Introduction
Breast cancer is the most prevalent type of cancer
globally, and accounts for >648,000 cancer-associated deaths
annually (1,2). In China, it is estimated that
~416,000 patients are diagnosed with breast cancer every year,
which results in >117,000 deaths annually (3,4).
Currently, the overall prognosis for patients with breast cancer is
unsatisfactory, partially due to a significant proportion of
patients being diagnosed with this cancer at an advanced stage
(5–7). In response to this, the
identification of novel biomarkers for the risk prediction and
early screening of breast cancer is of great importance.
Exosomes are spherical particles released by cells
that can carry a variety of molecules derived from the cells,
including DNAs, RNAs and proteins (8). Due to their stability and ability to
remain unaffected by the surrounding environment, it has been
suggested that the contents of exosomes exhibit potential as
biomarkers for breast cancer screening (9–11).
With the development of molecular biology, several
specific genes involved in the pathogenesis and progression of
breast cancer have been identified. For instance, previous studies
showed that enabled homolog (ENAH), an actin regulatory protein of
the enabled/vasodilator-stimulated phosphoprotein family, promoted
the proliferation, invasion and epithelial-mesenchymal transition
of breast cancer cells when overexpressed (12,13).
Also, other studies showed that septin 9 (SEPT9), an oncogenic
protein, was dysregulated in patients with breast cancer with lymph
node metastases and regulated the migration of breast cancer cells
via ras homolog family member A/focal adhesion kinase signaling
(14,15). Additionally, epidermal growth
factor (EGF) has been reported to interact with its receptor to
regulate the carcinogenesis and malignant behavior of breast cancer
cells (16,17). Moreover, matrix metalloproteinase-9
(MMP-9) has been revealed to critically increase the migration and
invasion abilities of breast cancer cells, thus reflecting the
aggressiveness of breast cancer (18,19).
C-X-C motif chemokine ligand 8 (CXCL8) has also been shown to
promote breast cancer progression and to be involved in the
immunosuppressive tumor microenvironment. Therefore, CXCL8 is
considered as a potential therapeutic target for breast cancer
(20–22). Accordingly, ENAH, SEPT9, EGF, MMP-9
and CXCL8 are critical genes for the pathogenesis and/or
progression of breast cancer (12–22).
The aforementioned findings indicate that these genes could be used
in the early screening of breast cancer.
The current study aimed to evaluate the association
between the exosomal levels of ENAH, SEPT9, EGF, MMP-9 and CXCL8 in
the blood and the risk of breast cancer, as well as the clinical
characteristics of patients with breast cancer.
Materials and methods
Subjects
Blood samples from 31 (first batch; age range, 30–87
years) and 16 (second batch; age range, 32–68 years) female
patients with breast cancer were collected between January 1 and
June 30, 2021 at Huashan Hospital Affiliated to Fudan University
(Shanghai, China). The inclusion criteria were as follows: i)
Patients diagnosed with breast cancer based on pathological tissue
and imaging examinations; ii) aged >18 years; and iii) willing
to voluntarily participate in the study and provide peripheral
blood (PB). The exclusion criteria were as follows: i) Patients
with other primary solid tumors or malignant hematological
disorders; and ii) female patients diagnosed with breast cancer
during pregnancy or breastfeeding. During the same period, 36
(first batch; age range, 15–85 years) and 27 (second batch; age
range, 23–85 years) patients with benign breast disease were also
enrolled as disease controls (DCs). Additionally, a total of 14
(first batch; age range, 26–73 years) and 19 (second batch; age
range, 28–70 years) healthy subjects were recruited as healthy
controls (HCs). The present study was approved by the Ethics
Committee of Huashan Hospital, Fudan University (Shanghai, China)
and all patients or the guardian for the patient who was <18
years old provided written informed consent prior to
enrollment.
Data documentation
The clinical data of patients with breast cancer,
including age, menopause status, histological type, molecular
subtype and tumor-node-metastasis (TNM) stage were recorded. The
molecular subtype of each patient was determined according to the
Chinese Anti-Cancer Association Breast Cancer Diagnosis and
Treatment Guidelines and Standards (2021 edition) (23). The patients received the
appropriate treatment based on disease stage, patient preferences
and physician recommendations, which were not affected by the
study. All treatments were also recorded.
Sample processing
A total of 4 ml PB was collected from each subject
in an EDTA tube. Plasma was then isolated from each sample using
Ficoll-Paque Plus Reagent (Cytiva) diluted with PBS at a ratio of
1:1, followed by centrifugation at 12,000 × g at 4°C for 15 min.
Subsequently, bind-elute size exclusion chromatography columns
(HiScreen Capto Core 700 column; Cytiva) connected to the ÄKTA Pure
25 chromatography system (Cytiva) were used to capture and purify
exosomes from 1.5 ml plasma at room temperature. The columns were
equilibrated with sterile PBS. The flow rate was 25 ml/min
according to the manufacturer's instruction. Following exosome
capture, reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was carried out to assess the mRNA expression
levels of ENAH and SEPT9 in the exosomes derived from the first
batch of subjects (31 patients with breast cancer, 36 DCs and 14
HCs) and EGF, MMP-9 and CXCL8 in exosomes derived from the second
batch of subjects (16 patients with breast cancer, 27 DCs and 19
HCs).
RT-qPCR
Total RNA was extracted from the exosomes using the
RNeasy Micro Kit (Qiagen GmbH) and then reverse transcribed into
cDNA using the ReverTra Ace® qPCR RT Kit (Toyobo Co.,
Ltd.). The conditions for reverse transcription comprised one cycle
of 37°C for 15 min and 98°C for 5 min. Subsequently, qPCR was
carried out using the KOD SYBR® qPCR Mix (Toyobo Co.,
Ltd.). The thermocycling conditions for qPCR comprised 1 cycle of
98°C for 2 min followed by 40 cycles of 98°C for 10 sec and 61°C
for 30 sec. The relative mRNA expression levels were calculated by
the 2−ΔΔCq method (24). The internal reference genes were
β-actin for ENAH and SEPT9, and glyceraldehyde-3-phosphate
dehydrogenase for EGF, MMP-9 and CXCL8. The primer sequences are
listed in Table SI.
Statistical analysis
SPSS 26.0 (IBM Corp.) and GraphPad Prism 7.01
(GraphPad Software Inc.) software were used for statistical
analysis and graph plotting, respectively. Differences among groups
were compared by Kruskal-Wallis H rank-sum and Wilcoxon rank-sum
tests. The ability of ENAH, SEPT9, EGF, MMP-9 or CXCL8 to
distinguish individuals from different groups was assessed using
receiver operating characteristic (ROC) curves. The normalized
partial area under the curve (AUC) was calculated as previously
described (25). Associations
between exosomal genes and age, menopause, hormone receptor status,
HER2 and Ki-67 were analyzed using Wilcoxon rank-sum tests.
Associations between exosomal genes and histological type and
molecular subtypes were analyzed using Kruskal-Wallis H rank-sum
tests. Associations of exosomal genes with TNM stage were analyzed
using Spearman's rank correlation test. Clinical characteristics
between the two batches were compared using an unpaired Student's
t-test for age and a Chi-square or Fisher's exact test for
categorical data. P<0.05 was considered to indicate a
statistically significant result.
Results
Characteristics of patients with
breast cancer
The mean age of the patients with breast cancer was
54.6±11.5 years, including 2 (4.3%), 7 (14.9%), 10 (21.3%), 17
(36.2%) and 11 (23.4%) patients with triple-negative, luminal A,
HER2-negative luminal B, HER2-positive luminal B and HER2-enriched
breast cancer, respectively. In terms of tumor stage, 4 (8.5%), 13
(27.7%), 22 (46.8%), 6 (12.8%) and 2 (4.3%) patients were diagnosed
with a TNM stage of 0, I, II, III and IV, respectively (Table I). Furthermore, comparative
analyses revealed that there were no differences in the demographic
and disease characteristics of patients with breast cancer between
the two batches (all P>0.05; Table
SII). In addition, the mean age of the DCs and HCs was
44.5±15.5 and 54.3±12.0 years, respectively (Table I).
 | Table I.Clinical characteristics of the study
participants. |
Table I.
Clinical characteristics of the study
participants.
| Items | HCs (n=33) | DCs (n=63) | Patients with
breast cancer (n=47) |
|---|
| Age (years),
mean±SD | 54.3±12.0 | 44.5±15.5 | 54.6±11.5 |
| Menopause, n
(%) |
|
|
|
| No | 25 (75.8) | 23 (36.5) | 36 (76.6) |
|
Yes | 8 (24.2) | 40 (63.5) | 11 (23.4) |
| Histological type,
n (%) |
|
|
|
| Ductal
carcinoma in situ | - | - | 3 (6.4) |
|
Invasive ductal carcinoma | - | - | 34 (72.3) |
|
Invasive lobular
carcinoma | - | - | 4 (8.5) |
|
Others | - | - | 6 (12.8) |
| Molecular subtypes,
n (%) |
|
|
|
|
Triple-negative | - | - | 2 (4.3) |
| Luminal
A | - | - | 7 (14.9) |
|
HER2-negative luminal B | - | - | 10 (21.3) |
|
HER2-positive luminal B | - | - | 17 (36.2) |
|
HER2-enriched | - | - | 11 (23.4) |
| Hormone receptor
status, n (%) |
|
|
|
| ER
negative and PR negative | - | - | 13 (27.7) |
| ER
positive and/or PR positive | - | - | 34 (72.3) |
| HER2, n (%) |
|
|
|
|
Negative | - | - | 19 (40.4) |
|
Positive | - | - | 28 (59.6) |
| Ki-67, n (%) |
|
|
|
|
<30% | - | - | 34 (72.3) |
|
≥30% | - | - | 13 (27.7) |
| TNM stage, n
(%) |
|
|
|
| 0 | - | - | 4 (8.5) |
| I | - | - | 13 (27.7) |
| II | - | - | 22 (46.8) |
|
III | - | - | 6 (12.8) |
| IV | - | - | 2 (4.3) |
| Surgical type, n
(%) |
|
| 47 (100.0) |
|
Modified radical
mastectomy | - | - | 24 (51.1) |
|
Sentinel lymph node
biopsy | - | - | 18 (38.3) |
| Radical
mastectomy | - | - | 15 (31.9) |
|
Breast-conserving surgery | - | - | 5 (10.6) |
| Neoadjuvant
therapy, n (%) | - | - | 9 (19.1) |
| Adjuvant therapy, n
(%) | - | - | 44 (93.6) |
Expression of exosomal ENAH, SEPT9,
EGF, MMP-9 and CXCL8
The exosomal mRNA expression level of ENAH was the
highest in patients with breast cancer, lower DCs and the lowest in
HCs (P<0.001; Fig. 1A).
Additionally, ROC curve analyses showed that exosomal ENAH
exhibited the ability to discriminate between patients with breast
cancer and the DCs (AUC, 0.841; Fig.
1B) and HCs (AUC, 0.859; Fig.
1C). However, it could not discriminate DCs from HCs (AUC,
0.523; Fig. 1D). By contrast, the
mRNA expression levels of exosomal SEPT9 were lowest in patients
with breast cancer, higher in DCs and the highest in HCs
(P<0.001; Fig. 1E). ROC curve
analyses revealed that exosomal SEPT9 was effectively able to
differentiate patients with breast cancer from DCs (AUC, 0.717;
Fig. 1F) and HCs (AUC, 0.830;
Fig. 1G), but not DCs from HCs
(AUC, 0.604; Fig. 1H).
Furthermore, the exosomal mRNA expression level of EGF was the
highest in patients with breast cancer, lower in DCs and the lowest
in HCs (P=0.021; Fig. 2A). ROC
curve analyses demonstrated that exosomal EGF failed to
discriminate patients with breast cancer from DCs (AUC, 0.664;
Fig. 2B). However, it showed an
acceptable ability to differentiate between patients with breast
cancer and HCs, with an AUC value of 0.776 (Fig. 2C), but not DCs from HCs (AUC,
0.607; Fig. 2D). Regarding the
exosomal mRNA expression levels of MMP-9 and CXCL8, no differences
were observed among the patients with breast cancer, DCs and HCs
(both P>0.05; Fig. 2E and I).
ROC curve analyses also revealed that exosomal MMP-9 and CXCL8
could not discriminate patients with breast cancer from DCs or HCs,
or DCs from HCs (Fig. 2F-H and
J-L).
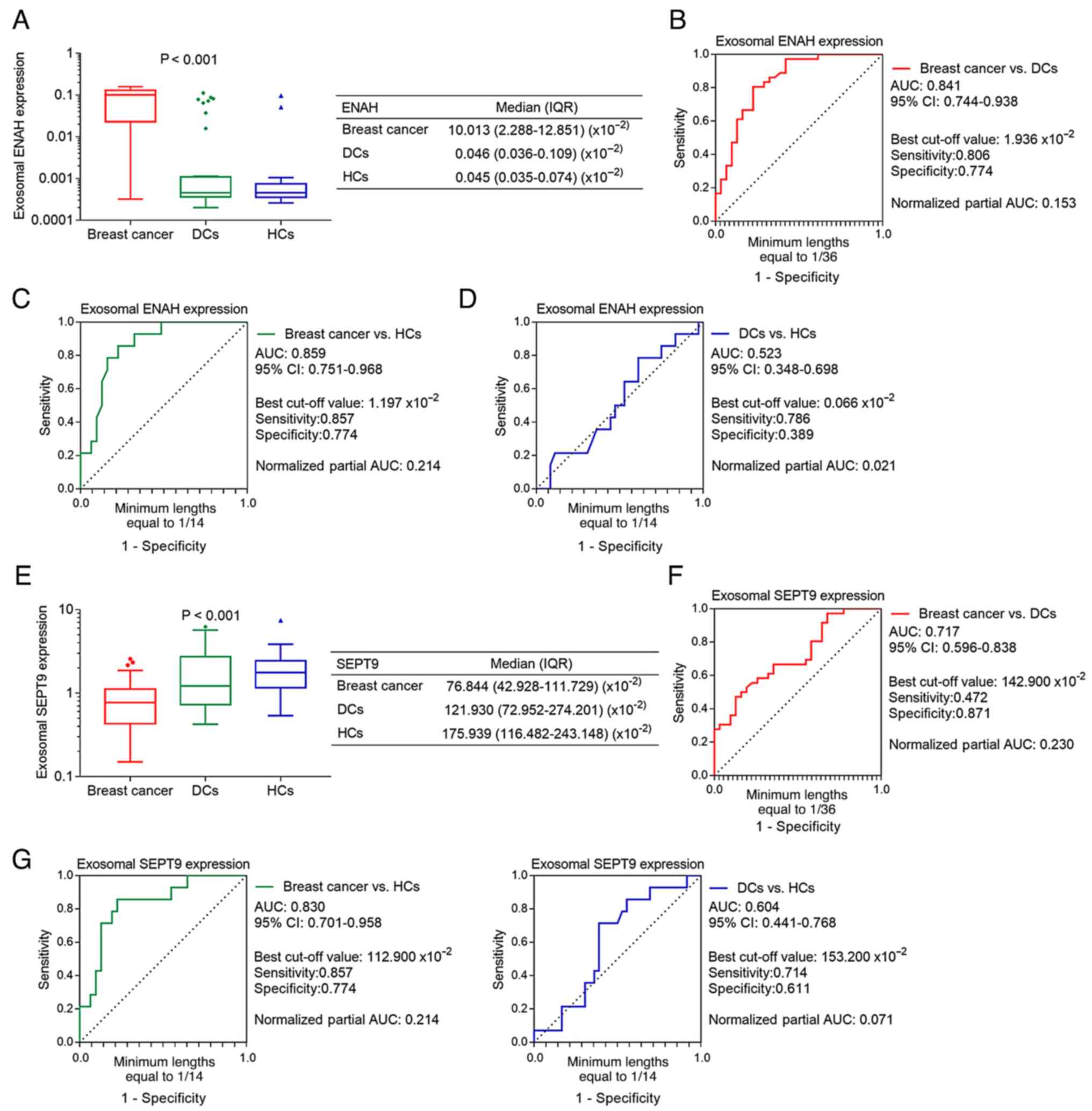 | Figure 1.Differential expression of exosomal
ENAH and SEPT9 among patients with breast cancer, DCs and HCs. (A)
Comparison of exosomal ENAH expression. ROC curve analysis of
exosomal ENAH expression for (B) patients with breast cancer vs.
DCs, (C) patients with breast cancer vs. HCs and (D) DCs vs. HCs.
(E) Comparison of exosomal SEPT9 expression. ROC curve analysis of
exosomal SEPT9 expression for (F) patients with breast cancer vs.
DCs, (G) patients with breast cancer vs. HCs and (H) DCs vs. HCs.
ENAH, enabled homolog; SEPT9, septin 9; DCs, disease controls; HCs,
healthy controls; ROC, receiver operating characteristic; AUC, area
under the curve; IQR, interquartile range; CI, confidence
interval. |
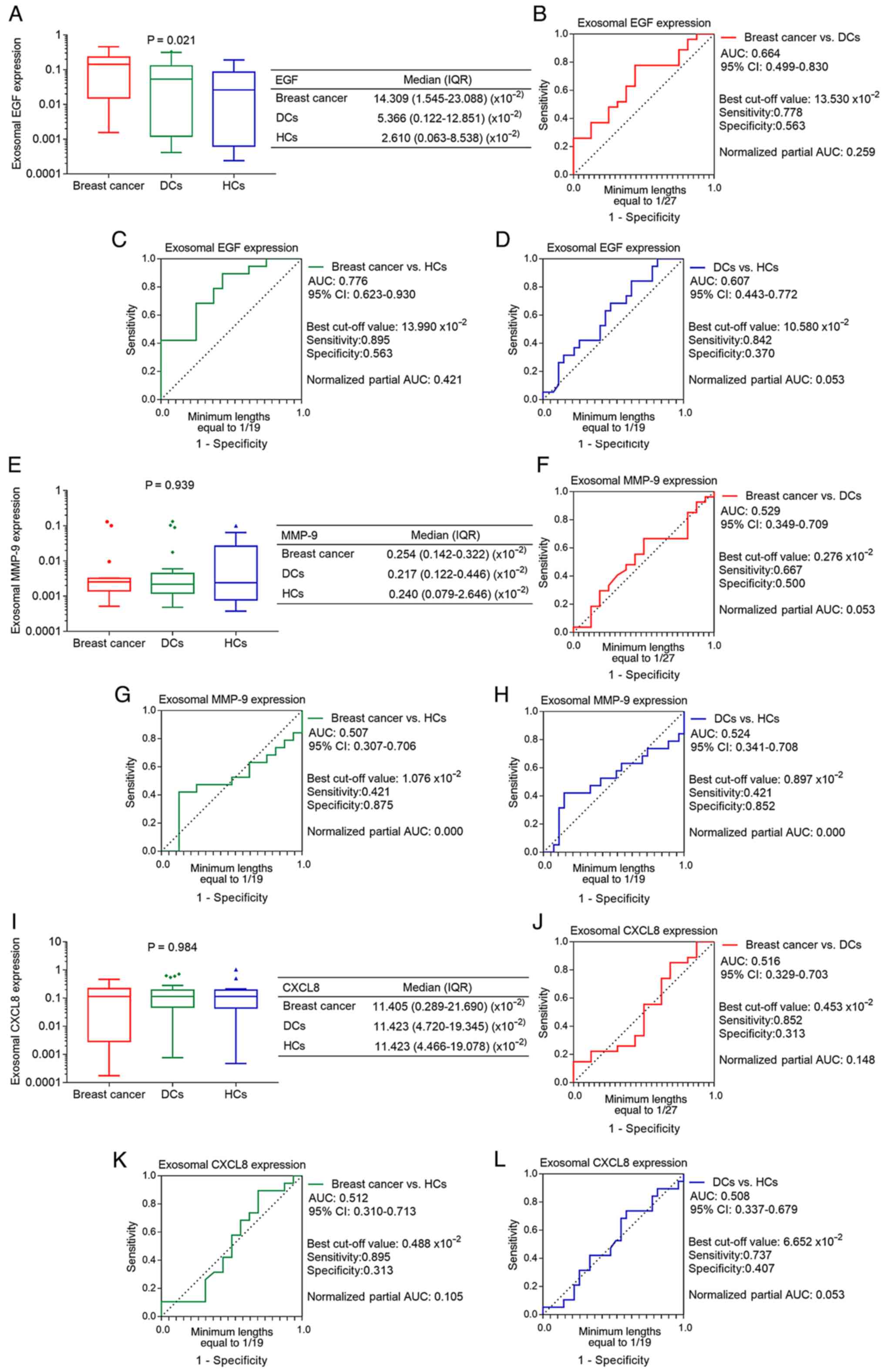 | Figure 2.Differential expression of exosomal
EGF, MMP-9 and CXCL8 among patients with breast cancer, DCs and
HCs. (A) Comparison of exosomal EGF expression. ROC curve analysis
of exosomal EGF expression for (B) patients with breast cancer vs.
DCs, (C) patients with breast cancer vs. HCs and (D) DCs vs. HCs.
(E) Comparison of exosomal MMP-9 expression. ROC curve analysis of
exosomal MMP-9 expression for (F) patients with breast cancer vs.
DCs, (G) patients with breast cancer vs. HCs and (H) DCs vs. HCs.
(I) Comparison of exosomal CXCL8 expression. ROC curve analysis of
exosomal CXCL8 expression for (J) patients with breast cancer vs.
DCs, (K) patients with breast cancer vs. HCs and (L) DCs vs. HCs.
EGF, epidermal growth factor; MMP-9, matrix metalloproteinase-9;
CXCL8, C-X-C motif chemokine ligand 8; DCs, disease controls; HCs,
healthy controls; ROC, receiver operating characteristic; AUC, area
under the curve; IQR, interquartile range; CI, confidence
interval. |
Association of exosomal ENAH, SEPT9,
EGF, MMP-9 and CXCL8 with the clinical characteristics of patients
with breast cancer
Exosomal ENAH was differentially expressed among
patients with different molecular subtypes of breast cancer
(P=0.010). More specifically, its expression level was increased in
patients with HER2-negative luminal B and HER2-enriched breast
cancer and reduced in those with triple-negative, luminal A and
HER2-positive luminal B breast cancer. Additionally, exosomal SEPT9
was not found to be associated with any of the clinical
characteristics of patients with breast cancer (all P>0.05;
Table II). Furthermore, the
exosomal expression of MMP-9 was associated with a Ki-67 index of
≥30% (P=0.011), but not with other clinical characteristics of the
patients with breast cancer (all P>0.05). Furthermore, the
exosomal expression levels of EGF and CXCL8 were also not found to
be associated with any of the clinical characteristics of the
patients with breast cancer (all P>0.05; Table III).
 | Table II.Association of exosomal ENAH and
SEPT9 with the clinical characteristics of patients with breast
cancer. |
Table II.
Association of exosomal ENAH and
SEPT9 with the clinical characteristics of patients with breast
cancer.
|
| Exosomal ENAH
expressiona | Exosomal SEPT9
expressiona |
|---|
|
|
|
|
|---|
| Items | Median (IQR) | Z/Χ2/ρ
value | P-value | Median (IQR) | Z/Χ2/ρ
value | P-value |
|---|
| Age (years) |
| −0.454 | 0.650 |
| −0.186 | 0.853 |
|
<60 | 0.102
(0.035-0.128) |
|
| 0.758
(0.437-0.976) |
|
|
|
≥60 | 0.067
(0.001-0.133) |
|
| 0.835
(0.232-1.395) |
|
|
| Menopause |
| −0.756 | 0.450 |
| −0.071 | 0.943 |
| No | 0.095
(0.006-0.126) |
|
| 0.766
(0.437-1.079) |
|
|
|
Yes | 0.111
(0.074-0.129) |
|
| 0.785
(0.293-1.197) |
|
|
| Histological
type |
| 2.790 | 0.425 |
| 3.353 | 0.340 |
| Ductal
carcinoma in situ | 0.123
(0.104-NA) |
|
| 1.197
(0.785-NA) |
|
|
|
Invasive ductal carcinoma | 0.074
(0.001-0.129) |
|
| 0.763
(0.325-1.117) |
|
|
|
Invasive lobular
carcinoma | NA |
|
| NA |
|
|
|
Others | 0.125
(0.031-0.139) |
|
| 0.758
(0.682-0.818) |
|
|
| Molecular
subtypes |
| 13.176 | 0.010 |
| 1.386 | 0.847 |
|
Triple-negative | 0.067
(0.000-NA) |
|
| 0.956
(0.763-NA) |
|
|
| Luminal
A | 0.067
(0.001-0.085) |
|
| 0.835
(0.607-1.256) |
|
|
|
HER2-negative luminal B | 0.125
(0.113-0.144) |
|
| 0.758
(0.380-0.926) |
|
|
|
HER2-positive luminal B | 0.060
(0.001-0.103) |
|
| 0.655
(0.335-0.976) |
|
|
|
HER2-enriched | 0.134
(0.090-0.145) |
|
| 0.616
(0.284-1.939) |
|
|
| Hormone receptor
status |
| −1.715 | 0.086 |
| −0.226 | 0.821 |
| ER
negative and PR negative | 0.131
(0.047-0.140) |
|
| 0.817
(0.334-1.687) |
|
|
| ER
positive and/or PR positive | 0.095
(0.001-0.123) |
|
| 0.768
(0.429-0.979) |
|
|
| HER2 |
| −0.360 | 0.719 |
| −0.621 | 0.535 |
|
Negative | 0.110
(0.034-0.130) |
|
| 0.785
(0.588-1.133) |
|
|
|
Positive | 0.098
(0.017-0.129) |
|
| 0.655
(0.317-1.201) |
|
|
| Ki-67 (%) |
| −0.993 | 0.321 |
| −0.248 | 0.804 |
|
<30 | 0.095
(0.023-0.127) |
|
| 0.763
(0.429-1.149) |
|
|
|
≥30 | 0.118
(0.012-0.142) |
|
| 0.802
(0.334-0.942) |
|
|
| TNM stage |
| −0.088 | 0.639 |
| 0.007 | 0.970 |
| 0 | 0.114
(0.026-0.140) |
|
| 0.991
(0.691-2.225) |
|
|
| I | 0.092
(0.025-0.121) |
|
| 0.540
(0.214-0.898) |
|
|
| II | 0.095
(0.034-0.129) |
|
| 0.768
(0.445-1.133) |
|
|
|
III | 0.100
(0.001-NA) |
|
| 0.763
(0.253-NA) |
|
|
| IV | NA |
|
| NA |
|
|
 | Table III.Association of exosomal EGF, MMP-9
and CXCL8 with the clinical characteristics of patients with breast
cancer. |
Table III.
Association of exosomal EGF, MMP-9
and CXCL8 with the clinical characteristics of patients with breast
cancer.
|
| Exosomal EGF
expressiona | Exosomal MMP-9
expressiona | Exosomal CXCL8
expressiona |
|---|
|
|
|
|
|
|---|
| Items | Median (IQR) | Z/Χ2/ρ
value | P-value | Median (IQR) | Z/Χ2/ρ
value | P-value | Median (IQR) | Z/Χ2/ρ
value | P-value |
|---|
| Age (years) |
| −0.623 | 0.533 |
| −0.963 | 0.336 |
| −0.623 | 0.533 |
|
<60 | 0.092
(0.003-0.254) |
|
| 0.003
(0.002-0.003) |
|
| 0.092
(0.003-0.230) |
|
|
|
≥60 | 0.144
(0.072-0.309) |
|
| 0.002
(0.001-0.052) |
|
| 0.186
(0.090-0.215) |
|
|
| Menopause |
| −1.091 | 0.275 |
| −1.334 | 0.182 |
| −0.121 | 0.903 |
| No | 0.145
(0.019-0.304) |
|
| 0.003
(0.002-0.008) |
|
| 0.136
(0.003-0.217) |
|
|
|
Yes | 0.073
(0.015-0.143) |
|
| 0.002
(0.001-0.003) |
|
| 0.114
(0.026-0.347) |
|
|
| Histological
type |
| 0.181 | 0.913 |
| 3.299 | 0.192 |
| 5.000 | 0.082 |
| Ductal
carcinoma in situ | NA |
|
| NA |
|
| NA |
|
|
|
Invasive ductal carcinoma | 0.092
(0.003-0.321) |
|
| 0.003
(0.002-0.010) |
|
| 0.102
(0.003-0.186) |
|
|
|
Invasive lobular
carcinoma | 0.144
(0.142-NA) |
|
| 0.001
(0.001-NA) |
|
| 0.218
(0.212-NA) |
|
|
|
Others | 0.099
(0.053-NA) |
|
| 0.003
(0.002-NA) |
|
| 0.046
(0.000-NA) |
|
|
| Molecular
subtypes |
| 3.287 | 0.349 |
| 2.592 | 0.459 |
| 1.522 | 0.677 |
|
Triple-negative | NA |
|
| NA |
|
| NA |
|
|
| Luminal
A | 0.036
(0.003-NA) |
|
| 0.006
(0.003-NA) |
|
| 0.117
(0.048-NA) |
|
|
|
HER2-negative luminal B | 0.098
(0.015-0.155) |
|
| 0.002
(0.001-0.003) |
|
| 0.172
(0.032-0.370) |
|
|
|
HER2-positive luminal B | 0.145
(0.073-0.356) |
|
| 0.003
(0.002-0.052) |
|
| 0.179
(0.048-0.338) |
|
|
|
HER2-enriched | 0.160
(0.047-0.353) |
|
| 0.002
(0.001-0.066) |
|
| 0.003
(0.002-0.166) |
|
|
| Hormone receptor
status |
| −1.076 | 0.282 |
| −0.623 | 0.533 |
| −1.190 | 0.234 |
| ER
negative and PR negative | 0.160
(0.047-0.353) |
|
| 0.002
(0.001-0.066) |
|
| 0.003
(0.002-0.166) |
|
|
| ER
positive and/or PR positive | 0.142
(0.003-0.159) |
|
| 0.003
(0.002-0.003) |
|
| 0.179
(0.048-0.218) |
|
|
| HER2 |
| −1.735 | 0.083 |
| −0.325 | 0.745 |
| −0.434 | 0.664 |
|
Negative | 0.061
(0.002-0.146) |
|
| 0.002
(0.001-0.005) |
|
| 0.156
(0.036-0.269) |
|
|
|
Positive | 0.153
(0.070-0.337) |
|
| 0.003
(0.001-0.028) |
|
| 0.097
(0.003-0.217) |
|
|
| Ki-67 (%) |
| −0.736 | 0.462 |
| −2.549 | 0.011 |
| −0.736 | 0.462 |
|
<30 | 0.142
(0.053-0.159) |
|
| 0.002
(0.001-0.003) |
|
| 0.102
(0.002-0.212) |
|
|
|
≥30 | 0.254
(0.002-0.421) |
|
| 0.003
(0.003-0.115) |
|
| 0.179
(0.003-0.347) |
|
|
| TNM stage |
| −0.248 | 0.354 |
| 0.013 | 0.962 |
| −0.215 | 0.423 |
| 0 | NA |
|
| NA |
|
| NA |
|
|
| I | 0.159
(0.003-NA) |
|
| 0.002
(0.001-NA) |
|
| 0.230
(0.002-NA) |
|
|
| II | 0.144
(0.036-0.320) |
|
| 0.003
(0.002-0.055) |
|
| 0.102
(0.026-0.182) |
|
|
|
III | 0.142
(0.002-NA) |
|
| 0.001
(0.001-NA) |
|
| 0.218
(0.001-NA) |
|
|
| IV | NA |
|
| NA |
|
| NA |
|
|
Discussion
Breast cancer screening is a currently focus of
attention, since it can diagnose patients with breast cancer at an
early stage of the disease, thus providing a satisfactory overall
prognosis (6,26). Mammography is recommended for the
screening of breast cancer in several countries. However, some
subjects may be unwilling to undergo mammography due to concerns
about radiation (27). Other
screening modalities include ultrasound, magnetic resonance imaging
and clinical breast examination. However, the above modalities may
have one or more of the following limitations: Low
sensitivity/specificity, increased cost and the potential influence
of demographic characteristics including age and body weight on
their effectiveness (28,29). Therefore, the exploration of novel
screening modalities for breast cancer is of great importance. It
has been recently reported that several RNAs and proteins exert a
great ability in predicting the risk of breast cancer and,
therefore, these molecules could be used in the early screening of
breast cancer (30–32). Among these biomarkers, exosomes and
their contents are of great interest. Due to the robust bilayer
lipid membrane of exosomes, their contents are protected from the
surrounding environment and can therefore provide accurate
information on the tumor (33–35).
Thus, exosomal contents could be considered as appropriate
biomarkers for the early screening of breast cancer.
The dysregulation of ENAH, SEPT9, EGF, MMP-9 and
CXCL8 in breast cancer tissues and/or cell lines is known to be of
considerable importance. For example, a study used data from the
ONCOMINE database to analyze the mRNA expression levels of ENAH in
breast cancer tissues and the results showed that ENAH was
upregulated in breast cancer tissues compared with normal tissues
(36). Furthermore, another study
revealed that a high level of SEPT9 methylation is present in
breast cancer cell lines and tissues (37). Additionally, the dysregulation of
EGF, MMP-9 and CXCL8 in breast cancer cell lines or tissues has
also been previously reported (38–40).
However, to the best of our knowledge, the expression levels of the
aforementioned genes in exosomes isolated from patients with breast
cancer have not been previously investigated. The present study
demonstrated that exosomal ENAH and EGF were notably upregulated,
while exosomal SEPT9 was downregulated in patients with breast
cancer. This finding suggests that high levels of ENAH and EGF as
well as reduced levels of SEPT9 could facilitate the growth of
breast cancer cells (12,14,16).
Furthermore, breast cancer cells may encapsulate these genes into
exosomes and release them into the circulatory system. This
assumption is consistent with the enhanced exosomal levels of ENAH
and EGF, and the reduced levels of exosomal SEPT9 observed in the
current study. In addition, ROC curve analysis showed that exosomal
ENAH exhibited good capacity for discriminating patients with
breast cancer from DCs and HCs, whereas exosomal SEPT9 and EGF each
had an acceptable capacity for this discrimination. These findings
indicate the potential of these exosomal biomarkers in the early
screening of breast cancer. A previous study demonstrated that ENAH
was elevated in pancreatic cancer tissues compared with tissues
from patients with pancreatitis or normal subjects (41). Additionally, another study revealed
that the methylation of SEPT9 was increased in colorectal cancer
tissues (42). Regarding MMP-9, a
previous study showed that it was aberrantly expressed in
osteosarcoma tissues, in which its expression was higher than that
in paracancerous tissues (43).
Furthermore, CXCL8 has been found to be significantly upregulated
in prostate cancer tissues (44).
The results of the present study demonstrated that
the exosomal levels of ENAH were partially associated with
HER2-negative luminal B and HER2-enriched breast cancer A possible
explanation for this could be that exosomal ENAH showed a tendency
to associate with estrogen receptor (ER)- and progesterone
(PR)-negative breast cancer, as well as with a Ki-67 index of ≥30%,
although this tendency did not reach statistical significance.
Based on the expression of ER, PR, HER2 and Ki-67, breast cancer is
classified in different molecular subtypes, namely HER2-negative
luminal B breast cancer, characterized by a lack of expression of
PR and upregulated expression of Ki-67, and HER2-enriched breast
cancer, characterized by the lack of PR and ER expression.
Therefore, exosomal ENAH showed a tendency to correlate with the
aforementioned breast cancer subtypes. The results of the current
study also showed that exosomal MMP-9 was associated with a Ki-67
index of ≥30%. This could be due to the expression of Ki-67
reflecting the proliferation ability of breast cancer cells
(45). Additionally, MMP-9
promotes the proliferation of breast cancer cells (18); therefore, it was also associated
with a Ki-67 index of ≥30%.
However, the present study has some limitations.
Firstly, the sample size was relatively small. Therefore, the
association of the expression levels of exosomal ENAH, SEPT9, EGF,
MMP-9 and CXCL8 with the risk of breast cancer should be further
investigated using a larger sample size. Secondly, a validation
cohort is required to verify the diagnostic value of exosomal ENAH,
SEPT9 and EGF in the early screening of breast cancer. Thirdly, due
to the single-center study design, there may be regional bias.
Fourthly, the expression levels of exosomal ENAH and SEPT9 were
detected in one batch of patients, while those of EGF, MMP-9 and
CXCL8 were detected in a different batch of patients. However, the
comparison of baseline characteristics revealed that the
demographic and disease features were comparable between the two
batches of patients, thus suggesting that there were no major
confounding factors. Although the treatment strategy varied between
batches, all samples were collected prior to treatment, i.e.,
before surgery or neoadjuvant therapy if the patients were due to
receive it. Therefore, different treatment approaches could not
significantly affect the main findings of the present study.
Fifthly, the current study lacked follow-up, and thus the
association between the expression levels of the aforementioned
exosomal genes and the prognosis of patients with breast cancer
requires investigation in further studies. Finally, further studies
are also required to evaluate the value of the expression of other
exosomal genes for the prediction of breast cancer risk.
In conclusion, the results of the present study
suggest that the expression levels of exosomal ENAH, SEPT9 and EGF
in blood possess the potential to identify patients with breast
cancer. However, this potential was not observed for MMP-9 and
CXCL8, possibly due to the small sample size. The results also
indicate that detection of the exosomal levels of ENAH, SEPT9 and
EGF in the blood could improve the early screening of breast
cancer. However, further validation experiments are necessary. In
addition, whether these exosomal genes could serve as potential
indicators for the prognosis of breast cancer merits further
investigation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ and SW substantially contributed to the
conception and the design of the study. ZZ, HW and YJ were
responsible for the acquisition and analysis of the data. CC, JB
and JH contributed to interpretation of the data. FT, LY and LZ
contributed to data interpretation and manuscript drafting. QZ, LY
and SW confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by Ethics Committee of
Huashan Hospital, Fudan University (Shanghai, China). Each patient
or guardian of the patient who was <18 years old signed a
written informed consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ENAH
|
enabled homolog
|
|
SEPT9
|
septin 9
|
|
EGF
|
epidermal growth factor
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
CXCL8
|
C-X-C motif chemokine ligand 8
|
|
PB
|
peripheral blood
|
|
HCs
|
healthy controls
|
|
DCs
|
disease controls
|
|
TNM
|
tumor-node-metastasis
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
ROC
|
receiver operating characteristic
|
|
IQR
|
interquartile range
|
|
CI
|
confidence interval
|
|
AUC
|
area under the curve
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smolarz B, Nowak AZ and Romanowicz H:
Breast cancer-epidemiology, classification, pathogenesis and
treatment (Review of Literature). Cancers (Basel). 14:25692022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao W, Chen HD, Yu YW, Li N and Chen WQ:
Changing profiles of cancer burden worldwide and in China: A
secondary analysis of the global cancer statistics 2020. Chin Med J
(Engl). 134:783–791. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan X, Zhang B, He Y, Zhou X, Zhang Y, Ma
L, Li X and Wu J: Burden of disease due to cancer-China, 2000–2019.
China CDC Wkly. 4:306–311. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mahanani MR, Valkov M, Agaeva A, Kaucher
S, Pikalova LV, Grishchenko MY, Zhuikova LD, Jaehn P and Winkler V:
Comparison of female breast cancer between Russia and Germany: A
population-based study on time trends and stage at diagnosis.
Cancer Epidemiol. 80:1022142022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miller KD, Nogueira L, Devasia T, Mariotto
AB, Yabroff KR, Jemal A, Kramer J and Siegel RL: Cancer treatment
and survivorship statistics, 2022. CA Cancer J Clin. 72:409–436.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jani C, Salcicciol I, Rupal A, Al Omari O,
Goodall R, Salciccioli JD, Marshall DC, Hanbury G, Singh H,
Weissmann L and Shalhoub J: Trends in breast cancer mortality
between 2001 and 2017: An observational study in the European Union
and the United Kingdom. JCO Glob Oncol. 7:1682–1693. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu W, Hurley J, Roberts D, Chakrabortty
SK, Enderle D, Noerholm M, Breakefield XO and Skog JK:
Exosome-based liquid biopsies in cancer: Opportunities and
challenges. Ann Oncol. 32:466–477. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu M, Mo F, Song X, He Y, Yuan Y, Yan J,
Yang Y, Huang J and Zhang S: Exosomal hsa-miR-21-5p is a biomarker
for breast cancer diagnosis. PeerJ. 9:e121472021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Qian T, Bao S, Zhao H, Chen H,
Xing Z, Li Y, Zhang M, Meng X, Wang C, et al: Circulating exosomal
miR-363-5p inhibits lymph node metastasis by downregulating PDGFB
and serves as a potential noninvasive biomarker for breast cancer.
Mol Oncol. 15:2466–2479. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Peng X, Liu Y, Hao R, Zhao R, Zhang
L, Zhao F, Liu Q, Liu Y and Qi Y: The diagnostic value of serum
exosomal Has_circ_0000615 for breast cancer patients. Int J Gen
Med. 14:4545–4554. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Modugno F, DeMonte L, Balsamo M, Bronzi
G, Nicotra MR, Alessio M, Jager E, Condeelis JS, Santoni A, Natali
PG and Nisticò P: Molecular cloning of hMena (ENAH) and its splice
variant hMena+11a: Epidermal growth factor increases their
expression and stimulates hMena+11a phosphorylation in breast
cancer cell lines. Cancer Res. 67:2657–2665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ahuja N, Ashok C, Natua S, Pant D, Cherian
A, Pandkar MR, Yadav P, Vishnu NSS, Mishra J, Samaiya A and Shukla
S: Hypoxia-induced TGF-β-RBFOX2-ESRP1 axis regulates human MENA
alternative splicing and promotes EMT in breast cancer. NAR Cancer.
2:zcaa0212020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng Y, Cao Y, Liu L, Zhao J, Zhang T,
Xiao L, Jia M, Tian Q, Yu H, Chen S and Cai Y: SEPT9_i1 regulates
human breast cancer cell motility through cytoskeletal and RhoA/FAK
signaling pathway regulation. Cell Death Dis. 10:7202019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Devlin L, Okletey J, Perkins G, Bowen JR,
Nakos K, Montagna C and Spiliotis ET: Proteomic profiling of the
oncogenic septin 9 reveals isoform-specific interactions in breast
cancer cells. Proteomics. 21:e21001552021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu C, Zhao Y, Wang J, Shi W, Dong F, Xin
Y, Zhao X and Liu C: Breast cancer cell-derived extracellular
vesicles transfer miR-182-5p and promote breast carcinogenesis via
the CMTM7/EGFR/AKT axis. Mol Med. 27:782021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kavarthapu R, Anbazhagan R and Dufau ML:
Crosstalk between PRLR and EGFR/HER2 signaling pathways in breast
cancer. Cancers (Basel). 13:46852021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong OY, Jang HY, Lee YR, Jung SH, Youn HJ
and Kim JS: Inhibition of cell invasion and migration by targeting
matrix metalloproteinase-9 expression via sirtuin 6 silencing in
human breast cancer cells. Sci Rep. 12:121252022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cancemi P, Buttacavoli M, Roz E and Feo S:
Expression of alpha-enolase (ENO1), Myc promoter-binding protein-1
(MBP-1) and matrix metalloproteinases (MMP-2 and MMP-9) reflect the
nature and aggressiveness of breast tumors. Int J Mol Sci.
20:39522019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mishra A, Suman KH, Nair N, Majeed J and
Tripathi V: An updated review on the role of the CXCL8-CXCR1/2 axis
in the progression and metastasis of breast cancer. Mol Biol Rep.
48:6551–6561. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ruffini PA: The CXCL8-CXCR1/2 axis as a
therapeutic target in breast cancer stem-like cells. Front Oncol.
9:402019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang RX, Ji P, Gong Y, Shao ZM and Chen S:
Value of CXCL8-CXCR1/2 axis in neoadjuvant chemotherapy for
triple-negative breast cancer patients: A retrospective pilot
study. Breast Cancer Res Treat. 181:561–570. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Breast Cancer Committee of Chinese
Anti-Cancer Association, . Chinese Anti-Cancer Association Breast
Cancer Diagnosis and Treatment Guidelines and Standards (2021).
China Oncology. 31:954–1040. 2021.
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han C, Bellone S, Siegel ER, Altwerger G,
Menderes G, Bonazzoli E, Egawa-Takata T, Pettinella F, Bianchi A,
Riccio F, et al: A novel multiple biomarker panel for the early
detection of high-grade serous ovarian carcinoma. Gynecol Oncol.
149:585–591. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo C, Wang L, Zhang Y, Lu M, Lu B, Cai J,
Chen H and Dai M: Advances in breast cancer screening modalities
and status of global screening programs. Chronic Dis Transl Med.
8:112–123. 2022.PubMed/NCBI
|
|
27
|
Ren W, Chen M, Qiao Y and Zhao F: Global
guidelines for breast cancer screening: A systematic review.
Breast. 64:85–99. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding R, Xiao Y, Mo M, Zheng Y, Jiang YZ
and Shao ZM: Breast cancer screening and early diagnosis in Chinese
women. Cancer Biol Med. 19:450–467. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mann RM, Hooley R, Barr RG and Moy L:
Novel approaches to screening for breast cancer. Radiology.
297:266–285. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang H, Shu L, Niu N, Zhao C, Lu S, Li Y,
Wang H, Liu Y, Zou T, Zou J, et al: Novel lncRNAs with diagnostic
or prognostic value screened out from breast cancer via
bioinformatics analyses. PeerJ. 10:e136412022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jia L, Li G, Ma N, Zhang A, Zhou Y, Ren L
and Dong D: Soluble POSTN is a novel biomarker complementing CA153
and CEA for breast cancer diagnosis and metastasis prediction. BMC
Cancer. 22:7602022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Veyssiere H, Bidet Y, Penault-Llorca F,
Radosevic-Robin N and Durando X: Circulating proteins as predictive
and prognostic biomarkers in breast cancer. Clin Proteomics.
19:252022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Na-Er A, Xu YY, Liu YH and Gan YJ:
Upregulation of serum exosomal SUMO1P3 predicts unfavorable
prognosis in triple negative breast cancer. Eur Rev Med Pharmacol
Sci. 25:154–160. 2021.PubMed/NCBI
|
|
34
|
Lakshmi S, Hughes TA and Priya S: Exosomes
and exosomal RNAs in breast cancer: A status update. Eur J Cancer.
144:252–268. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zokaei E, Darbeheshti F and Rezaei N:
Prospect of exosomal circular RNAs in breast cancer: Presents and
future. Mol Biol Rep. 49:6997–7011. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li QL, Su YL, Zeng M and Shen WX: Enabled
homolog shown to be a potential biomarker and prognostic indicator
for breast cancer by bioinformatics analysis. Clin Invest Med.
41:E186–E195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Matsui S, Kagara N, Mishima C, Naoi Y,
Shimoda M, Shimomura A, Shimazu K, Kim SJ and Noguchi S:
Methylation of the SEPT9_v2 promoter as a novel marker for the
detection of circulating tumor DNA in breast cancer patients. Oncol
Rep. 36:2225–2235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Khambri D, Suyuthie HD, Hilbertina N,
Yetti H and Purwanto DJ: Matrix metalloproteinase-9 as prognostic
factor for the treatment of HER-2 enriched breast cancer. Asian Pac
J Cancer Prev. 23:1013–1021. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yotsumoto F, Tokunaga E, Oki E, Maehara Y,
Yamada H, Nakajima K, Nam SO, Miyata K, Koyanagi M, Doi K, et al:
Molecular hierarchy of heparin-binding EGF-like growth
factor-regulated angiogenesis in triple-negative breast cancer. Mol
Cancer Res. 11:506–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fang QI, Wang X, Luo G, Yu M, Zhang X and
Xu N: Increased CXCL8 expression is negatively correlated with the
overall survival of patients with ER-Negative breast cancer.
Anticancer Res. 37:4845–4852. 2017.PubMed/NCBI
|
|
41
|
Melchionna R, Iapicca P, Di Modugno F,
Trono P, Sperduti I, Fassan M, Cataldo I, Rusev BC, Lawlor RT,
Diodoro MG, et al: The pattern of hMENA isoforms is regulated by
TGF-β1 in pancreatic cancer and may predict patient outcome.
Oncoimmunology. 5:e12215562016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wasserkort R, Kalmar A, Valcz G, Spisak S,
Krispin M, Toth K, Tulassay Z, Sledziewski AZ and Molnar B:
Aberrant septin 9 DNA methylation in colorectal cancer is
restricted to a single CpG island. BMC Cancer. 13:3982013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bai C, Ma X, Wang X and Chen X:
Correlation between pathological features and protein expressions
of TfR1, VEGF and MMP-9 in patients with osteosarcoma. Am J Transl
Res. 14:4562–4572. 2022.PubMed/NCBI
|
|
44
|
Kong L, Qi R, Zhou G and Ding S:
Correlation analysis of survivin, ING4, CXCL8 and VEGF expression
in prostate cancer tissue. Am J Transl Res. 13:13784–13790.
2021.PubMed/NCBI
|
|
45
|
Hacisalihoglu UP and Dogan MA: Expression
of estrogen and progesterone receptors, HER2 protein and Ki-67
proliferation index in breast carcinoma in both tumor tissue and
tissue microarray. Biotech Histochem. 97:298–305. 2022. View Article : Google Scholar : PubMed/NCBI
|
















