Introduction
Solitary fibrous tumors (SFTs) (1) are rare mesenchymal neoplasms of
fibroblastic type, which account for ~4% of all soft-tissue
sarcomas and mesenchymal tumors in France (2), with a reported incidence rate of
<1 case/million people/year in the United States (3). SFTs may occur at any anatomical
location and have a peak incidence age of between 40 and 70 years,
with no sex difference (1). SFTs
consist of a histologically random arrangement characterized by a
combination of hypercellular and hypocellular areas. Nuclear STAT6
protein expression and specific NGFI-A binding protein 2
(NAB2)-STAT6 gene fusion facilitates a definite diagnosis of SFT
(4–6). Although most cases are benign, the
features of malignant SFT may contain dense arrangements, evident
atypia, increased mitotic figures, necrosis, peripheral
infiltration, recurrence or metastasis (4,5).
Recurrence occurs in 10–30% of SFTs, and metastasis to the lymph
nodes is reported in <5% of malignant SFTs (7–9).
Surgical resection remains the main treatment modality, and
systematic adjuvant therapy or targeted treatment may also be used.
To the best of our knowledge, this is the first case report of a
patient who suffered two recurrences of retroperitoneal malignant
STF and lymph node metastases. This report mainly focused on the
samples of the second recurrence to identify the risk factors for
poor prognosis of SFT.
Case report
A 67-year-old male patient underwent retroperitoneal
benign SFT resection at the Tianjin Medical University Cancer
Institute and Hospital (Tianjin, China) in February 2009. The first
recurrence presented as a malignant SFT in January 2018 and the
second retroperitoneal tumor resection was performed in March 2018
at the Peking University International Hospital (Beijing, China). A
total of 4 months after the second surgery, the second recurrence
occurred and 40 months later the patient had hematochezia for 2
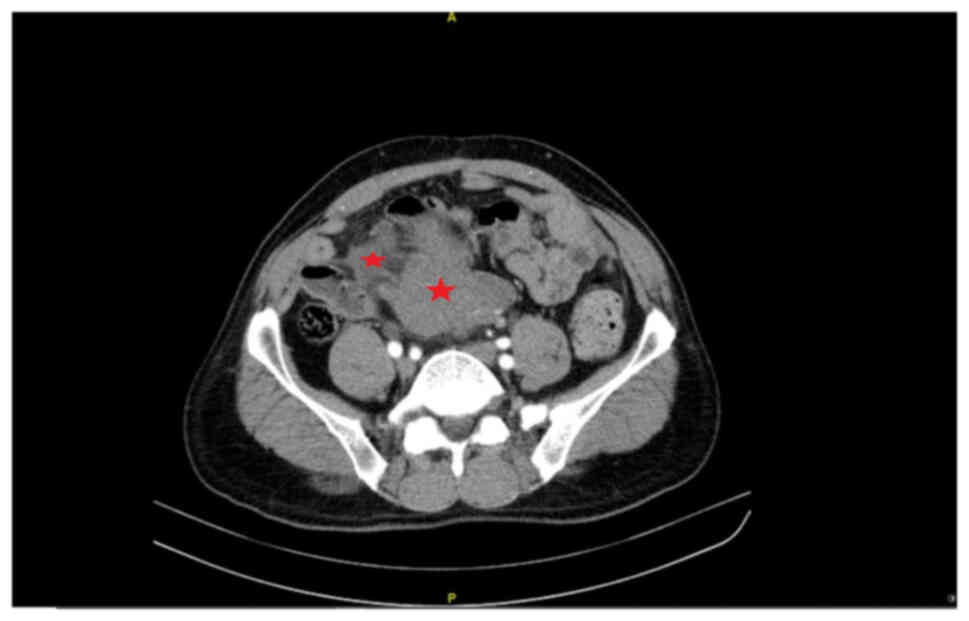
months. CT revealed multiple retroperitoneal masses involving the
intestinal wall (Fig. 1).
Immediately, the third retroperitoneal tumor resection (including
part liver, intestine, mesentery and omentum resection) was
performed at the Peking University International Hospital in
November 2021. However, a number of small lesions could not be
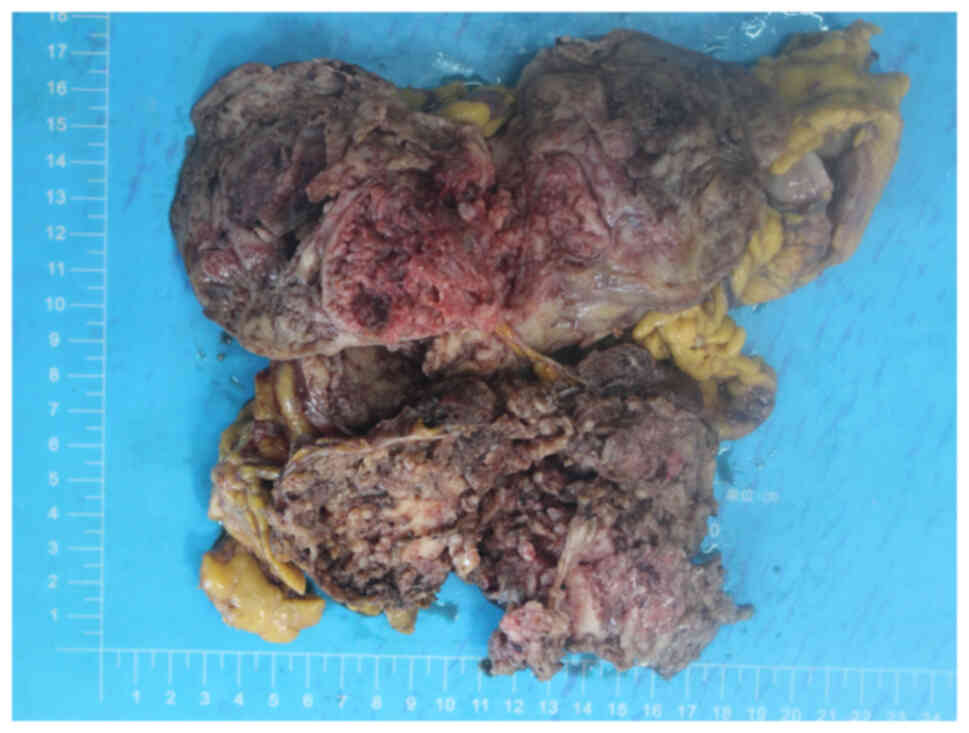
completely removed. The total size of the resected masses was
~18×18×8 cm, partially encapsulated with a smooth fibrous surface.
The cross-section of the tumor showed lobulated white-brown areas
(Fig. 2). Specimens were fixed
with 4% formalin at room temperature for 12 h, embedded in
paraffin, cut into 4-µm sections, stained for 5 min at room
temperature with hematoxylin and eosin, and observed under a light
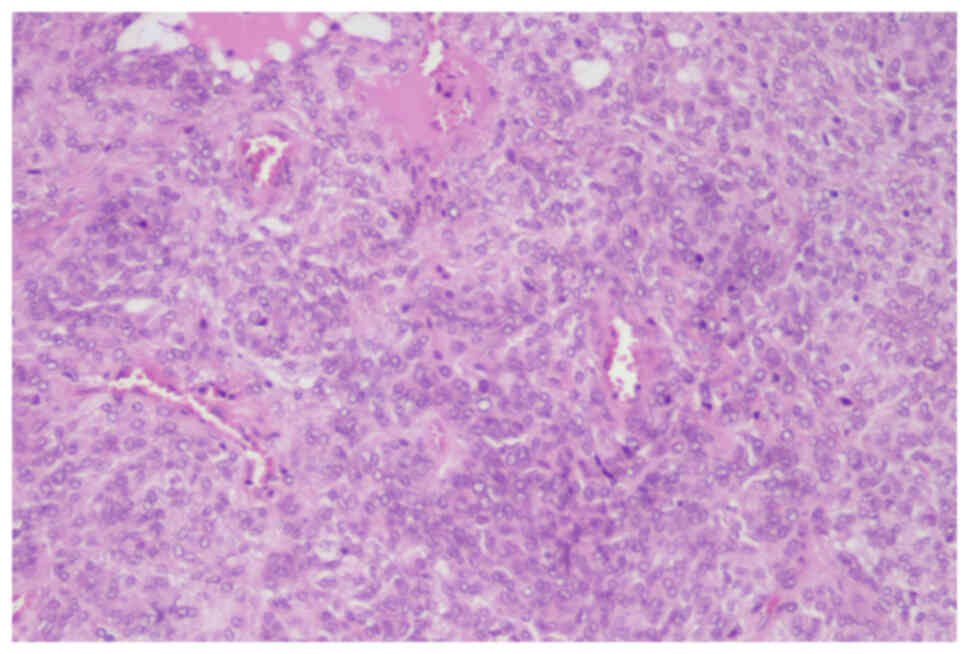
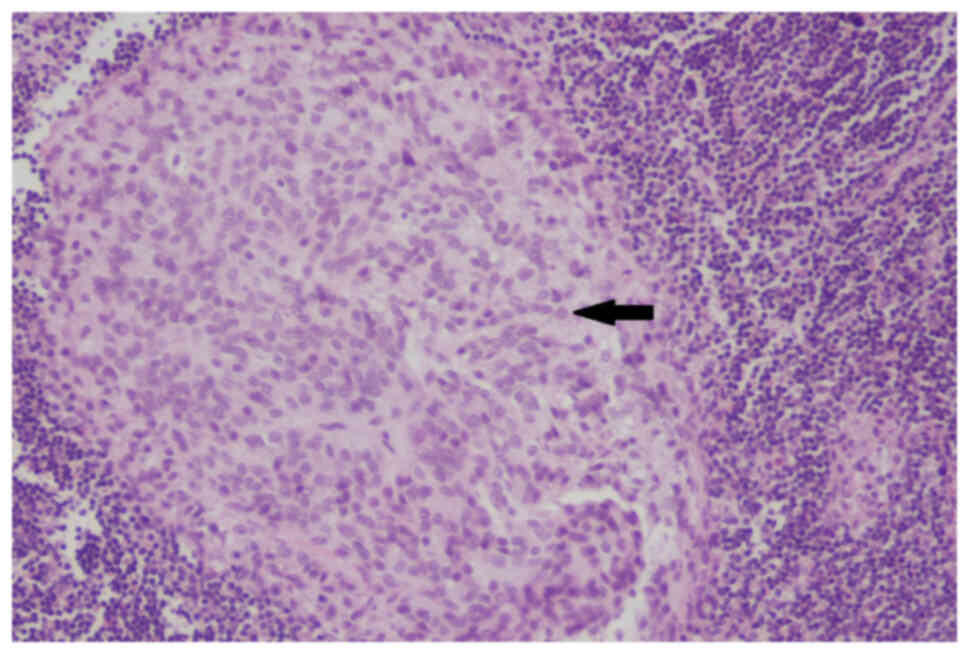
microscope (Nikon Corporation). At the microscopic level, the short
spindle-shaped tumor cells were arranged alternatively with
hypocellular and hypercellular patterns separated by thick collagen
fibers and blood vessels in the interstitium (Fig. 3). Compared with the previous
postoperative specimens from the Tianjin Medical University Cancer
Institute and Hospital, the hypercellular regions of the lesions
presented obvious cytological atypia, increased mitoses count of
6–8 per 10 high power fields, and focal necrosis. The tumor
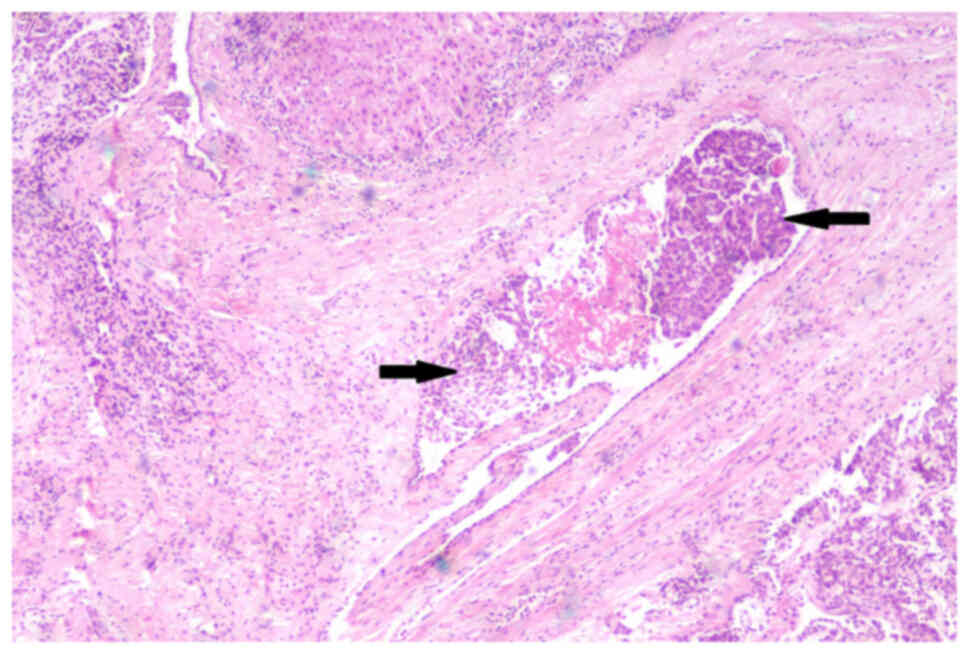
encroached the surrounding liver, the whole layer of the intestinal
wall and lymphatic vessels (Fig.
4). Two lymph nodes (2/18) showed the same histological finding
as the hypercellular area (Fig.
5). In addition, multiple tumor nodules were seen in the
mesentery and omentum.
For immunohistochemical staining, the tissue was
fixed with 4% neutral formalin at room temperature for 6–12 h, cut
into 2–3 mm sections and embedded in paraffin. Paraffin-embedded
tissues were cut into 4 µm sections and sealed with 3% hydrogen
peroxide at room temperature for 10 min. Antigen retrieval was
performed with EDTA at 100°C for 2.5 min, followed by washing with
PBS, primary antibody incubation at 37°C for 60 min and secondary
antibody incubation at 37°C for 20 min. The primary and the
secondary antibodies were purchased ready to use from OriGene
Technologies, Inc., with the exception of anti-CD117, which was
purchased from Leica Microsystems, Inc. The following primary
antibodies were used: STAT6 (cat. no. ZA-0647), CD34 (cat. no.
ZM-0046), CD99 (cat. no. ZM-0296), Bcl-2 (cat. no. ZA-0536), p16
(cat. no. ZM-0205), CDK4 (cat. no. ZA-0614), S-100 (cat. no.
ZA-0225), MDM2 (cat. no. ZM-0425), desmin (cat. no. ZA-0610),
smooth muscle actin (SMA; cat. no, ZM-0003), Myogenin (cat. no.
ZA-0592), CD117 (cat. no. PA0007), DOG-1 (cat. no. ZM-0371), p53
(cat. no. ZM-0408) and Ki-67 (cat. no. ZM-0166). Secondary
antibodies were obtained from OriGene Technologies, Inc. (cat. no.
PV-8000) and from Leica Microsystems, Ltd. (cat. no DS9800).
Finally, sections were stained with DAB at room temperature for 5
min, counterstained with hematoxylin at room temperature for 5 min
and observed under a Nikon light microscope (Nikon Corporation).
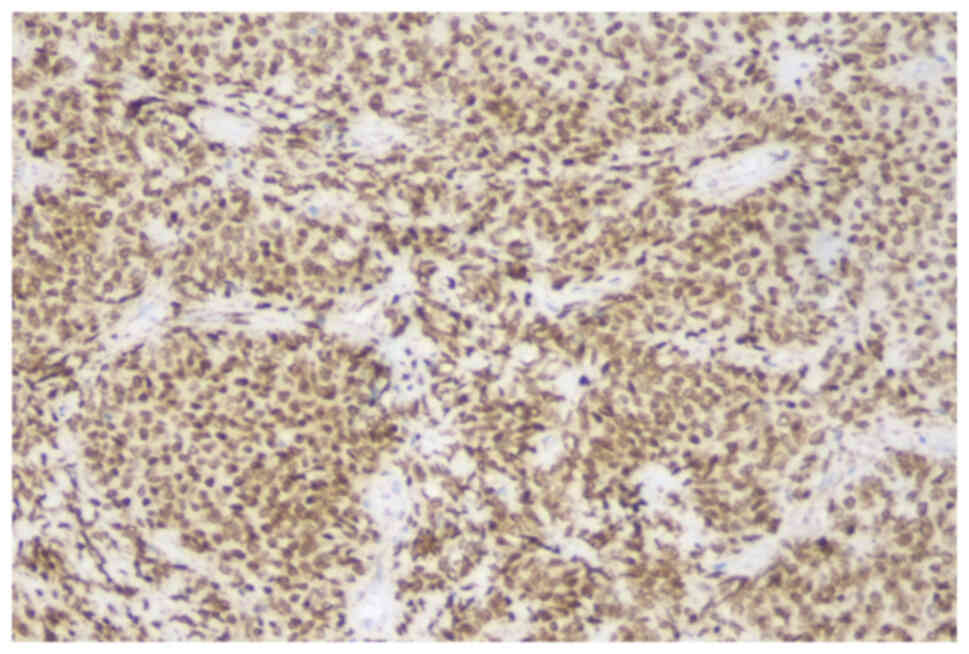
Immunohistochemical staining showed that the tumor cells were
diffusely positive for STAT6 (Fig.
6), Bcl-2 (Fig. S1), CD34
(Fig. S2) and CD99 (Fig. S3), focally positive for CDK4
(Fig. S4) and p16 (Fig. S5), and negative for S-100, MDM2,
desmin, SMA, Myogenin, CD117 and DOG-1 (data not shown). Wild-type
p53 was expressed (Fig. S6), and
Ki-67 index was ~20% (Fig. S7).
The final diagnosis was retroperitoneal malignant SFT; however, the
third recurrence was observed again by CT just 2 months after the
latest surgery. The patient is surviving to date having received no
further or additional treatment.
Discussion
Although the majority of SFTs are clinically benign,
SFTs can be malignant or can be transformed/dedifferentiated from a
benign to a malignant level during recurrence or metastasis. The
development in the present patient confirms the latter scenario.
Because the prognosis of SFTs is not well predicted by histological
grading, Demicco et al (10,11)
used the age of onset, tumor size, mitotic count and necrosis of
SFTs to evaluate the risk of metastasis and death, which greatly
enhanced the prediction for prognosis. According to the method for
risk stratification, Yuan et al (12) explored 31 cases of retroperitoneal
SFTs and revealed that patients in the high- or intermediate-risk
group were susceptible to metastasis and that the Ki-67 index ≥10%
could be used as an important reference to predict the prognosis.
In addition, considering the location of the tumor, a high risk of
recurrence has been reported when it is located in the
retroperitoneum (13), where
metastasis could enter the lung, liver or bone (12,14).
Ito et al (15) reported
the first case of primary retroperitoneal malignant SFT with
paraaortic lymph node metastasis, which belonged to the
non-high-risk group. In this previous study, only surgical
resection was performed and the patient did not develop recurrence
for 2.5 years. Comparatively, the present case was in the high-risk
group and the recurrent tumor morphology became denser and more
atypical compared with the primary tumor. Furthermore, organ
invasion, lymphatic tumor embolization and lymph node metastases
may be indicators of poor prognosis. Only 2 months after the latest
operation, CT scans showed new recurrence. The present case focused
on the pathology of the second recurrence. The specimens obtained
from the first operation are from the Tianjin Medical University
Cancer Institute and Hospital and no external hospital pictures are
presented here, which is a limitation of the present study.
The diagnosis of SFT should combine morphological
and immunophenotypic markers, as well as differentiation markers
from other mesenchymal tumors with spindle-shape cells.
Immunohistochemically, SFTs generally express STAT6, CD34, Bcl-2
and CD99, but rarely S-100, MDM2, desmin, SMA, Myogenin, CD117 and
DOG-1 (11,12). Moreover, GRIA2 and ALDH1 could be
used as novel markers of SFTs (16,17);
however, the absence of experimental results to support this claim
is a limitation of the present study. Notably, since liposarcomas
occasionally show STAT6 protein expression, the MDM2/CDK4 status
must also be evaluated by immunohistochemistry and/or genetic
amplification detection to exclude liposarcomas (18). Therefore, in this case, the
combined detection of these proteins helped to distinguish SFT from
myogenic/neurogenic tumors, gastrointestinal stromal tumors,
synovial sarcomas and liposarcomas.
The NAB2-STAT6 fusion gene is the driving gene
mutation of SFT; therefore, molecular detection of the NAB2-STAT6
fusion gene has high sensitivity and specificity for the diagnosis
of SFTs (19). Nonaka et al
(20) demonstrated for the first
time that downregulation of the NAB2-STAT6 fusion gene at the
transcriptional level was associated with malignant SFT, which
indicated that clinicians should be alerted to cases with a loss of
STAT6 (20). However, in this
case, STAT6 protein was diffusely positive but the patient refused
genetic testing due to financial constraints, which is a limitation
of this study.
Moreover, p53 mutation may be a potential molecular
mechanism promoting the malignancy of SFT (20,21).
Ito et al (15) detected
Bcl-2 positive staining only in the hypocellular area and deduced
that Bcl-2 may also be related to malignant transformation
(15). Nevertheless, the patient
in the present case expressed wild-type p53 and showed no notable
regional differences in Bcl-2 expression.
The first choice for the treatment of
retroperitoneal malignant SFT is surgery, but complete resection is
difficult, and incomplete resection can result in a high recurrence
rate. Notably, adjuvant methods, such as radiotherapy, chemotherapy
or targeted treatment, are currently under investigation (22). Mainly due to economic reasons and
physical fitness, the patient described in the present study will
not be receiving palliative care although the doctors strongly
recommended it. The patient never received chemotherapy or
radiotherapy and therefore long-term survival of the patient is not
expected. The patient has been subjected to regular follow-up
appointments during the past 13 years and is currently living with
the tumor. In conclusion, the course of retroperitoneal SFTs can
last >10 years and requires regular follow-up procedures.
Multiple masses, invasive growth and lymph node metastasis may
result in a poor prognosis.
Supplementary Material
Supporting Data
Acknowledgments
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL was responsible for the conception and design of
the study, and SC and LW contributed to the acquisition and
interpretation of the data. LL drafted the manuscript, and SC and
LW revised it. All authors read and approved the final manuscript,
and agree to be accountable for all aspects of the work to ensure
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved. LL and SC
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for publication was
obtained from the patient's family; due to the limited education
level and understanding ability of the patient, the specific
conditions of their disease was entrusted to their family.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Demicco EG, Fritchie KJ and Han A: WHO
classification of tumours: soft tissue and bone tumours. 5th ed.
International Agency for Research on Cancer; Lyon, France: 2020
|
|
2
|
de Pinieux G, Karanian M, Loarer FL,
Guellec SL, Chabaud S, Terrier P, Bouvier C, Batistella M, Neuville
A, Robin YM, et al: Nationwide incidence of sarcomas and connective
tissue tumors of intermediate malignancy over four years using an
expert pathology review network. PLoS One. 16:e02469582021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kinslow CJ and Wang TJC: Incidence of
extrameningeal solitary fibrous tumors. Cancer. 126:40672020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Machado I, Nieto Morales MG, Cruz J,
Lavernia J, Giner F, Navarro S, Ferrandez A and Llombart-Bosch A:
Solitary fibrous tumor: Integration of clinical, morphologic,
immunohistochemical and molecular findings in risk stratification
and classification may better predict patient outcome. Int J Mol
Sci. 22:94232021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim JM, Choi YL, Kim YJ and Park HK:
Comparison and evaluation of risk factors for meningeal, pleural,
and extrapleural solitary fibrous tumors: A clinicopathological
study of 92 cases confirmed by STAT6 immunohistochemical staining.
Pathol Res Pract. 213:619–625. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akaike K, Kurisaki-Arakawa A, Hara K,
Suehara Y, Takagi T, Mitani K, Kaneko K, Yao T and Saito T:
Distinct clinicopathological features of NAB2-STAT6 fusion gene
variants in solitary fibrous tumor with emphasis on the acquisition
of highly malignant potential. Hum Pathol. 46:347–356. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mearini E, Cochetti G, Barillaro F,
Fatigoni S and Roila F: Renal malignant solitary fibrous tumor with
single lymph node involvement: Report of unusual metastasis and
review of the literature. Onco Targets Ther. 7:679–685.
2014.PubMed/NCBI
|
|
8
|
Yang XJ, Zheng JW, Ye WM, Wang YA, Zhu HG,
Wang LZ and Zhang ZY: Malignant solitary fibrous tumors of the head
and neck: A clinicopathological study of nine consecutive patients.
Oral Oncol. 45:678–682. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Y, Miller CR, Clement C, Hes O and
Eyzaguirre E: Malignant solitary fibrous tumour of the kidney with
lymph node and liver metastases. Pathology. 49:450–453. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Demicco EG, Wagner MJ, Maki RG, Gupta V,
Iofin I, Lazar AJ and Wang WL: Risk assessment in solitary fibrous
tumors: Validation and refinement of a risk stratification model.
Mod Pathol. 30:1433–1442. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Demicco EG, Park MS, Araujo DM, Fox PS,
Bassett RL, Pollock RE, Lazar AJ and Wang WL: Solitary fibrous
tumor: A clinicopathological study of 110 cases and proposed risk
assessment model. Mod Pathol. 25:1298–1306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan X, Liu Y, Wang X, Chen Y, Zhang L and
Wei J: Clinicopathological analysis of retroperitoneal solitary
fibrous tumours: A study of 31 cases. Histol Histopathol. 37:43–50.
2022.PubMed/NCBI
|
|
13
|
Gholami S, Cassidy MR, Kirane A, Kuk D,
Zanchelli B, Antonescu CR, Singer S and Brennan M: Size and
Location are the most Important risk factors for malignant behavior
in resected solitary fibrous tumors. Ann Surg Oncol. 24:3865–3871.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuishi K, Eto K, Morito A, Hamasaki H,
Morita K, Ikeshima S, Horino K, Shimada S and Baba H:
Retroperitoneal fibrous tumor recurring as lung metastases after 10
years: A case report. Surg Case Rep. 7:1272021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ito H, Fukuda M, Imamura Y and Fuse H: A
malignant solitary fibrous tumor in the retroperitoneum. Int J Clin
Oncol. 13:173–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vivero M, Doyle LA, Fletcher CD, Mertens F
and Hornick JL: GRIA2 is a novel diagnostic marker for solitary
fibrous tumour identified through gene expression profiling.
Histopathology. 65:71–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ouladan S, Trautmann M, Orouji E, Hartmann
W, Huss S, Büttner R and Wardelmann E: Differential diagnosis of
solitary fibrous tumors: A study of 454 soft tissue tumors
indicating the diagnostic value of nuclear STAT6 relocation and
ALDH1 expression combined with in situ proximity ligation assay.
Int J Oncol. 46:2595–2605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Creytens D, Libbrecht L and Ferdinande L:
Nuclear expression of STAT6 in dedifferentiated liposarcomas with a
solitary fibrous tumor-like morphology: A diagnostic pitfall. Appl
Immunohistochem Mol Morphol. 23:462–463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bieg M, Moskalev EA, Will R, Hebele S,
Schwarzbach M, Schmeck S, Hohenberger P, Jakob J, Kasper B, Gaiser
T, et al: Gene expression in solitary fibrous tumors (SFTs)
correlates with anatomic localization and NAB2-STAT6 gene fusion
variants. Am J Pathol. 191:602–617. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nonaka H, Kandori S, Nitta S, Shiga M,
Nagumo Y, Kimura T, Kawahara T, Negoro H, Hoshi A, Kojima T, et al:
Case report: Molecular characterization of aggressive malignant
retroperitoneal solitary fibrous tumor: A case study. Front Oncol.
11:7369692021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park HK, Yu DB, Sung M, Oh E, Kim M, Song
JY, Lee MS, Jung K, Noh KW, An S, et al: Molecular changes in
solitary fibrous tumor progression. J Mol Med (Berl). 97:1413–1425.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Bernardi A, Dufresne A, Mishellany F,
Blay JY, Ray-Coquard I and Brahmi M: Novel therapeutic options for
solitary fibrous tumor: Antiangiogenic therapy and beyond. Cancers
(Basel). 14:10642022. View Article : Google Scholar : PubMed/NCBI
|