadevall J, Borràs JL,
Carmona-García MC, Ameijide A, Gonzalez-Vidal A, Ortiz MR, Bosch R,
Riu F, Parada D, Martí E, et al: Correlation between mutational
status and survival and second cancer risk assessment in patients
with gastrointestinal stromal tumors: A population-based study.
World J Surg Oncol. 13:472015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gerdes J, Schwab U, Lemke H and Stein H:
Production of a mouse monoclonal antibody reactive with a human
nuclear antigen associated with cell proliferation. Int J Cancer.
31:13–20. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Belev B, Brčić I, Prejac J, Golubić ZA,
Vrbanec D, Božikov J, Alerić I, Boban M and Razumović JJ: Role of
Ki-67 as a prognostic factor in gastrointestinal stromal tumors.
World J Gastroenterol. 19:523–527. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jia YF, Xiao DJ, Ma XL, Song YY, Hu R,
Kong Y, Zheng Y, Han SY, Hong RL and Wang YS: Differentiated
embryonic chondrocyte-expressed gene 1 is associated with
hypoxia-inducible factor 1α and Ki67 in human gastric cancer. Diagn
Pathol. 8:372013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Seidal T and Edvardsson H: Expression of
c-kit (CD117) and Ki67 provides information about the possible cell
of origin and clinical course of gastrointestinal stromal tumours.
Histopathology. 34:416–424. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nilsson B, Bümming P, Meis-Kindblom JM,
Odén A, Dortok A, Gustavsson B, Sablinska K and Kindblom LG:
Gastrointestinal stromal tumors: The incidence, prevalence,
clinical course, and prognostication in the preimatinib mesylate
era-a population-based study in western Sweden. Cancer.
103:821–829. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nagasako Y, Misawa K, Kohashi S, Hasegawa
K, Okawa Y, Sano H, Takada A and Sato H: Evaluation of malignancy
using Ki-67 labeling index for gastric stromal tumor. Gastric
Cancer. 6:168–172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gumurdulu D, Erdogan S, Kayaselcuk F,
Seydaoglu G, Parsak CK, Demircan O and Tuncer I: Expression of
COX-2, PCNA, Ki-67 and p53 in gastrointestinal stromal tumors and
its relationship with histopathological parameters. World J
Gastroenterol. 13:426–431. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wong NA, Young R, Malcomson RD, Nayar AG,
Jamieson LA, Save VE, Carey FA, Brewster DH, Han C and Al-Nafussi
A: Prognostic indicators for gastrointestinal stromal tumours: A
clinicopathological and immunohistochemical study of 108 resected
cases of the stomach. Histopathology. 43:118–126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kramer K, Knippschild U, Mayer B,
Bögelspacher K, Spatz H, Henne-Bruns D, Agaimy A, Schwab M and
Schmieder M: Impact of age and gender on tumor related prognosis in
gastrointestinal stromal tumors (GIST). BMC Cancer. 15:572015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hohenberger P, Ronellenfitsch U, Oladeji
O, Pink D, Ströbel P, Wardelmann E and Reichardt P: Pattern of
recurrence in patients with ruptured primary gastrointestinal
stromal tumour. Br J Surg. 97:1854–1859. 2010. View Article : Google Scholar : PubMed/NCBI
|