Introduction
Depending on various disease environments, the
presence of abnormal proteins may lead to antigenicity, which can
drive the humoral immune response to produce serum autoantibodies.
Several recent studies have demonstrated that serum autoantibodies
may be useful for detecting various cancers at early stages
(1–5). There are various subtype
classifications and treatments for HCC, and alternative
immunocombined approaches from a molecular pathological point of
view have been implemented, but their effects remain unclear
(6). Our research group has also
evaluated the usefulness of autoantibodies in detecting surgically
treatable hepatocellular carcinoma (HCC). Owing to their enhanced
signals, autoantibodies may be more useful as immunodiagnostic
markers for cancer detection than tumor-associated antigens (TAAs)
(7,8). The presence of autoantibodies has
been observed not only in cancer but also in autoimmune,
inflammatory, and fibrotic diseases (9–12).
Several studies, including those by our research
group, have demonstrated potential TAAs in HCC using enzyme-linked
immunosorbent assay (ELISA). In a test cohort, Okada et al
the showed potential benefits of using autoantibodies to detect
diseases (1). Moreover, in a
previous study of patients with surgically resected HCC,
autoantibodies against TAAs, including Sui1, RalA, p62, p53, c-myc,
and NY-ESO-1, had an additive effect with α-fetoprotein (AFP) on
the detection of stage I and II HCC (2). However, the clinical significance of
autoantibodies in liver cirrhosis (LC) or chronic hepatitis (CH)
has not been studied extensively. A single-institutional
retrospective study demonstrated the significance of autoantibodies
in distinguishing among HCC, LC, and CH; however, no prospective
multi-institutional study has been undertaken on this topic
(13,14). Patients with CH are at a high risk
of progression to LC and HCC, and an early diagnosis of LC is
essential for an early diagnosis of HCC.
This prospective multi-institutional study aimed to
cross-sectionally validate the positivity rates of six
autoantibodies to HCC. We also compared the positivity rates of
these six autoantibodies to LC and CH to evaluate whether the three
diseases could be differentiated through the measurement of these
autoantibodies.
Materials and methods
Patients
Patients enrolled in this study had primary HCC,
histologically proven, or either LC or CH according to ultrasound
findings. Eligible patients with LC had an aspartate
aminotransferase-to-platelet ratio index (APRI) of ≥1.0 or a
fibrosis-4 (FIB-4) score of ≥3.25, and eligible patients with CH
had an APRI of <1.0 and/or a FIB-4 score of <3.25 (15,16).
Patients with active co-cancers (co-cancers or metachronous cancers
within 5 years) were excluded to ensure that the previous cancer
had no effect on their antibody levels. Each research center
conducted central monitoring to confirm data submission, patient
eligibility, protocol compliance, safety, and on-schedule research
progress. We compared patients' clinicopathological variables,
demographic data, and tumor characteristics (positivity and
negativity for any of the six autoantibodies). The AFP cutoff value
was 10.0 ng/ml. Before enrollment, all participants provided
written informed consent to future analyses of their blood samples
for research purposes. The protocol for this prospective study was
approved by the ethics committee of Toho University, Tokyo, Japan
(approval numbers M19213, A18103, A17052, A16035, A16001, 26095,
25024, 24038, and 22047); the Chiba Cancer Center, Chiba, Japan
(H30-220, 21–26, and 20-1); and the institutional review boards of
each participating hospital (listed in the next section). The study
was conducted according to the guidelines of the Declaration of
Helsinki and the Japanese Ethical Guidelines for Clinical
Research.
Sample collection
Serum samples were obtained from 149 patients with
HCC, 76 patients with LC, and 103 patients with CH at the six
participating institutions (Chiba University School of Medicine,
Chiba Cancer Center, Japan Community Health care Organization Chiba
Hospital, Funabashi Municipal Medical Center, Kimitsu Chuo
Hospital, and Japan Community Health care Organization Funabashi
Central Hospital). Serum samples of 88 healthy controls who had no
previous malignant disease and no hepatitis B or hepatitis C
infection were also obtained from Biobank Japan. The average age of
the healthy control group was 48 years, and the male-to-female
ratio was 5:3. All serum samples were stored at −80°C until
analysis.
Patient variables
The HCC stage in each affected patient at the time
of the study was pathologically determined according to the TNM
Classification of Malignant Tumours, 8th edition (17). Preoperative resectability and local
or distant tumor enlargement were determined via computed
tomography. Tumors associated with distant metastasis, including
peritoneal dissemination, were considered nonresectable. The
hepatectomy procedure was performed according to the treatment
method described in the Japanese guidelines (18,19).
Isolation, purification, and
amplification of TAAs followed by ELISA of serum antibodies
Full-length complementary DNA of the TAAs Sui1
(GenBank accession number: JN545747), RalA (BM 560822), p62
(AF057352), p53 (AB082923), c-myc (K02276), and NY-ESO-1 (NM
001327) were amplified through polymerase chain reaction as
previously described (1,2). The recombinant proteins were
expressed in Escherichia coli BL21-CodonPlus (DE3)-RIL cells
(Agilent Technologies). Each TAA extract was added to Ni Sepharose
6 Fast Flow (GE Healthcare UK), and the column was washed with 50
mmol/L imidazole in phosphate-buffered saline. Purified TAA
recombinant proteins were eluted with 200 mmol/L imidazole in PBS.
DNA sequencing confirmed that the correct gene was inserted into
the constructed plasmid. Serum samples collected from patients and
controls were analyzed through ELISA as previously described
(2). Serum AFP was measured
through enzyme-linked fluorescent assay as previously described
(20).
Titration of autoantibodies
Using the serum from the patients with HCC, LC, and
CH and from the healthy controls, we measured the titers (means ±
standard deviations) of autoantibodies against the six TAAs. The
cutoff value for positive reactivity of each autoantibody was an
optical density greater than the mean plus three standard
deviations observed in the controls. We calculated the specificity
of the assay as the percentage of the controls in whom the
reactivity was negative. We also assessed the significance of
differences in each of the six autoantibody titers. Various serum
markers, clinicopathological characteristics, and AFP
concentrations were included in the analysis. Additionally, we
estimated the clinical utility of the combination of the six
autoantibodies in diagnosing HCC, LC, and CH.
Statistical analysis
Statistical analyses were performed using the JMP
statistical software (version 12; SAS Institute). We used Fisher's
exact test to determine whether the proportions of positive results
differed significantly between the patients with cancer and the
healthy controls and to determine associations between individual
and combined antibody assay results and clinical parameters. For
all tests, a P value of <0.05 (two-tailed) was considered
statistically significant.
Results
Serum anti-TAA antibody titers
The serum titers of the autoantibodies against TAAs
were higher in patients with HCC, LC, and CH than in healthy
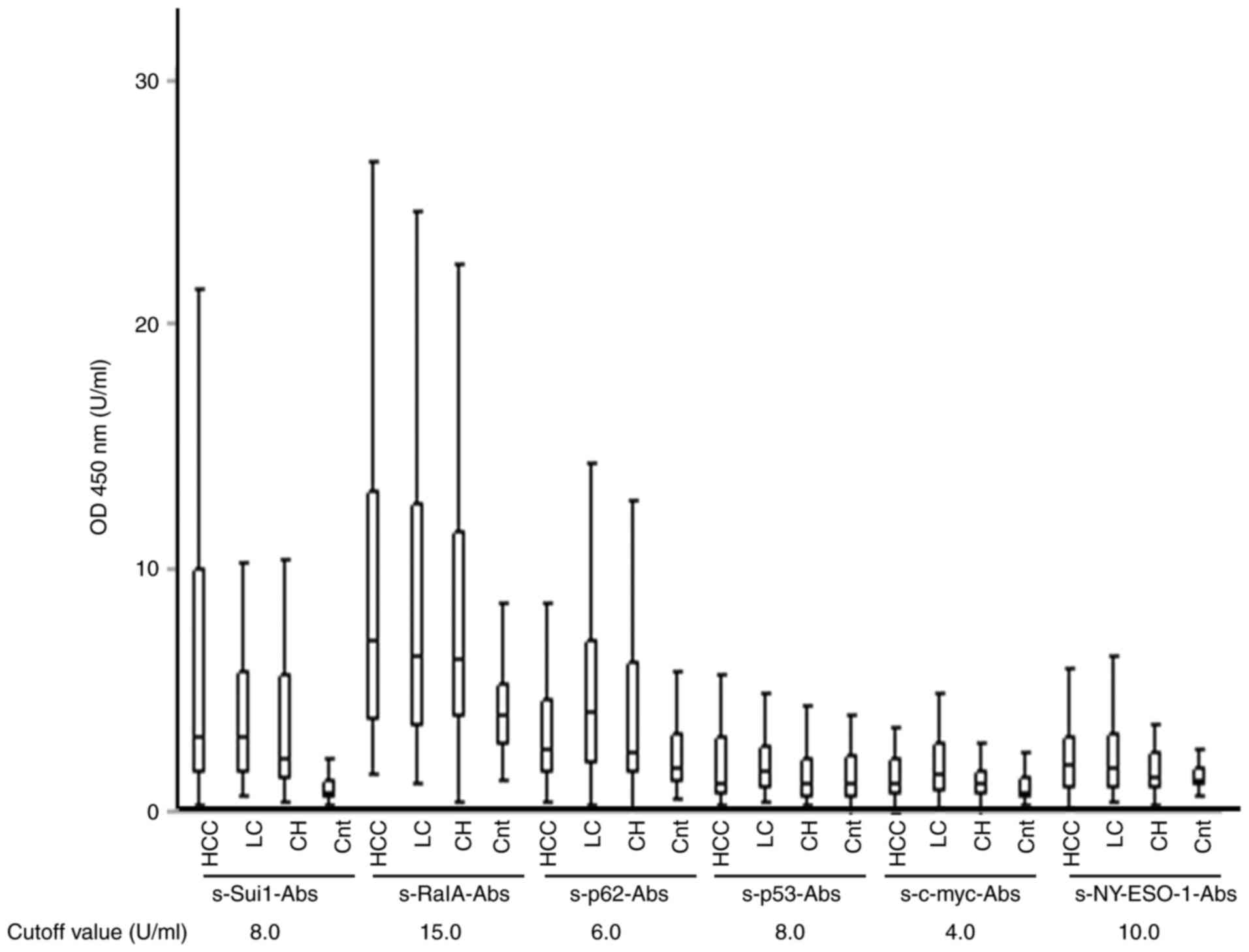
controls (P<0.05 for all). The cutoff titers were 8.0, 15.0,
6.0, 8.0, 4.0, and 10.0 U/ml for s-Sui1-Abs, s-RalA-Abs, s-p62-Abs,
s-p53-Abs, s-c-myc-Abs, and s-NY-ESO-1-Abs, respectively (Fig. 1).
Clinical characteristics and
autoantibody status of patients with HCC
Table I lists the
clinical characteristics of patients with HCC. Gender, age,
hepatitis virus infection type, liver disease type (CH vs. LC),
tumor size, tumor number, and TNM classification were not
significantly associated with autoantibody status. Autoantibody
status was also not associated with AFP status.
 | Table I.Patients' clinical characteristics
and serum tumor markers according to status of autoantibody panel
in 149 patients with HCC. |
Table I.
Patients' clinical characteristics
and serum tumor markers according to status of autoantibody panel
in 149 patients with HCC.
| Panel | Total | Autoantibody panel
(+) | Autoantibody panel
(−) |
P-valuea |
|---|
| Number | 149 | 98 | 51 |
|
| Sex, n (%) |
|
|
|
|
|
Male | 105 | 67 (68) | 38 (75) | 0.432 |
|
Female | 44 | 31 (32) | 13 (25) |
|
| Age, n (%) |
|
|
|
|
|
<65 | 28 | 16 (16) | 12 (24) | 0.291 |
|
≥65 | 121 | 82 (84) | 39 (76) |
|
| Hepatitis B virus
infection, n (%) |
|
|
|
|
|
Negative | 133 | 88 (90) | 45 (88) | 0.771 |
|
Positive | 16 | 10 (10) | 6 (12) |
|
| Hepatitis C virus
infection, n (%) |
|
|
|
|
|
Negative | 50 | 30 (31) | 20 (39) | 0.293 |
|
Positive | 99 | 68 (69) | 31 (61) |
|
| Liver disease, n
(%) |
|
|
|
|
| Chronic
hepatitis | 29 | 20 (20) | 9 (18) | 0.686 |
| Liver
cirrhosis | 120 | 78 (80) | 42 (82) |
|
| Tumor size, n
(%) |
|
|
|
|
| <36
mm | 109 | 70 (71) | 39 (76) | 0.500 |
| ≥36
mm | 40 | 28 (29) | 12 (24) |
|
| Tumor number, n
(%) |
|
|
|
|
| 1 | 52 | 33 (34) | 19 (37) | 0.664 |
| ≥2 | 97 | 65 (66) | 32 (63) |
|
| TNM stage, n
(%) |
|
|
|
|
| I | 50 | 33 (34) | 17 (33) | 0.967 |
| II,
III, IV | 99 | 65 (66) | 34 (67) |
|
| AFP, n (%) |
|
|
|
|
| <10
ng/ml | 79 | 52 (53) | 27 (53) | 0.989 |
| ≥10
ng/ml | 70 | 46 (47) | 24 (47) |
|
Positivity rates of each autoantibody
and AFP in patients with HCC at different TNM stages
The overall positivity rate of the serum
autoantibodies against the TAA panel (66%) was higher than that of
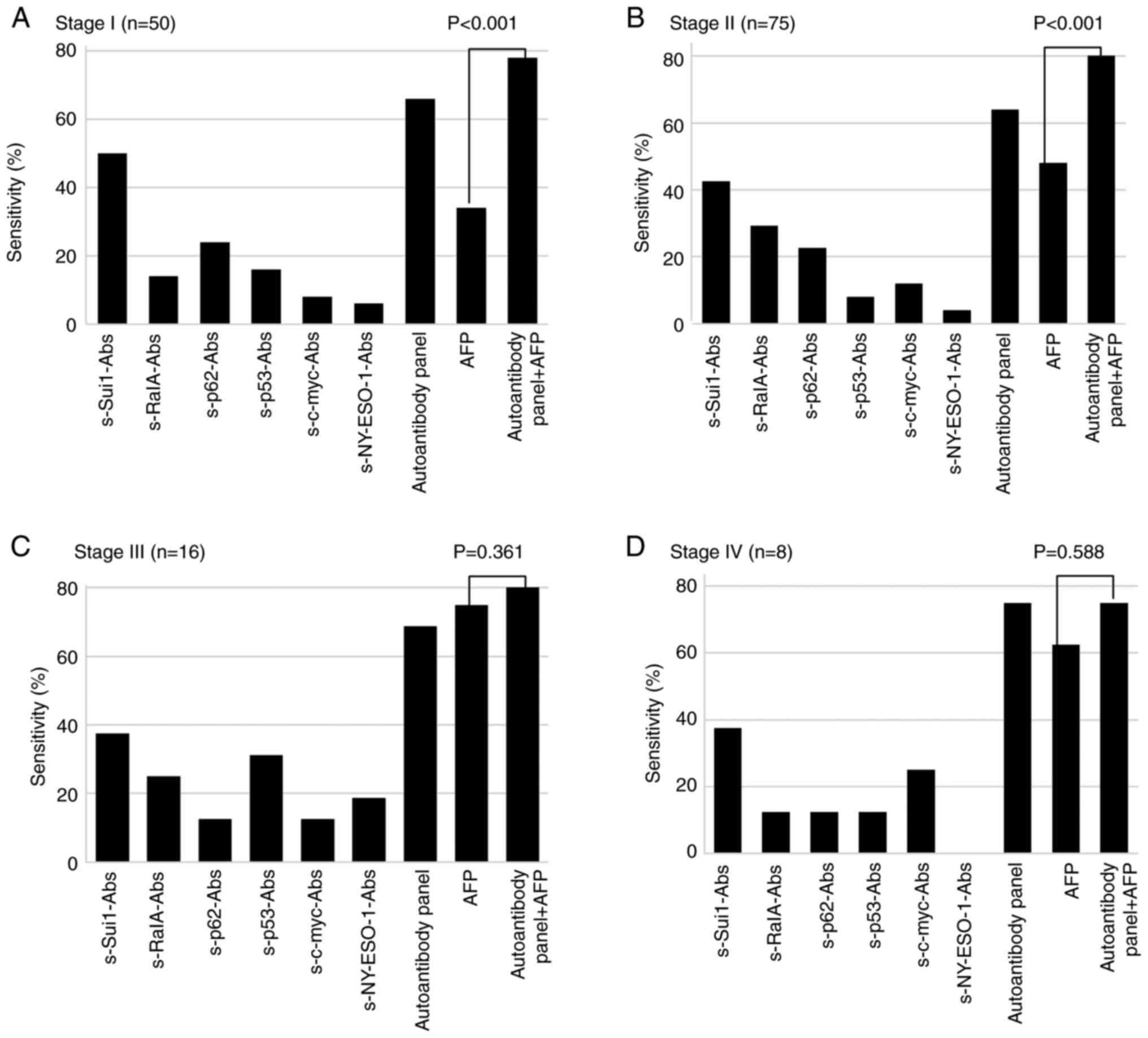
AFP (48%, P<0.05). Fig. 2 shows
the positivity rates of each autoantibody and AFP in patients with
HCC at different TNM stages. The corresponding positivity rates for
the autoantibody panel and AFP were 66 and 34%, respectively
(P=0.001), for TNM stage I disease; 64 and 48%, respectively
(P=0.047), for stage II; 69 and 75%, respectively (P=0.694), for
stage III; and 75 and 63%, respectively (P=0.588), for stage IV.
The combination of the positivity rates of the autoantibody panel
and AFP was significantly greater than the positivity rate of AFP
alone (P<0.001). The positivity rates of AFP and AFP + serum
autoantibodies were 34 and 78%, respectively (P<0.001), for
stage I disease; 48 and 84%, respectively (P<0.001), for stage
II; 75 and 88%, respectively (P=0.361), for stage III; and 63 and
75%, respectively (P=0.588), for stage IV.
Ability of autoantibody response to
TAAs to diagnose HCC
Table II lists the
positivity rates, specificities, positive predictive values,
negative predictive values, and accuracy of serum autoantibodies in
detecting HCC. Of the 149 patients with HCC, 66 (44%) had
s-Sui1-Abs; 34 (23%) had s-RalA-Abs; 32 (21%) had s-p62-Abs; 20
(13%) had s-p53-Abs; 17 (11%) had s-c-myc-Abs; and 9 (6%) had
s-NY-ESO-1-Abs. All autoantibodies had a specificity of >95%.
When the assay results for all six autoantibodies were combined,
the sensitivity, specificity, positive predictive value, negative
predictive value, and accuracy increased to 66% (95% confidence
interval [CI], 62–67%), 96% (95% CI, 90–98%), 96% (95% CI, 91–98%),
62% (95% CI, 58–64%), and 77% (95% CI, 73–79%), respectively.
 | Table II.Autoantibody responses to
tumor-associated antigens in 149 patients with hepatocellular
carcinoma. |
Table II.
Autoantibody responses to
tumor-associated antigens in 149 patients with hepatocellular
carcinoma.
| Group | s-Sui1-Abs | s-RalA-Abs | s-p62-Abs | s-p53-Abs | s-c-myc-Abs | s-NY-ESO-1-Abs | Autoantibody
panel |
|---|
| Sensitivity | 44 (42–45) | 23 (20–23) | 22 (20–23) | 14 (11–14) | 11 (9–13) | 6 (4–6) | 66 (62–67) |
| Specificity | 99 (94–100) | 99 (94–100) | 99 (94–100) | 99 (94–100) | 97 (92–99) | 99 (94–100) | 96 (90–98) |
| PPV | 99 (93–100) | 97 (86–100) | 97 (86–100) | 95 (78–99) | 85 (65–95) | 90 (60–98) | 96 (91–98) |
| NPV | 51 (49–52) | 43 (41–44) | 43 (41–44) | 40 (38–41) | 39 (37–40) | 38 (37–39) | 62 (58–64) |
| Accuracy | 65 (62–66) | 51 (48–52) | 50 (47–51) | 45 (42–46) | 43 (40–45) | 40 (38–41) | 77 (73–79) |
Clinical characteristics and
autoantibody status in patients with HCC, LC, and CH
Table III lists
the clinical characteristics of patients with HCC, LC, and CH. Male
sex, age of ≥65, APRI of ≥1.0, FIB-4 of ≥3.25, hepatitis virus
infection, and autoantibody positivity rates were significantly
associated with HCC. Autoantibody positivity rates in patients with
HCC were significantly higher than those in patients with CH
(P=0.033).
 | Table III.Clinical characteristics according to
status of autoantibody panel in patients with HCC, LC and CH. |
Table III.
Clinical characteristics according to
status of autoantibody panel in patients with HCC, LC and CH.
| Panel | HCC (n=149) | LC (n=76) |
P-valuea | CH (n=103) |
P-valueb |
|---|
| Gender, n (%) |
|
|
|
|
|
|
Male | 105 (70) | 41 (54) | 0.015 | 50 (49) | <0.001 |
|
Female | 44 (30) | 35 (46) |
| 53 (51) |
|
| Age, n (%) |
|
|
|
|
|
|
<65 | 28 (19) | 32 (42) | <0.001 | 59 (57) | <0.001 |
|
≥65 | 121 (81) | 44 (58) |
| 44 (43) |
|
| APRI, n (%) |
|
|
|
|
|
|
<1.0 | 55 (37) | 21 (28) | 0.160 | 103 (100) | <0.001 |
|
≥1.0 | 94 (63) | 55 (72) |
| 0 (0) |
|
| FIB-4, n (%) |
|
|
|
|
|
|
<3.25 | 30 (20) | 5 (7) | 0.005 | 103 (100) | <0.001 |
|
≥3.25 | 119 (80) | 71 (93) |
| 0 (0) |
|
| Hepatitis B virus
infection, n (%) |
|
|
|
|
|
|
Negative | 133 (89) | 74 (97) | 0.021 | 67 (65) | <0.001 |
|
Positive | 16 (11) | 2 (3) |
| 36 (35) |
|
| Hepatitis C virus
infection, n (%) |
|
|
|
|
|
|
Negative | 50 (34) | 28 (37) | 0.625 | 51 (50) | 0.011 |
|
Positive | 99 (66) | 48 (63) |
| 52 (50) |
|
| Autoantibody
panel |
|
|
|
|
|
|
Negative | 51 (34) | 32 (42) | 0.248 | 49 (48) | 0.033 |
|
Positive | 98 (62) | 44 (58) |
| 54 (52) |
|
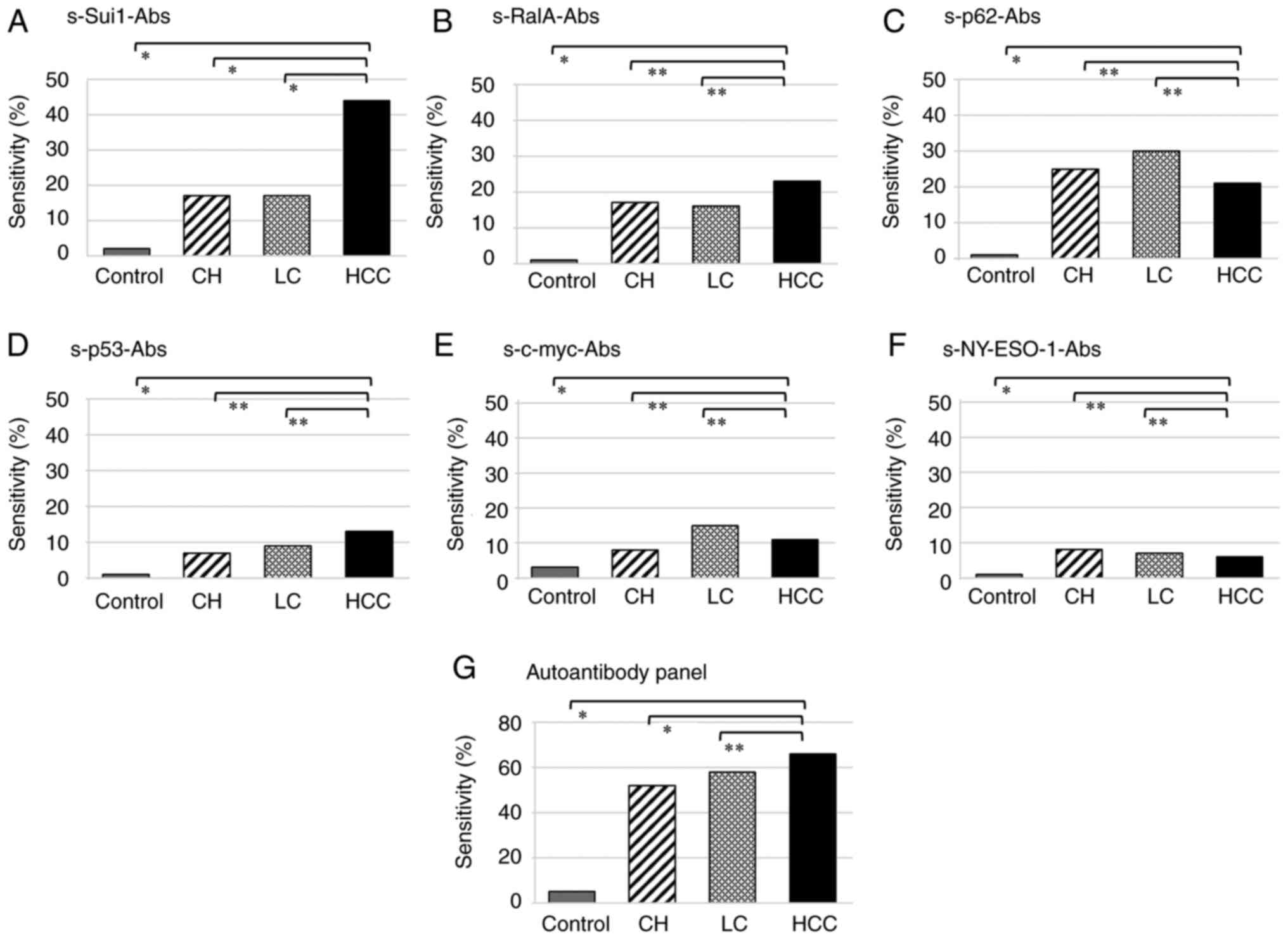
Positivity of autoantibodies in
patients with HCC, LC, and CH and in healthy controls
Table IV and
Fig. 3 present the autoantibody
positivity rates in patients with HCC, LC, and CH and in healthy
controls. Among the 149 patients with HCC, autoantibodies against
Sui1 were most common in 66 patients (44%); among the 76 patients
with LC, autoantibodies against p62 were most common in 23 patients
(30%); and among the 103 patients with CH, autoantibodies against
p62 were most common in 26 patients (25%). The positivity rates of
autoantibodies in patients with HCC, LC, and CH and in healthy
controls were 66, 58, 52, and 5%, respectively. Among patients with
HCC, LC, and CH, the autoantibodies s-Sui1-Abs, s-RalA-Abs, and
s-p53-Abs were more common than other autoantibodies.
 | Table IV.Positivity of autoantibodies to
tumor-associated antigens in patients with HCC, LC, and CH and in
controls. |
Table IV.
Positivity of autoantibodies to
tumor-associated antigens in patients with HCC, LC, and CH and in
controls.
| Group | s-Sui1-Abs | s-RalA-Abs | s-p62-Abs | s-p53-Abs | s-c-myc-Abs | s-NY-ESO-1-Abs | Autoantibody
panel |
|---|
| Control (n=88)
(%) | 2 (2) | 1 (1) | 1 (1) | 1 (1) | 3 (3) | 1 (1) | 4 (5) |
| CH (n=103) (%) | 18 (17) | 17 (17) | 26 (25) | 7 (7) | 8 (8) | 8 (8) | 54 (52) |
| LC (n=76) (%) | 13 (17) | 12 (16) | 23 (30) | 7 (9) | 11 (15) | 5 (7) | 44 (58) |
| HCC (n=149)
(%) | 66 (44) | 34 (23) | 32 (21) | 20 (13) | 17 (11) | 9 (6) | 98 (66) |
Association between hepatitis virus
infection and s-Sui1-autoantibody
Table SI presents
the association between hepatitis virus infection and s-Sui1-Abs
positivity in patients with HCC, LC, and CH. There was no
association between hepatitis B virus infection and s-Sui1-Abs. In
patients with hepatitis C virus, the s-Sui1-Abs positivity rate
gradually increased in CH, LC, and HCC. Patients with hepatitis C
HCC were significantly more positive for s-Sui1-Abs than those with
not hepatitis C HCC.
Discussion
The combination of positivity rates of
autoantibodies against the whole TAA panel and AFP was
significantly higher than that of AFP alone in stage I and II
disease. The positivity rates of the autoantibodies against the
whole TAA panel were significantly higher than those of AFP in
stage I and II disease. The positivity rates of the autoantibodies
against the whole TAA panel gradually increased (in order) in
patients with CH, LC, and HCC.
The fact that autoantibodies were effective in
identifying HCC in two different prospective studies indicates that
HCC induced serum immunoglobulin G autoantibodies against TAAs from
an early stage (2). However,
unlike other types of cancer, HCC comprises multistage carcinogenic
states that include CH and LC, and previous reports did not reveal
at what stage serum autoantibodies would appear. Zheng et al
reported that serum anti-SMP30 autoantibody titers were
significantly higher in patients with HCC than in healthy controls
or in patients with CH or LC (14). Furthermore, He et al
reported that serum anti-ACY1 autoantibody titers were
significantly higher in patients with LC than in healthy controls
or in patients with CH (13). In
this study, we found that serum autoantibody titers were elevated
not only in patients with HCC but also in those with CH and LC.
Sui1 may be a potential biomarker for HCC according
to Zhou et al, who reported that the positivity rate of
s-Sui1-Abs in patients with HCC was 15.5%, which was higher than
that in patients with LC (3.3%), those with CH (0%), and healthy
participants (0%) (21). Our
findings of relatively high positivity rates in patients with HCC,
LC, and CH confirmed their data. The differences may be explained
partly by the difference in the proportions of patients with
hepatitis B virus- or hepatitis C virus-related carcinogenesis.
Compared with other serum antibodies, only s-Sui1-Abs exhibited
significantly higher positivity rates in patients with HCC than in
patients with LC and CH. Moreover, the Suil positivity rate in this
study differed from that in previous studies (2). This may be partially explained by the
difference in the cutoff values calculated from two cohorts of
healthy subjects (8.0 U/ml vs. 4.4 U/ml) and the difference in the
proportion of early-stage cancer in this study.
RalA has also been reported to be a potential
biomarker for HCC as well as for esophageal, gastric, colorectal,
breast, and ovarian carcinomas (2,5,22–25).
This TAA has not been reported to be associated with fibrosis or
chronic inflammation. Because guanosine triphosphatase is
aberrantly induced during tumorigenesis by oncogenic Ras,
s-RalA-Abs positivity may indicate HCC development in patients with
LC and CH.
Previous studies have reported that s-p62-Abs are
positive not only for cancers such as HCC, colorectal cancer, and
breast cancer but also for conditions characterized by chronic
inflammation and fibrosis, such as primary biliary cholangitis
(2,5,26,27).
Even in our study, the positivity rate of s-p62-Abs was not
significantly higher in patients with HCC than in those with LC and
CH. Thus, s-p62-Abs is not a tumor-specific autoantibody.
NY-ESO-1 has been reported to be effective in
identifying cancers such as gastric cancer, esophageal cancer,
rectal cancer, HCC, and lung cancer; however, the identification of
inflammation and fibrosis has not been reported (2,4,5,28).
Because there are few reports on the positivity rate of
s-NY-ESO-1-Abs in precancerous conditions including LC and CH, the
mechanism of positive conversion during the carcinogenic process
remains unclear. However, considering that there is a report of
s-NY-ESO-1-Abs positivity in CH, s-NY-ESO-1-Abs positivity in
chronic liver disease may indicate the presence of microcancer that
is not represented in the image or the presence of precancerous
conditions (29). In addition, it
has been reported that p53 antibody is associated with autoimmune
hepatitis; therefore, the possibility that s-NY-ESO-1-Abs
positivity occurs as a result of concomitant autoimmune hepatitis
cannot be denied (30,31). It may be necessary to carefully
search for tumors if s-NY-ESO-1-Abs is positive in patients with LC
or CH.
Our study had some limitations. First, we did not
analyze the prognosis of the patients or changes in their
autoantibody titers. A previous study showed that only
s-NY-ESO-1-Abs was associated with poor overall survival in
patients with HCC after radical resection. Second, we could not
assess the risk of carcinogenesis in patients with
autoantibody-positive CH or LC. Third, the cases in this study were
not surgically resected, so we could not analyze tumor tissues
immunohistochemically. Serum autoantibodies are usually associated
with TAA expression in tumor tissue (32,33).
Similar to serum autoantibodies, Sui1, RalA, and p53 are reported
to have higher expression rates in HCC than in normal liver, CH, or
LC, as revealed through immunohistochemical studies (21,34,35).
Fourth, no pathological data were available for this study. The
diagnoses of CH and LC were based on ultrasound and hematological
findings. Finally, we were unable to investigate the genetic
mutations analysis of the cases in this study, including HCC. A
correlation between the protein expression of p53 and genetic
mutations of p53 has been demonstrated in human solid tumors,
including HCC (36–39). We could not clarify the association
between s-p53-Abs and p53 mutation, but we speculate that
the two are strongly correlated.
In conclusion, serum autoantibodies, including
s-Sui1-Abs, s-RalA-Abs, s-p62-Abs, s-p53-Abs, s-c-myc-Abs, and
s-NY-ESO-1-Abs, may be useful in differentiating patients with HCC
from healthy individuals. They are, however, not specific to HCC
and were also found to be positive in patients with CH and LC.
Possibly, the production of these autoantibodies is induced during
the development of HCC.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Akiko Kuwajima
(Medical & Biological Laboratories Co., Ltd., Nagoya, Japan)
for her assistance in preparing targeting peptides for the ELISA
system.
Funding
Hideaki Shimada received grants from Risk-Taking Fund for
Technology Development and Medical & Biological Laboratories
Co., Ltd., Nagoya, Japan.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
RO and HS designed and performed the experiments. YO
collected clinicopathological data. RO, OY, NK, FI, IH, NS, HM, RA
and KK analyzed the results. RO and YO generated the data, prepared
the panels and assembled the figures and tables. RO and HS wrote
the manuscript. RO and HS confirm the authenticity of all the raw
data. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The protocol of the present study, involving human
clinical samples, was approved by the Ethics Committee of Toho
University of Medicine, Tokyo, Japan (approval nos. M19213, A18103,
A17052, A16035, A16001, 26095, 25024, 24038 and 22047); the Chiba
Cancer Center, Chiba, Japan (approval nos. H30-220, 21–26 and
20-1); and the institutional review boards of each participating
hospital. Written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AFP
|
α-fetoprotein
|
|
CH
|
chronic hepatitis
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
HCC
|
hepatocellular carcinoma
|
|
LC
|
liver cirrhosis
|
|
TAA
|
tumor-associated antigen
|
References
|
1
|
Okada R, Shimada H, Tagawa M, Matsushita
K, Otsuka Y, Kuwajima A and Kaneko H: Profiling of serum
autoantibodies in Japanese patients with hepatocellular carcinoma.
Toho J Med. 3:84–92. 2017.
|
|
2
|
Okada R, Otsuka Y, Wakabayashi T, Shinoda
M, Aoki T, Murakami M, Arizumi S, Yamamoto M, Aramaki O, Takayama
T, et al: Six autoantibodies as potential serum biomarkers of
hepatocellular carcinoma: A prospective multicenter study. Int J
Cancer. 147:2578–2586. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoshino I, Nagata M, Takiguchi N, Nabeya
Y, Ikeda A, Yokoi S, Kuwajima A, Tagawa M, Matsushita K, Satoshi Y
and Hideaki S: Panel of autoantibodies against multiple
tumor-associated antigens for detecting gastric cancer. Cancer Sci.
108:308–315. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoshino I, Nabeya Y, Takiguchi N, Gunji H,
Ishige F, Iwatate Y, Shiratori F, Yajima S, Okada R and Shimada H:
Prognostic impact of p53 and/or NY-ESO-1 autoantibody induction in
patients with gastroenterological cancers. Ann Gastroenterol Surg.
4:275–282. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ushigome M, Nabeya Y, Soda H, Takiguchi N,
Kuwajima A, Tagawa M, Matsushita K, Koike J, Funahashi K and
Shimada H: Multi-panel assay of serum autoantibodies in colorectal
cancer. Int J Clin Oncol. 23:917–923. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kabashima A, Shimada S, Shimokawa M,
Akiyama Y, Tanabe M and Tanaka S: Molecular and immunological
paradigms of hepatocellular carcinoma: Special reference to
therapeutic approaches. J Hepatobiliary Pancreat Sci. 28:62–75.
2021. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan HT, Low J, Lim SG and Chung MC: Serum
autoantibodies as biomarkers for early cancer detection. FEBS J.
276:6880–6904. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lacombe J, Mangé A and Solassol J: Use of
autoantibodies to detect the onset of breast cancer. J Immunol Res.
2014:5749812014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Di Sabatino A, Biagi F, Lenzi M, Frulloni
L, Lenti MV, Giuffrida P and Corazza GR: Clinical usefulness of
serum antibodies as biomarkers of gastrointestinal and liver
diseases. Dig Liver Dis. 49:947–956. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pongratz G, Frieser R, Brinks R, Schneider
M, Hartung W, Fleck M and Ehrenstein B: Association between
autoantibody level and disease activity in rheumatoid arthritis is
dependent on baseline inflammation. Clin Exp Rheumatol. 38:691–698.
2020.PubMed/NCBI
|
|
11
|
Jodeleit H, Milchram L, Soldo R,
Beikircher G, Schönthaler S, Al-Amodi O, Wolf E, Beigel F,
Weinhäusel A, Siebeck M and Gropp R: Autoantibodies as diagnostic
markers and potential drivers of inflammation in ulcerative
colitis. PLoS One. 15:e02286152020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng B, Huang X, Nakayasu ES, Petersen JR,
Qiu S, Almeida IC and Zhang JY: Using immunoproteomics to identify
alpha-enolase as an autoantigen in liver fibrosis. J Proteome Res.
12:1789–1796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He X, Hong Y, Wang X, Zhang X, Long J, Li
H, Zhang B, Chen S, Liu Q, Li H, et al: Identification and clinical
significance of an elevated level of serum aminoacylase-1
autoantibody in patients with hepatitis B virus-related liver
cirrhosis. Mol Med Rep. 14:4255–4262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng SX, Xiang BD, Long JM, Qu C, Mo ZJ,
Li K, Zhuang Y, Lv ZL and Zhou SF: Diagnostic value of serum SMP30
and Anti-SMP30 antibody in hepatocellular carcinoma. Lab Med.
49:203–210. 2018.PubMed/NCBI
|
|
15
|
Kruger FC, Daniels CR, Kidd M, Swart G,
Brundyn K, van Rensburg C and Kotze M: APRI: A simple bedside
marker for advanced fibrosis that can avoid liver biopsy in
patients with NAFLD/NASH. S Afr Med J. 101:477–480. 2011.PubMed/NCBI
|
|
16
|
Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono
M, Fujii H, Eguchi Y, Suzuki Y, Aoki N, Kanemasa K, et al:
Validation of the FIB4 index in a Japanese nonalcoholic fatty liver
disease population. BMC Gastroenterol. 12:22012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brierley JD, Gospodarowicz MK and
Wittekind CH: TNM classification of malignant tumors. UICC
International Union Against Cancer; 8th edition. New York, USA: pp.
90–93. 2016
|
|
18
|
Makuuchi M and Kokudo N: Clinical practice
guidelines for hepatocellular carcinoma: The first evidence based
guidelines from Japan. World J Gastroenterol. 12:828–829. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kubota K, Makuuchi M, Kusaka K, Kobayashi
T, Miki K, Hasegawa K, Harihara Y and Takayama T: Measurement of
liver volume and hepatic functional reserve as a guide to
decision-making in resectional surgery for hepatic tumors.
Hepatology. 26:1176–1181. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jang ES, Jeong SH, Kim JW, Hoi YS,
Leissner P and Brechot C: Diagnostic performance of
alpha-fetoprotein, protein induced by vitamin K absence,
osteopontin, Dickkopf-1 and Its combinations for hepatocellular
carcinoma. PLoS One. 11:e01510692016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou JW, Li Y, Yue LX, Luo CL, Chen Y and
Zhang JY: Autoantibody response to Sui1 and its tissue-specific
expression in hepatocellular carcinoma. Tumour Biol. 37:2547–2553.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nanami T, Hoshino I, Ito M, Yajima S,
Oshima Y, Suzuki T, Shiratori F, Nabeya Y, Funahashi K and Shimada
H: Prevalence of autoantibodies against Ras-like GTPases, RalA, in
patients with gastric cancer. Mol Clin Oncol. 13:282020.PubMed/NCBI
|
|
23
|
Nanami T, Shimada H, Yajima S, Oshima Y,
Matsushita K, Nomura F, Nagata M, Tagawa M, Otsuka S, Kuwajima A
and Kaneko H: Clinical significance of serum autoantibodies against
Ras-like GTPases, RalA, in patients with esophageal squamous cell
carcinoma. Esophagus. 13:167–172. 2016. View Article : Google Scholar
|
|
24
|
Kubota Y, Ogata H, Otsuka S, Kuwajima A,
Saito F and Shimada H: Presence of autoantibodies against Ras-like
GTPases in Serum-in stage I/II breast cancer. Toho J Med.
3:125–130. 2017.
|
|
25
|
Sun H, Shi JX, Zhang HF, Xing MT, Li P,
Dai LP, Luo CL, Wang X, Wang P, Ye H, et al: Serum autoantibodies
against a panel of 15 tumor-associated antigens in the detection of
ovarian cancer. Tumour Biol. 39:10104283176991322017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu W, Li Y, Wang B, Dai L, Qian W and
Zhang JY: Autoimmune response to IGF2 mRNA-binding protein 2
(IMP2/p62) in breast cancer. Scand J Immunol. 81:502–507. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bauer A and Habior A: Detection of
autoantibodies against nucleoporin p62 in Sera of patients with
primary biliary cholangitis. Ann Lab Med. 39:291–298. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang J, Jiao S, Kang J, Li R and Zhang G:
Application of serum NY-ESO-1 antibody assay for early SCLC
diagnosis. Int J Clin Exp Pathol. 8:14959–14964. 2015.PubMed/NCBI
|
|
29
|
Zhang Z, Li FF, Lu MD, Zhang SX and Li YX:
Anti-NY-ESO-1 autoantibody may be a tumor marker for intrahepatic
cholangiocarcinoma. Oncotarget. 8:103283–103289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Herkel J, Modrow JP, Bamberger S, Kanzler
S, Rotter V, Cohen IR and Lohse AW: Prevalence of autoantibodies to
the p53 protein in autoimmune hepatitis. Autoimmunity. 35:493–496.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Himoto T, Yoneyama H, Kurokohchi K, Inukai
M, Masugata H, Goda F, Haba R, Watanabe S, Senda S and Masaki T:
Clinical significance of autoantibodies to p53 protein in patients
with autoimmune liver diseases. Can J Gastroenterol. 26:125–129.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okada R, Shimada H, Otsuka Y, Tsuchiya M,
Ishii J, Katagiri T, Maeda T, Kubota Y, Nemoto T and Kaneko H:
Serum p53 antibody as a potential tumor marker in extrahepatic
cholangiocarcinoma. Surg Today. 47:1492–1499. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ito M, Hiwasa T, Oshima Y, Yajima S,
Suzuki T, Nanami T, Sumazaki M, Shiratori F, Funahashi K, Li SY, et
al: Association of serum anti-PCSK9 antibody levels with favorable
postoperative prognosis in esophageal cancer. Front Oncol.
11:7080392021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Z, Gou W, Liu M, Sang W, Chu H and
Zhang W: Expression of P53 and HSP70 in chronic hepatitis, liver
cirrhosis, and early and advanced hepatocellular carcinoma tissues
and their diagnostic value in hepatocellular carcinoma: An
immunohistochemical study. Med Sci Monit. 21:3209–3215. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang K, Chen Y, Liu S, Qiu S, Gao S, Huang
X, Zhang J, Peng X, Qiani W and Zhang JY: Immunogenicity of Ra1A
and its tissue-specific expression in hepatocellular carcinoma. Int
J Immunopathol Pharmacol. 22:735–743. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu J, Li W, Deng M, Liu D, Ma Q and Feng
X: Immunohistochemical determination of p53 protein overexpression
for predicting p53 gene mutations in hepatocellular carcinoma: A
meta-analysis. PLoS One. 11:e01596362016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen L, Liu Y, Zhang Q, Zhang M, Han X, Li
Q, Xie T, Wu Q and Sui X: p53/PCDH17/Beclin-1 proteins as
prognostic predictors for urinary bladder cancer. J Cancer.
10:6207–6216. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bian C, Li Z, Xu Y, Wang J, Xu L and Shen
H: Clinical outcome and expression of mutant P53, P16, and Smad4 in
lung adenocarcinoma: A prospective study. World J Surg Oncol.
13:1282015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kashofer K and Regauer S: Analysis of full
coding sequence of the TP53 gene in invasive vulvar cancers:
Implications for therapy. Gynecol Oncol. 146:314–318. 2017.
View Article : Google Scholar : PubMed/NCBI
|