Introduction
Colorectal cancer is one of the leading causes of
cancer-related death globally, and ~30% of these cases are rectal
adenocarcinoma (READ). Although surgery is a principal approach for
READ treatment, preoperative concurrent chemoradiotherapy,
post-operative adjuvant chemotherapy and radiotherapy are often
used as a combined therapeutic regimen for high-risk patients and
those with advanced READ (1).
These multidisciplinary approaches not only decrease local
recurrence and distant metastasis rates, but also improve survival
outcome for patients with locally advanced rectal cancer (1,2).
Fluoropyrimidine-based postoperative adjuvant chemotherapy followed
by surgery is usually the standard treatment regimen for patients
with READ (3). However, drug
resistance quickly occurs within months, eventually followed by
distant metastasis, and is the major cause of treatment failure and
cancer-associated death in patients with advanced READ (4). Therefore, suitable prognostic factors
to stratify advanced READ for personalized therapeutic strategies
are urgently required.
Cell division cycle 27 (CDC27) is a crucial subunit
of the anaphase-promoting complex/cyclosome (APC/C) and regulates
the cell cycle by interacting with different coactivators through
targeting protein ubiquitination and degradation. APC/C is reported
to play a role in genome integrity, apoptosis, autophagy, energy
metabolism and tumorigenesis (5).
CDC27 also participates in the control of the mitotic checkpoint
and surveys the mitotic spindle to maintain chromosomal integrity.
CDC27 has been reported to be involved in apoptosis, stemness,
epithelial-mesenchymal transition (EMT) and efferocytosis
regulation (6,7). Previous studies have revealed that
overexpression of CDC27 promotes tumor cell proliferation, invasion
and metastasis in patients with colorectal cancer, breast cancer,
gastric cancer and lymphoma (8–11).
Downregulation of CDC27 has been reported to be associated with
clinical outcomes of patients with breast cancer and sensitivity to
radiotherapy (12–14). The alteration or mutation of CDC27
is also associated with tumorigenesis, tumor progression, drug
resistance and survival (6,15).
However, postoperative adjuvant chemotherapy is mostly used for
first-line treatment of patients with colorectal cancer, and these
chemotherapeutic drugs can interrupt DNA replication and cell cycle
processes. There is no direct clinical evidence to explain the
correlation between CDC27 expression and chemotherapeutic treatment
response, which is still controversial and uncertain.
Furthermore, the amount of tumor-infiltrating
lymphocytes (TILs) is an important prognostic factor for colorectal
cancer and in previous studies (16,17).
In the present study, in the TIMER database (http://timer.cistrome.org/), CDC27 expression was
associated with immune cell infiltration. Hence, the present study
aimed to investigate the expression profiles of CDC27 and
intratumor-infiltrating CD3+ lymphocytes by
immunohistochemical staining, and examine their clinical
significance and effect on survival outcome in patients with READ.
These findings provide new insights into the potential of CDC27 as
a prognostic factor in patients with READ.
Materials and methods
Patients, clinical staging and
pathological evaluation
Between January 2011 and December 2016, 290 patients
with rectal cancer were recruited from China Medical University
Hospital (CMUH) (Taichung, Taiwan). Among these patients, due to
insufficient tumor tissue, only 255 patients who received surgery
with or without postoperative chemotherapy were enrolled in this
study cohort. Resected specimens were reviewed by pathologists, and
pathological staging was based on the 7th American Joint Committee
on Cancer (AJCC) staging system (18). A postoperative chemotherapeutic
regimen was recommended for patients with high-risk stage II
disease and lymph node metastasis stage III identified in surgical
specimens, according to the status of the patients. The
clinicopathological characteristics were extracted from the
electronic medical records. This study was approved by the
Institutional Review Board (IRB) of CMUH (approval no.
CMUH105-REC2-072) and written informed consent was obtained from
all patients.
Tissue microarray (TMA)
construction
A TMA was constructed from the surgically resected
primary tumor tissues of patients with READ as previously described
(17,19), as approved by the IRB of CMUH
(approval no. CMUH105-REC2-073). Briefly, the tissues were fixed in
10% neutral buffered formalin (Leica Microsystems, Inc.) for 18–24
h at RT, dehydrated with ethanol, infiltrated with paraffin wax,
embedded into paraffin at 60°C and then cooled to become
formalin-fixed, paraffin-embedded (FFPE) blocks. Next, the sections
of FFPE blocks were cut by a microtome (Leica Microsystems, Inc.)
onto the slides. The slides were deparaffinization with xylene,
stained with hematoxylin for 3 min and eosin for 1 min, and then
mounted in resin. Next, the tumor areas were evaluated and marked
on hematoxylin and eosin-stained (H&E) slides by a pathologist
using light microscopy (Leica Microsystems, Inc.). Finally, the
corresponding area was identified, marked, and punched on the
matching FFPE block, and then transferred into a paraffin-embedded
recipient block for TMA construction using an AutoTiss 10C system
(EverBio Technology, Inc.). A single TMA block comprised a maximum
of 60 cores, 2 mm in diameter, and the sections were cut by a
microtome (Leica biosystems, Inc.) and mounted on capillary-gap
slides (Dako, Inc.).
Immunohistochemical (IHC) staining and
evaluation
IHC staining was performed using 3-µm sections of
TMAs as previously described (19). The antibodies used in this study
were anti-human CDC27 (1:100; cat. no. ab10538; Abcam) and
anti-human CD3 (1:100; cat. no. ab16669; Abcam). A HRP-conjugated
Vectastain Elite ABC Kit (Vector Laboratories, Inc.) and DAB
chromogen (Vector Laboratories, Inc.) were used for detection
according to the manufacturer's protocol. Briefly, the slides were
retrieved with sodium citrate buffer (pH 6.0) for 20 min at 80°C
and then incubated with 3% H2O2 in 1X PBS at
room temperature (RT) for 10 min to block endogenous peroxidase
activity. Next, the slides were incubated with primary antibodies
at RT for 2 h after blocking. According to the manufacturer's
protocol, blocking solution, biotinylated antibody and Vectastain
Elite ABC reagent were used and incubated in the slides at RT for
20, 30 and 30 min, respectively. The slides were incubated in a
peroxidase substrate solution with DAB chromogen for 10 min at RT
according to the manufacturer's protocol. Finally, the slides were
counterstained with hematoxylin for 1 min at RT, washed and mounted
in resin mount.
Evaluation of CDC27 expression in the cytoplasm and
nucleus was assessed through the histoscore (H-score) (20). The immunostaining was performed
using a semi-quantitative H-score. The intensity of the staining
was categorized as follows: Negative, weak, moderate and strong.
The H-score was determined as the intensity and the percentage of
the staining area of tumor cells at its intensity grade using the
following formula: H-score=[1 × (% of weak staining) + 2 × (% of
moderate staining) + 3 × (% of strong staining)]. The range of the
H-score was from 0 to 300. The CDC27 expression status was
categorized as low or high according to the mean value of the
H-score. Evaluation of CD3+ TILs was performed, with
counting at ×400 magnification [number of positively stained
TILs/high-power field (HPF)], as previously described (17). The mean count of CD3+
TILs in five HPFs was scored and graded as follows: 0, no
positively stained TILs/HPF; 1, 1–3 positively stained TILs/HPF; 2,
4–10 positively stained TILs/HPF; and 3, >10 positively stained
TILs/HPF. Finally, specimens with grade 2 or 3 were regarded as the
high group and those with grade 0 or 1 were regarded as the low
group.
TIMER database analysis. The Tumor Immune Estimation
Resource (TIMER) database (http://timer.cistrome.org/) is used for the systematic
analysis of immune infiltrates in the tumor microenvironment of
various cancer types (21). The
association between CDC27 expression and
CD4+/CD8+ T cells with purity adjustment was
analyzed via gene modules in patients with READ.
Statistical analysis
MP Pro statistical software version 12 (SAS
Institute, Inc.) was used to perform the statistical analysis. The
H-score of nuclear and cytoplasmic CDC27 expression was analyzed
with the Wilcoxon matched-pairs test, and the correlation was
examined with Spearman's analysis. Associations between CDC27
expression and clinicopathological variables were analyzed by a
χ2 test. The 5-year DFS and OS rates were estimated
using the Kaplan-Meier method, and survival differences were
analyzed with the log-rank test. The Cox proportional hazards model
was used for multivariate analysis to estimate the HR, 95% CI and
prognostic factors. P<0.05 (two-sided) was considered to
indicate a statistically significant difference.
Results
Clinicopathological characteristics in
patients with READ
Tissue specimens from 255 patients with READ who
underwent surgery were collected and the clinicopathological
characteristics are shown in Table
I. The majority of patients were male (67%), and the mean age
at diagnosis was 63.2±13.7 years (range, 25–97 years). The patients
were staged according to the 7th edition of the AJCC staging
system. There were 135 patients (53%) who had nodal metastasis, and
148 patients (58%) received fluorouracil-based postoperative
chemotherapy. KRAS mutations were assessed in 121 of the 255
patients (47%), and of these, 64 patients (53%) were determined to
have wild-type KRAS.
 | Table I.Clinicopathological characteristics
of patients with READ (n=255). |
Table I.
Clinicopathological characteristics
of patients with READ (n=255).
| Clinicopathological
parameters | Value |
|---|
| Sex, n (%) |
|
|
Male | 159 (62) |
|
Female | 96 (38) |
| Age, years |
|
| Mean
(range) | 63.2 (25–97) |
| <65,
n (%) | 142 (56) |
| ≥65, n
(%) | 113 (44) |
| Histological
grading, n (%) |
|
| Well to
moderate | 233 (94) |
|
Poor | 14 (6) |
| NA | 8 |
| pTMN stage (7th
AJCC), n (%) |
|
| I | 61 (24) |
| II | 56 (22) |
|
III | 104 (41) |
| IV | 34 (13) |
| pN, n (%) |
|
|
Negative | 117 (46) |
|
Positive | 138 (54) |
| Lymphovascular
invasion, n (%) |
|
|
Absent | 151 (61) |
|
Present | 98 (39) |
| NA | 6 |
| Perineural
invasion, n (%) |
|
|
Absent | 151 (61) |
|
Present | 98 (39) |
| NA | 6 |
| Preoperative CEA, n
(%) |
|
| <5
ng/ml | 138 (60) |
| ≥5
ng/ml | 92 (40) |
| NA | 25 |
| Postoperative
chemotherapy, n (%) |
|
| No | 107 (42) |
|
Yes | 148 (58) |
| Kras mutation, n
(%) |
|
|
Wild-type | 64 (53) |
|
Mutant | 57 (47) |
| NA | 134 |
Association between
clinicopathological characteristics and subcellular distribution of
CDC27 expression in patients with READ
To determine the expression profiles of CDC27 in
tumor tissues of patients with READ, CDC27 expression was examined
by IHC staining according to the H-scoring system. CDC27 protein
was present in the cytoplasm and/or nucleus with different
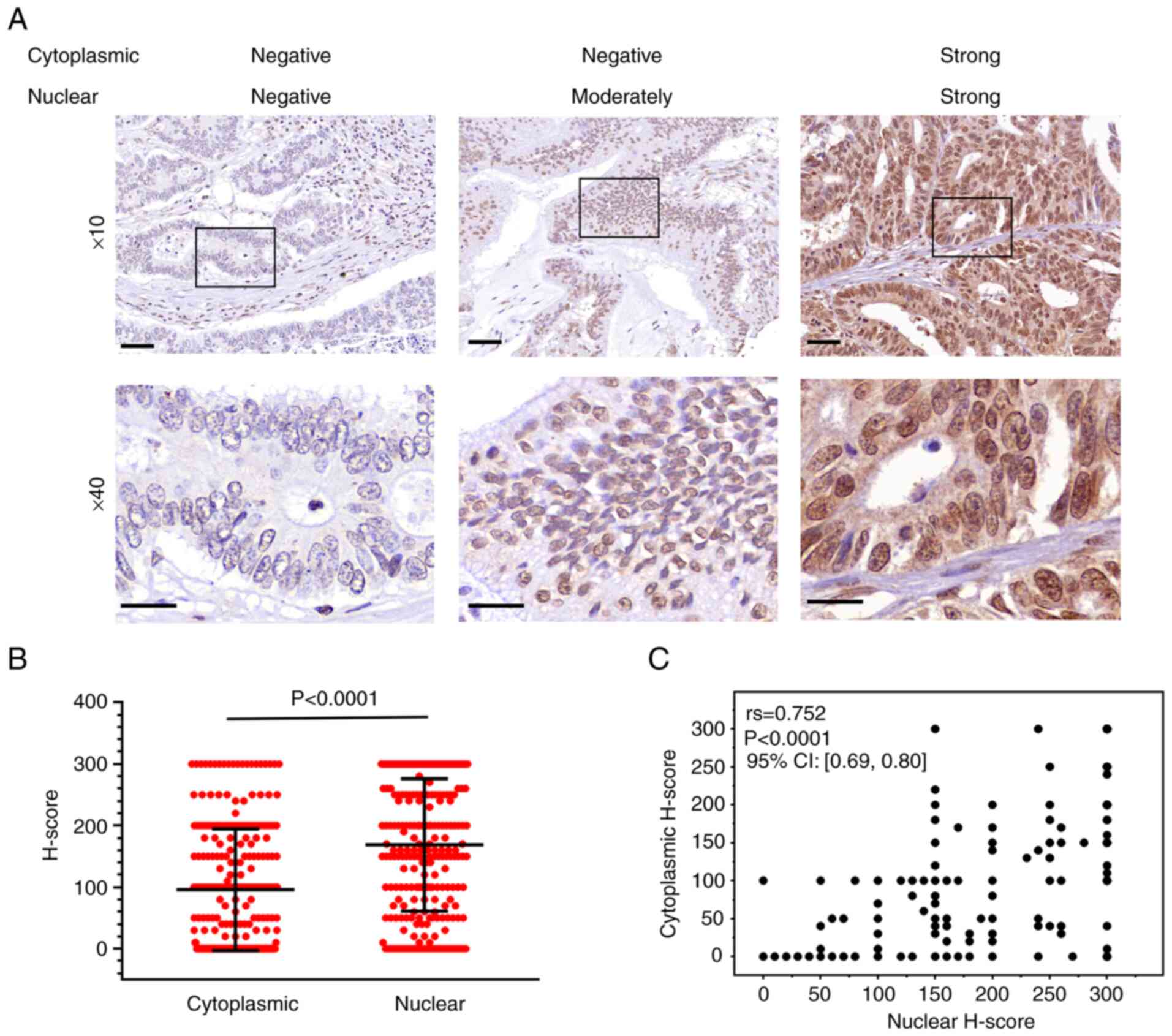
expression patterns (Fig. 1A). The
expression pattern of CDC27 was patchy or focally intense in the
nucleus, but was diffusely in the cytoplasm. There were 93 patients
(36%) with no cytoplasmic expression of CDC27, and 32 patients
(13%) with no nuclear expression of CDC27. Among these patients, 30
patients (12%) lacked CDC27 expression in the cytoplasm as well as
the nucleus. The distribution of CDC27 H-score in the cytoplasm and
nucleus is shown in Fig. 1B; the
mean H-scores of CDC27 expression in the cytoplasm and nucleus were
95.8 and 168.5, respectively. The H-score of nuclear CDC27
expression was significantly higher than that of cytoplasmic CDC27
(Wilcoxon matched-pairs test, P<0.0001). Spearman's correlation
analysis showed that the H-score of CDC27 in the nucleus was
positively correlated with that of the cytoplasm [correlation
coefficient (rs) = 0.752; P<0.0001] (Fig. 1C).
To evaluate whether the subcellular distribution of
CDC27 had different prognostic significance, the relationship
between the subcellular distribution of CDC27 and the
clinicopathological characteristics in patients with READ was
analyzed. READ patients were stratified into high and low CDC27
expression according to the mean H-score, and their relationship
with clinicopathological parameters was analyzed. As shown in
Table II, it was found that
cytoplasmic CDC27 expression was significantly associated with TNM
stage (P=0.021), pN stage (P=0.003), lymphovascular invasion (LVI)
(P<0.001), perineural invasion (PNI) (P=0.012) and distant
metastasis (P=0.043). Patients with high cytoplasmic CDC27
expression exhibited advanced stage, lymph node metastasis,
presence of LVI and PNI, and distant metastasis. Nuclear CDC27
expression was significantly associated with the presence of LVI
(P=0.002). There was no association between nuclear CDC27 and other
clinicopathological parameters. These findings suggest that
cytoplasmic CDC27 expression may be associated with tumor
progression and metastasis.
 | Table II.Association between
clinicopathological parameters and cell division cycle 27
expression in patients with READ. |
Table II.
Association between
clinicopathological parameters and cell division cycle 27
expression in patients with READ.
|
| Cytoplasmic
expression, n (%) |
| Nuclear expression,
n (%) |
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
parameters | Low | High | P-value | Low | High | P-value |
|---|
| Total patients | 129 (100) | 126 (100) |
| 127 (100) | 128 (100) |
|
| Sex |
|
|
|
|
|
|
|
Male | 77 (60) | 82 (65) | 0.374 | 78 (61) | 81 (63) | 0.758 |
|
Female | 52 (40) | 44 (35) |
| 49 (39) | 47 (37) |
|
| Age, years |
|
|
|
|
|
|
|
<65 | 75 (58) | 67 (53) | 0.424 | 71 (56) | 71 (55) | 0.944 |
|
≥65 | 54 (42) | 59 (47) |
| 56 (44) | 57 (45) |
|
| Histological
grading |
|
|
|
|
|
|
| Well to
moderate | 118 (95) | 115 (93) | 0.571 | 117 (94) | 116 (95) | 0.613 |
|
Poor | 6 (5) | 8 (7) |
| 8 (6) | 6 (5) |
|
| NA | 5 | 3 |
| 2 | 6 |
|
| pTNM stage (7th
AJCC) |
|
|
|
|
|
|
|
Early | 71 (55) | 46 (37) | 0.003a | 63 (50) | 54 (42) | 0.234 |
|
Late | 58 (45) | 80 (63) |
| 64 (50) | 74 (58) |
|
| pN |
|
|
|
|
|
|
|
Negative | 71 (55) | 46 (37) | 0.003a | 63 (50) | 54 (42) | 0.234 |
|
Positive | 58 (45) | 80 (63) |
| 64 (50) | 74 (58) |
|
| Lymphovascular
invasion |
|
|
|
|
|
|
|
Absent | 90 (71) | 61 (50) |
<0.001a | 88 (70) | 63 (51) | 0.002a |
|
Present | 36 (29) | 62 (50) |
| 38 (30) | 60 (49) |
|
| NA | 3 | 3 |
| 1 | 5 |
|
| Perineural
invasion |
|
|
|
|
|
|
|
Absent | 86 (68) | 65 (53) | 0.012a | 82 (65) | 69 (56) | 0.146 |
|
Present | 40 (32) | 58 (47) |
| 44 (35) | 54 (44) |
|
| NA | 3 | 3 |
| 1 | 5 |
|
| Preoperative CEA,
ng/ml |
|
|
|
|
|
|
|
<5 | 71 (60) | 67 (60) | 0.957 | 67 (58) | 61 (62) | 0.59 |
| ≥5 | 47 (40) | 45 (40) |
| 48 (42) | 44 (38) |
|
| NA | 11 | 14 |
| 12 | 13 |
|
| Kras mutation |
|
|
|
|
|
|
|
Wild-type | 28 (46) | 33 (49) | 0.796 | 32 (54) | 31 (49) | 0.707 |
|
Mutant | 24 (54) | 35 (51) |
| 27 (46) | 32 (51) |
|
| NA | 77 | 58 |
| 68 | 65 |
|
| CD3+
TILs |
|
|
|
|
|
|
|
High | 59 (46) | 66 (52) | 0.288 | 55 (43) | 70 (55) | 0.068 |
|
Low | 70 (54) | 60 (48) |
| 72 (57) | 58 (45) |
|
| Distant
metastasis |
|
|
|
|
|
|
| No | 111 (86) | 96 (76) | 0.043a | 106 (83) | 101 (79) | 0.351 |
|
Yes | 18 (14) | 30 (24) |
| 21 (17) | 27 (21) |
|
Association between CDC27 expression
and CD3+ TILs in patients with READ
From the TIMER database, it was found that CDC27
expression was associated with immune cells infiltration in
colorectal cancer (http://timer.cistrome.org/). To further explore these
results, the association between the subcellular distribution of
CDC27 and intratumor CD3+ TILs was analyzed in READ
tumor tissues. First, the association between CD3+ TILs
and clinicopathological parameters was analyzed, which found that
CD3+ TILs was markedly associated with TNM stage
(P<0.001), pN stage (P<0.001) and PNI (P<0.001) (Table SI). High-grade CD3+
TILs in tumor cells were associated with advanced stage, lymph node
metastasis and presence of PNI. However, it was also found that
intratumor CD3+ TILs had no association with cytoplasmic
or nuclear CDC27 expression (Table
II).
Nuclear CDC27 expression is associated
with survival outcomes in patients with READ
Next, the present study evaluated whether the
subcellular expression of CDC27 was associated with the clinical
outcomes of patients with READ using the Kaplan-Meier survival
analysis. As shown in Table III,
several clinicopathological parameters, including age (P=0.011 and
P<0.0001), TNM stage (P<0.0001 and P<0.0001), pN stage
(P<0.0001 and P<0.0001), PNI (P<0.0001 and P=0.0002),
preoperative CEA level (P<0.0001 and P<0.0001),
CD3+ TILs (P=0.002 and P=0.0043) and nuclear CDC27
expression (P=0.028 and P=0.028), were significantly associated
with 5-year disease-free survival (DFS) and 5-year overall survival
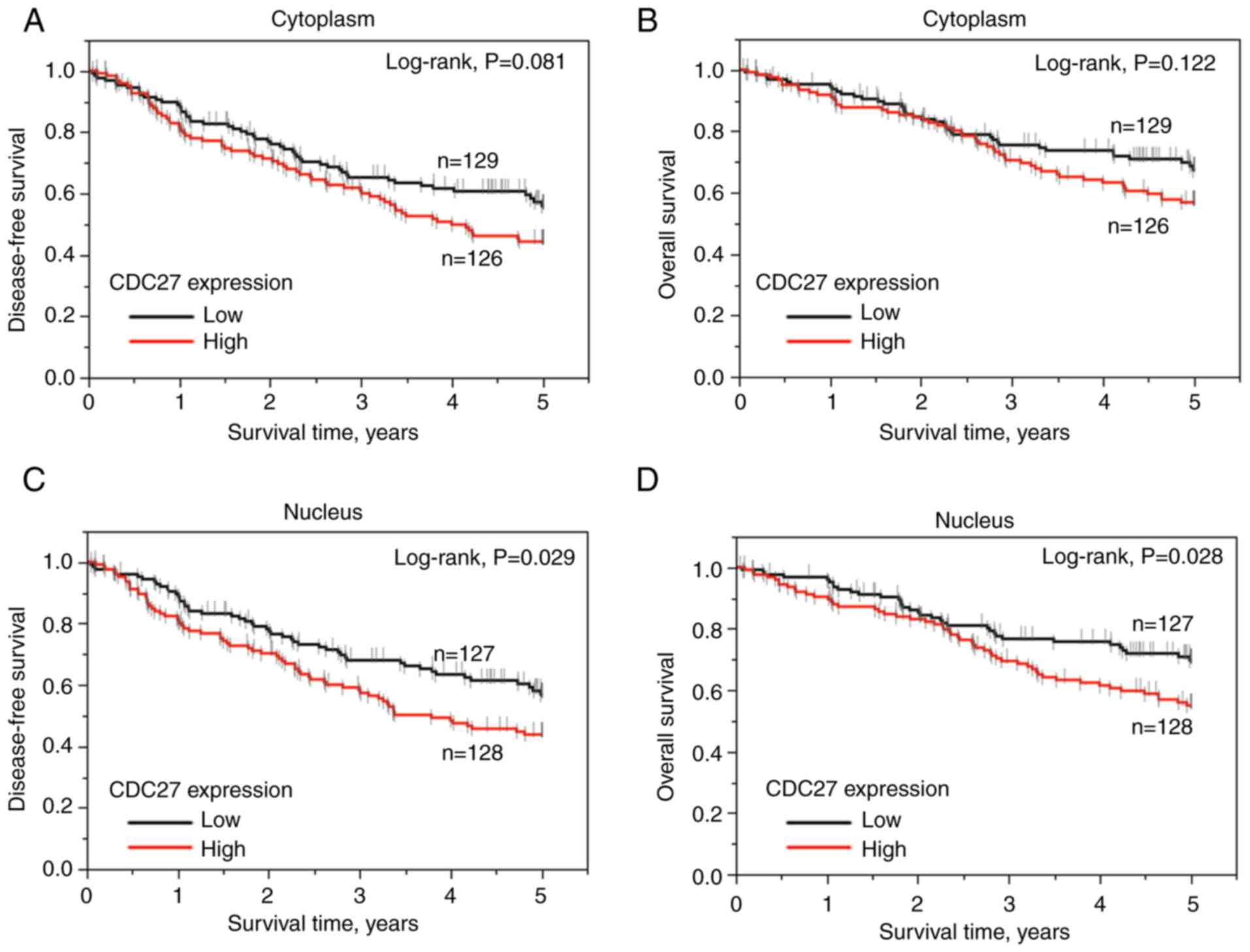
(OS), respectively. It is worth noting that cytoplasmic CDC27
expression had a non-significant tendency towards association with
5-year DFS (P=0.081, log-rank test; Fig. 2A) and 5-year OS (P=0.129, log-rank
test; Fig. 2B). Nuclear CDC27
expression was significantly associated with 5-year DFS and OS
(P=0.029 and P=0.028, respectively, log-rank test) in patients with
READ (Fig. 2C and D).
 | Table III.Association between
clinicopathological parameters and 5-year survival outcome in
patients with READ (n=255). |
Table III.
Association between
clinicopathological parameters and 5-year survival outcome in
patients with READ (n=255).
| Clinicopathological
parameters | Cases, n | 5-year DFS rate,
% | P-value | 5-year OS rate,
% | P-value |
|---|
| Total patients |
| 50.3 |
| 62.2 |
|
| Sex |
|
|
|
|
|
|
Male | 159 | 48.4 | 0.433 | 61.3 | 0.548 |
|
Female | 96 | 53.7 |
| 63.8 |
|
| Age, years |
|
|
|
|
|
|
<65 | 142 | 57.7 | 0.011a | 75.1 |
<0.0001a |
|
≥65 | 113 | 40.2 |
| 44.9 |
|
| Histological
grading |
|
|
|
|
|
| Well to
moderate | 14 | 50.8 | 0.310 | 55.0 | 0.449 |
|
Poor | 233 | 29.7 |
| 61.7 |
|
| NA | 8 |
|
|
|
|
| pTNM stage (7th
AJCC) |
|
|
|
|
|
|
Early | 117 | 65.3 |
<0.0001a | 77.2 |
<0.0001a |
|
Late | 138 | 37.8 |
| 49.6 |
|
| pN |
|
|
|
|
|
|
Negative | 117 | 65.3 |
<0.0001a | 77.2 |
<0.0001a |
|
Positive | 138 | 37.8 |
| 49.6 |
|
| Lymphovascular
invasion |
|
|
|
|
|
|
Absent | 151 | 53.4 | 0.213 | 66.2 | 0.121 |
|
Present | 98 | 43.9 |
| 55.0 |
|
| NA | 6 |
|
|
|
|
| Perineural
invasion |
|
|
|
|
|
|
Absent | 151 | 61.5 |
<0.0001a | 71.2 | 0.0002a |
|
Present | 98 | 31.5 |
| 47.6 |
|
| NA | 6 |
|
|
|
|
| Preoperative CEA,
ng/ml |
|
|
|
|
|
|
<5 | 138 | 63.2 |
<0.0001a | 72.8 |
<0.0001a |
| ≥5 | 92 | 32.6 |
| 45.5 |
|
| NA | 25 |
|
|
|
|
| CD3+
TILs |
|
|
|
|
|
|
High | 120 | 61.7 | 0.002a | 71.0 | 0.0043a |
|
Low | 135 | 41.0 |
| 53.3 |
|
| Cytoplasmic CDC27
expression |
|
|
|
|
|
|
Low | 129 | 55.9 | 0.08 | 67.4 | 0.121 |
|
High | 126 | 44.3 |
| 56.8 |
|
| Nuclear CDC27
expression |
|
|
|
|
|
|
Low | 127 | 56.8 | 0.028a | 69.6 | 0.028a |
|
High | 128 | 43.8 |
| 55.0 |
|
To further validate the prognostic values of nuclear
CDC27, Cox proportional hazards model and multivariate linear
regression analysis were used. As shown in Table IV, in the total patient group,
nuclear CDC27 expression was an independent risk factor for 5-year
DFS. Patients carrying high nuclear CDC27 expression had an
increased risk for a poorer 5-year DFS rate (HR, 1.590; 95% CI,
1.075-2.369; P=0.020). Taken together, these results indicated that
nuclear CDC27 expression acted as an independent prognostic factor
for patients with READ.
 | Table IV.Multivariate analysis of
clinicopathological parameters and nuclear CDC27 expression in
5-year DFS and OS. |
Table IV.
Multivariate analysis of
clinicopathological parameters and nuclear CDC27 expression in
5-year DFS and OS.
| A, All patients
(n=225)a |
|---|
|
|---|
|
| DFS | OS |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (≥65 years vs.
<65 years) | 1.499 | 1.015-2.216 | 0.042b | 2.735 | 1.733-4.394 |
<0.0001b |
| pTNM stage (late
vs. early) | - | - | - | - | - | - |
| pN stage (positive
vs. negative) | 1.610 | 0.988-2.669 | 0.055 | 2.151 | 1.242-3.843 | 0.005b |
| Perineural invasion
(present vs. absent) | 1.675 | 1.074-2.369 | 0.022b | 1.470 | 0.898-2.444 | 0.125 |
| CEA (abnormal vs.
normal) | 1.940 | 1.269-2.975 | 0.002b | 1.856 | 1.143-3.036 | 0.012b |
| CD3+
TILs (low vs. high) | 1.599 | 1.058-2.440 | 0.025b | 1.549 | 0.969-2.508 | 0.065 |
| Nuclear CDC27
expression (high vs. low) | 1.590 | 1.075-2.369 | 0.020b | 1.567 | 0.998-2.492 | 0.051 |
|
| B, No
chemotherapy (n=95) |
|
|
| DFS | OS |
|
|
|
|
|
Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| Age (≥65 years vs.
<65 years) | 2.644 | 1.209-6.413 | 0.013b | 6.214 | 2.332-21.550 |
<0.0001b |
| pTNM stage (late
vs. early) | - | - | - | - | - | - |
| pN stage (positive
vs. negative) | 2.005 | 0.795-4.842 | 0.137 | 2.557 | 1.039-6.331 | 0.041b |
| Perineural invasion
(present vs. absent) | 2.484 | 1.024-5.841 | 0.044b | 2.063 | 0.830-5.058 | 0.117 |
| CEA (abnormal vs.
normal) | 0.690 | 0.283-1.613 | 0.396 | 0.664 | 0.270-1.584 | 0.359 |
| CD3+
TILs (low vs. high) | 1.884 | 0.929-3.836 | 0.078 | 2.072 | 0.957-4.597 | 0.064 |
| Nuclear CDC27
expression (high vs. low) | 1.022 | 0.501-2.040 | 0.949 | 0.874 | 0.405-1.829 | 0.724 |
|
| C, Chemotherapy
(n=130) |
|
|
| DFS | OS |
|
|
Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| Age (≥65 years vs.
<65 years) | 1.045 | 0.626-1.708 | 0.862 | 1.64 | 0.904-2.947 | 0.102 |
| pTNM stage (late
vs. early) | - | - | - | - | - | - |
| pN stage (positive
vs. negative) | 1.734 | 0.862-3.885 | 0.127 | 3.638 | 1.284-15.267 | 0.012b |
| Perineural invasion
(present vs. absent) | 1.662 | 0.980-2.889 | 0.059 | 1.611 | 0.866-3.134 | 0.133 |
| CEA (abnormal vs.
normal) | 2.813 | 1.670-4.833 |
<0.0001b | 2.995 | 1.573-5.964 | 0.0007b |
| CD3+
TILs (low vs. high) | 1.545 | 0.921-2.646 | 0.099 | 1.433 | 0.774-2.708 | 0.254 |
| Nuclear CDC27
expression (high vs. low) | 2.106 | 1.275-3.570 | 0.003b | 2.369 | 1.272-4.681 | 0.005b |
Nuclear CDC27 expression as a
predictive biomarker for adjuvant chemotherapy treatment in
patients with READ
Furthermore, the present study examined whether the
subcellular expression of CDC27 was associated with the response to
postoperative adjuvant chemotherapy using Kaplan-Meier survival
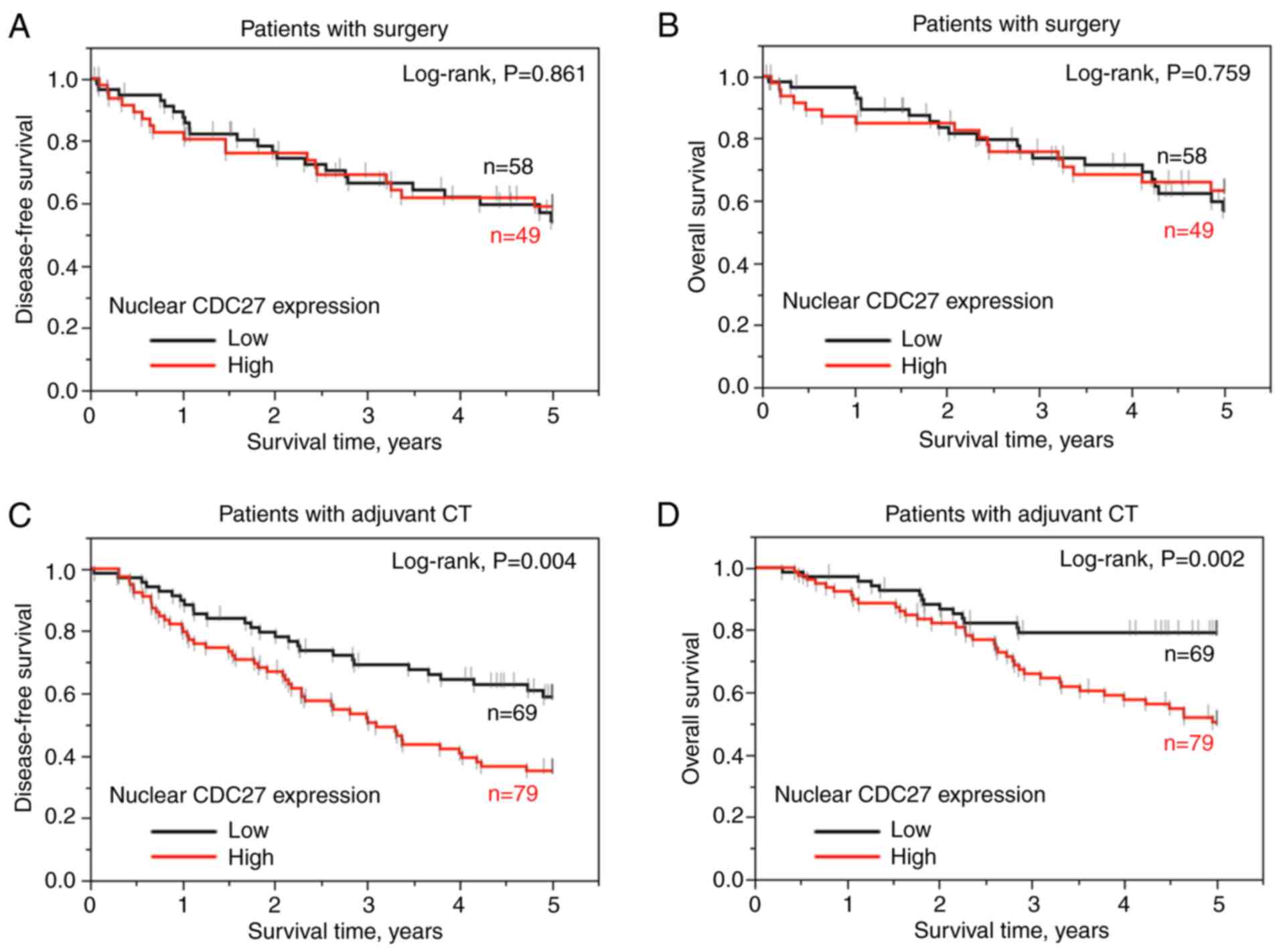
analysis. It was found that cytoplasmic CDC27 expression was not
associated with 5-year DFS or 5-year OS rates in the adjuvant
chemotherapy or surgery only groups (Fig. S1A-D). Nuclear CDC27 expression was
significantly associated with 5-year DFS (P=0.004, log-rank test)
and 5-year OS (P=0.002, log-rank test) in the adjuvant
chemotherapy-treated group (Fig. 3C
and D). According to the Cox proportional hazards model and
multivariate linear regression analysis, patients with high nuclear
CDC27 expression remarkably exhibited an increased risk for a
poorer 5-year DFS (HR, 2.106; 95% CI, 1.275-3.570; P=0.003) and OS
(HR, 2.369; 95% CI, 1.272-4.681; P=0.005) in the adjuvant
chemotherapy-treated group (Table
IV). These results indicated that nuclear CDC27 expression is
an independent prognostic factor for patients with READ, especially
those treated with postoperative adjuvant chemotherapy. The data
revealed that nuclear CDC27 expression may influence the response
to adjuvant chemotherapy.
Discussion
The present study evaluated the subcellular pattern
of CDC27 expression and intratumor-infiltrating CD3+
lymphocytes in resected tumors of a cohort of patients with READ
using IHC. It was found that cytoplasmic and nuclear CDC27
expression had different influences on clinicopathological
characteristics and survival outcomes. Cytoplasmic CDC27 expression
was associated with TNM stage, nodal metastasis and distant
metastasis. Nuclear CDC27 protein expression was significantly
associated with survival outcomes and acted as an independent
prognostic factor for patients with READ, especially in those
receiving post-operative adjuvant chemotherapy. In addition,
intratumor CD3+ TILs were associated with TNM stage,
nodal metastasis, PNI presence and survival outcomes in patients
with READ. However, cytoplasmic or nuclear CDC27 expression was not
associated with the density of intratumor CD3+ TILs.
Therefore, the data implied that cytoplasmic and nuclear CDC27
expression had different influences on tumor progression and the
response to chemotherapy, respectively.
CDC27 is an important subunit of APC/C that
regulates mitosis and chromosome segregation processes at the
G2/M and M/G1 transitions. Accumulating
studies revealed that the expression of CDC27 is associated with
tumorigenesis, but is still ambiguous in different cancer types
(6). For example, high CDC27
expression triggered cell proliferation, tumor growth,
epithelial-mesenchymal transition and metastasis in colorectal and
gastric cancer in vitro and in vivo (9,10,22).
High CDC27 expression caused chromosome instability and recurrence
in patients with breast cancer (8). However, downregulation of CDC27 was
also associated with a poor response to radiotherapy in cervical
and breast cancer (12,13). There are a number of isoforms of
CDC27, the main one having 824 amino acids, and the somatic
mutations of CDC27 have been found to disrupt APC/C activity in
various cancer types (6,15). The vast majority of CDC27 protein
is associated with APC/C and expressed in the nucleus, especially
in the nuclear envelope membrane and chromosome (23,24).
However, Huang et al (23)
showed that the mutated Cdc27 on Drosophila was expressed in
both nuclear and cytoplasmic locations. In the present study, an
antibody that recognized amino acids 814–823 at the extreme
C-terminus of CDC27 was selected for IHC detection. It was found
that CDC27 could be expressed in the nucleus and/or cytoplasm.
Hence, CDC27 was separated into cytoplasmic and nuclear expression
for exploring the roles of CDC27 in patients with READ. It was
found that cytoplasmic CDC27 was significantly associated with
tumor progression and distant metastasis, but not with survival
outcomes, while nuclear CDC27 was strongly associated with survival
outcomes and was an independent prognostic factor for this. The
results indicated that the location of CDC27 expression may have
different functions in tumor development. However, considering the
isoforms and somatic variants of CDC27 proteins, additional studies
are necessary to survey and evaluate the results in terms of CDC27
isoforms and mutations.
Chemotherapy is the most common treatment for
cancer, and 5-fluorouracil-based chemotherapeutic drugs combined
with oxaliplatin and/or irinotecan are commonly used to treat
patients with colorectal cancer to improve patient survival and
tumor response. Post-operative adjuvant chemotherapy is often used
after surgery to reduce the risk of distant metastasis and provide
additional survival benefits in high-risk colorectal cancer
patients (25,26). However, numerous deaths in patients
with cancer are due to the failure and resistance to chemotherapy.
Chemotherapeutic drugs often cause innate and acquired
chemoresistance by intrinsic and extrinsic factors (27–29).
Several studies have proven that dysfunction of the APC/C complex
is associated with chemoresistance and poor clinical outcomes, as
APC/C activity is reactivated and triggers cell cycle arrest for
repair under chemotherapeutic agent exposure (15,30).
Hu et al (31) demonstrated
that the stability of bromodomain-containing 7 protein was
regulated by APC/C and correlated with clinical outcomes in
patients with osteosarcoma. Inhibition of APC/C function improved
paclitaxel efficacy in breast cancer cells and increased the
sensitivity of osteosarcoma cells to cisplatin and doxorubicin
(31,32). Activation of APC/C led to
temozolomide resistance in glioma cells, docetaxel resistance in
the castration-resistant prostate cancer cell line and
chemoresistance to anti-microtubule drugs (33–35).
Moreover, altered expression or somatic mutation of CDC27 also
reduced chromosomal instability, dysfunction of APC/C and spindle
assembly checkpoint target therapy resistance (15). CDC27 also is a target of miRNA that
can predict therapy efficacy and modulate radiosensitivity
(36,37). In the present study, it was
discovered that nuclear CDC27 expression was an independent
prognostic factor in adjuvant chemotherapy-treated patients, but
not in patients only treated with surgery. These results revealed
that high nuclear CDC27 expression could attenuate the response to
chemotherapy treatment. This phenomenon may be as the overexpressed
CDC27 in the nucleus causes APC/C dysfunction and chemoresistance
in cancer cells. Further studies are important in order to
determine the roles of CDC27 in chemoresistance. Recently, the
subunits of APC/C have become attractive innovative therapies for
cancer treatment (5,15). Hence, CDC27 may act as a potential
therapeutic target in patients with READ.
The subset and amount of TILs, especially the
density of CD3+ TILs, have been reported to correlate
with antitumor immune response and survival outcome in patients
with colorectal cancer (16,38).
The present results revealed that the density of intratumor
CD3+ TILs was associated with TNM stage, pN stage and
PNI (P<0.001). The density of CD3+ TILs was also
associated with the 5-year DFS and OS rates in patients with READ.
In the present study, CD3+ TILs were a prognostic factor
for patients with READ in terms of 5-year DFS, but not for adjuvant
chemotherapy-treated patients in terms of either 5-year DFS or OS.
Furthermore, using TIMER database analysis, the expression of CDC27
was positively associated with CD4+ and CD8+
TILs in patients with READ in the present study. However, there was
no association between cytoplasmic/nuclear CDC27 expression and
intratumor CD3+ TILs in patients with READ. These
inconsistent results may be due to the difference in the study
population. Overall, there was no association between CDC27
expression and intratumor CD3+ TIL density, and
CD3+ TILs were associated with survival outcome in
patients with READ.
There are a number of different treatments for
patients with READ, such as surgery, preoperative concurrent
chemoradiotherapy, post-operative adjuvant chemotherapy,
radiotherapy and targeted therapy. In the present study cohort, the
resected specimens were only collected from patients with READ who
received surgery with or without postoperative chemotherapy, as
this group is more straight forward for exploring the effects of
CDC27 expression. However, the variants of CDC27 at the RNA, DNA
and protein levels are complex and may affect the functions of
CDC27 on tumorigenesis, prognosis, survival outcome and response to
treatment in patients with cancer. Also, only IHC staining was used
to detect CDC27 expression in this study. Further studies are
therefore needed to validate the present results using different
methods and retrospective cohorts, and to investigate the functions
of these CDC27 variants and their clinical significance. The
present study just indicates an easy method to evaluate the effects
of CDC27 in the clinic, and provides strong evidence that the
subcellular location of CDC27 protein expression in cancer cells
may have an impact on tumor progression, survival outcome and
chemotherapy efficacy in patients with READ. Thus, the expression
of CDC27 in the cytoplasm or nucleus may be used to predict
postoperative distant metastasis after surgery and the benefits of
adjuvant chemotherapy.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Professor Kevin CY
Huang (Translation Research Core of China Medical University
Hospital, Taichung, Taiwan) for providing tissue microarray
support.
Funding
This study was supported by grants from Feng Yuan Hospital (nos.
110-03 and 111-02), the Ministry of the Health and welfare (no.
MOHW110-12) and China Medical University Hospital (no.
DMR-110-239).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SFC, KCYH, YLL and CHK conducted and performed the
experiments. CLC, WTLC, TWC, HHH, KSCC and TWK enrolled the
patients with READ and performed the IHC evaluation. CLC and SFC
performed the statistical analysis. TWK and SFC supervised this
study. CLC, KCYH and SFC analyzed the data and wrote the
manuscript. KSCC and SFC provided the funds. All authors have read
and approved the manuscript. CLC, KCYH, TWK and SFC confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
This study was reviewed and approved by the Internal
Review Board of China Medical University Hospital (Taichung,
Taiwan; approval no. CMUH105-REC2-072). Informed consent was
obtained from all participants in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wilkinson N: Management of rectal cancer.
Surg Clin North Am. 100:615–628. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heald RJ, Moran BJ, Ryall RD, Sexton R and
MacFarlane JK: Rectal cancer: The Basingstoke experience of total
mesorectal excision, 1978–1997. Arch Surg. 133:894–899. 1988.
|
|
3
|
Petersen SH, Harling H, Kirkeby LT,
Wille-Jørgensen P and Mocellin S: Postoperative adjuvant
chemotherapy in rectal cancer operated for cure. Cochrane Database
Syst Rev. Mar 14–2012.(Epub ahead of print). View Article : Google Scholar
|
|
4
|
Stewart CL, Warner S, Ito K, Raoof M, Wu
GX, Kessler J, Kim JY and Fong Y: Cytoreduction for colorectal
metastases: Liver, lung, peritoneum, lymph nodes, bone, brain. When
does it palliate, prolong survival, and potentially cure? Curr
Probl Surg. 55:330–379. 2018.
|
|
5
|
Zhou Z, He M, Shah AA and Wan Y: Insights
into APC/C: From cellular function to diseases and therapeutics.
Cell Div. 11:92016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kazemi-Sefat GE, Keramatipour M, Talebi S,
Kavousi K, Sajed R, Kazemi-Sefat NA and Mousavizadeh K: The
importance of CDC27 in cancer: Molecular pathology and clinical
aspects. Cancer Cell Int. 21:1602021. View Article : Google Scholar
|
|
7
|
Lee J, Moon B, Lee DH, Lee G and Park D:
Identification of a novel protein interaction between Elmo1 and
Cdc27. Biochem Biophys Res Commun. 471:497–502. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Link LA, Howley BV, Hussey GS and Howe PH:
PCBP1/HNRNP E1 protects chromosomal integrity by translational
regulation of CDC27. Mol Cancer Res. 14:634–646. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiu L, Tan X, Lin J, Liu RY, Chen S, Geng
R, Wu J and Huang W: CDC27 induces metastasis and invasion in
colorectal cancer via the promotion of epithelial-to-mesenchymal
transition. J Cancer. 8:2626–2635. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiu L, Wu J, Pan C, Tan X, Lin J, Liu R,
Chen S, Geng R and Huang W: Downregulation of CDC27 inhibits the
proliferation of colorectal cancer cells via the accumulation of
p21Cip1/Waf1. Cell Death Dis. 7:e20742016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song Y, Song W, Li Z, Song W, Wen Y, Li J,
Xia Q and Zhang M: Corrigendum: CDC27 promotes tumor progression
and affects PD-L1 expression in T-cell lymphoblastic lymphoma.
Front Oncol. 10:5836982020. View Article : Google Scholar
|
|
12
|
Rajkumar T, Gopal G, Selvaluxmi G and
Rajalekshmy KR: CDC27 protein is involved in radiation response in
squamous cell cervix carcinoma. Indian J Biochem Biophys.
42:271–278. 2005.
|
|
13
|
Talvinen K, Karra H, Pitkänen R, Ahonen I,
Nykänen M, Lintunen M, Söderström M, Kuopio T and Kronqvist P: Low
cdc27 and high securin expression predict short survival for breast
cancer patients. APMIS. 121:945–953. 2013. View Article : Google Scholar
|
|
14
|
Wang C, Su Z, Hou H, Li D, Pan Z, Tian W
and Mo C: Inhibition of anaphase-promoting complex by silence
APC/C(Cdh1) to enhance radiosensitivity of nasopharyngeal carcinoma
cells. J Cell Biochem. 118:3150–3157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sansregret L, Patterson JO, Dewhurst S,
López-García C, Koch A, McGranahan N, Chao WCH, Barry DJ, Rowan A,
Instrell R, et al: APC/C dysfunction limits excessive cancer
chromosomal instability. Cancer Discov. 7:218–233. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Malka D, Lièvre A, André T, Taïeb J,
Ducreux M and Bibeau F: Immune scores in colorectal cancer: Where
are we? Eur J Cancer. 140:105–118. 2020. View Article : Google Scholar
|
|
17
|
Chen TW, Huang KC, Chiang SF, Chen WT, Ke
TW and Chao KSC: Prognostic relevance of programmed cell
death-ligand 1 expression and CD8+ TILs in rectal cancer
patients before and after neoadjuvant chemoradiotherapy. J Cancer
Res Clin Oncol. 145:1043–1053. 2019. View Article : Google Scholar
|
|
18
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. (7th edition).
Springer; New York, NY: 2010
|
|
19
|
Chiang SF, Huang CY, Ke TW, Chen TW, Lan
YC, You YS, Chen WT and Chao KSC: Upregulation of tumor PD-L1 by
neoadjuvant chemoradiotherapy (neoCRT) confers improved survival in
patients with lymph node metastasis of locally advanced rectal
cancers. Cancer Immunol Immunother. 68:283–296. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Detre S, Saclani Jotti G and Dowsett M: A
‘quickscore’ method for immunohistochemical semiquantitation:
Validation for oestrogen receptor in breast carcinomas. J Clin
Pathol. 48:876–878. 1995. View Article : Google Scholar
|
|
21
|
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q,
Li B and Liu XS: TIMER2.0 for analysis of tumor-infiltrating immune
cells. Nucleic Acids Res. 48:W509–W514. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xin Y, Ning S, Zhang L and Cui M: CDC27
facilitates gastric cancer cell proliferation, invasion and
metastasis via twist-induced epithelial-mesenchymal transition.
Cell Physiol Biochem. 50:501–511. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang JY, Morley G, Li D and Whitaker M:
Cdk1 phosphorylation sites on Cdc27 are required for correct
chromosomal localisation and APC/C function in syncytial Drosophila
embryos. J Cell Sci. 120:1990–1997. 2007. View Article : Google Scholar
|
|
24
|
Huang JY and Raff JW: The dynamic
localisation of the Drosophila APC/C: Evidence for the existence of
multiple complexes that perform distinct functions and are
differentially localised. J Cell Sci. 115:2847–2856. 2002.
View Article : Google Scholar
|
|
25
|
São Julião GP, Habr-Gama A, Vailati BB,
Araujo SE, Fernandez LM and Perez RO: New strategies in rectal
cancer. Surg Clin North Am. 97:587–604. 2017. View Article : Google Scholar
|
|
26
|
Liu Z, Meng X, Zhang H, Li Z, Liu J, Sun
K, Meng Y, Dai W, Xie P, Ding Y, et al: Predicting distant
metastasis and chemotherapy benefit in locally advanced rectal
cancer. Nat Commun. 11:4308–4318. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Galluzzi L, Vitale I, Michels J, Brenner
C, Szabadkai G, Harel-Bellan A, Castedo M and Kroemer G: Systems
biology of cisplatin resistance: Past, present and future. Cell
Death Dis. 5:e12572014. View Article : Google Scholar
|
|
28
|
Köberle B and Schoch S: Platinum complexes
in colorectal cancer and other solid tumors. Cancers (Basel).
13:20732021. View Article : Google Scholar
|
|
29
|
Vodenkova S, Buchler T, Cervena K,
Veskrnova V, Vodicka P and Vymetalkova V: 5-fluorouracil and other
fluoropyrimidines in colorectal cancer: Past, present and future.
Pharmacol Ther. 206:1074472020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bassermann F, Frescas D, Guardavaccaro D,
Busino L, Peschiaroli A and Pagano M: The Cdc14B-Cdh1-Plk1 axis
controls the G2 DNA-damage-response checkpoint. Cell. 134:256–267.
2008. View Article : Google Scholar
|
|
31
|
Hu K, Liao D, Wu W, Han AJ, Shi HJ, Wang
F, Wang X, Zhong L, Duan T, Wu Y, et al: Targeting the
anaphase-promoting complex/cyclosome (APC/C)-bromodomain containing
7 (BRD7) pathway for human osteosarcoma. Oncotarget. 5:3088–3100.
2014. View Article : Google Scholar
|
|
32
|
Giovinazzi S, Bellapu D, Morozov VM and
Ishov AM: Targeting mitotic exit with hyperthermia or APC/C
inhibition to increase paclitaxel efficacy. Cell Cycle.
12:2598–2607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Zhou F, Li Y, Li Q, Wu Z, Yu L,
Yuan F, Liu J, Tian Y, Cao Y, et al: Cdc20 overexpression is
involved in temozolomide-resistant glioma cells with
epithelial-mesenchymal transition. Cell Cycle. 16:2355–2365. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu F, Lin Y, Cui P, Li H, Zhang L, Sun Z,
Huang S, Li S, Huang S, Zhao Q and Liu Q: Cdc20/p55 mediates the
resistance to docetaxel in castration-resistant prostate cancer in
a Bim-dependent manner. Cancer Chemother Pharmacol. 81:999–1006.
2018. View Article : Google Scholar
|
|
35
|
Zhang S, Shen Y, Li H, Bi C and Sun Y,
Xiong X, Wei W and Sun Y: The negative cross-talk between
SAG/RBX2/ROC2 and APC/C E3 ligases in regulation of cell cycle
progression and drug resistance. Cell Rep. 32:1081022020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee SJ and Langhans SA: Anaphase-promoting
complex/cyclosome protein Cdc27 is a target for curcumin-induced
cell cycle arrest and apoptosis. BMC Cancer. 12:442012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ren YQ, Fu F and Han J: MiR-27a modulates
radiosensitivity of triple-negative breast cancer (TNBC) cells by
targeting CDC27. Med Sci Monit. 21:1297–1303. 2015. View Article : Google Scholar
|
|
38
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar
|