Introduction
The treatment and prognosis of tumors has been a
major focus of research in tumor-related studies, and the study of
tumor pathogenesis and mechanisms of tumorigenesis is even more
difficult than the study of tumor treatment. In the 1970s, Pierce
and colleagues proposed that tumors are a developmental biology
problem, and he believed that tumorigenesis is closely related to
developmental biology (1,2). In 1892, Lobstein and Recamier
presented a fundamental discussion on whether tumors are embryonic
physiological disorders and their origin (3–5).
They argued that tumors are formed by the continuous proliferation
of embryonic cells stored in the body for a long time and that
there is a high degree of similarity between tumors and embryos
(6). Moreover, a study has shown
that genes related to tumors can affect the normal development and
differentiation of cells (7).
Tumors are the products of embryonic gene expression and the result
of the activation and expression of numerous oncogenes in the body
(7). Early studies have confirmed
that interconversion between tumor cells and early embryonic cells
is possible under specific conditions (8,9). In
2000, Hanahan and Weinberg (2)
proposed six major characteristics of tumor cells, including
unlimited replication, tissue invasion, insensitivity to growth
resistance, self-sufficient growth, evasion of apoptosis and
sustained angiogenesis. Subsequently, in 2011, they added four more
features of tumor cells, including genomic instability, promotion
of inflammation, avoidance of immune response and energy
dysregulation (10). These
characteristics are very similar to the biological features of
early embryonic cells. For example, gene methylation and
demethylation, cell implantation, functional gene expression,
cellular immune evasion and other such aspects in the early
embryonic growth and development are strikingly like the biological
function and behavior of tumor cells (11–14).
Both embryonic and tumor cells can be deprogrammed to achieve a
proliferative stem cell state with potential for apoptosis and
invasiveness. Therefore, it is hypothesized that the set of genes
expressed in tumor cells may be the same as those expressed in
embryonic cells, particularly those genes involved in
deprogramming, proliferation and undifferentiation (4,15–21).
The present study focused on the similarity of gene
expression related to early embryonic development and tumor growth.
Nine factors highly associated with tumor regulation, including
MYC, MYB, BCL-2, BCL-2-interacting protein 3 (BNIP3), p53, PTEN,
PI3K, AKT and mTOR were selected as experimental research subjects.
These nine factors occupy important positions in the large
regulatory network of the body (22–27).
Changing of their expression may lead to changes or even loss of
control of the regulatory network (22,26–37).
In addition, these factors are highly related to the development
and growth, regulatory mechanisms and microenvironment maintenance
of early embryos (4,6,17,30,38–42).
MYC (29,31,43),
MYB (26), BCL-2 (44–46),
BNIP3 (47), p53 (27,48),
PI3K (22,28), AKT (23,49)
and mTOR (32,50–53)
are regarded as proto-oncogenes that serve important roles in cell
proliferation and differentiation, apoptosis, cell cycle regulation
and metabolic processes. In previous studies of mouse embryonic
development, high mRNA and protein expression levels of these
proto-oncogenes were also detected in mouse embryos. The expression
of these proto-oncogenes was highly associated with the successful
implantation of fertilized eggs into the uterus, which could be
determined by observing whether the mice became pregnant (6,30,38–42,45,54–56).
The main function of the anti-oncogenic biomarker,
PTEN, is to promote apoptosis and hinder cell proliferation,
migration and local adhesion (57). Downregulation or loss of PTEN
expression was found in a variety of tumors, such as non-small cell
lung cancer and glioma (37,57).
A previous study showed that PTEN has low expression levels in the
zygote and blastocyst stages in early embryonic development of mice
(58).
Since the early embryos used in the present study
were those before gastrulation, it was impossible to obtain human
or large primate early embryos due to ethical restrictions.
Furthermore, retrieving the early embryos of mice could be painful
for the mice (59). Therefore, the
insect model was chosen in the present study. Drosophila
could be a good model as much of the research on humans has been
conducted with Drosophila (60). However, the eggs of
Drosophila are too small to clearly distinguish the
embryonic development stage within them (61).
Spodoptera litura is an omnivorous and
gluttonous lepidopteran pest and is closely related to
Drosophila (62). Their
eggs are ideal for early embryonic studies, as they are flat and
hemispherical, with a diameter of about 0.4-0.5 mm; they are yellow
and white when they are newly laid, turning black before hatching,
and the eggs are neatly stacked together (63,64).
S. litura early embryonic development occurs between 1 and 8
h after egg laying, with the earliest divisions generally occurring
at 2 h after egg laying (63,64).
In the present study, the newly laid eggs of S. litura were
used to analyze the expression of genes related to tumor
metabolism, to understand the similarities and differences between
these early embryos and tumors.
Materials and methods
Materials
Experiments involved two different cell lines, T98G
human glioma (65) and human
astrocyte (HA) (66), which were
provided by the Kunming Institute of Zoology, Chinese Academy of
Sciences (Kunming, China). The original T98G cells were purchased
from American Type Culture Collection (CRL-1690) and the HA cells
were purchased from ScienCell Research Laboratories, Inc. (#1800).
S. litura was from the Key Laboratory of the University in
Yunnan Province for International Cooperation in Intercellular
Communications and Regulations, Yunnan University (Kunming,
China).
S. litura breeding. S. litura were maintained
in an artificial climate incubator in the following conditions:
Temperature, 27±1°C; humidity, 60–80%; and 12-h light/dark cycle
(67). Larvae were feed with
artificial synthetic diet and adult moths were feed with 10% honey
solution. The formulation of the artificial diet was the same as
that used by the Key Laboratory of the University in Yunnan
Province for International Cooperation in Intercellular
Communications and Regulations (67). Provision of food and water was
ad libitum. Both the larvae and adult moths were fed with a
diet containing a large amount of water, so additional drinking
water was not provided. The feed and honey solution were refreshed
every 2 days. Larvae between first and sixth instar were kept in
breathable boxes until pupation began. Larvae were transferred to a
fine sand box for pupation. The pupae were separated from the male
and female according to the position of the cloaca on the pupae.
After emergence, the adult S. litura were placed in glass
boxes with a 1:1 sex ratio to mate and lay eggs; they were fed with
honey solution at the bottom of the boxes. The mating and spawning
of the eggs were recorded. Two hours after oviposition, the eggs
for RNA extraction were harvested and put into liquid nitrogen for
preservation. The hemocytes of 3rd instar larvae were extracted for
use as a control. The hemolymph was allowed to escape by puncturing
the hematopoietic cavity from the larval gastropods and collecting
it in a 1.5-ml Eppendorf tube containing 5 µl 5% reduced
glutathione. After obtaining 1 ml hemolymph, it was gently pipetted
and centrifuged at 10,000 × g for 5 min at 4°C. The precipitated
fraction was hemocytes.
Cell culture
T98G and HA cells were thawed at 37°C, centrifuged
at 500 × g for 1 min at room temperature to remove DMSO, and
cultured as follows: The T98G cells were cultured at 37°C in a 5%
CO2 atmosphere in Eagle's Minimum Essential Medium
containing L-glutamine (Gibco; Thermo Fisher Scientific, Inc.) with
the addition of 10% (v/v) fetal bovine serum (FBS) (Lonza Group
Ltd.) and 1% (v/v) solution of penicillin and streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) (65). HA cells were cultured in HA medium
containing DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.), 10%
(v/v) FBS (Gibco; Thermo Fisher Scientific, Inc.), 2% B27
supplements (Gibco; Thermo Fisher Scientific, Inc), 3.5 mM glucose
(Sigma-Aldrich), 10 ng/ml fibroblast growth factor 2 (Alomone
Labs), 10 ng/ml epidermal growth factor (Alomone Labs), and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.)
(66).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA samples for qPCR were extracted with RNA
extraction kit (R6934-01; Omega Bio-Tek, Inc.), reverse
transcription was carried out using a PrimeScript™ RT
reagent Kit (RR047Q; Takara Bio, Inc.) according to the
manufacturer's protocol and the concentration was determined. The
relative expression levels of nine tumor-related genes and β-actin
in early embryos and hemocytes of S. litura, the T98G and HA
cell lines were measured by qPCR using an Applied Biosystems 7500
Fast Real-Time PCR System (Thermo Fisher Scientific, Inc.). The
qPCR reaction procedure was as follows: 2 min at 50°C and 10 min at
95°C to activate the enzyme; 5 sec at 95°C and 35 sec at 60°C for
40 PCR cycles; 15 sec at 95°C, 1 min at 60°C, 30 sec at 95°C and 15
sec at 60°C to determine the melt curve. mRNA levels were
quantified using the 2−ΔΔCq method (68) and normalized to the internal
quantitative reference gene β-actin. The primers used for T98G and
HA cells are listed in Table SI,
and those used for S. litura are listed in Table SII.
Artemether (ART) treatment
Hatching rate measurement, head width measurement
and ART treatment were conducted under S. litura breeding
conditions. Newly laid eggs of S. litura were soaked for 10
sec at 27°C in 1 ml ART solution [300 ng/µl dissolved in 0.1%
(v/v)] three times each day until eggs started hatching. As a blank
control, newly laid eggs were soaked for 10 sec at 27°C in 1 ml
H2O, and as a negative control, newly laid eggs were
soaked for 10 sec at 27°C in in 1 ml 0.1% DMSO solution. ART itself
is slightly soluble in water and 0.1% DMSO was added to the
solution to increase the solubility of ART, so a 0.1% DMSO negative
control group was set up in this experiment. The hatching rate of
eggs was counted after 2 days of treatment. For the experiment on
larvae ART treatment, 270 healthy newly hatched larvae were
reselected and were divided into three groups. The groups of larvae
were fed with a normal diet, a diet containing 0.1% DMSO and a diet
containing 300 ng/µl ART, respectively, for 14 days until they
pupated. During the feeding period, the width of the larvae's head
capsule was measured once a day. All ART treatment experiments
included three independent replicates.
Neighbor joining (NJ) tree
construction
The gene sequences of MYB, MYC, BCL-2, BNIP3, p53,
PI3K, AKT, mTOR and PTEN of S. litura, Homo sapiens and 18
other invertebrates and vertebrates were downloaded from the NCBI
database (https://www.ncbi.nlm.nih.gov/gene/?term=). The
accession numbers of all downloaded sequences are listed in
Table SIII. The FASTA file was
analyzed with MEGA7.0 (69), and
the systematic cluster tree was constructed by NJ.
Statistical analysis
mRNA expression levels between T98G cells and HA
cells, and between early embryos and larval hemocytes were compared
by unpaired Student's t-test. mRNA expression of
H2O-treated, DMSO-treated and artemether (ART)-treated
eggs was compared using one-way ANOVA and Tukey. The hatch rate of
H2O-treated, DMSO-treated and ART-treated eggs was
compared using Fisher's test. The 14-day measurements of larvae
head capsule width in the H2O-treated, DMSO-treated and
ART-treated larvae were compared using two-way ANOVA and Tukey. The
14-day measurements of larvae head capsule width were also compared
within all three groups using two-way ANOVA and Tukey to confirm
normal larval growth within the group (70). GraphPad Prism 9.0 (GraphPad
Software, Inc.) was used for data analysis and graph plotting.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Gene building sequence alignment of S.
litura
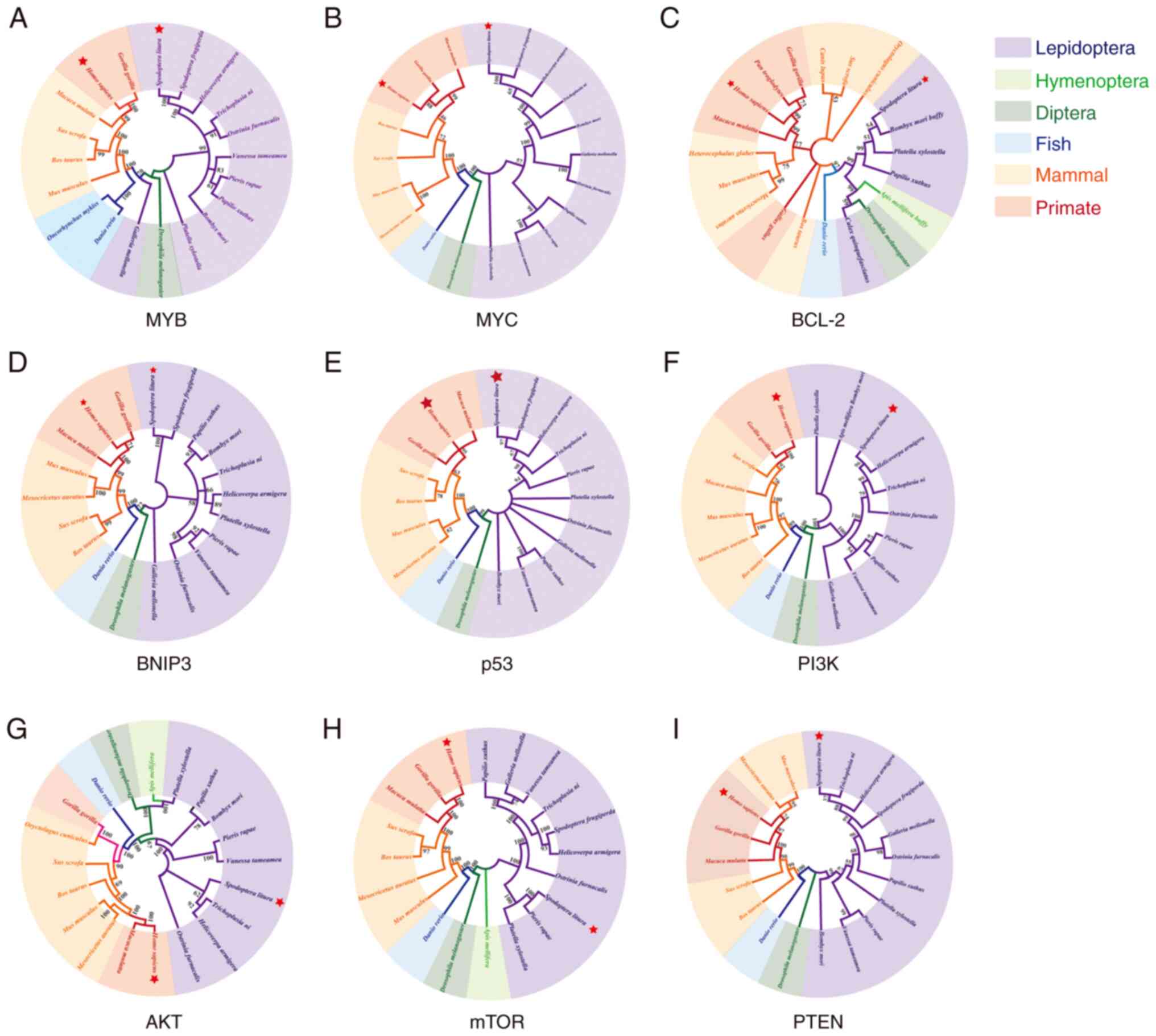
As shown in Fig. 1,
MYB, MYC, BCL-2, BNIP3, p53, PI3K, AKT, mTOR and PTEN genes are
related between the 20 species, indicating the evolutionary
developmental conservation of the nine tumor-related factors.
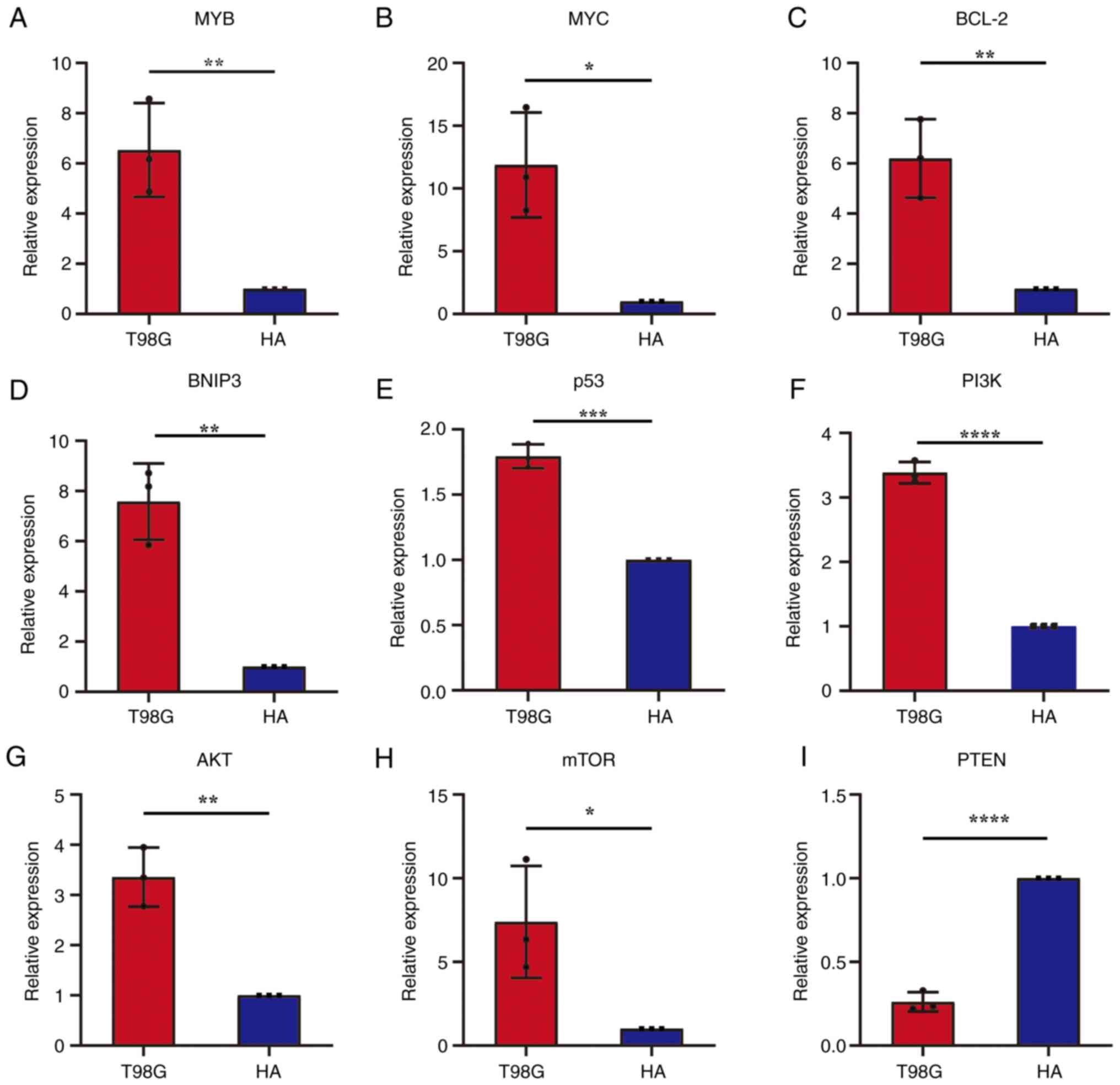
mRNA expression levels of tumor marker
genes in T98G cells
The mRNA expression levels of eight oncogenes
including MYB, MYC, BCL-2, BNIP3, p53, PI3K, AKT and mTOR in T98G
and HA cells were detected (Fig.
2A-H). The results demonstrated that the mRNA expression levels
of the eight oncogenes in T98G cells were significantly higher
compared with that in the control group. The mRNA expression level
of the anti-oncogene, PTEN, in T98G cells was significantly lower
compared with that in the HA cell control group (Fig. 2I). These results demonstrated that
seven of these nine genes may be excellent indicators of tumor
cells. As soon as the expression of these marker genes is detected,
it is possible to distinguish cells which are more like tumor
cells, and which are not.
mRNA expression of tumor marker genes
in early embryos and larval hemocytes of S. litura
The mRNA expression levels of the eight oncogenes
(MYB, MYC, BCL-2, BNIP3, p53, PI3K, AKT and mTOR) and the
anti-oncogene (PTEN) were determined in S. litura early
embryos and larval hemocytes (Fig.
3). The results demonstrated that the mRNA expressions of the
eight oncogenes in early embryos were significantly higher compared
with that in the larval control group (Fig. 3A-H). mRNA expression of the
anti-oncogene, PTEN, in early embryos was significantly lower
compared with that in the larval hemocytes control group (Fig. 3I). The expression levels of these
oncogenes in early embryos of S. litura showed the same
trend in T98G cells, suggesting that the metabolisms of tumor cells
are more like S. litura early embryos than differentiated
cells such as hemocyte.
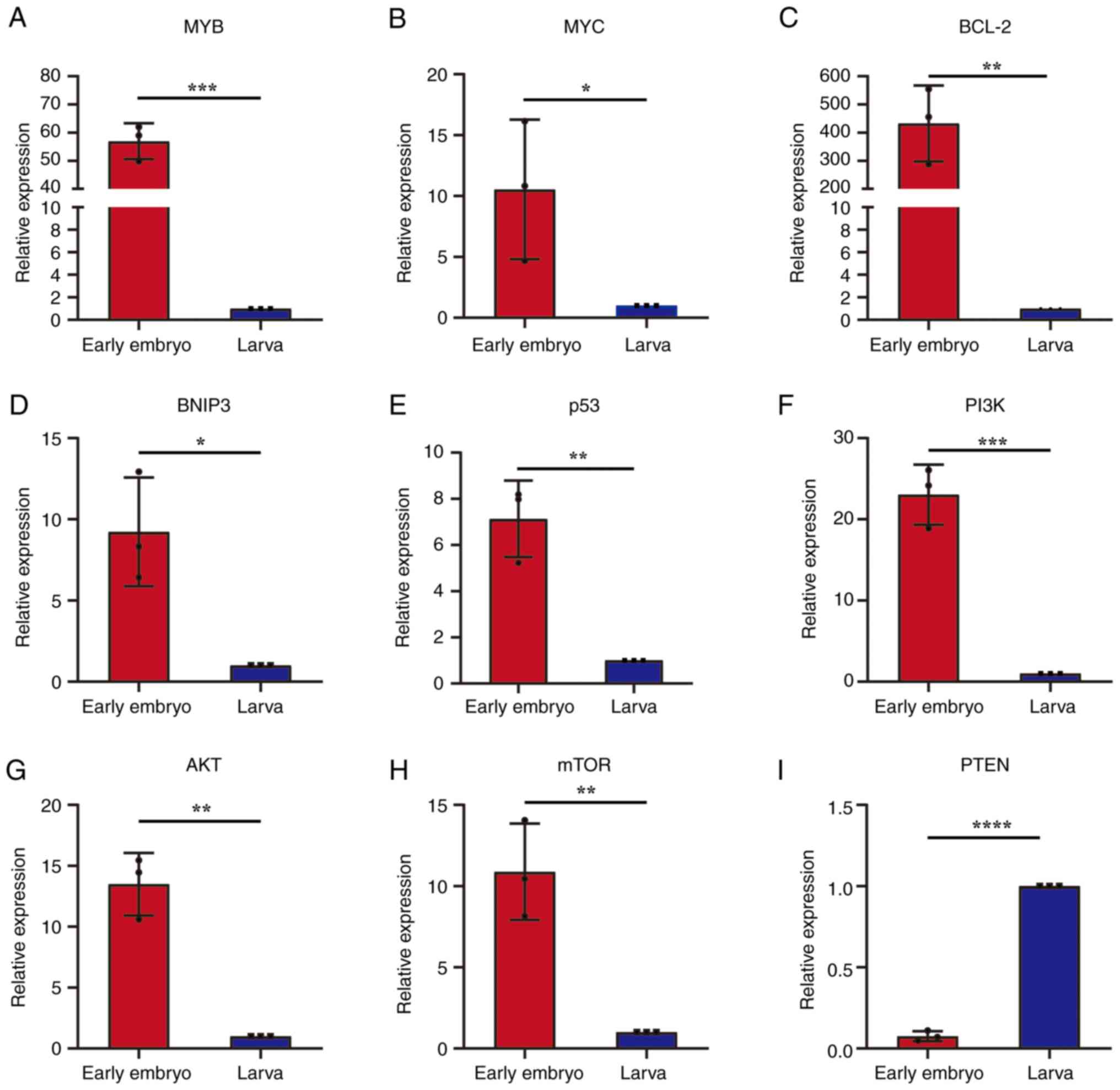 | Figure 3.Relative mRNA expression of eight
oncogenes and one anti-oncogene in early embryos and larval
hemocytes of Spodoptera litura. mRNA expression levels of
the oncogenes (A) MYB, (B) MYC, (C) BCL-2, (D) BNIP3, (E) p53, (F)
pI3K, (G) AKT and (H) mTOR, as well as (I) the anti-oncogene, PTEN,
in early embryonic and larval hemocyte cells. *P<0.05;
**P<0.01; ***P<0.001; ****P<0.0001. BNIP3,
BCL-2-interacting protein 3. |
Anti-cancer drugs cause the death of
eggs but not fully developed larvae
In our previous studies, ART was demonstrated to
exhibit excellent antitumor effects in vitro and in
vivo via targeting several oncogenes and anti-tumor genes
(36,71–75).
The results demonstrated that the hatching rate of eggs was
significantly decreased after ART treatment compared with
H2O treatment and DMSO treatment (Fig. 4A). Furthermore, following ART
treatment, the mRNA expression levels of PI3K, AKT, mTOR and p53 of
early embryos were also significantly downregulated (Fig. 4B). However, when S. litura
1st instar larvae, which had completed their embryonic development
and emerged from eggs, were fed the same concentration of ART
solution for 14 days, the growth and development of larvae were not
significantly affected (Fig. 4C).
These results suggested that embryonic development may share some
similarities with tumor cells in terms of gene expression, which
can be altered by ART.
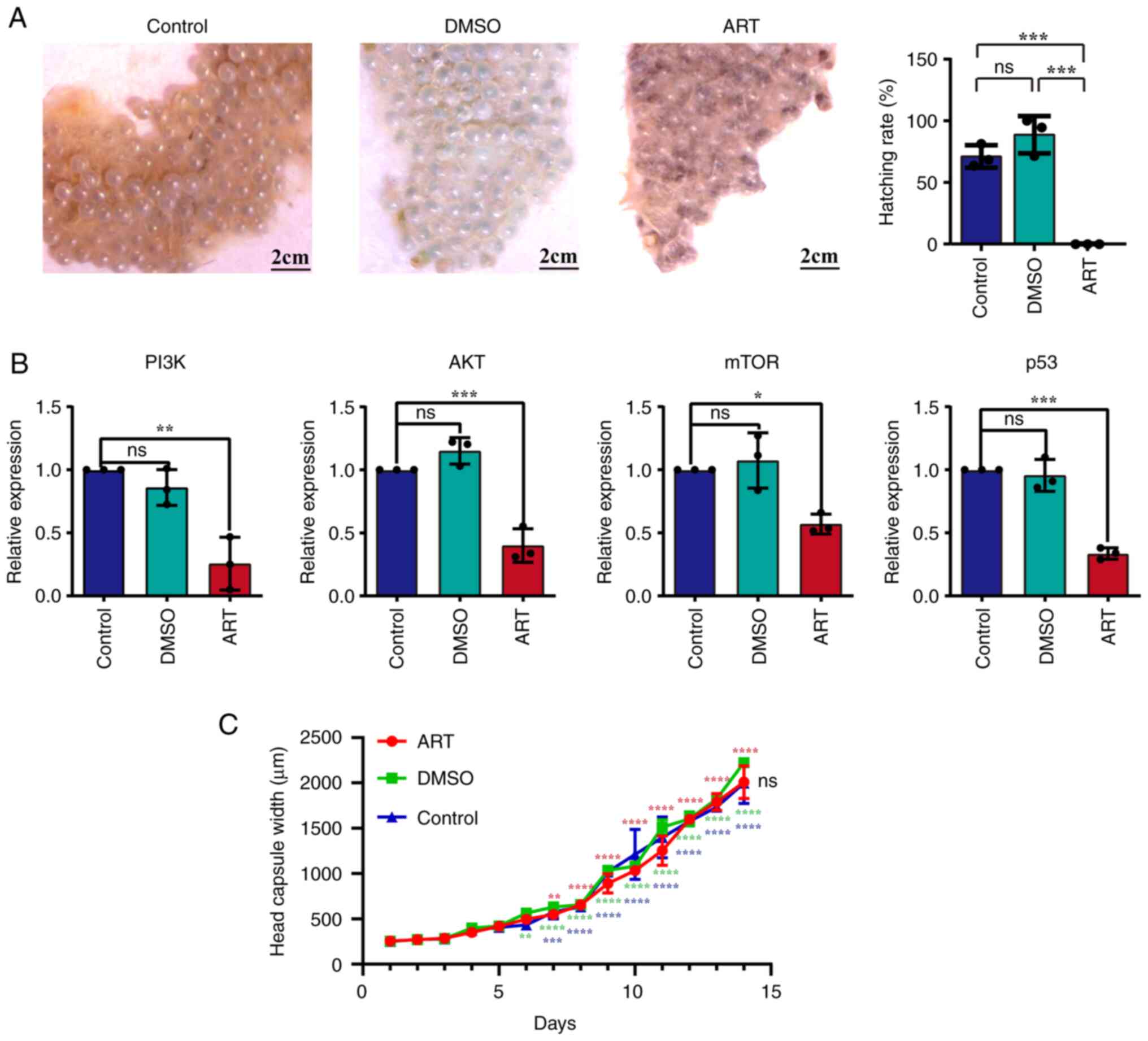 | Figure 4.Viability of Spodoptera litura
eggs and embryos following treatment with the anti-tumor compound,
ART. (A) Eggs looked still healthy and glossy after treatment with
H2O and DMSO, but the eggs became no longer glossy and
gradually turned black after treatment with ART, indicating that
the eggs had started to die. The hatch rates of S. litura
eggs were significantly decreased after ART treatment.
***P<0.001. (B) The mRNA expression levels of PI3K, AKT, mTOR
and p53 in S. litura embryos treated with ART were reduced.
*P<0.05; **P<0.01; ***P<0.001. (C) Head capsule width was
measured and analyzed to evaluate the growth and the development of
S. litura larvae in H2O-treated, DMSO-treated and
ART-treated larvae. To indicate normal larval growth within the
group, the larval head capsule widths within a single group were
also compared with those of newly hatched 1st instar larvae on day
1. All three groups of larvae showed the same growth trend.
**P<0.01, ***P<0.001 and ****P<0.0001 vs. day 1 (same
group). No significant differences in growth trends were observed
among the larvae of the ART, DMSO and control groups. ART,
artemether; ns, not significant. |
Discussion
Malignant glioma is one of the most serious tumors,
yet little is known about the pathogenesis of malignant glioma and
other tumors (36). The causes of
tumor formation are a matter of developmental biology. In our
perspective of view, oncogenic factors in the environment and
oncogenes in cells may initiate the rapid cell division, an ability
obtained by cells after they became embryonic stem cells to ensure
survival (76). In the absence of
oncogenic stimulation, the rate of cell division would be reduced.
Therefore, simply looking for carcinogenic factors and cancer
suppressing drugs is not a solution to the cancer treatment
problems such as side effects and drug resistance. From this
perspective, the present study focused mainly on the similarity of
the regulatory mechanisms between early embryonic development and
tumor growth.
A number of in vitro systems have been
established for the study of malignant gliomas, including the
well-known U87, U251 and T98G cell lines. The glioma cell line used
in this study was T98G because, morphologically, it is a fibroblast
and is more likely to form cell clusters, which are more similar to
the cellular division and proliferation of early embryos (77). In addition, this study detected
expression levels of p53. Therefore, T98G cells, which could stably
express p53, was an excellent candidate (78). Since p53 in S. litura has
two opposite functions of both promoting and inhibiting apoptosis
(79,80), we hypothesized that it could be
determined whether similar mutations had occurred in p53 in early
embryos and hemocytes of S. litura using the sequences of
wild-type and mutant p53 in T98G cells as a reference. However, as
the mutation sites could not be accurately identified by Sanger
sequencing, transcriptome, proteome and SNP analyses will be
performed in future studies.
Since the early embryos used in this study were
those before gastrula, it was not possible to obtain human early
embryos, as it is contrary to ethics and the original intention of
treating diseases. The most commonly used human embryonic cell
lines are not at this stage. Therefore, human embryonic cell lines
were not selected. Retrieving the early embryos of mice could also
be extremely painful for mice, which should be avoided. Other
vertebrates such as chicken were also considered; however, due to
the lack of suitable husbandry facilities and larger breeding
sites, and the difficulty and expense of obtaining other vertebrate
materials, an insect model organism was finally chosen for the
present research. Therefore, we chose the insect S. litura,
which is closely related to Drosophila, as it grows fast, is
inexpensive and is easy to obtain. Besides, S. litura lay
larger eggs and it is easy to observe the embryonic stage in the
eggs with a low magnification dissecting microscope (62,67).
However, S. litura is mainly used for specific innate
immunity studies (67,70,79,81–83),
and, to the best of our knowledge, this is the first time that
S. litura is being used in oncology research. Therefore,
organisms, such as Drosophila, are also included in the NJ
tree to show that S. litura is related to Drosophila
and could potentially be used in oncology research.
Nine factors highly associated with tumor
regulation, including MYC, MYB, BCL-2, BNIP3, p53, PTEN, PI3K, AKT
and mTOR, were selected for investigation. These nine factors
occupy very important positions in the large regulatory network as
changes in their expression and function may lead to changes or
even loss of control of the regulatory network (22–27).
There is an obvious difference between the present and previous
studies-the use of invertebrates as experimental materials
(23,25–27,29,37,45,84)
According to the NJ phylogenetic trees of 20 species presented in
this study, these nine tumor-related key regulators are
evolutionarily conserved in these species, suggesting that their
functions may also be conserved.
The results of the present study revealed high
expression levels of MYC, MYB, BCL-2 and BNIP3 mRNA in T98G cells,
which was also observed in early embryos. The oncogenes MYC and MYB
may serve similar roles in the growth-promoting regulation
mechanisms of early embryogenesis and tumor growth (25,26,29),
and the oncogenes BCL-2 and BNIP3 may serve anti-apoptotic and
microenvironmental roles in both early embryonic stage and tumor
growth (25,46,85,86).
This conclusion supports the embryogenic concept of a tumor and
indicated that functional genes that serve a dominant role in the
tumor and early embryos may be identical.
The high mRNA expression level of the BNIP3 in early
embryos and tumor cells suggested that the microenvironment of the
early embryo may be highly similar to that of a tumor. BNIP3 is a
downstream target protein of hypoxia-inducible factor, HIF-1α
(24). High expression of HIF-1α
in hypoxic environments can directly promote the high expression of
BNIP3. HIF-1α and BNIP3 serve important roles in the maintenance of
the hypoxic microenvironment in early embryos and tumors (24,47,84).
In a previous study, it was demonstrated that embryonic development
and embryonic cell growth require a good healthy microenvironment
rather than a hypoxic tumor-like microenvironment (87). Therefore, the high similarity of
the microenvironment between early embryos and tumors suggests that
there may be a high degree of similarity between the gene
expression in early embryos and tumor cells, as the similar
microenvironments are regulated by the similar expression of
genes.
p53 mRNA expression in the early embryos of S.
litura was also examined. Previous studies have reported that
bracovirus could upregulate p53 in S. litura larval
hemocytes to induce apoptosis, suggesting that p53 in hemocytes
might function similarly to human wild-type p53 (67,80).
However, p53 mRNA expression in early embryos of S. litura
and T98G cells were higher compared with larval hemocytes and HA
cells, respectively, suggesting that p53 in early embryos,
consistent with tumors, lost its apoptotic function and serves a
role as growth promoter (48). The
high expression of p53 in early embryos suggested similarities in
the expression and function of p53 between early embryos and
tumors.
The expression of the anti-oncogene, PTEN mRNA in
T98G cells and early embryos of S. litura exhibited the same
trend of low expression, which suggested that PTEN is expressed at
a very low level or not expressed at all in the early embryonic
stage or in tumors, and the proapoptotic effect is inhibited.
Previous studies demonstrated that PTEN expression was low in tumor
cells compared with normal cells (37,57).
The present results suggested that PTEN expression was also low in
S. litura early embryos. The similarity of the early embryo
and the tumor cell was further demonstrated by the low expression
of PTEN in both compared with that in normal somatic cells.
The high expression levels of the PI3K, AKT and mTOR
in early embryos and T98G cells suggested that this signaling
pathway may serve an important regulatory role in both, confirming
the high similarity of signaling pathway regulation between early
embryos and tumors. Previous studies have demonstrated that PI3K,
AKT and mTOR expression was high in tumor cells compared with
normal cells (23). The present
results suggested that these genes were also highly expressed in
S. litura early embryos. mTOR is an important node at which
multiple signaling pathways intersect and is therefore an important
link in the regulatory network (23,88,89).
In the present study, the similarity in the
expression trends of functionally important genes between the early
embryos and tumor cells was discussed by comparing the mRNA
expression levels of nine evolutionarily conserved tumor-associated
regulatory factors in early embryos and T98G cell lines. In
addition, when early embryos and developed larvae of S.
litura were treated with artemether [It was considered a
typical antitumor compound in our previous studies, which could
cause apoptosis by inhibiting the expression of oncogenes such as
mTOR and BCL-2 and increasing the expression of oncogenes such as
PTEN in cancer cells; however, artemether has no effect on normal
cells (36,72–75)], our results demonstrated that
artemether killed the early embryos but not the larvae. In
addition, the present study revealed that the expression of
oncogenes was reduced, and the expression of the anti-tumor gene
was increased in S. litura early embryos after treatment
with artemether, and the hatching rate of eggs was reduced, and the
mortality rate increased after treatment with artemether, which was
similar to the increase in apoptosis of tumor cells after treatment
with artemether (36). These data
suggested that gene expression and metabolism of early embryos and
glioma cells are extremely similar.
The results of the present experiments preliminarily
confirm the concept of the embryonic origin of tumors, and place
tumors from a cancer-based perspective into the perspective of
individual development and evolution, that is, tumor-related
regulatory factors are used to protect and promote early embryonic
development and ensure the normal growth of living individuals in
the early stages of their development (4,5).
However, towards the end of an individual's life, tumor-associated
regulatory factors are reactivated to create an embryonic-like
mechanism, which competes strongly with the host and eventually
outcompetes the host (90,91). The tumor is a life-regulating
mechanism that has evolved over a long period of time and has been
selected by natural selection and is both the beginning and the end
of life (91,92).
The present study will help to reveal the gene
expression regulating early embryonic development, expand the study
of tumorigenesis, enrich the discourse that tumor-associated
regulators are products of individual development and population
evolution, and further contribute to the exploration of the nature
of life and tumors and the complex relationship between them.
Supplementary Material
Supporting Data
Acknowledgements
The authors are would like to thank Professor Li
Wang for helpful discussions and revision of the manuscript
(Department of Medicine, Oncological Sciences and Huntsman Cancer
Institute, University of Utah, UT, USA). The authors would also
like to thank Dr Xi-Cai Wang, Dr Cong-Guo Jin and Dr Xiao-qun Chen
for their technical assistance (Yunnan Tumor Institute, The Third
Affiliated Hospital of Kunming Medical University; Kunming, China)
and Kunming Pharmaceutical Commercial Co., Ltd. for provision of
the artemether.
Funding
This research was supported by The Science and Technology
Project of the Yunnan Health Department (grant no. 2011WS0065) and
The Research and Talent Training Open Foundation of Life Science
College, Yunnan University (Grant no. 2013S213).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QCC, QSZ and CHC conceived and designed the
experiments. DLL performed the qPCR of Spodoptera litura
samples and tumor cell samples. PF and YYZ bred Spodoptera
litura for all experiments, harvested hemocytes and performed
mRNA extraction of hemocytes. YKY and CH performed head capsule
width measurement and collected all measurement data. CWG and SQZ
prepared the ART solution for ART treatment of newly laid eggs and
larvae and performed ART treatment of newly laid eggs. YZ and YYL
analyzed all sequences, calculated genetic distances, variability
and conservation between species, and constructed NJ trees in the
article. QCC, DLL, YZ and YYL analyzed the data. QCC and DLL
drafted the manuscript. CWG, QSZ and CHC and revised the
manuscript. CWG, QSZ and CHC reviewed the manuscript. CWG, QSZ and
CHC confirmed the authenticity of the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pierce GB: The cancer cell and its control
by the embryo. Rous-Whipple Award lecture. Am J Pathol.
113:117–124. 1983.PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krebs ET: Cancer and the embryonal
hypothesis. Calif Med. 66:270–271. 1947.PubMed/NCBI
|
|
4
|
Ma YL, Zhang P, Wang F, Yang JJ, Yang Z
and Qin HL: The relationship between early embryo development and
tumourigenesis. J Cell Mol Med. 14:2697–2701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cofre J and Abdelhay E: Cancer is to
embryology as mutation is to genetics: Hypothesis of the cancer as
embryological phenomenon. Sci World J. 2017:35780902017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murray MJ and Lessey BA: Embryo
implantation and tumor metastasis: Common pathways of invasion and
angiogenesis. Semin Reprod Endocrinol. 17:275–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vogelstein B and Kinzler KW: Cancer genes
and the pathways they control. Nat Med. 10:789–799. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Williams JW III, Carlson DL, Gadson RG,
Rollins-Smith L, Williams CS and McKinnell RG: Cytogenetic analysis
of triploid renal carcinoma in Rana pipiens. Cytogenet Cell Genet.
64:18–22. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bignold LP, Coghlan BL and Jersmann HP:
Hansemann, Boveri, chromosomes and the gametogenesis-related
theories of tumours. Cell Biol Int. 30:640–644. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nordstrom L, Andersson E, Kuci V,
Gustavsson E, Holm K, Ringnér M, Guldberg P and Ek S: DNA
methylation and histone modifications regulate SOX11 expression in
lymphoid and solid cancer cells. BMC Cancer. 15:2732015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gibadulinova A, Tothova V, Pastorek J and
Pastorekova S: Transcriptional regulation and functional
implication of S100P in cancer. Amino Acids. 41:885–892. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carosella ED, Rouas-Freiss N, Tronik-Le
Roux D, Moreau P and LeMaoult J: HLA-G: An immune checkpoint
molecule. Adv Immunol. 127:33–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bagley RG, Honma N, Weber W, Boutin P,
Rouleau C, Shankara S, Kataoka S, Ishida I, Roberts BL and Teicher
BA: Endosialin/TEM 1/CD248 is a pericyte marker of embryonic and
tumor neovascularization. Microvasc Res. 76:180–188. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Monk M and Holding C: Human embryonic
genes re-expressed in cancer cells. Oncogene. 20:8085–8091. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Monk M: Variation in epigenetic
inheritance. Trends Genet. 6:110–114. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stojanov T and O'Neill C: In vitro
fertilization causes epigenetic modifications to the onset of gene
expression from the zygotic genome in mice. Biol Reprod.
64:696–705. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wrenzycki C and Niemann H: Epigenetic
reprogramming in early embryonic development: Effects of in-vitro
production and somatic nuclear transfer. Reprod Biomed Online.
7:649–656. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen HM, Egan JO and Chiu JF: Regulation
and activities of alpha-fetoprotein. Crit Rev Eukar Gene. 7:11–41.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y and Steinbeisser H: Molecular basis
of morphogenesis during vertebrate gastrulation. Cell Mol Life Sci.
66:2263–2273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Katoh M: Networking of WNT, FGF, Notch,
BMP, and Hedgehog signaling pathways during carcinogenesis. Stem
Cell Rev. 3:30–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou JS, Yang ZS, Cheng SY, Yu JH, Huang
CJ and Feng Q: miRNA-425-5p enhances lung cancer growth via the
PTEN/PI3K/AKT signaling axis. BMC Pulm Med. 20:2232020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fattahi S, Amjadi-Moheb F, Tabaripour R,
Ashrafi GH and Akhavan-Niaki H: PI3K/AKT/mTOR signaling in gastric
cancer: Epigenetics and beyond. Life Sci. 262:1185132020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu L, Qi BX and Hou DR: Roles of HIF1α-
and HIF2α-regulated BNIP3 in hypoxia-induced injury of neurons.
Pathol Res Pract. 215:822–827. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Wang H, Ren C, Yu H, Fang W,
Zhang N, Gao S and Hou Q: Correlation Between C-MYC, BCL-2, and
BCL-6 protein expression and gene translocation as biomarkers in
diagnosis and prognosis of diffuse large B-cell lymphoma. Front
Pharmacol. 9:017492019. View Article : Google Scholar
|
|
26
|
Mitra P: Transcription regulation of MYB:
A potential and novel therapeutic target in cancer. Ann Transl Med.
6:4432018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yue X, Zhao Y, Xu Y, Zheng M, Feng Z and
Hu W: Mutant p53 in cancer: Accumulation, Gain-of-Function, and
therapy. J Mol Biol. 429:1595–1606. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang Y, Weng X, Liu C, Li X and Chen C:
Hypoxia enhances activity and malignant behaviors of colorectal
cancer cells through the STAT3/MicroRNA-19a/PTEN/PI3K/AKT axis.
Anal Cell Pathol (Amst). 2021:41324882021.PubMed/NCBI
|
|
29
|
Pennanen M, Hagstrom J, Heiskanen I, Sane
T, Mustonen H, Arola J and Haglund C: C-myc expression in
adrenocortical tumours. J Clin Pathol. 71:129–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
En-Wu Y, Yin-Fang W, Jin-Fang X, Guang-Wei
Y, Li-Huan S and Yan-Peng D: Expressions of HIF-1α, BNIP3, LC3 in
villi from with women early pregnancy missed abortion. J Zhengzhou
Univ (Med Sci). 52:52017.
|
|
31
|
Scognamiglio R, Cabezas-Wallscheid N,
Thier MC, Altamura S, Reyes A, Prendergast ÁM, Baumgärtner D,
Carnevalli LS, Atzberger A, Haas S, et al: Myc depletion induces a
pluripotent dormant state mimicking diapause. Cell. 164:668–680.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mayer IA and Arteaga CL: The PI3K/AKT
pathway as a target for cancer treatment. Annu Rev Med. 67:11–28.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu LF, Wu ZP, Chen Y, Zhu QS, Hamidi S and
Navab R: MicroRNA-21 (miR-21) regulates cellular proliferation,
invasion, migration, and apoptosis by targeting PTEN, RECK and
Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS One.
9:e1036982014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen Y, Yang JL, Xue ZZ, Cai QC, Hou C, Li
HJ, Zhao LX, Zhang Y, Gao CW, Cong L, et al: Effects and mechanism
of microRNA-218 against lung cancer. Mol Med Rep.
23:282021.PubMed/NCBI
|
|
35
|
Chen Y, Hou C, Zhao LX, Cai QC, Zhang Y,
Li DL, Tang Y, Liu HY, Liu YY, Zhang YY, et al: The association of
microRNA-34a with high incidence and metastasis of lung cancer in
gejiu and xuanwei yunnan. Front Oncol. 11:6193462021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu QS, Cao CH, Yang JL, Li HJ, Zhang Y,
Cai QC, Chen Y, Gao CW, Hou C, Li X, et al: Biological effects of
artemether in U251 Glioma cells. Jap J Oncol Clin Res. 2:1–10.
2021.
|
|
37
|
Alvarez-Garcia V, Tawil Y, Wise HM and
Leslie NR: Mechanisms of PTEN loss in cancer: It's all about
diversity. Semin Cancer Biol. 59:66–79. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Elahi F, Lee H, Lee J, Lee ST, Park CK,
Hyun SH and Lee E: Effect of rapamycin treatment during
post-activation and/or in vitro culture on embryonic development
after parthenogenesis and in vitro fertilization in pigs. Reprod
Domest Anim. 52:741–748. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee GK, Shin H and Lim HJ: Rapamycin
influences the efficiency of in vitro fertilization and development
in the mouse: A role for autophagic activation. Asian-Australas J
Anim Sci. 29:1102–1110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Murakami M, Ichisaka T, Maeda M, Oshiro N,
Hara K, Edenhofer F, Kiyama H, Yonezawa K and Yamanaka S: mTOR is
essential for growth and proliferation in early mouse embryos and
embryonic stem cells. Mol Cell Biol. 24:6710–6718. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li Y, Yao Y, Yao B, Huang W and Yang M:
Expression of apoptosis modulation gene bcl-2 and p53 in mouse
preimplantation embryos. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
16:493–494,515. 2000.PubMed/NCBI
|
|
42
|
Pal SK, Crowell R, Kiessling AA and Cooper
GM: Expression of proto-oncogenes in mouse eggs and preimplantation
embryos. Mol Reprod Dev. 35:8–15. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang J, Ma X, Jones HM, Chan LL, Song F,
Zhang W, Bae-Jump VL and Zhou C: Evaluation of the antitumor
effects of c-Myc-Max heterodimerization inhibitor 100258-F4 in
ovarian cancer cells. J Transl Med. 12:2262014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chami M, Prandini A, Campanella M, Pinton
P, Szabadkai G, Reed JC and Rizzuto R: Bcl-2 and bax exert opposing
effects on Ca2+ signaling, which do not depend on their putative
pore-forming region. J Biol Chem. 279:54581–54589. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Singh R, Letai A and Sarosiek K:
Regulation of apoptosis in health and disease: The balancing act of
BCL-2 family proteins. Nat Rev Mol Cell Bio. 20:175–193. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Radha G and Raghavan SC: BCL2: A promising
cancer therapeutic target. Biochim Biophys Acta Rev Cancer.
1868:309–314. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Farrall AL and Whitelaw ML: The
HIF1α-inducible pro-cell death gene BNIP3 is a novel target of
SIM2s repression through cross-talk on the hypoxia response
element. Oncogene. 28:3671–3680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Levine AJ and Oren M: The first 30 years
of p53: Growing ever more complex. Nat Rev Cancer. 9:749–758. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lien EC, Dibble CC and Toker A: PI3K
signaling in cancer: Beyond AKT. Curr Opin Cell Biol. 45:62–71.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xia Z, Gao T, Zong Y, Zhang X, Mao Y, Yuan
B and Lu G: Evaluation of subchronic toxicity of GRD081, a dual
PI3K/mTOR inhibitor, after 28-day repeated oral administration in
Sprague-Dawley rats and beagle dogs. Food Chem Toxicol. 62:687–698.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee DH, Szczepanski MJ and Lee YJ:
Magnolol induces apoptosis via inhibiting the EGFR/PI3K/Akt
signaling pathway in human prostate cancer cells. J Cell Biochem.
106:1113–1122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen H, Zhou L, Wu X, Li R, Wen J, Sha J
and Wen X: The PI3K/AKT pathway in the pathogenesis of prostate
cancer. Front Biosci (Landmark Ed). 21:1084–1091. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xu K, Liu P and Wei W: mTOR signaling in
tumorigenesis. Biochim Biophys Acta. 1846:638–654. 2014.PubMed/NCBI
|
|
54
|
Yan-Hong L, Yuan-Qing Y, Bing Y, Wei-Quan
H and Meng-Geng Y: Expression of the proto-oncogene c-myc products
in early mouse embryos. J Fourth Military Med Univ. 2:253–254.
2000.
|
|
55
|
Jieping C, Clarke D and Bonifer C: Effect
of c-myb on hematopoietic differentiation and shaping of embryonic
stem cells in vitro. J Third Military Med Univ. 27:52005.
|
|
56
|
Hu W, Feng Z, Teresky AK and Levine AJ:
p53 regulates maternal reproduction through LIF. Nature.
450:721–724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gkountakos A, Sartori G, Falcone I, Piro
G, Ciuffreda L, Carbone C, Tortora G, Scarpa A, Bria E, Milella M,
et al: PTEN in lung cancer: Dealing with the problem, building on
new knowledge and turning the game around. Cancers (Basel).
11:11412019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xu W: Localization and expression of PTEN
during early embryonic development and its effects Northwest A
& F University. 2010.
|
|
59
|
Moreno-Moya JM, Ramirez L, Vilella F,
Martínez S, Quiñonero A, Noguera I, Pellicer A and Simón C:
Complete method to obtain, culture, and transfer mouse blastocysts
nonsurgically to study implantation and development. Fertil Steril.
101:e132014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pandey UB and Nichols CD: Human disease
models in Drosophila melanogaster and the role of the fly in
therapeutic drug discovery. Pharmacol Rev. 63:411–436. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Markow TA, Beall S and Matzkin LM: Egg
size, embryonic development time and ovoviviparity in Drosophila
species. J Evol Biol. 22:430–434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cheng T, Wu J, Wu Y, Chilukuri RV, Huang
L, Yamamoto K, Feng L, Li W, Chen Z, Guo H, et al: Genomic
adaptation to polyphagy and insecticides in a major East Asian
noctuid pest. Nat Ecol Evol. 1:1747–1756. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Perveen F, Ahmed H, Abbasi FM, Siddiqui NY
and Gul A: Characterization of Embryonic Stages through Variations
in the Egg's Contents in Spodoptera litura. J Agricultural Sci
Technol. 4:24–36. 2010.(In Chinese).
|
|
64
|
Bi HL, Xu J, Tan AJ and Huang YP:
CRISPR/Cas9-mediated targeted gene mutagenesis in Spodoptera
litura. Insect Sci. 23:469–477. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Abate M, Scotti L, Nele V, Caraglia M,
Biondi M, De Rosa G, Leonetti C, Campani V, Zappavigna S and Porru
M: Hybrid Self-assembling nanoparticles encapsulating zoledronic
acid: A strategy for fostering their clinical use. Int J Mol Sci.
23:51382022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yin JC, Zhang L, Ma NX, Wang Y, Lee G, Hou
XY, Lei ZF, Zhang FY, Dong FP, Wu GY and Chen G: Chemical
conversion of human fetal astrocytes into neurons through
modulation of multiple signaling pathways. Stem Cell Rep.
12:488–501. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhou GF, Chen CX, Cai QC, Yan X, Peng NN,
Li XC, Cui JH, Han YF, Zhang Q, Meng JH, et al: Bracovirus sneaks
into apoptotic bodies transmitting immunosuppressive signaling
driven by integration-mediated eIF5A hypusination. Front Immunol.
13:9015932022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rao X, Huang X, Zhou Z and Lin X: An
improvement of the 2ˆ(−delta delta CT) method for quantitative
real-time polymerase chain reaction data analysis. Biostat
Bioinforma Biomath. 3:71–85. 2013.PubMed/NCBI
|
|
69
|
Kumar S, Stecher G and Tamura K: MEGA7:
Molecular evolutionary genetics analysis version 7.0 for bigger
datasets. Mol Biol Evol. 33:1870–1874. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kou TC, Liu YT, Li M, Yang Y, Zhang W, Cui
JH, Zhang XW, Dong SM, Xu S, You S, et al: Identification of
β-chain of Fo F1-ATPase in apoptotic cell
population induced by Microplitis bicoloratus bracovirus and its
role in the development of Spodoptera litura. Arch Insect Biochem
Physiol. 952017.doi: 10.1002/arch.21389. PubMed/NCBI
|
|
71
|
Wu ZP, Gao CW, Wu YG, Zhu QS, Yan Chen,
Xin Liu and Chuen Liu: Inhibitive effect of artemether on tumor
growth and angiogenesis in the rat C6 orthotopic brain gliomas
model. Integr Cancer Ther. 8:88–92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wu ZP, Gao CW, Wang XC, Wu YG, Zhu QS and
Hu WY: Anti-tumor Effect of artemether in CT-26 colorectal cancer
bearing BALB/c mice. China Cancer. 16:22007.
|
|
73
|
Wu ZP, Zhu QS, Gao CW, Wang XC, Wu YG and
Hu WY: Experiment of inhibitive effect of artemether in different
stages on colorectal cancer growth in BALB/c mice. Chin Clin Oncol.
12:743–745. 2007.
|
|
74
|
Wu ZP, Zhu QS, Wei WL, Huang J, Shen HM
and Tong SY: Study on inhibit ory effects of artemet her on brain
glioma growth and angiogenesis in SD rats. J Kunming Med Univ.
4:16–21. 2012.
|
|
75
|
Zhu QS, Wu ZP, Gao CW, Wu YG and Wang XC:
Experiment of inhibitive efect of artemether on colorectal cancer
growth and angiogenesis in BALB/c mice. Chin J Cancer Prev Treat.
15:189–192. 2008.
|
|
76
|
Liu G, David BT, Trawczynski M and Fessler
RG: Advances in pluripotent stem cells: History, mechanisms,
technologies, and applications. Stem Cell Rev Rep. 16:3–32. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Morimoto T, Nakazawa T, Matsuda R,
Nishimura F, Nakamura M, Yamada S, Nakagawa I, Park YS, Tsujimura T
and Nakase H: Evaluation of comprehensive gene expression and NK
cell-mediated killing in glioblastoma cell line-derived spheroids.
Cancers (Basel). 13:48962021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Park CM, Park MJ, Kwak HJ, Moon SI, Yoo
DH, Lee HC, Park IC, Rhee CH and Hong SI: Induction of p53-mediated
apoptosis and recovery of chemosensitivity through p53 transduction
in human glioblastoma cells by cisplatin. Int J Oncol. 28:119–125.
2006.PubMed/NCBI
|
|
79
|
Li M, Pang Z, Xiao W, Liu X, Zhang Y, Yu
D, Yang M, Yang Y, Hu J and Luo K: A transcriptome analysis
suggests apoptosis-related signaling pathways in hemocytes of
Spodoptera litura after parasitization by Microplitis bicoloratus.
PLoS One. 9:e1109672014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang P: A study on apoptosis in host
hemocytes induced by CypD-p53 interactions promoted by parasitic
Microplitis bicoloratus of Spodoptera litura. Yunnan University;
2019
|
|
81
|
Dong SM, Cui JH, Zhang W, Zhang XW, Kou
TC, Cai QC, Xu S, You S, Yu DS, Ding L, et al: Inhibition of
translation initiation factor eIF4A is required for apoptosis
mediated by Microplitis bicoloratus bracovirus. Arch Insect Biochem
Physiol. 962017.doi: 10.1002/arch.21423. PubMed/NCBI
|
|
82
|
Cai QC, Chen CX, Liu HY, Zhang W, Han YF,
Zhang Q, Zhou GF, Xu S, Liu T, Xiao W, et al: Interactions of Vank
proteins from Microplitis bicoloratus bracovirus with host Dip3
suppress eIF4E expression. Dev Comp Immunol. 118:1039942021.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chen CX, He HJ, Cai QC, Zhang W, Kou TC,
Zhang XW, You S, Chen YB, Liu T, Xiao W, et al: Bracovirus-mediated
innexin hemichannel closure in cell disassembly. iScience.
24:1022812021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gorbunova AS, Yapryntseva MA, Denisenko TV
and Zhivotovsky B: BNIP3 in Lung cancer: To kill or rescue? Cancers
(Basel). 12:33902020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wu Y and Tang L: Bcl-2 family proteins
regulate apoptosis and epithelial to mesenchymal transition by
calcium signals. Curr Pharm Des. 22:4700–4704. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Dlamini Z, Tshidino SC and Hull R:
Abnormalities in alternative splicing of apoptotic genes and
cardiovascular diseases. Int J Mol Sci. 16:27171–27190. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Gu Z, Guo J, Wang H, Wen Y and Gu Q:
Bioengineered microenvironment to culture early embryos. Cell
Prolif. 53:e127542020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Norambuena A, Wallrabe H, McMahon L, Silva
A, Swanson E, Khan SS, Baerthlein D, Kodis E, Oddo S, Mandell JW
and Bloom GS: mTOR and neuronal cell cycle reentry: How impaired
brain insulin signaling promotes Alzheimer's disease. Alzheimers
Dement. 13:152–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Song L, Liu S, Zhang L, Yao H, Gao F, Xu D
and Li Q: MiR-21 modulates radiosensitivity of cervical cancer
through inhibiting autophagy via the PTEN/Akt/HIF-1α feedback loop
and the Akt-mTOR signaling pathway. Tumor Biol. 37:12161–12168.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Somarelli JA: The hallmarks of cancer as
ecologically driven phenotypes. Front Ecol Evol. 9:6615832021.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Merlo LMF, Pepper JW, Reid BJ and Maley
CC: Cancer as an evolutionary and ecological process. Nat Rev
Cancer. 6:924–935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Dujon AM, Aktipis A, Alix-Panabieres C,
Amend SR, Boddy AM, Brown JS, Capp JP, DeGregori J, Ewald P,
Gatenby R, et al: Identifying key questions in the ecology and
evolution of cancer. Evol Appl. 14:877–892. 2021. View Article : Google Scholar : PubMed/NCBI
|