Introduction
Prostate cancer (PCa) is the most common type of
cancer in men aged >50 years and the fifth most common cause of
cancer-associated mortality (1).
Androgens are essential for prostate development, growth and
secretory functions, and the development of PCa is primarily
androgen dependent (2). However,
some studies have indicated that estrogens may also play a role in
the development of PCa (3).
Estrogens exert their effects at the cellular level
through two receptors: Estrogen receptor-α (ERα) and estrogen
receptor-β (ERβ) (3). Various
mechanisms for ERα and ERβ in PCa have been identified. These
include the fusion of transmembrane protease serine 2 with v-ets
avian erythroblastosis virus E26 oncogene homolog, the most common
gene fusion in the pathogenesis of PCa, which is increased through
ERα activity and decreased via ERβ activity (4). In addition, the ERβ receptor has been
reported to inhibit cell growth, decrease epithelial-mesenchymal
transition-related aggressive behavior and induce apoptosis in PCa
cells (5,6). Although the results in the literature
are conflicting, ERα is associated with malignant transformation
from high-grade prostatic intraepithelial neoplasia (PIN) to PCa,
while ERβ has antiproliferative, antiinvasive and pro-apoptotic
effects (7–14).
To the best of our knowledge, while numerous
preclinical studies have evaluated the effects of ERα and ERβ on
PCa, only three studies have investigated their impact on patients
with non-metastatic PCa (15–17).
Moreover, these three studies have conflicting results and certain
limitations. Therefore, the present study aimed to clarify whether
estrogen receptors affect the development of biochemical recurrence
(BCR) after prostatectomy by balancing well-known risk factors for
BCR in patients with non-metastatic PCa.
Materials and methods
Study population
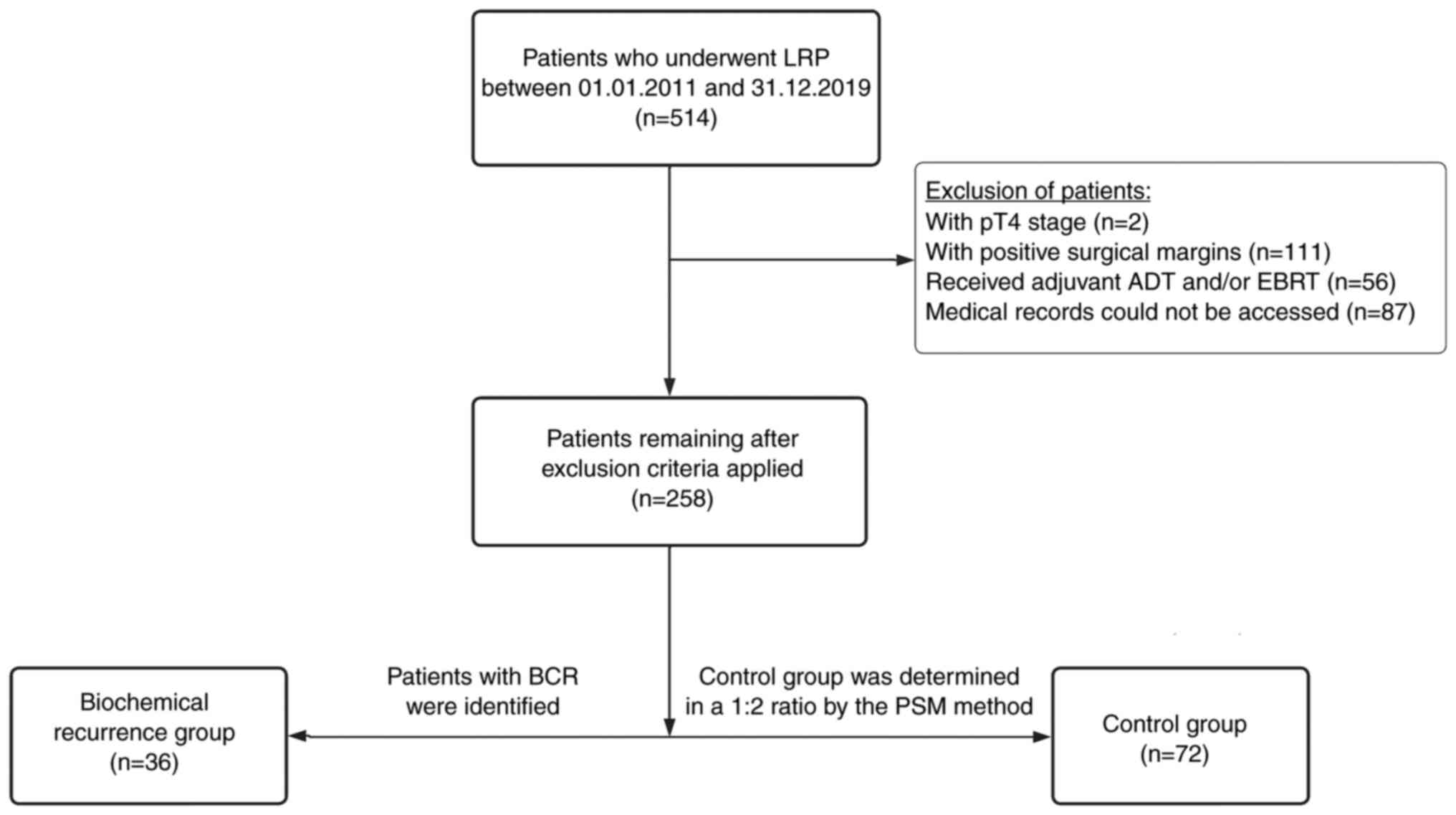
The electronic medical records of 514 patients who
underwent laparoscopic radical prostatectomy (LRP) for PCa between
January 2011 and December 2019 were reviewed in the Department of
Urology, Bursa Uludag University (Bursa, Turkey). Patients who had
incomplete medical records (n=87), pathological T4 stage disease
after LRP (n=2), positive surgical margins (n=111) and adjuvant
androgen deprivation therapy (ADT) or external beam radiotherapy
(EBRT) due to high risk (n=56) were excluded. Lymph node
involvement, which was planned to be an exclusion criterion, was
not observed in any of the remaining 258 patients. BCR was defined
as a prostate-specific antigen (PSA) level >0.2 ng/ml in at
least two serial measurements 3 weeks apart (18). Patients with BCR (n=36) were
identified and constituted the BCR group. The control group (n=72)
was established by the propensity score matching (PSM) method among
the other 222 patients in a 1:2 ratio. The 150 patients that
remained after PSM were excluded from the study (Fig. 1). The Institutional Review Board of
Bursa Uludag University approved the study (approval no.
2021-2/15). The study protocol complied with the tenets of the
Declaration of Helsinki.
Immunohistochemical (IHC) staining and
pathological assessment
IHC staining and pathological reevaluation were
performed by two independent pathologists, one of whom had 26 years
of experience in uropathology. The tissue sections of the patients
stained with hematoxylin and eosin (H&E) were examined, and for
each case, the slides best representing the morphology of the
lesion and Gleason score (GS) were selected for evaluation.
Paraffin blocks of these specimens were obtained from the pathology
archive. From these blocks, 4-µm sections were prepared for the IHC
staining of ERα and ERβ. Paraffin sections were baked overnight at
50°C. The sections to be used for the IHC method were prepared on
positively charged slides. The deparaffinization step was performed
using the Ventana Discovery XT platform with EZ prep solution
(catalogue number 950–100; Roche Diagnostics) at 75°C for 8 min.
The standard antigen retrieval method was heat-induced epitope
retrieval in Tris-Ethylene diamine tetra acetic acid buffer (pH
7.8) at 95°C for 64 min [standard cell conditioning solution
(CC1)], performed using the Ventana Discovery XT (catalogue number
950–124; Roche Diagnostics). For blocking endogenous peroxides and
protein, the Ventana Discovery XT platform was used with Inhibitor
ChromoMap at 37°C for 4 min. The primary antibodies used were ERα
(1:250 dilution; F-10; catalogue number sc-8002) and Erβ (1:250
dilution; B-1; catalogue number sc-390243) (both Santa Cruz
Biotechnology, Inc.). ERα primary antibody was incubated at 37°C
for 20 min, while Erβ primary antibody was incubated at 37°C for 32
min.
The ultra views Universal diaminobenzidine (DAB)
Detection Kit solution (Roche Diagnstics) was used. The kit
contains 5 pre-diluted and ready-to-use dispensers comprising DAB
inhibitor, horseradish peroxidase (HRP) multimer, DAB chromogen,
DAB H2O2 and copper. Slides were lightly
counterstained with hematoxylin (catalogue number 760–2021; Roche
Diagnostics) and incubated at 37°C for 12 min. Post-counterstain
slides were incubated for 8 min with Bluing Reagent (catalogue
number 760–2037; Roche Diagnostics). Slides were washed in warm tap
water with detergent, and after dehydration in graded ethanol and
xylene, they were coverslipped. Endocervical tissue was used as a
positive control for the ERα antibody, and testicular tissue served
as a positive control for the ERβ antibody. Finally, tumor
specimens were observed under a light microscope (model BX51TF;
Olympus Corporation).
The IHC stained slides were analyzed simultaneously
with H&E stained slides from the archive for each case to avoid
the false positivity that may occur due to intense background
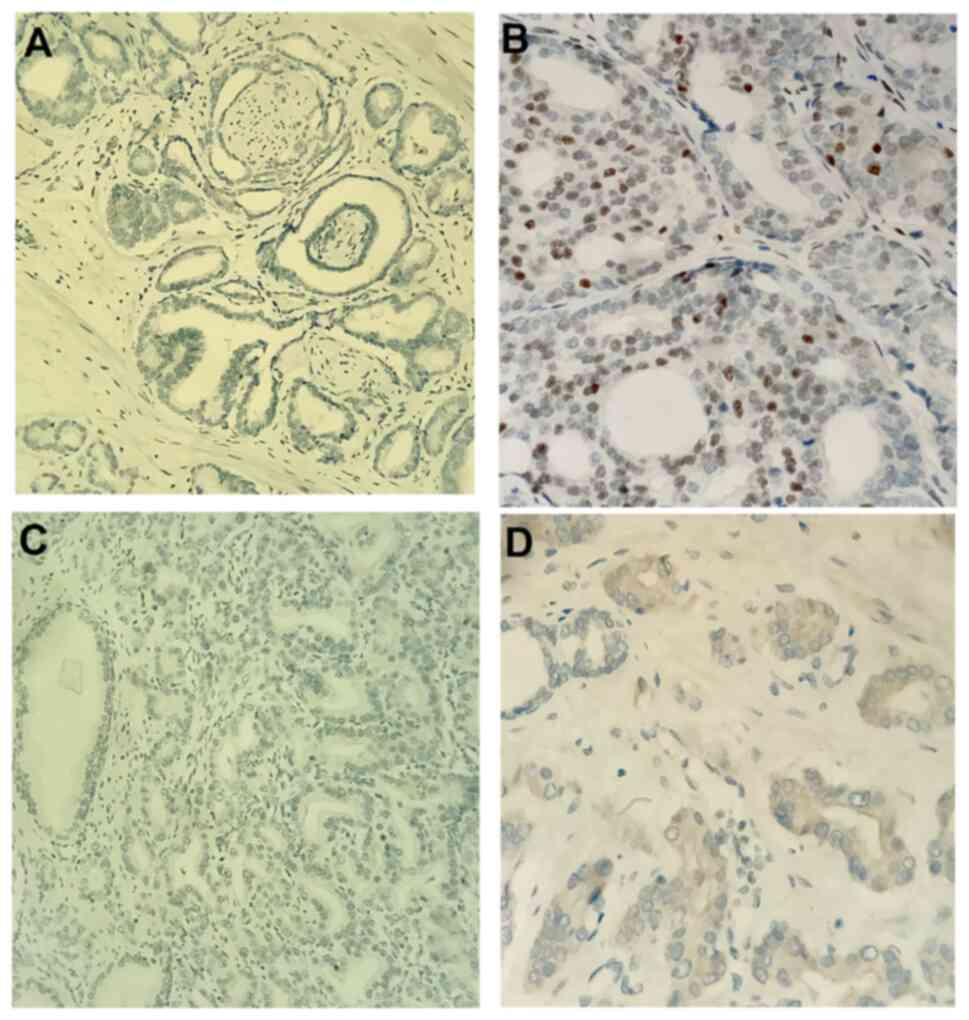
staining during evaluation. The ERα antibody was evaluated with
regard to the nuclear staining of tumor epithelial cells, and ERβ
antibody was evaluated with regard to the cytoplasmic staining of
tumor epithelial cells. The percentage and intensity of the tumor
epithelial cells staining with ERα and ERβ antibodies were
evaluated. The staining percentage was calculated by counting the
number of cells positively stained with antibodies in 100 cells at
×10 optical magnification. For IHC staining, ER expression levels
≥1% were considered positive (+). The strength of staining was
scored as follows: -, no staining, +, weak staining, ++, moderate
staining and +++, strong staining (Fig.
2).
Statistical analysis
The 1:2 matching by PSM was performed considering
the preoperative PSA level, International Society of Urological
Pathology (ISUP) grade and pathological T stage (pT) as matching
parameters. During PSM, ISUP grade groups were divided into three
categories: ISUP grade group 1 was the first group, ISUP grade
groups 2 and 3 were the second group and ISUP grade groups 4 and 5
were the third group. Categorical variables were analyzed using
χ2 and Fisher's exact tests. The Shapiro-Wilk test was
used to test whether the quantitative data were normally
distributed. The Mann-Whitney U test was used to compare the
non-normally distributed quantitative data by group. Quantitative
data are expressed as the median (range). Four groups were defined
according to estrogen staining status: ERα(+)/ERβ(+),
ERα(+)/ERβ(−), ERα(−)/ERβ(+) and ERα(−)/ERβ(−). Each group was
compared with the other three groups. Univariate and multivariate
logistic regression analyses were performed to determine the
independent risk factors for BCR. Multivariate logistic regression
analysis was performed with variables with P<0.25 in the
univariate analysis. Survival analysis was performed using
Kaplan-Meier and the log-rank test. P<0.05 was considered to
indicate a statistically significant result. SPSS software (IBM
SPSS Statistics for Windows, version 25.0; IBM Corp) was used for
the analyses.
Results
Characteristics of the cohort
The clinicopathological characteristics of the
entire study population are presented in Table I. The median age at diagnosis was
62.1 years in the BCR group and 63.9 years in the control group.
ISUP Grade 2/3 patients comprised more than three-quarters of each
group. Patients with pT3 cancer predominated in the two groups, and
most patients had perineural invasion. The median follow-up time
was 74.3 months (range, 30–127.5 months) in the BCR group and 66.6
months (range, 31.5-130 months) in the control group. No
significant difference was found in patient characteristics between
the two groups (P>0.05).
 | Table I.Clinicopathological characteristics of
all patients. |
Table I.
Clinicopathological characteristics of
all patients.
| Characteristics | BCR group (n=36) | Control group
(n=72) | P-value |
|---|
| Age, median
(range) | 62.1 (53.5-73.5) | 63.9 (49.6-77.5) | 0.391 |
| BMI, median (range),
kg/m2 | 26.9 (22.1-35.6) | 26.5 (20.2-40.1) | 0.632 |
| Preop PSA level,
median (range), ng/ml | 8.2 (3.1-40.0) | 9.2 (4.1-23.0) | 0.736 |
| ISUP grade, n
(%) |
|
| 0.863a |
| 1 | 5 (13.9) | 11 (15.3) |
|
| 2,3 | 28 (77.8) | 57 (79.1) |
|
| 4,5 | 3 (8.3) | 4 (5.6) |
|
| pT, n (%) |
|
| 1.000b |
| pT2 | 6 (16.7) | 13 (18.1) |
|
| pT3 | 30 (83.3) | 59 (81.9) |
|
| PNI, n (%) |
|
| 0.378a |
|
Negative | 3 (8.3) | 11 (15.3) |
|
|
Positive | 33 (91.7) | 61 (84.7) |
|
| LVI, n (%) |
|
| 0.257a |
|
Negative | 34 (94.4) | 71 (98.6) |
|
|
Positive | 2 (5.6) | 1 (1.4) |
|
| Follow-up time,
median (range) | 74.3
(30–127.5) | 66.6
(31.5-130) | 0.160 |
IHC staining
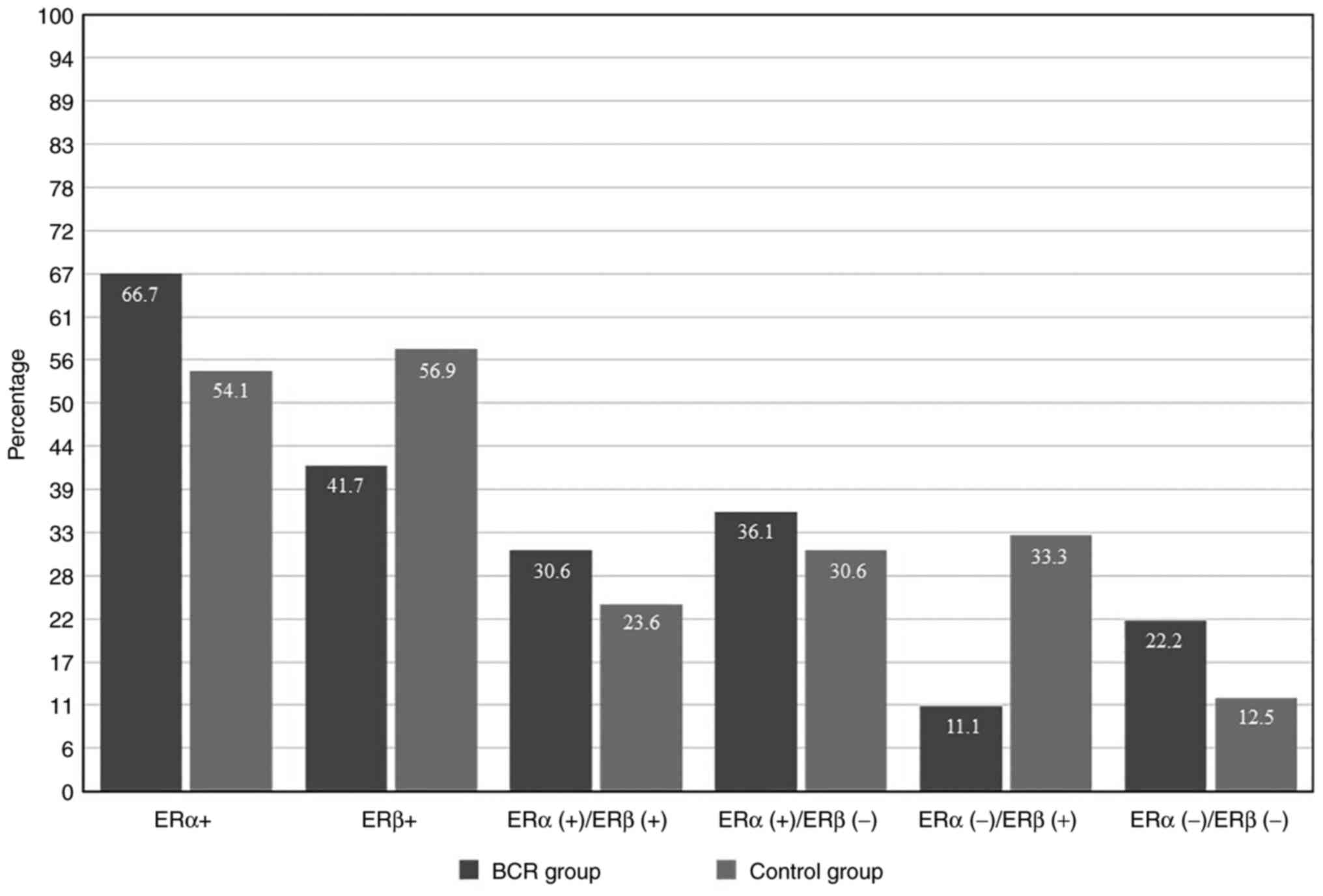
ERα staining was positive in 24 patients (66.7%) in
the BCR group and 39 patients (54.1%) in the control group. ERβ
staining was positive in 15 patients (41.7%) in the BCR group and
41 patients (56.9%) in the control group. Fig. 3 displays the percentages of patients
with positive staining of the ER receptors. No difference was
detected between the two groups in ERα staining status, ERα
strength of staining, ERα staining percentage, ERβ staining status,
ERβ strength of staining and ERβ staining percentage (P>0.05).
Only patients in the ERα(−)/ERβ(+) group had significantly fewer
BCRs among the four ERα/ERβ groups (P=0.024; Table II).
 | Table II.Immunohistochemical staining results
for ERα and ERβ. |
Table II.
Immunohistochemical staining results
for ERα and ERβ.
| Type of staining
result | BCR group
(n=36) | Control group
(n=72) |
P-valuea |
|---|
| ERa staining, n
(%) |
|
| 0.301 |
|
Positive | 24 (66.7) | 39 (54.1) |
|
|
Negative | 12 (33.3) | 33 (45.9) |
|
| ERa strength of
staining, n (%) |
|
| 0.098b |
|
Negative | 12 (33.3) | 33 (45.9) |
|
|
Weak | 20 (55.6) | 23 (31.8) |
|
|
Moderate | 4 (11.1) | 15 (20.9) |
|
|
Strong | 0 (0) | 1 (1.4) |
|
| ERa staining
percentage, median (range), | 1 (0–40) | 1 (0–30) | 0.107 |
| ERb staining, n
(%) |
|
| 0.196 |
|
Positive | 15 (41.7) | 41 (56.9) |
|
|
Negative | 21 (58.3) | 31 (43.1) |
|
| ERb strength of
staining, n (%) |
|
| 0.392b |
|
Negative | 21 (58.3) | 31 (43.1) |
|
|
Weak | 9 (25.0) | 26 (36.1) |
|
|
Moderate | 5 (13.9) | 14 (19.4) |
|
|
Strong | 1 (2.8) | 1 (1.4) |
|
| ERb staining
percentage, median (range), | 0 (0–20) | 1 (0–60) | 0.085 |
| ERa/ERb staining
groups, n (%) |
|
|
|
|
ERα(+)/ERβ(+) | 11 (30.6) | 17 (23.6) | 0.587 |
|
Non-ERα(+)/ERβ(+) | 25 (69.4) | 55 (76.4) |
|
|
ERα(+)/ERβ(−) | 13 (36.1) | 22 (30.6) | 0.716 |
|
Non-ERα(+)/ERβ(−) | 23 (63.9) | 50 (69.4) |
|
|
ERα(−)/ERβ(+) | 4 (11.1) | 24 (33.3) | 0.024 |
|
Non-ERα(−)/ERβ(+) | 32 (88.9) | 48 (66.7) |
|
|
ERα(−)/ERβ(−) | 8 (22.2) | 9 (12.5) | 0.304 |
|
Non-ERα(−)/ERβ(−) | 28 (77.8) | 63 (87.5) |
|
Outcomes
In the univariate logistic regression analysis to
determine the risk factors for BCR, the P-values for ERα(+), ERβ(−)
and the ERα(−)/ERβ(+) group were 0.216, 0.136 and 0.018,
respectively (Table III).
Multivariate logistic regression analysis revealed that only
ERα(−)/ERβ(+) staining was an independent risk factor for BCR
(P=0.048). The risk of BCR was 5.8-fold lower in this group
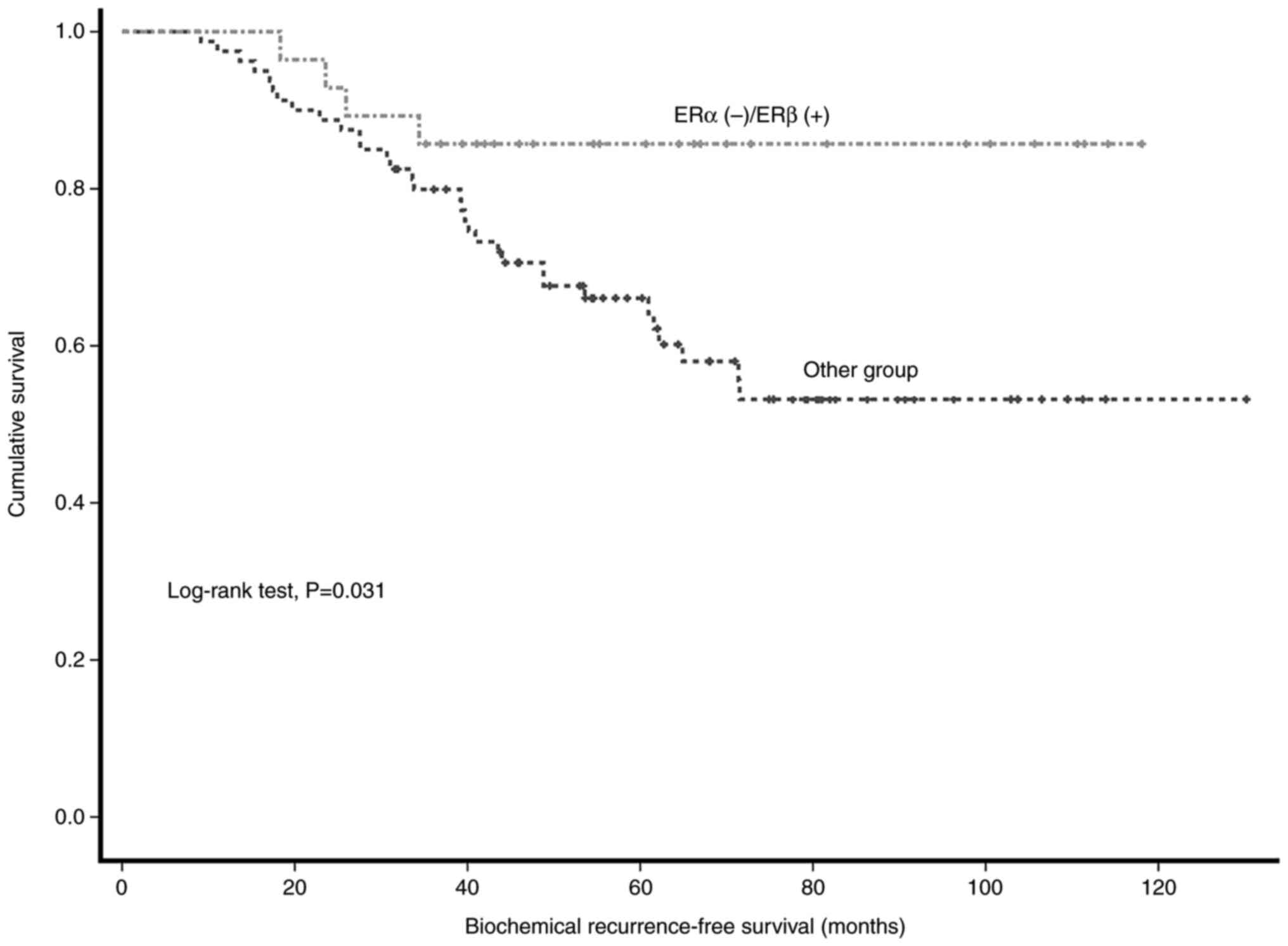
(OR=5.840). The log-rank test revealed that the 5-year BCR-free
survival (BFS) rate was significantly higher in the ERα(−)/ERβ(+)
staining group than in other patients (85.7 vs. 66.1%; P=0.031;
Fig. 4).
 | Table III.Logistic regression analysis for
predictors of biochemical recurrence. |
Table III.
Logistic regression analysis for
predictors of biochemical recurrence.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | OR | P-value | 95% CI lower | 95% CI higher | OR | P-value | 95% CI lower | 95% CI higher |
|---|
| Preop PSA level,
ng/ml | 1.026 | 0.511 | 0.956 | 1.109 | - | - | - | - |
| Age, years | 0.972 | 0.401 | 0.908 | 1.039 | - | - | - | - |
| BMI,
kg/m2 | 0.994 | 0.920 | 0.890 | 1.111 | - | - | - | - |
| ISUP grade |
|
|
|
|
|
|
|
|
| 1 | - | 0.853 | - | - | - | - | - | - |
|
2,3 | 1.081 | 0.895 | 0.342 | 3.412 | - | - | - | - |
|
4,5 | 1.650 | 0.592 | 0.264 | 10.313 | - | - | - | - |
| pT, T2 (R) vs.
pT3 | 1.102 | 0.858 | 0.381 | 3.188 | - | - | - | - |
| PNI, negative (R)
vs. positive | 1.984 | 0.318 | 0.517 | 7.614 | - | - | - | - |
| LVI, negative (R)
vs. positive | 2.059 | 0.480 | 0.278 | 15.248 | - | - | - | - |
| ERα, negative (R)
vs. positive | 1.692 | 0.216 | 0.735 | 3.895 | 0.665 | 0.495 | 0.206 | 2.149 |
| ERβ, positive (R)
vs. negative | 1.852 | 0.136 | 0.823 | 4.164 | 0.913 | 0.862 | 0.329 | 2.539 |
| ERα(−)/ER(+)β (R)
vs. other groups | 4.0 | 0.018 | 1.268 | 12.622 | 5.840 | 0.048 | 1.012 | 33.706 |
| ERα(+)/ERβ (−) (R)
vs. other groups | 1.285 | 0.516 | 0.552 | 2.990 | - | - | - | - |
Discussion
The role of estrogen receptors in PCa has been
studied extensively, but remains a matter of debate (19,20).
Considering that PCa tumor cells may use pathways other than those
associated with androgen receptors as resistance mechanisms in
advanced disease (21), it may be
reasonable to evaluate the role of estrogen receptors in
early-stage patients. A limited number of studies have investigated
the role of estrogen receptors in PCa in non-metastatic
castration-naive disease, and their results are conflicting
(15–17). The present study evaluated ERα and
ERβ receptors using PSM analysis, in order to reduce the effect of
well-known risk factors on BCR, and the results revealed that
patients with ERα(−)/ERβ(+) PCa had a 5.8-fold lower risk of BCR
than other patients and the 5-year BFS rate was significantly
higher in patients with ERα(−)/ERβ(+) staining than in other
patients (85.7 vs. 66.1%).
Megas et al (15) reported that ERα upregulation
increased the risk of BCR 4.04-fold and low ERβ expression
increased the risk of disease progression 6.59-fold. The authors
also found that patients with concurrent ERα negative and ERβ
positive staining had longer BFS than other patients, which is
consistent with the present study. However, the study group in the
previous study was heterogeneous, since 29% of patients received
adjuvant ADT + EBRT and 33% received adjuvant ADT. Moreover,
although patients with non-metastatic locally advanced PCa were
included, the distribution of parameters such as preoperative PSA
level and ISUP grades was not explicitly presented. Since PSM was
performed in the present study, this is likely to have reduced the
effect of these parameters on BCR and revealed the impact of ERα
and ERβ more clearly. Although no significant differences regarding
ERα and ERβ staining, the strength of staining and staining
percentage were detected between the groups in the present study,
significant differences were observed when combinations of ERα and
ERβ results were considered; this may be due to the limited number
of patients.
Horvath et al (17) reported that patients with
ERβ-positive tumors had shorter survival times than those with
ERβ-negative tumors, but ERα staining was not evaluated. In
addition, the authors performed a multivariate analysis, which
showed that ERβ expression, pT and GS were independent predictors
of PCa prognosis while the preoperative PSA level and surgical
margin positivity were not. Grindstad et al (16) analyzed ERα and ERβ staining in the
normal stroma, tumoral stroma, normal epithelial cells and tumoral
epithelial cells in 535 patients undergoing radical prostatectomy
in a multicenter study. The authors reported that upregulation of
ERα in the tumor stroma increased clinical progression-free
survival (CPFS) and cancer-specific survival, and that the
upregulation of ERβ in the tumor stroma reduced BFS (16). Unlike the present study, ER receptor
staining was performed on the tumor stroma and epithelium, and no
analysis of ERα-ERβ groups was performed. In addition, patients who
had received adjuvant EBRT or ADT and had pelvic lymph node
involvement and positive surgical margins were included in the
study, although patients who received EBRT and ADT before surgery
were excluded (16). Furthermore,
patients in these two studies were not homogeneous in terms of
preoperative PSA level, ISUP grade, pT, pelvic lymph node
involvement and surgical margins. Pelvic lymph node involvement and
surgical margin positivity are the most important independent risk
factors for disease progression (22,23).
Several studies have demonstrated that adjuvant ADT plus EBRT and
adjuvant ADT monotherapy increase BFS, CPFS and overall survival
(OS) (24–27). The inclusion of patients with these
poor prognostic features and those who have received adjuvant
therapy may have confounded the effect of ERα and ERβ on oncologic
outcomes and could explain the differences in BFS, CPFS and OS. In
the current study, this effect has been minimized by the exclusion
of patients with positive surgical margins and lymph nodes after
LRP and those who received adjuvant therapy.
ERα is most commonly localized in the prostatic
stroma but is also found in the prostatic utricle and periurethral
epithelium of the male reproductive system (28). Risbridger et al (7) and Prins et al (8) showed that ERα plays a role in the
development of prostatic stromal hyperplasia, inflammatory cell
infiltration, squamous metaplasia and PIN in studies with ERα and
ERβ knockout mice, and Bonkhoff et al (9) indicated that ERα expression increases
in luminal cells during the malignant transformation from
high-grade PIN to PCa. ERβ is expressed in human prostate tissue,
specifically in stromal and epithelial cells (29). Cheng et al (12) induced ERβ-negative PCa cells to
express ERβ using an ERβ-encoding adenoviral vector and showed that
ERβ inhibited the growth and invasion of the cells and increased
their apoptosis. It has been suggested that the anti-proliferative
effect of ERβ is achieved via the prevention of androgenic
stimulation (30). Based on the
findings of these studies, it can be concluded that ERα plays a
role in the pathophysiological processes of chronic prostatitis,
BPH and cancer development, and ERβ has anti-proliferative,
anti-invasive, anti-inflammatory and pro-apoptotic effects.
The application of adjuvant treatment modalities or
early salvage strategies has been a subject of debate in patients
with a high risk of recurrence, such as those with a positive
surgical margin and ≥pT3 cancer after radical prostatectomy
(31). In this context, the use of
estrogen receptor status in combination with other well-studied
clinicopathological prognostic markers may help clinicians to
select patients with a poor prognosis for adjuvant therapy.
The strengths of the present study are the pertinent
exclusion-inclusion criteria that were applied and the homogeneous
control group determined by the PSM method considering well-known
risk factors for BCR. The retrospective design of the study, the
lack of CPFS and radiological recurrence-free survival data and the
limited number of patients due to the strict inclusion-exclusion
criteria are limitations of the study. In addition, an OS analysis
could not be performed because there were insufficient
PCa-associated deaths.
In conclusion, estrogen receptors may have
prognostic value for non-metastatic PCa. Moreover, the 5-year BFS
rate of patients with ERα(−)/ERβ(+) stained PCa is significantly
improved compared with that of other patients.
Acknowledgements
The authors would like to thank Ms. Züleyha
Sarıkaya, Ms. Nihan Genç, Ms. Fatma Aydın Yazıcı, Ms. Zeliha Altun,
Ms. Elif Bayazit, Ms. Pelin Bilir and Ms. Şerife Kirez (Department
of Pathology, Bursa Uludag University) for their technical support
in this study.
Funding
The Institution for Scientific Research Projects of Bursa Uludag
University financially supported this study (project no.
TTU-2021-420).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YMA was responsible for investigation, data
curation, writing the original draft of the manuscript and
visualization (designing tables and figures). ABS was responsible
for conceptualization, methodology and for writing, reviewing and
editing the manuscript. BAV and RD performed the histopathological
examination and revised the manuscript critically for important
intellectual content. GO was responsible for formal analysis and
methodology. HV and IY provided substantial contributions to the
design of the study, the interpretation of data, drafting the work
and providing final approval of the version to be published. BC was
responsible for substantial contributions to the design of the
study, reviewing and editing the manuscript, as well as drafting
the work and final approval of the version to be published. All
authors read and approved the final version of the manuscript. YMA
and BC confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The Institutional Review Board of Bursa Uludag
University approved the study (approval number: 2021-2/15). The
study protocol complied with the tenets of the Declaration of
Helsinki. The Clinical Research Ethics Committee of The Bursa
Uludag University Faculty of Medicine waived informed consent due
to the retrospective nature of the study.
Patient consent for publication
Not applicable.
Authors' information
The ORCID iD of Dr Yavuz Mert Aydın is
0000-0002-6287-6767. The ORCID iD of Ahmet Bilgehan Sahin is
0000-0002-7846-0870. The ORCID iD of Rabia Dolek is
0000-0002-1751-7693. The ORCID iD of Berna Aytac Vuruskan is
0000-0001-9549-8435. The ORCID iD of Gokhan Ocakoglu is
0000-0002-1114-6051. The ORCID iD of Hakan Vuruskan is
0000-0002-3917-4847. The ORCID iD of İsmet Yavascaoglu is
0000-0002-1788-1997. The ORCID iD of Burhan Coskun is
0000-0001-7206-6648.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of ıncidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bostwick DG, Burke HB, Djakiew D, Euling
S, Ho SM, Landolph J, Morrison H, Sonawane B, Shifflett T, Waters
DJ and Timms B: Human prostate cancer risk factors. Cancer. 101 (10
Suppl):S2371–S2490. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dobbs RW, Malhotra NR, Greenwald DT, Wang
AY, Prins GS and Abern MR: Estrogens and prostate cancer. Prostate
Cancer Prostatic Dis. 22:185–194. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Setlur SR, Mertz KD, Hoshida Y, Demichelis
F, Lupien M, Perner S, Sboner A, Pawitan Y, Andrén O, Johnson LA,
et al: Estrogen-dependent signaling in a molecularly distinct
subclass of aggressive prostate cancer. J Natl Cancer Inst.
100:815–825. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McPherson SJ, Hussain S, Balanathan P,
Hedwards SL, Niranjan B, Grant M, Chandrasiri UP, Toivanen R, Wang
Y, Taylor RA and Risbridger GP: Estrogen receptor-beta activated
apoptosis in benign hyperplasia and cancer of the prostate is
androgen independent and TNFalpha mediated. Proc Natl Acad Sci USA.
107:3123–3128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mak P, Leav I, Pursell B, Bae D, Yang X,
Taglienti CA, Gouvin LM, Sharma VM and Mercurio AM: ERβ ımpedes
prostate cancer EMT by destabilizing HIF-1α and inhibiting
VEGF-mediated snail nuclear localization: Implications for gleason
grading. Cancer Cell. 17:319–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Risbridger G, Wang H, Young P, Kurita T,
Wang YZ, Lubahn D, Gustafsson JA and Cunha G: Evidence that
epithelial and mesenchymal estrogen receptor-α mediates effects of
estrogen on prostatic epithelium. Dev Biol. 229:432–442. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prins GS, Birch L, Couse JF, Choi I,
Katzenellenbogen B and Korach KS: Estrogen imprinting of the
developing prostate gland is mediated through stromal estrogen
receptor alpha: Studies with alphaERKO and betaERKO mice. Cancer
Res. 61:6089–6097. 2001.PubMed/NCBI
|
|
9
|
Bonkhoff H, Fixemer T, Hunsicker I and
Remberger K: Estrogen receptor expression in prostate cancer and
premalignant prostatic lesions. Am J Pathol. 155:641–647. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu X, Leav I, Leung YK, Wu M, Liu Q, Gao
Y, McNeal JE and Ho SM: Dynamic regulation of estrogen
receptor-beta expression by DNA methylation during prostate cancer
development and metastasis. Am J Pathol. 164:2003–2012. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang X, Leung YK and Ho SM: AP-2
regulates the transcription of estrogen receptor (ER)-beta by
acting through a methylation hotspot of the 0N promoter in prostate
cancer cells. Oncogene. 26:7346–7354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng J, Lee EJ, Madison LD and Lazennec
G: Expression of estrogen receptor beta in prostate carcinoma cells
inhibits invasion and proliferation and triggers apoptosis. FEBS
Lett. 566:169–172. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weihua Z, Warner M and Gustafsson JA:
Estrogen receptor beta in the prostate. Mol Cell Endocrinol.
193:1–5. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McPherson SJ, Ellem SJ, Simpson ER,
Patchev V, Fritzemeier KH and Risbridger GP: Essential role for
estrogen receptor beta in stromal-epithelial regulation of
prostatic hyperplasia. Endocrinology. 148:566–574. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Megas G, Chrisofos M, Anastasiou I,
Tsitlidou A, Choreftaki T and Deliveliotis C: Estrogen receptor (α
and β) but not androgen receptor expression is correlated with
recurrence, progression and survival in post prostatectomy T3N0M0
locally advanced prostate cancer in an urban Greek population.
Asian J Androl. 17:98–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grindstad T, Skjefstad K, Andersen S, Ness
N, Nordby Y, Al-Saad S, Fismen S, Donnem T, Khanehkenari MR, Busund
LT, et al: Estrogen receptors α and β and aromatase as independent
predictors for prostate cancer outcome. Sci Rep. 6:331142016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Horvath LG, Henshall SM, Lee CS, Head DR,
Quinn DI, Makela S, Delprado W, Golovsky D, Brenner PC, O'Neill G,
et al: Frequent loss of estrogen receptor-β expression in prostate
cancer. Cancer Res. 61:5331–5335. 2001.PubMed/NCBI
|
|
18
|
Nelson JB and Lepor H: Prostate cancer:
Radical prostatectomy. Urol Clin North Am. 30:703–723. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bonkhoff H: Estrogen receptor signaling in
prostate cancer: Implications for carcinogenesis and tumor
progression. Prostate. 78:2–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qu LG, Wardan H, Davis ID, Pezaro C and
Sluka P: Effects of estrogen receptor signaling on prostate cancer
carcinogenesis. Transl Res. 222:56–66. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He Y, Xu W, Xiao YT, Huang H, Gu D and Ren
S: Targeting signaling pathways in prostate cancer: Mechanisms and
clinical trials. Signal Transduct Target Ther. 7:1982022.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mottet N, Vice-Chair PC, Bergh RCN, Van
Den, Mottet N, et al: Guidelines on Prostate Cancer. Update.
53:31–45. 2021.
|
|
23
|
Briganti A, Karnes JR, Da Pozzo LF,
Cozzarini C, Gallina A, Suardi N, Bianchi M, Freschi M, Doglioni C,
Fazio F, et al: Two positive nodes represent a significant Cut-off
value for cancer specific survival in patients with node positive
prostate cancer. A new proposal based on a Two-ınstitution
experience on 703 consecutive N+ patients treated with radical
prostatectomy, extended pelvic lymph node dissection and adjuvant
therapy. Eur Urol. 55:261–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thompson IM, Tangen CM, Paradelo J, Lucia
MS, Miller G, Troyer D, Messing E, Forman J, Chin J, Swanson G, et
al: Adjuvant radiotherapy for pathological T3N0M0 prostate cancer
significantly reduces risk of metastases and ımproves survival:
Long-term followup of a randomized clinical trial. J Urol.
181:956–962. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bolla M, Van Poppel H, Tombal B, Vekemans
K, Da Pozzo L, de Reijke TM, Verbaeys A, Bosset JF, van Velthoven
R, Colombel M, et al: Postoperative radiotherapy after radical
prostatectomy for high-risk prostate cancer: Long-term results of a
randomised controlled trial (EORTC trial 22911). Lancet.
380:2018–2027. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wiegel T, Bartkowiak D, Bottke D, Bronner
C, Steiner U, Siegmann A, Golz R, Störkel S, Willich N, Semjonow A,
et al: Adjuvant radiotherapy versus wait-and-see after radical
prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95
trial. Eur Urol. 66:243–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hackman G, Taari K, Tammela TL, Matikainen
M, Kouri M, Joensuu T, Luukkaala T, Salonen A, Isotalo T, Pétas A,
et al: Randomised trial of adjuvant radiotherapy following radical
prostatectomy versus radical prostatectomy alone in prostate cancer
patients with positive margins or extracapsular extension. Eur
Urol. 76:586–595. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shapiro E, Huang H, Masch RJ, McFadden DE,
Wilson EL and Wu XR: Immunolocalization of estrogen receptor α and
β in human fetal prostate. J Urol. 174:2051–2053. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prins GS and Korach KS: The role of
estrogens and estrogen receptors in normal prostate growth and
disease. Steroids. 73:233–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weihua Z, Makela S, Andersson LC, Salmi S,
Saji S, Marketon JW, Jensen EV, Nilsson S, Warner M and Gustafsson
JA: A role for estrogen receptor? in the regulation of the ventral
prostate. Proce National Acad Sci. 98:6330–6335. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Terlizzi M, Limkin EJ, Moukasse Y and
Blanchard P: Adjuvant or salvage radiation therapy for prostate
cancer after prostatectomy: Current status, controversies and
perspectives. Cancers (Basel). 14:16882022. View Article : Google Scholar : PubMed/NCBI
|