Introduction
Vulvar malignant melanoma (VMM) often arises from
the malignant transformation of a vulvar pigmented nevus and augurs
a poor prognosis (1). VMM is
characterized by the presence of a vulvar mass that may be
accompanied by persistent itching, pain, tenderness, ulcers and
bleeding, and the melanoma may spread directly, through local lymph
nodes or give rise to distant metastases via the bloodstream. The
most commonly affected primary sites are the labia minora, clitoris
and perineum, and most lesions are hyperpigmented (2–4). In
fact, melanosis plays an important role in melanoma progression, as
it can attenuate the efficacy of radiation, chemotherapy and
immunotherapy (5).
VMM is a rare gynecological malignancy that has been
understudied due to its extremely low incidence and lack of clear
high-risk factors. Surgery, including both wide local excision and
radical surgery, is the primary treatment method for VMM (6,7).
However, due to the location of the lesions, it is occasionally
difficult to meet the negative margin distance for resection.
Radiotherapy is only recommended for patients with advanced
inoperable disease or for patients with recurrence of metastasis
after surgery. However, melanoma is generally considered to be
insensitive to radiotherapy and disease remission rates are higher
than expected (8). Strategies
under investigation to improve response rates include simultaneous
immune checkpoint inhibitor (ICI) therapy. ICIs are effective in
the treatment of metastatic vulvar and vaginal melanomas (1). Preclinical studies have shown that
the combination of radiotherapy with ICIs is more effective than
either treatment modality alone (9–13).
In addition, targeted therapy continues to be important for
patients with advanced or metastatic tumors with well-defined
target mutations (13). For
example, encorafenib in combination with binimetinib is considered
to be the first-line treatment for unresectable or advanced VMM
with BRAF V600E/K mutations.
In the present study, a case of advanced giant VMM
is reported for which treatment included hypo-fractionated
radiotherapy (HFRT) combined with ICIs. The patient was evaluated
for 4 months after HFRT and a near complete remission (CR; CR is
defined by 100% resolution of the lesion) of the melanoma in the
radiotherapy target area was observed. This result is contrary to
the general notion that melanoma is insensitive to radiotherapy, so
the feasibility of combining ICIs with HFRT is also briefly
discussed.
Case report
Patient
A 77-year-old woman presented to the Department of
Oncology, Affiliated Hospital of Southwest Medical University
(Luzhou, China) with discomfort in the vaginal opening, recurrent
bloody discharge and vulvar masses in September 2019. Pathology
confirmed the vulvar mass were a malignant melanoma with
hyperpigmentation, which did not carry BRAF mutations. The
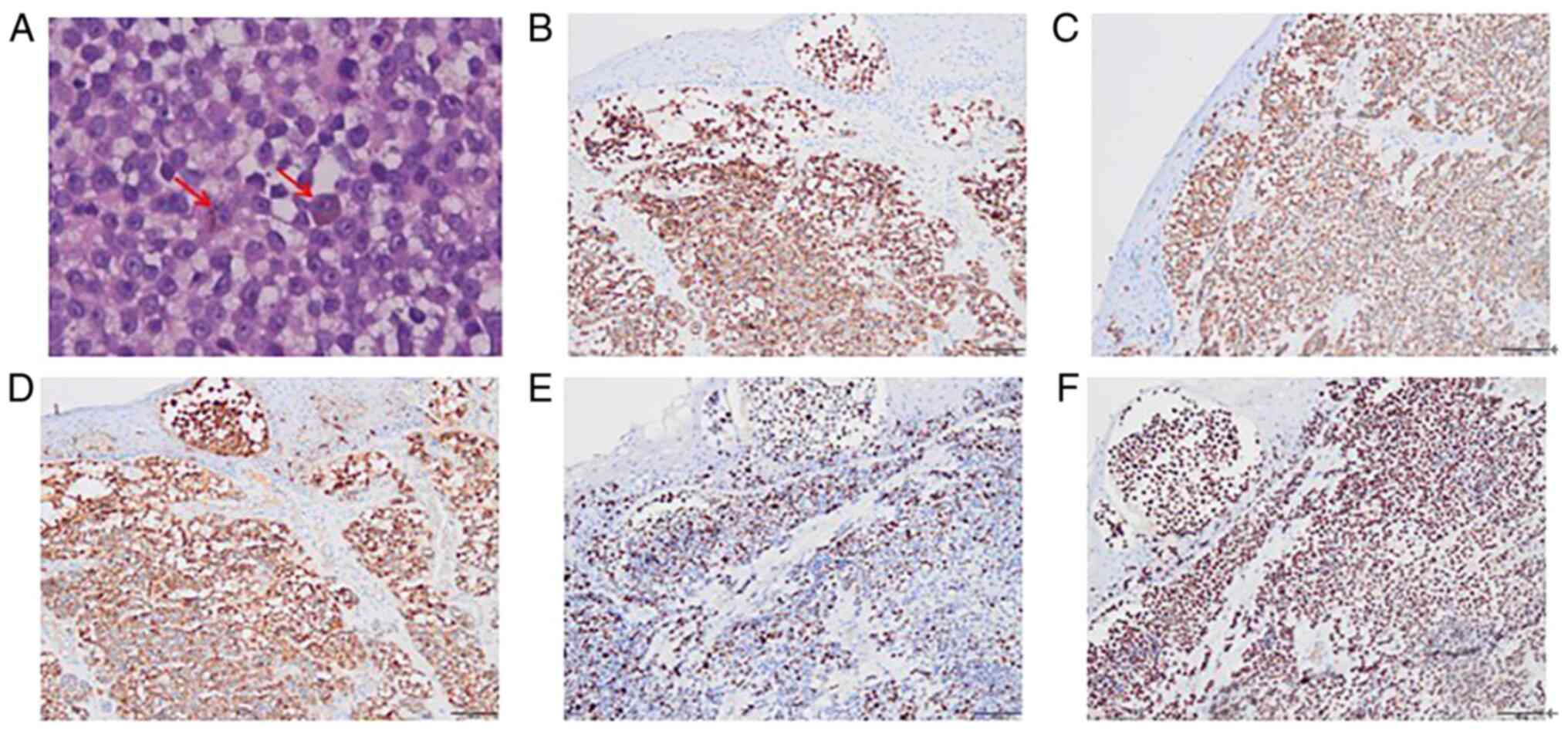
immunohistochemical profile was HMB45+,
Melan-A+, S-100+, Ki-67+ (30%) and
p53+ (90%) (Fig. 1).
The patient did not undergo treatment for financial reasons and the
vulvar mass enlarged, with the patient also experiencing vulvar
ulceration, vaginal bleeding and paroxysmal pain in the lower
abdomen. The melanoma was staged as T4N0Mx using the American Joint
Committee on Cancer Melanoma Tumor-Node-Metastasis Staging System
(8th Edition) (14) and could not
be treated surgically.
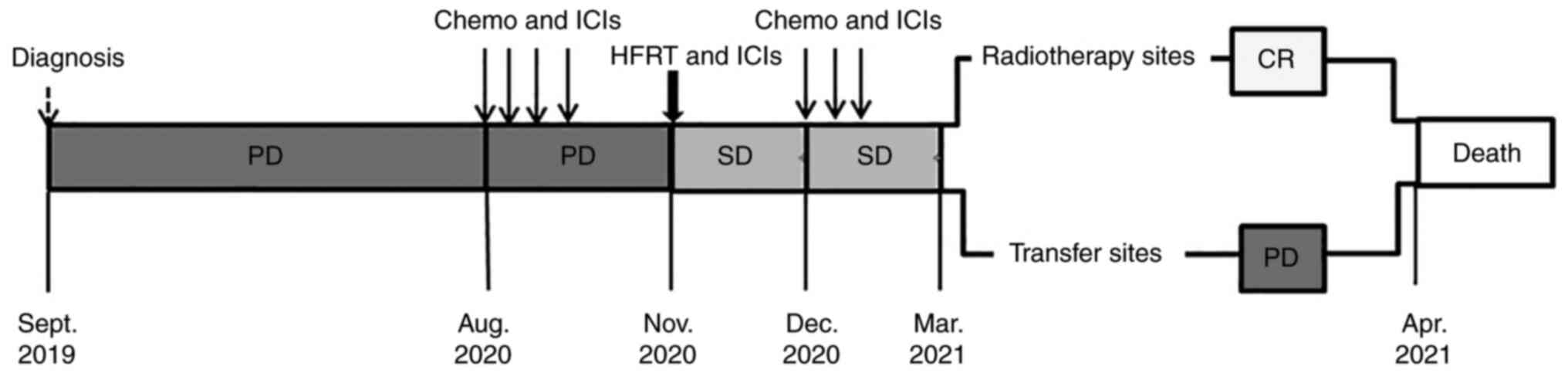
In August 2020, the patient was prescribed four
cycles of triprizumab (240 mg on day 1), dacarbazine (300 mg on
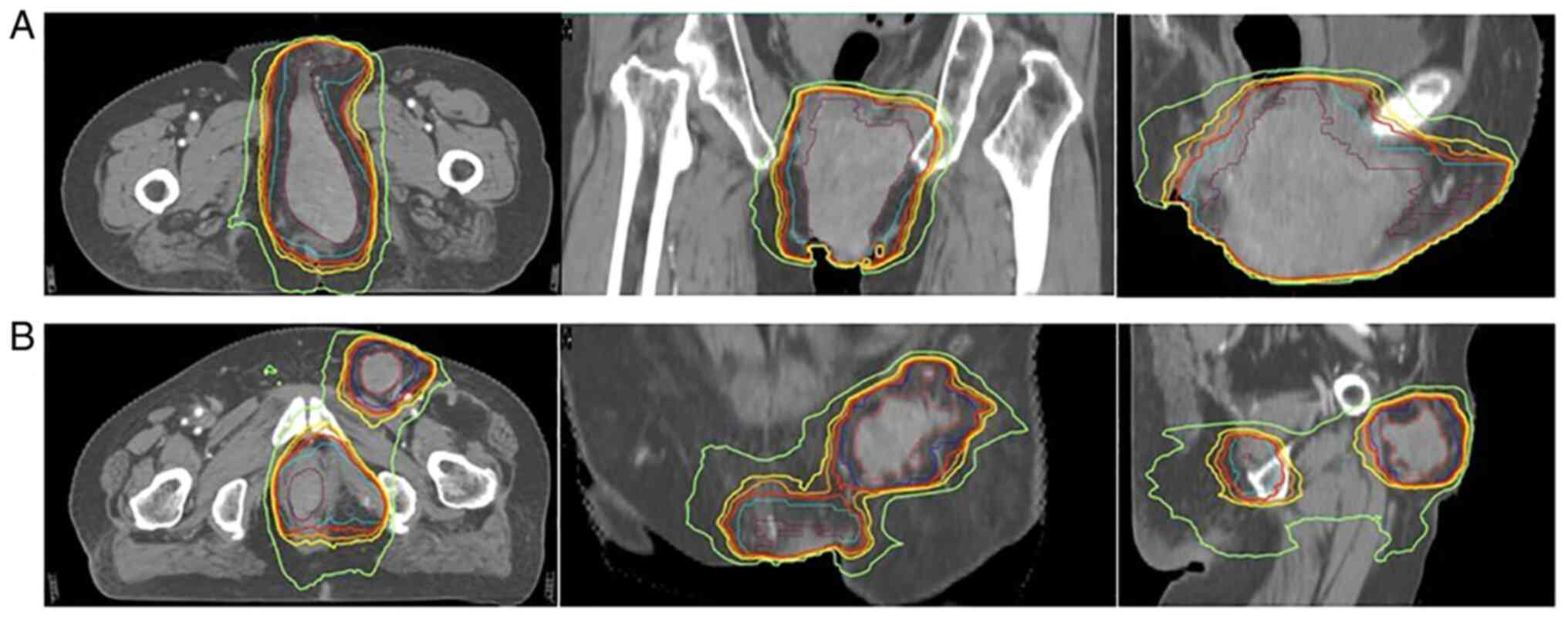
days 1–5) and vincristine (2 mg on day 1) (Fig. 2). In November 2020, the patient
received 3,000 cGy (at six fractions over 12 days, every other day)
of radiation therapy to the perineal tumors and the left inguinal
lymph nodes (Fig. 3), and was also
treated with intensity-modulated radiation therapy (IMRT). The
primary tumor of the vulva corresponded to the gross target volume
(GTV), and the left inguinal lymph nodes were referred to by the
GTV of the nodes; the maximal dose (Dmax) delivered was 3,000 cGy,
with target coverage of 95%. Regarding the organs at risk, the Dmax
deliveries were 393 cGy to the right femur, 1,264 cGy to the left
femur, 3,389 cGy to the pelvis, 3,396 cGy to the bladder and 3,242
cGy to the rectum. After 3 days, two cycles of triprizumab (240 mg,
administered every 2 weeks) were prescribed. In December 2020, the
patient was prescribed three cycles of triprizumab (240 mg on day
1), paclitaxel (210 mg on day 1) and nedaplatin (90 mg on day
1).
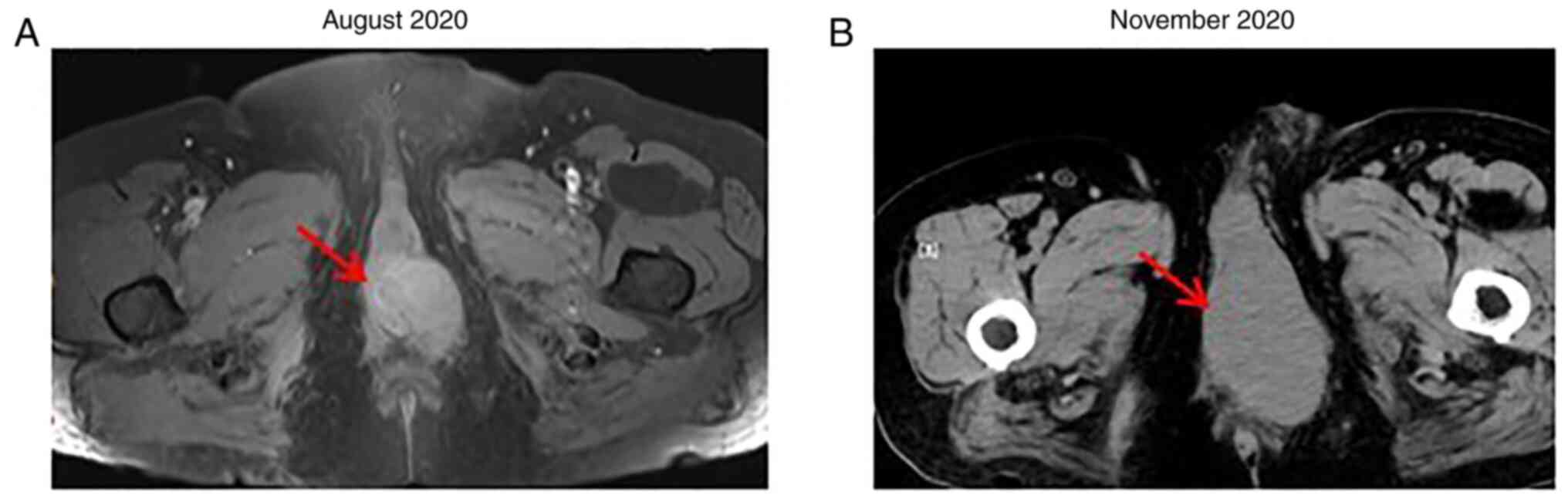
After four cycles of triprizumab, dacarbazine and
vincristine, the CT scan showed that the perineal mass (63 mm in
diameter) (Fig. 4) and the left
inguinal lymph node (34 mm in diameter) were enlarged, with
irregular soft tissue density shadowing. Multiple solid nodules of
different sizes were also noted in both the lungs and in the liver
(the diameter range is provided in Table I). The disease was evaluated as
progressive disease (PD; as defined by a target lesion with a
maximal diameter that increased at least ≥20%, or new lesions).
 | Table I.Change in size of the perineal tumor,
inguinal lymph node tumor, and liver and lungs metastases before
and after treatment. |
Table I.
Change in size of the perineal tumor,
inguinal lymph node tumor, and liver and lungs metastases before
and after treatment.
|
|
|
| Liver | Lungs |
|---|
|
|
|
|
|
|
|---|
| Clinical stage | Perineal tumor,
mm | Inguinal lymph node
tumor, mm | Diameter range,
mm | Severity of
metastases | Diameter range,
mm | Severity of
metastases |
|---|
| At diagnosis | 31 | 10 | / | / | / | / |
| Before chemotherapy
and ICIs | 55 | 29 | 8-15 | + | 3-4 | + |
| After chemotherapy
and ICIs (before HFRT and ICIs) | 63 | 34 | 6-24 | +++ | 3-8 | +++ |
| After HFRT and
ICIs | 58 | 26 | 4-27 | ++++ | 1-14 | ++++ |
| After 4 months of
HFRT and ICIs | 12 | 4 | 1-31 | +++++ | 1-17 | +++++ |
After HFRT and two cycles of triprizumab, a
reduction in the size of the masses (among them, the perineal tumor
was ~58 mm, while the inguinal lymph node tumor was ~26 mm) was
noted, and the disease was evaluated as stable disease (where the
target lesion reduction did not reach a partial response or its
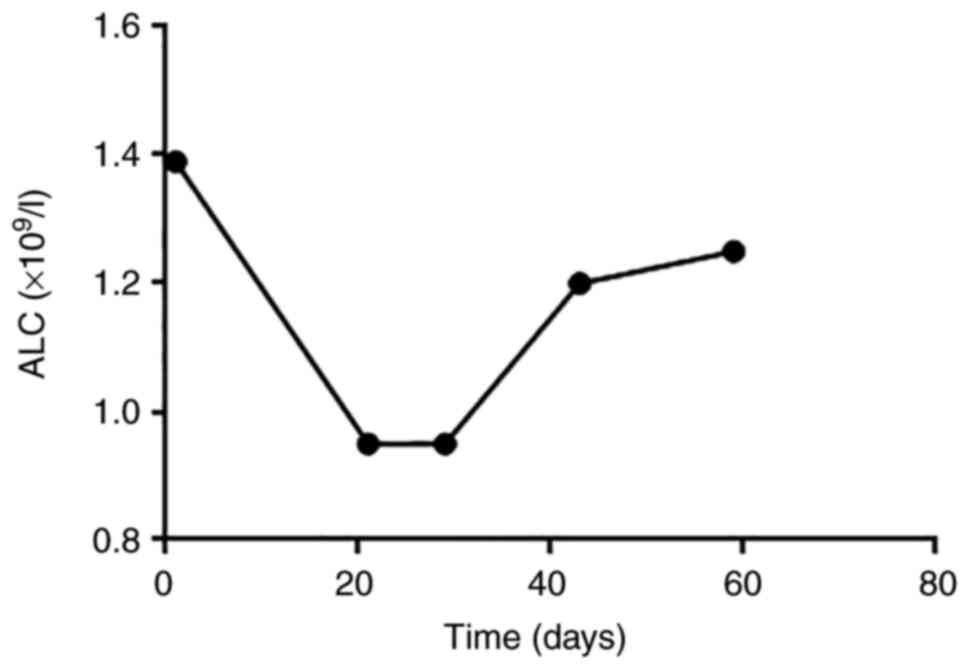
increase did not reach PD). The absolute lymphocyte count (ALC) was
markedly elevated after HFRT and ICIs (Fig. 5) and the patient experienced severe
gastrointestinal adverse reactions.
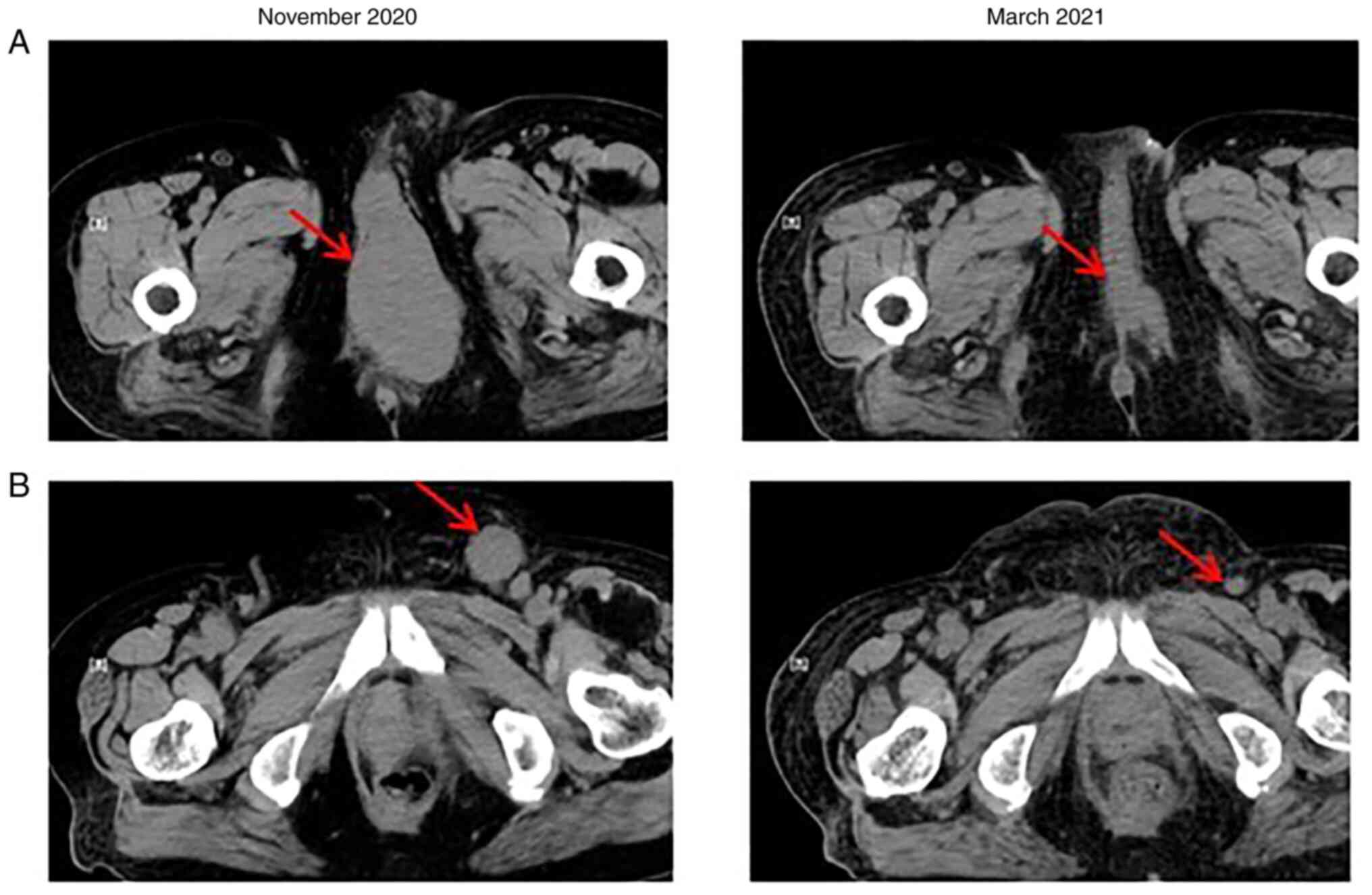
After 4 months of HFRT, a CT scan revealed that the
tumors that had received HFRT experienced marked shrinkage (the
perineal tumor was ~12 mm, while the inguinal lymph node tumor was
~4 mm), which was evaluated as near CR (Fig. 6). However, the metastases in the
liver and lungs continued to grow, new metastases appeared in the
abdominal subcutaneous tissues and enlarged lymph nodes were
observed in the pelvic area; therefore, overall disease state was
evaluated as PD. The patient then received palliative care and died
from liver failure after 1 month.
Pathological methods
Hematoxylin and eosin staining
The tissues were fixed in 10% formalin at 37°C for
1–2 h and sectioned to 3–4 mm. The tissues were then stained with
hematoxylin at 37°C for 8–15 min and with eosin at 37°C for 2–5
min. The tissues were observed with a light microscope at ×100
magnification.
Immunohistochemistry
Tissues were embedded in paraffin and fixed in 10%
formalin at 37°C for 1–2 h, before sectioning to 3–4 mm. Methanol
hydrogen peroxide (3%) was used as the blocking reagent at 37°C for
10 min. The following primary antibodies were used at 37°C for 60
min: HMB45 (1:1,000; cat. no. ZM-0187), MelanA (1:1,000; cat. no.
ZM-0398), S-100 (1:1,000; cat. no. ZM-0224), Ki-67 (1:1,000; cat.
no. ZM-0166), p53 (1:1,000; cat. no. ZM-0408) (all OriGene
Technologies, Inc.). Goat anti-mouse IgG polymer III (1:200; cat.
no. 220426S935c; Fuzhou Maixin Biotech. Co., Ltd.) was used as the
secondary antibody at 37°C for 30 min. The results were observed
using a light microscope at ×200 magnification.
Discussion
Radiotherapy is able to control local lesions, and
the recommended modality for VMM is IMRT (15). The conventional fractionation
schedule is 1.8-2.0 Gy/session, five times/week, with a clinical
target volume (CTV) of 45–50 Gy/25 sessions in the area of the
vulvar lesions and a local push volume of 60–70 Gy to the primary
visible lesions and metastatic lymph nodes (16). Due to issues such as the
lubrication of the vulva, the poor tolerance of skin mucosa to
radiation, a large vulvar tumor and metastases to the lymph nodes,
it is difficult to achieve a satisfactory dose distribution for
radiotherapy. As the aforementioned factors make it difficult for
vulvar cancer to receive the appropriate radiation dose, the effect
of simple radiotherapy for vulvar cancer is poor and the local
recurrence rate is high (8).
The treatment landscape for advanced and metastatic
melanoma has changed markedly with the introduction of ICIs. Trials
with programmed cell death protein 1 (PD-1) inhibitors have shown
significantly improved response rate (28.5%) in patients with
un-resectable or metastatic melanoma (4,17).
The principal function of these inhibitors is to block the
interaction between immune cells and tumor cells that express
immune checkpoint proteins, thus hampering the inhibitory effect of
tumor cells on immune cell function. ALCs and absolute eosinophil
counts are important biomarkers for melanoma treatment that
encompasses ICIs (13,18). Despite promising outcomes in a
phase III clinical trial, a complete response is infrequent, and
most patients who respond eventually exhibit progression (18). Thus, outcomes still can be improved
in these patients.
Previous studies demonstrated that tumor size was
significantly correlated with the efficacy of immunotherapy (i.e.,
smaller tumor loads correlated with higher immunotherapy efficacy)
(19,20), and that HFRT reduced tumor loads
and exhibited specific immunogenicity (21,22).
Thus, the combination of HFRT and ICIs to enhance the
radiotherapy-induced antitumor T-cell response comprises a viable
modality. This approach has been applied to numerous tumor types
with satisfactory results, particularly lung cancer (23–25).
Shaverdian et al (23)
reported that patients with advanced lung cancer who had undergone
radiotherapy prior to immunotherapy manifested longer
progression-free survival (4.4 vs. 2.1 months) and overall survival
(10.7 vs. 5.3 months) times. A study by Theelen et al
(24) on non-small cell lung
cancer revealed that the 1-year survival rate from immunotherapy
combined with radiotherapy increased from 18 to 36% compared with
the immunotherapy-only group. However, compared with other tumor
types, melanoma is not sensitive to radiotherapy, so the same
radiotherapy and immunotherapy combination may have different
therapeutic effects.
In previous years, there have been some reports on
the combination of radiotherapy and immunotherapy in melanoma, but
the segmentation modes and doses have been different. A consensus
on the use of combined therapy has not yet been formed (26–30).
A retrospective study showed higher response rates (33% compared
with 23%) in patients with immune concurrent radiotherapy compared
with immunotherapy alone (29).
Ahmed et al (30) showed
that stereotactic radiotherapy combined with PD-1 may have a
synergistic effect on brain metastatic melanoma. Compared with
non-reproductive melanomas, vulvar and vaginal melanomas have
unique features in terms of their molecular biology and type of
gene mutation, so the prognosis is very different.
There are few reports concerning this combination
therapy for vaginal melanoma. Parisi et al (31) reported that a patient with vaginal
melanoma achieved CR after HFRT combined with ICIs. Schonewolf
et al (32) presented two
cases of vaginal melanoma, where both patients achieved CR. The
patients were treated at different comprehensive cancer centers
using a multimodality approach of immunotherapy and stereotactic
body radiation therapy, with or without surgical therapy. However,
there have been no reports on combination therapy for vulvar
melanoma treatment.
Based on the aforementioned theoretical basis and
the present research results, we propose to improve treatment
efficacy for advanced melanoma through use of local radiotherapy in
combination with ICIs. In the present case, the patient received
chemotherapy combined with ICIs, but the results revealed that the
combined therapy did not achieve satisfactory results. The patient
did not consent to gross pathology images being captured of the
results at this point. The patient then received HFRT combined with
ICIs, and the results showed that the perineal tumor and inguinal
lymph node tumors that underwent HFRT shrank significantly, almost
reaching CR, and indicating that this combination exerted a marked
local effect. As aforementioned, melanoma is insensitive to
radiotherapy, with an α/β value of only 0.6 (8). According to the present segmentation
method, the 2 Gy fractionated radiation equivalent dose was ~64 Gy,
which was not enough to allow the melanoma mass to regress.
Therefore, the patient reached near CR of the perineal and left
inguinal lymph node tumors. This may be since HFRT caused presumed
antigen release and stimulated the ICIs to exert better efficacy,
in contrast to follow-up chemotherapy and ICIs. After treatment
with HFRT and ICIs, there was an increase in ALC, one biomarker
that is associated with improved survival rates in patients with
ICI-treated melanoma. This indicated that HFRT modulated the
efficacy of ICIs, as was also shown in previous studies (9–12).
In contrast to the perineal and inguinal lymph node
tumors, the metastases of the liver and lungs increased, the lymph
nodes in the pelvic area enlarged and new metastases appeared in
the abdominal subcutaneous tissue. These observations indicated the
absence of an abscopal effect, which may have been due to the
heterogeneity of the tumor and the tumor microenvironment. Thus,
the release of neoantigens and effector T cells produced by
radiotherapeutic application to a single lesion does not appear
sufficient to produce effects on all metastases, and it is
questionable as to whether it is possible to stimulate the abscopal
effect by combining other treatments with HFRT and ICIs. It is
clear that additional studies need to be conducted to confirm this.
In addition, the present patient was initially diagnosed with
vulvar melanoma and had no melanoma elsewhere. Whether there is a
difference in the efficacy of HFRT combined with ICIs in melanomas
of different organs/areas also requires further study.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SC made substantial contributions to the acquisition
and analysis of data, and drafted the manuscript. CX and QD
contributed to implementing the treatment and the collection of
case information. XD made substantial contributions to the
acquisition and analysis of data, and revised the study critically
for important intellectual content. HY made substantial
contributions to conception and agreed to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved. SC and HY confirm the authenticity of
all the raw data. All authors have all read and approved the final
manuscript, and agree with its submission.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient's husband for publication of this manuscript and all
accompanying images after the patient had succumbed to the
disease.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wohlmuth C, Wohlmuth-Wieser I and
Laframboise S: Clinical characteristics and treatment response with
checkpoint inhibitors in malignant melanoma of the vulva and
vagina. J Low Genit Tract Dis. 25:146–151. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wohlmuth C, Wohlmuth-Wieser I, May T,
Vicus D, Gien LT and Laframboise S: Malignant melanoma of the vulva
and vagina: A US population-based study of 1863 patients. Am J Clin
Dermatol. 21:285–295. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu Y, Tse KY, Lee HHY, Chow KL, Tsang HW,
Wong RWC, Cheung ETY, Cheuk W, Lee VWK, Chan WK, et al: Predictive
biomarkers and tumor microenvironment in female genital melanomas:
A multi-institutional study of 55 cases. Mod Pathol. 33:138–152.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wohlmuth C and Wohlmuth-Wieser I: Vulvar
malignancies: An interdisciplinary perspective. J Dtsch Dermatol
Ges. 17:1257–1276. 2019. View Article : Google Scholar
|
|
5
|
Slominski RM, Sarna T, Plonka PM, Raman C,
Brozyna AA and Slominski AT: Melanoma, melanin, and melanogenesis:
The yin and yang relationship. Front Oncol. 12:8424962022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garbe C, Amaral T, Peris K, Hauschild A,
Arenberger P, Bastholt L, Bataille V, Del Marmol V, Dréno B,
Fargnoli MC, et al: European consensus-based interdisciplinary
guideline for melanoma. Part 2: Treatment-update 2019. Eur J
Cancer. 126:159–177. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garbe C, Amaral T, Peris K, Hauschild A,
Arenberger P, Bastholt L, Bataille V, Del Marmol V, Dréno B,
Fargnoli MC, et al: European consensus-based interdisciplinary
guideline for melanoma. Part 1: Diagnostics-update 2019. Eur J
Cancer. 126:141–158. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strojan P: Role of radiotherapy in
melanoma management. Radiol Oncol. 44:1–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Komatsu-Fujii T, Nomura M, Otsuka A,
Ishida Y, Doi K, Matsumoto S, Muto M and Kabashima K: Response to
imatinib in vaginal melanoma with KIT p.Val559Gly mutation
previously treated with nivolumab, pembrolizumab and ipilimumab. J
Dermatol. 46:e203–e204. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anko M, Nakamura M, Kobayashi Y, Tsuji K,
Nakada S, Nakamura Y, Funakoshi T, Banno K and Aoki D: Primary
malignant melanoma of the uterine cervix or vagina which were
successfully treated with nivolumab. J Obstet Gynaecol Res.
46:190–195. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin L, Xue J, Li R, Zhou L, Deng L, Chen
L, Zhang Y, Li Y, Zhang X, Xiu W, et al: Effect of low-dose
radiation therapy on abscopal responses to hypofractionated
radiation therapy and anti-PD1 in mice and patients with non-small
cell lung cancer. Int J Radiat Oncol Biol Phys. 108:212–224. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mohamad O, Diaz de Leon A, Schroeder S,
Leiker A, Christie A, Zhang-Velten E, Trivedi L, Khan S, Desai NB,
Laine A, et al: Safety and efficacy of concurrent immune checkpoint
inhibitors and hypofractionated body radiotherapy. Oncoimmunology.
7:e14401682018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takahashi J and Nagasawa S:
Immunostimulatory effects of radiotherapy for local and systemic
control of melanoma: A review. Int J Mol Sci. 21:93242020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gershenwald JE, Scolyer RA, Hess KR,
Sondak VK, Long GV, Ross MI, Lazar AJ, Faries MB, Kirkwood JM,
McArthur GA, et al: Melanoma staging: Evidence-based changes in the
American joint committee on cancer eighth edition cancer staging
manual. CA Cancer J Clin. 67:472–492. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gaffney DK, King B, Viswanathan AN,
Barkati M, Beriwal S, Eifel P, Erickson B, Fyles A, Goulart J,
Harkenrider M, et al: Consensus recommendations for radiation
therapy contouring and treatment of vulvar carcinoma. Int J Radiat
Oncol Biol Phys. 95:1191–1200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rao YJ, Chundury A, Schwarz JK,
Hassanzadeh C, DeWees T, Mullen D, Powell MA, Mutch DG and Grigsby
PW: Intensity modulated radiation therapy for squamous cell
carcinoma of the vulva: Treatment technique and outcomes. Adv
Radiat Oncol. 2:148–158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Indini A, Di Guardo L, Cimminiello C,
Lorusso D, Raspagliesi F and Del Vecchio M: Investigating the role
of immunotherapy in advanced/recurrent female genital tract
melanoma: A preliminary experience. J Gynecol Oncol. 30:e942019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bence C, Hofman V, Chamorey E, Long-Mira
E, Lassalle S, Albertini AF, Liolios I, Zahaf K, Picard A,
Montaudié H, et al: Association of combined PD-L1 expression and
tumour-infiltrating lymphocyte features with survival and treatment
outcomes in patients with metastatic melanoma. J Eur Acad Dermatol
Venereol. 34:984–994. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Theelen WSME, Chen D, Verma V, Hobbs BP,
Peulen HMU, Aerts JGJV, Bahce I, Niemeijer ALN, Chang JY, de Groot
PM, et al: Pembrolizumab with or without radiotherapy for
metastatic non-small-cell lung cancer: A pooled analysis of two
randomised trials. Lancet Respir Med. 9:467–475. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang AC, Postow MA, Orlowski RJ, Mick R,
Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, et al: T-cell
invigoration to tumour burden ratio associated with anti-PD-1
response. Nature. 545:60–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Welsh J, Menon H, Chen D, Verma V, Tang C,
Altan M, Hess K, de Groot P, Nguyen QN, Varghese R, et al:
Pembrolizumab with or without radiation therapy for metastatic
non-small cell lung cancer: A randomized phase I/II trial. J
Immunother Cancer. 8:e0010012020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Quéreux G, Wylomanski S, Bouquin R,
Saint-Jean M, Peuvrel L, Knol AC, Hanf M and Dréno B: Are
checkpoint inhibitors a valuable option for metastatic or
unresectable vulvar and vaginal melanomas? J Eur Acad Dermatol
Venereol. 32:e39–e40. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shaverdian N, Lisberg AE, Bornazyan K,
Veruttipong D, Goldman JW, Formenti SC, Garon EB and Lee P:
Previous radiotherapy and the clinical activity and toxicity of
pembrolizumab in the treatment of non-small-cell lung cancer: A
secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol.
18:895–903. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Theelen WSME, Peulen HMU, Lalezari F, van
der Noort V, de Vries JF, Aerts JGJV, Dumoulin DW, Bahce I,
Niemeijer AN, de Langen AJ, et al: Effect of pembrolizumab after
stereotactic body radiotherapy vs pembrolizumab alone on tumor
response in patients with advanced non-small cell lung cancer:
Results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA
Oncol. 5:1276–1282. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pierini S, Mishra A, Perales-Linares R,
Uribe-Herranz M, Beghi S, Giglio A, Pustylnikov S, Costabile F,
Rafail S, Amici A, et al: Combination of vasculature targeting,
hypofractionated radiotherapy, and immune checkpoint inhibitor
elicits potent antitumor immune response and blocks tumor
progression. J Immunother Cancer. 9:e0016362021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Escorcia FE, Postow MA and Barker CA:
Radiotherapy and immune checkpoint blockade for melanoma: A
promising combinatorial strategy in need of further investigation.
Cancer J. 23:32–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shabason JE and Minn AJ: Radiation and
immune checkpoint blockade: From bench to clinic. Semin Radiat
Oncol. 27:289–298. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yin G, Guo W, Huang Z and Chen X: Efficacy
of radiotherapy combined with immune checkpoint inhibitors in
patients with melanoma: A systemic review and meta-analysis.
Melanoma Res. 32:71–78. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barker CA, Postow MA, Kronenberg SA, Ma Y,
Yamada Y, Beal K, Chan TA, Callahan MK and Wolchok JD: Concurrent
radiation therapy (RT), ipilimumab (Ipi) and/or nivolumab (Nivo) on
a phase 1 clinical trial. Int J Radiat Oncol Biol Phys. 93
(Suppl):S210–S211. 2015. View Article : Google Scholar
|
|
30
|
Ahmed KA, Stallworth DG, Kim Y, Johnstone
PA, Harrison LB, Caudell JJ, Yu HH, Etame AB, Weber JS and Gibney
GT: Clinical outcomes of melanoma brain metastases treated with
stereotactic radiation and anti-PD-1 therapy. Ann Oncol. 27:434–41.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Parisi S, Lillo S, Cacciola A,
Santacaterina A, Palazzolo C, Platania A, Settineri N, Franchina T,
Tamburella C and Pergolizzi S: Vaginal mucosal melanoma: A complete
remission after immunotherapy and '0-7-21'
radiotherapy regimen (24 Gy/3 fractions/21 days). Folia Med
(Plovdiv). 62:605–609. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schonewolf CA, Jaworski EM, Allen SG,
McLean K, Lao CD, Schuchter LM, Tanyi J and Taunk NK: Complete
response after stereotactic body radiation therapy with concurrent
immunotherapy for vaginal melanoma. Adv Radiat Oncol. 7:1008392021.
View Article : Google Scholar : PubMed/NCBI
|