Introduction
Patients with cancer frequently experience
nutritional disorders, with reports showing that these disorders
occur in ~40% of cases (1).
Nutritional disorders in patients with cancer affect the
progression of the cancer and the treatment outcome, and are
considered to worsen the cancer prognosis (2). Weight loss in patients with cancer is
the result of reduced food intake due to a loss of appetite.
However, cachexia secondary to inflammation may also be involved.
Cancer cachexia promotes protein catabolism. This tends to reduce
muscle mass and increase the likelihood of the development of
sarcopenia (3). There have also
been reports that cachexia promotes resting energy expenditure
(REE) (4). Therefore, ensuring
adequate food intake and controlling inflammation is essential in
patients with cancer.
Inflammatory cytokines are elevated in cancer
cachexia due to the immune response of the body and the production
of cytokines by cancer cells (3).
The severity of inflammation differs depending on the type and
stage of the cancer (5,6), and the degree of invasiveness
(7). Inflammatory cytokines, such
as interleukin-6 (IL-6), promote catabolism of muscle proteins
(8) and act on the central nervous
system, leading to a reduced appetite (9). In addition to inflammatory cytokines,
tumors produce proteolysis-inducing factor and lipid-mobilising
factor, which promote the breakdown of skeletal muscle and adipose
tissue (10,11). Increasing skeletal muscle mass by
supplementation with nutrition alone is difficult; it is also
essential to address the inflammatory state (12).
Under inflammatory conditions, C-reactive protein
(CRP) synthesis predominates over albumin synthesis in the liver.
As a consequence, CRP and albumin are considered to reflect the
extent of the inflammation. The Glasgow Prognostic Score (GPS),
advocated by Forrest et al (13), is a simple index involving CRP and
albumin as prognostic predictors based on systemic inflammation
evaluations. The GPS CRP cut-off value has been adjusted from 1.0
to 0.5 mg/dl for patients in Japan and this modified GPS (mGPS) has
been used as the benchmark for cancer cachexia in our previous
study (14).
Eicosapentaenoic acid (EPA) and docosahexaenoic acid
(DHA) in fish oil exert anti-inflammatory effects. EPA is an n-3
fatty acid that antagonises the n-6 fatty acid arachidonic acid
(ARA), and inhibits the production of inflammatory eicosanoids such
as prostaglandin E2 (15). It has been reported that the EPA
and DHA metabolites (resolvins) also have anti-inflammatory effects
(16). Reports indicate that they
inhibit inflammatory cytokine production and skeletal muscle
catabolism (17,18). EPA and DHA are biosynthesized from
the n-3 fatty acid α-linolenic acid; however, their conversion rate
is limited. Therefore, the direct consumption of EPA and DHA is a
more efficient means of increasing EPA and DHA levels in the body
(19). A previous meta-analysis
has reported the use of EPA and DHA to inhibit inflammation in
patients with gastric cancer (20). While clinical studies have often
used high amounts of fish oil to verify the anti-inflammatory
effects of EPA and DHA, Mocellin et al (21) administered low amounts of fish oil
(EPA 360 mg, DHA 240 mg) instead to patients with colorectal cancer
during chemotherapy and reported a reduction in CRP. There is
currently no consensus on the appropriate amount of EPA and DHA for
patients with cancer and cachexia.
Cancer immuno-nutrition therapy using high-energy,
high-protein products containing elevated levels of EPA has been
used for anorexic patients with cancer cachexia to supplement their
reduced food intake. In our previous study, it was reported that a
combination of high-energy, high-protein dietary supplements with 2
g EPA not only increased skeletal muscle and lean body mass, but
also improved the prognosis of patients with an mGPS of 1 or 2 who
were administered prolonged chemotherapy (22). However, Aoyama et al
(23) administered EPA-enriched
dietary supplements to patients with cancer perioperatively and
reported low patient compliance.
It is considered that high patient compliance could
be maintained with low amounts of dietary supplements, in addition
to fish oil capsules. The present study aimed to investigate the
effects of combining relatively low amounts of EPA, DHA and dietary
supplements on the inflammatory and nutritional status of cancer
outpatients.
Materials and methods
Study design
This study was conducted between July 2018 and
December 2019 at Iga City General Hospital (Iga, Japan) as a
single-arm interventional study. The protocol was approved by the
Ethics Review Committee of Iga City General Hospital (approval
date: 4 June, 2018; approval no. 210). Written informed consent was
obtained from all participants prior to the study. The study was
conducted in accordance with the tenets of the Declaration of
Helsinki (Fortaleza revision) and the Ethical Guidelines for
Medical and Health Research Involving Human Subjects (Ministry of
Education, Culture, Sports, Science and Technology and the Ministry
of Health, Labour and Welfare Notice No. 3 of 2014, partially
revised on 28 February 2017). The study was registered in the
University Hospital Medical Information Network Clinical Trials
Registry (UMIN-CTR; 06/07/2018, UMIN000033309).
Subjects
The subjects were recruited from Iga City General
Hospital. The inclusion criteria were an outpatient status, an age
≥20 years, a diagnosis of epithelial cancer, a CRP level ≥0.30
mg/dl, the capability for oral intake and at least 6 months of
expected hospital visits as an outpatient. The exclusion criteria
included food allergies (fish, milk, soya and gelatine) or possible
food allergies, consumption of fish oil supplements, EPA/DHA
products or steroid anti-inflammatories, participation in another
clinical trial, the expectation of developing serious adverse
events during the clinical trial period, being regarded as a
difficult patient and being deemed unsuitable by the principal
investigator. Patients using steroids for purposes other than
anti-inflammatory agents such as antiemetics, were not excluded
from the study. Surgical treatment, the use of EPA/DHA products,
and the use of supplements containing EPA and DHA, and EPA and DHA
fortified products was prohibited during the study period. The use
of steroids as anti-inflammatory agents was prohibited.
Dietary interventions
The subjects were provided with fish oil capsules
(Umi no Genki EPA; Nippon Suisan Kaisha, Ltd.) and dietary
supplements (Enjoy Small High-calorie Jelly; Morinaga Milk Industry
Co., Ltd.) for 8 weeks, consisting of six fish oil capsules (498 mg
EPA and 213 mg DHA) and one small high-calorie jelly (content, 40
g; energy, 100 kcal; protein, 5 g) once per day. The high-calorie
jelly contained collagen peptides and whey proteins. Additionally,
valine, leucine and isoleucine were added as branched-chain amino
acids (BCAAs). The test food (fish oil capsules and small
high-calorie jelly) was provided directly by the dietitian at the
time of nutritional guidance. The time of the test food intake was
not stipulated and subjects were asked to record their daily intake
in a diary. The test food intake rate was calculated as [100×
actual intake (number)/planned intake (number)]. Nutritional
guidance was provided prior to the study (baseline) and at 4 and 8
weeks after commencement of the study. A 3-day dietary survey was
conducted prior to the study period.
Measurements
Body weight, body composition, REE and vital sign
measurements (blood pressure, heart rate and body temperature),
blood tests, and blood biochemical examinations were performed at
baseline (week 0), and after 4 and 8 weeks of intake. CRP, IL-6,
albumin and pre-albumin levels were measured as
inflammation-related indicators. The inflammation-related
indicators were measured by a clinical examination company (SRL,
Inc.). Body composition was measured using a body composition
analyzer (In Body S10; InBody Japan, Inc.), and REE was measured
using an indirect calorimeter (MedGem; MP Japan Co., Ltd.).
Statistical analysis
The target number of cases was set to 20, which is
considered the maximum number of cases collected during the study.
The test food intake rate is expressed as the mean ± standard
error. Continuous variables were analyzed using linear mixed model
analysis with a Tukey-Kramer post hoc test, and were expressed as
the least-mean square values ± standard error. Additionally,
individual data on inflammation-related indicators are shown as box
plots. P<0.05 was used to indicate a statistically significant
difference. The observation time point was defined as a fixed
effect and the subject ID as a random effect. Logarithmic
transformation was performed when continuous variables were
non-normally distributed. Pearson's product-moment correlation
coefficient was used to evaluate the inflammation-related
indicators. All statistical analyses were performed using JMP13.2.1
software (SAS Institute, Inc.).
Results
Subject background
characteristics
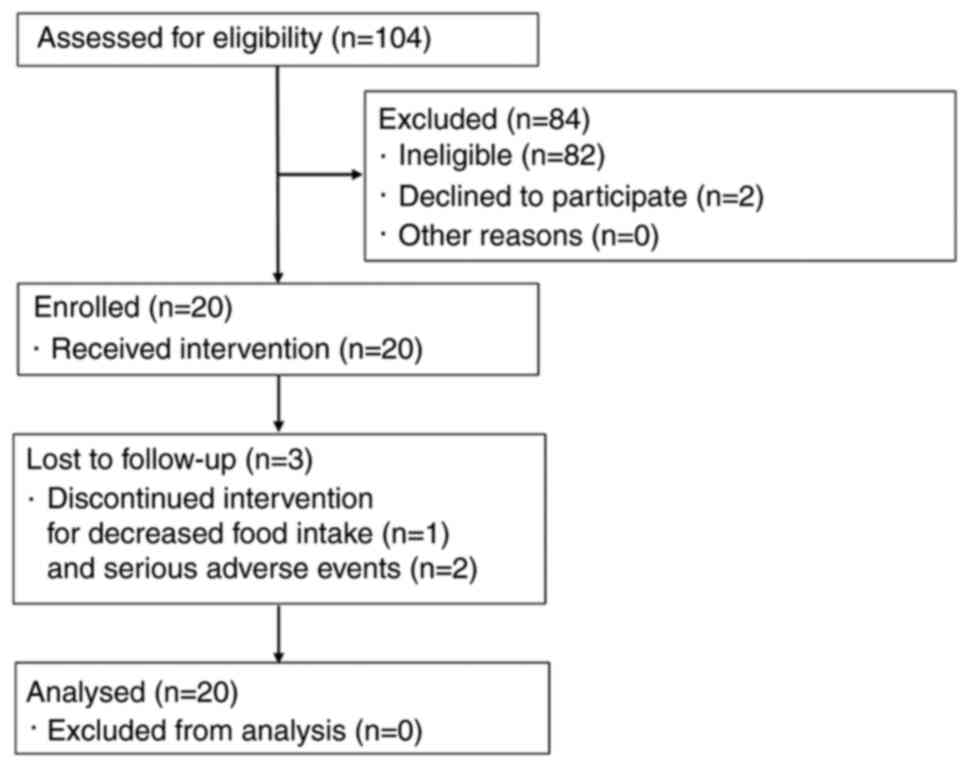
A flow diagram of participants is shown in Fig. 1. Almost all ineligible patients had
a CRP level <0.30 mg/dl. In total, 20 patients were enrolled in
this study. Among them, 17 patients completed the trial, while 1
patient violated compliance requirements due to decreased food
intake, and 2 patients withdrew due to serious adverse events. All
20 subjects were included in the efficacy analysis set. The diaries
of 2 patients were not collected due to drop-out. These were
considered compliance violations. A single patient violated the
eligibility criteria due to the use of EPA preparations. In
addition, 2 patients had findings suggestive of inflammation
secondary to infection due to their sudden increase in CRP levels
at week 8 and their good response to antibacterial drugs, and were
excluded from the subgroup evaluation of inflammation-related
indicators. In 1 of these excluded cases, the observed adverse
events included lower extremity peripheral neuropathy, high
cholesterol, cracked skin on the hands, reduced magnesium, chills,
fever, elevated CRP level, slightly elevated white blood cell
count, and reduced pre-albumin level. In the other case, adverse
events included malaise, peripheral neuropathy, cheilitis,
stomatitis, elevated CRP level and dysphagia. A causal relationship
between such adverse events and test food was ruled out.
The baseline characteristics of the subjects and the
results of the 3-day dietary survey are shown in Table I. The median age of the subjects
was 72 years (range, 44–94 years). The types of cancer included
epithelial colorectal (n=16), stomach (n=2), lung (n=1) and
pancreatic (n=1) cancer. In total, 17 subjects underwent
chemotherapy at baseline. Test food intake rates of fish oil
capsules and dietary supplements were 97.3±0.9% (n=18) and
95.6±1.1% (n=18), respectively.
 | Table I.Subject baseline characteristics. |
Table I.
Subject baseline characteristics.
| Characteristic | Value |
|---|
| Age, years | 69.6±2.6 |
| Sex (male:female),
n | 17:3 |
| Height, cm | 165.2±1.6 |
| Stage (III:IV),
n | 3:17 |
| Energy intake,
kcal/day | 1909±108.2 |
| Protein intake,
g/day | 72.3±4.7 |
| Fat intake,
g/day | 66.0±6.2 |
| Carbohydrate
intake, g/day | 239±10 |
| EPA intake,
mg/day | 320±94 |
| DHA intake,
mg/day | 598±143 |
| Vitamin D intake,
µg/day | 9.0±1.4 |
| Zinc intake,
mg/day | 8.1±0.6 |
Body composition, vital signs and
REE
The changes in body weight and body composition are
shown in Table II. No significant
changes were observed in any of the measurements. The changes in
vital signs (blood pressure, heart rate and body temperature) and
REE are shown in Table III. No
significant changes were observed in any of the measurements.
 | Table II.Changes in body weight and body
composition. |
Table II.
Changes in body weight and body
composition.
| Test element | Week 0 (n=20) | Week 4 (n=18) | Week 8 (n=17) | P-value |
|---|
| Body weight,
kg | 61.5±2.6 | 62.1±2.7 | 62.9±2.7 | 0.1367 |
| BMI, kg/m2 | 22.6±1.0 | 22.8±1.0 | 23.1±1.0 | 0.1566 |
| Muscle mass,
kg | 43.9±1.2 | 43.7±1.2 | 44.6±1.2 | 0.4374 |
| Body fat
percentage | 23.1±2.0 | 24.2±2.0 | 23.8±2.0 | 0.2003 |
| Body water, l | 34.3±0.9 | 34.2±0.9 | 34.9±0.9 | 0.3804 |
| ECW/TBW | 0.391±0.003 | 0.391±0.003 | 0.395±0.003 | 0.2527 |
| TBW/FFM | 73.8±0.1 | 73.8±0.1 | 73.8±0.1 | 0.7479 |
| Skeletal muscle
mass, kg | 25.3±0.8 | 25.1±0.8 | 25.5±0.8 | 0.7369 |
| Protein amounts,
kg | 9.0±0.3 | 9.0±0.3 | 9.1±0.3 | 0.6595 |
| Bone mineral
content, kg | 2.55±0.09 | 2.58±0.09 | 2.69±0.09 | 0.1173 |
| Body fat, kg | 15.0±2.1 | 15.8±2.1 | 15.7±2.1 | 0.0629 |
| Intracellular
water, l | 20.9±0.6 | 20.8±0.6 | 21.1±0.6 | 0.7540 |
| Extracellular
water, l | 13.4±0.4 | 13.4±0.4 | 13.8±0.4 | 0.1109 |
 | Table III.Changes in vital signs and REE. |
Table III.
Changes in vital signs and REE.
| Measured
outcomes | Week 0 (n=20) | Week 4 (n=19) | Week 8 (n=17) | P-value |
|---|
| Systolic blood
pressure, mmHg | 129±3 | 135±3 | 129±3 | 0.1670 |
| Diastolic blood
pressure, mmHg | 80±2 | 77±2 | 77±2 | 0.4093 |
| Heart rate,
bpm | 87±3 | 87±3 | 85±3 | 0.8706 |
| Body temperature,
°C | 36.4±0.1 | 36.4±0.1 | 36.4±0.1 | 0.9705 |
| REE, kcala | 1278.8±55.5 | 1293.2±60.0 | 1345.7±61.9 | 0.5708 |
Clinical test items
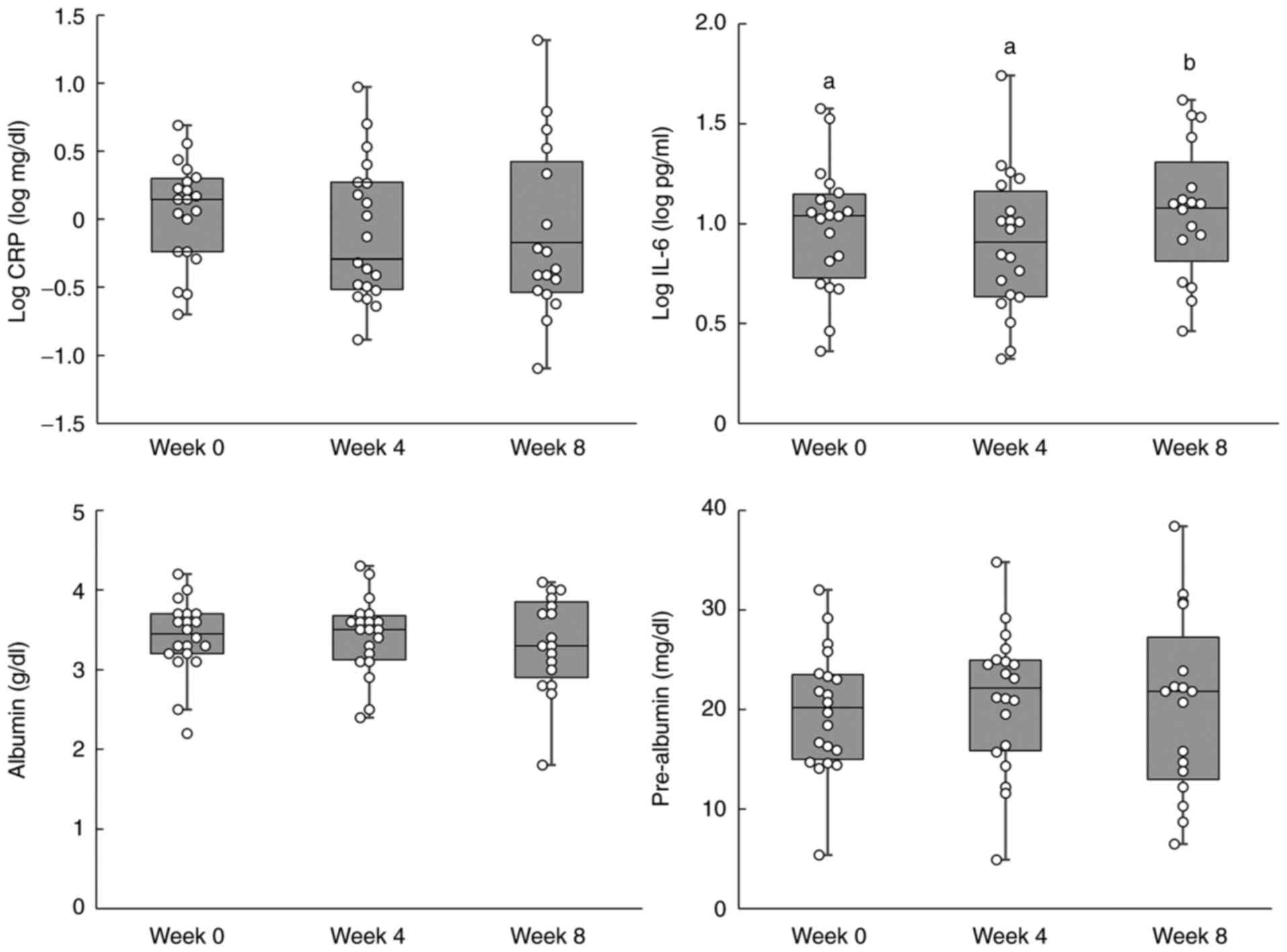
The results for the inflammation-related indicators
are shown in Table IV, and the
individual data are shown in Fig.
2. No significant differences were observed in log-transformed
(log) CRP levels. A significant increase in log IL-6 levels was
observed after 8 weeks. Furthermore, no significant differences
were observed in albumin and pre-albumin levels. The results of the
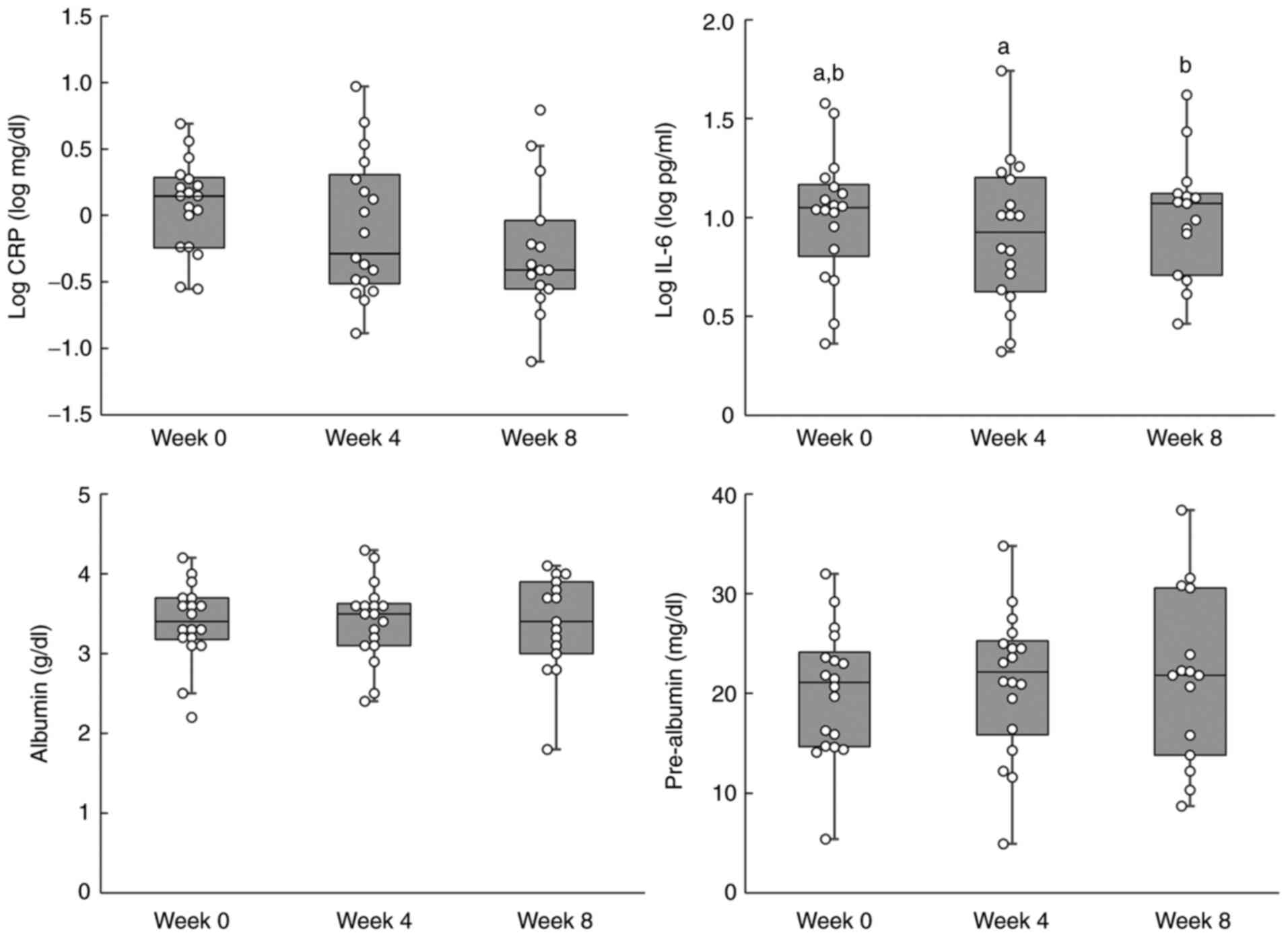
subgroup analysis, which excluded the 2 cases with suspected acute
inflammation secondary to infection, are shown in Table V. Furthermore, individual data for
the sub-group analysis are shown in Fig. 3. The log CRP level showed a
significant trend in the subgroup analysis, which was performed
using a linear mixed model. A decreasing trend was also observed in
the subgroup analysis performed using the Tukey-Kramer post-hoc
test (week 0 vs. week 8; P=0.0632). However, post-hoc analyses are
not routinely performed when no significant differences are
observed in data. A significant difference in log IL-6 levels was
not observed between weeks 0 and 8, although a significant increase
was observed between weeks 4 and 8. The blood tests and biochemical
examination results are shown in Table VI. With regard to urea nitrogen
levels, significant changes were observed in urea nitrogen levels
between weeks 0 and 4, although no significant differences were
observed between weeks 0 and 8. The correlation coefficients of
inflammation-related indicators are listed in Table VII. A positive correlation was
observed between log CRP and log IL-6, and between albumin and
pre-albumin, while a negative correlation was observed between log
CRP and albumin, and between log CRP and pre-albumin.
 | Table IV.Inflammation-related indicators. |
Table IV.
Inflammation-related indicators.
| Test element | Week 0 (n=20) | Week 4 (n=20) | Week 8 (n=17) | P-value |
|---|
| Log CRP, log
mg/dl | 0.05±0.12
(1.12) | −0.10±0.12
(0.79) | −0.04±0.12
(0.91) | 0.4338 |
| Log IL-6, log
pg/dl | 0.98±0.08a
(9.5) | 0.90±0.08a
(7.9) | 1.13±0.08b
(13.5) | 0.0072 |
| Albumin, g/dl | 3.41±0.12 | 3.43±0.12 | 3.26±0.12 | 0.1117 |
| Pre-albumin,
mg/dl | 19.89±1.68 | 21.05±1.68 | 19.65±1.74 | 0.5205 |
 | Table V.Inflammation-related indicators in
subgroup analysis. |
Table V.
Inflammation-related indicators in
subgroup analysis.
| Test element | Week 0 (n=18) | Week 4 (n=18) | Week 8 (n=15) | P-value |
|---|
| Log CRP, log
mg/dl | 0.078±0.111
(1.20) | −0.093±0.111
(0.81) | −0.173±0.117
(0.67) | 0.0637 |
| Log IL-6, log
pg/dl | 1.01±0.08a,b
(10.2) | 0.91±0.08a
(8.1) | 1.08±0.08b
(12.0) | 0.0324 |
| Albumin, g/dl | 3.39±0.13 | 3.41±0.13 | 3.30±0.13 | 0.4156 |
| Pre-albumin,
mg/dl | 20.14±1.79 | 21.13±1.79 | 20.81±1.85 | 0.7344 |
 | Table VI.Blood tests and biochemical
examinations. |
Table VI.
Blood tests and biochemical
examinations.
| Test element | Week 0 (n=20) | Week 4 (n=20) | Week 8 (n=17) | P-value |
|---|
| White blood cell
count, cells ×103/µl | 5.5±0.5 | 5.8±0.5 | 6.5±0.5 | 0.2694 |
| Red blood cell
count, cells ×106/µl | 4.06±0.13 | 4.09±0.13 | 3.94±0.13 | 0.2471 |
| Haemoglobin,
g/dl | 11.9±0.4 | 12.2±0.4 | 11.9±0.4 | 0.6102 |
| Haematocrit, % | 36.2±1.1 | 37.0±1.1 | 35.9±1.1 | 0.3331 |
| Platelet count,
cells ×103/µl | 214±20 | 214±20 | 208±21 | 0.8996 |
| Total protein,
g/dl | 6.4±0.1 | 6.4±0.1 | 6.1±0.1 | 0.1170 |
| Log AST, log
IU/l | 1.48±0.04 | 1.51±0.04 | 1.49±0.05 | 0.6123 |
|
| (30) | (32) | (31) |
|
| Log ALT, log
IU/l | 1.39±0.05 | 1.38±0.05 | 1.39±0.06 | 0.9942 |
|
| (25) | (24) | (25) |
|
| Urea nitrogen,
mg/dl | 14.5±1.5a | 17.6±1.5b | 15.9±1.5a,b | 0.0346 |
| Log creatinine, log
mg/dl | −0.08±0.02 | −0.07±0.02 | −0.08±0.03 | 0.7352 |
|
| (0.83) | (0.85) | (0.83) |
|
| Log triglycerides,
log mg/dlc | 2.13±0.05 | 2.09±0.05 | 2.14±0.05 | 0.4444 |
|
| (135) | (123) | (138) |
|
| Total cholesterol,
mg/dlc | 212±13 | 231±13 | 214±13 | 0.1786 |
| LDL cholesterol,
mg/dl) | 132±11 | 146±11 | 136±11 | 0.1118 |
| HDL cholesterol,
mg/dl | 51±3 | 57±3 | 54±4 | 0.0897 |
| 25-OHVD, ng/ml | 13.2±1.2 | 13.4±1.2 | 12.4±1.3 | 0.1948 |
| Total carnitine,
µmol/l | 45.5±4.8 | 50.8±4.8 | 48.1±4.9 | 0.3456 |
| Free carnitine,
µmol/l | 36.1±3.8 | 39.5±3.8 | 38.4±3.9 | 0.4614 |
| Acyl carnitine,
µmol/l | 9.5±1.2 | 11.0±1.2 | 9.3±1.3 | 0.4554 |
 | Table VII.Correlation between
inflammation-related indicators. |
Table VII.
Correlation between
inflammation-related indicators.
| Variable | vs. variable | Correlation
coefficient | P-value |
|---|
| Log CRP | Log IL-6 | 0.7323 | 0.0008 |
| Log CRP | Alb | −0.5585 | 0.0198 |
| Log CRP | PreAlb | −0.7117 | 0.0014 |
| Log IL-6 | Alb | −0.3852 | 0.1268 |
| Log IL-6 | PreAlb | −0.2952 | 0.2499 |
| Alb | PreAlb | 0.6359 | 0.0061 |
Serum fatty acid composition
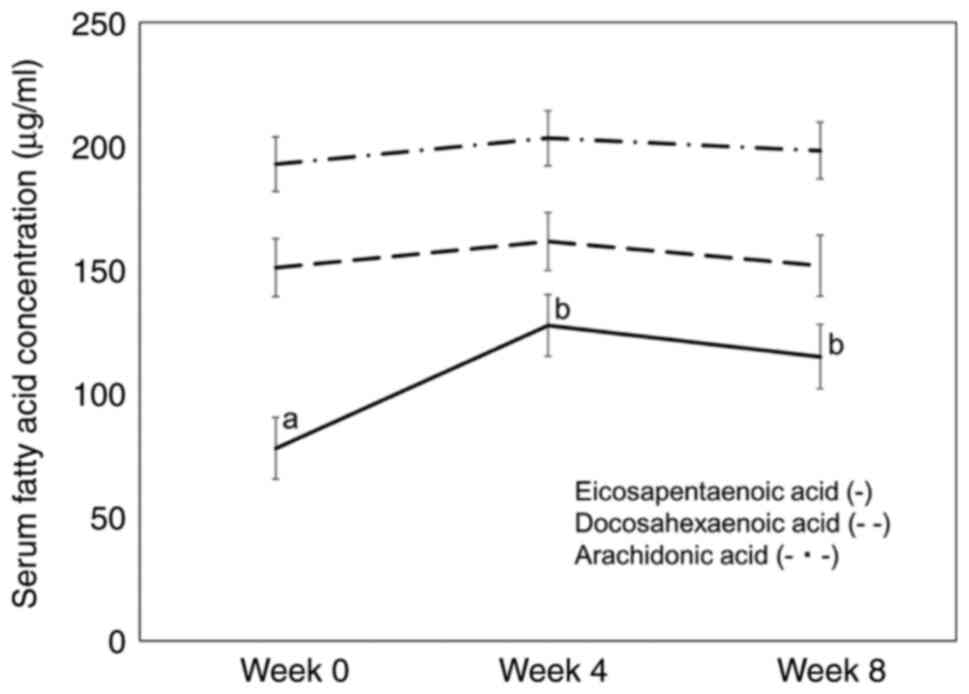
The main changes in fatty acid composition (%) are
shown in Table VIII. Serum EPA,
docosapentaenoic acid, and EPA/ARA ratios significantly increased
at weeks 4 and 8 compared with week 0. Serum oleic acid level
significantly decreased at week 4 compared with that at week 0 and
8. Serum EPA, DHA and ARA concentrations are shown in Fig. 4. Serum EPA concentrations
significantly increased in weeks 4 and 8 compared with those in
week 0.
 | Table VIII.Serum fatty acid composition. |
Table VIII.
Serum fatty acid composition.
| Test element | Week 0 (n=20) | Week 4 (n=20) | Week 8 (n=17) | P-value |
|---|
| Myristic acid,
% | 0.97±0.12 | 0.85±0.12 | 0.90±0.12 | 0.3862 |
| Palmitic acid,
% | 23.52±0.44 | 23.00±0.44 | 23.48±0.45 | 0.2569 |
| Palmitoleic acid,
% | 2.11±0.17 | 1.98±0.17 | 2.07±0.17 | 0.4703 |
| Stearic acid,
% | 7.17±0.15 | 7.02±0.15 | 7.25±0.16 | 0.1033 |
| Oleic acid, % | 22.33±0.70a | 19.80±0.70b | 21.35±0.73a | 0.0004 |
| Linoleic acid,
% | 25.87±0.80 | 27.15±0.80 | 25.69±0.84 | 0.1458 |
| Linolenic acid,
% | 0.82±0.05 | 0.79±0.06 | 0.74±0.06 | 0.3038 |
| Dihome-γ-linolenic
acid, % | 1.10±0.07 | 1.01±0.07 | 0.99±0.07 | 0.0992 |
| ARA, % | 5.42±0.36 | 5.74±0.36 | 5.69±0.37 | 0.4062 |
| EPA, % | 2.19±0.37a | 3.71±0.37b | 3.24±0.38b | <0.0001 |
| Docosapentaenoic
acid, % | 0.58±0.04a | 0.74±0.04b | 0.72±0.04b | 0.0002 |
| DHA, % | 4.21±0.34 | 4.61±0.34 | 4.30±0.35 | 0.2500 |
| Other, % | 3.71±0.12 | 3.62±0.12 | 3.57±0.12 | 0.1167 |
| EPA/ARA | 0.39±0.06a | 0.66±0.06b | 0.61±0.06b | <0.0001 |
Discussion
No significant changes in log CRP, albumin and
pre-albumin levels were observed for patients following intake of
the test food for 8 weeks. Log IL-6 levels significantly increased
between week 0 and week 8. The test food intake rates remained high
and the general nutritional status of the patients was well
maintained. CRP levels in the subgroup analysis showed a downward
trend at week 8 using linear mixed-model analysis with a
Tukey-Kramer post hoc test. In addition, a significant difference
from week 0 was not observed at week 8 for log IL-6 levels. These
findings indicate that although suppression of acute inflammation
secondary to infection is difficult with the study intake amounts
of EPA and DHA, they may exert suppressive effects on mild chronic
inflammation in patients with epithelial cancer without
infection.
It has been suggested that the anti-inflammatory
effects of n-3 fatty acids present in EPA and DHA in patients with
colorectal cancer may differ depending on the factors involved in
the perioperative period and during chemotherapy. A meta-analysis
reported a reduction in IL-6 inflammatory cytokines and an increase
in albumin in the overall analysis. The intake of a relatively
small amount of EPA and DHA during chemotherapy decreased CRP
levels, but not IL-6 levels (24).
By contrast, another meta-analysis involving patients with gastric
cancer, in which 8 out of the 9 included trials involved surgical
patients, found no changes in CRP levels but a reduction in IL-6
levels (20).
IL-6 is known to increase markedly in the
perioperative period due to invasive surgery (7). Intake of high-dose fish oil is
considered essential to inhibit IL-6 production. By contrast,
chemotherapy causes inflammation due to the immune response of the
body to cancer and the inflammatory substances produced by the
cancer cells. If cancer cells can be controlled with chemotherapy,
then IL-6 production is assumed to be relatively suppressed,
resulting in the persistence of a chronic mild inflammatory state.
However, even when inflammation is mild, chronic persistence may
result in muscle mass reduction. Therefore, it is preferable to
suppress inflammation completely. In these instances, even a
relatively small amount of fish oil was assumed to exert an
anti-inflammatory effect.
Cancer patients are at a high risk of infection due
to their reduced immunity during both the perioperative and
chemotherapy periods. CRP levels are markedly elevated during
infection (25,26). Consequently, when using CRP as an
inflammation indicator, it is essential to distinguish primary
inflammation from that secondary to infection. This lack of
distinction is considered to be one of the reasons for the
difficulty in verifying the inflammatory suppression effects of
fish oil. As in the present clinical study, the present study had
some cases with suspected acute inflammation secondary to either an
infection or acute exacerbation of cancer. Only 2 cases were
excluded in the subgroup analysis due to their good response to
antibacterial drugs. However, it cannot be denied that the
infection might have affected CRP levels in the other cases. In the
present study, a downward trend in log CRP was observed after
excluding the 2 cases in which infection was strongly suspected.
This suggested that the intake of fish oil may work to suppress
mild chronic inflammation; however, the difference was not
statistically significant. In this study, the suppression of acute
inflammation caused by infection was considered challenging. Hence,
further research is required to investigate the effects of fish oil
on the suppression of inflammation triggered by cancer cells during
chemotherapy.
A number of the subjects from the present study
consumed fish daily; hence, the serum levels of EPA and DHA were
relatively high. Particularly, the levels of DHA were high, and
they did not increase with the intake of fish oil capsules. Fish
oil capsules have a high EPA content, and serum EPA levels were
significantly elevated. Both EPA and DHA are known to have
anti-inflammatory effects, although in this study, it was assumed
that the effects were primarily elicited by EPA. Plasma DHA levels
were maintained at a relatively high state from baseline, which may
reflect a constant anti-inflammatory effect. Antagonism of ARA,
which promotes inflammation, is known to be an anti-inflammatory
action of EPA (15). Therefore,
the EPA/ARA and ARA/EPA ratios are considered useful inflammatory
indicators (27). Although no
significant changes in ARA were observed in this study, the EPA/ARA
ratio was found to be significantly elevated. EPA and DHA may also
exert anti-inflammatory actions through metabolites such as
resolvins that also have anti-inflammatory actions (16). These actions are considered to lead
to a reduction in inflammatory cytokines and CRP levels. A study by
Mocellin et al (21) found
an increase in plasma EPA and DHA levels, and a decrease in ARA
levels, when lower amounts of fish oil (360 mg EPA and 240 mg DHA)
were ingested compared with the value observed in the present
study. Given that the baseline plasma EPA level was low and the ARA
level was high, it is assumed that the diets of their subjects
contained mainly n-6 fatty acids and that the elevated plasma DHA
and reduced ARA levels may have also contributed to the reduction
of CRP. Hamazaki et al (28) reported that a relatively low amount
of fish oil (600 mg EPA and 260 mg DHA) decreased serum
triglyceride and remnant-like particle-cholesterol in
normolipidemic and hypertriglyceridemic subjects. In the study, the
participants habitually consumed fish, and the intake of DHA was
estimated at 670–830 mg/day. In addition, the EPA levels in red
blood cells increased, but the DHA levels did not. The relatively
small amount of fish oil (~500 mg EPA) may be physiologically
meaningful for mild metabolic disorders, including inflammation, in
Japanese patients who habitually consume fish.
In a comparison analysis of 3 studies focusing on
reducing triglycerides levels by plasma EPA concentrations, the
baseline values increased from 7.9, 28.1 and 63.6 µg/ml to 108.9,
123.8 and 185.0 µg/ml, respectively with the administration of a
2-g EPA preparation (29–32). In the present study, as a number of
the subjects consumed fish daily, baseline plasma EPA
concentrations were high, and DHA intake levels, including intake
from fish oil capsules, were also high. This suggests that high
plasma EPA concentrations could be maintained even with a
relatively small amount of EPA supplementation. The high daily
intake of fish was also considered to be one of the contributing
factors to the downward trend in CRP.
In the present study, a decline in log CRP at week 8
was observed in all the patients, except for the 2 patients with
suspected infection, although no changes in log IL-6 were observed
at week 8 compared with week 0. A decline in CRP levels was also
reported by Mocellin et al (21), with no changes in inflammatory
cytokines such as TNF-α. Silva Jde et al (33) also found that the intake of small
amounts of fish oil (EPA 360 mg, DHA 240 mg) produced no
differences in the levels of inflammatory cytokines, such as IL-6,
when compared to a group without fish oil intake. However, there
was a decrease in the trends of CRP in the fish oil-supplemented
group compared with those in the control group. IL-6 induces CRP
production in the liver. A previous report suggested that EPA may
also inhibit CRP production in the liver through IL-6 (34). This suggests that the reduction of
CRP levels due to EPA may also be involved in the downstream
inhibition of IL-6.
Chronic inflammation in patients with cancer is one
of the causes of cachexia, and EPA-enriched dietary supplements are
utilized to improve the nutritional status of patients. A previous
study reported that intake of high-energy, high-protein dietary
supplements with a high EPA content may be useful for inhibiting
caloric intake-dependent weight loss in patients with inoperable
pancreatic cancer (35). By
contrast, a study on perioperative conditions found that although
compliance was good, no changes in body weight or improvement in
the nutritional status were observed with nutritional
supplementation (36). High-dose
dietary supplements also present a problem in terms of a tendency
for low compliance (23).
Maintaining a high continuation rate of test food intake is
important in immuno-nutrition therapy. The present study combined
the use of fish oil capsules with low-content, high-energy
products, both of which have high compliance rates. However, no
significant changes in the nutritional status of the patients were
observed. Supplement intake (calories and protein) might not be
sufficient for weight and muscle increase. The main purpose of this
study was to evaluate the levels of cancer-related
anti-inflammatory markers. An improvement in nutritional status was
expected to accompany an anti-inflammatory response. Therefore,
very small and high-calorie commercial supplements were chosen to
avoid the impact of food intake. The dietary intake of the study
subjects was good, and the body mass index (BMI) was maintained at
~22 kg/m2. Mocellin et al (21) also reported high baseline BMI and
no weight loss, even in the control group. By contrast, Silva Jde
et al (33) reported weight
loss in the control group and a significant increase in the fish
oil-supplemented group compared with that of the control group. If
the dietary intake is adequate, there should be no weight loss even
in mildly inflammatory states. This makes verifying effects on the
improvement in nutritional status difficult. In the present study,
no significant changes in muscle mass were demonstrated. However,
mild inflammatory conditions are known to cause sarcopenia.
Therefore, a longitudinal clinical study is essential to verify
this finding.
Albumin is also used to evaluate the nutritional
status. The mean albumin value in the present study was slightly
lower than the mGPS reference standard (3.5 mg/dl). The synthesis
of albumin is known to decrease under inflammatory conditions and a
negative correlation was found between log CRP and albumin levels
in this study. Considering the changes in dietary intake and body
weight, this suggests that the decrease in albumin is due more to
the effects of inflammation, rather than due to energy or protein
deficiency. A declining trend in log CRP was seen by excluding the
2 subjects strongly suspected of having an infection, although no
increase in albumin levels was demonstrated.
The limitations of the present study include the
single-arm interventional design and the small sample size. The
inflammatory status is often increased in patients with cancer
during chemotherapy and their nutritional status may worsen. In
this study, there was no deterioration in albumin and pre-albumin
levels. However, as this was a single-arm interventional study, it
was not possible to rule out the possibility that the deterioration
of the nutritional status may have been inhibited. Mocellin et
al (21) reported that albumin
levels did not change in the fish oil intake group but declined in
the control group. Therefore, the possibility that the progression
of the inflammatory condition may have been inhibited cannot be
ruled out. Large-scale randomized clinical trials are required to
make the final decision regarding efficacy.
The present study excluded perioperative patients
and targeted outpatients. The study also did not restrict the
inclusion of patients undergoing chemotherapy, as the main reason
for an outpatient visit among patients with cancer is for
chemotherapy. There was also no restriction on the type or stage of
the cancer. We predicted that an anti-inflammatory effect would be
expected regardless of the type of cancer in cases of mild chronic
inflammation. As a result, the majority of the subjects were
patients undergoing chemotherapy for colorectal cancer. The study
subjects had good dietary intake and BMI values, and the
significance of adding dietary supplements was not obtained from
this study. It may be better to restrict the evaluation of dietary
supplementation to patients with a poor nutritional status.
Patients with cancer cachexia are prone to malnutrition due to
reduced dietary intake associated with anorexia and due to their
increased inflammatory status. Dietary supplements, small amounts
of high EPA content for energy and protein supplementation are
considered useful for improving malnutrition in patients with
cancer cachexia. However, there is no established evidence.
Large-scale, randomized clinical trials are required to investigate
the appropriate amounts of EPA and DHA, as well as the nutritional
intake of cancer patients.
In conclusion, the present results suggest that
although suppressing acute inflammation associated with infection
is challenging, the intake of relatively small amounts of EPA and
DHA may be effective for mild chronic inflammation in patients with
epithelial cancer without infection. Large-scale randomized
clinical trials are required to make the final decision regarding
efficacy. The participants in this study had a relatively good
nutritional status, although no improvement was observed. Further
studies are required to determine the appropriate levels of EPA,
DHA and dietary intake to inhibit inflammation and improve
malnutrition in patients with cancer.
Acknowledgements
Not applicable.
Funding
This study was funded by Morinaga Milk Industry Co., Ltd.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS, SM, MY, HI, CM and KT conceived and designed the
study. YS, TI, HN and KT acquired the data. YS, SM, TI, HN, KY, HI,
KM, YO, CM and KT analyzed and interpreted the data. YS, SM, KM,
HI, KM, YO, CM and KT drafted the manuscript. YS, TI and SM confirm
the authenticity of all the raw data. All authors have edited, read
and approved the final manuscript.
Ethics approval and consent to
participate
The protocol was approved by the Ethics Review
Committee of Iga City General Hospital (approval date: 4 June,
2018; approval no. 210). Written informed consent was obtained from
all participants prior to the study. The study was conducted in
accordance with the tenets of the Declaration of Helsinki
(Fortaleza revision) and the Ethical Guidelines for Medical and
Health Research Involving Human Subjects (Ministry of Education,
Culture, Sports, Science and Technology and the Ministry of Health,
Labour and Welfare Notice No. 3 of 2014, partially revised on 28
February 2017).
Patient consent for publication
Not applicable.
Competing interests
Enjoy Small High-calorie Jelly was manufactured by
Morinaga Milk Industry Co., Ltd., and the study was funded by
Morinaga Milk Industry Co., Ltd. SM, MY, KY, HI and KM are
employees of Morinaga Milk Industry Co., Ltd. All other authors
declare no competing interests.
References
|
1
|
Hébuterne X, Lemarié E, Michallet M, de
Montreuil CB, Schneider SM and Goldwasser F: Prevalence of
malnutrition and current use of nutrition support in patients with
cancer. JPEN J Parenter Enteral Nutr. 38:196–204. 2014. View Article : Google Scholar
|
|
2
|
Ryan AM, Prado CM, Sullivan ES, Power DG
and Daly LE: Effects of weight loss and sarcopenia on response to
chemotherapy, quality of life, and survival. Nutrition. 67–68.
1105392019.
|
|
3
|
Arends J, Baracos V, Bertz H, Bozzetti F,
Calder PC, Deutz NEP, Erickson N, Laviano A, Lisanti MP, Lobo DN,
et al: ESPEN expert group recommendations for action against
cancer-related malnutrition. Clin Nutr. 36:1187–1196. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu J, Huang C, Xiao H, Tang Q and Cai W:
Weight loss and resting energy expenditure in male patients with
newly diagnosed esophageal cancer. Nutrition. 29:1310–1314. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li G, Zhang K, Gong F and Jin H: A study
on changes and clinical significance of blood glucose, blood lipid
and inflammation in patients with ovarian cancer. J BUON.
24:2322–2326. 2019.PubMed/NCBI
|
|
6
|
So AR, Si JM, Lopez D and Pellegrini M:
Molecular signatures for inflammation vary across cancer types and
correlate significantly with tumor stage, sex and vital status of
patients. PLoS One. 15:e02215452020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakamoto K, Arakawa H, Mita S, Ishiko T,
Ikei S, Egami H, Hisano S and Ogawa M: Elevation of circulating
interleukin 6 after surgery: Factors influencing the serum level.
Cytokine. 6:181–186. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carson JA and Baltgalvis KA: Interleukin 6
as a key regulator of muscle mass during cachexia. Exerc Sport Sci
Rev. 38:168–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Banks WA: Anorectic effects of circulating
cytokines: Role of the vascular blood-brain barrier. Nutrition.
17:434–437. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tisdale MJ: Mechanisms of cancer cachexia.
Physiol Rev. 89:381–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suzuki H, Asakawa A, Amitani H, Nakamura N
and Inui A: Cancer cachexia-pathophysiology and management. J
Gastroenterol. 48:574–594. 2013. View Article : Google Scholar
|
|
12
|
Bosaeus I: Nutritional support in
multimodal therapy for cancer cachexia. Support Care Cancer.
16:447–451. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Forrest LM, McMillan DC, McArdle CS,
Angerson WJ and Dunlop DJ: Evaluation of cumulative prognostic
scores based on the systemic inflammatory response in patients with
inoperable non-small-cell lung cancer. Br J Cancer. 89:1028–1030.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koike Y, Miki C, Okugawa Y, Yokoe T,
Toiyama Y, Tanaka K, Inoue Y and Kusunoki M: Preoperative
C-reactive protein as a prognostic and therapeutic marker for
colorectal cancer. J Surg Oncol. 98:540–544. 2008. View Article : Google Scholar
|
|
15
|
Gorjao R, Dos Santos CM, Serdan TD, Diniz
VL, Alba-Loureiro TC, Cury-Boaventura MF, Hatanaka E, Levada-Pires
AC, Sato FT, Pithon-Curi TC, et al: New insights on the regulation
of cancer cachexia by N-3 polyunsaturated fatty acids. Pharmacol
Ther. 196:117–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duvall MG and Levy BD: DHA- and
EPA-derived resolvins, protectins, and maresins in airway
inflammation. Eur J Pharmacol. 785:144–155. 2016. View Article : Google Scholar
|
|
17
|
Wang H, Li TL, Hsia S, Su IL, Chan YL and
Wu CJ: Skeletal muscle atrophy is attenuated in tumor-bearing mice
under chemotherapy by treatment with fish oil and selenium.
Oncotarget. 6:7758–7773. 2015. View Article : Google Scholar
|
|
18
|
Whitehouse AS, Smith HJ, Drake JL and
Tisdale MJ: Mechanism of attenuation of skeletal muscle protein
catabolism in cancer cachexia by eicosapentaenoic acid. Cancer Res.
61:3604–3609. 2001.PubMed/NCBI
|
|
19
|
Flock MR, Harris WS and Kris-Etherton PM:
Long-chain omega-3 fatty acids: Time to establish a dietary
reference intake. Nutr Rev. 71:692–707. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mocellin MC, Fernandes R, Chagas TR and
Trindade E: A meta-analysis of n-3 polyunsaturated fatty acids
effects on circulating acute-phase protein and cytokines in gastric
cancer. Clin Nutr. 37:840–850. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mocellin MC, Pastore e Silva Jde A,
Camargo Cde Q, Fabre ME, Gevaerd S, Naliwaiko K, Moreno YM, Nunes
EA and Trindade EB: Fish oil decreases C-reactive protein/albumin
ratio improving nutritional prognosis and plasma fatty acid profile
in colorectal cancer patients. Lipids. 48:879–888. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shirai Y, Okugawa Y, Hishida A, Ogawa A,
Okamoto K, Shintani M, Morimoto Y, Nishikawa R, Yokoe T, Tanaka K,
et al: Fish oil-enriched nutrition combined with systemic
chemotherapy for gastrointestinal cancer patients with cancer
cachexia. Sci Rep. 7:48262017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aoyama T, Yoshikawa T, Ida S, Cho H,
Sakamaki K, Ito Y, Fujitani K, Takiguchi N, Kawashima Y, Nishikawa
K, et al: Effects of perioperative Eicosapentaenoic acid-enriched
oral nutritional supplement on lean body mass after total
gastrectomy for gastric cancer. J Cancer. 10:1070–1076. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mocellin MC, Camargo CQ, Nunes EA, Fiates
GMR and Trindade EBSM: A systematic review and meta-analysis of the
n-3 polyunsaturated fatty acids effects on inflammatory markers in
colorectal cancer. Clin Nutr. 35:359–369. 2013. View Article : Google Scholar
|
|
25
|
Póvoa P, Coelho L, Almeida E, Fernandes A,
Mealha R, Moreira P and Sabino H: C-reactive protein as a marker of
infection in critically ill patients. Clin Microbiol Infect.
11:101–108. 2005. View Article : Google Scholar
|
|
26
|
Sproston NR and Ashworth JJ: Role of
C-reactive protein at sites of inflammation and infection. Front
Immunol. 9:7542018. View Article : Google Scholar
|
|
27
|
Tutino V, De Nunzio V, Caruso MG, Veronese
N, Lorusso D, Masi MD, Benedetto ML and Notarnicola M: Elevated
AA/EPA ratio represents an inflammatory biomarker in tumor tissue
of metastatic colorectal cancer patients. Int J Mol Sci.
20:20502019. View Article : Google Scholar
|
|
28
|
Hamazaki K, Itomura M, Huan M, Nishizawa
H, Watanabe S, Hamazaki T, Sawazaki S, Terasawa K, Nakajima S,
Terano T, et al: n-3 long-chain FA decrease serum levels of TG and
remnant-like particle-cholesterol in humans. Lipids. 38:353–358.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bays HE, Ballantyne CM, Doyle RT Jr,
Juliano RA and Philip S: Icosapent ethyl: Eicosapentaenoic acid
concentration and triglyceride-lowering effects across clinical
studies. Prostaglandins Other Lipid Mediat. 125:57–64. 2016.
View Article : Google Scholar
|
|
30
|
Bays HE, Ballantyne CM, Kastelein JJ,
Isaacsohn JL, Braeckman RA and Soni PN: Eicosapentaenoic acid ethyl
ester (AMR101) therapy in patients with very high triglyceride
levels (from the Multi-center, plAcebo-controlled, Randomized,
double-blINd, 12-week study with an open-label Extension [MARINE]
trial). Am J Cardiol. 108:682–690. 2011. View Article : Google Scholar
|
|
31
|
Ballantyne CM, Bays HE, Kastelein JJ,
Stein E, Isaacsohn JL, Braeckman RA and Soni PN: Efficacy and
safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in
statin-treated patients with persistent high triglycerides (from
the ANCHOR study). Am J Cardiol. 110:984–992. 2012. View Article : Google Scholar
|
|
32
|
Braeckman RA, Stirtan WG and Soni PN:
Pharmacokinetics of eicosapentaenoic acid in plasma and red blood
cells after multiple oral dosing with icosapent ethyl in healthy
subjects. Clin Pharmacol Drug Dev. 3:101–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Silva Jde A, Trindade EB, Fabre ME,
Menegotto VM, Gevaerd S, Buss Zda S and Frode TS: Fish oil
supplement alters markers of inflammatory and nutritional status in
colorectal cancer patients. Nutr Cancer. 64:267–273. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang TM, Hsieh SC, Chen JW and Chiang AN:
Docosahexaenoic acid and eicosapentaenoic acid reduce C-reactive
protein expression and STAT3 activation in IL-6-treated HepG2
cells. Mol Cell Biochem. 377:97–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fearon KC, Von Meyenfeldt MF, Moses AG,
Van Geenen R, Roy A, Gouma DJ, Giacosa A, Van Gossum A, Bauer J,
Barber MD, et al: Effect of a protein and energy dense N-3 fatty
acid enriched oral supplement on loss of weight and lean tissue in
cancer cachexia: A randomised double blind trial. Gut.
52:1479–1486. 2003. View Article : Google Scholar
|
|
36
|
Hanai N, Terada H, Hirakawa H, Suzuki H,
Nishikawa D, Beppu S and Hasegawa Y: Prospective randomized
investigation implementing immunonutritional therapy using a
nutritional supplement with a high blend ratio of ω-3 fatty acids
during the perioperative period for head and neck carcinomas. Jpn J
Clin Oncol. 48:356–361. 2018. View Article : Google Scholar
|