Introduction
Ovarian cancer (OC) is one of the 10 most common
types of cancer in women in the world. In 2020, ovarian cancer was
ranked eighth in terms of incidence and mortality with over 313 000
new cases and over 207 000 deaths (1).
Among ovarian cancers (carcinomas), there are
cancers originating from epithelial cells (the most common), germ
cells and stromal cells (2).
According to the World Health Organization (WHO) classification of
female genital tumours from 2020, at least five main types of
ovarian carcinomas are identified based on histopathology,
immunoprofile and molecular analysis. Among them, high-grade serous
carcinoma (HGSC) is the most common ovarian cancer accounting for
about 70% of all ovarian carcinomas, the second most common
histotype is endometroid carcinoma (EC, 10%), clear cell carcinoma
(CCC, 6–10%), low-grade serous carcinoma (LGSC, 5%) and mucinous
carcinoma (MC, 3–4%) (3).
An important diagnostic and therapeutic problem is a
diagnosis of ovarian cancer patients at advanced stages with
metastatic sites within the peritoneal cavity, retroperitoneum and
even in distant organs (4). In
such cases patients have a much lower chance of recovery and the
five-year survival rate is less than 30% (5,6).
Early diagnosis of ovarian cancer is difficult due to the lack of
appropriate markers and definitive screening tools as well as
non-specific symptoms accompanying this cancer. These include:
bloating and abdominal pain, early satiety or fullness, changes in
bowel habits or frequent urination. Women with those symptoms may
seek medical help too late and even be treated without identifying
the specific causes of their symptoms (7).
Treatment depends on the diagnosed stage of ovarian
cancer (8). The standard therapy
involves surgical treatment, which is maximal cytoreductive
debulking, and the platinum-based chemotherapy (8,9).
Unfortunately, tumours relapse in over 70% of cases, despite an
initially good response to treatment by the majority of patients
(4). In recent years,
Poly(ADP-ribose) polymerase inhibitors (PARPi) have been approved
for the treatment of ovarian cancer as drugs that maintain therapy
following the completion of first-line platinum-based chemotherapy
(10,11).
It is believed that cancer stem cells (CSCs) that
have not been eliminated during treatment and are responsible for
the development of resistance to chemotherapy and are able to
replenish their population, may contribute to recurrence of the
cancer, which might be even more aggressive (4). Hence, effective methods of cancer
treatment based on the elimination of CSCs are being sought. High
hopes are raised by CSCs-targeting therapies, which in combination
with traditional methods of treatment may give a better therapeutic
effect (12).
Cancer stem cells (CSCs)
Models of tumorigenesis
The two main models try to explain the origin,
progression and heterogeneity of tumours: the stochastic model (or
clonal evolution model) and the hierarchical (or CSC) model
(13–15). According to the stochastic model,
each tumour cell is biologically homogeneous and has the same
developmental potential as well as the ability to promote tumour
progression (16,17). This model assumes that the
acquisition of oncogenic mutations in normal differentiated somatic
cells results in hyperplasia and contributes to clonal expansion
(14,17). The accumulation of genetic and
epigenetic alterations in cells may increase tumour aggressiveness,
invasiveness and treatment resistance which in turn leads to tumour
progression and increases tumour heterogeneity (13).
The hierarchical model states that only a distinct
population of cancer cells features tumorigenic potential-these
cells are referred to as cancer stem cells (CSCs). According to
this model, tumour initiation starts when a normal stem cell
escapes regulation and becomes cancer stem cell-the first abnormal
cell assumed to be the cell-of-origin (14,17).
Moreover, this model says that there is a differentiation hierarchy
of cells in tumour that includes CSCs responsible for maintaining
the whole populations of cells in tumour (13,15,16,18).
However, there is an alternative model of cellular
plasticity that combines these two models by assuming that cancer
cells can interconvert between stem cell and differentiated states
(13). Cell dedifferentiation
capacity may be inherited (hierarchical model) or acquired through
mutations (stochastic model) (14). According to the plasticity model,
differentiated tumour cells can reacquire stem cell characteristics
by intrinsic processes of these cells and/or stimuli within the
tumour microenvironment (13).
The origin of cancer stem cells
Cancer stem cells (CSCs) represent a small
subpopulation of cells in tumour mass (19). The origin of these cells has still
not been clearly elucidated. One of the hypotheses suggests that
CSCs originate from normal adult stem cells that have acquired
epigenetic and genetic changes (20). On the other hand, CSCs may derive
from mature differentiated cells through various mechanisms,
including genomic instability, horizontal gene transfer and
microenvironmental changes (21).
A differentiated cancer cells may de-differentiate into CSCs in
response to different factors, such as stress and hypoxia, wounding
or ionizing radiation (22).
Various studies suggest that the epithelial-mesenchymal transition
(EMT) is involved in dedifferentiation and cells that have
undergone this process exhibit a more CSC-like phenotype allowing
them to self-renew and differentiate into all cell types in the
tumour (23). It is believed that
CSCs can also arise as a result of cell fusion and metabolic
reprogramming of non-CSCs into CSCs during the cancer development
(21,22).
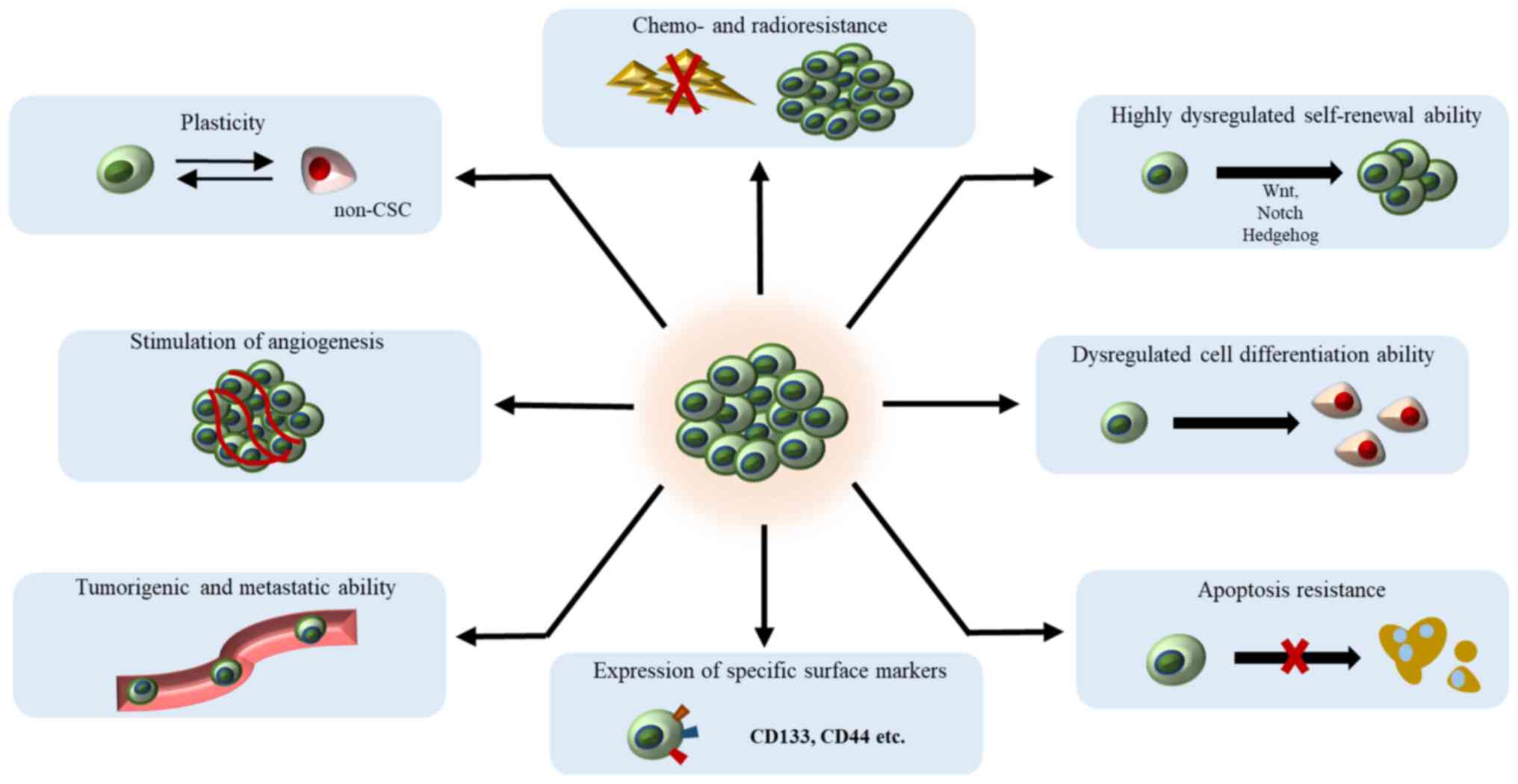
Characteristics of cancer stem
cells
The assumption that CSCs originate from normal stem
cells (NSCs) that have accumulated transforming mutations may be
supported by the fact that these cells share many features
(13). Both CSCs and NSCc have the
capacity for self-renewal through mitotic divisions. Symmetric
divisions give rise to two sister stem cells, while asymmetric
divisions give rise to one daughter stem cell and one
differentiated cell (24). The
ability of CSCs to divide asymmetrically enables these cells to
both self-renew their population and initiate a neoplastic process
(25). However, NSCs are able to
control and regulate self-renewal, while CSCs have lost this
capacity (26). Moreover, CSCs and
NSCs are regulated by similar signalling pathways such as Wnt,
Hedgehog or Notch (26). Most of
these pathways are essential for stemness properties of NSCs, such
as the ability to self-renew, differentiate, proliferate and
develop various organs during embryogenesis. However, genetic
mutations and epigenetic changes may cause dysregulation of these
pathways in the CSCs, leading to uncontrolled self-renewal and
impaired differentiation of these cells (27).
Both CSCs and NSCs have the ability to differentiate
into multiple progenitor cell types. However, CSCs can replicate
and differentiate in an uncontrolled manner into populations of
molecularly and phenotypically altered progenitor cells that may
have limitless proliferative and survival potential with more
plasticity than progeny of NSCs (13). Additionally, CSCs and NSCs possess
high telomerase activity that prolongs their life span, express
similar surface receptors and can stimulate angiogenesis (28).
In addition to impaired self-renewal and
differentiation abilities, CSCs also have other characteristics
that distinguish them from NSCs, such as the ability to form
tissues and organs. NSCs develop through organogenesis to form
internal organs, while CSCs have tumorigenic properties and form
tumour tissues. Moreover, NSCs have normal karyotyping, whereas
CSCs have abnormal karyotyping with genetic alterations (28). Characteristics of CSCs are
summarised in Fig. 1.
The importance of tumour
microenvironment for CSCs
Stem cells division and differentiation take place
in a specialised microenvironment (the niche) that regulates
self-renew of these cells through cell-cell communication or
secretion of paracrine factors (28,29).
CSCs are population of cancer cells within the cancer
microenvironment that consists of various cells, including
cancer-associated fibroblasts (CAFs), mesenchymal stem cells
(MSCs), endothelial cells (ECs) and immune cells (the macrophages,
T-cells and natural killer (NK) cells, factors secreted by these
cells, such as cytokines and growth factors as well as the
extracellular matrix (ECM) (28,29).
ECM is a noncellular component of tumour microenvironment composed
of glycosaminoglycans, collagens, metalloproteases, hyaluronic
acid, polysaccharides, glycoproteins, proteoglycans and other
proteins (30).
The components of tumour microenvironment provide
ideal conditions for maintaining the properties of CSCs, such as
self-renewal, proliferation and differentiation as well as
generation of heterogeneous cancer population (30). Moreover, the tumour niche supports
initiation, growth, invasion and metastasis of tumour cells and
also plays an important role in therapy resistance, mostly by
supporting stem-related signaling pathway maintenance in CSCs
(29). The CSCs are surrounded by
cancer niche cells, which secrete factors promoting survival and
plasticity of CSCs, as well as increasing drug resistance.
Additionally, ECM is a physical barrier that protects CSCs from
chemotherapeutic agents (29).
However, the relation between CSCs and their niche
can be bidirectional. It is suggested that CSCs may promote the
recruitment and activation of niche components by producing factors
such as proinflammatory cytokines and chemokines. Furthermore, it
has been shown that CSCs may differentiate into functional ECs,
which in turn may transdifferentiate into MSCs (29).
Within tumours CSCs are usually located near hypoxic
regions. Hypoxia plays a role in the maintenance of CSCs
characteristics. It is also involved in chemo- and radioresistance
(29). In hypoxic conditions CSCs
produce vascular endothelial growth factor (VEGF), which further
induces angiogenesis (14).
Methods of CSCs identification and
isolation
The specific properties of CSCs are used in methods
of isolating these cells from a tumour mass or cell culture
(31). There are several in
vitro assays to identify CSCs, such as the detection of surface
markers, assessment of the activity of aldehyde dehydrogenase
(ALDH)-Aldefluor assay, sphere-forming assay or Hoechst dye
exclusion assay (32). Methods
that use the expression of surface markers of CSCs to isolate these
cells include fluorescence-activated cell sorting (FACS), based on
flow cytometry and using fluorescently labeled antibodies, and
magnetic activated cell sorting (MACS) using antibodies coupled
with superparamagnetic nanoparticles (31). Moreover, polymerase chain reaction
analysis is also used to isolate CSCs by identifying the markers
expressed on the cell surface (21,32).
On the other hand, CSCs may also be identified by
the flow cytometry-based ALDEFLUOR assay which measure the activity
of intracellular marker-ALDH, which is increased in these cells
(31–33).
The ability of CSCs to form spheres is also used to
identify and isolate these cells. Culturing cells harvested from
tumour specimens in a serum-free medium supplemented with basic
fibroblast growth factor (bFGF) and epithelial growth factor (EGF)
results in the formation of non-adherent spheres by immature cells
(21,31,32).
Another method of isolating CSCs is based on the ability of cells,
termed as the side population (SP), to export a fluorescent dye,
such as Hoechst 33342 or Rhodamine 123, by ABC transporters
(21,31,32).
However, some ABC transporters expressed on CSCs, such as ABCB1 or
ABCG2, are also expressed on non-CSCs (21). Moreover, this method is limited by
the toxicity of the dyes (21,32).
When identifying and isolating CSCs, it should be
kept in mind, that CSCs share many features with NSCs and are
similar e.g., in the expression of specific surface markers or the
utilization of common signaling pathways. In contrast, when CSCs
are transplanted into animals they can form a tumour, while NSCs do
not have this ability (32). In
the method of isolating CSCs in vivo, that is serial
transplantation assay in animal model, tumour cells are
transplanted into immunocompromised mouse. Such in vivo
assays are regarded as the gold standard in the identification of
CSCs (32).
CSCs in ovarian cancer
Cancer stem cells (CSCs) are a small subpopulation
of cancer cells within ovarian tumour tissue (24). Bapat et al (34) were the first to confirm the
existence of cells with the characteristics of NSCs in ovarian
cancer, which are capable of driving tumorigenesis. Ovarian CSCs
are thought to be responsible for tumour growth, metastasis and
recurrence, as well as resistance to standard treatments such as
chemotherapy (35). It is believed
that the involvement of CSCs in metastasis in ovarian cancer is
related to their ability to resist anoikis, which allows
them to survive in non-adherent conditions and then to adhere in
other than primary locations and create secondary tumours there
(36). Moreover, the metastasis
formation may be influenced by the ability of CSCs to undergo the
process of EMT which is an example of the plasticity of these cells
(36).
An important therapeutic problem in patients with
ovarian cancer is the frequent recurrence of the disease, even if
an initial response to the treatment is promising. Moreover,
patients may become resistant to chemotherapy, which results in
treatment failure or even death (37). The mechanisms underlying the
development of chemoresistance are not entirely clear, but it is
suggested that CSCs may play a role in cancer recurrence following
chemotherapy. There are several mechanisms implicated in
chemoresistance of ovarian CSCs, including increased drug effects,
CSCs quiescence (essential for self-renewal function), enhanced DNA
repair, autophagy, etc. (37).
Various markers are used to identify CSCs in ovarian
cancer. However, due to a tumour heterogeneity it is difficult to
describe ovarian CSCs phenotype. Among the characteristic markers
of CSCs in ovarian cancer, there are: CD133, CD44, CD24, CD117, or
ALDH1 (4,38). Recent findings indicate that some
markers may be of diagnostic and prognostic importance in ovarian
cancer (38). In addition,
scientists' attention is drawn to the use of CSCs markers in
targeted personalised therapies (39).
Clinical significance of CSCs markers in
ovarian cancer
This review presents selected CSCs markers used in
ovarian cancer research with particular emphasis on their
prognostic value and association with chemoresistance in this
cancer.
CD133
CD133 is one of the most well-known markers of CSCs,
used to isolate and study these cells in different types of cancer,
including ovarian cancer (40).
Zhou et al (41) performed
meta-analysis of eight studies including a total of 1051 women with
ovarian cancer to investigate the association between the
expression of CD133 and clinicopathological outcomes as well as to
determine the prognostic value of CD133 in ovarian cancer. Their
analysis showed that the presence of CD133 expression was highly
correlated with poor two-year overall survival (OS), which may
indicate the prognostic importance of this marker related to the
worse prognosis in patients with ovarian cancer. Moreover, they
showed that the expression of CD133 correlated with tumour stage,
but was not associated with other clinical parameters, such as
patients' age, tumour grade, histological type and response to
treatment (41). Another
meta-analysis performed by Tao et al (42) indicated that the expression of
CD133 correlated with FIGO stage and was statistically associated
with tumour differentiation grade, which may suggest the
involvement of CD133 in the malignant progression of ovarian
cancer.
Different results were obtained in the study of
Onisim et al (43), who did
not observe an association between the expression of CD133 and
progression free survival (PFS) or OS in patients with serous
ovarian carcinoma. They also found that the expression of CD133 in
tumour cells was not significantly associated with
clinicopathological parameters, such as age, serum CA125,
peritoneal carcinomatosis, malignant ascites or tumour grade
(43).
In the study by Ruscito et al (44) it was shown that there was a
significant shift from higher frequency of CD133+ cells in patients
with primary high-grade serous ovarian cancer (HGSOC) to lower
levels in the paired recurrent samples. Moreover, all primary
ovarian cancer CD133+ patients were diagnosed at FIGO III/IV stage
and had significantly worse progression-free survival (PFS) as well
as OS (44). In turn, in the study
by Steg et al (45), who
examined matched primary and recurrent tumour pairs from patients
with high grade ovarian adenocarcinomas, it was shown that the
average number of CD133-positive cells was significantly higher in
the samples of recurrent tumours than in primary tumours. Moreover,
the expression of CD133 was significantly increased in tumours
collected from recurrent platinum-resistant patients (45). Liu et al (46) showed that the absence of CD133
expression in patients with primary epithelial ovarian cancer was
significantly associated with high platinum sensitivity in patients
with and without central nervous system (CNS) metastases. Their
results also indicated a positive association between the
expression of CD133 in primary tumours and increased risk of CNS
metastases (46). The association
between the expression of CD133 and chemoresistance was also shown
in another study (47).
The presented results may indicate a relationship
between the expression of CD133 and chemoresistance in women with
ovarian cancer and the potential use of this marker in personalized
targeted therapy.
CD44
The prognostic value and clinical significance of
CSCs surface marker CD44 in patients with ovarian cancer is
controversial. Different authors in their reviews point out that
there are some conflicted data on CD44 expression and its
correlation with prognosis in ovarian cancer (48,49).
The meta-analysis performed by Lin and Ding
(50) included 18 studies
conducted in total on over 2,000 patients with ovarian cancer.
Their study revealed that the expression of CD44 in ovarian cancers
was significantly associated with a high TMN stage and with a poor
five-year OS, while was not significantly correlated with
disease-free survival (DFS). They also showed that there was no
significant correlation between the expression of CD44 and tumour
grade, lymphatic metastasis, patients' age, residual tumour size,
ascites volume as well as response to chemotherapy (50). Another meta-analysis conducted by
Tao et al (42) showed that
overexpression of isoform CD44s was associated with poor OS and
worse DFS as well as with chemotherapy resistance in ovarian cancer
patients. However, there was no association between overexpression
of isoform CD44v6 and poor OS (42).
In the studies of Zhou et al (51) it was found that in patients with
ovarian cancer the high expression of CD44 was associated with
higher histological grade and more advanced FIGO stage. Moreover,
they showed that high expression of CD44 was significantly
associated with worse OS and DFS suggesting that CD44 may be a
potential prognostic marker (51).
High expression of CD44 has also been demonstrated in the samples
of chemotherapy resistant epithelial ovarian cancer tissue, which
may indicate the usefulness of this marker in targeted therapy
(52).
Zhu et al (53) showed that CD44/myeloid
differentiation factor 88 (MyD88) co-expression in patients with
epithelial ovarian carcinoma (EOC) was associated with tumour
progression, metastasis and recurrence. Moreover, the authors'
findings suggest that CD44/MyD88 co-expression is an independent
prognostic factor related to poor DFS and OS (53).
The researchers' attention is also focused on the
clinical significance of CD44 variant 6 (CD44v6). It was found that
CD44v6 is highly expressed in ovarian cancer patients, suggesting
that CD44v6 may promote incidence and progression of this cancer
(54). In addition, the study by
Tjhay et al (55) showed
that an increased number of CD44v6-positive cancer cells in primary
tumours was associated with a shortened OS in patients with
advanced epithelial ovarian cancer (stage III–IV). The authors also
found that CD44v6-positive cancer cells show metastatic potential
and they are associated with tumour chemoresistance (55). Motohara et al (56) found that the expression of CD44v6
was an independent risk factor for distant metastatic recurrence in
patients with ovarian cancer. Moreover, increased expression of
this marker in primary ovarian tumours was associated with shorter
OS (56).
ALDH1
Different studies results indicate a relationship
between high expression of ALDH1 and poor prognosis and clinical
outcome in patients with ovarian cancer (57–59).
However, there is also a study in which the expression of ALDH1 was
associated with favourable prognosis in ovarian cancer (60). The long-term follow-up
retrospective study by Huang et al (61) showed that high expression of ALDH1
in ovarian cancer cells was associated with histological subtypes,
early FIGO stage, well differentiation grade and better survival.
However, in multivariate analysis, the expression of ALDH1 in
tumour cells was not an independent risk factor for OS. Their study
revealed that high expression of ALDH1 in ovarian cancer cells may
portends favourable prognosis (61).
The clinicopathological characteristics and
prognostic significance of ALDH1 in ovarian cancer were evaluated
by Zhao et al (62) in a
meta-analysis of 18 studies including over 2 500 patients. Their
results indicated that elevated expression of ALDH1 was
significantly associated with poor OS but not with DFS. They also
found that ALDH1 was most frequently elevated in patients with poor
clinicopathological characteristics and was associated with FIGO
stage, lymph node metastasis and distant metastasis (62). Another meta-analysis, published in
the same year, showed that overexpression of ALDH1 was correlated
with poor OS as well as with worse DFS (42).
Ayub et al (63) demonstrated that in patients with
advanced epithelial ovarian cancer the enrichment of ALDH1
expression after treatment was associated with poor response to
chemotherapy. Another study showed that the expression of isoform
ALDH1A1 was associated with poor response to platinum-based therapy
in patients with high-grade ovarian serous carcinoma (64).
CD133/ALDH1
The study conducted by Ricci et al (65) found that neither CD133 expression
nor ALDH enzymatic activity were correlated with response to
therapy, PFS and OS in ovarian cancer. The authors suggest that
those markers do not provide additional predictive/prognostic
information in ovarian cancer patients (65). On the other hand, Silva et
al (66) showed that the
presence of ALDH+CD133+ cells in debulked
primary tumour specimens correlated with reduced disease-free
survival and OS in ovarian cancer patients. Similarly, in the
aforementioned study by Ruscito et al (44) it was found that the co-expression
of CD133/ALDH1 in patients with primary HGSOC, rather than the
expression of a single marker, was an independent prognostic factor
associated with poor PFS and OS.
CD24
CD24 is a sialoglycoprotein that has been identified
as an independent prognostic marker of survival in patients with
ovarian cancer (67). CD24 is
localised in lipid rafts through its glycosylphosphatidylinositol
anchor, but also its diffuse cytoplasmic accumulation is observed
in cancer cells (67). Kristiansen
et al (68) found that
cytoplasmic expression of CD24 was a prognostic factor for poor
survival in ovarian cancer, while membranous expression had no
influence on patients survival. In the study by Nakamura et
al (69) it was shown that the
expression of CD24 was significantly associated with
progression-free survival and overall survival in patients with
ovarian cancer. Moreover, the authors found that the expression of
CD24 was correlated with the FIGO stage and the presence of
peritoneal and lymph node metastasis. Additionally, CD24 induced
the EMT phenomenon in ovarian cancer, which was involved in
resistance to chemotherapy (69).
Also, according to Soltész et al (70) high expression of CD24 in serous
ovarian cancer patients' tissue samples was associated with
advanced FIGO stages.
CD117
Meta-analysis conducted by Yang et al
(71) included seven studies
enrolling over 1200 patients with epithelial ovarian cancer. They
showed that the expression of CD117 was significantly correlated
with FIGO stage, histological type, tumour differentiation grade
and age. Moreover, high expression of CD117 was significantly
correlated with poor OS, but there was no statistically significant
association between this marker expression and DFS (71). The study by Luo et al
(72) showed that the expression
of CD117 is also statistically correlated with response to
chemotherapy and CD117+ patients were less sensitive to
chemotherapy than CD117− patients.
CD105 (endoglin)
It has been shown that the expression of CD105 was
associated with poor survival in patients with ovarian cancer
(73). Furthermore, it is
suggested that CD105 plays a role in ovarian cancer metastasis
(74). Zhang et al
(52) found that moderately and
highly differentiated ovarian cancer tissue samples exhibited
decreased expression of CD105 compared with poorly differentiated
samples. Moreover, early-stage (I and II) ovarian cancer tissue
samples exhibited decreased expression of CD105 compared with
advanced stage (III) samples. Additionally, there were increased
protein expression of CD105 in drug-resistant epithelial ovarian
cancer tissue samples compared with drug-sensitive samples
(52). Ziebarth et al
(75) found that inhibition of
CD105 increased cisplatin sensitivity in epithelial ovarian
cancer.
CD106 (VCAM-1)
The study conducted by Huang et al (76) showed that overexpression of VCAM-1
in high grade serous ovarian cancer cells was associated with poor
prognosis. Moreover, the authors found that high expression of
VCAM-1 was related to advanced age at diagnosis and poor response
to surgery and chemotherapy. Their data suggest that VCAM-1 may be
a prognostic factor and novel therapeutic target for ovarian cancer
(76). Scalici et al
(77) found that mesothelium
expression of VCAM-1 in patients with epithelial ovarian cancer was
associated with shorter PFS and OS. In the study by Zhang et
al (52) it was shown that
high expression of CD106 was associated with drug resistance.
EpCAM
The study by Tayama et al (78) showed that an increased expression
of EpCAM was associated with poor prognosis in patients with
ovarian cancer and correlated with shortened PFS and OS. Moreover,
they also found that EpCAM was associated with chemoresistance to
platinum-based chemotherapy (78).
Spizzo et al (79) also
showed that overexpression of EpCAM was significantly correlated
with decreased OS in patients with epithelial ovarian cancer.
However, different results were obtained by Woopen et al
(80) who showed that epithelial
ovarian cancer patients with overexpression of EpCAM had better
prognosis than patients with a weak or no expression of this
marker. EpCAM overexpression was associated with a more favourable
OS, better PFS and high response to platinum-based chemotherapy
(80).
SOX2
The association between the expression of SOX2 and
poor prognosis in ovarian cancer was shown by Zhang et al
(81). They found that the
expression of SOX2 was associated with decreased DFS durations, but
there was no association between SOX2 expression and OS. Moreover,
there was significant association between the expression of SOX2
and high-grade serous carcinoma. Their data showed that there was
no significant correlation between the expression of SOX2 and
response to chemotherapy (81).
Bååth et al (82) found
that within the group of patients with non-radical debulking
surgery, there were shorter OS and PFS for patients with
SOX2-positive tumours. Moreover, Li et al (83) investigated that the SOX2 was
overexpressed in paclitaxel-resistant cells.
Nestin
The study by Onisim et al (43) showed that the expression of nestin
in tumour cells was associated with poorer PFS and OS in patients
with ovarian cancer. In another study by Czekierdowski et al
(84) it was found that in high
grade serous ovarian cancer patients with high expression of nestin
had worse OS and DFS rates than patients with low expression of
nestin. Qin et al (85)
found that in serous ovarian cancer nestin-positive patients had
significantly shorter OS. Moreover, overexpression of nestin was
associated with the cisplatin-based chemotherapy resistance
(85).
SSEA1
SSEA1 was studied by Davidson et al (86) in metastatic high grade serous
carcinoma. They found that higher expression of SSEA1 was
significantly associated with shorter OS and poorer PFS. Moreover,
SSEA1 was significantly overexpressed in post-chemotherapy
effusions compared with pre-chemotherapy specimens tapped at
diagnosis (86).
Thy-1 (CD90)
In the study conducted by Chen et al
(87) it was found that the
expression of CD90 was significantly decreased in ovarian tumour
tissues and lower expression of CD90 was correlated with poor
survival rate. Moreover, the authors investigated that CD90
decreased the expression of other CSCs markers, such as CD133 and
CD24 (87). Different results were
obtained by Connor et al (88), who found that the expression of
Thy-1 (CD90) was associated with poorer clinical outcome in women
with ovarian cancer. Their study showed that in high expression of
Thy-1 was associated with poorer OS and PFS in women with serous
ovarian cancer, while the expression of Thy-1 in endometroid
ovarian cancer was associated only with poorer PFS. Moreover, they
demonstrated that the expression of Thy-1 is associated with
increased proliferative and self-renewal capacity of ovarian cancer
cells (88).
All CSCs markers selected for this review are also
presented in Fig. 2, according to
their surface or intracellular presence. Additionally, association
of chemoresistance with type of ovarian cancer is presented in
Table I.
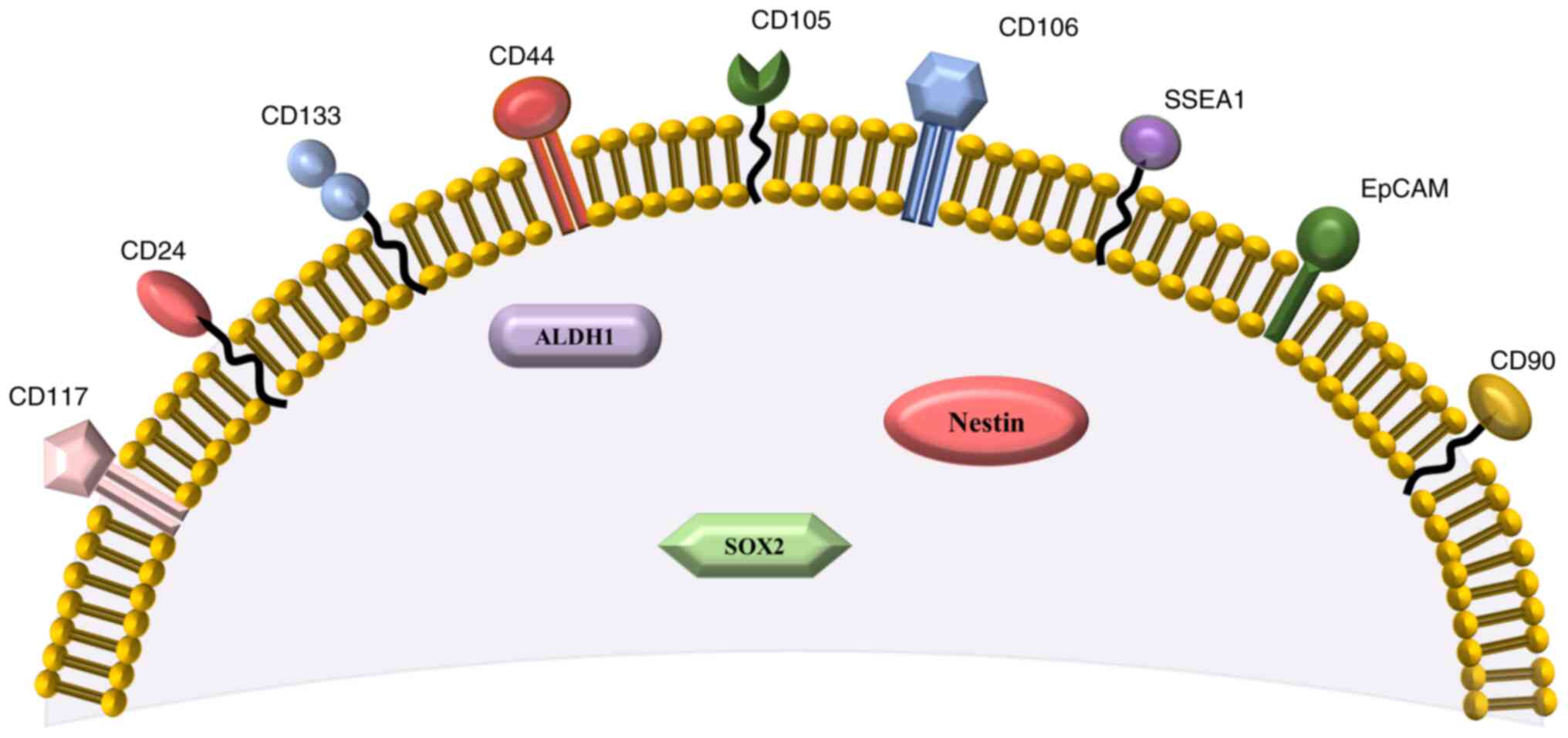 | Figure 2.Surface and intracellular markers of
ovarian cancer stem cells presented in the present review. The
surface markers include: CD133, CD44, CD117, CD24, CD105, CD106,
CD90, SSEA1 and EpCAM. The intracellular markers include: ALDH1,
SOX2 and Nestin (39,52,84,90).
SSEA1, stage-specific embryonic antigen-1; EpCAM, epithelial cell
adhesion molecule; ALDH1, aldehyde dehydrogenase 1; SOX2,
sex-determining region Y-box 2. |
 | Table I.Association of chemoresistance with
type of ovarian cancer. |
Table I.
Association of chemoresistance with
type of ovarian cancer.
| CSCs marker | (Refs.) | Type of ovarian
cancer |
|---|
| CD133 | Steg et al
(45) | High grade ovarian
adenocarcinomas |
|
| Liu et al
(46) | Epithelial ovarian
cancer (serous, mucinous, endometroid, clear cell, mixed
epithelial, undifferentiated) with and without CNS metastases |
|
| Liu et al
(47) | Ovarian cancer cell
lines (CSC-like SKOV3 spheres, CSC-like IGROV1-spheres) |
| CD44 | Tao et al
(42) | Meta-analysis
(patients with different types of ovarian cancer) |
|
| Zhang et al
(52) | Epithelial ovarian
cancer, OVCAR3 cell line, PTX-resistant OC3/TAX300 cells |
| ALDH1 | Ayub et al
(63) | Epithelial ovarian
cancer |
|
| Roy et al
(64) | High grade serous
ovarian cancer |
| CD24 | Nakamura et
al (69) | Caov-3 (human
ovarian mucinous adenocarcinoma cancer cell line) |
| CD117 | Luo et al
(72) | Ovarian serous
adenocarcinoma |
| CD105 | Zhang et al
(52) | Epithelial ovarian
cancer, OVCAR3 cell line, PTX-resistant OC3/TAX300 cells |
|
| Ziebarth et
al (75) | Epithelial ovarian
cancer (cell lines) |
| CD106 | Zhang et al
(52) | Epithelial ovarian
cancer, OVCAR3 cell line, PTX-resistant OC3/TAX300 cells |
|
| Huang et al
(76) | High grade serous
ovarian cancer |
| EpCAM | Tayama et al
(78) | Epithelial ovarian
cancer-tissue samples (serous, clear cell, endometroid, mucinous,
other). Human ovarian cancer cell lines. Animal study |
| SOX2 | Li et al
(83) | Tissue specimens
(patients diagnosed with ovarian cancer). SKOV3 and SKOV3/TAX cells
(paclitaxel-resistant human ovarian adenocarcinoma cell line) |
| Nestin | Qin et al
(85) | Serous ovarian
cancer |
Therapeutic importance of CSCs markers
Targeting CSCs markers remains a challenge. Most of
currently known CSCs surface markers are also expressed on normal
stem cells (embryonic and/or adult stem cells) and they are rarely
or considerably expressed on various normal tissue cells (89,90).
Markers CD133, CD24, CD117, CD90 are expressed on the surface of
human embryonic stem cells (hESC) and adult stem cells (89). CD133 is also expressed in
epithelial and non-epithelial cells as well as it can be found in
many cancers such as breast, lung, ovarian, melanoma, pancreatic,
colon, prostate, glioma and hepatocellular cancers (91). EpCAM has been used as an
undifferentiated hESC marker and it is also expressed on some
normal epithelial cells (89).
SSEA-1 is a surface marker for neural stem cells and is related to
lung and renal tumours (89).
Marker CD44 has been detected in human hematopoietic, mesenchymal
and adipose-derived stem cells. Moreover, it is ubiquitously
expressed in many normal tissue cells (89). CD106 is expressed by mesenchymal
and neural stem cells (52).
Monoclonal antibodies (mAb) that target specific
CSCs markers are a promising therapeutic option. Yang et al
(92) reviewed agents that have
been used to target CSCs markers in recent years. For example,
anti-CD44mAb (bivatuzumab) was used for the treatment of head and
neck squamous cell carcinoma, and EpCAM antibody (adecatumumab) was
used in patients with hormone-resistant prostate cancer (92). CSCs markers could also be a target
for chimeric antigen receptor (CAR)-T cell therapy (93,94).
Conclusion
The role of CSCs in the development and progression
of ovarian cancer as well as their association with therapy
resistance is still the subject of numerous studies. Unfortunately,
due to the heterogeneity and plasticity of these cells, finding a
specific phenotype of CSCs that would allow for their better
identification remains a challenge. Moreover, identification of
such phenotypes could also be helpful in developing new diagnostic
and therapeutic strategies in ovarian cancer.
Despite the ambiguous results, the usefulness of
CSCs markers in the assessment of prognosis and their relationship
with the development of chemoresistance in ovarian cancer patients
has been demonstrated. In our review we found that the expression
of ovarian CSCs markers CD133, CD44, ALDH1, CD24, CD117, CD105,
CD106, SOX2, Nestin and SSEA1 may have a prognostic significance
associated with poor prognosis for patients with ovarian cancer.
Moreover, the expression of CD133, CD44, ALDH1, CD24, CD117, CD105,
CD106, EpCAM, SOX2 and Nestin could be associated with resistance
to chemotherapy in ovarian cancer. However, it is advisable to
perform further studies that will allow the use of CSCs markers
especially in the aspect of tumour recurrence and in the
development of personalised targeted therapies.
Acknowledgements
Not applicable.
Funding
The present review was funded by the Medical University of
Silesia in Katowice, Poland (grant no. PCN-1-069/K/1/O).
Availability of data and materials
Not applicable.
Authors' contributions
AMP and PKD conceptualised this review. PKD, DW,
MSK and SS searched and selected literature. AMP and PKD prepared
and reviewed the original draft. PKD, DW, MSK and SS designed the
table and figures. All authors read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kroeger PT Jr and Drapkin R: Pathogenesis
and heterogeneity of ovarian cancer. Curr Opin Obstet Gynecol.
29:26–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Leo A, Santini D, Ceccarelli C,
Santandrea G, Palicelli A, Acquaviva G, Chiarucci F, Rosini F,
Ravegnini G, Pession A, et al: What is new on ovarian carcinoma:
Integrated morphologic and molecular analysis following the new
2020 World Health Organization classification of female genital
tumors. Diagnostics (Basel). 11:6972021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kenda Suster N and Virant-Klun I: Presence
and role of stem cells in ovarian cancer. World J Stem Cells.
11:383–397. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nebgen DR, Lu KH and Bast RC Jr: Novel
approaches to ovarian cancer screening. Curr Oncol Rep. 21:752019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kujawa KA and Lisowska KM: Ovarian
cancer-from biology to clinic. Postepy Hig Med. Dosw (online).
69:1275–1290. 2015.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stewart C, Ralyea C and Lockwood S:
Ovarian cancer: An integrated review. Semin Oncol Nurs. 35:151–156.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ottevanger PB: Ovarian cancer stem cells
more questions than answers. Semin Cancer Biol. 44:67–71. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cortez AJ, Tudrej P, Kujawa KA and
Lisowska KM: Advances in ovarian cancer therapy. Cancer Chemother
Pharmacol. 81:17–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Valabrega G, Scotto G, Tuninetti V, Pani A
and Scaglione F: Differences in PARP inhibitors for the treatment
of ovarian cancer: Mechanisms of action, pharmacology, safety, and
efficacy. Int J Mol Sci. 22:42032021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu L, Cai S, Han C, Banerjee A, Wu D, Cui
T, Xie G, Zhang J, Zhang X, McLaughlin E, et al: ALDH1A1
contributes to PARP inhibitor resistance via enhancing DNA repair
in BRCA2−/− ovarian cancer cells. Mol Cancer Ther.
19:199–210. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan Y, Ma S, Cao K, Zhou S, Zhao A, Li M,
Qian F and Zhu C: Therapeutic approaches targeting cancer stem
cells. J Cancer Res Ther. 14:1469–1475. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rich JN: Cancer stem cells: Understanding
tumour hierarchy and heterogeneity. Medicine (Baltimore). 95 (Suppl
1):S2–S7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Plaks V, Kong N and Werb Z: The cancer
stem cell niche: How essential is the niche in regulating stemness
of tumor cells? Cell Stem Cell. 16:225–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Afify SM and Seno M: Conversion of stem
cells to cancer stem cells: Undercurrent of cancer initiation.
Cancers (Basel). 11:3452019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szaryńska M and Kmieć Z: The role of
cancer stem cells in pathogenesis and therapy of cancer. Forum Med
Rodz. 5:47–56. 2011.
|
|
17
|
Melzer C, von der Ohe J, Lehnert H,
Ungefroren H and Hass R: Cancer stem cell niche models and
contribution by mesenchymal stroma/stem cells. Mol Cancer.
16:282017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang T, Shigdar S, Gantier MP, Hou Y, Wang
L, Li Y, Shamaileh HA, Yin W, Zhou SF, Zhao X and Duan W: Cancer
stem cell targeted therapy: Progress amid controversies.
Oncotarget. 6:44191–44206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Markowska J, Kojs Z and Twardawa D: Cancer
stem cells in targeted therapy. Curr Gynecol Oncol. 16:96–100.
2018. View Article : Google Scholar
|
|
20
|
Islam F, Qiao B, Smith RA, Gopalan V and
Lam AK: Cancer stem cell: fundamental experimental pathological
concepts and updates. Exp Mol Pathol. 98:184–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Atashzar MR, Baharlou R, Karami J,
Abdollahi H, Rezaei R, Pourramezan F and Zoljalali Moghaddam SH:
Cancer stem cells: A review from origin to therapeutic
implications. J Cell Physiol. 235:790–803. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nimmakayala RK, Batra SK and Ponnusamy MP:
Unraveling the journey of cancer stem cells from origin to
metastasis. Biochim Biophys Acta Rev Cancer. 1871:50–63. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang H and Unternaehrer JJ:
Epithelial-mesenchymal transition and cancer stem cells: At the
crossroads of differentiation and dedifferentiation. Dev Dyn.
248:10–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bar JK, Grelewski P, Lis-Nawara A and
Drobnikowska K: The role of cancer stem cells in progressive growth
and resistance of ovarian cancer: True or fiction? Postepy Hig Med
Dosw (Online). 69:1077–1086. 2015.(In Polish). PubMed/NCBI
|
|
25
|
Huang R and Rofstad EK: Cancer stem cells
(CSCs), cervical CSCs and targeted therapies. Oncotarget.
8:35351–35367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Al-Alem LF, Pandya UM, Baker AT, Bellio C,
Zarrella BD, Clark J, DiGloria CM and Rueda BR: Ovarian cancer stem
cells: What progress have we made? Int J Biochem Cell Biol.
107:92–103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lathia JD and Liu H: Overview of cancer
stem cells and stemness for community oncologists. Target Oncol.
12:387–399. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wan Kamarul Zaman WS, Nurul AA and Nordin
F: Stem cells and cancer stem cells: The Jekyll and Hyde scenario
and their implications in stem cell therapy. Biomedicines.
9:12452021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prieto-Vila M, Takahashi RU, Usuba W,
Kohama I and Ochiya T: Drug resistance driven by cancer stem cells
and their niche. Int J Mol Sci. 18:25742017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bighetti-Trevisan RL, Sousa LO, Castilho
RM and Almeida LO: Cancer stem cells: Powerful targets to improve
current anticancer therapeutics. Stem Cells Int. 2019:96180652019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Helbrecht I, Szymanski Ł, Fiedorowicz M,
Matak D, Bartnik E, Golik P, Szczylik C and Czarnecka AM: Isolation
of renal cancer stem cells. Postępy Biologii Komórki. 45:115–134.
2018.
|
|
32
|
Bandhavkar S: Cancer stem cells: A
metastasizing menace! Cancer Med. 5:649–655. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Codd AS, Kanaseki T, Torigo T and Tabi Z:
Cancer stem cells as targets for immunotherapy. Immunology.
153:304–314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bapat SA, Mali AM, Koppikar CB and Kurrey
NK: Stem and progenitor-like cells contribute to the aggressive
behavior of human epithelial ovarian cancer. Cancer Res.
65:3025–3029. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lupia M and Cavallaro U: Ovarian cancer
stem cells: Still an elusive entity? Mol Cancer. 16:642017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bregenzer ME, Horst EN, Mehta P, Novak CM,
Repetto T and Mehta G: The role of cancer stem cells and mechanical
forces in ovarian cancer metastasis. Cancers (Basel). 11:10082019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li SS, Ma J and Wong AST: Chemoresistance
in ovarian cancer: Exploiting cancer stem cell metabolism. J
Gynecol Oncol. 29:e322018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Klemba A, Purzycka-Olewiecka JK, Wcisło G,
Czarnecka AM, Lewicki S, Lesyng B, Szczylik C and Kieda C: Surface
markers of cancer stem-like cells of ovarian cancer and their
clinical relevance. Contemp Oncol (Pozn). 22:48–55. 2018.PubMed/NCBI
|
|
39
|
Walcher L, Kistenmacher AK, Suo H, Kitte
R, Dluczek S, Strauß A, Blaudszun AR, Yevsa T, Fricke S and
Kossatz-Boehlert U: Cancer stem cells-origins and biomarkers:
Perspectives for targeted personalized therapies. Front Immunol.
11:12802020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liou GY: CD133 as a regulator of cancer
metastasis through the cancer stem cells. Int J Biochem Cell Biol.
106:1–7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou Q, Chen A, Song H, Tao J, Yang H and
Zuo M: Prognostic value of cancer stem cell marker CD133 in ovarian
cancer: A meta-analysis. Int J Clin Exp Med. 8:3080–3088.
2015.PubMed/NCBI
|
|
42
|
Tao Y, Li H, Huang R, Mo D, Zeng T, Fang M
and Li M: Clinicopathological and prognostic significance of cancer
stem cell markers in ovarian cancer patients: Evidence from 52
studies. Cell Physiol Biochem. 46:1716–1726. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Onisim A, Iancu M, Vlad C, Kubelac P,
Fetica B, Fulop A, Achimas-Cadariu A and Achimas-Cadariu P:
Expression of Nestin and CD133 in serous ovarian carcinoma. J BUON.
21:1168–1175. 2016.PubMed/NCBI
|
|
44
|
Ruscito I, Cacsire Castillo-Tong D,
Vergote I, Ignat I, Stanske M, Vanderstichele A, Ganapathi RN,
Glajzer J, Kulbe H, Trillsch F, et al: Exploring the clonal
evolution of CD133/aldehyde-dehydrogenase-1 (ALDH1)-positive cancer
stem-like cells from primary to recurrent high-grade serous ovarian
cancer (HGSOC). A study of the ovarian cancer therapy-innovative
models prolong survival (OCTIPS) consortium. Eur J Cancer.
79:214–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Steg AD, Bevis KS, Katre AA, Ziebarth A,
Dobbin ZC, Alvarez RD, Zhang K, Conner M and Landen CN: Stem cell
pathways contribute to clinical chemoresistance in ovarian cancer.
Clin Cancer Res. 18:869–881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu BL, Liu SJ, Baskys A, Cheng H, Han Y,
Xie C, Song H, Li J and Xin XY: Platinum sensitivity and CD133
expression as risk and prognostic predictors of central nervous
system metastases in patients with epithelial ovarian cancer. BMC
Cancer. 14:8292014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu CL, Chen YJ, Fan MH, Liao YJ and Mao
TL: Characteristics of CD133-sustained chemoresistant cancer
stem-like cells in human ovarian carcinoma. Int J Mol Sci.
21:64672020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ween MP, Oehler MK and Ricciardelli C:
Role of versican, hyaluronan and CD44 in ovarian cancer metastasis.
Int J Mol Sci. 12:1009–1029. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sacks JD and Barbolina MV: Expression and
function of CD44 in epithelial ovarian carcinoma. Biomolecules.
5:3051–3066. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lin J and Ding D: The prognostic role of
the cancer stem cell marker CD44 in ovarian cancer: A
meta-analysis. Cancer Cell Int. 17:82017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou J, Du Y, Lu Y, Luan B, Xu C, Yu Y and
Zhao H: CD44 expression predicts prognosis of ovarian cancer
patients through promoting epithelial-mesenchymal transition (EMT)
by regulating snail, ZEB1, and caveolin-1. Front Oncol. 9:8022019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang J, Yuan B, Zhang H and Li H: Human
epithelial ovarian cancer cells expressing CD105, CD44 and CD106
surface markers exhibit increased invasive capacity and drug
resistance. Oncol Lett. 17:5351–5360. 2019.PubMed/NCBI
|
|
53
|
Zhu Y, Zhang H, Zhang G, Shi Y and Huang
J: Co-expression of CD44/MyD88 is a poor prognostic factor in
advanced epithelial ovarian cancer. Ann Transl Med. 7:912019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang HF, Hu P and Fang SQ: Understanding
the role of CD44V6 in ovarian cancer. Oncol Lett. 14:1989–1992.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tjhay F, Motohara T, Tayama S, Narantuya
D, Fujimoto K, Guo J, Sakaguchi I, Honda R, Tashiro H and Katabuchi
H: CD44 variant 6 is correlated with peritoneal dissemination and
poor prognosis in patients with advanced epithelial ovarian cancer.
Cancer Sci. 106:1421–1428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Motohara T, Fujimoto K, Tayama S,
Narantuya D, Sakaguchi I, Tashiro H and Katabuchi H: CD44 variant 6
as a predictive biomarker for distant metastasis in patients with
epithelial ovarian cancer. Obstet Gynecol. 127:1003–1011. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Deng S, Yang X, Lassus H, Liang S, Kaur S,
Ye Q, Li C, Wang LP, Roby KF, Orsulic S, et al: Distinct expression
levels and patterns of stem cell marker, aldehyde dehydrogenase
isoform 1 (ALDH1), in human epithelial cancers. PLoS One.
5:e102772010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kuroda T, Hirohashi Y, Torigoe T, Yasuda
K, Takahashi A, Asanuma H, Morita R, Mariya T, Asano T, Mizuuchi M,
et al: ALDH1-high ovarian cancer stem-like cells can be isolated
from serous and clear cell adenocarcinoma cells, and ALDH1 high
expression is associated with poor prognosis. PLoS One.
8:e651582013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang YC, Yo YT, Lee HY, Liao YP, Chao TK,
Su PH and Lai HC: ALDH1-bright epithelial ovarian cancer cells are
associated with CD44 expression, drug resistance, and poor clinical
outcome. Am J Pathol. 180:1159–1169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chang B, Liu G, Xue F, Rosen DG, Xiao L,
Wang X and Liu J: ALDH1 expression correlates with favorable
prognosis in ovarian cancers. Mod Pathol. 22:817–823. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Huang R, Li X, Holm R, Trope CG, Nesland
JM and Suo Z: The expression of aldehyde dehydrogenase 1 (ALDH1) in
ovarian carcinomas and its clinicopathological associations: A
retrospective study. BMC Cancer. 15:5022015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhao W, Zang C, Zhang T, Li J, Liu R, Feng
F, Lv Q, Zheng L, Tian J and Sun C: Clinicopathological
characteristics and prognostic value of the cancer stem cell marker
ALDH1 in ovarian cancer: A meta-analysis. Onco Targets Ther.
11:1821–1831. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ayub TH, Keyver-Paik MD, Debald M,
Rostamzadeh B, Thiesler T, Schröder L, Barchet W, Abramian A,
Kaiser C, Kristiansen G, et al: Accumulation of ALDH1-positive
cells after neoadjuvant chemotherapy predicts treatment resistance
and prognosticates poor outcome in ovarian cancer. Oncotarget.
6:16437–16448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Roy M, Connor J, Al-Niaimi A, Rose SL and
Mahajan A: Aldehyde dehydrogenase 1A1 (ALDH1A1) expression by
immunohistochemistry is associated with chemo-refractoriness in
patients with high-grade ovarian serous carcinoma. Hum Pathol.
73:1–6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ricci F, Bernasconi S, Porcu L, Erba E,
Panini N, Fruscio R, Sina F, Torri V, Broggini M and Damia G: ALDH
enzymatic activity and CD133 positivity and response to
chemotherapy in ovarian cancer patients. Am J Cancer Res.
3:221–229. 2013.PubMed/NCBI
|
|
66
|
Silva IA, Bai S, McLean K, Yang K,
Griffith K, Thomas D, Ginestier C, Johnston C, Kueck A, Reynolds
RK, et al: Aldehyde dehydrogenase in combination with CD133 defines
angiogenic ovarian cancer stem cells that portend poor patient
survival. Cancer Res. 71:3991–4001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tarhriz V, Bandehpour M, Dastmalchi S,
Ouladsahebmadarek E, Zarredar H and Eyvazi S: Overview of CD24 as a
new molecular marker in ovarian cancer. J Cell Physiol.
234:2134–2142. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kristiansen G, Denkert C, Schlüns K, Dahl
E, Pilarsky C and Hauptmann S: CD24 is expressed in ovarian cancer
and is a new independent prognostic marker of patient survival. Am
J Pathol. 161:1215–1221. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Nakamura K, Terai Y, Tanabe A, Ono YJ,
Hayashi M, Maeda K, Fujiwara S, Ashihara K, Nakamura M, Tanaka Y,
et al: CD24 expression is a marker for predicting clinical outcome
and regulates the epithelial-mesenchymal transition in ovarian
cancer via both the Akt and ERK pathways. Oncol Rep. 37:3189–3200.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Soltész B, Lukács J, Szilágyi E, Márton É,
Szilágyi Bónizs M, Penyige A, Póka R and Nagy B: Expression of CD24
in plasma, exosome and ovarian tissue samples of serous ovarian
cancer patients. J Biotechnol. 298:16–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yang B, Yan X, Liu L, Jiang C and Hou S:
Overexpression of the cancer stem cell marker CD117 predicts poor
prognosis in epithelial ovarian cancer patients: Evidence from
meta-analysis. Onco Targets Ther. 10:2951–2961. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Luo L, Zeng J, Liang B, Zhao Z, Sun L, Cao
D, Yang J and Shen K: Ovarian cancer cells with the CD117 phenotype
are highly tumorigenic and are related to chemotherapy outcome. Exp
Mol Pathol. 91:596–602. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Taskiran C, Erdem O, Onan A, Arisoy O,
Acar A, Vural C, Erdem M, Ataoglu O and Guner H: The prognostic
value of endoglin (CD105) expression in ovarian carcinoma. Int J
Gynecol Cancer. 16:1789–1793. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Bai S, Zhu W, Coffman L, Vlad A, Schwartz
LE, Elishaev E, Drapkin R and Buckanovich RJ: CD105 is expressed in
ovarian cancer precursor lesions and is required for metastasis to
the ovary. Cancers (Basel). 11:17102019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ziebarth AJ, Nowsheen S, Steg AD, Shah MM,
Katre AA, Dobbin ZC, Han HD, Lopez-Berestein G, Sood AK, Conner M,
et al: Endoglin (CD105) contributes to platinum resistance and is a
target for tumor-specific therapy in epithelial ovarian cancer.
Clin Cancer Res. 19:170–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Huang J, Zhang J, Li H, Lu Z, Shan W,
Mercado-Uribe I and Liu J: VCAM1 expression correlated with
tumorigenesis and poor prognosis in high grade serous ovarian
cancer. Am J Transl Res. 5:336–346. 2013.PubMed/NCBI
|
|
77
|
Scalici JM, Arapovic S, Saks EJ, Atkins
KA, Petroni G, Duska LR and Slack-Davis JK: Mesothelium expression
of vascular cell adhesion molecule-1 (VCAM-1) is associated with an
unfavorable prognosis in epithelial ovarian cancer (EOC). Cancer.
123:977–984. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tayama S, Motohara T, Narantuya D, Li C,
Fujimoto K, Sakaguchi I, Tashiro H, Saya H, Nagano O and Katabuchi
H: The impact of EpCAM expression on response to chemotherapy and
clinical outcomes in patients with epithelial ovarian cancer.
Oncotarget. 8:44312–44325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Spizzo G, Went P, Dirnhofer S, Obrist P,
Moch H, Baeuerle PA, Mueller-Holzner E, Marth C, Gastl G and Zeimet
AG: Overexpression of epithelial cell adhesion molecule (Ep-CAM) is
an independent prognostic marker for reduced survival of patients
with epithelial ovarian cancer. Gynecol Oncol. 103:483–488. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Woopen H, Pietzner K, Richter R,
Fotopoulou C, Joens T, Braicu EI, Mellstedt H, Mahner S, Lindhofer
H, Darb-Esfahani S, et al: Overexpression of the epithelial cell
adhesion molecule is associated with a more favorable prognosis and
response to platinum-based chemotherapy in ovarian cancer. J
Gynecol Oncol. 25:221–228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang J, Chang DY, Mercado-Uribe I and Liu
J: Sex-determining region Y-box 2 expression predicts poor
prognosis in human ovarian carcinoma. Hum Pathol. 43:1405–1412.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Bååth M, Westbom-Fremer S, Martin de la
Fuente L, Ebbesson A, Davis J, Malander S, Måsbäck A, Kannisto P
and Hedenfalk I: SOX2 is a promising predictor of relapse and death
in advanced stage high-grade serous ovarian cancer patients with
residual disease after debulking surgery. Mol Cell Oncol.
7:18050942020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li Y, Chen K, Li L, Li R, Zhang J and Ren
W: Overexpression of SOX2 is involved in paclitaxel resistance of
ovarian cancer via the PI3K/Akt pathway. Tumour Biol. 36:9823–9828.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Czekierdowski A, Stachowicz N,
Czekierdowska S, Łoziński T, Gurynowicz G and Kluz T: Prognostic
significance of TEM7 and nestin expression in women with advanced
high grade serous ovarian cancer. Ginekol Pol. 89:135–141. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Qin Q, Sun Y, Fei M, Zhang J, Jia Y, Gu M,
Xia R, Chen S and Deng A: Expression of putative stem marker nestin
and CD133 in advanced serous ovarian cancer. Neoplasma. 59:310–315.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Davidson B, Holth A and Dong HP:
Expression of the cancer stem cell marker SSEA1 is associated with
poor survival in metastatic high-grade serous carcinoma. Virchows
Arch. 477:677–685. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chen WC, Hsu HP, Li CY, Yang YJ, Hung YH,
Cho CY, Wang CY, Weng TY and Lai MD: Cancer stem cell marker CD90
inhibits ovarian cancer formation via β3 integrin. Int J Oncol.
49:1881–1889. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Connor EV, Saygin C, Braley C, Wiechert
AC, Karunanithi S, Crean-Tate K, Abdul-Karim FW, Michener CM, Rose
PG, Lathia JD and Reizes O: Thy-1 predicts poor prognosis and is
associated with self-renewal in ovarian cancer. J Ovarian Res.
12:1122019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kim WT and Ryu CJ: Cancer stem cell
surface markers on normal stem cells. BMB Rep. 50:285–298. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yang W, Kim D, Kim DK, Choi KU, Suh DS and
Kim JH: Therapeutic strategies for targeting ovarian cancer stem
cells. Int J Mol Sci. 22:50592021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Barzegar Behrooz A, Syahir A and Ahmad S:
CD133: Beyond a cancer stem cell biomarker. J Drug Target.
27:257–269. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang
J, Zhang G, Wang X, Dong Z, Chen F and Cui H: Targeting cancer stem
cell pathways for cancer therapy. Signal Transduct Target Ther.
5:82020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Masoumi J, Jafarzadeh A, Abdolalizadeh J,
Khan H, Philippe J, Mirzaei H and Mirzaei HR: Cancer stem
cell-targeted chimeric antigen receptor (CAR)-T cell therapy:
Challenges and prospects. Acta Pharm Sin B. 11:1721–1739. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Huang B, Miao L, Liu J, Zhang J and Li Y:
A promising antitumor method: Targeting CSC with immune cells
modified with CAR. Front Immunol. 13:9373272022. View Article : Google Scholar : PubMed/NCBI
|