Certain studies have indicated that cancer cells
have an enhanced ability to proliferate and invade caused by
changes in their metabolism, among which abnormal cholesterol
metabolism has a key role. It is mainly manifested as abnormally
increased or abnormally accumulated cholesterol in cancer cells,
and inhibiting the increase of cholesterol levels may slow down the
proliferation of cancer cells. An important factor affecting
intracellular cholesterol levels is protein transport (1). ATP-binding cassette (ABC)
transporters are localized to the plasma membrane and function as a
class of lipid transporters. Energy is obtained by hydrolyzing ATP,
thereby mediating the efflux of intracellular phospholipids and
free cholesterol, which combine with non-fat or low-fat
apolipoprotein A-I (apo A-I) on the cell surface to form
high-density lipoprotein (HDL) to then initiate the reverse
cholesterol transport process (2,3).
When exploring the association between cholesterol and malignant
cancers, it was observed that the accumulation of excessive
intracellular cholesterol may promote the development of cancer
cells to a certain extent (4).
Phillips (5) indicated that high
cholesterol levels were positively associated with the
aggressiveness of prostate cancer. Abnormally active cholesterol
metabolism in cancer cells leads to proliferation, survival,
invasion, metastasis and enhanced adaptation to the cancer
microenvironment (6). ABCA1 is
also closely related to cancer resistance mechanisms (7); thus, inducing the expression of ABCA1
may provide novel therapeutic strategies for cancer prevention,
inhibition and even treatment, bringing new hope for the treatment
of cancer patients (8).
As mentioned earlier in the introduction,
cholesterol has an important role in the development of cancers,
such as participation in the proliferation, migration and invasion
of cancer cells. Luo et al (9) indicated that the abnormal cholesterol
metabolism of cancer cells leads to proliferation, survival,
invasion, metastasis and enhanced adaptability to the cancer
microenvironment, thereby promoting the occurrence and development
of cancers. Changes in membrane cholesterol and cholesterol-rich
membranes have been observed to affect the progression and invasion
of cancers (9). Cholesterol
homeostasis is critical for cellular function and survival, and
dysregulated cholesterol homeostasis is known to be associated with
a variety of cancers, including prostate cancer (10), and alterations in lipid metabolism
are increasingly recognized as a hallmark of prostate cancer cells
(11). A large number of studies
suggested that a variety of genes related to cholesterol synthesis
exhibit increased activity in cancer cells, thereby promoting the
growth, migration and metastasis of various cancer cell types,
including gastric cancer, glioma and prostate cancer (12–14).
In addition, it was reported that the expression of low-density
lipoprotein receptors (LDLR) is upregulated in various cancer
cells, which was significantly associated with poor clinical
prognosis in patients with small cell lung cancer (15–18).
Elevated expression of LDLR in patients with pancreatic cancer was
associated with an increased recurrence rate. However, silencing of
HDLR by short hairpin RNA in pancreatic cancer cells significantly
reduced the cholesterol uptake, thereby inhibiting the
proliferation of pancreatic cancer cells. Treatments related to the
synthesis, uptake and esterification of cholesterol may alleviate
cancer progression (19–21). It was reported that altering the
intracellular lipid microenvironment by affecting various metabolic
pathways of cholesterol has an important role in the proliferation
and invasion of certain types of cancer cell (22,23).
Table I summarizes the specific
effects of intracellular cholesterol levels on cancer; as one of
the major efflux pathways of cellular cholesterol, ABCA1 may be an
important determinant of prostate cancer aggressiveness and a
potential therapeutic target (24,25).
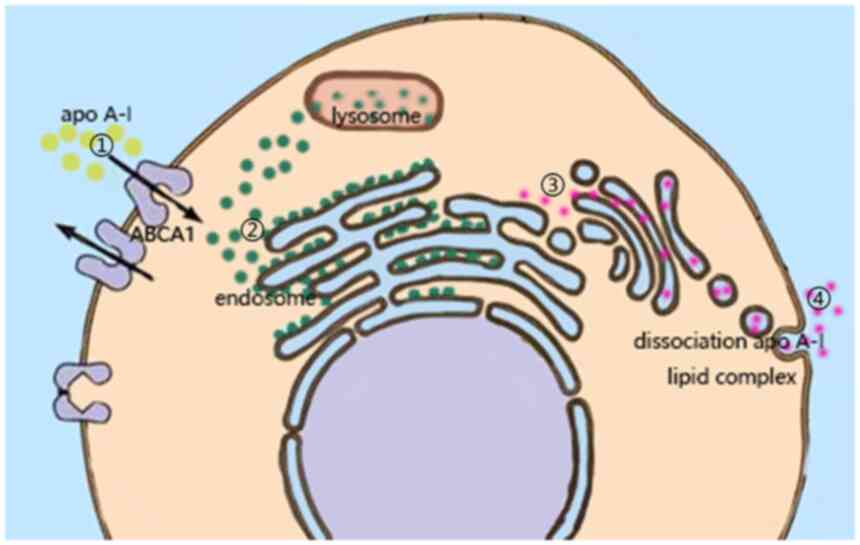
ABCA1 is known as the main efflux channel of
intracellular cholesterol. Although there is still no perfect model
to explain the mechanism, the latest efflux mechanism is widely
recognized (8). Multiple studies
suggested that the binding of apo A-I to ABCA1 may lead to the
redistribution of phospholipids and cholesterol on the outside of
the plasma membrane, thereby changing the intracellular lipid
microenvironment and remodeling the plasma membrane, which
facilitated the binding of apo A-I to lipids and maintained
intracellular cholesterol homeostasis (26–29).
After the combination of the two, the plasma membrane is remodeled
in two ways: Horizontal and vertical. The vertical remodeling
promotes the net transport of phospholipids to the outer leaflets
of the plasma membrane through ABCA1, resulting in an imbalance in
the packing density of the two leaflets of the phospholipid
bilayer, thereby regulating the lipid density of the plasma
membrane (30). In horizontal
remodeling, ABCA1 is enabled by hydrolysis of ATP, causing the
phospholipid and cholesterol molecules that constitute the plasma
membrane to translocate laterally on the cell membrane, resulting
in the redistribution of lipids from non-lipid rafts to lipid
rafts, which in turn changes the lipid distribution of the entire
plasma membrane (31–33). Therefore, it may be speculated that
ABCA1 changes the intracellular lipid microenvironment through
plasma membrane remodeling and lipid redistribution.
All cells in the body are able to synthesize
cholesterol, but most of them lack effective metabolic pathways and
may only be excreted from cells through a series of transporters
(34). Among them, ABCA1 is able
to use the energy provided by ATP to promote the efflux of free
cholesterol and phospholipids in cells, and combine with apo A-I on
the cell surface to form new HDL, which in turn initiates the
reverse cholesterol transport process to transport cholesterol from
peripheral tissues back to the liver (35–37).
At this stage, it has been indicated that numerous factors (such as
IGF-1), are able to directly or indirectly regulate the expression
level of ABCA1 to affect the intracellular cholesterol level
(38). The importance of ABCA1 for
cholesterol efflux is currently widely recognized, but the pathway
to the plasma membrane remains to be fully elucidated (39). Yoshioka et al (40) provided three models of cholesterol
efflux mechanisms. They are the channel transport model, the
mushroom-like protrusion model and the endocytosis-exocytosis
transport model. The channel transport model is widely accepted and
is presented in Fig. 1 to indicate
how ABCA1 is able to regulate the intracellular microenvironment by
transporting intracellular lipids.
The above studies proved that a series of life
activities of cancer cells are closely related to the level of
intracellular cholesterol and ABCA1 indirectly affects the
proliferation, metastasis and invasion of cancer cells by
regulating the level of intracellular cholesterol (41–44).
ABCA1 mediates the transmembrane transport of free intracellular
cholesterol and phospholipids to apo A-I, which has an important
role in maintaining the normal metabolism of intracellular
cholesterol. As indicated by Prochazka et al (45), in non-small cell lung carcinoma
H1299 cells, overexpression of ABCA1 enhanced drug resistance.
ABCA1 is strongly expressed in normal breast epithelium and the
reduced expression of ABCA1 in breast cancer appears to be
associated with poor prognosis (46). Activated liver X receptor and
overexpression of ABCA1 have been reported to reduce cholesterol
levels and cancer growth in mouse prostate cancer xenografts
(47). Maslyanko et al
(48) also indicated that high
cholesterol levels may promote the proliferation of breast cancer
cells in a mouse transgenic model, specifically by upregulating the
protein expression of ABCA1 to maintain the cholesterol balance in
the body and inhibit the proliferation of cancer cells. It was also
indicated that cholesterol efflux has an important role in the
treatment of lung cancer. Downregulation of ABCA1 was evident in
all prostate cancers. It has been reported that when prostate
cancer metastasizes, the cells contain high levels of cholesterol
(49). Studies suggested that
cancer-specific ABCA1 methylation and loss of protein expression
directly led to elevated intracellular cholesterol levels in cancer
cells, thus forming a microenvironment favorable for cancer
progression (50). Liu et
al (51) indicated that
microRNA-200b-3p acts as an oncogene in lung adenocarcinoma samples
and in the human lung adenocarcinoma cell lines A549 and H1299 by
targeting ABCA1, and overexpression of ABCA1 significantly
inhibited the proliferation, migration and invasion of lung
adenocarcinoma cells. Huang et al (52) demonstrated that ABCA1 may protect
the normal function of cells by maintaining low levels of
cholesterol in mitochondria and have a certain inhibitory effect on
the proliferation of cancer cells. Prostate cancer bone metastases
exhibit clear metabolic differences from bone metastases of other
cancer types, including increased levels of cholesterol. Regulation
of cholesterol in the plasma membrane has been indicated to
modulate the ability of cells to migrate (53). Furthermore, cholesterol-rich lipid
rafts were reported to have an important role in the adhesion and
migration of cancer cells (54,55).
The activity of phosphoinositide 3-kinase is able to regulate the
surface protein expression of ABCA1, which significantly increases
the risk of cancer cells entering the blood to form metastases
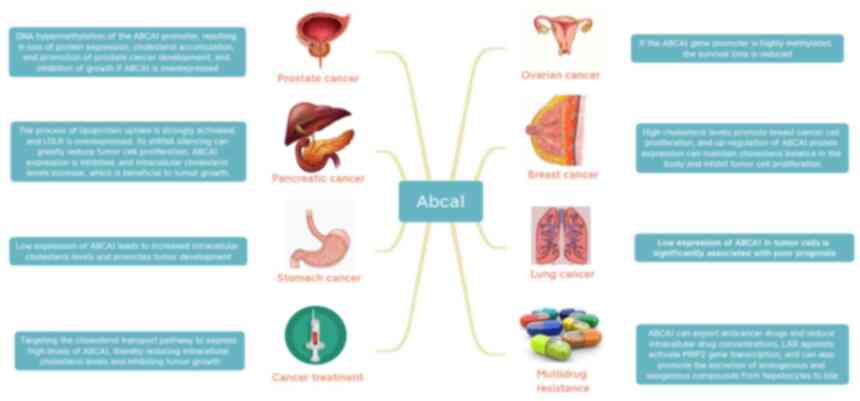
(56). In Tables I and II, the specific effects of ABCA1 in
cancer cells are summarized, linking abnormal cholesterol levels to
aberrant ABCA1 expression, regulation of ABCA1 expression levels,
which may be utilized for inhibiting cancer cells to proliferate,
metastasize and invade.
It is promising to treat cancer by regulating the
expression level of ABCA1, thereby regulating the level of
intracellular cholesterol. Of note, cancer cells require excess
cholesterol to maintain high levels of proliferation, which has
been widely accepted (57).
Available epidemiological data indicated that low serum cholesterol
as well as statins reduce the risk of prostate cancer,
demonstrating that cholesterol metabolism has a role in the
development of aggressive prostate cancer (58). Therefore, it may be speculated that
high expression of ABCA1 is able to mediate cholesterol efflux,
resulting in the reduction of intracellular cholesterol in prostate
cancer cells to achieve the purpose of inhibiting cancer growth.
Ovarian cancer is a common gynecological malignancy. Although
chemotherapy is able to delay the disease, the post-operative
survival rate is not high. Studies have indicated that ABCA1 has a
key role in drug resistance and prognosis (59). According to the latest research,
patients with ovarian cancer with high methylation of RASSF1C
(functions as an oncogene in cancer cells) and low ABCA1 expression
have shorter survival (60). In
conclusion, existing studies suggest that moderately high
expression of ABCA1 has a positive effect on the treatment of
prostate cancer and breast cancer.
ABCA1, as a research hotspot in recent years, has
received extensive attention due to its function, and as a major
transporter regulating intracellular cholesterol, its role in
cancer resistance has attracted much attention (8). Chemotherapy, as one of the most
important cancer treatment methods, is still not able to completely
remove cancer cells. Chemotherapy tolerance is one of the most
common problems in chemotherapy failure. Therefore, overcoming the
resistance of cancer cells to chemotherapeutic drugs is an
important issue in cancer treatment (61). Multidrug resistance generally
refers to the adaptability of cancer cells to increase drug output
and decrease drug uptake, and drug transporters have a key role in
pre-targeted drug resistance (62). Existing studies suggested found
that chemotherapy failure is directly related to ABC transporters
and preventing the induction of ABC transporters in cancer cells
may help avoid drug resistance (63). The high expression of transporters
in cancer cells results in a variety of anticancer drugs being
transported out of cells and unable to exert their anticancer
effects (64,65), which has been observed in the drug
resistance to curcumin, doxorubicin and nitidine in the treatment
of breast and lung cancer (66–68).
Chen et al (69) indicated
that ABC transporters have a certain role in cancer stem cells
through the reversal of inhibition of ABC transporter expression.
There are numerous ways to overcome the drug resistance that
depends on high expression of ABCA1, such as valproic acid
downregulating the expression level of ABCA1 through histone
deacetylase 2, thereby enhancing the sensitivity of non-small cell
lung cancer cells to cisplatin (70). ABCA1-dependent resistance to
α-tocopheryl succinate in mitochondria-targeted therapy of lung
cancer was also reported (71).
However, certain studies also indicated that when the expression
level of ABCA1 in cancer cells was generally decreased, cholesterol
accumulated and increased the order of bilayer phospholipids,
thereby reducing the permeability of the membrane and finally
promoting the resistance of cancers to membrane-active anticancer
drugs (72). As indicated in
Fig. 2, the role of ABCA1 in
multidrug resistance has been widely recognized and measuring the
expression of ABCA1 may be used to predict the response to
anticancer drugs.
Cholesterol efflux minimizes the potential harm to
cells by excess cholesterol, but cancer cells have evolved to
exploit this link to promote malignancy. As one of the main
transporters of intracellular cholesterol efflux, ABCA1 has been
widely recognized and used in anti-atherosclerosis treatment
(73); its ability to transport
cholesterol also has great potential in cancer treatment (74). Further research on ABCA1 will help
elucidate its transport function and mechanisms to inhibit the
transport of chemotherapeutic drugs, as well as the related effects
of its reduced expression level on cancer cells, so as to achieve
the purpose of cancer treatment. By modifying and inhibiting this
gene, mechanisms of cancer cell growth and efflux of
chemotherapeutic drugs may be interfered with. This provides novel
directions for research on lipid metabolism and offers new
effective targets for cancer prevention, inhibition and treatment.
ABCA1 in cancer and provides a new avenue for drug discovery and
therapy.
Not applicable.
This work was funded by the Hengyang City Science and Technology
Planning Project (grant no. 202150063473), the Scientific Research
Project of Hunan Provincial Health Commission (grant no.
202202044140), the Scientific Research Project of Hunan Provincial
Education Department (grant no. 21B0438) and the Undergraduate
Research-based Learning and Innovative Experimental Program of the
University of South China (grant no. X202110555457).
Not applicable.
XY was responsible for the design of the review. XL,
XY and LZ were responsible for revising the draft. KW drafted the
manuscript, all authors approved the final draft. All authors read
and approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bian X, Liu R, Meng Y, Xing D, Xu D and Lu
Z: Lipid metabolism and cancer. J Exp Med. 218:e202016062021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang YC, Lee TS and Chiang AN: Quercetin
enhances ABCA1 expression and cholesterol efflux through a
p38-dependent pathway in macrophages. J Lipid Res. 53:1840–1850.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang J, Wang WJ, Zhai L and Zhang DF:
Association of cholesterol with risk of pancreatic cancer: A
meta-analysis. World J Gastroenterol. 21:3711–3719. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee BH, Taylor MG, Robinet P, Smith JD,
Schweitzer J, Sehayek E, Falzarano SM, Magi-Galluzzi C, Klein EA
and Ting AH: Dysregulation of cholesterol homeostasis in human
prostate cancer through loss of ABCA1. Cancer Res. 73:1211–1218.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Phillips MC: Molecular mechanisms of
cellular cholesterol efflux. J Biol Chem. 289:24020–24029. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chushi L, Wei W, Kangkang X, Yongzeng F,
Ning X and Xiaolei C: HMGCR is up-regulated in gastric cancer and
promotes the growth and migration of the cancer cells. Gene.
587:42–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang W, Lokman NA, Noye TM, Macpherson AM,
Oehler MK and Ricciardelli C: ABCA1 is associated with the
development of acquired chemotherapy resistance and predicts poor
ovarian cancer outcome. Cancer Drug Resist. 4:485–502.
2021.PubMed/NCBI
|
|
8
|
Jacobo-Albavera L, Domínguez-Pérez M,
Medina-Leyte DJ, González-Garrido A and Villarreal-Molina T: The
role of the ATP-binding cassette A1 (ABCA1) in human disease. Int J
Mol Sci. 22:15932021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo X, Cheng C, Tan Z, Li N, Tang M, Yang
L and Cao Y: Emerging roles of lipid metabolism in cancer
metastasis. Mol Cancer. 16:762017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adlakha YK, Khanna S, Singh R, Singh VP,
Agrawal A and Saini N: Pro-apoptotic miRNA-128-2 modulates ABCA1,
ABCG1 and RXRα expression and cholesterol homeostasis. Cell Death
Dis. 4:e7802013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yue S, Li J, Lee SY, Lee HJ, Shao T, Song
B, Cheng L, Masterson TA, Liu X, Ratliff TL and Cheng JX:
Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT
activation underlies human prostate cancer aggressiveness. Cell
Metab. 19:393–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiu Z, Yuan W, Chen T, Zhou C, Liu C,
Huang Y, Han D and Huang Q: HMGCR positively regulated the growth
and migration of glioblastoma cells. Gene. 576:22–27. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ashida S, Kawada C and Inoue K: Stromal
regulation of prostate cancer cell growth by mevalonate pathway
enzymes HMGCS1 and HMGCR. Oncol Lett. 14:6533–6542. 2017.PubMed/NCBI
|
|
14
|
Xiong T, Xu G, Huang XL, Lu KQ, Xie WQ,
Yin K and Tu J: ATP-binding cassette transporter A1: A promising
therapy target for prostate cancer. Mol Clin Oncol. 8:9–14.
2018.PubMed/NCBI
|
|
15
|
Guo D, Reinitz F, Youssef M, Hong C,
Nathanson D, Akhavan D, Kuga D, Amzajerdi AN, Soto H, Zhu S, et al:
An LXR agonist promotes glioblastoma cell death through inhibition
of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov.
1:442–456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Touvier M, Fassier P, His M, Norat T, Chan
DS, Blacher J, Hercberg S, Galan P, Druesne-Pecollo N and
Latino-Martel P: Cholesterol and breast cancer risk: A systematic
review and meta-analysis of prospective studies. Br J Nutr.
114:347–357. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou P, Li B, Liu B, Chen T and Xiao J:
Prognostic role of serum total cholesterol and high-density
lipoprotein cholesterol in cancer survivors: A systematic review
and meta-analysis. Clin Chim Acta. 477:94–104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guillaumond F, Bidaut G, Ouaissi M,
Servais S, Gouirand V, Olivares O, Lac S, Borge L, Roques J, Gayet
O, et al: Cholesterol uptake disruption, in association with
chemotherapy, is a promising combined metabolic therapy for
pancreatic adenocarcinoma. Proc Natl Acad Sci USA. 112:2473–2478.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Menter DG, Ramsauer VP, Harirforoosh S,
Chakraborty K, Yang P, His L, Newman RA and Krishnan K:
Differential effects of pravastatin and simvastatin on the growth
of tumor cells from different organ sites. PLoS One. 6:e288132011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Gu D, Lee SS, Song B, Bandyopadhyay
S, Chen S, Konieczny SF, Ratliff TL, Liu X, Xie J and Cheng JX:
Abrogating cholesterol esterification suppresses growth and
metastasis of pancreatic cancer. Oncogene. 35:6378–6388. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou T, Zhan J, Fang W, Zhao Y, Yang Y,
Hou X, Zhang Z, He X, Zhang Y, Huang Y and Zhang L: Serum
low-density lipoprotein and low-density lipoprotein expression
level at diagnosis are favorable prognostic factors in patients
with small-cell lung cancer (SCLC). BMC Cancer. 17:2692017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cruz PM, Mo H, McConathy WJ, Sabnis N and
Lacko AG: The role of cholesterol metabolism and cholesterol
transport in carcinogenesis: A review of scientific findings,
relevant to future cancer therapeutics. Front Pharmacol. 4:1192013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Folkerd EJ and Dowsett M: Influence of sex
hormones on cancer progression. J Clin Oncol. 28:4038–4044. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Phillips MC: Is ABCA1 a lipid transfer
protein? J Lipid Res. 59:749–763. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saxena K and Shipley GG: Structural
studies of detergent-solubilized and vesicle-reconstituted
low-density lipoprotein (LDL) receptor. Biochemistry.
36:15940–15948. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vedhachalam C, Duong PT, Nickel M, Nguyen
D, Dhanasekaran P, Saito H, Rothblat GH, Lund-Katz S and Phillips
MC: Mechanism of ATP-binding cassette transporter A1-mediated
cellular lipid efflux to apolipoprotein A-I and formation of high
density lipoprotein particles. J Biol Chem. 282:25123–25130. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagao K, Tomioka M and Ueda K: Function
and regulation of ABCA1-membrane meso-domain organization and
reorganization. FEBS J. 278:3190–3203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takahashi Y and Smith JD: Cholesterol
efflux to apolipoprotein AI involves endocytosis and resecretion in
a calcium-dependent pathway. Proc Natl Acad Sci USA.
96:11358–11363. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu R, Arakawa R, Ito-Osumi C, Iwamoto N
and Yokoyama S: ApoA-I facilitates ABCA1 recycle/accumulation to
cell surface by inhibiting its intracellular degradation and
increases HDL generation. Arterioscler Thromb Vasc Biol.
28:1820–1824. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hamon Y, Broccardo C, Chambenoit O,
Luciani MF, Toti F, Chaslin S, Freyssinet JM, Devaux PF, McNeish J,
Marguet D and Chimini G: ABC1 promotes engulfment of apoptotic
cells and transbilayer redistribution of phosphatidylserine. Nat
Cell Biol. 2:399–406. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Landry YD, Denis M, Nandi S, Bell S,
Vaughan AM and Zha X: ATP-binding cassette transporter A1
expression disrupts raft membrane microdomains through its
ATPase-related functions. J Biol Chem. 281:36091–36101. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Santamarina-Fojo S, Remaley AT, Neufeld EB
and Brewer HB Jr: Regulation and intracellular trafficking of the
ABCA1 transporter. J Lipid Res. 42:1339–1345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koseki M, Hirano K, Masuda D, Ikegami C,
Tanaka M, Ota A, Sandoval JC, Nakagawa-Toyama Y, Sato SB, Kobayashi
T, et al: Increased lipid rafts and accelerated
lipopolysaccharide-induced tumor necrosis factor-alpha secretion in
Abca1-deficient macrophages. J Lipid Res. 48:299–306. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang SL, Chen WJ, Yin K, Zhao GJ, Mo ZC,
Lv YC, Ouyang XP, Yu XH, Kuang HJ, Jiang ZS, et al: PAPP-A
negatively regulates ABCA1, ABCG1 and SR-B1 expression by
inhibiting LXRα through the IGF-I-mediated signaling pathway.
Atherosclerosis. 222:344–354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang N, Silver DL, Thiele C and Tall AR:
ATP-binding cassette transporter A1 (ABCA1) functions as a
cholesterol efflux regulatory protein. J Biol Chem.
276:23742–23747. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smith JD, Le Goff W, Settle M, Brubaker G,
Waelde C, Horwitz A and Oda MN: ABCA1 mediates concurrent
cholesterol and phospholipid efflux to apolipoprotein A-I. J Lipid
Res. 45:635–644. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tall AR: An overview of reverse
cholesterol transport. Eur Heart J. 19 (Suppl A):A31–A35.
1998.PubMed/NCBI
|
|
38
|
Qian H, Zhao X, Cao P, Lei J, Yan N and
Gong X: Structure of the human lipid exporter ABCA1. Cell.
169:1228–1239.e10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kang MH, Singaraja R and Hayden MR:
Adenosine-triphosphate-binding cassette transporter-1 trafficking
and function. Trends Cardiovasc Med. 20:41–49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yoshioka Y, Sasaki J, Yamamoto M, Saitoh
K, Nakaya S and Kubokawa M: Quantitation by (1)H-NMR of dolichol,
cholesterol and choline-containing lipids in extracts of normal and
phathological thyroid tissue. NMR Biomed. 13:377–383. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ding X, Zhang W, Li S and Yang H: The role
of cholesterol metabolism in cancer. Am J Cancer Res. 9:219–227.
2019.PubMed/NCBI
|
|
42
|
Kitahara CM, Berrington de González A,
Freedman ND, Huxley R, Mok Y, Jee SH and Samet JM: Total
cholesterol and cancer risk in a large prospective study in Korea.
J Clin Oncol. 29:1592–1598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang B, Song BL and Xu C: Cholesterol
metabolism in cancer: Mechanisms and therapeutic opportunities. Nat
Metab. 2:132–141. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tabas I: Consequences of cellular
cholesterol accumulation: Basic concepts and physiological
implications. J Clin Invest. 110:905–911. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Prochazka L, Koudelka S, Dong LF, Stursa
J, Goodwin J, Neca J, Slavik J, Ciganek M, Masek J, Kluckova K, et
al: Mitochondrial targeting overcomes ABCA1-dependent resistance of
lung carcinoma to α-tocopheryl succinate. Apoptosis. 18:286–299.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schimanski S, Wild PJ, Treeck O, Horn F,
Sigruener A, Rudolph C, Blaszyk H, Klinkhammer-Schalke M, Ortmann
O, Hartmann A and Schmitz G: Expression of the lipid transporters
ABCA3 and ABCA1 is diminished in human breast cancer tissue. Horm
Metab Res. 42:102–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dufour J, Viennois E, De Boussac H, Baron
S and Lobaccaro JM: Oxysterol receptors, AKT and prostate cancer.
Curr Opin Pharmacol. 12:724–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Maslyanko M, Harris RD and Mu D:
Connecting cholesterol efflux factors to lung cancer biology and
therapeutics. Int J Mol Sci. 22:72092021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Silvente-Poirot S and Poirot M: Cancer.
Cholesterol and cancer, in the balance. Science. 343:1445–1446.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Smith B and Land H: Anticancer activity of
the cholesterol exporter ABCA1 gene. Cell Rep. 2:580–590. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu K, Zhang W, Tan J, Ma J and Zhao J:
MiR-200b-3p functions as an oncogene by targeting ABCA1 in lung
adenocarcinoma. Technol Cancer Res Treat. 18:15330338198925902019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huang CX, Zhang YL, Wang JF, Jiang JY and
Bao JL: MCP-1 impacts RCT by repressing ABCA1, ABCG1, and SR-BI
through PI3K/Akt posttranslational regulation in HepG2 cells. J
Lipid Res. 54:1231–1240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Thysell E, Surowiec I, Hörnberg E, Crnalic
S, Widmark A, Johansson AI, Stattin P, Bergh A, Moritz T, Antti H
and Wikström P: Metabolomic characterization of human prostate
cancer bone metastases reveals increased levels of cholesterol.
PLoS One. 5:e141752010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Murai T, Maruyama Y, Mio K, Nishiyama H,
Suga M and Sato C: Low cholesterol triggers membrane
microdomain-dependent CD44 shedding and suppresses tumor cell
migration. J Biol Chem. 286:1999–2007. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ramprasad OG, Srinivas G, Rao KS, Joshi P,
Thiery JP, Dufour S and Pande G: Changes in cholesterol levels in
the plasma membrane modulate cell signaling and regulate cell
adhesion and migration on fibronectin. Cell Motil Cytoskeleton.
64:199–216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Plösch T, Gellhaus A, van Straten EM, Wolf
N, Huijkman NC, Schmidt M, Dunk CE, Kuipers F and Winterhager E:
The liver X receptor (LXR) and its target gene ABCA1 are regulated
upon low oxygen in human trophoblast cells: A reason for
alterations in preeclampsia? Placenta. 31:910–918. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ohno Y, Ohori M, Nakashima J, Okubo H,
Satake N, Hashimoto T and Tachibana M: Association between
preoperative serum total cholesterol level and biochemical
recurrence in prostate cancer patients who underwent radical
prostatectomy. Mol Clin Oncol. 4:1073–1077. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Buchwald H: Cholesterol inhibition,
cancer, and chemotherapy. Lancet. 339:1154–1156. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cerovska E, Elsnerova K, Vaclavikova R and
Soucek P: The role of membrane transporters in ovarian cancer
chemoresistance and prognosis. Expert Opin Drug Metab Toxicol.
13:741–753. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Amaar YG and Reeves ME: RASSF1C regulates
miR-33a and EMT marker gene expression in lung cancer cells.
Oncotarget. 10:123–132. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Di Nicolantonio F, Mercer SJ, Knight LA,
Gabriel FG, Whitehouse PA, Sharma S, Fernando A, Glaysher S, Di
Palma S, Johnson P, et al: Cancer cell adaptation to chemotherapy.
BMC Cancer. 5:782005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shang Y, Zhang Z, Liu Z, Feng B, Ren G, Li
K, Zhou L, Sun Y, Li M, Zhou J, et al: miR-508-5p regulates
multidrug resistance of gastric cancer by targeting ABCB1 and
ZNRD1. Oncogene. 33:3267–3276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
64
|
Moitra K: Overcoming multidrug resistance
in cancer stem cells. Biomed Res Int. 2015:6357452015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bachmeier BE, Iancu CM, Killian PH,
Kronski E, Mirisola V, Angelini G, Jochum M, Nerlich AG and Pfeffer
U: Overexpression of the ATP binding cassette gene ABCA1 determines
resistance to Curcumin in M14 melanoma cells. Mol Cancer.
8:1292009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hou H, Kang Y, Li Y, Zeng Y, Ding G and
Shang J: miR-33a expression sensitizes Lgr5+ HCC-CSCs to
doxorubicin via ABCA1. Neoplasma. 64:81–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Iwasaki H, Okabe T, Takara K, Yoshida Y,
Hanashiro K and Oku H: Down-regulation of lipids transporter ABCA1
increases the cytotoxicity of nitidine. Cancer Chemother Pharmacol.
66:953–959. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sun M, Yang C, Zheng J, Wang M, Chen M, Le
DQS, Kjems J and Bünger CE: Enhanced efficacy of chemotherapy for
breast cancer stem cells by simultaneous suppression of multidrug
resistance and antiapoptotic cellular defense. Acta Biomater.
28:171–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chen JH, Zheng YL, Xu CQ, Gu LZ, Ding ZL,
Qin L, Wang Y, Fu R, Wan YF and Hu CP: Valproic acid (VPA) enhances
cisplatin sensitivity of non-small cell lung cancer cells via HDAC2
mediated down regulation of ABCA1. Biol Chem. 398:785–792. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ma Y, Li X, Cheng S, Wei W and Li Y:
MicroRNA-106a confers cisplatin resistance in non-small cell lung
cancer A549 cells by targeting adenosine triphosphatase-binding
cassette A1. Mol Med Rep. 11:625–632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Heilos D, Röhrl C, Pirker C, Englinger B,
Baier D, Mohr T, Schwaiger M, Iqbal SM, van Schoonhoven S, Klavins
K, et al: Altered membrane rigidity via enhanced endogenous
cholesterol synthesis drives cancer cell resistance to destruxins.
Oncotarget. 9:25661–25680. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wali VB, Bachawal SV and Sylvester PW:
Suppression in mevalonate synthesis mediates antitumor effects of
combined statin and gamma-tocotrienol treatment. Lipids.
44:925–934. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen W, Li L, Wang J, Zhang R, Zhang T, Wu
Y, Wang S and Xing D: The ABCA1-efferocytosis axis: A new strategy
to protect against atherosclerosis. Clin Chim Acta. 518:1–8. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Srivastava N: ATP binding cassette
transporter A1-key roles in cellular lipid transport and
atherosclerosis. Mol Cell Biochem. 237:155–164. 2002. View Article : Google Scholar : PubMed/NCBI
|