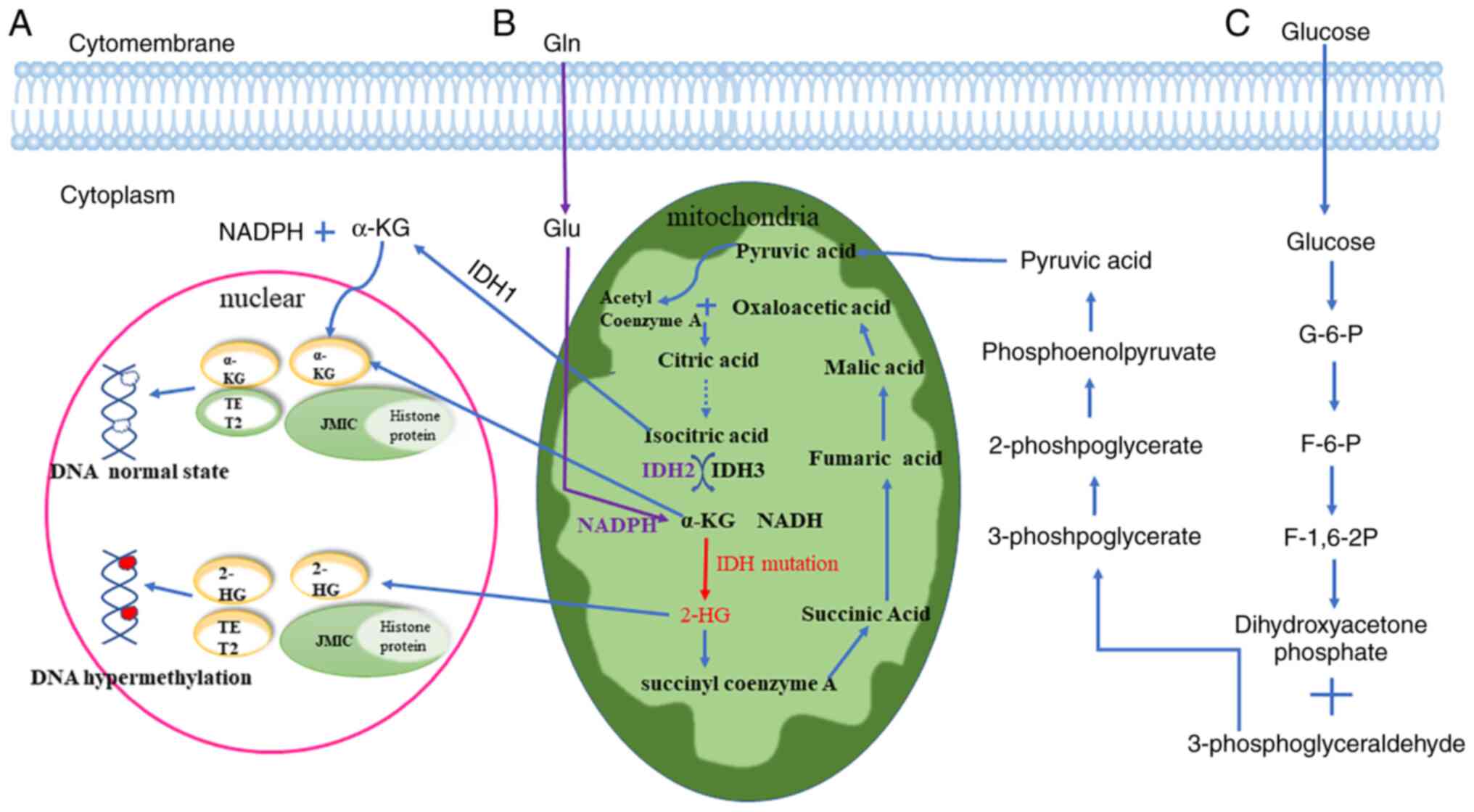
The expression of IDH2 is affected by exposure
factors such as ionizing radiation. IDH2 plays a crucial role in
disorders caused by exposure factors (8,9).
Therefore, the present study provides an overview of the effect of
IDH2 on circulatory system diseases and other system diseases with
regard to exposure factors. IDH2 is a mitochondrial enzyme that
catalyzes the oxidative decarboxylation of isocitrate to α-KG
(10) and protects the body from
oxidative stress by converting nicotinamide adenine dinucleotide
phosphate (NADP+) to reduced NADP (NADPH) (11). Multiple internal and external
factors may contribute to mutations of the IDH2 gene, which may
lead to the loss of enzyme activity or obtaining new enzymatic
functions (change-of-function) (12). The mutant IDH2 consumes a-KG and
NADPH, and produces 2-HG and NADP+ (12) (Fig.
1). Mutations in the IDH2 potein often occur in arginine
residues R140 and R172, and are closely associated with the
development and progression of AML and glioma (13). Moreover, one study has revealed the
effect of wild-type IDH2 on the molecular mechanism of Epstein Barr
virus-mediated disease. EBV can cause nasopharyngeal carcinoma in
humans, and wild-type IDH2 promotes the survival of nasopharyngeal
carcinoma cells (14). Details on
the different functions of IDH2 are expounded in the following
sections.
Over past decades, studies have shown that IDH2 is
associated with hematological tumorigenesis or disorders, including
AML, myelodysplastic syndrome (MDS) and chronic myelomonocytic
leukemia (CMML). The frequencies of IDH2 mutations in diseases of
the hematopoietic system, such as AML (15–17),
MDS (18,19) and chronic mononuclear leukemia, are
8.0–19, 4–4.6 and 7.8–8.8%, respectively (18,20).
Although the frequency of diseases with an IDH2 gene mutation is
low, it is crucial to understand the association between IDH2
mutations and disease occurrence. Therefore, the following sections
will review the role of IDH2 in various physiological or
pathological processes.
IDH2 gene mutations contribute to hematopoietic
neoplasms, such as AML and CMML (21,22).
AML is a hazardous, enervating and invasive disease with a poor
prognosis (23,24). IDH2 and PHD finger protein 6
mutations exert synergistic effects on leukemia formation through
excessive production of 2-HG and damage to DNA repair (25). The mutated IDH2 enzyme exhibits
gain-of-function activity and catalyzes the conversion of α-KG to
2-HG, which is involved in the pathogenesis of AML (26,27).
The 2-HG metabolite is oncogenic and closely associated with the
hypermethylation of DNA and histones, which alters the expression
of different mRNAs and hematopoietic cell differentiation (28,29).
IDH2 gene mutations are crucial for the maintenance of AML
progenitor cells, but these mutations may not be the key
preliminary step for AML development (30,31).
Therefore, further studies are needed to confirm the role of IDH2
mutations in the occurrence of AML. IDH2-mutated cells have
significantly increased sensitivity to IL-1β signaling, which may
be a potential therapeutic target (32). Furthermore, there are drugs in
various stages of clinical development for the treatment of AML
with mutated IDH2 proteins, such as AG-221 (enasidenib) (33), AGI-6780 (34), CP-17 (34), TQ05310 (35) and AG-881 (36). AG-221 is an oral, valid, selective
inhibitor of mutated IDH2 that has been approved by the US Food and
Drug Administration for the treatment of relapsed or refractory
leukemia with IDH2 gene mutations. AG-221 binds to the IDH2 dimer
interface and blocks the production and accumulation of 2-HG,
thereby allowing hematopoietic cells to differentiate from terminal
or ancestral mutant clones (37,38).
For patients who are unable to benefit from treatment with AG-221
alone, the addition of all-trans-retinoic acid may enhance the
response rate of AG-221 therapy (39). Additionally, AG-221 in combination
with azacitidine is more effective than azacitidine alone, and is a
tolerated and effective treatment option for relapsed or refractory
AML that promotes cell differentiation and overall response rates
(33,40). There are usually no clinical signs
of concurrent infection or clinical features of IDH
inhibitor-associated differentiation syndrome, which is
characterized by dyspnea, hypoxia, fluid retention and weight gain
(41). AG-221 was found to exhibit
a poor inhibitory effect on the IDH2R140Q mutation.
However, AGI-6780, a preclinical inhibitor, is a selective
inhibitor of the IDH2R140Q protein and may be an
effective targeted drug therapy for this mutation (34,42,43).
In addition, SH1573 is a potential inhibitor of
IDH2R140Q, and has been demonstrated to be novel, safe
and effective, and is currently in clinical trials (44). Furthermore, CP-17 acts as a potent
inhibitor of the IDH2R140Q mutant and may be a lead
compound for developing drugs against AML (34). Furthermore, TQ05310 showed
selective specificity that targeted both IDH2R140Q and
IDH2R172K mutant enzymes but had no effect on wild-type
IDH or mutated IDH1 (35). AG-881
has lower specificity than the aforementioned drugs and may be
useful as an inhibitor of IDH1 and IDH2 mutations for treating
associated diseases (37). It is
important to note that prognosis can be judged according to the
IDH2 mutation site and whether other genes are mutated (2). Moreover, it has been reported that an
IDH2 mutation is a marker for poor prognosis in patients with AML,
and the overall survival (OS) time of the patients with wild-type
IDH2 was greater than that of patients with an IDH2 mutation
(45). However, another study
found that patients with IDH2R172K mutations have
improved relapse-free survival (RFS) and OS times compared with
patients with the wild-type (46).
Therefore, this topic remains controversial, and more experimental
data are needed to confirm which view is correct. CMML is a
myelodysplastic/myeloproliferative neoplasm. IDH2 can be used as an
indicator of poor prognosis in CMML patients to a lesser extent.
One study indicated that patients with IDH2 mutations in CMML had
inferior OS rates than wild-type IDH2 patients (17,47).
IDH2 status not only plays an important role in
hematopoietic neoplasms, but also regulates the development of
other diseases in the hematopoietic system. Previous studies have
discovered that a small number of patients with MDS have IDH2
mutations. The most common IDH2 mutant subtype in MDS is
IDH2R140Q, and as aforementioned, this mutation results
in enzymatic gain-of-function and inhibition of hemopoietic cell
differentiation (48,49). The IDH2 mutation rate in patients
with advanced MDS is higher than in those with early MDS (14). Mutations in IDH1 and IDH2 are
mutually exclusive (50).
Similarly, analysis of patients with MDS showed IDH2 and tet
methylcytosine dioxygenase 2 (TET2) gene mutations do not exist
simultaneously (51). IDH2
mutations may be involved in the development but not the
progression of MDS (52).
Moreover, high 2-HG serum levels predict the presence of IDH2
mutations; thus, mutated IDH2 proteins can be used as targets for
pre-transplantation and post-transplantation treatment of MDS
(53,54). For effective treatment of MDS with
IDH2 gene mutations, the mutated protein can be specifically
targeted (19). For example,
AG-221 is an effective drug for the treatment of MDS with IDH2
mutations, including use in those patients for whom the use of
hypomethylating agents has been unsatisfactory (55). There is a poor OS time among
patients with MDS and IDH2 mutations under low-risk stratification
in the International Prognostic Scoring System (53,56).
In summary, IDH2 plays an extremely important role in the
generation and progression of some diseases of the hematopoietic
system, and can be used as a molecular marker and target for
therapy.
IDH2 is closely associated with therapeutic
effectiveness and sensitivity. IDH2 is not only associated with
blood circulation disorders, but also with other diseases, such as
glioma (57), non-small cell lung
cancer (58), solid papillary
carcinoma with reverse polarity (SPCRP) (59), angioimmunoblastic T-cell lymphoma
(AITL) (60), high-grade
chondrosarcoma (61) and
undifferentiated sinus carcinoma (62).
IDH2 mutations play an important role in some
diseases of the nervous system, such as gliomas and
2-hydroxyglutaric aciduria. Studies have shown that IDH2 mutations
are a driving factor in gliomas, especially in low-grade gliomas
and glioblastomas (57,63). Glioma subtypes with IDH2 gene
mutations have unique clinical characteristics. For instance, IDH2
mutations are more common in younger individuals and are more
likely to occur in low-risk surgical areas (64). Furthermore, IDH2 can be used as a
biomarker with diagnostic, prognostic and predictive implications
(65,66). In gliomas, IDH2 mutations were
found to be mutually exclusive with IDH1, phosphatase and tensin
homolog, cellular tumor antigen p53 and α-thalassemia retardation
syndrome X-linked mutations (63).
Injecting mutated IDH2 into glioma mice accelerated tumor growth
and increased mortality rate compared with that in mice with
wild-type IDH2 (67). Moreover,
IDH2 mutations are significantly correlated with the incidence of
preoperative glioma-related epilepsy, and affect the surgical
resectability of gliomas (68,69).
IDH2 mutations may also trigger the development of
2-hydroxyglutaric aciduria and Parkinson's disease (70,71).
In addition to playing an important role in the
progression of neurological diseases, IDH2 gene status promotes the
development of certain respiratory diseases. Studies have shown
that the IDH2 protein plays a role in specific lung cancer types;
for example, IDH2 plays an important role in non-small cell lung
cancer (58). The expression level
of IDH2 in the serum of patients with non-small cell lung cancer is
higher than that of the normal population, and after treatment, the
expression of serum IDH2 in patients with non-small cell lung
cancer decreases (58). Notably,
the IDH2 rs11540478 genetic variant represents a novel
susceptibility locus for lung cancer; it can change the level of
IDH2 protein, and affect cancer cell viability and disease
evolution (72). Compared with
patients with lung cancer, healthy individuals have lower levels of
IDH2 mRNA in peripheral blood lymphocytes (66). Wild-type IDH2 promotes the
development of lung cancer, and an increased level of IDH2 protein
is a characteristic of poor survival (73). Overall, IDH2 may represent a target
for lung cancer treatment. Furthermore, IDH2 is important for the
progression of lung injury. IDH2 has different effects depending on
the cause of the lung injury. IDH2 can reduce acrolein-induced lung
injury by providing NADPH (74),
and as a provider of the pro-inflammatory metabolite α-KG, IDH2 is
indirectly involved in lipopolysaccharide-induced acute lung
injury. Therefore, targeting the IDH2 enzyme may be a treatment
strategy for systemic inflammatory response syndrome (75).
AITL is a subtype of peripheral T-cell lymphoma that
is affected by IDH2 gene status. It has been reported that IDH2
expression is significantly upregulated in patients with
myeloproliferative neoplasia (MPN)-AITL compared with that in AITL
patients without MPN (76).
Meanwhile, it has also been reported that mutated IDH2 and TET2
proteins usually occur together in AITL (60,77).
In one study, AITL with the IDH2R172 mutation displayed
a limited gene expression profile that correlated with cellular
differentiation, and genes associated with interleukin-12
stimulation were significantly enriched (78). Moreover, an AITL case with mutated
IDH2 protein presented with high intracellular levels of 2-HG
without an increase in circulating 2-HG. This case suggests that
levels of circulating 2-HG may not accurately reflect the presence
of IDH2 gene mutations (79). In
20–30% of patients with AITL, IDH2 has diagnostic value and is a
potential therapeutic target. Next-generation sequencing technology
and allele-specific quantitative polymerase chain reaction show
good sensitivity for the detection and diagnosis of IDH2 mutations
(80). As expected, compared with
the TET2 gene mutation alone, TET2/IDH2 co-mutations conferred
longer progression-free survival times to affected patients
(81).
IDH2 status is important in other diseases, such as
SPCRP, prostate and colon cancer. A previous clinical study
determined that 77% of SPCRP cases had an IDH2 hot spot mutation at
amino acid site 172 (59). One
study indicated that the IDH2R172 mutation was highly
specific for SPCRP in various subtypes of breast cancer, and that
it may be a suitable diagnostic marker for SPCRP and guide
effective treatment (82).
Furthermore, high expression levels of IDH2 mRNA or protein were
associated with a poor outcome in patients with invasive breast
cancer (83). Enzymatic
malfunction of IDH2 in prostate cancer cells disrupted oxidative
bioenergetics, enhanced reactive oxygen species (ROS) generation
and increased mitochondrial dynamics (84). Frequent IDH2 gene mutations have
recently found in central chondrosarcomas, which resulted in longer
RFS and metastasis-free survival times in high-grade
chondrosarcomas; however, no association with OS was observed
(61). Undifferentiated sinus
carcinoma is an infrequent, aggressive, highly malignant tumor with
finite therapy choices (85,86),
and women with this disease have a higher frequency of IDH2
mutations compared with men with the disease (87). IDH2 mutated undifferentiated sinus
carcinoma subtypes have high levels of DNA methylation and a poor
prognosis (62). As a prime
component in anti-oxidative damage, wild-type IDH2 protects
cochlear hair cells from ROS and prevents age-related hearing loss
by providing NADPH (88,89). Moreover, IDH2 is vital for
conditions that develop from ROS exposure or mechanical damage
(90). For example, IDH2 has a
protective effect against ultraviolet B radiation-induced skin
damage and is involved in skin wound healing (90,91).
Downregulation of IDH2 protein is likely one of the mechanisms
underlying 5-hydroxymethylcytosine loss in melanoma (92). Studies have shown that IDH2
knockout can inhibit the growth of colon cancer cell lines. Thus,
the IDH2 enzyme has an important impact on the generation and
progression of colon carcinoma (93,94).
IDH2 may be a target to treat skin pigmentation, as IDH2 deficiency
can cause skin pigmentation (95).
Additionally, IDH2 may be a therapeutic target for adipose
inflammation (96,97). In summary, IDH2 gene status drives
the process of multiple diseases and may serve as a biomarker
and/or therapeutic target (Table
I).
IDH2 not only greatly contributes to the progression
and treatment of diseases, but it is also involved in the
maintenance of cellular metabolism under conditions of
environmentally induced damage. IDH2 can protect cells from damage
caused by excessive ROS production after cells are exposed to
ionizing radiation (98). ROS are
natural products of mitochondrial metabolism and can trigger
oxidative damage (99). The
protective effect of IDH2 on cells is based on the reduction of
NADP+ to NADPH, which is a precondition for some
cellular defense systems to reduce oxidative damage (100,101). Imbalance between mitochondrial
oxidation and reduction reactions causes cell death in organisms
exposed to ionizing radiation. When organisms are exposed to
ionizing radiation, the level of ROS increases and the NADPH
regulated by IDH2 to the antioxidant system decreases (102,103). Wild-type IDH2 protects cells
against γ-irradiation by maintaining NADPH levels that buffer
against radiation-induced ROS and protect cells from apoptosis. The
latest studies and clinical data from the European Organization for
Research on Treatment of Cancer trial have verified that gliomas
with IDH2 gene mutations are extremely sensitive to radiotherapy
(104–106). Furthermore, IDH2 not only
protects the cells from ionizing radiation, but also protects the
cells from non-ionizing radiation. Under the non-ionizing radiation
environment, IDH2 has a protective effect on ultraviolet B-induced
skin damage (90).
Environmental heavy metals affect the enzymatic
activity of IDH2. When organisms are exposed to heavy metals, such
as cadmium (Cd2+) (107), CoCl2 (108), mercury (109) or copper (8), the expression of IDH2 may be altered.
Cd2+ ions have a dual effect on cells. Cd2+
activates IDH2, and activated IDH2 provides NADPH for cellular
defense, as aforementioned. However, Cd2+ ions have a
high affinity for thiols and contribute to the combination between
Cd2+ ions and cysteine residues, which trigger IDH2
inactivation. Therefore, cells exposed to cadmium are prone to
apoptosis (107,110). Removing cadmium from the
environment with microorganisms is difficult due to its toxicity.
The yeast Pichia kudriavzevii has been used as a model
organism for cadmium removal, and acid stress can reduce the
toxicity of Cd2+ and upregulate genes associated with
ATP synthesis, such as IDH2 (111). CoCl2 can indirectly
affect IDH2 expression. Hypoxia was induced by CoCl2
treatment increasing the expression of IDH2 in breast cancer cells
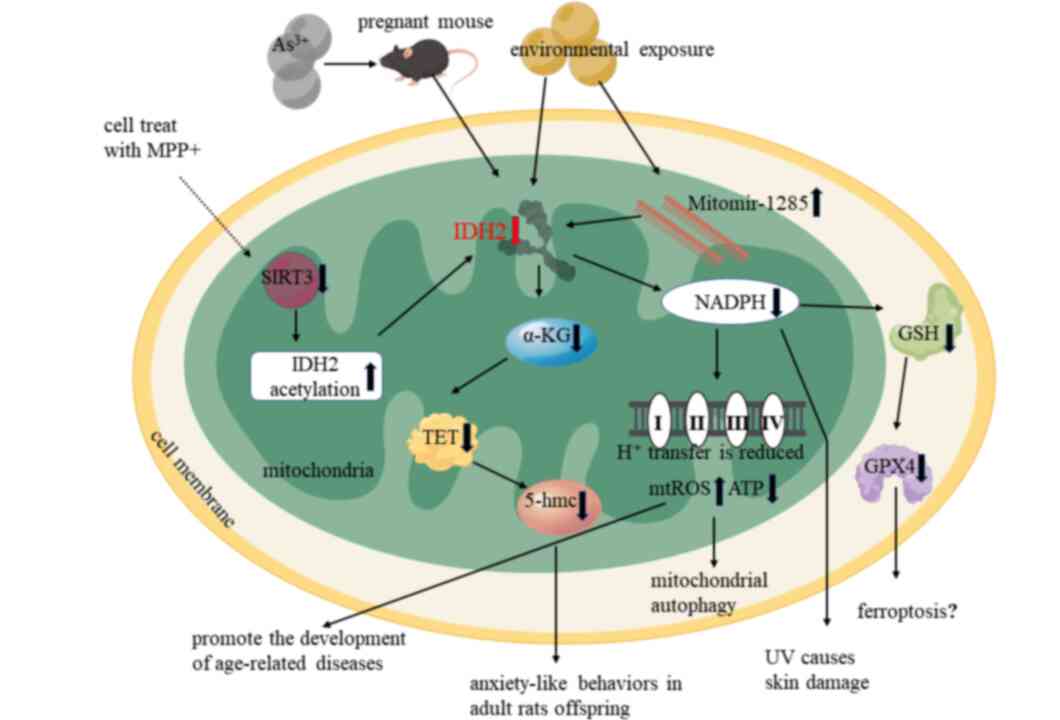
after radiation (108). Jejunal
epithelial cells induce mitochondrial dysfunction and mitosis
through the Mitomir-1285-IDH2 axis during copper exposure
Mitomir-1285 aggravates copper-induced mitochondrial dysfunction by
inhibiting IDH2 expression (8)
(Fig. 2). Exposure to arsenic in
pregnant rats indirectly induces anxiety-like behavior in adult
offspring through downregulation of IDH2 expression in the fetal
brain (9). Moreover, some lower
animals, such as bivalves and corals, may also be affected by heavy
metals, resulting in a change in IDH2 activity. For example,
bivalves exposed to mercury alone showed a reduction in IDH2
activity and a subsequent alteration in cellular energy production
(109). When corals were exposed
to copper, IDH2 activity was inhibited, and aerobic and oxidative
metabolism was reduced (112)
(Fig. 2) (Table II).
IDH2 is regulated by SIRT3 and other factors. SIRT3
is regulated by acetylation, which is a post-translational
modification that alters its activity (115,116). IDH2 activity is increased
significantly after deacetylation by SIRT3, which is a deacetylase
in mitochondria. A decrease in SIRT3 protein reduces IDH2 enzymatic
activity by decreasing IDH2 dimer formation (117,118) (Fig.
2). For example, in one study, during therapy for multiple
myeloma, the combination of carfilzomib with SIRT3 inhibitors
decreased IDH2 activity and increased multiple myeloma cell death
(118). Overexpression of
nicotinamide mononucleotide adenylate transferase 3 in bone marrow
mesenchymal stem cells enhanced the ability of specific antioxidant
stress by enhancing SIRT3 activity and decreasing IDH2 acetylation
level (119).
To avoid damage caused by oxidative stress, the
antioxidant activity of IDH2 can be enhanced by SUMOylation
(120). A previous study showed
that the activity of IDH2 is affected by post-translational
modification. Under oxidative stress, IDH2 ubiquitination deficient
cells had more apoptosis than normal cells, suggesting that
ubiquitination is an important means of regulating IDH2 activity
(120). Furthermore,
IDH2R140Q and cytoplasmic nucleophosmin mutation
increase myeloid ecotropic viral integration site 1 and homeobox A9
gene expression, respectively, which activates the hypoxia pathway
in AML cells (121). Cells
expressing the mutant IDH2 protein are deficient in their capacity
for reductive carboxylation and the ability to produce acetyl-CoA
may be impaired under hypoxic conditions. Acetyl-CoA is involved in
certain metabolic processes in the body, such as cholesterol
synthesis, fatty acid generation and glucose metabolism (122). In addition, decreased IDH2
expression may promote ferroptosis through the coordination of
erastin (ferroptosis inhibitor) with the
NADPH-glutathione-glutathione peroxidase 4 (GPX4) axis (Fig. 2) (123). Taken together, these factors
provide new insight into the treatment of diseases involving the
IDH2 gene.
The present review summarizes the existing knowledge
relating to various aspects of IDH2 expression and activity. IDH2
and its associated diseases, mechanisms of action and alterations
after exposure to radiation and heavy metals are described. A
number of environmental exposure factors can inhibit IDH2
expression or activity, indirectly leading to decreased GPX4
expression, which may lead to ferroptosis, but the specific
mechanism is not clear. Further research may provide therapeutic
methods for some diseases so that patients can obtain more accurate
and effective treatment plans. In addition, it is possible to find
other factors that regulate normal levels of IDH2 and maintain
homeostasis in response to radiation exposure, a strategy that may
have great application value. Furthermore, IDH2 is promising as a
marker of environmental exposure for circulatory diseases. On the
basis of the important role of IDH2 in blood circulatory system
disorders, it is feasible to recommend IDH2 as a novel biomarker
for environmental exposure in blood circulatory system disorders
both theoretically and practically.
Not applicable.
This study was supported by the Hunan Natural Science Foundation
(grant no. 2019JJ40238), the Key Scientific Research Project of
Hunan Health Commission (grant no. 202102051816), the Hunan Health
Commission (grant no. C2019096), the Project of Hengyang Science
and Technology Bureau (grant no. 2020jh042) and the Hunan
Provincial Natural Science Foundation of China (grant no.
2019JJ50509).
Not applicable.
YQG and SW completed the writing and proofreading of
the manuscript. YYW, YLC, and JC revised the manuscirpt critically
for intellectual content and created the figures and tables. YQY,
XL, HXY, and HQ made corrections to the original manuscript and
also performed literature searches. LY was involved in the
conception, directed the writing of the article, and made partial
revisions. All authors have read and approved the final manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Dang L, Yen K and Attar EC: IDH mutations
in cancer and progress toward development of targeted therapeutics.
Ann Oncol. 27:599–608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Montalban-Bravo G and DiNardo CD: The role
of IDH mutations in acute myeloid leukemia. Future Oncol.
14:979–993. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma H: Development of novel
therapeutics targeting isocitrate dehydrogenase mutations in
cancer. Curr Top Med Chem. 18:505–524. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Willander K, Falk IJ, Chaireti R, Paul E,
Hermansson M, Gréen H, Lotfi K and Söderkvist P: Mutations in the
isocitrate dehydrogenase 2 gene and IDH1 SNP 105C > T have a
prognostic value in acute myeloid leukemia. Biomark Res. 2:182014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu X, Zhao J, Xu Z, Peng B, Huang Q,
Arnold E and Ding J: Structures of human cytosolic NADP-dependent
isocitrate dehydrogenase reveal a novel self-regulatory mechanism
of activity. J Biol Chem. 279:33946–33957. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Medeiros BC, Fathi AT, DiNardo CD, Pollyea
DA, Chan SM and Swords R: Isocitrate dehydrogenase mutations in
myeloid malignancies. Leukemia. 31:272–281. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clark O, Yen K and Mellinghoff IK:
Molecular pathways: Isocitrate dehydrogenase mutations in cancer.
Clin Cancer Res. 22:1837–1842. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liao J, Li Q, Hu Z, Yu W, Zhang K, Ma F,
Han Q, Zhang H, Guo J, Hu L, et al: Mitochondrial miR-1285
regulates copper-induced mitochondrial dysfunction and mitophagy by
impairing IDH2 in pig jejunal epithelial cells. J Hazard Mater.
422:1268992022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lv JW, Song YP, Zhang ZC, Fan YJ, Xu FX,
Gao L, Zhang XY, Zhang C, Wang H and Xu DZ: Gestational arsenic
exposure induces anxiety-like behaviors in adult offspring by
reducing DNA hydroxymethylation in the developing brain. Ecotoxicol
Environ Saf. 227:1129012021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bergaggio E, Riganti C, Garaffo G, Vitale
N, Mereu E, Bandini C, Pellegrino E, Pullano V, Omedè P, Todoerti
K, et al: IDH2 inhibition enhances proteasome inhibitor
responsiveness in hematological malignancies. Blood. 133:156–167.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park JB, Nagar H, Choi S, Jung SB, Kim HW,
Kang SK, Lee JW, Lee JH, Park JW, Irani K, et al: IDH2 deficiency
impairs mitochondrial function in endothelial cells and
endothelium-dependent vasomotor function. Free Radic Biol Med.
94:36–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lang F, Jha A, Meuter L, Pacak K and Yang
C: Identification of isocitrate dehydrogenase 2 (IDH2) mutation in
carotid body paraganglioma. Front Endocrinol (Lausanne).
12:7310962021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cairns RA and Mak TW: Oncogenic isocitrate
dehydrogenase mutations: Mechanisms, models, and clinical
opportunities. Cancer Discov. 3:730–741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi F, He Y, Li J, Tang M, Li Y, Xie L,
Zhao L, Hu J, Luo X, Zhou M, et al: Wild-type IDH2 contributes to
Epstein-Barr virus-dependent metabolic alterations and
tumorigenesis. Mol Metab. 36:1009662020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cerchione C, Romano A, Daver N, DiNardo C,
Jabbour EJ, Konopleva M, Ravandi-Kashani F, Kadia T, Martelli MP,
Isidori A, et al: IDH1/IDH2 inhibition in acute myeloid leukemia.
Front Oncol. 11:6393872021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stein EM, DiNardo CD, Fathi AT, Pollyea
DA, Stone RM, Altman JK, Roboz GJ, Patel MR, Collins R, Flinn IW,
et al: Molecular remission and response patterns in patients with
mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood.
133:676–687. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abou Dalle I and DiNardo CD: The role of
enasidenib in the treatment of mutant IDH2 acute myeloid leukemia.
Ther Adv Hematol. 9:163–173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rocquain J, Carbuccia N, Trouplin V,
Raynaud S, Murati A, Nezri M, Tadrist Z, Olschwang S, Vey N,
Birnbaum D, et al: Combined mutations of ASXL1, CBL, FLT3, IDH1,
IDH2, JAK2, KRAS, NPM1, NRAS, RUNX1, TET2 and WT1 genes in
myelodysplastic syndromes and acute myeloid leukemias. BMC Cancer.
10:4012010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Montoro J, Yerlikaya A, Ali A and Raza A:
Improving treatment for myelodysplastic syndromes patients. Curr
Treat Options Oncol. 19:662018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gelsi-Boyer V, Trouplin V, Roquain J,
Adélaïde J, Carbuccia N, Esterni B, Finetti P, Murati A, Arnoulet
C, Zerazhi H, et al: ASXL1 mutation is associated with poor
prognosis and acute transformation in chronic myelomonocytic
leukaemia. Br J Haematol. 151:365–375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Woods BA and Levine RL: The role of
mutations in epigenetic regulators in myeloid malignancies. Immunol
Rev. 263:22–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Molenaar RJ, Thota S, Nagata Y, Patel B,
Clemente M, Przychodzen B, Hirsh C, Viny AD, Hosano N, Bleeker FE,
et al: Clinical and biological implications of ancestral and
non-ancestral IDH1 and IDH2 mutations in myeloid neoplasms.
Leukemia. 29:2134–2142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buege MJ, DiPippo AJ and DiNardo CD:
Evolving treatment strategies for elderly leukemia patients with
IDH mutations. Cancers (Basel). 10:1872018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amaya ML and Pollyea DA: Targeting the
IDH2 pathway in acute myeloid leukemia. Clin Cancer Res.
24:4931–4936. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen TC, Yao CY, Chen YR, Yuan CT, Lin CC,
Hsu YC, Chuang PH, Kao CJ, Li YH, Hou HA, et al: Oncogenesis
induced by combined Phf6 and Idh2 mutations through increased
oncometabolites and impaired DNA repair. Oncogene. 41:1576–1588.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gross S, Cairns RA, Minden MD, Driggers
EM, Bittinger MA, Jang HG, Sasaki M, Jin S, Schenkein DP, Su SM, et
al: Cancer-associated metabolite 2-hydroxyglutarate accumulates in
acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2
mutations. J Exp Med. 207:339–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen C, Liu Y, Lu C, Cross JR, Morris JP
IV, Shroff AS, Ward PS, Bradner JE, Thompson C and Lowe SW:
Cancer-associated IDH2 mutants drive an acute myeloid leukemia that
is susceptible to Brd4 inhibition. Genes Dev. 27:1974–1985. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Waitkus MS, Diplas BH and Yan H:
Biological role and therapeutic potential of IDH mutations in
cancer. Cancer Cell. 34:186–195. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Upadhyay VA, Brunner AM and Fathi AT:
Isocitrate dehydrogenase (IDH) inhibition as treatment of myeloid
malignancies: Progress and future directions. Pharmacol Ther.
177:123–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kats LM, Reschke M, Taulli R, Pozdnyakova
O, Burgess K, Bhargava P, Straley K, Karnik R, Meissner A, Small D,
et al: Proto-oncogenic role of mutant IDH2 in leukemia initiation
and maintenance. Cell Stem Cell. 14:329–341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chotirat S, Thongnoppakhun W,
Wanachiwanawin W and Auewarakul CU: Acquired somatic mutations of
isocitrate dehydrogenases 1 and 2 (IDH1 and IDH2) in preleukemic
disorders. Blood Cells Mol Dis. 54:286–291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sunthankar KI, Jenkins MT, Cote CH, Patel
SB, Welner RS and Ferrell PB: Isocitrate dehydrogenase mutations
are associated with altered IL-1β responses in acute myeloid
leukemia. Leukemia. 36:923–934. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Venugopal S, Takahashi K, Daver N, Maiti
A, Borthakur G, Loghavi S, Short NJ, Ohanian M, Masarova L, Issa G,
et al: Efficacy and safety of enasidenib and azacitidine
combination in patients with IDH2 mutated acute myeloid leukemia
and not eligible for intensive chemotherapy. Blood Cancer J.
12:102022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen J, Yang J, Wei Q, Weng L, Wu F, Shi
Y, Cheng X, Cai X, Hu C and Cao P: Identification of a selective
inhibitor of IDH2/R140Q enzyme that induces cellular
differentiation in leukemia cells. Cell Commun Signal. 18:552020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao M, Zhu H, Fu L, Li Y, Bao X, Fu H,
Quan H, Wang L and Lou L: Pharmacological characterization of
TQ05310, a potent inhibitor of isocitrate dehydrogenase 2 R140Q and
R172K mutants. Cancer Sci. 110:3306–3314. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma R and Yun CH: Crystal structures of
pan-IDH inhibitor AG-881 in complex with mutant human IDH1 and
IDH2. Biochem Biophys Res Commun. 503:2912–2917. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yen K, Travins J, Wang F, David MD, Artin
E, Straley K, Padyana A, Gross S, DeLaBarre B, Tobin E, et al:
AG-221, a first-in-class therapy targeting acute myeloid leukemia
harboring oncogenic IDH2 mutations. Cancer Discov. 7:478–493. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Quek L, David MD, Kennedy A, Metzner M,
Amatangelo M, Shih A, Stoilova B, Quivoron C, Heiblig M, Willekens
C, et al: Clonal heterogeneity of acute myeloid leukemia treated
with the IDH2 inhibitor enasidenib. Nat Med. 24:1167–1177. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim Y, Jeung HK, Cheong JW, Song J, Bae
SH, Lee JI and Min YH: All-trans retinoic acid synergizes with
enasidenib to induce differentiation of IDH2-mutant acute myeloid
leukemia cells. Yonsei Med J. 61:762–773. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
DiNardo CD, Schuh AC, Stein EM, Montesinos
P, Wei AH, de Botton S, Zeidan AM, Fathi AT, Kantarjian HM, Bennett
JM, et al: Enasidenib plus azacitidine versus azacitidine alone in
patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia
(AG221-AML-005): A single-arm, phase 1b and randomised, phase 2
trial. Lancet Oncol. 22:1597–1608. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stein EM, DiNardo CD, Pollyea DA, Fathi
AT, Roboz GJ, Altman JK, Stone RM, DeAngelo DJ, Levine RL, Flinn
IW, et al: Enasidenib in mutant-IDH2 relapsed or refractory acute
myeloid leukemia. Blood. 130:722–731. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang F, Travins J, DeLaBarre B,
Penard-Lacronique V, Schalm S, Hansen E, Straley K, Kernytsky A,
Liu W, Gliser C, et al: Targeted inhibition of mutant IDH2 in
leukemia cells induces cellular differentiation. Science.
340:622–626. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen J, Yang J, Sun X, Wang Z, Cheng X, Lu
W, Cai X, Hu C, Shen X and Cao P: Allosteric inhibitor remotely
modulates the conformation of the orthestric pockets in mutant
IDH2/R140Q. Sci Rep. 7:164582017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang Z, Zhang Z, Li Y, Sun L, Peng D, Du
D, Zhang X, Han L, Zhao L, Lu L, et al: Preclinical efficacy
against acute myeloid leukaemia of SH1573, a novel mutant IDH2
inhibitor approved for clinical trials in China. Acta Pharm Sin B.
11:1526–1540. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Aref S, Kamel Areida el S, Abdel Aaal MF,
Adam OM, El-Ghonemy MS, El-Baiomy MA and Zeid TA: Prevalence and
clinical effect of IDH1 and IDH2 mutations among cytogenetically
normal acute myeloid leukemia patients. Clin Lymphoma Myeloma Leuk.
15:550–555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Middeke JM, Metzeler KH, Röllig C, Krämer
M, Eckardt JN, Stasik S, Greif PA, Spiekermann K,
Rothenberg-Thurley M, Krug U, et al: Differential impact of IDH1/2
mutational subclasses on outcome in adult AML: Results from a large
multicenter study. Blood Adv. 6:1394–1405. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Itzykson R, Kosmider O, Renneville A,
Gelsi-Boyer V, Meggendorfer M, Morabito M, Berthon C, Adès L,
Fenaux P, Beyne-Rauzy O, et al: Prognostic score including gene
mutations in chronic myelomonocytic leukemia. J Clin Oncol.
31:2428–2436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Willekens C, Rahme R, Duchmann M, Vidal V,
Saada V, Broutin S, Delahousse J, Renneville A, Marceau A, Clappier
E, et al: Effects of azacitidine in 93 patients with IDH1/2 mutated
acute myeloid leukemia/myelodysplastic syndromes: A French
retrospective multicenter study. Leuk Lymphoma. 62:438–445. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hosono N: Genetic abnormalities and
pathophysiology of MDS. Int J Clin Oncol. 24:885–892. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lin J, Yao DM, Qian J, Chen Q, Qian W, Li
Y, Yang J, Wang CZ, Chai HY, Qian Z, et al: IDH1 and IDH2 mutation
analysis in Chinese patients with acute myeloid leukemia and
myelodysplastic syndrome. Ann Hematol. 91:519–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kosmider O, Gelsi-Boyer V, Slama L,
Dreyfus F, Beyne-Rauzy O, Quesnel B, Hunault-Berger M, Slama B, Vey
N, Lacombe C, et al: Mutations of IDH1 and IDH2 genes in early and
accelerated phases of myelodysplastic syndromes and
MDS/myeloproliferative neoplasms. Leukemia. 24:1094–1096. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lin CC, Hou HA, Chou WC, Kuo YY, Liu CY,
Chen CY, Lai YJ, Tseng MH, Huang CF, Chiang YC, et al: IDH
mutations are closely associated with mutations of DNMT3A, ASXL1
and SRSF2 in patients with myelodysplastic syndromes and are stable
during disease evolution. Am J Hematol. 89:137–144. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lin P, Luo Y, Zhu S, Maggio D, Yang H, Hu
C, Wang J, Zhang H, Ren Y, Zhou X, et al: Isocitrate dehydrogenase
2 mutations correlate with leukemic transformation and are
predicted by 2-hydroxyglutarate in myelodysplastic syndromes. J
Cancer Res Clin Oncol. 144:1037–1047. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kharfan-Dabaja MA, Komrokji RS, Zhang Q,
Kumar A, Tsalatsanis A, Perkins J, Nishihori T, Field T, Al Ali N,
Mishra A, et al: TP53 and IDH2 somatic mutations are associated
with inferior overall survival after allogeneic hematopoietic cell
transplantation for myelodysplastic syndrome. Clin Lymphoma Myeloma
Leuk. 17:753–758. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Stein EM, Fathi AT, DiNardo CD, Pollyea
DA, Roboz GJ, Collins R, Sekeres MA, Stone RM, Attar EC, Frattini
MG, et al: Enasidenib in patients with mutant IDH2 myelodysplastic
syndromes: A phase 1 subgroup analysis of the multicentre,
AG221-C-001 trial. Lancet Haematol. 7:e309–e319. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Greenberg P, Cox C, LeBeau MM, Fenaux P,
Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, et al:
International scoring system for evaluating prognosis in
myelodysplastic syndromes. Blood. 89:2079–2088. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Turkalp Z, Karamchandani J and Das S: IDH
mutation in glioma: New insights and promises for the future. JAMA
Neurol. 71:1319–1325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li JJ, Li R, Wang W, Zhang B, Song X,
Zhang C, Gao Y, Liao Q, He Y, You S, et al: IDH2 is a novel
diagnostic and prognostic serum biomarker for non-small-cell lung
cancer. Mol Oncol. 12:602–610. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chiang S, Weigelt B, Wen HC, Pareja F,
Raghavendra A, Martelotto LG, Burke KA, Basili T, Li A, Geyer FC,
et al: IDH2 mutations define a unique subtype of breast cancer with
altered nuclear polarity. Cancer Res. 76:7118–7129. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Steinhilber J, Mederake M, Bonzheim I,
Serinsöz-Linke E, Müller I, Fallier-Becker P, Lemonnier F, Gaulard
P, Fend F and Quintanilla-Martinez L: The pathological features of
angioimmunoblastic T-cell lymphomas with IDH2R172 mutations. Mod
Pathol. 32:1123–1134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhu GG, Nafa K, Agaram N, Zehir A, Benayed
R, Sadowska J, Borsu L, Kelly C, Tap WD, Fabbri N, et al: Genomic
profiling identifies association of IDH1/IDH2 mutation with longer
relapse-free and metastasis-free survival in high-grade
chondrosarcoma. Clin Cancer Res. 26:419–427. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Libera L, Ottini G, Sahnane N, Pettenon F,
Turri-Zanoni M, Lambertoni A, Chiaravalli AM, Leone F, Battaglia P,
Castelnuovo P, et al: Methylation drivers and prognostic
implications in sinonasal poorly differentiated carcinomas. Cancers
(Basel). 13:50302021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Miller JJ, Shih HA, Andronesi OC and
Cahill DP: Isocitrate dehydrogenase-mutant glioma: Evolving
clinical and therapeutic implications. Cancer. 123:4535–4546. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Qi S, Yu L, Li H, Ou Y, Qiu X, Ding Y, Han
H and Zhang X: Isocitrate dehydrogenase mutation is associated with
tumor location and magnetic resonance imaging characteristics in
astrocytic neoplasms. Oncol Lett. 7:1895–1902. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Picca A, Berzero G, Di Stefano AL and
Sanson M: The clinical use of IDH1 and IDH2 mutations in gliomas.
Expert Rev Mol Diagn. 18:1041–1051. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gusyatiner O and Hegi ME: Glioma
epigenetics: From subclassification to novel treatment options.
Semin Cancer Biol. 51:50–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ludwig N, Rao A, Sandlesh P, Yerneni SS,
Swain AD, Bullock KM, Hansen KM, Zhang X, Jaman E, Allen J, et al:
Characterization of systemic immunosuppression by IDH mutant glioma
small extracellular vesicles. Neuro Oncol. 24:197–209. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Phan K, Ng W, Lu VM, McDonald KL, Fairhall
J, Reddy R and Wilson P: Association between IDH1 and IDH2
mutations and preoperative seizures in patients with low-grade
versus high-grade glioma: A systematic review and meta-analysis.
World Neurosurg. 111:e539–e545. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chen R, Ravindra VM, Cohen AL, Jensen RL,
Salzman KL, Prescot AP and Colman H: Molecular features assisting
in diagnosis, surgery, and treatment decision making in low-grade
gliomas. Neurosurg Focus. 38:E22015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Thirumal Kumar D, Jerushah Emerald L,
George Priya Doss C, Sneha P, Siva R, Charles Emmanuel Jebaraj W
and Zayed H: Computational approach to unravel the impact of
missense mutations of proteins (D2HGDH and IDH2) causing
D-2-hydroxyglutaric aciduria 2. Metab Brain Dis. 33:1699–1710.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kim H, Kim SH, Cha H, Kim SR, Lee JH and
Park JW: IDH2 deficiency promotes mitochondrial dysfunction and
dopaminergic neurotoxicity: Implications for Parkinson's disease.
Free Radic Res. 50:853–860. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li J, Lu J, He Y, Wu Y, Wu Y, Song X,
Jiang Y, Tang M, Weng X, Yi W, et al: A new functional IDH2 genetic
variant is associated with the risk of lung cancer. Mol Carcinog.
56:1082–1087. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Li J, He Y, Tan Z, Lu J, Li L, Song X, Shi
F, Xie L, You S, Luo X, et al: Wild-type IDH2 promotes the Warburg
effect and tumor growth through HIF1α in lung cancer. Theranostics.
8:4050–4061. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Park JH, Ku HJ, Lee JH and Park JW: Idh2
deficiency exacerbates acrolein-induced lung injury through
mitochondrial redox environment deterioration. Oxid Med Cell
Longev. 2017:15951032017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Park JH, Ku HJ, Lee JH and Park JW:
Disruption of IDH2 attenuates lipopolysaccharide-induced
inflammation and lung injury in an α-ketoglutarate-dependent
manner. Biochem Biophys Res Commun. 503:798–802. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Holst JM, Enemark MB, Pedersen MB,
Lauridsen KL, Hybel TE, Clausen MR, Frederiksen H, Møller MB,
Nørgaard P, Plesner TL, et al: Proteomic profiling differentiates
lymphoma patients with and without concurrent myeloproliferative
neoplasia. Cancers (Basel). 13:55262021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lemonnier F, Cairns RA, Inoue S, Li WY,
Dupuy A, Broutin S, Martin N, Fataccioli V, Pelletier R, Wakeham A,
et al: The IDH2 R172K mutation associated with angioimmunoblastic
T-cell lymphoma produces 2HG in T cells and impacts lymphoid
development. Proc Natl Acad Sci USA. 113:15084–15089. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wang C, McKeithan TW, Gong Q, Zhang W,
Bouska A, Rosenwald A, Gascoyne RD, Wu X, Wang J, Muhammad Z, et
al: IDH2R172 mutations define a unique subgroup of patients with
angioimmunoblastic T-cell lymphoma. Blood. 126:1741–1752. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Churchill H, Naina H, Boriack R, Rakheja D
and Chen W: Discordant intracellular and plasma
D-2-hydroxyglutarate levels in a patient with IDH2 mutated
angioimmunoblastic T-cell lymphoma. Int J Clin Exp Pathol.
8:11753–11759. 2015.PubMed/NCBI
|
|
80
|
Dupuy A, Lemonnier F, Fataccioli V,
Martin-Garcia N, Robe C, Pelletier R, Poullot E, Moktefi A,
Mokhtari K, Rousselet MC, et al: Multiple ways to detect IDH2
mutations in angioimmunoblastic T-cell lymphoma from
immunohistochemistry to next-generation sequencing. J Mol Diagn.
20:677–685. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ye Y, Ding N, Mi L, Shi Y, Liu W, Song Y,
Shu S and Zhu J: Correlation of mutational landscape and survival
outcome of peripheral T-cell lymphomas. Exp Hematol Oncol.
10:92021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Pareja F, da Silva EM, Frosina D, Geyer
FC, Lozada JR, Basili T, Da Cruz Paula A, Zhong E, Derakhshan F,
D'Alfonso T, et al: Immunohistochemical analysis of IDH2 R172
hotspot mutations in breast papillary neoplasms: Applications in
the diagnosis of tall cell carcinoma with reverse polarity. Mod
Pathol. 33:1056–1064. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Aljohani AI, Toss MS, Kurozumi S, Joseph
C, Aleskandarany MA, Miligy IM, Ansari RE, Mongan NP, Ellis IO,
Green AR and Rakha EA: The prognostic significance of wild-type
isocitrate dehydrogenase 2 (IDH2) in breast cancer. Breast Cancer
Res Treat. 179:79–90. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wang Y, Agarwal E, Bertolini I, Ghosh JC,
Seo JH and Altieri DC: IDH2 reprograms mitochondrial dynamics in
cancer through a HIF-1α-regulated pseudohypoxic state. FASEB J.
33:13398–13411. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Jo VY, Chau NG, Hornick JL, Krane JF and
Sholl LM: Recurrent IDH2 R172X mutations in sinonasal
undifferentiated carcinoma. Mod Pathol. 30:650–659. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Dogan S, Chute DJ, Xu B, Ptashkin RN,
Chandramohan R, Casanova-Murphy J, Nafa K, Bishop JA, Chiosea SI,
Stelow EB, et al: Frequent IDH2 R172 mutations in undifferentiated
and poorly-differentiated sinonasal carcinomas. J Pathol.
242:400–408. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Riobello C, López-Hernández A, Cabal VN,
García-Marín R, Suárez-Fernández L, Sánchez-Fernández P, Vivanco B,
Blanco V, López F, Franchi A, et al: IDH2 mutation analysis in
undifferentiated and poorly differentiated sinonasal carcinomas for
diagnosis and clinical management. Am J Surg Pathol. 44:396–405.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kim YR, Kim KH, Lee S, Oh SK, Park JW, Lee
KY, Baek JI and Kim UK: Expression patterns of members of the
isocitrate dehydrogenase gene family in murine inner ear. Biotech
Histochem. 92:536–544. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
White K, Kim MJ, Han C, Park HJ, Ding D,
Boyd K, Walker L, Linser P, Meneses Z, Slade C, et al: Loss of IDH2
accelerates age-related hearing loss in male mice. Sci Rep.
8:50392018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ku HJ, Park JH, Kim SH and Park JW:
Isocitrate dehydrogenase 2 deficiency exacerbates dermis damage by
ultraviolet-B via ΔNp63 downregulation. Biochim Biophys Acta Mol
Basis Dis. 1864:1138–1147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kim SH and Park JW: IDH2 deficiency
impairs cutaneous wound healing via ROS-dependent apoptosis.
Biochim Biophys Acta Mol Basis Dis. 1865:1655232019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lian CG, Xu Y, Ceol C, Wu F, Larson A,
Dresser K, Xu W, Tan L, Hu Y, Zhan Q, et al: Loss of
5-hydroxymethylcytosine is an epigenetic hallmark of melanoma.
Cell. 150:1135–1146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lv Q, Xing S, Li Z, Li J, Gong P, Xu X,
Chang L, Jin X, Gao F, Li W, et al: Altered expression levels of
IDH2 are involved in the development of colon cancer. Exp Ther Med.
4:801–806. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Teicher BA, Linehan WM and Helman LJ:
Targeting cancer metabolism. Clin Cancer Res. 18:5537–5545. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Park JH, Ku HJ, Lee JH and Park JW: IDH2
deficiency accelerates skin pigmentation in mice via enhancing
melanogenesis. Redox Biol. 17:16–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Liu Z, Gan L, Zhang T, Ren Q and Sun C:
Melatonin alleviates adipose inflammation through elevating
α-ketoglutarate and diverting adipose-derived exosomes to
macrophages in mice. J Pineal Res. 64:e124552018. View Article : Google Scholar
|
|
97
|
Gong F, Gao L and Ding T: IDH2 protects
against nonalcoholic steatohepatitis by alleviating dyslipidemia
regulated by oxidative stress. Biochem Biophys Res Commun.
514:593–600. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chen X, Zhuo S, Xu W, Chen X, Huang D, Sun
X and Cheng Y: Isocitrate dehydrogenase 2 contributes to radiation
resistance of oesophageal squamous cell carcinoma via regulating
mitochondrial function and ROS/pAKT signalling. Br J Cancer.
123:126–136. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lee SJ, Cha H, Lee S, Kim H, Ku HJ, Kim
SH, Park JH, Lee JH, Park KM and Park JW: Idh2 deficiency
accelerates renal dysfunction in aged mice. Biochem Biophys Res
Commun. 493:34–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Lee SH, Jo SH, Lee SM, Koh HJ, Song H,
Park JW, Lee WH and Huh TL: Role of NADP+-dependent isocitrate
dehydrogenase (NADP+-ICDH) on cellular defence against oxidative
injury by gamma-rays. Int J Radiat Biol. 80:635–642. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lee JH, Go Y, Kim DY, Lee SH, Kim OH, Jeon
YH, Kwon TK, Bae JH, Song DK, Rhyu IJ, et al: Isocitrate
dehydrogenase 2 protects mice from high-fat diet-induced metabolic
stress by limiting oxidative damage to the mitochondria from brown
adipose tissue. Exp Mol Med. 52:238–252. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lee JH, Kim SY, Kil IS and Park JW:
Regulation of ionizing radiation-induced apoptosis by mitochondrial
NADP+-dependent isocitrate dehydrogenase. J Biol Chem.
282:13385–13394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Kim SY, Yoo YH and Park JW: Silencing of
mitochondrial NADP(+)-dependent isocitrate dehydrogenase gene
enhances glioma radiosensitivity. Biochem Biophys Res Commun.
433:260–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Li S, Chou AP, Chen W, Chen R, Deng Y,
Phillips HS, Selfridge J, Zurayk M, Lou JJ, Everson RG, et al:
Overexpression of isocitrate dehydrogenase mutant proteins renders
glioma cells more sensitive to radiation. Neuro Oncol. 15:57–68.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
van den Bent MJ, Dubbink HJ, Marie Y,
Brandes AA, Taphoorn MJ, Wesseling P, Frenay M, Tijssen CC, Lacombe
D, Idbaih A, et al: IDH1 and IDH2 mutations are prognostic but not
predictive for outcome in anaplastic oligodendroglial tumors: A
report of the European organization for research and treatment of
cancer brain tumor group. Clin Cancer Res. 16:1597–1604. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Bleeker FE, Atai NA, Lamba S, Jonker A,
Rijkeboer D, Bosch KS, Tigchelaar W, Troost D, Vandertop WP,
Bardelli A and Van Noorden CJ: The prognostic IDH1 (R132) mutation
is associated with reduced NADP+-dependent IDH activity in
glioblastoma. Acta Neuropathol. 119:487–494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Cho HJ, Cho HY, Park JW, Kwon OS, Lee HS,
Huh TL and Kang BS: NADP+-dependent cytosolic isocitrate
dehydrogenase provides NADPH in the presence of cadmium due to the
moderate chelating effect of glutathione. J Biol Inorg Chem.
23:849–860. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhao CB, Shi L, Pu HH and Zhang QY: The
promoting effect of radiation on glucose metabolism in breast
cancer cells under the treatment of cobalt chloride. Pathol Oncol
Res. 23:47–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Oliveira P, Barboza LGA, Branco V,
Figueiredo N, Carvalho C and Guilhermino L: Effects of
microplastics and mercury in the freshwater bivalve corbicula
fluminea (Müller, 1774): Filtration rate, biochemical biomarkers
and mercury bioconcentration. Ecotoxicol Environ Saf. 164:155–163.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Kil IS, Shin SW, Yeo HS, Lee YS and Park
JW: Mitochondrial NADP+-dependent isocitrate dehydrogenase protects
cadmium-induced apoptosis. Mol Pharmacol. 70:1053–1061. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Li C, Xu Y, Li L, Yang X and Wang Y: Acid
stress induces cross-protection for cadmium tolerance of
multi-stress-tolerant Pichia kudriavzevii by regulating
cadmium transport and antioxidant defense system. J Hazard Mater.
366:151–159. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
da Silva Fonseca J, de Barros Marangoni
LF, Marques JA and Bianchini A: Energy metabolism enzymes
inhibition by the combined effects of increasing temperature and
copper exposure in the coral mussismilia harttii. Chemosphere.
236:1244202019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Pan JH, Kim HS, Beane KE, Montalbano AM,
Lee JH, Kim YJ, Kim JH, Kong BC, Kim S, Park JW, et al: IDH2
deficiency aggravates fructose-induced NAFLD by modulating hepatic
fatty acid metabolism and activating inflammatory signaling in
female mice. Nutrients. 10:6792018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chae U, Park JW, Lee SR, Lee HJ, Lee HS
and Lee DS: Reactive oxygen species-mediated senescence is
accelerated by inhibiting Cdk2 in Idh2-deficient conditions. Aging
(Albany NY). 11:7242–7256. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Xu Y, Liu L, Nakamura A, Someya S,
Miyakawa T and Tanokura M: Studies on the regulatory mechanism of
isocitrate dehydrogenase 2 using acetylation mimics. Sci Rep.
7:97852017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Yu W, Dittenhafer-Reed KE and Denu JM:
SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and
regulates mitochondrial redox status. J Biol Chem. 287:14078–14086.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zou X, Zhu Y, Park SH, Liu G, O'Brien J,
Jiang H and Gius D: SIRT3-mediated dimerization of IDH2 directs
cancer cell metabolism and tumor growth. Cancer Res. 77:3990–3999.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Smolková K, Špačková J, Gotvaldová K,
Dvořák A, Křenková A, Hubálek M, Holendová B, Vítek L and Ježek P:
SIRT3 and GCN5L regulation of NADP+- and NADPH-driven reactions of
mitochondrial isocitrate dehydrogenase IDH2. Sci Rep. 10:86772020.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wang T, Zhang F, Peng W, Wang L, Zhang J,
Dong W, Tian X, Ye C, Li Y and Gong Y: Overexpression of NMNAT3
improves mitochondrial function and enhances antioxidative stress
capacity of bone marrow mesenchymal stem cells via the NAD+-Sirt3
pathway. Biosci Rep. 42:BSR202110052022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yu Y, Chen Y, Liu K, Cheng J and Tu J:
SUMOylation enhances the activity of IDH2 under oxidative stress.
Biochem Biophys Res Commun. 532:591–597. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Ogawara Y, Katsumoto T, Aikawa Y, Shima Y,
Kagiyama Y, Soga T, Matsunaga H, Seki T, Araki K and Kitabayashi I:
IDH2 and NPM1 mutations cooperate to activate Hoxa9/Meis1 and
hypoxia pathways in acute myeloid Leukemia. Cancer Res.
75:2005–2016. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Leonardi R, Subramanian C, Jackowski S and
Rock CO: Cancer-associated isocitrate dehydrogenase mutations
inactivate NADPH-dependent reductive carboxylation. J Biol Chem.
287:14615–14620. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Kim H, Lee JH and Park JW: Down-regulation
of IDH2 sensitizes cancer cells to erastin-induced ferroptosis.
Biochem Biophys Res Commun. 525:366–371. 2020. View Article : Google Scholar : PubMed/NCBI
|