Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common type of cancer and the third leading cause of cancer-related
death worldwide (1,2). Although significant progress in the
diagnosis and treatment of HCC has been achieved in recent decades,
the survival of patients with HCC after resection remains poor.
Tumor relapse and metastasis are the major complications of
hepatectomy, occurring in >70% of patients within 5 years of
follow-up (3). Therefore,
non-invasive preoperative tumor biomarkers that can better predict
HCC relapse and metastasis are urgently required. Consequently,
early detection of patients with a high risk of recurrence or
mortality and timely intervention may improve the postoperative
survival of patients with HCC.
Alkaline phosphatase (ALP) is a hydrolase enzyme
widely distributed in the human tissues of the liver, bile duct,
intestine, bone, and kidney, but most ALP in serum is primarily
from the liver (4). A previous
study has demonstrated that ALP is an independent prognostic risk
factor for patients with HCC (5).
ALP is an enzyme typically used to evaluate liver damage (6). Albumin (ALB) is a key component of
serum proteins and is closely related to long-term malnutrition and
systemic immune responses (7).
Moreover, tumor-mediated malnutrition and systemic inflammatory
responses can affect the long-term postoperative survival of
patients with HCC (8). To date, the
significance of the preoperative ALP-to-ALB ratio (APAR) in
predicting tumor relapse and survival of postoperative patients
with HCC has yet to be established, although the preoperative
ALP-to-platelet ratio index (APPRI) (9), aspartate aminotransferase
(AST)-to-lymphocyte ratio (10),
and ALB-to-globulin ratio (AGR) (11) have been demonstrated to be
independent risk factors for poor survival. The APPRI,
AST-to-lymphocyte ratio, and AGR indicators are single feedback
indicators for either liver function impairment or nutritional
status, but none of these are useful markers for both. As an
indicator of liver function, serum ALP levels are frequently used
as a biomarker to determine the progression of liver diseases
(6) and are significantly
associated with poor survival in patients with HCC (12). Compared with APPRI, the
AST-to-lymphocyte ratio, AGR, and APAR can reflect the systemic
inflammatory response, immunity level, and nutritional status of
patients under the influence of tumors. Therefore, this novel
indicator is significantly important for the prognosis of patients
with HCC after surgery.
This study aimed to investigate the correlation
between preoperative APAR and the clinicopathological features of
HCC and to evaluate the prognostic value of APAR after curative
resection in patients with HCC.
Materials and methods
Study population
The clinical and pathological data of 370 patients
with HCC treated with radical hepatectomy between November 2010 and
January 2014 were retrospectively analyzed. The criteria for
admission were as follows: i) radical hepatectomy, ii)
pathologically proven HCC after surgery, and iii) absence of
anti-tumor treatment before surgery. Patients were excluded if they
i) died during the perioperative period, ii) were diagnosed with
intrahepatic cholangiocarcinoma or non-primary liver cancer, iii)
were positive for human immunodeficiency virus, iv) did not have
complete clinical and pathological data, or v) had severe
infections or immune system diseases or used hematology-related
drugs within 1 month before enrollment in this study. Among the 370
patients, 3 died during the perioperative period, 8 had
intrahepatic cholangiocarcinoma, 13 did not have primary liver
cancer, and 16 did not have complete clinical data. Thus, 330
patients (271 men and 59 women) were enrolled in this study. Their
ages ranged from 19 to 79 years, and the median age was 52 years.
Written informed consent was obtained from all enrolled patients.
The clinicopathological characteristics of these patients,
including age, sex, clinical symptoms, hepatitis B surface antigen
(HBsAg) level, serum α-fetoprotein (AFP) level, tumor diameter,
tumor number, liver cirrhosis, macrovascular invasion or tumor
thrombus, family history of cancer, and tumor-node-metastasis (TNM)
stage, are displayed in Table
I.
 | Table I.Clinicopathological data of the 330
patients with hepatocellular carcinoma. |
Table I.
Clinicopathological data of the 330
patients with hepatocellular carcinoma.
| Parameters | Mean ± SD |
|---|
| Age, years | 50.95±0.83 |
| α-fetoprotein,
ng/ml |
3,221.76±942.84 |
| Tumor size, cm | 4.52±0.15 |
| Hemoglobin,
g/l | 141.82±0.90 |
| White blood cell,
×109/l | 6.29±0.70 |
| Platelets,
×109/l | 160.09±3.26 |
| Alkaline
phosphatase, U/l | 85.14±1.89 |
| Aspartate
aminotransferase, U/l | 44.43±6.01 |
| Alanine
aminotransferase, U/l | 45.59±6.04 |
| Albumin, g/l | 42.50±1.17 |
| Total bilirubin,
µmol/l | 12.49±0.80 |
| Direct bilirubin,
µmol/l | 4.97±0.62 |
| Alkaline
phosphatase-to-albumin ratio | 2.11±0.06 |
Receiver operating characteristic
(ROC) curve
ROC curve analysis was performed to determine the
best cut-off value of APAR to predict the prognosis of patients
with HCC after the surgery (ALP unit, U/l albumin unit, g/l). The
optimal cut-off value was determined as the value closest to the
point with maximum specificity and sensitivity according to the
correct index. The correct index, also known as the Youden's index,
is the sum of the sensitivity and specificity minus 1. The best
critical point of the cut-off value was determined using the
following equation: Youden's index=sensitivity +
specificity-1=sensitivity-(1-specificity). The best cut-off value
was determined based on the maximum value of the Youden's
index.
Follow-up
All patients were regularly followed up every 3
months during the first 3 postoperative years and every 6 months
thereafter. Patients were followed up through outpatient reviews,
phone calls, or house visits. The follow-up primarily consisted of
liver function tests (ALT, ALP, γ-glutamyltransferase, total
bilirubin, direct bilirubin, total protein, ALB, globulin, AGR,
triglyceride, cholesterol, high-density lipoprotein cholesterol,
low-density lipoprotein cholesterol, and glucose levels), tumor
marker tests [AFP, carcinoembryonic antigen (CEA), and CA19-9],
chest radiography, and abdominal ultrasound (US). Computed
tomography (CT) or magnetic resonance imaging (MRI) was performed
when clinical recurrence was suspected. Clinical relapse was
confirmed if i) the AFP level increased again (AFP level ≥400
µg/l), ii) new lesions were detected by US, CT, or MRI, and iii)
the patient was proven to have HCC after reoperation. Disease-free
survival (DFS) was defined as the interval between the date of
surgery and recurrence, metastasis, or death, whereas overall
survival (OS) was defined as the interval between the date of
surgery and death. Any missing data were treated as censored data
for survival analysis.
Statistical analysis
Statistical analysis was performed using SPSS
version 21.0 (IBM Corp.). A χ2 test was used to compare
the categorical variables. ROC curve analysis was performed to
determine the best APAR cut-off value. Univariate analysis was
performed to determine the significance of parameters found to be
significant in the log-rank test for survival. The Cox proportional
hazards model was used to perform multivariate survival analyses.
Survival curves for patients with HCC were plotted using the
Kaplan-Meier method. P<0.05 was considered to indicate a
statistically significant difference.
Results
Biochemical and clinicopathological
characteristics of the enrolled patients
Hemoglobin, AFP, ALT, ALP, ALB, globulin, total
bilirubin, direct bilirubin, preoperative APAR values, and white
blood cell and platelet counts are presented in Table I. The preoperative APAR value in
this study was calculated using the following formula: (ALP
value/ALB value) × g/U.
Optimal cut-off value of APAR for
survival analysis
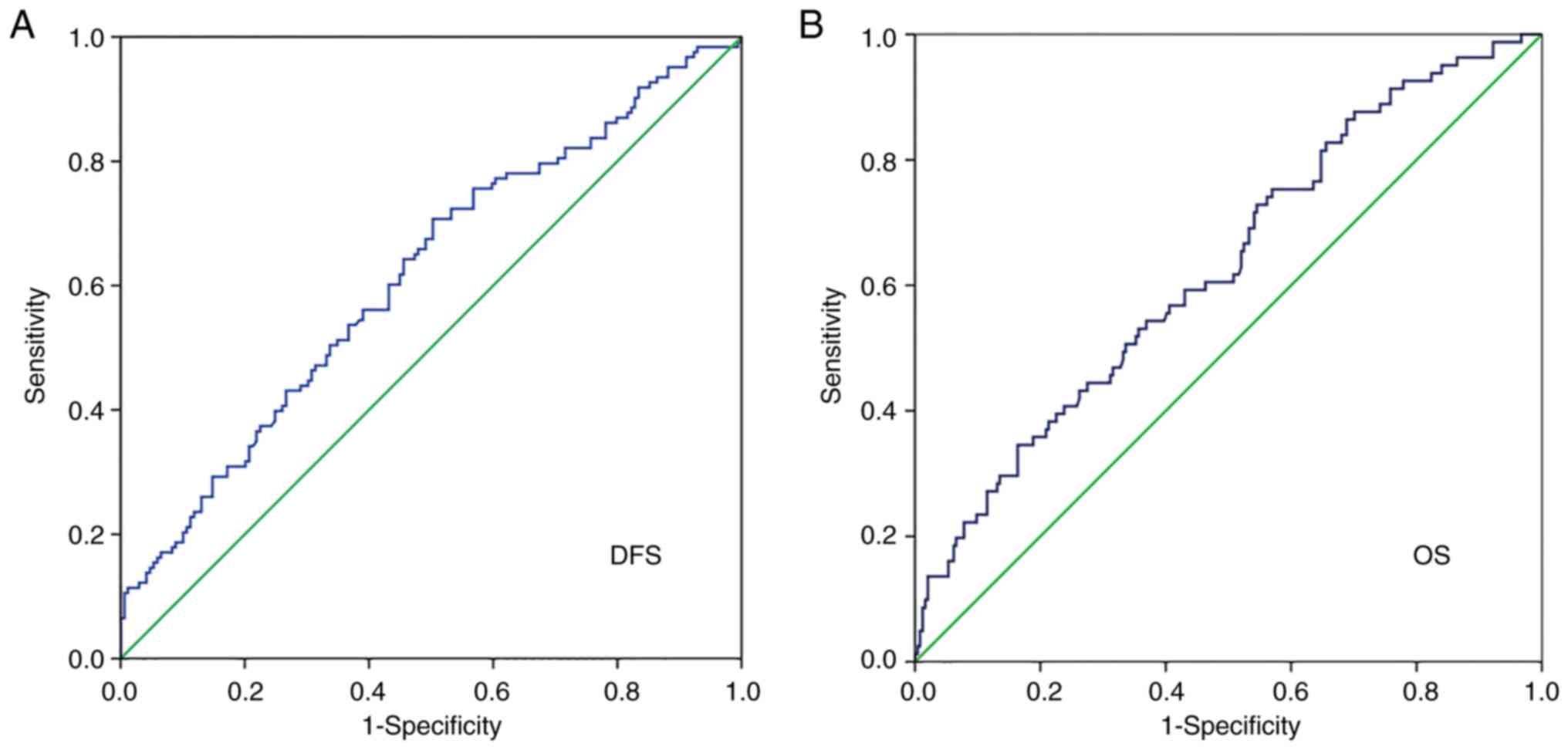
ROC curve analysis showed that the optimal cut-off
value of APAR for DFS and OS was 1.74. It was considered the
uniform point for survival analysis (Fig. 1A and B). The area under the curve
(AUC) of APAR was 0.616 [95% confidence interval (CI):
0.550-0.681]. The optimal cut-off value of 1.74 presented a
sensitivity of 0.707 and a specificity of 0.503.
Correlation between preoperative APAR
and the clinicopathological characteristics of patients with
HCC
The correlation between preoperative APAR and the
clinicopathological factors of patients with HCC is shown in
Table II. Based on the cut-off
value of APAR, all patients were divided into a high APAR group
(≥1.74, n=195) and a low APAR group (<1.74, n=135). The results
demonstrated that a high preoperative APAR value was closely
associated with positive HBsAg levels (P=0.041), tumor diameter (≥5
cm, P<0.001), and TNM stage (III/IV, P<0.044). No significant
association was noted between APAR and age, sex, clinical symptoms,
AFP level, tumor number, macrovascular invasion or tumor thrombus,
or a family history of cancer (P>0.05).
 | Table II.Correlation between
clinicopathological parameters and preoperative APAR. |
Table II.
Correlation between
clinicopathological parameters and preoperative APAR.
|
|
|
| APAR |
|---|
|
|
|
|
|
|---|
| Clinical
factor | Variable | No. of patients
(n=330) | <1.74
(n=135) | ≥1.74 (n=195) | P-value |
|---|
| Age, years | <50 | 101 | 47 | 54 | 0.167 |
|
| ≥50 | 229 | 88 | 141 |
|
| Sex | Female | 59 | 23 | 36 | 0.740 |
|
| Male | 271 | 112 | 159 |
|
| Clinical
symptoms | No | 267 | 113 | 154 | 0.282 |
|
| Yes | 63 | 22 | 41 |
|
| Hepatitis B surface
antigen | Negative | 96 | 31 | 65 | 0.041a |
|
| Positive | 234 | 104 | 130 |
|
| α-fetoprotein,
ng/ml | <20 | 194 | 81 | 113 | 0.710 |
|
| ≥20 | 136 | 54 | 82 |
|
| Tumor diameter,
cm | <5 | 217 | 107 | 110 |
<0.001b |
|
| ≥5 | 113 | 28 | 85 |
|
| Tumor number | Single | 299 | 125 | 174 | 0.303 |
|
| Multiple | 31 | 10 | 21 |
|
| Macrovascular
invasion or tumor thrombus | No | 280 | 116 | 164 | 0.65 |
|
| Yes | 50 | 19 | 31 |
|
| Liver
cirrhosis | No | 127 | 56 | 71 | 0.352 |
|
| Yes | 203 | 79 | 124 |
|
| Indocyanine green
retention rate at 15 min | <10% | 172 | 76 | 96 | 0.219 |
|
| ≥10% | 158 | 59 | 99 |
|
| Family history of
cancer | No | 254 | 104 | 150 | 0.981 |
|
| Yes | 76 | 31 | 45 |
|
| TNM stage | I/II | 320 | 134 | 186 | 0.044a |
|
| III/IV | 10 | 1 | 9 |
|
Univariate analysis of prognostic
factors in patients with HCC
Univariate analysis showed that a preoperative APAR
value ≥1.74 (P=0.005) and macrovascular invasion or tumor thrombus
(P=0.001) were associated with the median DFS of the patients. The
significant predictors of OS were APAR ≥1.74 (P=0.008), clinical
symptoms (P=0.002), AFP level ≥20 ng/ml (P=0.001), macrovascular
invasion or tumors thrombus (P<0.001), and a family history of
cancer (P=0.015) (Table III).
 | Table III.Univariate analysis of the
clinicopathological characteristics influencing prognosis. |
Table III.
Univariate analysis of the
clinicopathological characteristics influencing prognosis.
|
|
|
| Disease-free
survival, months | Overall survival,
months |
|---|
| Clinical
factor | Variable | No. of patients
(n=330) |
|
|
|---|
| Mean | 95% CI | P-value | Mean | 95% CI | P-value |
|---|
| Age, years | <50 | 101 | 49.48 | 44.08-54.88 | 0.566 | 59.98 | 56.24-63.71 | 0.431 |
|
| ≥50 | 229 | 46.99 | 43.31-50.67 |
| 57.22 | 54.54-59.91 |
|
| Sex | Female | 59 | 48.56 | 41.28-55.83 | 0.814 | 58.64 | 53.48-63.80 | 0.879 |
|
| Male | 271 | 48.07 | 44.67-51.47 |
| 58.62 | 56.17-61.08 |
|
| Clinical
symptoms | No | 267 | 49.23 | 45.89-52.58 | 0.116 | 60.18 | 57.82-62.54 | 0.002b |
|
| Yes | 63 | 43.29 | 35.58-50.99 |
| 52.48 | 46.86-58.10 |
|
| Hepatitis B surface
antigen | Negative | 96 | 46.99 | 41.27-52.71 | 0.78 | 57.14 | 52.82-61.46 | 0.843 |
|
| Positive | 234 | 48.42 | 44.79-52.05 |
| 58.90 | 56.34-61.45 |
|
| α-fetoprotein,
ng/ml | <20 | 194 | 49.75 | 45.96-53.55 | 0.282 | 61.78 | 59.28-64.28 | 0.001c |
|
| ≥20 | 136 | 45.72 | 40.54-50.90 |
| 54.34 | 50.47-58.21 |
|
| Tumor diameter,
cm | <5 | 217 | 49.90 | 46.17-53.62 | 0.135 | 59.69 | 57.10-62.27 | 0.238 |
|
| ≥5 | 113 | 44.45 | 39.15-49.74 |
| 55.77 | 51.71-59.84 |
|
| Tumor number | Single | 299 | 48.27 | 45.05-51.49 | 0.776 | 58.89 | 56.56-61.23 | 0.265 |
|
| Multiple | 31 | 46.13 | 35.97-56.30 |
| 54.40 | 47.77-61.03 |
|
| Macrovascular
invasion or tumor thrombus | No | 280 | 50.10 | 46.88-53.32 | 0.001c | 60.44 | 58.24-62.63 |
<0.001c |
|
| Yes | 50 | 33.97 | 26.13-41.82 |
| 46.60 | 39.42-53.77 |
|
| Liver
cirrhosis | No | 127 | 49.17 | 44.33-54.01 | 0.333 | 57.84 | 54.03-61.66 | 0.482 |
|
| Yes | 203 | 47.18 | 43.25-51.11 |
| 58.60 | 55.95-61.26 |
|
| Family history of
cancer | No | 254 | 49.58 | 46.13-53.04 | 0.080 | 59.87 | 57.46-62.29 | 0.015a |
|
| Yes | 76 | 42.48 | 36.10-48.86 |
| 52.78 | 47.76-57.80 |
|
| TNM stage | I/II | 320 | 48.04 | 44.92-51.16 | 0.582 | 58.87 | 56.63-61.11 | 0.102 |
|
| III/IV | 10 | 52.71 | 35.21-70.22 |
| 48.29 | 34.67-61.91 |
|
| Alkaline
phosphatase-to-albumin | <1.74 | 135 | 53.53 | 49.13-57.93 | 0.004b | 62.07 | 59.04-65.11 | 0.008b |
| ratio | ≥1.74 | 195 | 44.38 | 40.24-48.52 |
| 56.23 | 53.17-59.29 |
|
Multivariate analysis of prognostic
factors in patients with HCC
The Cox proportional hazards regression model was
used to examine the independent risk factors for the survival of
postoperative patients with HCC. A stepwise multivariate Cox
proportional hazards model revealed that a preoperative APAR value
≥1.74 [hazard ratio (HR): 1.781; 95% CI: 1.192-2.661; P=0.013] and
macrovascular invasion or tumor thrombus (HR: 2.080; 95% CI:
1.284-3.368; P=0.003) were independent prognostic factors for DFS
in patients with HCC. A preoperative APAR value ≥1.74 (HR: 1.828;
95% CI: 1.063-3.141; P=0.029), clinical symptoms (HR: 1.747; 95%
CI: 1.045-2.918; P=0.046), AFP ≥20 ng/ml (HR: 1.739; 95% CI:
1.057-2.862; P=0.029), macrovascular invasion or tumor thrombus
(HR: 2.216; 95% CI: 1.269-3.869; P=0.005), and a family history of
cancer (HR: 1.833; 95% CI: 1.099-3.059; P=0.020) were independent
prognostic predictors of OS in postoperative patients with HCC
(Table IV).
 | Table IV.Multivariate analysis of
clinicopathological characteristics influencing prognosis in 330
patients with hepatocellular carcinoma. |
Table IV.
Multivariate analysis of
clinicopathological characteristics influencing prognosis in 330
patients with hepatocellular carcinoma.
|
| Disease-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
| Clinicopathological
parameters | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Clinical symptoms,
yes vs. no | 1.286
(0.813-2.035) | 0.283 | 1.747
(1.045-2.918) | 0.046a |
| α-fetoprotein,
ng/ml, ≥20 vs. <20 | 1.029
(0.697-1.520) | 0.887 | 1.739
(1.057-2.862) | 0.029a |
| Macrovascular
invasion or tumor thrombus, yes vs. no | 2.080
(1.284-3.368) | 0.003b | 2.216
(1.269-3.869) | 0.005b |
| Family history of
cancer, yes vs. no | 1.438
(0.948-2.180) | 0.087 | 1.833
(1.099-3.059) | 0.020a |
| APAR, <1.74 vs.
≥1.74 | 1.781
(1.192-2.661) | 0.005b | 1.828
(1.063-3.141) | 0.029a |
Correlation between preoperative APAR
and postoperative survival in patients with HCC
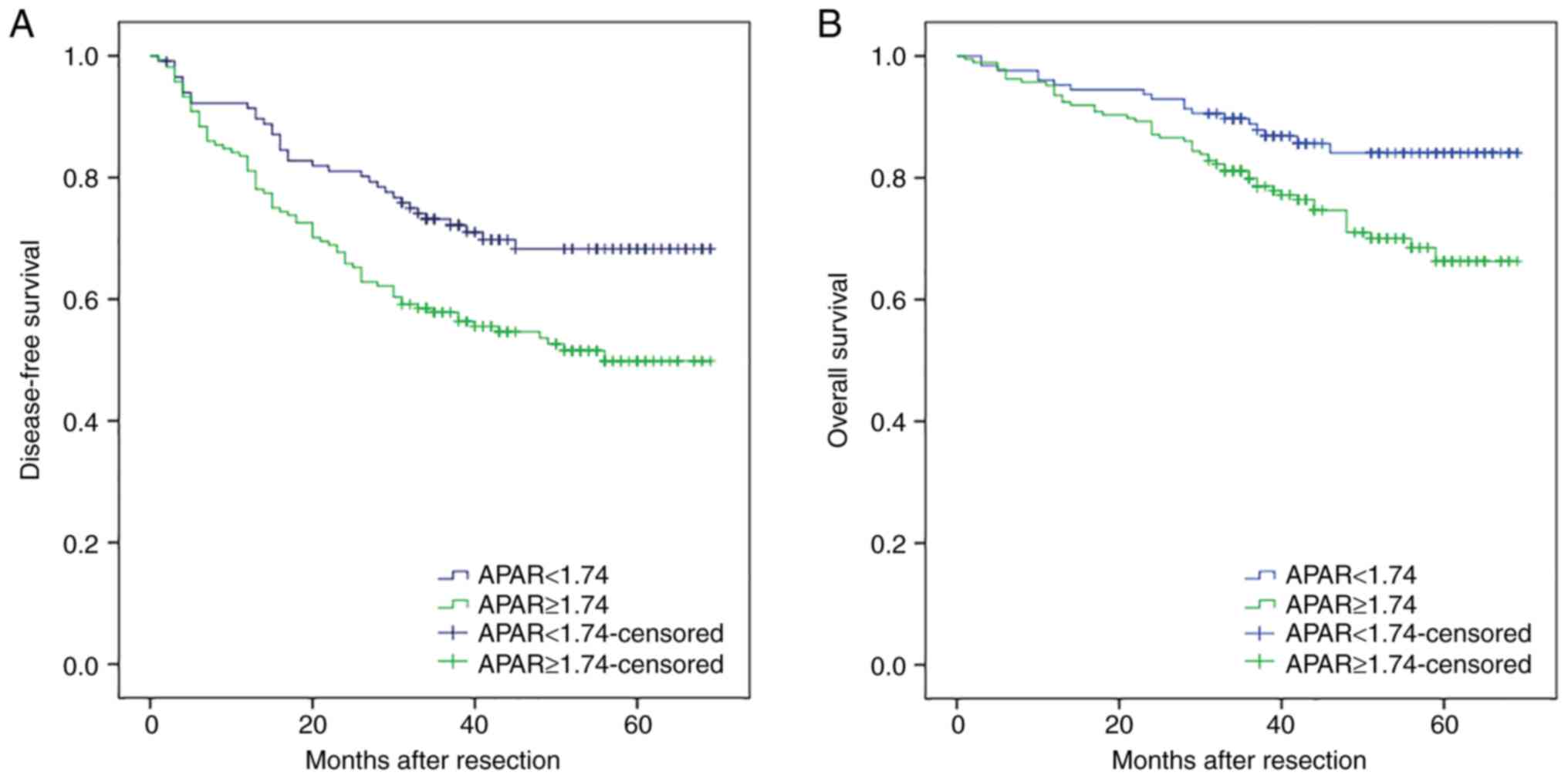
The above data confirmed that a preoperative APAR
value ≥1.74 was significantly associated with a shorter DFS
(P=0.005 and P=0.013, respectively) and OS (P=0.029 and P=0.001,
respectively) in patients with HCC (Fig. 2A and B). Kaplan-Meier analysis
showed that the 1, 3, and 5-year DFS rates of the APAR <1.74
group were significantly higher than those of the APAR ≥1.74 group
(91.4, 73.2 and 68.3% vs. 81.1, 57.9 and 49.8% respectively,
P=0.004). The 1, 3, and 5-year OS rates of the APAR <1.74 group
were also markedly higher than those of the APAR ≥1.74 group (95.3,
88.8 and 84.1% vs. 93.5, 79.9 and 66.3%, respectively,
P=0.008).
Discussion
Predicting the postoperative survival of patients
with HCC plays a key role in HCC treatment. To improve the accuracy
and reliability of predictions, significant efforts have been made
to identify effective prognostic indicators of HCC. Currently,
clinicians and researchers commonly rely on conventional
clinicopathological parameters, such as serum AFP, CEA, CA19-9
level, tumor size, tumor number, vascular invasion, and TNM stage.
However, the sensitivity and specificity of AFP, CEA, and CA19-9
for predicting the prognosis of patients with HCC are limited. For
example, serum AFP levels are not elevated in ~30% of patients
(13), and various confounding
factors can influence the reliability and accuracy of CEA in
predicting the prognosis of patients with cholangiocarcinoma
(14). Elevated serum CA19-9 levels
are frequently found in normal bile secreted by a healthy biliary
tract (15). Therefore, identifying
less invasive and effective tumor biomarkers is important for the
prognostic evaluation of HCC.
In this study, the clinicopathological parameters
and prognosis of 330 patients with HCC who underwent radical
hepatectomy were retrospectively analyzed. To avoid empirical
selection bias, a reliable and objective cut-off value of 1.74 for
APAR was determined via ROC curve analysis. Univariate analysis
revealed that APAR ≥1.74 and macrovascular invasion or tumor
thrombus was significantly correlated with DFS and OS after
operation in patients with HCC. Meanwhile, Cox multivariate
regression analysis showed that APAR ≥1.74, and macrovascular
invasion or tumor thrombus were independent prognostic risk factors
for DFS and OS in the overall cohort. In addition, univariate and
Cox multivariate analyses showed that existing clinical symptoms,
AFP level ≥20 ng/ml, and a family history of cancer were
significantly associated with OS in these patients. The prognostic
value of clinical symptoms, AFP level, macrovascular invasion or
tumor thrombus, and a family history of cancer in patients with HCC
has been reported in previous studies (16–18).
Interestingly, an APAR ≥1.74 was found to be a novel prognostic
indicator in patients with HCC after hepatectomy. The Cox
regression model demonstrated that this indicator has an important
prognostic value as an independent prognostic factor.
ALP is a hydrolase enzyme that plays a crucial role
in the dephosphorylation of various biomolecules, including nucleic
acids, proteins, and alkaloids. It is widely distributed in human
tissues of the liver, intestine, kidney, and bone. However, serum
ALP is primarily present in the liver (19). Several reports have demonstrated
increased secretion of ALP in the blood during some pathological
conditions, such as pregnancy, urinary system diseases, and hepatic
malignant tumors (20–22). In the correlation analysis, ALP
levels were closely correlated with HBsAg levels. Preliminary
statistical data showed that ~95% of patients with HCC in China
also had hepatitis B virus (HBV) infection and liver cirrhosis
(23). The three-step process of
HBV infection, liver cirrhosis, and progression to HCC is well
established (24). Chronic HBV
infection is the most severe factor causing progression to HCC, and
the degree of liver fibrosis is correlated with tumor recurrence
and OS in postoperative patients with small and solitary
HBV-related HCC (25,26). This indicates that elevated serum
ALP levels are closely correlated with HBV status and liver
cirrhosis, which is consistent with the results of the present
study in which high serum APAR was significantly associated with
HBsAg positively in patients with HCC. Meanwhile, the correlation
analysis showed that ALP was closely correlated with tumor size and
TNM stage. ALP is related to the differentiation of embryonic cells
and other stem cells derived from adipose tissue and bone (27). Proliferating tumor cells primarily
induce aerobic glycolysis and elevated amino acid metabolism to
maintain nucleotide biosynthesis and the transfer of amino groups,
which are catalyzed by ALP (28).
In addition, a study found that almost all cultured cancer cells,
including HCC cells, had high ALP activity in the nucleolus, and
the localization of ALP in cancer cells changed during the cell
cycle (29). ALP may be involved in
the proliferation and progression of malignant cells, indicating
its role in tumor size and TNM stage (30). Moreover, advanced liver diseases,
such as HCC, are typically accompanied by mitochondrial damage,
which can substantially promote the release of ALP (31). However, liver fibrosis and cirrhosis
reduce the plasma clearance of ALP, which also increases serum ALP
levels (32). Therefore, high serum
ALP levels are associated with HCC progression and patient
survival.
In addition to ALP, the levels of serum liver
enzymes, such as ALB, are also commonly elevated in patients with
HCC and play crucial roles in the evaluation of the status of liver
damage (33). ALB, the major
component of serum proteins, is a nutritional indicator of the
functions of stabilizing cell proliferation and DNA replication,
buffering various biochemical variations, and exhibiting an
antioxidant role against carcinogens, including nitrosamines and
aflatoxins (34,35). Moreover, ALB is a reliable
prognostic indicator in various malignant tumors, including HCC,
colorectal, renal, and prostate cancers (36–38).
The possible mechanisms for the association between low levels of
serum ALB and poor survival of patients with HCC are systemic
inflammatory response and malnutrition. Systemic inflammation and
oxidative stress are pivotal in tumor progression (39–41).
ALB is a reliable indicator of the host inflammatory response,
which is important for tumorigenesis (42). Malnutrition, which is reflected by
hypoproteinemia, can subvert the host's cellular and humoral immune
response, resulting in an increased risk of infection and poor
sensitivity to anti-cancer therapy (43,44).
Furthermore, hypoalbuminemia in patients with HCC is caused by
hepatic injury due to potential chronic liver disease, and a
sustained systemic inflammatory reaction, either from the neoplasm
itself or as a host response (45).
Finally, malnutrition and systemic inflammatory response cause an
imbalance in the tumor microenvironment, which can promote tumor
growth, invasion, and metastasis (46). Therefore, decreased serum ALB levels
may be closely associated with systemic inflammatory response and
malnutrition, which may increase the risk of relapse and thus
induce adverse survival in patients with HCC.
Taken together, high serum ALP levels are
significantly associated with poorer clinical outcomes in patients
with HCC. Conversely, a significant decrease in serum ALB levels is
closely correlated with the adverse survival of patients with HCC.
Therefore, high APAR is a reasonable indicator of poor survival in
postoperative patients with HCC. Previous studies have demonstrated
that a high preoperative APPRI (10), AST to lymphocyte ratio (11), and AGR (12) are associated with a high recurrence
rate and poor survival in patients with HCC. In contrast to
previous studies, the present study showed that a high preoperative
APAR was associated with poor outcomes in patients with HCC after
resection. As mentioned above, it is hypothesized that the host
systemic immune response, inflammatory state, viral infection,
liver fibrosis, liver cirrhosis, and liver function play key roles
in promoting recurrence and poor clinical outcomes in patients with
HCC (47–49). Accordingly, it is emphasized that a
focus should be placed on not only hepatic tumors alone, but also
on the host liver function, preoperative systemic response, and
nutritional status of patients with HCC after resection.
Interestingly, the analysis showed that an APAR <1.74 had a
better prognostic value than APAR ≥1.74. Compared to patients with
APAR ≥1.74, those with APAR<1.74 had better DFS and OS rates,
with 3- and 5-year DFS rates of 72.2 and 68.3%, respectively, and
3- and 5-year OS rates of 88.8 and 84.1%, respectively. However,
the outcomes of patients with APAR ≥1.74 were worse, with 3- and
5-year DFS rates of only 57.9 and 49.8%, respectively, and 3- and
5-year OS rates of only 79.9 and 66.3%, respectively. From the
above data, an APAR ≥1.74 indicates a high risk of recurrence and
mortality, whereas an APAR <1.74 indicates a low risk of
recurrence and mortality.
Accurately predicting the prognosis of patients with
HCC who undergo curative resection is important. Concurrently, APAR
is important in developing follow-up and further treatment plans
for patients after hepatectomy. Going forward, in our daily
practice, every patient will have their own table of APAR scores,
and physicians will determine their APAR values. For patients with
an APAR ≥1.74, follow-up will be performed at close intervals for
the early detection of tumor recurrence and progression. For
example, once-a-month follow-up is recommended for such patients,
and more adjuvant therapies are recommended, such as transarterial
chemoembolization, systemic chemotherapy, and cellular
immunotherapy. Notably, antiviral therapy may reduce the host
inflammatory response, enhance liver functional reserves, and
increase the survival time of patients with HCC who have chronic
hepatitis infection (50,51).
The present study has several strengths. First, the
prognostic significance of APAR in postoperative patients with HCC
and its correlation with other clinicopathological parameters were
retrospectively analyzed, which has not been performed in previous
studies. Second, the APAR values can be readily and objectively
determined from the peripheral blood of the host. Finally, APAR
<1.74 as an indicator for prognosis demonstrated a better
prognostic value than the simple summation of ALP and ALB. However,
this study has some limitations. First, this was a single-center
study comprising only Chinese patients. Second, this was a
retrospective study with an inherent bias. In the DFS analysis,
there were 49 patients with censored data. Thus, the statistical
analysis for DFS included was of only 281 patients. Moreover, in
the OS analysis, 17 patients were lost to follow-up. For patients
who were lost to follow-up, additional attempts to contact them or
their families will be made through their phone numbers, email
addresses, and family contact information left in the medical
record system. Additionally, local hospitals or local health
authorities will be contacted for home follow-up, hoping to obtain
this part of the missing data. The statistical data used for the
final analysis were obtained from 313 patients. Third, a stratified
analysis was not performed to evaluate the prognostic value of APAR
during the different tumor stages given the relatively small
cohorts after subcategorization. In addition, the optimal cut-off
value of APAR also requires external validation. In this study, due
to the limited number of patients enrolled from a single center,
all the cases were utilized to determine the cut-off value to
ensure that we could obtain the most accurate cut-off value with
the highest sensitivity and specificity. As a result, the
validation queue for the cut-off value was lost. Therefore, future
prospective clinical trials and larger multicenter studies are
warranted to validate the prognostic significance of APAR in
further studies.
In conclusion, the best preoperative APAR cut-off
value for predicting the survival of patients with HCC is 1.74.
APAR is a novel prognostic indicator in patients with HCC after
radical surgery. Moreover, a preoperative APAR of ≥1.74 is closely
correlated with HBsAg positivity, a larger tumor size, and a more
advanced TNM stage, whereas a preoperative APAR of <1.74 is
significantly related to improved clinicopathological
characteristics. Hence, patients with HCC with a higher APAR should
be closely followed up and timely postoperative therapeutic
intervention is required to improve their survival and quality of
life. Finally, the mechanisms for the potential correlation between
high preoperative APAR and poor prognosis in postoperative patients
with HCC need to be further investigated.
Acknowledgements
Not applicable.
Funding
This work was financially supported by the National High-tech
R&D (863) Program of China (grant no. SS2015AA020408),
Fundamental Research Funds of Central Universities (grant no.
2042022kf1076), Natural Science Foundation of Hubei Province (grant
on. 2020CFB659), and the Research Foundation of Hubei Provincial
Health Commission (grant no. WJ2021M139).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding authors upon reasonable
request.
Authors' contributions
YKW performed the research and drafted the
manuscript. YJR and JQC designed the study and revised the
manuscript. XYB, HZ, ZYL, JJZ, JGZ, ZH, YFZ, XC, and CDZ analyzed
the data and revised the article for important intellectual
content. YKW collected the data and participated in data
interpretation. All authors have read and approved the final
manuscript. YJR and JQC confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Ethical approval was granted by the Ethical
Committee of the Cancer Hospital, Chinese Academy of Medical
Sciences and Peking Union Medical College (Cancer Hospital,
approval no. CAMS 15-121/1048). Written consent was obtained from
all examined patients or their guardians prior to surgery.
Patient consent for publication
Informed consent for publication was obtained from
all individual participants included in this study.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
APAR
|
alkaline phosphatase-to-albumin
ratio
|
|
HCC
|
hepatocellular carcinoma
|
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
|
ALP
|
alkaline phosphatase
|
|
AST
|
aspartate aminotransferase
|
|
ALB
|
albumin
|
|
APPRI
|
alkaline phosphatase-to-platelet ratio
index
|
|
AGR
|
albumin-to-globulin ratio
|
|
AFP
|
α-fetoprotein
|
|
ROC
|
receiver operating characteristic
|
|
AUC
|
area under the curve
|
|
CI
|
confidence interval
|
|
HBsAg
|
hepatitis B surface antigen
|
|
CEA
|
carcinoembryonic antigen
|
|
CA19-9
|
carbohydrate antigen 19-9
|
|
HBV
|
hepatitis B virus
|
|
US
|
ultrasound
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gluer AM, Cocco N, Laurence JM, Johnston
ES, Hollands MJ, Pleass HC, Richardson AJ and Lam VW: Systematic
review of actual 10-year survival following resection for
hepatocellular carcinoma. HPB (Oxford). 14:285–290. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Greene PJ and Sussman HH: Structual
comparison of ectopic and normal placental alkaline phosphatase.
Proc Natl Acad Sci USA. 70:2936–2940. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu MC, Chan KM, Lee CF, Lee YS, Eldeen FZ,
Chou HS, Lee WC and Chen MF: Alkaline phosphatase: Does it have a
role in predicting hepatocellular carcinoma recurrence? J
Gastrointest Surg. 15:1440–1449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liao FL, Peng DH, Chen W, Hu HN, Tang P,
Liu YY, Luo Y and Yao T: Evaluation of serum hepatic enzyme
activities in different COVID-19 phenotypes. J Med Virol.
93:2365–2373. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McMillan DC, Watson WS, O'Gorman P,
Preston T, Scott HR and McArdle CS: Albumin concentrations are
primarily determined by the body cell mass and the systemic
inflammatory response in cancer patients with weight loss. Nutr
Cancer. 39:210–213. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Dai Y, Zhou F, Long Z, Li Y, Liu B,
Xie D, Tang J, Tan J, Yao K, et al: The prognostic role of
preoperative serum albumin/globulin ratio in patients with bladder
urothelial carcinoma undergoing radical cystectomy. Urol Oncol.
34:484.e1–484.e8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu YQ, Li J, Liao Y, Chen Q, Liao WJ and
Huang J: The preoperative alkaline phosphatase-to-platelet ratio
index is an independent prognostic factor for hepatocellular
carcinoma after hepatic resection. Medicine (Baltimore).
95:e57342016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin J, Zhu P, Liao Y, Li J, Liao W and He
S: Elevated preoperative aspartate aminotransferase to lymphocyte
ratio index as an independent prognostic factor for patients with
hepatocellular carcinoma after hepatic resection. Oncotarget.
6:19217–19227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suh B, Park S, Shin DW, Yun JM, Keam B,
Yang HK, Ahn E, Lee H, Park JH and Cho B: Low albumin-to-globulin
ratio associated with cancer incidence and mortality in generally
healthy adults. Ann Oncol. 25:2260–2266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou M, Luo J, Chen M, Yang H, Learned RM,
DePaoli AM, Tian H and Ling L: Mouse species-specific control of
hepatocarcinogenesis and metabolism by FGF19/FGF15. J Hepatol.
66:1182–1192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu AX, Abbas AR, de Galarreta MR, Guan Y,
Lu S, Koeppen H, Zhang W, Hsu CH, He AR, Ryoo BY, et al: Molecular
correlates of clinical response and resistance to atezolizumab in
combination with bevacizumab in advanced hepatocellular carcinoma.
Nat Med. 28:1599–1611. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Izquierdo-Sanchez L, Lamarca A, La Casta
A, Buettner S, Utpatel K, Klümpen HJ, Adeva J, Vogel A, Lleo A,
Fabris L, et al: Cholangiocarcinoma landscape in Europe:
Diagnostic, prognostic and therapeutic insights from the ENSCCA
Registry. J Hepatol. 76:1109–1121. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singhi AD, Nikiforova MN, Chennat J,
Papachristou GI, Khalid A, Rabinovitz M, Das R, Sarkaria S, Ayasso
MS, Wald AI, et al: Integrating next-generation sequencing to
endoscopic retrograde cholangiopancreatography (ERCP)-obtained
biliary specimens improves the detection and management of patients
with malignant bile duct strictures. Gut. 69:52–61. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao
C, Huang F, Tang R, Cheng Y, Huang Z, et al: Lenvatinib combined
with transarterial chemoembolization as first-line treatment for
advanced hepatocellular carcinoma: A phase III, randomized clinical
trial (LAUNCH). J Clin Oncol. 41:117–127. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lyu N, Wang X, Li JB, Lai JF, Chen QF, Li
SL, Deng HJ, He M, Mu LW and Zhao M: Arterial chemotherapy of
oxaliplatin plus fluorouracil versus sorafenib in advanced
hepatocellular carcinoma: A biomolecular exploratory, randomized,
phase III trial (FOHAIC-1). J Clin Oncol. 40:468–480. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rimassa L, Personeni N, Czauderna C,
Foerster F and Galle P: Systemic treatment of HCC in special
populations. J Hepatol. 74:931–943. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng D, Xu W, Gong X, Yuan L and Zhang
XB: Design strategy of fluorescent probes for live drug-induced
acute liver injury imaging. Acc Chem Res. 54:403–415. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Aerts RMM, van de Laarschot LFM,
Banales JM and Drenth JPH: Clinical management of polycystic liver
disease. J Hepatol. 68:827–837. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu Y, Huang S, Wang J, Sun L, Zeng F and
Wu S: Activatable probes for diagnosing and positioning liver
injury and metastatic tumors by multispectral optoacoustic
tomography. Nat Commun. 9:39832018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kemeny NE, Chou JF, Capanu M, Chatila WK,
Shi H, Sanchez-Vega F, Kingham TP, Connell LC, Jarnagin WR and
D'Angelica MI: A randomized phase II trial of adjuvant hepatic
arterial infusion and systemic therapy with or without panitumumab
after hepatic resection of KRAS Wild-type colorectal cancer. Ann
Surg. 274:248–254. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seto WK, Lo YR, Pawlotsky JM and Yuen MF:
Chronic hepatitis B virus infection. Lancet. 392:2313–2324. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim HS, Yu X, Kramer J, Thrift AP,
Richardson P, Hsu YC, Flores A, El-Serag HB and Kanwal F:
Comparative performance of risk prediction models for hepatitis
B-related hepatocellular carcinoma in the United States. J Hepatol.
76:294–301. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kulik L and El-Serag HB: Epidemiology and
management of hepatocellular carcinoma. Gastroenterology.
156:477–491.e1. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shih C, Yang CC, Choijilsuren G, Chang CH
and Liou AT: Hepatitis B Virus. Trends Microbiol. 26:386–387. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zou Y, Qazvini NT, Zane K, Sadati M, Wei
Q, Liao J, Fan J, Song D, Liu J, Ma C, et al: Mgelatin-derived
graphene-silicate hybrid materials are biocompatible and
synergistically promote BMP9-Induced osteogenic differentiation of
mesenchymal stem cells. ACS Appl Mater Interfaces. 9:15922–15932.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
al-Rashida M and Iqbal J: Iqbal,
Inhibition of alkaline phosphatase: An emerging new drug target.
Mini Rev Med Chem. 15:41–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamamoto K, Awogi T, Okuyama K and
Takahashi N: Nuclear localization of alkaline phosphatase in
cultured human cancer cells. Med Electron Microsc. 36:47–51. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Muñoz-González JI, Álvarez-Twose I,
Jara-Acevedo M, Zanotti R, Perkins C, Jawhar M, Sperr WR,
Shoumariyeh K, Schwaab J, Greiner G, et al: Proposed global
prognostic score for systemic mastocytosis: A retrospective
prognostic modelling study. Lancet Haematol. 8:e194–e204. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou H, Du W, Li Y, Shi C, Hu N, Ma S,
Wang W and Ren J: Effects of melatonin on fatty liver disease: The
role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and
mitophagy. J Pineal Res. 642018.
|
|
32
|
Villa-Bellosta R, González-Parra E and
Egido J: Alkalosis and dialytic clearance of phosphate increases
phosphatase activity: A hidden consequence of hemodialysis. PLoS
One. 11:e01598582016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takagi A, Hawke P, Tokuda S, Toda T,
Higashizono K, Nagai E, Watanabe M, Nakatani E, Kanemoto H and Oba
N: Serum carnitine as a biomarker of sarcopenia and nutritional
status in preoperative gastrointestinal cancer patients. J Cachexia
Sarcopenia Muscle. 13:287–295. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Garcia-Martinez R, Caraceni P, Bernardi M,
Gines P, Arroyo V and Jalan R: Albumin: Pathophysiologic basis of
its role in the treatment of cirrhosis and its complications.
Hepatology. 58:1836–1846. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vincent JL, Russell JA, Jacob M, Martin G,
Guidet B, Wernerman J, Ferrer R, McCluskey SA and Gattinoni L:
Albumin administration in the acutely ill: What is new and where
next? Crit Care. 18:2312014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
China L, Freemantle N, Forrest E, Kallis
Y, Ryder SD, Wright G, Portal AJ, Becares Salles N, Gilroy DW and
O'Brien A; ATTIRE Trial Investigators, : A randomized trial of
albumin infusions in hospitalized patients with cirrhosis. N Engl J
Med. 384:808–817. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wong F, Pappas SC, Curry MP, Reddy KR,
Rubin RA, Porayko MK, Gonzalez SA, Mumtaz K, Lim N, Simonetto DA,
et al: Terlipressin plus albumin for the treatment of type 1
hepatorenal syndrome. N Engl J Med. 384:818–828. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boada M, López OL, Olazarán J, Núñez L,
Pfeffer M, Paricio M, Lorites J, Piñol-Ripoll G, Gámez JE, Anaya F,
et al: A randomized, controlled clinical trial of plasma exchange
with albumin replacement for Alzheimer's disease: Primary results
of the AMBAR Study. Alzheimers Dement. 16:1412–1425. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Carr BI and Guerra V: Serum albumin levels
in relation to tumor parameters in hepatocellular carcinoma
patients. Int J Biol Markers. 32:e391–e396. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bağırsakçı E, Şahin E, Atabey N, Erdal E,
Guerra V and Carr BI: Role of albumin in growth inhibition in
hepatocellular carcinoma. Oncology. 93:136–142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ram AK, Pottakat B and Vairappan B:
Increased systemic zonula occludens 1 associated with inflammation
and independent biomarker in patients with hepatocellular
carcinoma. BMC Cancer. 18:5722018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lv LL, Feng Y, Wen Y, Wu WJ, Ni HF, Li ZL,
Zhou LT, Wang B, Zhang JD, Crowley SD and Liu BC: Exosomal CCL2
from tubular epithelial cells is critical for albumin-induced
tubulointerstitial inflammation. J Am Soc Nephrol. 29:919–935.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sapienza C and Issa JP: Nutrition, and
cancer epigenetics. Annu Rev Nutr. 36:665–681. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kaymak I, Williams KS, Cantor JR and Jones
RG: Immunometabolic interplay in the tumor microenvironment. Cancer
Cell. 39:28–37. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Esper DH and Harb WA: The cancer cachexia
syndrome: A review of metabolic and clinical manifestations. Nutr
Clin Pract. 20:369–376. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Atasheva S, Emerson CC, Yao J, Young C,
Stewart PL and Shayakhmetov DM: Systemic cancer therapy with
engineered adenovirus that evades innate immunity. Sci Transl Med.
12:eabc66592020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gola A, Dorrington MG, Speranza E, Sala C,
Shih RM, Radtke AJ, Wong HS, Baptista AP, Hernandez JM, Castellani
G, et al: Commensal-driven immune zonation of the liver promotes
host defence. Nature. 589:131–136. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ringelhan M, Pfister D, O'Connor T,
Pikarsky E and Heikenwalder M: The immunology of hepatocellular
carcinoma. Nat Immunol. 19:222–232. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Limketkai BN, Mehta SH, Sutcliffe CG,
Higgins YM, Torbenson MS, Brinkley SC, Moore RD, Thomas DL and
Sulkowski MS: Relationship of liver disease stage and antiviral
therapy with liver-related events and death in adults coinfected
with HIV/HCV. JAMA. 308:370–378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Carrat F, Fontaine H, Dorival C, Simony M,
Diallo A, Hezode C, De Ledinghen V, Larrey D, Haour G, Bronowicki
JP, et al: Clinical outcomes in patients with chronic hepatitis C
after direct-acting antiviral treatment: A prospective cohort
study. Lancet. 393:1453–1464. 2019. View Article : Google Scholar : PubMed/NCBI
|