Introduction
Cervical cancer (CC) is a malignant tumor that
occurs in the cervical region and it severely endangers a woman's
health. CC was the fourth most common gynecological malignancy in
developing countries in 2019 (1,2).
Carbohydrates provide energy for essential biochemical processes,
and glycolysis is the main process through which organisms,
including cancer cells, obtain energy (3). Even though cellular oxygen is
adequate, tumor cells rely on aerobic glycolysis, and not on
mitochondrial oxidative phosphorylation to generate energy
(4). Glycolysis and adenosine
triphosphate (ATP) generation are crucial to the development of
cancers, including growth, metastasis and chemoresistance (5,6). A
negative effect of the glycolysis pathway is that it leads to the
abnormal growth and proliferation of cancer cells (7). Tumor glycolysis has also been
reported to be a target for cancer therapy (6). Therefore, it is of utmost importance
to determine the specific molecules regulating glycolysis in
CC.
Studies have demonstrated that a number of crucial
intracellular mechanisms are related to the proliferation and
apoptosis of cancer cells (8,9).
MicroRNAs (miRNAs/miRs) are a type of non-coding RNA, which can
bind to target mRNAs to inhibit their translation (10,11).
miRNAs are widely involved in various physiological and
pathological processes of tumor cells by regulating the expression
of oncogenes and tumor suppressors (12,13).
It has been demonstrated that miRNA expression levels are
dysregulated in CC tissues and cell lines, and this dysregulated
expression plays crucial roles in the pathological process of CC
(14,15). In 2018, a bioinformatics study
indicated that the downregulation of miR-99a was closely related to
the 5-year survival rate of patients with CC (16). In 2019, miR-99a was reported to be
decreased in cervical intraepithelial neoplasia (CIN) vs. normal
tissue, and in cervical squamous cell carcinoma (CSCC) vs. CIN,
suggesting that miR-99a may be involved in the pathogenesis of CIN
and in the progression of CIN to CSCC (17). However, the distinct roles of
miR-99a-5p in glycolysis in CC have not yet been fully elucidated.
Thus, the present study aimed to explore the potential functions of
this miRNA in glycolysis in CC.
Materials and methods
Antibodies and reagents
The primary and secondary antibodies used in the
present study were as follows: Ras-related GTP-binding protein D
(RRAGD; cat. no. A304-301A-T; Thermo Fisher Scientific, Inc.),
GAPDH (cat. no. A300-642A-T; Thermo Fisher Scientific, Inc.), goat
anti-rabbit (cat. no. 7074; Cell Signaling Technology, Inc.) and
horse anti-mouse (cat. no. 7076; Cell Signaling Technology,
Inc.).
The reagents used were as follows: FBS, DMEM,
penicillin-streptomycin, Lipofectamine 2000®,
TRIzol® reagent (all from Thermo Fisher Scientific,
Inc.); the PrimeScript qRT Reagent kit (Takara Bio, Inc.),
SYBR-Green Mix (Roche Diagnostics), the Dual Luciferase Assay
System (Promega Corporation), protease inhibitor cocktail
(Sigma-Aldrich; Merck KGaA), RIPA buffer (Roche Diagnostics),
chemical HRP substrate (MilliporeSigma), the Annexin-V/Dead Cell
Apoptosis kit, glucose uptake colorimetric assay kit (BioVision,
Inc.),
2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose
(2-NBDG; Sigma-Aldrich; Merck KGaA), Krebs-ringer-phosphate-HEPES
(KRPH; Thermo Fisher Scientific, Inc.), the lactate colorimetric
assay kit (BioVision, Inc.) and the XF Glycolysis Stress Test kit
(Seahorse Bioscience).
Specimens
In total, 21 pairs of CC tissues and matched normal
tissues were obtained from patients (47±6 years; range, 41–59
years) with CC at the Wuhan Third Hospital (Tongren Hospital of
Wuhan University, Wuhan, China) between February 2018 and March
2019. Patients who received chemotherapy or radiotherapy were
excluded. Written informed consent was provided by all participants
prior to the initiation of the study. The present study was
approved by the Ethics Committee of Wuhan Third Hospital (approval
no. WHTH-2018-07). Tissues were collected for use in subsequent
experiments or stored in −80°C if not used immediately.
Cell culture and transfection
The Ect1 (cat. no. CRL-2614), HeLa (cat. no.
CRM-CCL-2), C33A (cat. no. HTB-31) and SiHa (cat. no. HTB-35) cells
were obtained from the American Type Culture Collection (ATCC) and
cultured in DMEM containing 10% FBS, incubated with 5%
CO2 and 37°C. miR-99a-5p mimic and miR-NC mimic were
purchased from Shanghai GenePharma Co., Ltd. Transfection of
pcDNA3.1-RRAGD plasmid (30 µg), pcDNA3.1 plasmid (30 µg),
miR-99a-5p mimic (100 nM; 5′-AACCCGUAGAUCCGAUCUUGUG-3′) and miR-NC
mimic (100 nM; 5′-UUGUACUACACAAAAGUACUG-3′) was performed at 37°C
for 48 h using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.).
At 48 h after transfection, cells were collected for the subsequent
experimentation.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from CC tissues or Ect1,
HeLa, C33A and SiHa cell lines using TRIzol® reagent and
reverse transcribed into cDNA using the PrimeScript™ qRT reagent
kit with the following thermocycling conditions: A total of three
cycles at 37°C for 15 min, termination at 85°C for 5 sec, and
maintenance at 4°C. qPCR was performed using SYBR-Green mix on an
ABI Prism 7500 system (Thermo Fisher Scientific, Inc.) with the
following thermocycling conditions: 95°C for 5 min (initial
denaturation), followed by 40 cycles at 95°C for 30 sec, 55°C for
30 sec, 72°C for 45 sec, with a final extension at 72°C for 10 min.
GAPDH and U6 served as an internal control for RRAGD and
miR-99a-5p, respectively. The sequences of all primers used are
listed in Table I. The relative
expression levels were analyzed using the 2−∆∆Cq method
(18).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene name | Primer sequences
(5′-3′) |
|---|
| miR-99a-5p | F:
AACCCGTAGATCCGATCTTGTG |
|
| R:
CACAAGATCGGATCTACGGGTT |
| U6 | F:
CTCGCTTCGGCAGCACATA |
|
| R:
AACGATTCACGAATTTGCGT |
| RRAGD | F:
CTAGCGGACTACGGAGACG |
|
| R:
ATGAGCAGGATTCTCGGCTTC |
| GAPDH | F:
GAAGGTGAAGGTCGGAGTC |
|
| R:
GAAGATGGTGATGGGATTTC |
Western blot analysis
The Ect1 and SiHa cells were washed in PBS, and
harvested before being lysed using RIPA buffer (Beyotime Institute
of Biotechnology) containing protease inhibitor cocktail. Protein
concentration was evaluated using a Pierce BCA protein assay kit
(Thermo Fisher Scientific, Inc.). The protein samples (15 µg) were
then mixed with SDS loading buffer, before separating by 8%
SDS-PAGE, and electro-transferring onto a PVDF filter. The filter
was then blocked with 5% BSA (Thermo Fisher Scientific, Inc.) in
TBST (0.2% Tween-20) at room temperature for 2 h and incubated
overnight at 4°C with anti-RRAGD (1:2,000) or anti-GAPDH (1:2,000)
antibodies in TBST. This was followed by washing and incubation
with appropriate HRP-labeled secondary antibodies (1:1,000) for 1 h
at room temperature. After washing thoroughly, the HRP signals were
detected using an ECL Prime detection reagent (Thermo Fisher
Scientific, Inc.). Band intensity was quantified using ImageJ
software (version 1.8.0; National Institutes of Health).
MTT assay
Following culture for 24, 48 and 72 h, each well of
SiHa cells (3×104 cells/well) was incubated at 37°C for
3 h with 20 µl (5 mg/ml) MTT solution. Following incubation for 3
h, 100 µl dimethyl sulfoxide (DMSO) was added to each well for dye
extraction. After shaking for 15 min, the absorbance of the cells
was recorded at 570 nm using a microplate reader.
Flow cytometric analysis of
apoptosis
Each group of cultured SiHa cells was washed and
harvested with PBS. The cells (1×106) were then
suspended in 100 µl PBS and mixed with Annexin V-FITC and propidium
iodide (PI), and then incubated for 10–15 min in a dark at room
temperature. After washing with PBS, the cells were then analyzed
using a BD FACSCalibur™ flow cytometer (BD Biosciences), and
Annexin V-FITC positive cells were considered apoptotic (early +
late apoptosis was assessed). Data were analyzed using FlowJo
(version 7.6.3; FlowJo LLC).
Glucose uptake and lactate production
measurement
For the measurement of glucose uptake, SiHa cells
were measured using a glucose uptake colorimetric assay kit. A
total of 2×103 cells were placed in a 96-well plate and
starved with serum-free DMEM for 12 h. The SiHa cells were then
incubated at room temperature with KRPH with 2% BSA. After 30 min,
10 µl 2-NBDG were added to each well and incubated for 20 min at
room temperature. Nicotinamide adenine dinucleotide phosphate
generation was determined using an enzymatic recycling
amplification reaction. The absorbance at 412 nm was recorded using
a GloMax®Discover Microplate Reader (Promega
Corporation).
For the measurement of lactate production, the SiHa
cells were harvested and suspended in fresh DMEM without pyruvic
acid and seeded in a 96-well plate at a density of 2×103
cells/well. After 6 h, 100 µl culture medium was collected and
diluted with 0.5 ml KRPH buffer. The lactate concentration was
determined using a lactate colorimetric assay kit according to the
manufacturer's protocol. The absorbance at 450 nm was also recorded
using a GloMax®Discover Microplate Reader and normalized
to the protein concentration.
Extracellular acidification rate
(ECAR) assay
The SiHa cells were placed in an XFe96 plate at a
density of 1×104 cells/well and incubated for 12 h at
37°C. At 1 h before measurement, the medium was replaced with XF
medium. The XF Glycolysis Stress Test kit was used to detect the
glycolytic capacity according to the manufacturer's protocol. After
20, 50 and 80 min, glucose (10 mM), oligomycin (1 mM) and 2-deoxy
glucose (2-DG; 50 mM) were diluted into XF medium and the ECAR
assay was performed using a Seahorse XFe96 extracellular flux
analyzer (Seahorse Bioscience).
Dual luciferase reporter assay
To explore the targets of miR-99a-5p in regulating
CC cell apoptosis, the TargetScan 7.1 website (TargetScanHuman 7.1)
was searched. Wild-type (WT) or mutant type (Mut) RRAGD 3′
untranslated region (3′-UTR) sequences were constructed into the
pGL3-luciferase reporter plasmid (Promega Corporation), and
co-transfected with miR-99a-5p/NC mimics (100 nM) into SiHa cells
(1×105/well) using Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.). At 48 h post-transfection, the cells were
transferred to a 24-well culture dish. The Dual Luciferase Assay
System (Promega Corporation) was used to measure the luciferase
activities. Renilla luciferase activity was used as the
reference control.
Statistical analysis
All data were analyzed using GraphPad Prism 6.0
(GraphPad Software, Inc.) and are presented as the mean ± SD.
Kaplan-Meier analysis and log-rank testing were used to analyze the
association between miR-99a and the prognosis of patients with CC
from the Cancer Genome Atlas database. Differences between two
groups were compared using a paired (tissues) and unpaired (cell
lines) Student's t-test, and differences among three groups were
compared using one-way ANOVA followed by a Tukey's post hoc test.
Pearson's correlation analysis was used to analyze the correlation
between miR-99a-5p and RRAGD expression in CC tissues. A value of
P<0.05 was considered to indicate a statistically significant
difference. Each experiment was replicated at least three
times.
Results
miR-99a-5p expression is decreased in
CC tissues and cell lines
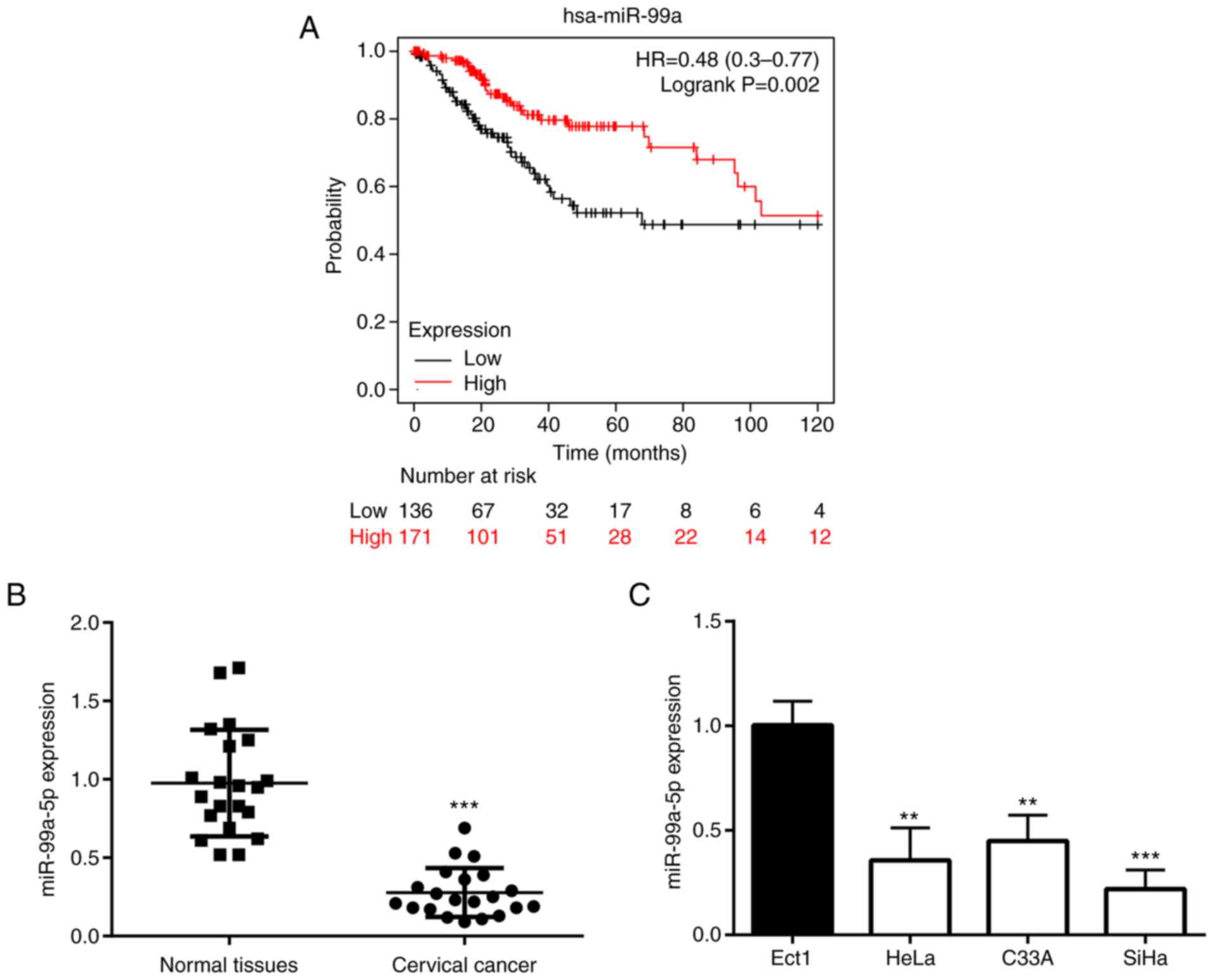
Firstly, the present study aimed to determine
whether the expression level of miR-99a was related to the survival
rate of patients with CC; thus, Kaplan-Meier analysis was
performed. The results revealed that the patients with a high
expression of miR-99a had a higher survival rate than those in the
low expression group (Fig. 1A). In
order to further confirm the association between the miR-99a-5p
expression level and CC, its expression was examined using RT-qPCR
in CC tissues and various cell lines. The results demonstrated that
the expression level of miR-99a-5p was significantly decreased in
CC tissues (Fig. 1B) and cell
lines, including HeLa, C33A and SiHa cells (Fig. 1C). Among the three different cell
lines, the SiHa cells exhibited the lowest expression of
miR-99a-5p, and were thus used in the following experiments.
Moreover, in the collected tumor tissues from
patients with CC, the miR-99a-5p level was found to be associated
with the FIGO stage, but not with age or tumor size (Table II).
 | Table II.Association between miR-99a-5p
expression levels and clinicopathological characteristics of
patients with cervical cancer. |
Table II.
Association between miR-99a-5p
expression levels and clinicopathological characteristics of
patients with cervical cancer.
|
| Expression level of
miR-99a-5p |
|
|---|
|
|
|
|
|---|
| Clinicopathological
characteristics | Low (n=10) | High (n=11) | P-value |
|---|
| Age, years |
|
| 0.659 |
|
<45 | 3 | 5 |
|
| ≥45 | 7 | 6 |
|
| Tumor size, cm |
|
| 0.395 |
|
<4 | 6 | 4 |
|
| ≥4 | 4 | 7 |
|
| FIGO stages |
|
| 0.031 |
|
I–II | 2 | 8 |
|
|
III–IV | 8 | 3 |
|
miR-99a-5p promotes the apoptosis and
reduces the glycolysis of CC cells
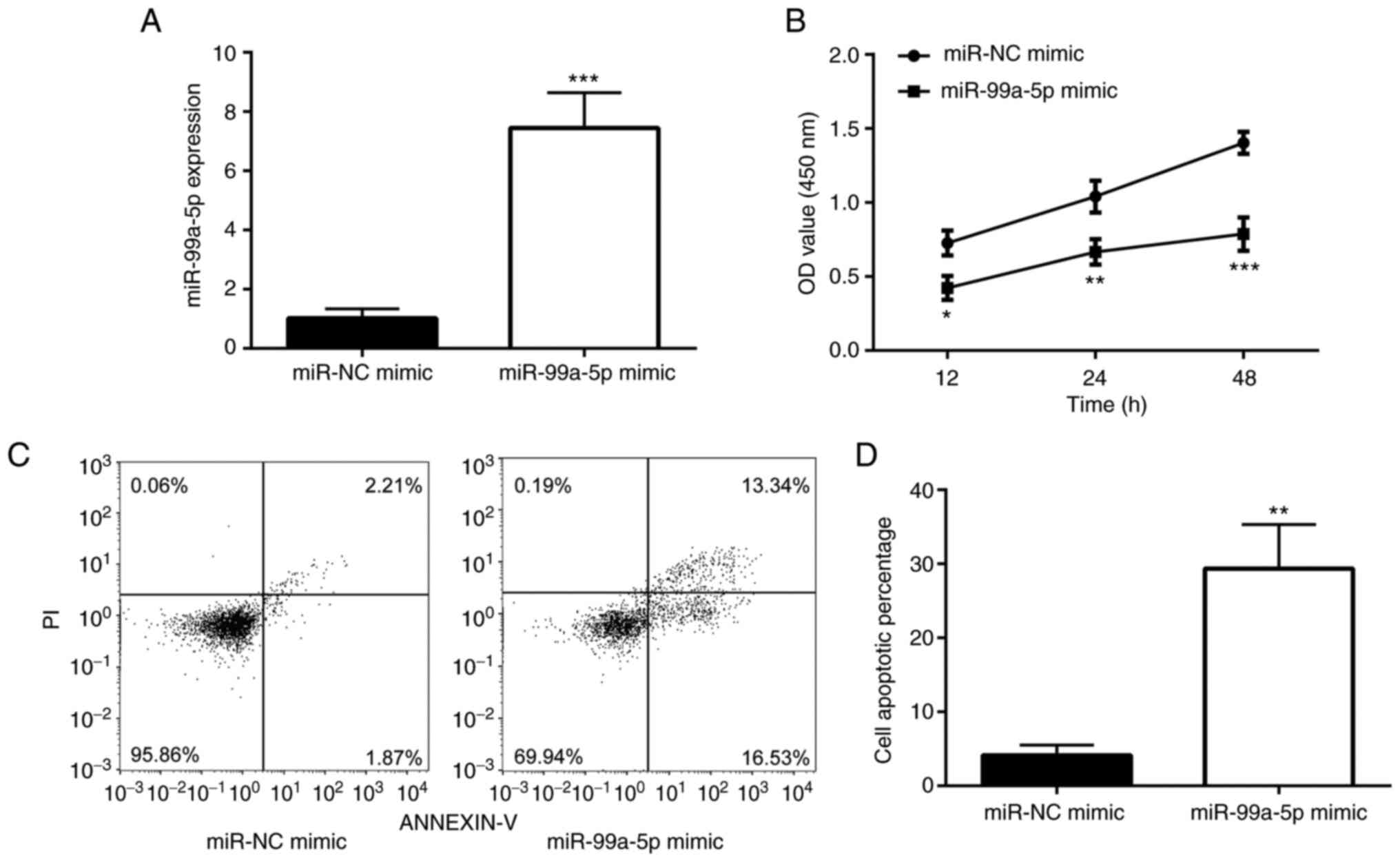
In order to examine whether miR-99a-5p affects the
survival of SiHa cells, miR-99a-5p was overexpressed in SiHa cells
using a miRNA mimic (Fig. 2A). It
was found that transfection with miR-99a-5p mimic significantly
reduced the number of SiHa cells (Fig.
2B). To determine whether miR-99a-5p affects the apoptosis of
SiHa cells, flow cytometry was performed. As shown in Fig. 2C and D, transfection with
miR-99a-5p mimic significantly induced SiHa cell apoptosis when
compared with the cells transfected with miR-NC mimic.
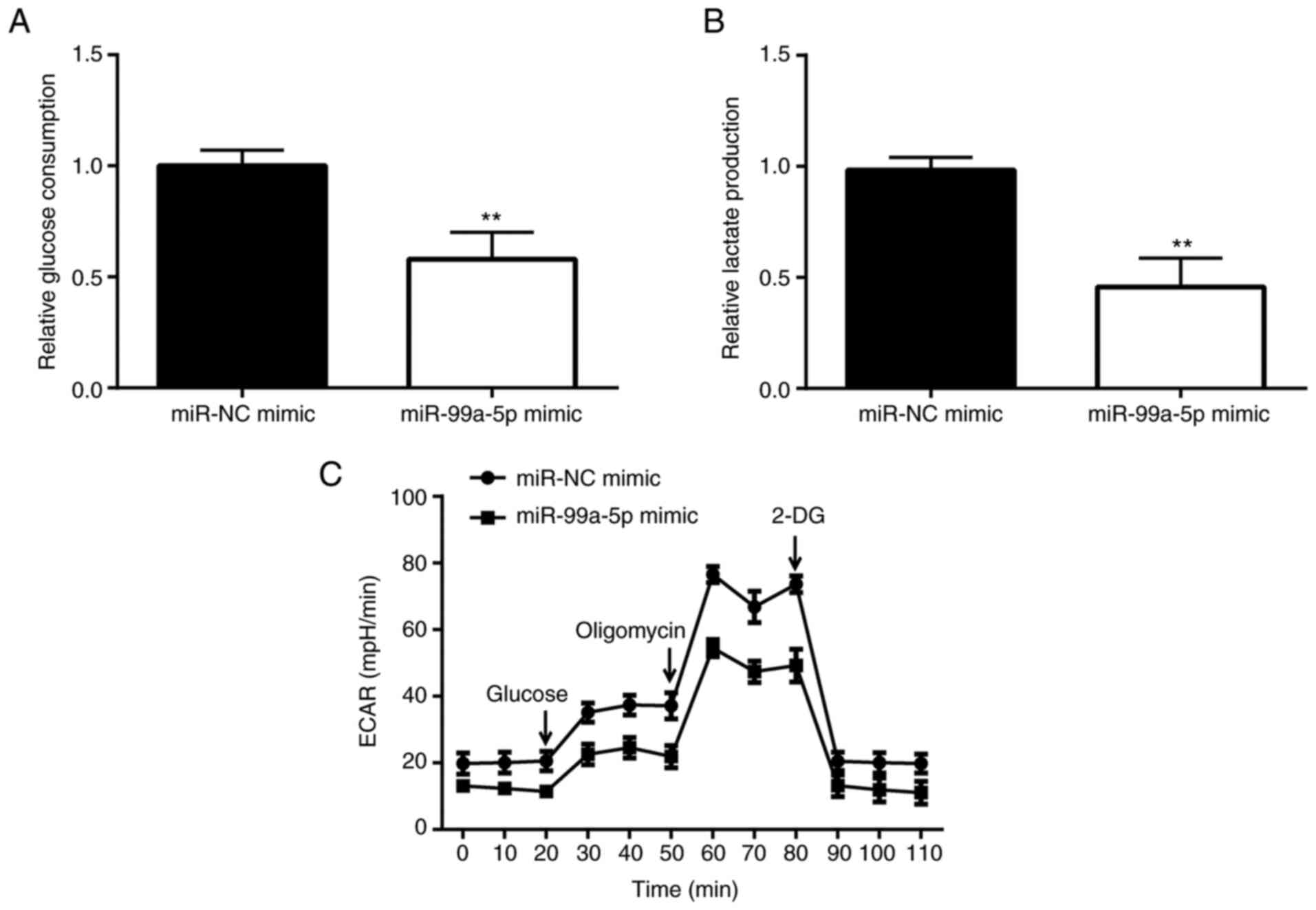
In order to examine the mechanisms underlying the
promotion of cell apoptosis by miR-99a-5p, the glucose consumption
of SiHa cells was detected. The results revealed that following the
overexpression of miR-99a-5p, the glucose consumption of SiHa cells
significantly decreased (Fig. 3A),
and the production of glycolytic product lactate also significantly
decreased compared with the miR-NC mimic group (Fig. 3B). Subsequently, the ECAR was
examined and the results revealed that the ECAR value of the
miR-99a-5p mimic group was significantly lower than that of the
miR-NC mimic group, particularly following treatment with the ATP
synthesis inhibitor, oligomycin (Fig.
3C).
RRAGD is targeted by miR-99a-5p, and
its expression is decreased in CC tissues and cells
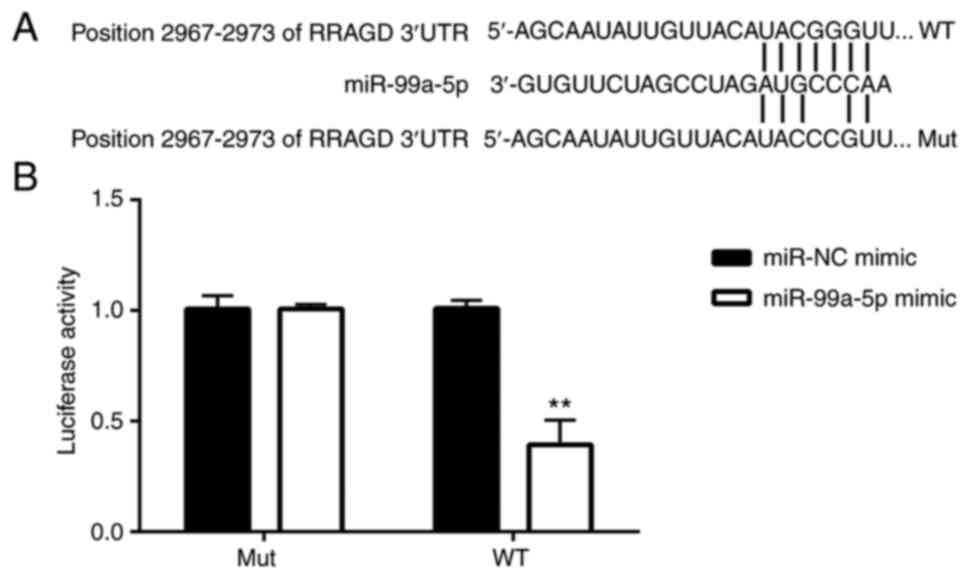
To explore the targets of miR-99a-5p in regulating
CC cell apoptosis, the TargetScan 7.1 website was searched. It was
found that the 3′-UTR of RRAGD was potentially targeted by
miR-99a-5p (Fig. 4A). RRAGD is a
monomeric GTP/GDP binding protein and plays critical roles in the
mTOR signaling pathway (19). In
the present study, to confirm this hypothesis, a dual luciferase
reporter assay was performed. The results demonstrated that in the
WT RRAGD 3′-UTR-transfected cells, the luciferase activity was
significantly lower in the miR-99a-5p mimic group in comparison
with the miR-NC mimic group; however, no significant difference was
observed between the miR-NC mimic group and miR-99a-5p mimic group
in the MUT RRAGD 3′-UTR-transfected cells (Fig. 4B). These results indicated that
RRAGD was targeted by miR-99a-5p.
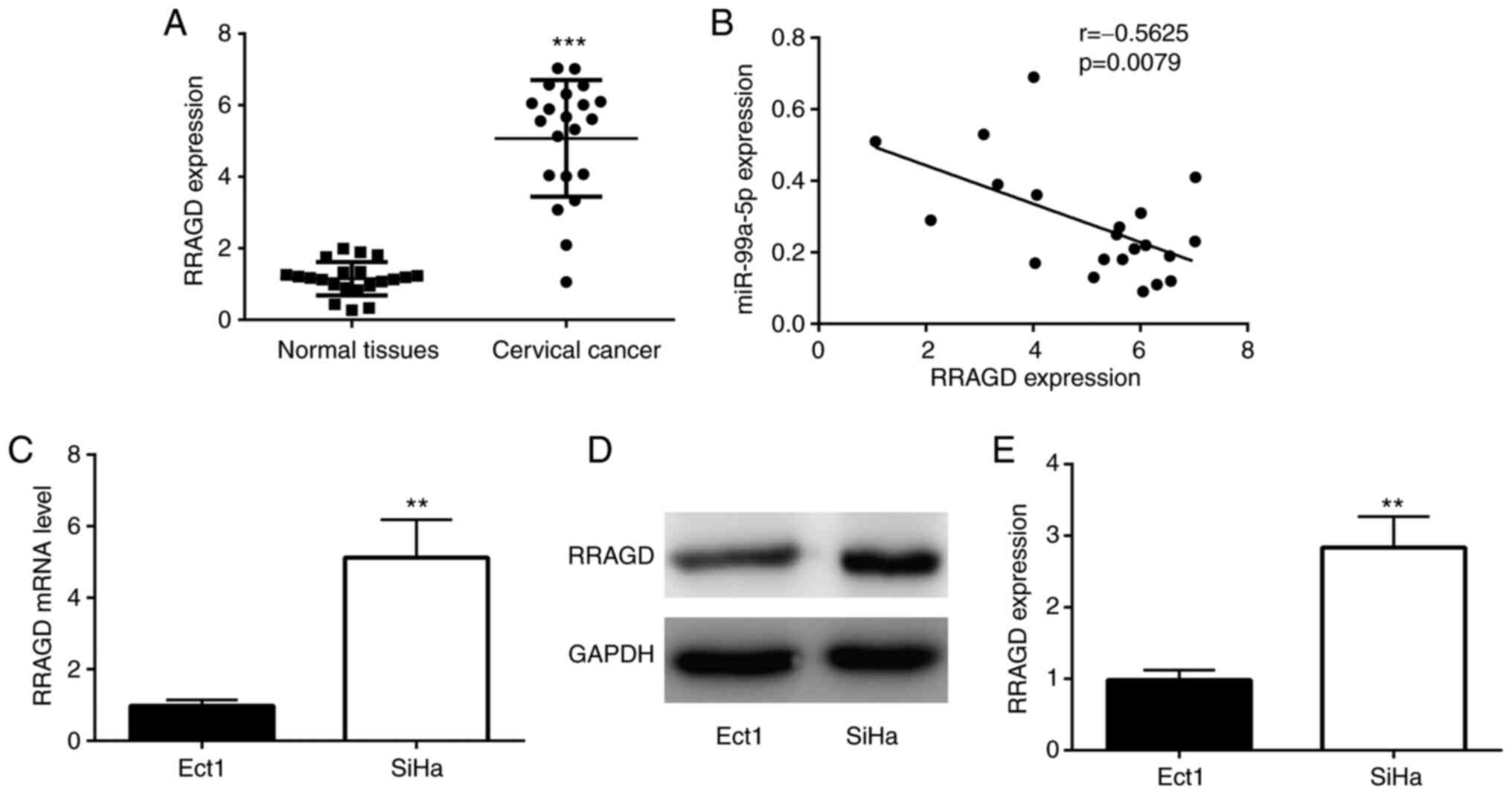
Subsequently, the expression of RRAGD was examined
in CC tissues, and it was found that the mRNA level of RRAGD was
significantly higher in CC tissues compared with normal tissues
(Fig. 5A). In addition, RRAGD
expression was negatively correlated with the expression of
miR-99a-5p in CC tissues (Fig.
5B). The expression of RRAGD was also detected in CC cell
lines. As demonstrated in Fig.
5C-E, both the mRNA and protein levels of RRAGD were higher in
the SiHa cells than in the Ect1 control cells.
miR-99a-5p regulates CC cell apoptosis
and glycolysis by targeting RRAGD
The present study then determined whether miR-99a-5p
plays a role in the regulation of cell apoptosis and glycolysis in
CC by targeting RRAGD. To explore whether the overexpression of
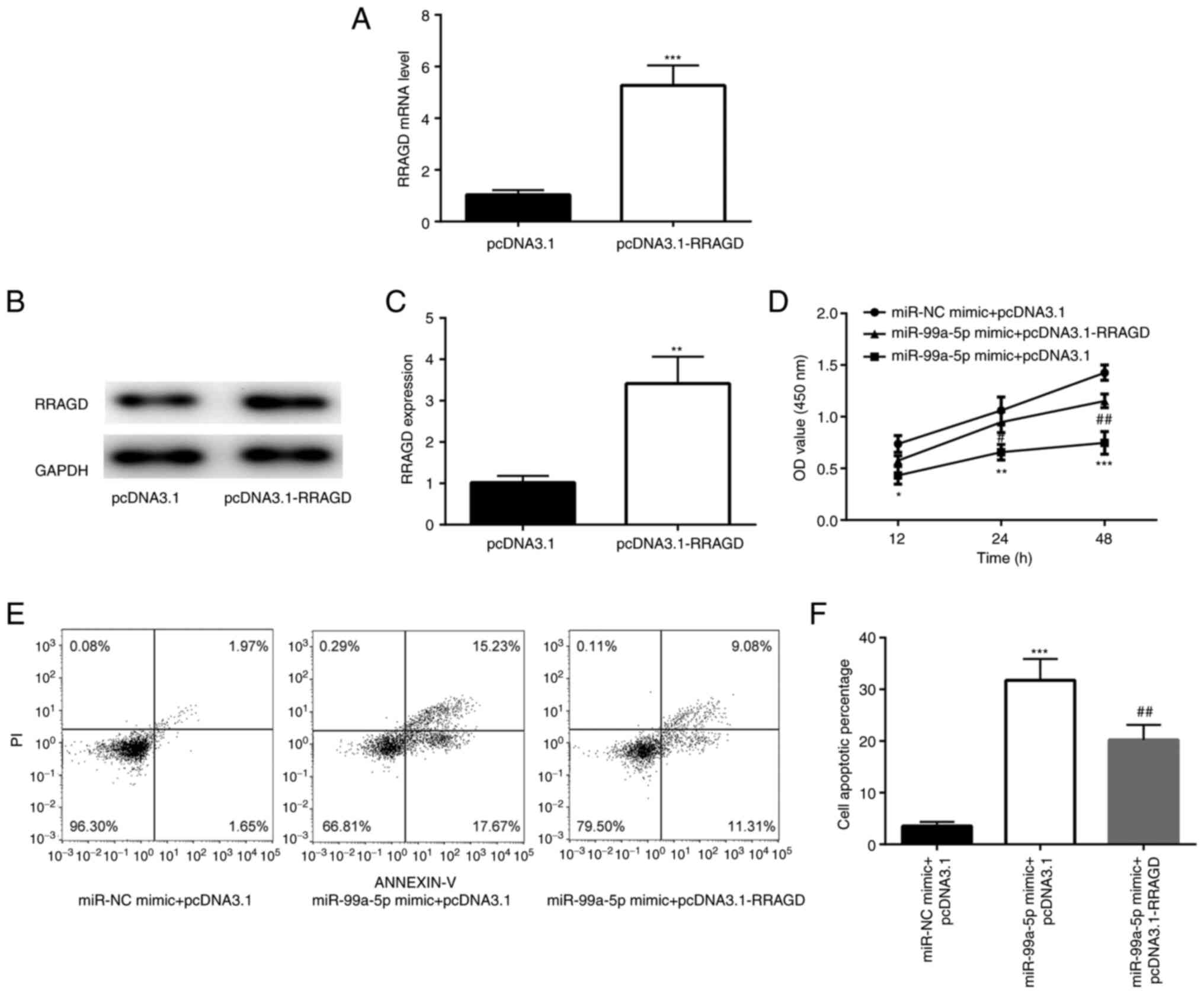
RRAGD could reverse the effects of overexpression of miR-99a-5p, a
pcDNA3.1-RRAGD plasmid was constructed, which could increase the
RRAGD mRNA and protein level effectively (Fig. 6A-C). The effects of RRAGD on the
miR-99a-5p-induced alteration of cell survival and apoptosis were
then examined. As shown in Fig.
6D, the overexpression of RRAGD in SiHa cells effectively
reversed the decrease in cell number induced by miR-99a-5p mimic.
In addition, flow cytometry also revealed that the overexpression
of RRAGD effectively reduced cell apoptosis induced by miR-99a-5p
mimic (Fig. 6E and F).
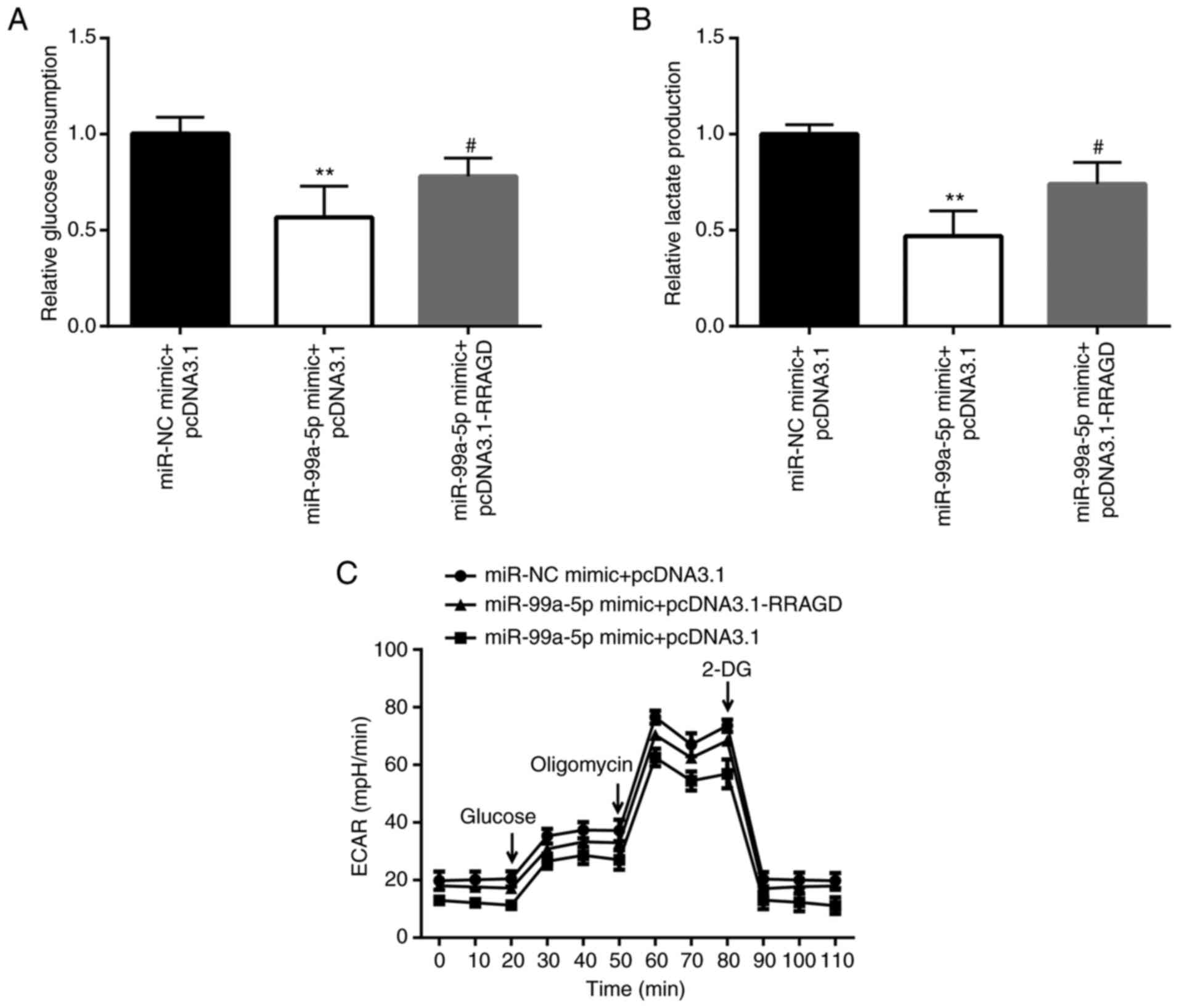
In order to investigate whether RRAGD is involved in
the effects of miR-99a-5p on glycolysis, glucose consumption,
lactate production and ECAR were detected in RRAGD and
miR-99a-5p-overexpressing SiHa cells. The results revealed that
miR-99a-5p mimic induced a decrease of glucose consumption and
lactate production, which was attenuated by the overexpression of
RRAGD (Fig. 7A and B). In
addition, ECAR detection assay also demonstrated that RRAGD
overexpression attenuated the decrease in the ECAR value induced by
miR-99a-5p mimic (Fig. 7C).
Discussion
By analyzing the information from The Cancer Genome
Atlas database, the present study found that patients with CC with
a higher expression level of miR-99a had a higher survival rate;
moreover, in the collected CC tissues and cell lines, miR-99a-5p
expression was downregulated. These results indicated that
miR-99a-5p is a potential tumor suppressor in CC.
Subsequently, it was revealed that the
overexpression of miR-99a-5p reduced the proliferation of SiHa
cells; this finding is in accordance with that of a previous study
demonstrating that miR-99a-5p reduced the proliferation of HeLa
cells (20); in addition, the
present study found that the overexpression of miR-99a-5p promoted
the apoptosis of SiHa cells. A negative effect of the glycolysis
pathway is that it leads to the abnormal growth and proliferation
of cancer cells (6). Furthermore,
tumor cell apoptosis is closely related to glucose consumption
(21). In further experiments, the
present study explored the effects of miR-99a-5p mimic on glucose
consumption, and it was found that the overexpression of miR-99a-5p
impaired the glycolysis of CC cells by decreasing glucose
consumption, lactate production and ECAR. It is considered that in
patients with CC, miR-99a-5p may play a role in inhibiting the
glycolysis process of tumor cells and promoting tumor cell
apoptosis, so as to achieve an anticancer effect. In follow-up
studies, the unknown mechanism was further explored.
Subsequently, it was found that RRAGD was a target
of miR-99a-5p. However, to the best of our knowledge, there is no
report available to date of the role of RRAGD or the
miR-99a-5p/RRAGD axis in CC. In the present study, RRAGD expression
was increased in CC tissues and cell lines, which was also
negatively correlated with miR-99a-5p expression in CC tissues. It
is thus suggested that RRAGD is a potential oncogene in CC.
Furthermore, it was identified that RRAGD was an important
substrate for miR-99a-5p to regulate apoptosis and glycolysis.
Additionally, during the process of the present study, an article
published in 2021 demonstrated that RRAGD expression was elevated
in patients with hepatocellular carcinoma who had a poor prognosis,
and RRAGD was an important cancer-promoting factor for cancer
progression and aerobic glycolysis in HCC (22); this further confirmed the findings
of the present study, suggesting that RRAGD is an important
oncogenic factor for cancer progression of CC. It may also prove to
be a potential therapeutic target for CC intervention.
However, the overexpression of RRAGD only partially
reversed the effects of miR-99a-5p mimic on cell apoptosis and
glycolysis, rather than completely blocking them. It is considered
that this may be due to the following reasons: Firstly, previous
studies have demonstrated that miR-99a-5p can target the mRNAs of
other important kinases or proteins, such as mTOR (20,23);
thus, the overexpression of RRAGD was not sufficient to eliminate
all the effects of miR-99a; secondly, the overexpression of RRAGD
mRNA was also under the regulation of miR-99a, and the
overexpression of miR-99a-5p was sufficient to attenuate the
effects of RRAGD.
In conclusion, the present study found that the
expression of miR-99a-5p was decreased in CC tissues and cell
lines. miR-99a-5p was found to play a role in inducing cell
apoptosis and reducing cell glycolysis by targeting RRAGD in CC.
These findings revealed a possible mechanism of miR-99a-5p in CC,
and provided potential biomarkers and therapeutic targets for the
diagnosis and treatment of CC. However, the effects of miR-99a-5p
and RRAGD on tumor growth in nude mice were not verified in the
present study. Therefore, such experiments will be performed in
future studies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GW, YL, SD, FJ and MX performed the experiments and
analyzed the data. XD conceived the project, supervised the study,
and performed data interpretation and manuscript preparation. GW
and XD confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent. The
present study was approved by the Ethics Committee of Wuhan Third
Hospital (Tongren Hospital of Wuhan University, Wuhan, China;
approval no. WHTH-2018-07).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cohen PA, Jhingran A, Oaknin A and Denny
L: Cervical cancer. Lancet. 393:169–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lunt SY and Vander Heiden MG: Aerobic
glycolysis: Meeting the metabolic requirements of cell
proliferation. Annu Rev Cell Dev Biol. 27:441–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwartz L, Supuran CT and Alfarouk KO:
The Warburg effect and the Hallmarks of cancer. Anticancer Agents
Med Chem. 17:164–170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ganapathy-Kanniappan S and Geschwind JF:
Tumor glycolysis as a target for cancer therapy: Progress and
prospects. Mol Cancer. 12:1522013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Z, Peng Y, Li J, Chen Z, Chen F, Tu J,
Lin S and Wang H: N6-methyladenosine regulates
glycolysis of cancer cells through PDK4. Nat Commun. 11:25782020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kroemer G and Pouyssegur J: Tumor cell
metabolism: Cancer's Achilles' heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: Metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bushati N and Cohen SM: MicroRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sadri Nahand J, Moghoofei M, Salmaninejad
A, Bahmanpour Z, Karimzadeh M, Nasiri M, Mirzaei HR, Pourhanifeh
MH, Bokharaei-Salim F, Mirzaei H and Hamblin MR: Pathogenic role of
exosomes and microRNAs in HPV-mediated inflammation and cervical
cancer: A review. Int J Cancer. 146:305–320. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pardini B, De Maria D, Francavilla A, Di
Gaetano C, Ronco G and Naccarati A: MicroRNAs as markers of
progression in cervical cancer: A systematic review. BMC Cancer.
18:6962018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao C, Zhou C, Zhuang J, Liu L, Liu C, Li
H, Liu G, Wei J and Sun C: MicroRNA expression in cervical cancer:
Novel diagnostic and prognostic biomarkers. J Cell Biochem.
119:7080–7090. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun D, Han L, Cao R, Wang H, Jiang J, Deng
Y and Yu X: Prediction of a miRNA-mRNA functional synergistic
network for cervical squamous cell carcinoma. FEBS Open Bio.
9:2080–2092. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Di Malta C, Siciliano D, Calcagni A,
Monfregola J, Punzi S, Pastore N, Eastes AN, Davis O, De Cegli R,
Zampelli A, et al: Transcriptional activation of RagD GTPase
controls mTORC1 and promotes cancer growth. Science. 356:1188–1192.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L, Chang L, Li Z, Gao Q, Cai D, Tian
Y, Zeng L and Li M: MiR-99a and −99b inhibit cervical cancer cell
proliferation and invasion by targeting mTOR signaling pathway. Med
Oncol. 31:9342014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ribas V, García-Ruiz C and Fernández-Checa
JC: Mitochondria, cholesterol and cancer cell metabolism. Clin
Transl Med. 5:222016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding L and Liang X: Ras related GTP
binding D promotes aerobic glycolysis of hepatocellular carcinoma.
Ann Hepatol. 23:1003072021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Li B, Yang X and Zhang C:
MiR-99a-5p inhibits bladder cancer cell proliferation by directly
targeting mammalian target of rapamycin and predicts patient
survival. J Cell Biochem. 120:19330–19337. 2019. View Article : Google Scholar : PubMed/NCBI
|