Introduction
The American Cancer Society estimated that 17,960
and 2,870 patients in the United States would be newly diagnosed
with, and die as a result of tongue cancer (TC), respectively, in
2021 (1). While advances in
surgical and radiation therapies have increased the average 5-year
survival rate for patients with oropharyngeal cancer to 66%, this
is still markedly lower than the >90% 5-year survival rate of
patients with other cancer types, such as prostate and breast
cancer (1). Most often, patient
death is caused by regional and/or distant metastasis; thus,
metastasis is indicative of a poor prognosis (2–5).
Squamous cell carcinoma (SCC) accounts for approximately 90% of
oral and oropharyngeal malignancies in the United States (6), and commonly develops in the tongue
(7). Notably, the average rate of
nodal metastasis has been reported to be approximately 30% among
patients with TC at initial evaluation, which is markedly higher
than that of patients with other oral cavity cancers (8,9).
Moreover, several studies have identified a high rate of occult
nodal metastasis (20-40%) in patients with TC who showed no
evidence of regional spread during clinical or radiographic
evaluation (8,10–15).
Combined with the fact that the rate of TC has increased among
young women over the last 20-30 years (16–18),
these data highlight the urgent need for a novel approach to
predict metastasis and start treatments at the early stage in
patients with TC.
Angiopoietin-like 4 (ANGPTL4) belongs to a family of
proteins that are structurally similar to the angiopoietins but do
not bind to the angiopoietin receptors, tyrosine kinase with
immunoglobulin-like and EGF-like domain 1 (TIE 1) and
endothelial-specific receptor tyrosine kinase (TEK or TIE 2)
(19). ANGPTL4 is a critical
mediator of transmigration (20),
and promotes trans-endothelial migration by up-regulating the
expression of vascular endothelial adhesion molecule-1 (VCAM-1) in
endothelial cells (21). Increased
VCAM-1 expression, in turn, promotes the attachment of circulating
cancer cells to the vessel walls, and facilitates extravasation and
tumor establishment in other tissues. Clinically, ANGPTL4
expression is correlated with venous and lymphatic invasion in
human SCC (22), and increased
ANGPTL4 gene expression has been reported to promote lung
metastasis in breast cancer (23).
Recently, we identified a robust increase in ANGPTL4 mRNA
expression in lung-metastasized TC cells (24) that we induced to become highly
metastatic to lymph nodes by repeating the passage in which the
cells were injected into a nude mouse tongue and harvested from
metastasized cervical lymph nodes (25). Together, these data suggest that
ANGPTL4 is associated with TC lung metastasis.
To determine whether ANGPTL4 levels are predictive
of TC lung metastasis, we investigated the clinical association of
ANGPTL4 with TC lung metastasis and prognosis of the patients.
Materials and methods
Tissue samples, immunohistochemistry,
and retrospective patient analysis
TC tissue samples were obtained via surgical
resection from 48 Japanese patients (male 27, female 21, ranging
23~91 years old) with TC who complained of mostly uncurable tongue
aphtha or ulcer and were admitted to the Kumamoto University
Hospital between 2003 and 2015. Tissue samples were used with the
approval of the internal ethics committee and all patients provided
written informed consent. ICD-10 codes of TC comprise 2 C020, 28
C021, 7 C022 and 1 C028. In total, 23 of the patients with TC
subsequently had lung metastasis, of which 13 also had lymph node
metastasis, as diagnosed via computed tomography (CT) scans and
pathological tissue examination of resected lymph nodes,
respectively.
Deparaffinized 3-µm-thick tissue sections were
pretreated (20 min) with 0.3% H2O2 in
methanol before treatment (20 min) with Serum-Free Protein Block
(Dako Cytomation). Sections were then incubated (4°C overnight)
with a rabbit polyclonal antibody to ANGPTL4 (20 µg/m1; ab196746,
Abcam) and stained at room temperature for 20 min using EnVision+
solution (Dako Cytomation) and 3,3′-diaminobenzidine
tetrahydrochloride solution containing 0.006%
H2O2, according to the manufacturer's
instructions. Nuclei were counterstained with hematoxylin. After
counting TC cells in five random high-power fields (BX40, Olympus)
in each tissue section, the percentage of TC cells expressing
AGPTL4 was determined. Patients were resultantly classified into
‘low’ (0-30% positivity) and ‘high’ (>30% positivity) ANGPTL4
expression groups according to previous methods (26–28).
Patients' clinical parameters were compared between the two
groups.
Plasma ANGPTL4 assay
Blood samples were collected from 40 patients with
TC who were admitted to the Kumamoto University Hospital between
2003 and 2017. Of these, 20 patients subsequently had lung
metastasis later, and 13 also had lymph node metastasis. One
patient developed lymph node, but not lung, metastasis. Heparinized
plasma samples were obtained by centrifugation, and plasma ANGPTL4
levels were measured using a human ARP4 ELISA kit (ab99974, Abcam)
according to the manufacturer's instructions. Briefly, ANGPTL4
standards and plasma samples were pipetted into each well of a
96-well plate precoated with a human ANGPTL4-specific antibody.
After ANGPTL4 capture, the plate was washed, and biotinylated
anti-human ANGPTL4 antibody was added to each well. The plate was
again washed to remove unbound biotinylated antibody before
HRP-conjugated streptavidin was added to each well. After further
washing, a 3,3,5,5-tetramethylbenzidine (TMB) substrate solution
was added to each well to initiate a color reaction that was
proportional to the original amount of bound ANGPTL4. Stop Solution
was used to change the resultant color from blue to yellow, and the
intensity of the converted color was measured at 450 nm with a
microplate reader (Model 550; Bio-Rad Laboratories).
Statistical analysis
Fisher's exact test was used to analyze potential
associations between ANGPTL4 expression levels and all patients'
clinicopathological parameters except age, which was instead
analyzed via unpaired Student's t-test. Overall patient survival
rates were evaluated using the Kaplan-Meier method and verified
using the log-rank test. The Cox proportional hazards model was
used to calculate the hazard ratio (HR) and 95% confidence interval
(CI) for overall 5-year survival rate of patients in univariate and
multivariate analyses. Plasma ANGPTL4 concentration values were
analyzed using the unpaired Student's t-test. Values were expressed
as the average ± standard deviation (SD) (n=20). An optimal
cut-point of the plasma ANGPTL4 concentration for screening lung
metastasis of TC was identified by bootstrapped ROC analysis under
Liu's method using 1,000 bootstrap samples. The 95% confidence
interval of the optimal cut-point was determined by normal
distribution, under the ROC curve by binomial distribution, and
sensitivity and specificity by binomial distribution. All
statistical analyses were performed using the Stata Statistical
Software: Release 17 for Windows (StataCorp LLC). A P-value
<0.05 was considered to indicate statistically significance.
Results
ANGPTL4 expression in TC cells
To determine whether ANGPTL4 is involved in TC
progression, tongue tissues were examined for ANGPTL4 expression in
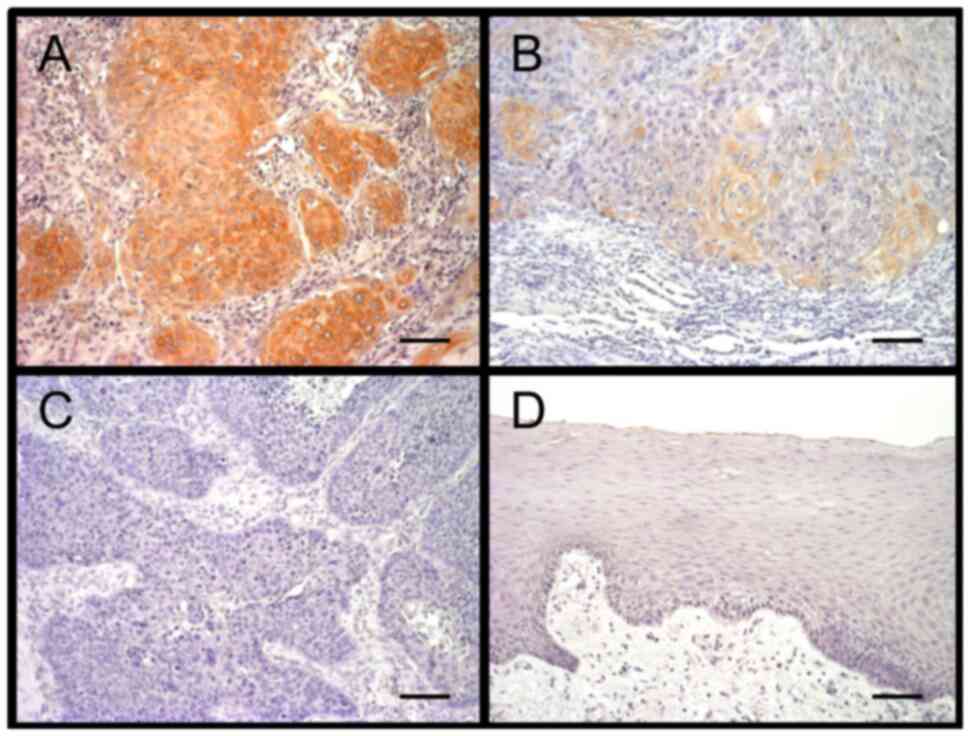
TC cells. Only a subset of TC cells expressed ANGPTL4. Of 48
patients analyzed, 33 (69%) and 15 (31%) were then classified into
‘low’ and ‘high’ ANGPTL4 expression groups, respectively (Fig. 1A-C), according to the percentage of
ANGPTL4-expressing TC cells. ANGPTL4 was not expressed in
noncancerous tongue epithelial cells (Fig. 1D).
Association of TC ANGPTL4 expression
with lung metastasis and poor prognosis
To evaluate the impact of ANGPTL4 on TC lung
metastasis, TC cells from patients with or without subsequent
metastasis were examined for ANGPTL4 expression. No significant
differences in patient age, sex, tumor histological grade, vascular
invasion, or lymph node metastasis were observed between the high
and low ANGPTL4 expression groups (Table I). In addition to patients at
advanced pathological stage (P=0.031) and clinical stage (P=0.043),
a significant greater proportion of patients with lung metastasis
exhibited a high percentage of ANGPTL4 expressing cancer cells as
compared to those without lung metastasis (P=0.029) (Table I). These findings suggested an
association between high level of TC ANGPTL4 expression and lung
metastasis.
 | Table I.Association between cancer-cell
ANGPTL4 expression and patient clinicopathological parameters in
tongue cancer. |
Table I.
Association between cancer-cell
ANGPTL4 expression and patient clinicopathological parameters in
tongue cancer.
|
| ANGPTL4
expression |
|
|---|
|
|
|
|
|---|
| Parameter | Low | High | P-value |
|---|
| Patients, n | 33 | 15 |
|
| Average |
|
|
|
| age ± SD,
years | 61.0±15.4 | 61.8±14.3 | 0.860a |
| Sex, n |
|
|
|
|
Male | 16 | 11 | 0.129b |
|
Female | 17 | 4 |
|
| Histological
gradec, n |
|
|
|
|
Well | 30 | 13 | 0.642b |
|
Moderate | 2 | 2 |
|
|
Poor | 1 | 0 |
|
| Pathological
staged, n |
|
|
|
| T1 | 21 | 2 | 0.031b |
| T2 | 7 | 6 |
|
| T3 | 4 | 5 |
|
| T4 | 1 | 2 |
|
| Clinical
stagee, n |
|
|
|
| I | 21 | 2 | 0.043b |
| II | 5 | 5 |
|
|
III | 6 | 4 |
|
| IV | 1 | 4 |
|
| Vascular invasion,
n |
|
|
|
|
(+) | 4 | 1 | 0.497b |
|
(−) | 29 | 14 |
|
| Lymph node
metastasis, n |
|
|
|
|
(+) | 6 | 7 | 0.077b |
|
(−) | 27 | 8 |
|
| Lung
metastasis,n |
|
|
|
|
(+) | 12 | 11 | 0.029b |
|
(−) | 21 | 4 |
|
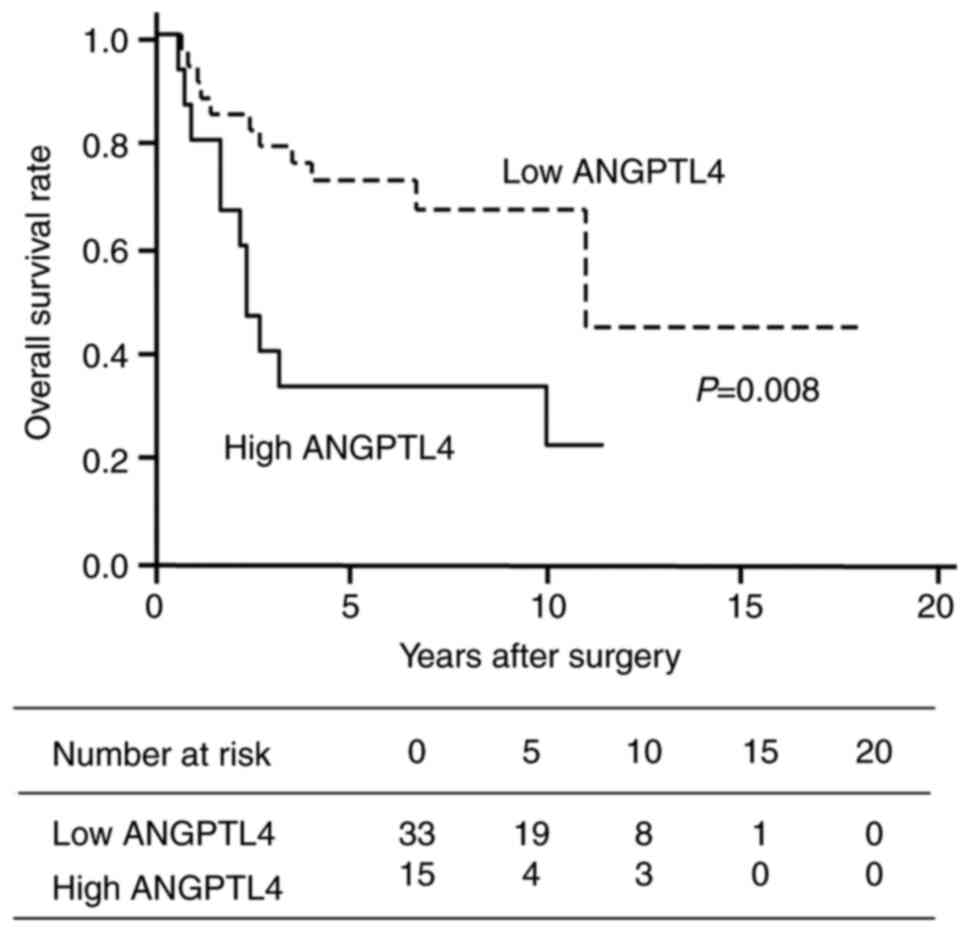
Furthermore, the overall survival (OS) rate of
patients with TC high rate of ANGPTL4 expression was significantly
lower than that of patients with low ANGPTL4 expression (Fig. 2). The overall 5-year survival rate
was more than twofold higher in patients in the low (68%) as
compared to the high (27%) ANGPTL4 expression group (Table II). The median survival period of
the patients in the two groups was 132 and 28 months, respectively.
Univariate and multivariate analyses revealed that the OS rate of
patients with high ANGPTL4-expressing TC was significantly lower
than that of the patients with low ANGPTL4-expressing TC [hazard
ratio (HR), 2.99; 95% confidence interval (CI), 1.34-6.69; P=0.08
and HR, 2.72; 95% CI, 1.14-6.51; P=0.024, respectively]. However,
no significant difference in OS rate was identified in pathological
and clinical stages in multivariate analysis. These results
indicated that high expression of ANGPTL4 in TC cells is an
independent predictor for poor prognosis and may suggest that
ANGPTL4 promotes lung metastasis and poor patient outcomes in
TC.
 | Table II.Univariate and multivariate analysis
of overall survival in 48 patients with tongue cancer. |
Table II.
Univariate and multivariate analysis
of overall survival in 48 patients with tongue cancer.
|
| 5-year survival
rate | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameter | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Pathological stage
(T1-T2/T3-T4) | 0.71/0.08 | 4.45 | 1.98-9.98 | <0.001 | 0.27 | 0.07-1.12 | 0.071 |
| Clinical stage
(I–II/III–IV) | 0.73/0.08 | 5.87 | 2.60-13.3 | <0.001 | 1.05 | 0.23-4.86 | 0.954 |
| Lymph node
metastasis (−/+) | 0.78/0.07 | 6.67 | 2.89-15.4 | <0.001 | 18.9 | 2.17-163 | 0.008 |
| ANGPTL4 expression
(low/high) | 0.68/0.27 | 2.99 | 1.34-6.69 | 0.008 | 2.72 | 1.14-6.51 | 0.024 |
Increase of plasma ANGPTL4
concentrations in TC patients with lung metastasis and poor
prognosis
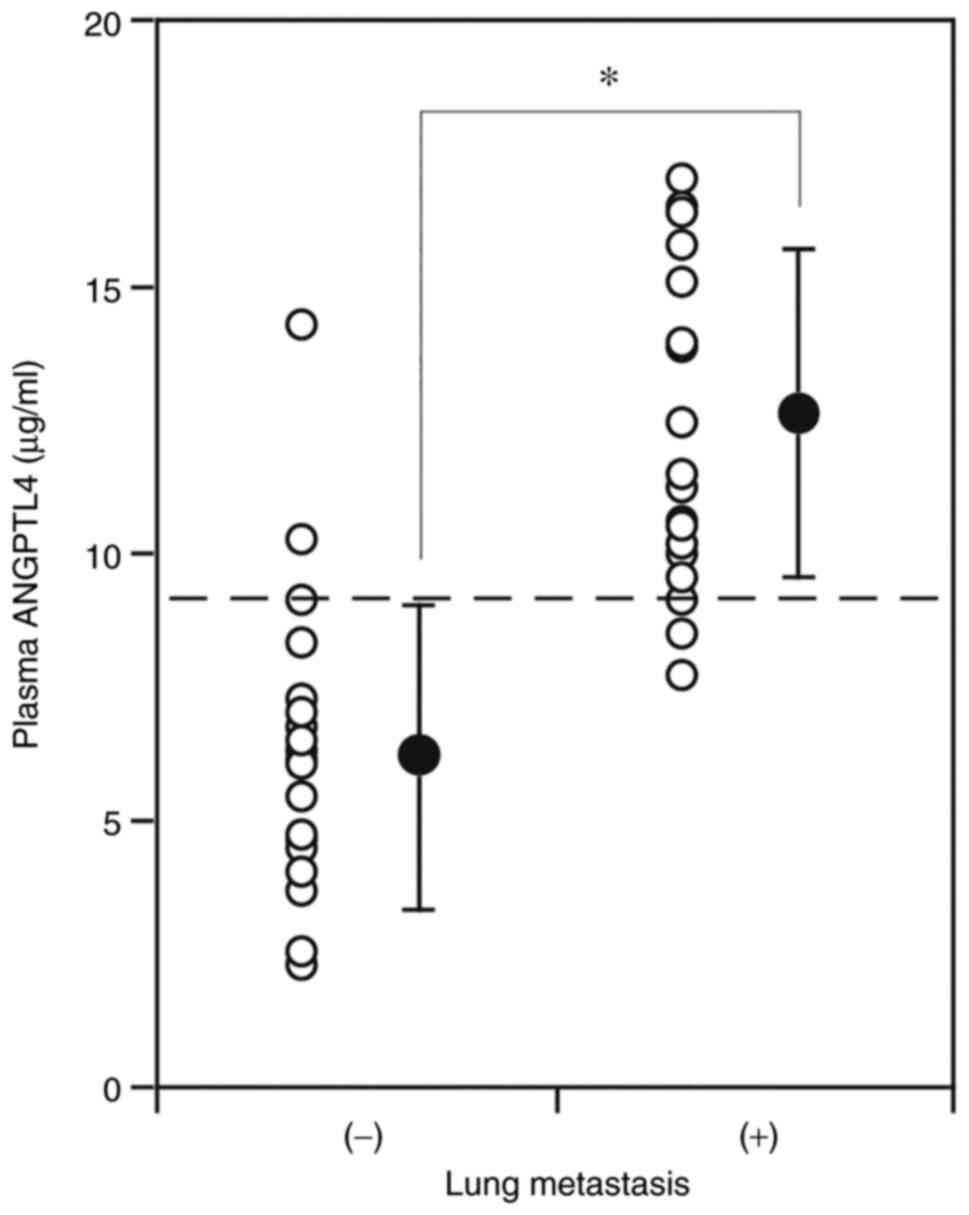
To further explore the relationship between ANGPTL4
and TC lung metastasis, ANGPTL4 concentrations in plasma obtained
on the first day of admission was measured. The plasma ANGPTL4
concentrations of the patients who subsequently developed lung
metastasis later (12.6±3.1 ng/ml) were significantly higher than
those of the patients without lung metastasis (6.2±2.8 ng/ml)
(P<0.001) (Fig. 3). This result
supports the likely association of high ANGPTL4 concentrations with
lung metastasis in TC. ANGPTL4 levels in plasma/serum of controls
(individuals without cancer or other disease) varied in reports.
ANGPTL4 concentrations in the present study were comparable to
those reported by Smart-Halajko et al (29); the median concentration was 7.7
(interquartile range, 5.9 to 11.0) ng/ml.
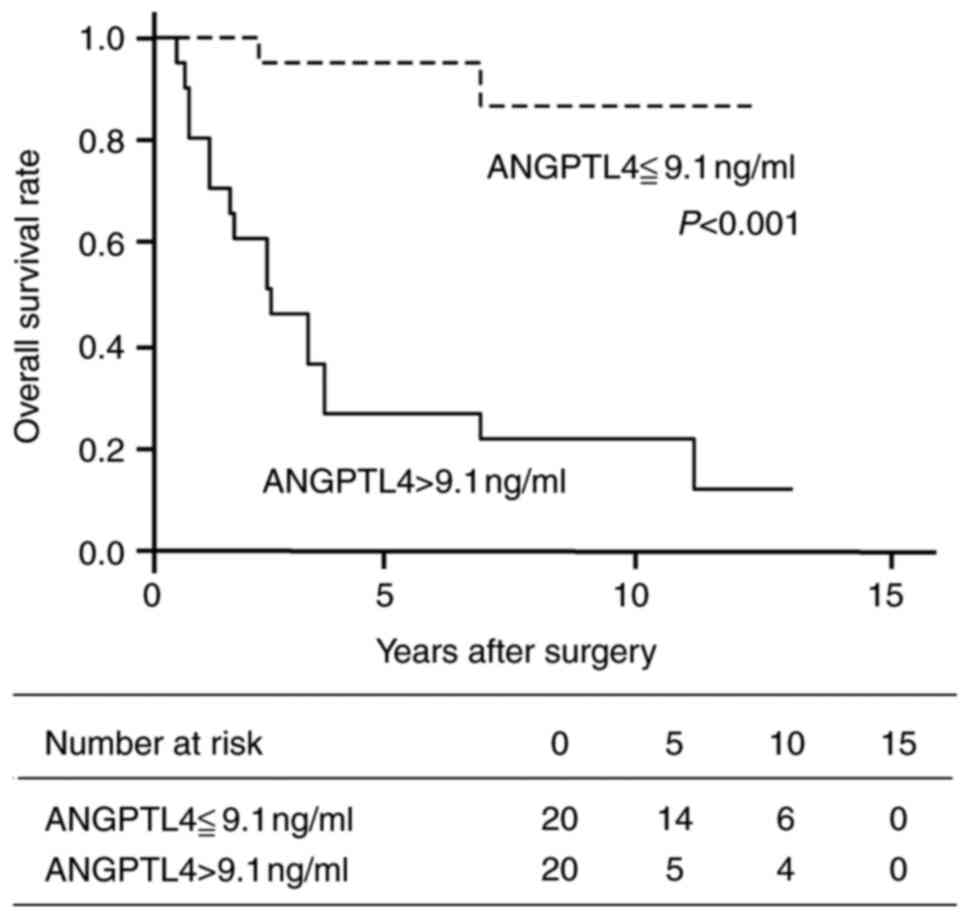
An optimal cut-point of plasma ANGPTL4 concentration
for prediction of TC lung metastasis was determined to be 9.1 ng/ml
(P<0.001; 95% CI: 7.2-10.9) with a sensitivity of 90.0% and
specificity of 90.0% (Fig. 3;
Figs. S1 and S2; Table
SI, Table SII, Table SIII, Table SIV, Table V). The OS rate of patients with
plasma ANGPTL4 concentrations above the cut-point was significantly
lower than that of patients with plasma ANGPTL4 less than or equal
to the cut-point (Fig. 4).
Twenty-eight of the TC patients were examined both cellular
expression and plasma concentration of ANGPTL4. These were no
significant difference in plasma ANGPTL4 concentrations of patients
whose TC cells had high (10.51±4.70 ng/ml; n=9) or low (9.36±4.75
ng/ml; n=19) ANGPTL4 expression (P=0.55).
Discussion
ANGPTL4 has been reported to be involved in various
processes required for cancer progression and metastasis. For
example, ANGPTL4 mediates the induction of neovascularization
(30) and increases cancer cell
proliferation and tumor growth (31,32)
through enabling cancer cells to evade apoptosis and acquire
anoikis resistance (33).
Moreover, ANGPTL4 has been reported to enhance vascular invasion
(22,34,35).
In fact, cancer cell ANGPTL4 expression has been reported to
correlate with lymph node metastasis in esophageal (22,36),
gastric (34), and oral squamous
cell cancers (37). Consistent
with these findings, we herein demonstrated for the first time that
both a high rate of ANGPTL4 expression in TC cells and high plasma
ANGPTL4 concentration of TC patients are associated with lung
metastasis (Table I and Fig. 3).
Only cancer cells in the collected TC tissues
expressed ANGPTL4 (Fig. 1);
accordingly, ANGPTL4 mRNA expression in TC tissues is derived from
TC cells. The low survival rate of TC patients with high cellular
ANGPTL4 protein expression (Fig.
2) agrees with a previous report of poor prognosis in patients
with high ANGPTL4 mRNA expression in TC tissues (38). Given that ANGPTL4 has been shown to
possess multiple cancer promoting effects, the high ANGPTL4
expression rates reported herein in TC, and the high ANGPTL4 mRNA
expression levels previously identified in lung-metastasized breast
cancer cells (23) and TC cells
(24), strongly suggest that
AMPTL4 promotes the metastasis of cancer cells, which is supported
by finding that the OS rate of patients with high TC cell
expression of ANGPTL4 was significantly lower than that of patients
with low TC cell expression in multivariate analysis (Table II). Thus, high ANGPTL4 expression
is likely an indicative marker for lung metastasis and poor
prognosis in TC. Furthermore, OS rate of patients with plasma
ANGPTL4 concentrations above the cut-point 9.1 ng/ml was
significantly lower than that of patients with plasma ANGPTL4
concentrations at or below 9.1 ng/ml (Fig. 4). Using the tentative cut-point
identified in the present study, TC patients with a plasma ANGPTL4
concentration above 9.1 ng/ml may be treated at an earlier stage,
thereby enhancing survival with a lessened risk of lung
metastasis.
Previous studies have shown that serum ANGPTL4
concentrations are approximately threefold higher in patients with
esophageal cancer than in those with benign esophageal diseases.
Furthermore, serum ANGPTL4 levels in esophageal cancer patients are
ameliorated after surgical resection of the cancer tissues
(36). Similarly, serum ANGPTL4
concentrations in patients with renal cell cancer have been
reported to be twofold higher than in healthy controls and are
associated with advanced clinical disease stages and metastasis
(39). It is likely that in both
cases, increased serum concentrations of ANGPTL4 enhanced cancer
cell proliferation and tumor growth (31). Thus, ANGPTL4 concentrations may be
indicative of disease progression in other types of cancers besides
TC lung metastasis. This may also be supported by the fact that
plasma ANGPTL4 levels were higher in cachectic cancer patients than
in weight-stable cancer patients (40).
Hypoxia-inducible factor-1α (HIF-1α) induces ANGPTL4
expression in hepatocellular carcinoma (21) and HIF-1α expression is positively
correlated with advanced clinical stages and metastasis in TC
(41). Thus, it is presumed that
HIF-1α-driven upregulation of TC cell ANGPTL4 secretion in
combination with an increase of ANGPTL4-secreting TC cells
synergistically elevated plasma ANGPTL4 concentrations in TC
patients with lung metastasis (Fig.
3). ANGPTL4 promotes cancer cell growth (31,32)
but there is a delay from an elevation of TC cell ANGPTL4 secretion
to an increase in TC cells, and even a low level of ANGPTL4
secretion can lead to a significant increase in TC cells after a
relatively long time. This may explain why there is no correlation
of plasma ANGPTL4 concentrations with TC cell ANGPTL4 expression
levels. Thus, assessing ANGPTL4 levels in both TC cells and in
plasma may increases confidence in predicting lung metastasis and
poor outcomes of patients with TC.
The present study demonstrated that lung metastasis
and low OS rate in TC are associated with high rates of ANGPTL4
expression in TC cells (Table I
and Fig. 2). This finding suggests
that ANGPTL4 secretion promotes lung metastasis and mortality in TC
patients, leading to increased plasma ANGPTL4 concentrations
(Fig. 3), which induce an increase
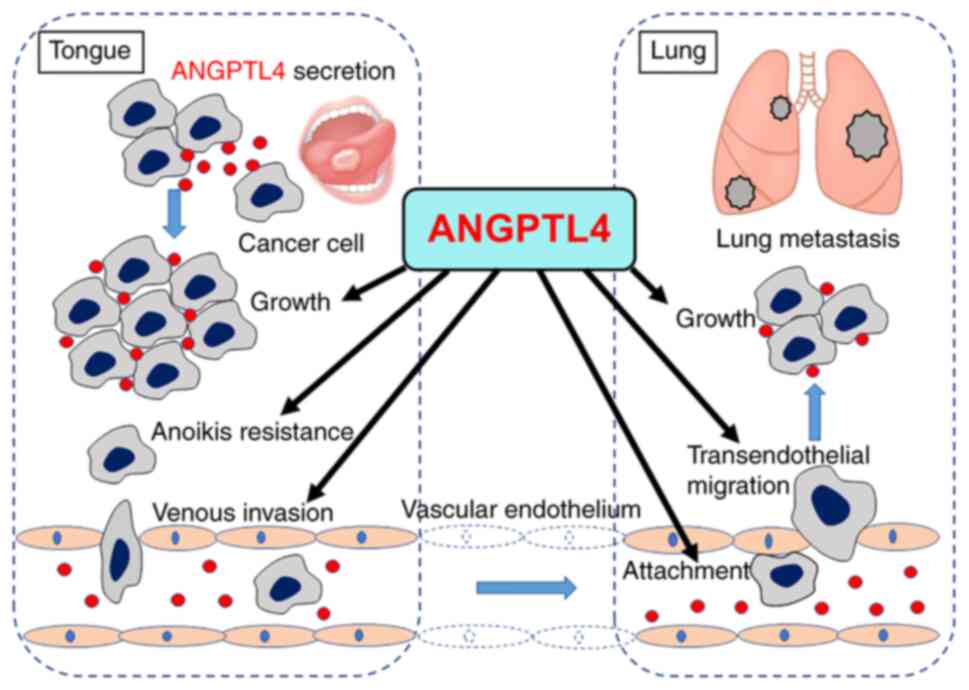
in ANGPTL4-driven cancer promoting effects (20-22,31-35). A
schematic illustrating the possible promoting effects of ANGPTL4 on
TC lung metastasis are presented in Fig. 5. ANGPTL4 secreted from TC cells
promotes TC growth and anoikis resistance in the tongue
facilitating TC cell migration to blood vessels and venous invasion
into the circulation, followed by attachment to endothelial cells
in the lung vessels, transendothelial migration and cancer nest
growth in the lung. Future mechanistic studies to delineate the
role of ANGPTL4 in TC in vitro using TC cells and in vivo using a
mouse model would elucidate the association between ANGPTL4 and
lung metastasis. Interestingly, a higher histological grade is a
well-established predictor of low overall survival rates in TC
(42); however, while the OS rate
of the high ANGPTL4 expression group was much lower than that of
the low ANGPTL4 expression group (Fig.
2), the histological grade exhibited by patients in the two
groups were not different (Table
I). Thus, high rates of ANGPTL4-expressing cancer cells and
high plasma ANGPTL4 concentrations may be reliable predictive
factors for lung metastasis and poor patient prognosis in TC.
ANGPTL4-driven cancer promoting activities suggest a therapeutic
effect of lowering AGPTL4; therefore, AGPTL4 is a potential
therapeutic target for TC.
There are some limitations on the present study.
Data from patients who had heterogeneous therapies were analyzed,
which may affect prognostic evaluation for AGPTL4. Because the
survey consisted of a homogenous ethnic group and in a relatively
small patient number, the generalizability of the present results
is potentially limited.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Dr Ameya Mahayan for
English editing.
Funding
The present study was supported in part by a KAKENHI grant
(17K11912) awarded by the Japan Society for the Promotion of
Science.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TT and TI made substantial contributions to the
conception, design and intellectual content of the present study.
RI and TK performed immunohistochemistry. TI, MY and HO interpreted
immunohistochemical staining results for patient classification.
TT, MY, HO and HN collected tongue cancer tissue samples and
patients' plasmas, and analyzed patients' clinicopathological data.
TT, AI and SK contributed to the ELISA of ANGPTL4 in plasma. TT and
KK performed statistical analysis of data. TT prepared the
manuscript and TI and HN revised it critically for important
intellectual content. MY, HO and HN confirmed the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Written informed consent for tissue usage was
obtained from the patients, and the use of these tissues was
approved by The Internal Review Board of Kumamoto University
Hospital (Rinri no. 1427; Kumamoto, Japan).
Patient consent for publication
Written informed consent for publication was
obtained from the patients.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ANGPTL4
|
angiopoietin-like 4
|
|
SCC
|
squamous cell carcinoma
|
|
TC
|
tongue cancer
|
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kalnins IK, Leonard AG, Sako K, Razack MS
and Shedd DP: Correlation between prognosis and degree of lymph
node involvement in carcinoma of the oral cavity. Am J Surg.
134:450–454. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schuller DE, McGuirt WF, McCabe BF and
Young D: The prognostic significance of metastatic cervical lymph
nodes. Laryngoscope. 90:557–570. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Snow GB, Annyas AA, van Slooten EA,
Bartelink H and Hart AA: Prognostic factors of neck node
metastasis. Clin Otolaryngol Allied Sci. 7:185–192. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grandi C, Alloisio M, Moglia D, Podrecca
S, Sala L, Salvatori P and Molinari R: Prognostic significance of
lymphatic spread in head and neck carcinomas: Therapeutic
implications. Head Neck Surg. 8:67–73. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chi AC, Day TA and Neville BW: Oral cavity
and oropharyngeal squamous cell carcinoma-an update. CA Cancer J
Clin. 65:401–421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sano D and Myers JN: Metastasis of
squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev.
26:645–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ho CM, Lam KH, Wei WI, Lau SK and Lam LK:
Occult lymph node metastasis in small oral tongue cancers. Head
Neck. 14:359–363. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Myers EN and Simental AA Jr: Cancer of the
oral cavity. Cancer of the Head and Neck. 4th edition. Myers EN,
Suen JY, Myers JN and Hanna EY: Saunders; Philadelphia, PA: pp.
279–319. 2003
|
|
10
|
Teichgraeber JF and Clairmont AA: The
incidence of occult metastases for cancer of the oral tongue and
floor of the mouth: Treatment rationale. Head Neck Surg. 7:15–21.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cunningham MJ, Johnson JT, Myers EN,
Schramm VL Jr and Thearle PB: Cervical lymph node metastasis after
local excision of early squamous cell carcinoma of the oral cavity.
Am J Surg. 152:361–366. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fakih AR, Rao RS and Patel AR:
Prophylactic neck dissection in squamous cell carcinoma of oral
tongue: A prospective randomized study. Semin Surg Oncol.
5:327–330. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lydiatt DD, Robbins KT, Byers RM and Wolf
PF: Treatment of stage I and II oral tongue cancer. Head Neck.
15:308–312. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuen AP, Wei WI, Wong YM and Tang KC:
Elective neck dissection versus observation in the treatment of
early oral tongue carcinoma. Head Neck. 19:583–588. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuen AP, Lam KY, Chan AC, Wei WI, Lam LK,
Ho WK and Ho CM: Clinicopathological analysis of elective neck
dissection for N0 neck of early oral tongue carcinoma. Am J Surg.
177:90–92. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Müller S, Pan Y, Li R and Chi AC: Changing
trends in oral squamous cell carcinoma with particular reference to
young patients: 1971-2006. The Emory University experience. Head
Neck Pathol. 2:60–66. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patel SC, Carpenter WR, Tyree S, Couch ME,
Weissler M, Hackman T, Hayes DN, Shores C and Chera BS: Increasing
incidence of oral tongue squamous cell carcinoma in young white
women, age 18 to 44 years. J Clin Oncol. 29:1488–1494. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toporcov TN, Znaor A, Zhang ZF, Yu GP,
Winn DM, Wei Q, Vilensky M, Vaughan T, Thomson P, Talamini R, et
al: Risk factors for head and neck cancer in young adults: A pooled
analysis in the INHANCE consortium. Int J Epidemiol. 44:169–185.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Santulli G: Angiopoietin-like proteins: A
comprehensive look. Front Endocrinol (Lausanne). 5:42014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang RL, Teo Z, Chong HC, Zhu P, Tan MJ,
Tan CK, Lam CR, Sng MK, Leong DT, Tan SM, et al: ANGPTL4 modulates
vascular junction integrity by integrin signaling and disruption of
intercellular VE-cadherin and claudin-5 clusters. Blood.
118:3990–4002. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li H, Ge C, Zhao F, Yan M, Hu C, Jia D,
Tian H, Zhu M, Chen T, Jiang G, et al: Hypoxia-inducible factor 1
alpha-activated angiopoietin-like protein 4 contributes to tumor
metastasis via vascular cell adhesion molecule-1/integrin β1
signaling in human hepatocellular carcinoma. Hepatology.
54:910–919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shibata K, Nakayama T, Hirakawa H, Hidaka
S and Nagayasu T: Clinicopathological significance of
angiopoietin-like protein 4 expression in oesophageal squamous cell
carcinoma. J Clin Pathol. 63:1054–1058. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Minn AJ, Gupta GP, Padua D, Bos P, Nguyen
DX, Nuyten D, Kreike B, Zhang Y, Wang Y, Ishwaran H, et al: Lung
metastasis genes couple breast tumor size and metastatic spread.
Proc Natl Acad Sci USA. 104:6740–6745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka T, Imamura T, Yoneda M, Irie A, Ogi
H, Nagata M, Yoshida R, Fukuma D, Kawahara K, Shinohara M and
Nakayama H: Enhancement of active MMP release and invasive activity
of lymph node metastatic tongue cancer cells by elevated signaling
via the TNF-α-TNFR1-NF-кB pathway and a possible involvement of
angiopoietin-like 4 in lung metastasis. Int J Oncol. 49:1377–1384.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanaka T, Nakayama H, Yoshitake Y, Irie A,
Nagata M, Kawamura K, Takamune Y, Yoshida R, Nakagawa Y, Ogi H, et
al: Selective inhibition of nuclear factor-κB by nuclear factor-κB
essential modulator-binding domain peptide suppresses the
metastasis of highly metastatic oral squamous cell carcinoma.
Cancer Sci. 103:455–463. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koomägi R and Volm M: Expression of Fas
(CD95/APO-1) and Fas ligand in lung cancer, its prognostic and
predictive relevance. Int J Cancer. 84:239–243. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rahman MA, Dhar DK, Yamaguchi E, Maruyama
S, Sato T, Hayashi H, Ono T, Yamanoi A, Kohno H and Nagasue N:
Coexpression of inducible nitric oxide synthase and COX-2 in
hepatocellular carcinoma and surrounding liver: Possible
involvement of COX-2 in the angiogenesis of hepatitis C
virus-positive cases. Clin Cancer Res. 7:1325–1332. 2001.PubMed/NCBI
|
|
28
|
Yoneda M, Imamura R, Nitta H, Taniguchi K,
Saito F, Kikuchi K, Ogi H, Tanaka T, Katabuchi H, Nakayama H and
Imamura T: Enhancement of cancer invasion and growth via the
C5a-C5a receptor system: Implications for cancer promotion by
autoimmune diseases and association with cervical cancer invasion.
Oncol Lett. 17:913–920. 2019.PubMed/NCBI
|
|
29
|
Smart-Halajko MC, Robciuc MR, Cooper JA,
Jauhiainen M, Kumari M, Kivimaki M, Khaw KT, Boekholdt SM, Wareham
NJ, Gaunt TR, et al: The relationship between plasma
angiopoietin-like protein 4 levels, angiopoietin-like protein 4
genotype, and coronary heart disease risk. Arterioscler Thromb Vasc
Biol. 30:2277–2282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ito Y, Oike Y, Yasunaga K, Hamada K,
Miyata K, Matsumoto S, Sugano S, Tanihara H, Masuho Y and Suda T:
Inhibition of angiogenesis and vascular leakiness by
angiopoietin-related protein 4. Cancer Res. 63:6651–6657.
2003.PubMed/NCBI
|
|
31
|
Kim SH, Park YY, Kim SW, Lee JS, Wang D
and DuBois RN: ANGPTL4 induction by prostaglandin E2 under hypoxic
conditions promotes colorectal cancer progression. Cancer Res.
71:7010–7020. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang Z, Xie J, Lin S, Li S, Huang Z, Wang
Y and Ye J: The downregulation of ANGPTL4 inhibits the migration
and proliferation of tongue squamous cell carcinoma. Arch Oral
Biol. 71:144–149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu P, Tan MJ, Huang RL, Tan CK, Chong HC,
Pal M, Lam CR, Boukamp P, Pan JY, Tan SH, et al: Angiopoietin-like
4 protein elevates the prosurvival intracellular
O2(−):H2O2 ratio and confers
anoikis resistance to tumors. Cancer Cell. 19:401–415. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakayama T, Hirakawa H, Shibata K, Abe K,
Nagayasu T and Taguchi T: Expression of angiopoietin-like 4 in
human gastric cancer: ANGPTL4 promotes venous invasion. Oncol Rep.
24:599–606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakayama T, Hirakawa H, Shibata K, Nazneen
A, Abe K, Nagayasu T and Taguchi T: Expression of angiopoietin-like
4 (ANGPTL4) in human colorectal cancer: ANGPTL4 promotes venous
invasion and distant metastasis. Oncol Rep. 25:929–935. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yi J, Pan BZ, Xiong L and Song HZ:
Clinical significance of angiopoietin-like protein 4 expression in
tissue and serum of esophageal squamous cell carcinoma patients.
Med Oncol. 30:6802013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tanaka J, Irié T, Yamamoto G, Yasuhara R,
Isobe T, Hokazono C, Tachikawa T, Kohno Y and Mishima K: ANGPTL4
regulates the metastatic potential of oral squamous cell carcinoma.
J Oral Pathol Med. 44:126–133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Z, Han B, Zhang Z, Pan J and Xia H:
Expression of angiopoietin-like 4 and tenascin C but not cathepsin
C mRNA predicts prognosis of oral tongue squamous cell carcinoma.
Biomarkers. 15:39–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dong D, Jia L, Zhou Y, Ren L, Li J and
Zhang J: Serum level of ANGPTL4 as a potential biomarker in renal
cell carcinoma. Urol Oncol. 35:279–285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Neto NIP, Boldarine VT, Hachul ACL, Oyama
LM, Lima JDCC, Fernandez ES, Otoch JP, de Alcântara PSM, Tokeshi F,
Seelaender MC and Oller do Nascimento CMDP: Association between
ANGPTL-4 and the proinflammatory process in cancer cachexia
patients. Oncotarget. 10:6444–6455. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vasconcelos MG, Vasconcelos RG, Pereira de
Oliveira DH, de Moura Santos E, Pinto LP, da Silveira ÉJ and
Queiroz LM: Distribution of hypoxia-inducible factor-1α and glucose
transporter-1 in human tongue cancers. J Oral Maxillofac Surg.
73:1753–1760. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bell RB, Kademani D, Homer L, Dierks EJ
and Potter BE: Tongue cancer: Is there a difference in survival
compared with other subsites in the oral cavity? J Oral Maxillofac
Surg. 65:229–236. 2007. View Article : Google Scholar : PubMed/NCBI
|