Introduction
Thyroid cancer is one of the most prevalent tumors
of the endocrine system, and remains a global problem (1). Considerable progression has been made
in the therapeutic approaches against this cancer type, such as
radio/chemotherapy, surgical resection and target therapy (2). In addition, combination therapy with
Lenvatinib and Radioactive Iodine in preclinical models also gained
favorable clinical outcomes for patients with advanced thyroid
cancer (3). However, the long-time
prognosis of patients with thyroid cancer and tumor metastasis is
poor due to resistance to radio/chemotherapy and/or post-surgical
recurrence (4,5). Therefore, exploring new diagnostic
biomarkers or therapeutic objectives is an urgent need to increase
the survival rate of patients with thyroid cancer.
One of the main subcategories of non-coding RNA is
long non-coding RNA (lncRNA). lncRNAs have no protein-coding
capability and are >200 nucleotides in length (6). Previous evidence suggested that
lncRNAs were involved in various biological processes, including
cancer cell proliferation, cycle, apoptosis, migration, invasion
(7,8). A number of lncRNAs were identified to
act as oncogenes or tumor suppressors in thyroid cancer (9–11),
suggesting that lncRNAs could be implemented as therapy targets and
markers of diagnosis for this cancer type.
lncRNAs exert their biological function by sponging
microRNAs (miRNAs or miRs) (12).
miRNAs were reported to be involved in the progression of various
cancer types, including cancer cell proliferation, migration, cell
cycle and apoptosis (13,14). miRNAs were shown to serve as
diagnostic and therapy targets for thyroid cancer (15,16).
lncRNA Down syndrome cell adhesion
molecule-antisense 1 (DSCAM-AS1) was reported to affect cancer cell
proliferation, migration and invasion in multiple cancers, such as
breast cancer (17), hepatocellular
carcinoma (18), ovarian cancer
(19), non-small cell lung
carcinoma (20), cervical cancer
(21) and melanoma (21). However, the clinical significance,
functions and strategies associated with DSCAM-AS1 in the context
of thyroid cancer have remained mainly ambiguous. The present study
investigated the expression of DSCAM-AS1 within samples of patients
with thyroid cancer, as well as its clinical significance, and
assessed the biological application and potential therapeutic
strategy of DSCAM-AS1 in thyroid cancer by conducting a number of
in vivo and in vitro experiments.
Materials and methods
Patient samples
A total of 48 paired adjacent normal tissues and
thyroid cancer tissues were harvested from patients (age range,
18–72 years) who underwent surgery at Ningbo Medical Centre Lihuili
Hospital (Ningbo, China) between January 2019 and January 2020.
Clinical stage classification was conducted according to the World
Health Organization categorization. None of the patients had
received any anticancer treatment prior to the operation. The
clinicopathological factors of patients are shown in Table I. A written informed consent was
obtained from all patients, and the study was approved (approval
no. KY2022SL376-01) by the Ethics Committee of Ningbo Medical
Centre Lihuili Hospital (Ningbo, China).
 | Table I.Association of DSCAM-AS1 expression
with clinicopathologic factors of 48 patients with thyroid
cancer. |
Table I.
Association of DSCAM-AS1 expression
with clinicopathologic factors of 48 patients with thyroid
cancer.
|
|
| DSCAM-AS1
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
factors | Cases, n | High | Low | P-value |
|---|
| Age, years |
|
|
| 0.2461 |
|
<40 | 22 | 9 | 13 |
|
|
≥40 | 26 | 16 | 10 |
|
| TNM stage |
|
|
| 0.0088 |
|
I–II | 35 | 14 | 21 |
|
|
III–IV | 13 | 11 | 2 |
|
| Tumor size, cm |
|
|
| 0.2209 |
| ≤1 | 33 | 15 | 18 |
|
|
>1 | 15 | 10 | 5 |
|
| Lymphatic
metastasis |
|
|
| 0.0002 |
| No | 36 | 14 | 22 |
|
|
Yes | 12 | 11 | 1 |
|
Cell culture and transfection
The human normal thyroid cell line Nthy-ori3-1 and
human thyroid cancer TPC-1 cells were obtained from the
Conservation Genetics Shanghai Cell Bank. The purchased cells were
cultured in RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.) in the presence of 5% CO2 at
37°C.
A total of 3 small hairpin RNAs (shRNAs) suppressing
DSCAM-AS1 expression (namely sh-DSCAM-AS1#1,
5′-CCTCCTCCAACTGCCATTTT-3′; sh-DSCAM-AS1#2,
5′-GTTCTGGTCTCATCATGATT-3′ and sh-DSCAM-AS1#3,
5′-CACATAGGCATGACATACTTT-3′), and corresponding negative control
(sh-NC, 5′-TTCTCCGAACGTGTCACGTTT-3′) were prepared by Shanghai
GenePharma Co., Ltd., and were cloned into pGPU6/Hygro vector.
miR-211 mimics (miR-211, 5′-UUCCCUUUGUCAUCCUUCGCCU-3′), miR-211
inhibitor (anti-miR-211, 5′-AGGCGAAGGAUGACAAAGGGAA-3′) and their
negative controls (miR-NC, 5′-AGAAGCUGUUCCAAGGUGGGCC-3′ and
anti-miR-NC, 5′-GAACAUCCAGGGUCCCGUUCCU-3′ respectively), were
obtained from Guangzhou RiboBio Co., Ltd. To perform cell
transfection, 5×105 TPC-1 cells were plated in 96-well
plates and incubated for 24 h at 37°C. Transfection of shRNAs (100
nM), inhibitors (100 nM) and mimics (100 nM) into the TPC-1 cells
was conducted with Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. After 24 h of transfection at 37°C, the cells were used
for follow-up experiments. G418 (0.5 mg/ml; Sigma-Aldrich; Merck
KGaA) was utilized to select stably transfected cells.
Extraction of RNA and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from cell lines and tissues
by using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the protocol of the manufacturer. RT
was carried out to synthesize cDNA using the PrimeScript RT Reagent
Kit (Takara Biotechnology Co., Ltd.) according to the
manufacturer's protocol. For the quantification of DSCAM-AS1
expression, qPCR was performed with SYBR® Premix Ex Taq™
II kit (Takara Biotechnology Co., Ltd.) on a 7500 Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). For
the quantification of miR-211, qPCR was conducted by using TaqMan™
MicroRNA Assays (Applied Biosystems; Thermo Fisher Scientific,
Inc.) on a 7500 Real-Time PCR System. The reaction conditions were
as follows: 95°C for 2 min, and 40 cycles of 95°C for 35 sec and
56°C for 40 sec. The sequences of the primers were as follows: U6
forward, 5′-AAAGCAAATCATCGGACGACC-3′ and reverse,
5′-GTACAACACATTGTTTCCTCGGA-3′; miR-211 forward,
5′-TCGGCAGGTCCCTTTGTCATCC-3′ and reverse,
5′-AGGCGAAGGATGACAAAGGGTT-3′; GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′; and DSCAM-AS1 forward,
5′-ACAGAGATGGACGACGGATC-3′, and reverse,
5′-TTGTGAGCCTGAGAGATCCC-3′. The levels of DSCAM-AS and miR-211 were
normalized to those of GAPDH and small nuclear RNA U6,
respectively. The 2−ΔΔCq method was utilized to assess
gene expression (22).
Cell proliferation assessment
The ability of cell proliferation in thyroid cancer
cell lines was assessed via Cell Counting Kit-8 (CCK-8; Dojindo
Laboratories, Inc.) assay based on our previous study (19).
Colony formation assay
The ability of TPC-1 cells to form colonies was
assessed as described in our previous study (19).
Transwell cell invasion assay
A total of 5×104 transfected cells in 200
µl serum-free medium were placed in the upper chamber of a
Transwell plate (pore size of the insert, ~8 µm; EMD Millipore)
pre-coated with Matrigel (precoating at 37°C for 2 h; BD
Biosciences), while RPMI-1640 containing 10% FBS was added to the
lower chamber. After 24 h, the invasive cells located in the lowest
part of the surface were fixed with 4% polyoxymethylene at 25°C for
30 min and subsequently stained by using 0.1% crystal violet
(Sigma-Aldrich; Merck KGaA) at 25°C for 30 min. The invasive cells
in 5 randomly selected visual fields were counted under a light
microscope (X71; Olympus Corporation).
Wound healing assay
Transfected cells were added to six-well culture
plates and cultured in serum-free medium until 100% confluence.
Next, a wound area was created by scratching the cell monolayer
with a 10-µl pipette tip. Scratch wounds at 0 and 24 h were
depicted by utilizing a microscope (X71; Olympus Corporation).
Isolation of cytoplasmic/nuclear
RNA
The Cytoplasmic & Nuclear RNA Purification Kit
(Norgen Biotek Corp) was utilized to isolate cytoplasmic/nuclear
RNA from TPC-1 cells according to the manufacturer's instructions.
DSCAM-AS1 expression was assessed by RT-qPCR, as aforementioned. U6
was utilized as the nuclear control, while GAPDH was utilized as
the cytoplasmic control.
Luciferase reporter assay
The possible sites of binding among DSCAM-AS1 and
miR-211 were estimated using ENCORI (http://starbase.sysu.edu.cn/). The DSCAM-AS1 sequences
with the estimated wild-type (Wt) or mutant (Mut) miR-211 binding
sites were prepared through chemical synthesis and subsequently
cloned into the luciferase reporter vector psi-CHECK-2 (Promega
Corporation). The recombinant plasmids were named Wt-DSCAM-AS1
(5′-CUUGUGAUUCUUUCAAAGGGAA-3′) and Mut-DSCAM-AS1
(5′-CUUGUGAUUCUUUGUUUCCCUA-3′), respectively. For calculating the
luciferase reporter activity, TPC-1 cells were co-transfected with
recombinant reporter plasmids and miR-211 mimics or miR-NC with
Lipofectamine 3000® (Invitrogen; Thermo Fisher
Scientific, Inc.). After 24 h, the luciferase activities were
determined with a Dual-Luciferase Reporter Assay (Promega
Corporation) according to the manufacturer's instructions, and
normalized to Renilla luciferase activity.
RNA immunoprecipitation (RIP)
assay
RIP experiments were implemented to investigate the
binding between miR-211 and endogenous DSCAM-AS1 in TPC-1 cells by
using the Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit
(cat. no. 17-701; Merck KGaA) according to the manufacturer's
instructions. In brief, cells were lysed with RIP lysis buffer, and
then incubated with RIP buffer containing 50 µl magnetic beads
conjugated with human anti-argonaute-2 (Ago2) antibody (EMD
Millipore) or negative control IgG (EMD Millipore). Upon protein
removal, the RNA precipitates were isolated using
TRIzol® reagent according to the manufacturer's
protocol, and then subjected to RT-qPCR for detection of DSCAM-AS1
and miR-211 expression.
Statistical analysis
Statistical analysis was performed with SPSS 20.0
(IBM Corp.) and GraphPad Prism 5.0 (GraphPad Software, Inc.).
Results are presented as the mean ± standard deviation of ≤3
replicates. An unpaired or paired Student's t-test was used to
analyze the comparisons between two groups, while one-way ANOVA
followed by the Tukey's post hoc test was utilized for
multiple-group comparisons. The association between DSCAM-AS1 and
clinical pathology criteria was determined by Pearson's
c2 test. The correlation between miR-211 and DSCAM-AS1
expression was assessed with Spearman's correlation test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Elevated DSCAM-AS1 expression is
correlated with weak prognosis of patients with thyroid cancer
To explore the relevance of DSCAM-AS1 in the
development of thyroid cancer, the expression of DSCAM-AS1 was
detected in 48 pairs of thyroid cancer tissues and adjacent normal
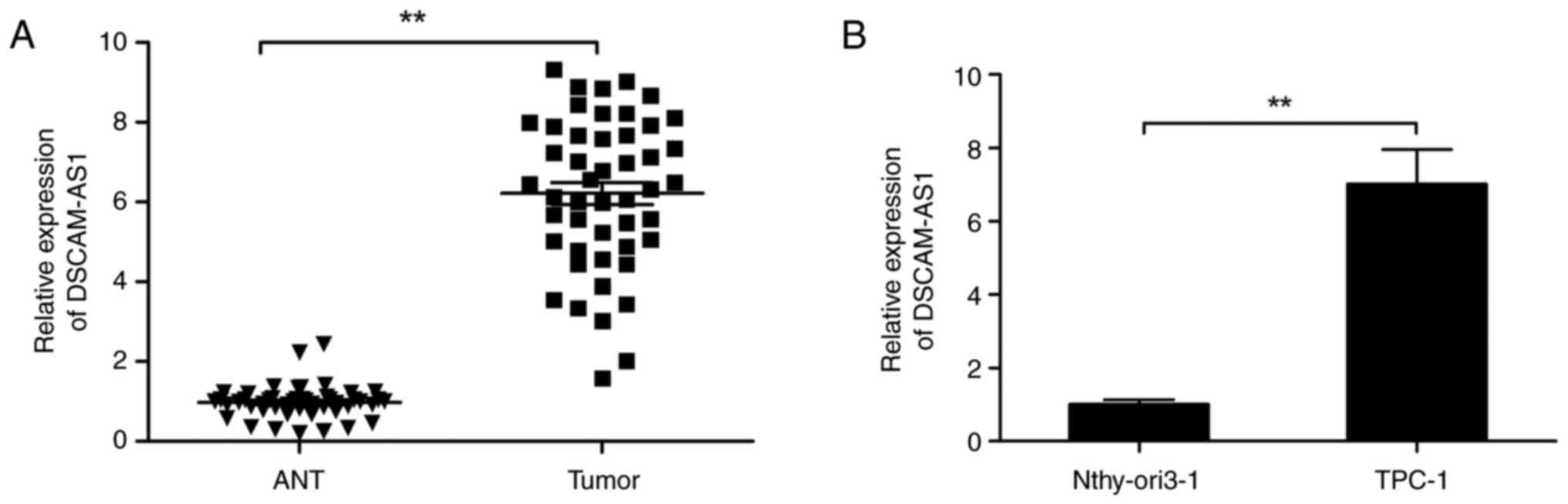
tissues. The expression of DSCAM-AS1 was considerably increased in
thyroid cancer tissues in comparison with that in adjacent normal
tissues (Fig. 1A). The expression
of DSCAM-AS1 in human thyroid cancer cell lines was also examined,
and its was found that the expression of DSCAM-AS1 in TPC-1 cells
was considerably greater than that in normal thyroid cells
Nthy-ori3-1 (Fig. 1B).
The association of DSCAM-AS1 with the
clinicopathological characteristics of patients with thyroid cancer
was investigated. The 48 cases were divided into two categories: i)
DSCAM-AS1-low group (n=22) and ii) DSCAM-AS1-high group (n=26)
based on the mean value of DSCAM-AS1 expression in thyroid cancer.
As illustrated in Table I, high
expression of DSCAM-AS1 was positively correlated with lymph node
metastasis and advanced clinical stage.
DSCAM-AS1 knockdown inhibits cell
proliferation and colony formation of thyroid cancer cells
To explore the role of DSCAM-AS1 in thyroid cancer
cells, loss-of function assays were performed in TPC-1 cells by
transfection with three shRNAs specific for DSCAM-AS1 (namely
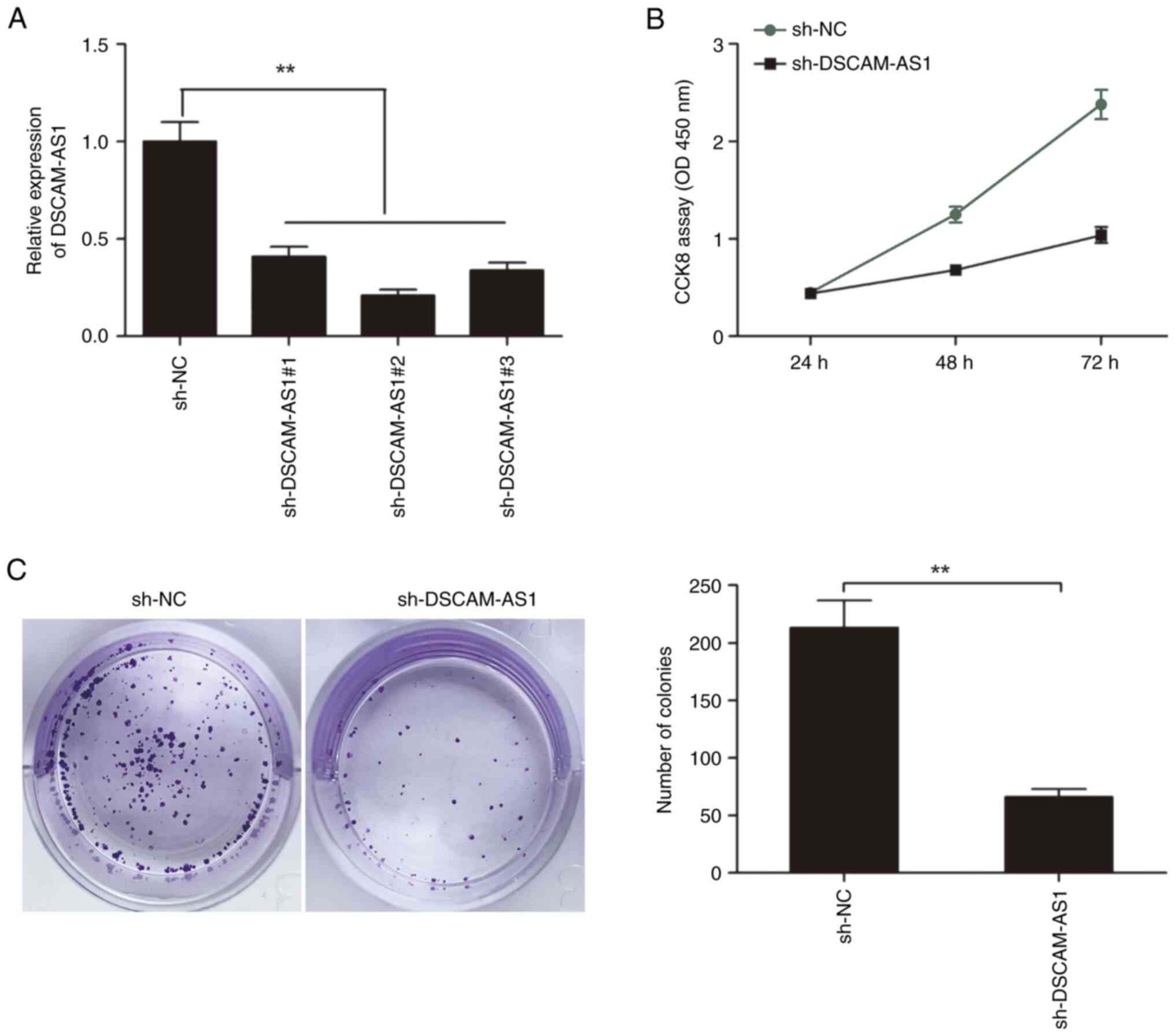
sh-DSCAM-AS1#1, sh-DSCAM-AS1#2 and sh-DSCAM-AS1#3). It was found
that the three shRNAs against DSCAM-AS1 were all able to decrease
DSCAM-AS1 expression in TPC-1 cells (Fig. 2A), and sh-DSCAM-AS1#2 displayed the
most effectiveness, and was selected for subsequent experiments
(referred to as sh-DSCAM-AS1).
CCK-8 assay revealed that DSCAM-AS1 depletion led to
a notable decrease in cell proliferation in TPC-1 cells (Fig. 2B). In addition, colony formation
assay revealed that DSCAM-AS1 depletion led to a significant
decrease in colony formation in TPC-1 cells (Fig. 2C).
DSCAM-AS1 knockdown inhibits the
invasion and migration of thyroid cancer cells
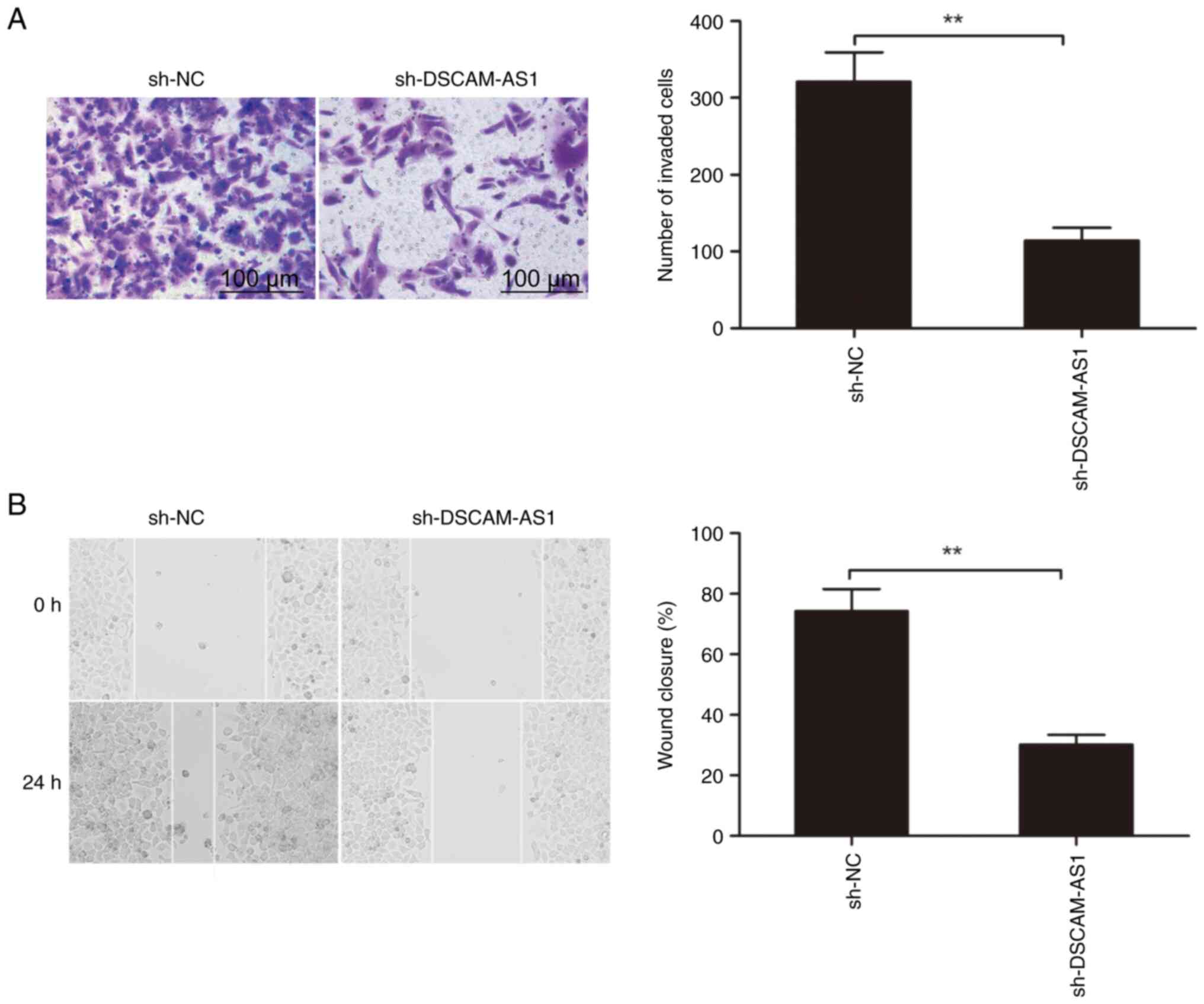
The impact of DSCAM-AS1 depletion on cell invasion
and migration in thyroid cancer cells was studied by Transwell and
wound healing assays, respectively. It was revealed that the
invasion and migration capabilities of TPC-1 cells were
significantly reduced when DSCAM-AS1 was downregulated (Fig. 3A and B).
DSCAM-AS1 binds to miR-211 in thyroid
cancer cells
To ascertain the mechanism of DSCAM-AS1 in cancer
progression, miR-211 was determined to be a potential target of
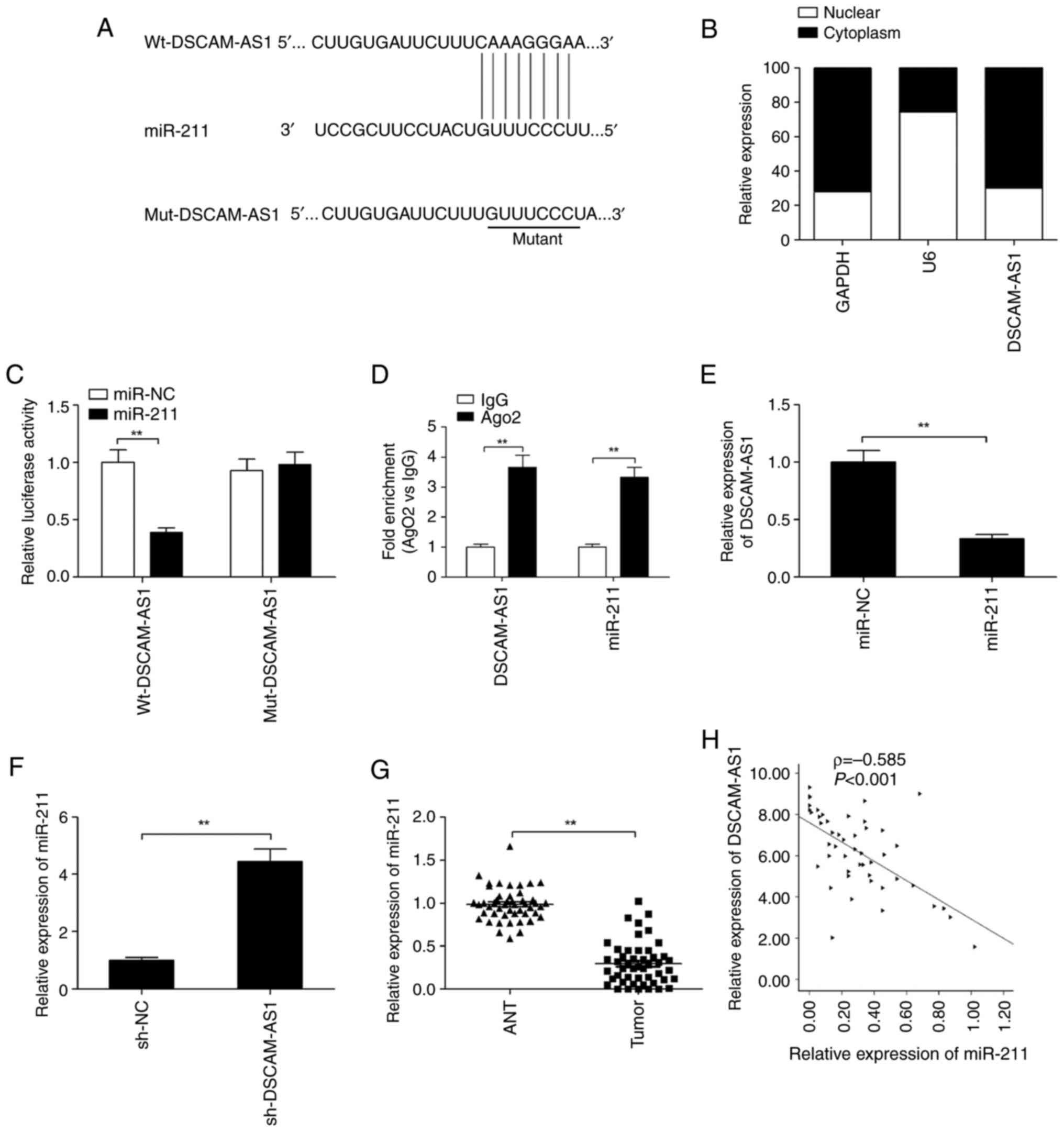
DSCAM-AS1 by using ENCORI software. DSCAM-AS1 has a binding site on
miR-211 (Fig. 4A). In addition,
DSCAM-AS1 was mainly located in the cytoplasm of TPC-1 cells
(Fig. 4B); thus, DSCAM-AS1 may
regulate miRNAs as a sponge. To examine this hypothesis, a
luciferase assay was conducted. As revealed in Fig. 4C, overexpression of miR-211 in TPC-1
cells by transfection using miR-211 mimics could reduce
Wt-DSCAM-AS1 luciferase activity (Wt-DSCAM-AS1). However, it did
not affect the mutant type of DSCAM-AS1 (Mut-DSCAM-AS1) (Fig. 4C). To further confirm this result,
RIP assay was performed. The results demonstrated that both
DSCAM-AS1 and miR-211 expression were increased in Ago2-rich beads
in thyroid cancer cells (Fig.
4D).
Moreover, it was found that overexpression of
miR-211 significantly suppressed DSCAM-AS1 expression (Fig. 4E), while DSCAM-AS1 depletion
enhanced miR-211 expression in TPC-1 cells (Fig. 4F). The expression of miR-211 was
also examined within tissue samples from 48 clinical patients with
thyroid cancer via RT-qPCR. The results demonstrated that miR-211
expression was considerably downregulated (Fig. 4G), and was negatively associated
with DSCAM-AS1 in these tissues (ρ=−0.585; Fig. 4H). These data demonstrated that
miR-211 was a target of DSCAM-AS1 in thyroid cancer cells.
DSCAM-AS1 knockdown suppresses the
development of thyroid cancer via regulation of miR-211
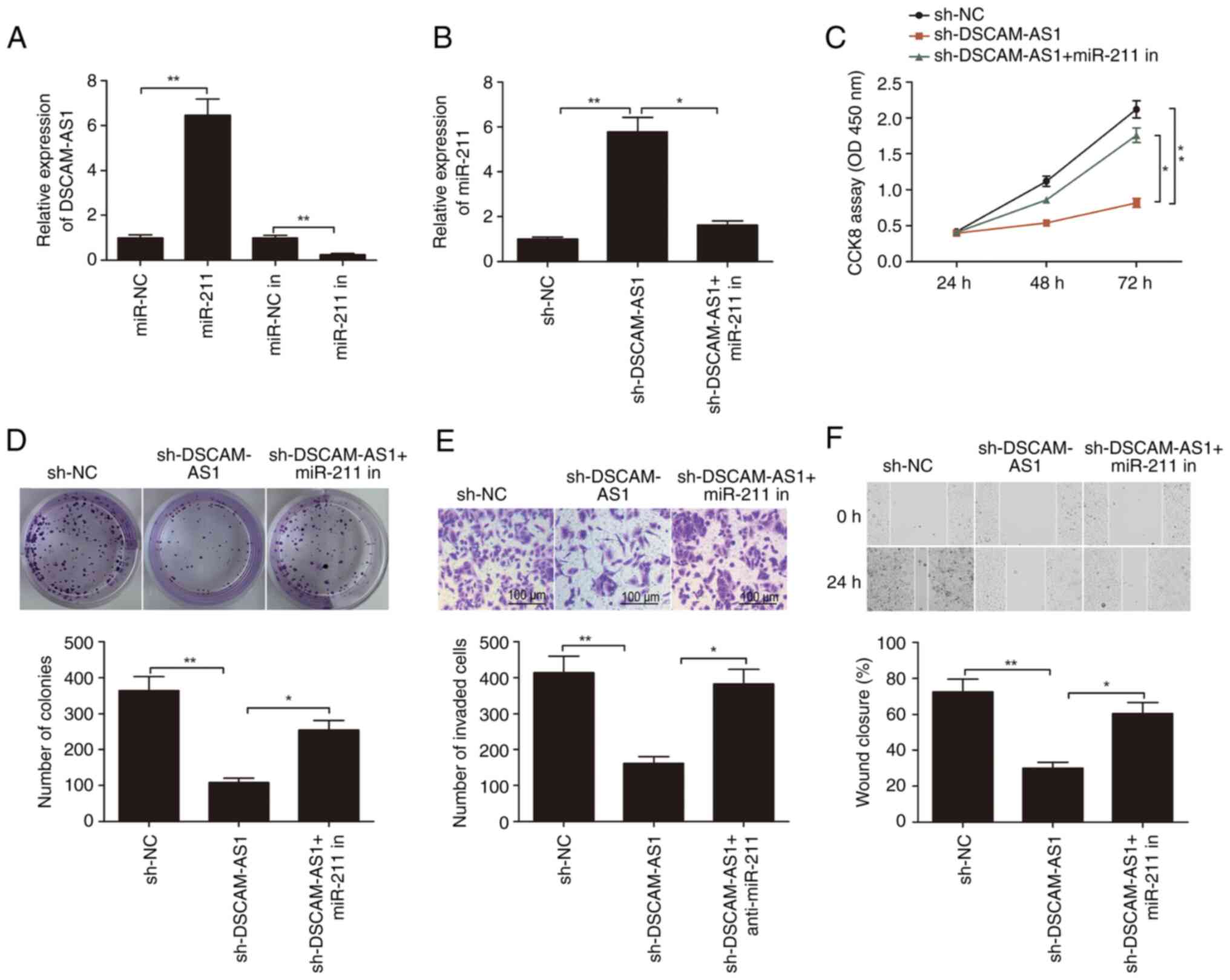
The association between DSCAM-AS1 and miR-211 was
investigated. Mimics and inhibitors of miR-211 were transfected
into TPC-1 cells, and it was found that transfection of miR-211
mimics significantly increased the expression of miR-211 in TPC-1
cells (Fig. 5A), while transfection
of miR-211 inhibitors significantly decreased the expression of
miR-211 in TPC-1 cells (Fig. 5A).
It was observed that knockdown of DSCAM-AS1 significantly increased
the expression of miR-211 in TPC-1 cells, while miR-211 inhibitor
rescued the effects of DSCAM-AS1 knockdown (Fig. 5B). Furthermore, it was found that
miR-211 downregulation partially reversed the effects of DSCAM-AS1
knockdown on the proliferation, migration and invasion of TPC-1
cells (Fig. 5C-F). Thus, it was
suggested that DSCAM-AS1 knockdown suppressed the development of
thyroid cancer by regulating miR-211.
Discussion
Numerous lncRNAs have been found to be dysregulated
in various cancer types, and to perform an essential role in tumor
development, including thyroid cancer (9–11). For
instance, Wang et al (23)
reported that lncRNA maternally expressed 3 inhibited the migration
and invasion of thyroid cancer cell by targeting Rac1 protein. Liu
et al (24) reported that
lncRNA-X-inactive specific transcript enhanced the invasion and
proliferation of thyroid cancer cells by acting as a competing
endogenous RNA to regulate MET-PI3K-AKT signaling via sponging
miR-34a. Guo et al (25)
indicated that lncRNA myocardial infarction associated transcript
enhanced the development of thyroid cancer through modulation of
the miR-150-5P/enhancer of zeste homolog 2 axis. The present study
investigated a new regulatory network of miR-211 and DSCAM-AS1
involved in the proliferation and invasion of thyroid cancer. The
current results demonstrated that DSCAM-AS1 knockdown hindered the
development of thyroid cancer via regulation of miR-211, suggesting
that DSCAM-AS1 could be a therapeutic target for thyroid
cancer.
Recent studies have suggested an oncogenic role for
lncRNA DSCAM-AS1 in multiple cancer types (18–21).
However, the detailed roles and fundamental mechanism of DSCAM-AS1
in thyroid cancer remain largely unclear. The current study
demonstrated that DSCAM-AS1 was highly expressed in thyroid cancer
tissues, and was positively correlated with lymph node metastasis
and advanced clinical stage. Functional experiments revealed that
DSCAM-AS1 depletion considerably diminished the invasion and
proliferation abilities of thyroid cancer cells in vitro.
These results showed that DSCAM-AS1 functioned as an oncogenic
lncRNA in thyroid cancer.
Several studies have reported that lncRNAs can
affect the tumorigenesis and progression of various cancer types by
serving as miRNA sponges to restore the expression of target genes
(26,27). DSCAM-AS1 was reported to be able to
bind to several miRNAs, such as miR-338-3p (18), miR-136 (21), miR-137 (28) and miR-204-5p (29). Whether DSCAM-AS1 regulates the
development of thyroid cancer through a similar mechanism remains
unknown. In the present study, bioinformatics suggested that
DSCAM-AS1 could interact with miR-211 in thyroid cancer, which was
demonstrated by luciferase activity reporter and RIP assays.
Furthermore, miR-211 was reported to be downregulated and to play a
tumor suppressive role in thyroid cancer (30,31).
In the present study, it was also confirmed that the expression of
miR-211 was notably reduced in thyroid cancer tissues, which was in
consistency with previous studies (30,31).
Moreover, there was a negative association between miR-211 and
DSCAM-AS1 expression in thyroid cancer tissues. These data
indicated that DSCAM-AS1 could interact with miR-211 in thyroid
cancer cells.
Several limitations exist in the present study: i)
The effects of DSCAM-AS1 on cell cycle and apoptosis were not
investigated, which is performed in the related ongoing study; ii)
The effect of DSCAM-AS1 on change of related genes expression of
cell proliferation, cycle, apoptosis, migration and invasion should
be studied, iii) The animal study was not performed, which is
performed in the related ongoing study; and iv) The effect of
overexpression of DSCAM-AS1 on thyroid cancer cell proliferation,
migration and invasion was not investigated, which will be
performed in the future study.
Briefly, the present study revealed that DSCAM-AS1
was upregulated in thyroid cancer cells and tissues, and was
correlated with lymph node metastasis and clinical stage.
Functional assays demonstrated that knockdown of DSCAM-AS1 hindered
thyroid cancer cell invasion, proliferation and migration.
Mechanistically, DSCAM-AS1 promoted thyroid cancer aggressiveness
via regulating miR-211. These results may provide a molecular basis
for potential roles of DSCAM-AS1 in the treatment and prognosis of
thyroid cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TH, YL and JY designed the study, performed the
experiments and wrote the manuscript. YZ performed the statistical
analysis. All authors have read and approved the final manuscript.
TH and YL confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All experimental protocols were approved (approval
no. KY2022SL376-01) by the Ethics Committee of Ningbo Medical
Centre Lihuili Hospital (Ningbo, China). Written informed consent
was provided by all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pratilas CA and Solit DB: Therapeutic
strategies for targeting BRAF in human cancer. Rev Recent Clin
Trials. 2:121–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki K, Iwai H, Utsunomiya K, Kono Y,
Watabe T, Kobayashi Y, Bui DV, Sawada S, Yun Y, Mitani A, et al:
Efficacy of combination therapy with lenvatinib and radioactive
iodine in thyroid cancer preclinical model. Int J Mol Sci.
23:98722022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li M, Dal Maso L and Vaccarella S: Global
trends in thyroid cancer incidence and the impact of overdiagnosis.
Lancet Diabetes Endocrinol. 8:468–470. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guttman M, Russell P, Ingolia NT, Weissman
JS and Lander ES: Ribosome profiling provides evidence that large
noncoding RNAs do not encode proteins. Cell. 154:240–251. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun T: Long noncoding RNAs act as
regulators of autophagy in cancer. Pharmacol Res. 129:151–155.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sedaghati M and Kebebew E: Long noncoding
RNAs in thyroid cancer. Curr Opin Endocrinol Diabetes Obes.
26:275–281. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Javed Z, Ahmed Shah F, Rajabi S, Raza Q,
Iqbal Z, Ullah M, Ahmad T, Salehi B, Sharifi-Rad M, Pezzani R, et
al: LncRNAs as potential therapeutic targets in thyroid cancer.
Asian Pac J Cancer Prev. 21:281–287. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao J, Zhang M, Zhang L, Lou J, Zhou F and
Fang M: Non-coding RNA in thyroid cancer-Functions and mechanisms.
Cancer Lett. 496:117–126. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Niknafs YS, Han S, Ma T, Speers C, Zhang
C, Wilder-Romans K, Iyer MK, Pitchiaya S, Malik R, Hosono Y, et al:
The lncRNA landscape of breast cancer reveals a role for DSCAM-AS1
in breast cancer progression. Nat Commun. 7:127912016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Winkle M, El-Daly SM, Fabbri M and Calin
GA: Noncoding RNA therapeutics - challenges and potential
solutions. Nat Rev Drug Discov. 20:629–651. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hill M and Tran N: miRNA interplay:
Mechanisms and consequences in cancer. Dis Model Mech.
14:dmm0476622021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li B, Cao Y, Sun M and Feng H: Expression,
regulation, and function of exosome-derived miRNAs in cancer
progression and therapy. FASEB J. 35:e219162021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghafouri-Fard S, Shirvani-Farsani Z and
Taheri M: The role of microRNAs in the pathogenesis of thyroid
cancer. Noncoding RNA Res. 5:88–98. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Geropoulos G, Psarras K, Papaioannou M,
Giannis D, Meitanidou M, Kapriniotis K, Symeonidis N, Pavlidis ET,
Pavlidis TE, Sapalidis K, et al: Circulating microRNAs and
clinicopathological findings of papillary thyroid cancer: A
systematic review. In vivo. 36:1551–1569. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji D, Hu G, Zhang X, Yu T and Yang J: Long
non-coding RNA DSCAM-AS1 accelerates the progression of
hepatocellular carcinoma via sponging miR-338-3p. Am J Transl Res.
11:4290–4302. 2019.PubMed/NCBI
|
|
18
|
Li Y, Hao J, Jiang YM, Liu Y and Zhang SH:
Long non-coding RNA DSCAM-AS1 indicates a poor prognosis and
modulates cell proliferation, migration and invasion in ovarian
cancer via upregulating SOX4. Eur Rev Med Pharmacol Sci.
24:109152020.PubMed/NCBI
|
|
19
|
Hua T and Luo Y: Circular RNA PVT1
promotes progression of thyroid cancer by competitively binding
miR-384. Exp Ther Med. 24:6292022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang X, Wang Z, Hou S, Yue C, Li Z, Hu W
and Lu H: Long non-coding RNA DSCAM-AS1 promotes pancreatic cancer
progression via regulating the miR-136-5p/PBX3 axis. Bioengineered.
13:4153–4165. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang J, Zhang S, Wang W, Xu Y, Kawuli A,
Lu J and Xiu X: Long non-coding RNA DSCAM-AS1 contributes to the
tumorigenesis of cervical cancer by targeting miR-877-5p/ATXN7L3
axis. Biosci Reps. 40:BSR201920612020. View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta DeltaC(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang C, Yan G, Zhang Y, Jia X and Bu P:
Long non-coding RNA MEG3 suppresses migration and invasion of
thyroid carcinoma by targeting of Rac1. Neoplasma. 62:541–549.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu H, Deng H, Zhao Y, Li C and Liang Y:
LncRNA XIST/miR-34a axis modulates the cell proliferation and tumor
growth of thyroid cancer through MET-PI3K-AKT signaling. J Exp Clin
Cancer Res. 37:2792018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo K, Qian K, Shi Y, Sun T and Wang Z:
LncRNA-MIAT promotes thyroid cancer progression and function as
ceRNA to target EZH2 by sponging miR-150-5p. Cell Death Dis.
12:10972021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi X, Zhang DH, Wu N, Xiao JH, Wang X and
Ma W: ceRNA in cancer: Possible functions and clinical
implications. J Med Genet. 52:710–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma Y, Bu D, Long J, Chai W and Dong J:
LncRNA DSCAM-AS1 acts as a sponge of miR-137 to enhance Tamoxifen
resistance in breast cancer. J Cell Physiol. 234:2880–2894. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liang WH, Li N, Yuan ZQ, Qian XL and Wang
ZH: DSCAM-AS1 promotes tumor growth of breast cancer by reducing
miR-204-5p and up-regulating RRM2. Mol Carcinog. 58:461–473. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang L, Shen YF, Shi ZM, Shang XJ, Jin DL
and Xi F: Overexpression miR-211-5p hinders the proliferation,
migration, and invasion of thyroid tumor cells by downregulating
SOX11. J Clin Lab Anal. 32:e222932018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang M, Jia J, Chen L, Wei B, Guan Q,
Ding Z, Yu J, Pang R and He G: LncRNA MCM3AP-AS1 promotes
proliferation and invasion through regulating miR-211-5p/SPARC axis
in papillary thyroid cancer. Endocrine. 65:318–326. 2019.
View Article : Google Scholar : PubMed/NCBI
|