Introduction
Urothelial carcinoma is the most common type of
malignant tumor of the bladder, but collision tumors of the bladder
are rare; they may included urothelial carcinoma and squamous
carcinoma collision, urothelial carcinoma and small cell carcinoma
collision, and urothelial carcinoma and lymphoma collision
(1–3). Certain studies have reported on
primary malignant melanoma of the bladder (4–7), but
to the best of our knowledge, there has been no previous report of
collision between malignant melanoma of the bladder and high-grade
non-invasive urothelial papillary carcinoma. The clinical
manifestations of collision tumor of the bladder include gross
hematuria, urinary tract irritation symptoms, dysuria and urinary
tract infection (1).
Ultrasonography, CT and cystoscopy may be used to reveal
space-occupying lesions in the bladder. Pathology may indicate
different histological morphology and immunohistochemical
expression results. Among them, malignant melanoma of the bladder
is composed of diffuse infiltrating heterotypes of cells, such as
large cell epithelioid, small cell, spindle, clear cell, rhabdoid
or mixed type (4–6). The cytoplasm mostly contains obvious
pigmentation, while certain cases may have no pigmentation.
Urothelial carcinoma may feature high- or low-grade changes.
Immunohistochemistry may indicate that S-100, Melan-A and HMB45 are
expressed in melanomas. Urothelial carcinoma expresses cytokeratin
(CK)pan and GATA3. In the present study, a case of primary
malignant melanoma of the bladder colliding with high-grade
non-invasive urothelial papillary carcinoma was reported. The
clinicopathological characteristics, immunohistochemistry and
treatment prognosis were provided.
Case report
A 74-year-old male patient was admitted to the First
People's Hospital in Xiaoshan District (Hangzhou, China) in May
2018 due to ‘gross hematuria for 3 months and aggravation for 1
week’. The patient reported to have intermittent gross hematuria
without any obvious inducement for 3 months and the hematuria had
aggravated significantly in the past week, occasionally accompanied
by blood clots, but there was no obvious increased frequency of
urination, pain or fever when urinating. There was no history of
malignant melanoma or cellular nevus in the skin or mucous
membranes of other organs, including the penis and urethra. B
ultrasound indicated a substantial mass in the bladder with a size
of 3.1×2.4×2.1 cm; the blood flow signal was rich and the prostate
was enlarged with intense light spots. Contrast-enhanced urological
CT indicated focal thickening of the left posterior wall of the
bladder and a cauliflower-shaped soft-tissue density shadow
protrusive into the bladder was observed. On the contrast-enhanced
scan, the lesion was mild to moderately enhanced, with
space-occupying lesions in the bladder, which was considered
bladder cancer. At three days after the first presentation,
electrosurgical transurethral resection of bladder injury was
performed and cautery was used to stop the bleeding. During the
operation, the cauliflower-shaped tumor was found 1 cm lateral to
the left ureteral opening in the bladder, with a scope of
~3.0×2.0×2.0 cm, which was locally solid and broad, and the tumor
easily bled when touched.
Gross pathological examination indicated a mass of
gray and white broken tissue with a volume of 3.0×2.0×2.0 cm.
Certain sections had a solid structure with a medium-firm texture,
accompanied by clots. The tissue was fixed in 4% neutral formalin
(24 h at 25°C) and embedded in paraffin, and 4-µm serial sections
were prepared and subjected to hematoxylin-eosin staining
(according to a standard protocol) and Envision immunohistochemical
staining (Beijing Jinqiao Zhongshan Biological Co. Ltd.) according
to the standard protocol, as well as fluorescence quantitative PCR
assay.
At low magnification, the tumor was observed to
consist of two components with no tightly linked or translocated
regions (Fig. 1A). At high
magnification, one of the major tumor components was solid and had
lamellar structures. The tumor cells were medium in size and round
or oval in shape, with obvious atypia. Most had a small amount of
cytoplasm, a high nucleoplasmic ratio, hyperchromatic nuclei and
mitosis was obvious. In certain cells of different sizes, the
nuclei were signet-ring cell-like and the cytoplasm had red
staining. Pigmentation was present in the focal area, clear
cytoplasm was observed in the cell and a small number of scattered
aberrant multinucleated giant cells were present, accompanied by a
small amount of necrotic cells. There were obvious apoptotic bodies
in the region, thin-walled or fibrous vessels in the interstitium,
and tumor infiltration into the muscularis propria (Fig. 1B-F). A small fraction of the tumors
exhibited a typical noninvasive high-grade papillary urothelial
carcinoma structure (Fig. 1G),
with a papillary fibrous vascular axis covered by multiple layers
of urothelial cells with atypia, nucleoli and mitotic figures.
Focally, urothelial carcinoma in situ was observed (Fig. 1H).
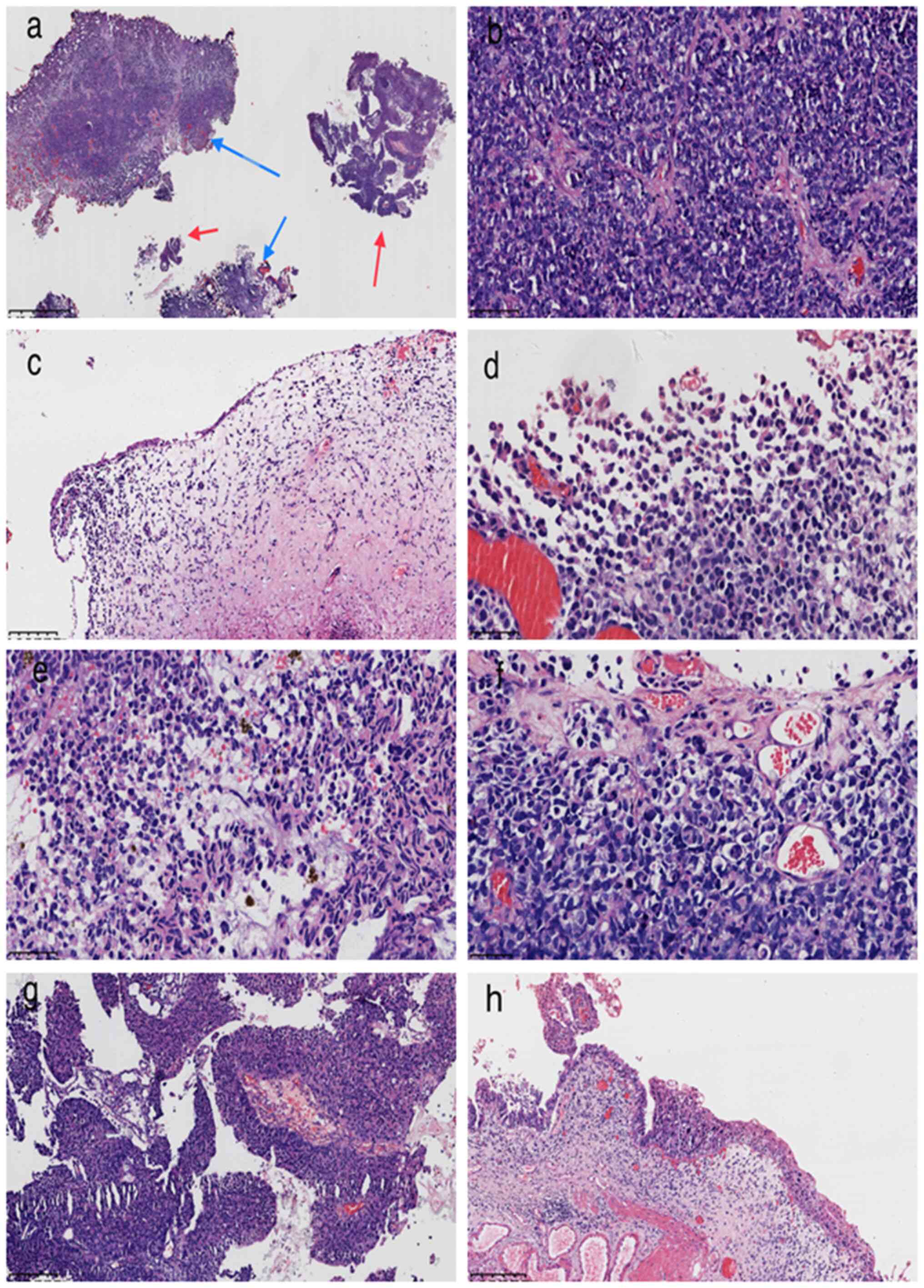 | Figure 1.Tumor histology. (a) The two tumor
components were not closely related or migrated into each other
(blue arrows indicate malignant melanoma and red arrows carcinoma;
magnification, ×1.25; scale bar, 16,000 µm; H&E staining). (b)
Solid growth area of malignant melanoma with round and oval cells
and hyperchromatic nuclei (magnification, ×200; scale bar, 100 µm;
H&E staining). (c) Malignant melanoma tumor cells are located
below the atrophic urothelium; the cells are round and a small
number of scattered multinucleated giant cells are present
(magnification, ×100; scale bar, 200 µm; H&E staining). (d)
Malignant melanoma cells of different sizes, nuclear deviation,
signet-ring cell-like appearance, cytoplasmic red staining
(magnification, ×400; scale bar, 50 µm; H&E staining). (e)
Melanin was found in the cytoplasm of certain tumor cells
(magnification, ×400; scale bar, 50 µm; H&E staining). (f)
Certain malignant melanoma cells have clear cytoplasm
(magnification, ×400; scale bar, 50 µm; H&E staining). (g)
Urothelial papillary carcinoma; the papillary structure is
surrounded by multiple layers of atypical urothelial cells, the
stroma is a component of the fibrous blood vessels (magnification,
×100; scale bar, 200 µm; H&E staining). (h) Focally, urothelial
carcinoma in situ was observed (magnification, ×100; scale
bar, 200 µm; H&E staining). |
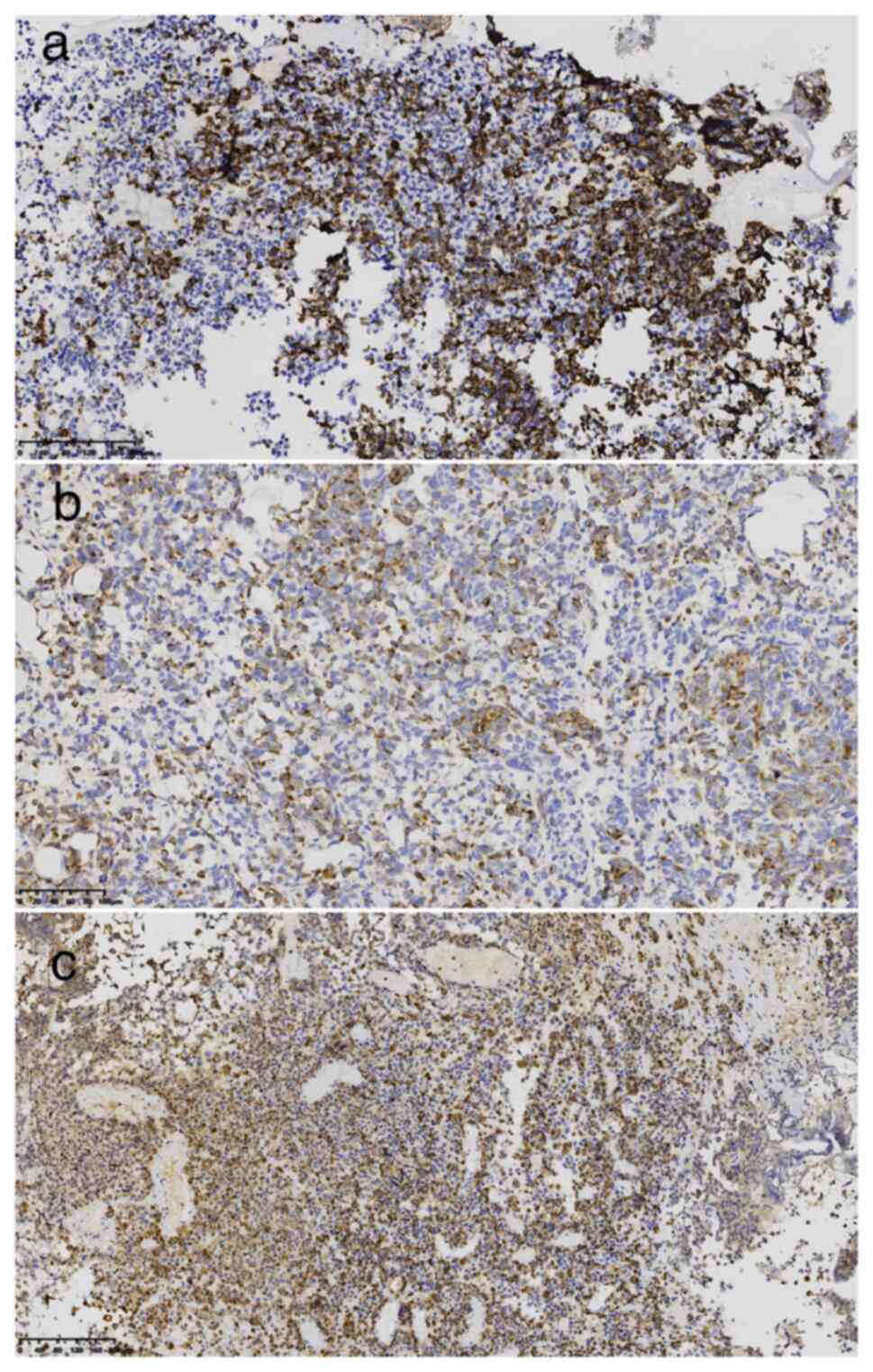
Immunohistochemistry performed with reagents
purchased from Beijing Jinqiao Zhongshan Biological Co. Ltd.
(pre-diluted working solutions unless otherwise indicated)
indicated the following: Mesenchymal tumor cells: HMB45+ (cat. no.
21065615), Melan-A+ (cat. no. 2106160275b), S-100+ (cat. no.
2012240585C8) (Fig. 2A-C), CD56+
(cat. no. 21082702), Ki-67 positive index, 60% (1:200 dilution;
cat. no. 21030436), CKpan- (cat. no. 21061509), leukocyte common
antigen- (cat. no. 0385), Desmin- (cat. no. 20092713), P504S- (cat.
no. 2101200546 a), synaptophysin- (cat. no. 2105130742 c),
Chromogranin A- (cat. no. 21060816); urothelial carcinoma: CKpan+
(cat. no. 21061509), CK20+ (cat. no. 20083095), GATA3+ (cat. no.
19122616). DAB staining solution (polymer method; KIT-0014; Beijing
Jinqiao Zhongshan Biological Co. Ltd.) was applied at 25°C for 20
min.
The fluorescence quantitative PCR assay performed by
Shanghai Keyi Lianchuang Medical Laboratory, Co., Ltd. according to
a standard protocol indicated that BRAF V600E was negative (V600E
forward primer, 5′-GGACCCACTCCATCGAGATTACT-3′ and reverse primer,
5′-TGTTTTCCTTTACTTACTACACCTCAGA-3′; the probe:
FAM-5′-CTGTGAGGTCTTCATGAA-3′-MGB).
The pathological diagnosis was as follows: Primary
malignant melanoma of bladder colliding with high-grade
non-invasive urothelial papillary carcinoma; malignant melanoma
involving muscularis. The patient underwent total cystectomy at an
external hospital without any chemotherapy, immunization or gene
therapy. The patient died of systemic metastasis 31 months after
the first operation.
Discussion
Collision tumors are rare neoplasms consisting of
two or more distinct cell populations that maintain clear
boundaries. They may be composed of two benign tumors, one benign
and one malignant tumor or two malignant tumors (8). The collision of urothelial carcinoma
with squamous cell carcinoma or adenocarcinoma is relatively common
in literature reports, including reports of urothelial carcinoma
with small cell carcinoma or lymphoma (1–3).
Among them, malignant melanoma occurring in the bladder is also
rare (4–7), accounting for <1% of primary
melanoma. The present study reported, for the first time, a case of
collision tumor between malignant melanoma of the bladder and
urothelial carcinoma, and reviewed the literature in order to
deepen the understanding of this disease.
The pathogenesis of bladder collision tumor may be
the result of a variety of pathogenic factors, which have been
reported to include chronic stimulation, smoking and germline
radiotherapy (1). Regarding their
histogenesis, scholars have proposed that the reasonable
explanation is pluripotent stem cells derived from normal
urothelium (9), which also
explains the presence of melanoma and urothelial carcinoma
components in the current case. Histological origins of bladder
malignant melanomas are thought to be melanocyte remnants,
agrophilic cells in normal urothelium or metaplasia of urothelium
(10). At the same time, detailed
general examination and review of the patient's history are
required to exclude metastasis of malignant melanoma in the skin
and other parts of the internal organs. The diagnosis of primary
collision bladder tumor (malignant melanoma and urothelial
carcinoma) was established after the exclusion of systemic
pigmentotic lesions in the present case.
The clinical manifestations include gross hematuria,
urinary tract irritation symptoms, dysuria and urinary tract
infection (1). Imaging
examinations such as B-mode ultrasound and CT may indicate
space-occupying lesions in the bladder, as well as the presence or
absence of invasive changes outside the bladder. Cystoscopy may
indicate single or multiple lesions, a broad-base
cauliflower-shaped mass, papillary or microprotuberant structures
and invasive growth. Non-invasive urine cytology may reveal the
presence of tumor cells and a definite diagnosis may be made only
when combined with certain immunohistochemistry features (11). The pathological characteristics of
bladder collision tumor are different histological morphology and
immunohistochemical expression results according to different
components. Among them, bladder malignant melanoma is composed of
diffuse infiltrating malignant tumor cells, such as large cell
epithelioid, small cell, spindle, clear cell, rhabdoid or mixed
type (4–6). Most of them contain obvious pigment
in the cytoplasm, while certain cases have no pigmentation. Cell
atypia is obvious with large nucleoli and infiltrative growth.
Urothelial carcinoma has no specific morphology and may present
with high-grade or low-grade changes. Immunohistochemistry may
indicate that S-100, Melan-A and HMB45 are expressed in melanoma
and CK was also reported to be puncta-positive around the nuclei of
the tumor cells (5). Urothelial
carcinoma expresses CKpan and GATA3. Molecular examination
indicated that B-raf mutation exists in certain melanoma cases
(5). In the present case, a
collision between a malignant melanoma with a small amount of
pigment and a high-grade noninvasive urothelial papillary carcinoma
was identified, which was confirmed by clinical and pathological
examination.
The differential diagnoses include the following: i)
High-grade urothelial carcinoma with malignant melanin
differentiation (12): The tumor
is rare, and is a high-grade poorly differentiated tumor. The
majority of the tumor cells are morphologically and
immunohistochemically consistent with melanoma, a minority of cells
are positive for urothelial markers and rare cells coexpress both
melanocytic and urothelial markers. Cells that express melanocytic
markers or urothelial markers are closely admixed together. A minor
component of high-grade papillary urothelial carcinoma and
carcinoma in situ is also present. ii) Small round cell
malignant tumors of the bladder, including lymphoma, primitive
neuroectodermal tumor (PNET) and small cell carcinoma. When the
tumor cells exhibit diffuse growth of uniform size,
immunohistochemical staining is necessary for further differential
diagnosis. Immunohistochemistry was positive for lymphoma, PNET or
small cell carcinoma, but negative for melanoma markers. iii)
Metastatic malignant melanoma of the bladder: The primary lesion
may be found mainly by dermoscopy examination of the whole body
skin, or CT and magnetic resonance imaging examination of the whole
body system. iv) Metastatic renal clear cell carcinoma of the
bladder: Primary malignant melanoma of the bladder should be
differentiated when it is of the clear cell type. Morphologically,
renal clear cell carcinoma has small atypia, clear cytoplasm and
round or oval nuclei located in the center of the cell.
Immunohistochemistry is positive for Vim, CK and CD10, while
melanoma markers are negative.
Regarding treatment and prognosis, different
surgical plans may be made according to the different conditions of
patients with collision bladder tumor. If the patient's condition
is good, complete cystectomy is recommended, and if the condition
is generally poor, partial cystectomy is feasible. Whether to
receive radiotherapy and chemotherapy after surgery is still a
controversial issue, which should be determined according to the
pathological type of the collision cancer. Chemotherapy for
malignant melanoma and urothelial carcinoma mainly refers to the
chemotherapy regimens of cutaneous or mucosal malignant melanoma
and urothelial carcinoma. In recent years, breakthrough progress
was made in the targeted therapy of advanced malignant melanoma.
Schindler et al (4) first
reported that Ipilimumab was used to treat patients and achieved
partial response. However, the survival time of patients with
malignant melanoma of the bladder is <3 years (13). The patient of the present study was
diagnosed by electrosurgical resection and then received total
cystectomy at another hospital without chemotherapy, immunization
or gene therapy. The patient died of systemic metastasis 31 months
after surgery.
In conclusion, primary malignant melanoma of the
bladder with high-grade non-invasive urothelial papillary carcinoma
collision is a rare tumor type. The diagnosis depends on clinical
and pathological examinations. The degree of malignancy is high and
recurrence and metastasis occur easily. In general, comprehensive
treatment, mainly surgery, immunotherapy and targeted therapy, may
help improve the prognosis of patients, but the prognosis is poor.
Due to the small number of reported cases reported to date, the
clinical and pathological features, treatment and prognosis require
to be further explored.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
BH and XC drafted the manuscript and conceived the
study. HL and XC were responsible for the collection and analysis
of case data and literature. HG, JY and XC revised the manuscript
and interpreted the data. BH and HL confirm the authenticity of all
the raw data. All authors agreed on the journal to which the
article has been submitted and agreed to be accountable for all
aspects of the work. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the case study to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu D, He D, Nan X, et al: Collision
carcinoma of bladder (report of 9 cases). Chin J Cancer Clin.
33:382006.(In Chinese).
|
|
2
|
Okumura K, Kato K, Furuhashi K, Suzuki K
and Murase T: A collision cancer between urothelial carcinoma and
malignant lymphoma of the urinary bladder: A case report. Hinyokika
Kiyo. 53:649–651. 2007.(In Japanese). PubMed/NCBI
|
|
3
|
Qu Z, Chen J, Qin X, Chen B and Zhuo Y:
Collision carcinoma of bladder: A case report. Guangdong Med.
35:5272014.(In Chinese).
|
|
4
|
Schindler K, Schicher N, Kunstfeld R,
Pehamberger H, Toepker M, Haitel A, Hoeller C and Harmankaya K: A
rare case of primary rhabdoid melanoma of the urinary bladder
treated with ipilimumab, an anti-CTLA 4 monoclonal antibody.
Melanoma Res. 22:320–325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karabulut YY, Erdogan S, Sayar H, Ergen A
and Ertoy Baydar D: Primary malignant melanoma of the urinary
bladder: Clinical, morphological, and molecular analysis of five
cases. Melanoma Res. 26:616–624. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barillaro F, Camilli M, Dessanti P, Gorji
N, Chiesa F, Villa A, Pastorino A, Aschele C and Conti E: Primary
melanoma of the bladder: Case report and review of the literature.
Arch Ital Urol Andro. l90:224–226. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Snajdar E, Ajo AR, Rosen K, Miller R,
Mohammed S, Gordon C, Pui JC and McIntosh G: Primary malignant
melanoma of the urinary bladder. Cureus. 13:e140672021.PubMed/NCBI
|
|
8
|
Bulte CA, Hoegler KM and Khachemoune A:
Collision tumors: A review of their types, pathogenesis, and
diagnostic challenges. Dermatol Ther. 33:e142362020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gandhi JS, Pasricha S and Gupta G:
Collision tumor of the urinary bladder comprising large cell
neuroendocrine carcinoma and adenocarcinoma. Asian J Oncol.
3:144–146. 2017. View Article : Google Scholar
|
|
10
|
Eble JN, Sauter G, Epstein JI and
Sesterhenn IA: WHO Classication of Tumours. Pathology and Genetics:
Tumors of the Urinary System and Male Genital Organs. IARC Press;
Lyon: 2006
|
|
11
|
Hori T, Kato T, Komiya A, Fuse H, Nunomura
S, Fukuoka J and Nomoto K: Primary melanoma of the urinary bladder
identified by urine cytology: A rare case report. Diagn Cytopathol.
42:1091–1095. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu H, Genega EM, Zhuang L and Zhou M:
High-grade urothelial carcinoma with malignant melanocytic
differentiation. Int J Surg Pathol. 29:794–797. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bejrananda T, Sawasdee A, Boonchai S and
Tanthanuch M: Primary malignant melanoma of the bladder: A rare
case report in asia and review of the literature. Res Rep Urol.
13:833–839. 2021.PubMed/NCBI
|