Introduction
Head and neck squamous cell carcinoma (HNSCC) has
been identified as the sixth most common cancer in the world, with
approximately 600,000 new cases diagnosed annually (1). Two-thirds of patients are diagnosed at
an advanced stage, and the 5-year survival rate is 39–65%; thus,
HNSCC has one of the worst prognoses compared to other cancer types
(2). Worldwide, the standard
treatments are surgery and chemoradiation (3–7).
Unfortunately, recurrence and metastasis often occur even after
these therapies. Recently, immune checkpoint inhibitors, such as
nivolumab and pembrolizumab, have exhibited better performance than
standard chemotherapy in the Phase III CHECK-MATE-141 and
KEYNOTE-040 studies (8,9). However, the response rate was only
about 15%, and the median overall survival was 1.4-2.4 months,
which is deemed unsatisfactory (10).
The L-type amino acid transporter (LAT1, SLC7A5) has
been attracting the recent attention as a therapeutic target. Among
the 50 types of mammalian cell membrane amino acid transporters,
the expression of the following transporters is upregulated in
malignant tumors: LAT1 (SLC7A5) (11), LAT3 (SLC43A1) (12), ASCT2 (SLC1A5) (13), ATB0+ (SLC6A14) (14), and xCT (SLC7A11) (15). LAT1 forms a heterodimeric complex
with CD98 heavy chain (CD98hc) (4F2hc, SLC3A2) (16) and transports large neutral amino
acids, such as leucine, isoleucine, valine, phenylalanine,
tyrosine, tryptophan, methionine, and histidine. LAT1 is often
upregulated in human cancer tissues, including colon, lung,
prostate, gastric, breast, kidney, esophageal, and brain cancers.
In non-small cell lung cancer, pancreatic cancer, brain tumor,
prostate cancer, and breast cancer, high expression of LAT1 is
associated with a poor prognosis, suggesting that the expression of
LAT1 is related to cancer malignancy (17–22).
LAT1 is upregulated in cancers, and its expression is highly
specific to cancers. Inhibition of LAT1 often blocks the amino acid
supply to tumor cells and evokes antitumor effects. Thus, LAT1
inhibitors are currently being developed as antitumor drugs.
Intravenous administration of the LAT1 inhibitor, JPH203, inhibits
tumor growth in nude mice (23).
Despite the high expression of LAT1 in various
cancers, the function of LAT1 has not yet been elucidated in HNSCC.
Therefore, this study aimed to characterize LAT1 in HNSCC and to
investigate the relationship between LAT1 and prognosis in clinical
samples. If LAT1 is a prognostic factor, the use of JPH203 will be
improved significantly. If LAT1 can be used as a prognostic factor,
tailor-made treatment based on the patient's background can be
implemented instead of the current standard of care, which includes
platinum-based agents combined with radiation therapy and surgery.
For refractory and recurrent tumors, JPH203 may be an alternative
treatment to nivolumab and pembrolizumab.
Materials and methods
Cell culture
Sa3 (gingiva), HSC2 (oral), and HSC4 (tongue) cell
lines were used. Cells were grown in RPMI1640 (Nissui
Pharmaceutical, Tokyo, Japan) supplemented with 10% fetal bovine
serum (FBS) (Clontech Laboratories, Mountain View, CA) and 2 mM
L-glutamine. The radioresistant cell lines were described
previously (24).
Flow cytometry
For the analysis of the LAT1-positive fraction, cell
pellets were incubated with FITC-conjugated SLC7A5/LAT1 antibody
(BU53) (Novus Biologicals, Littleton). After washing with PBS
twice, the cells were resuspended in 2 µg/ml propidium
iodide/Hank's balanced salt solution and filtered through a cell
strainer (BD Biosciences Discovery Labware, Bedford, MA). Flow
cytometry was performed using a BD FACS Aria III instrument
(Becton, Dickinson and Company Japan, Tokyo, Japan). To determine
the negative fraction, FITC-conjugated mouse IgG2a isotype control
(M2A) (Novus, Biologicals, Littleton, USA) was used.
Sphere formation assay
To avoid adhesion and subsequent development to
non-CSCs, cells were cultured in serum-free semisolid medium.
LAT1-positive and LAT1-negative cells (1×103 cells each)
were seeded in 100 µl of PromoCell 3D Tumorsphere Medium XF
(PromoCell GmbH, Heidelberg, Germany), containing 0.33% agar. The
cells were then incubated for 14 days at 37°C in a humidified
atmosphere containing 5% CO2.
Invasion assay
Invasion assays were performed using an 8 µm Boyden
chamber (Falcon, USA). The filter was coated with 100 µl of
Matrigel (1 mg/ml). The upper chamber was filled with 500 µl of
serum-free RPMI 1640 medium with 5×103 cells, whereas
the bottom chamber was filled with 1,000 µl of 10%-FBS-supplemented
RPMI 1640 medium. After 72 h of incubation, the upside of the
filter was swabbed off and fixed with formalin. Cells were stained
with hematoxylin-eosin and counted under an optical microscope
(Olympus, Tokyo, Japan).
Wound healing assay
Cells were grown to confluency with growth medium in
a 60-mm dish, and a straight line was drawn with a 200-µl pipet
tip. After 24 h of incubation with serum-free RPMI medium, the
cells were fixed in formalin, and the residual area of wound gap
after migration was analyzed using ImageJ and compared to that of
the corresponding initial wound gap (set at 1).
Immunostaining
Immunohistochemical staining was performed on 173
preoperative untreated HNSCC biopsy specimens collected at our
hospital from 2010 to 2019 for clinicopathological studies. The
median follow-up period was 34 (range: 2–6) months. The anti-LAT-1
antibody was a monoclonal antibody purified from rabbit. Tissue
sections (3 µm) were deparaffinized and treated with 3% hydrogen
peroxide methanol for 10 min to inhibit endogenous peroxidase
activity. After washing with the buffer, the sections were
incubated with anti-LAT1 antibody (1:1,000; ab208776, Abcam,
Cambridge, MA) for 90 min. The sections were then incubated with
Dako EnVision+ System HRP and colorized with DAB
(3,3-diaminobenzidine). The results were classified into four
levels according to the degree of staining: score 0 (negative),
score 1 (weak), score 2 (moderate), and score 3 (strong). A score
of ≥2 was considered positive for LAT1 expression. The evaluation
method was adopted from a study by Rietbergen et al
(25). At least two skilled
pathologists scored the staining while blinded to the clinical
information.
Experiment using JPH203
JPH203 (Namiki, Tokyo, Japan) was used to inhibit
LAT1 at a concentration of 100 µM, referring to the concentration
used by Choi et al (26).
Statistical analysis
Significant differences in OS and PFS were assessed
using the Kaplan-Meier method and log-rank test. The univariate and
multivariate Cox proportional hazards modeling was used to evaluate
prognostic significance. One-way ANOVA followed by Tukey's post hoc
test was used to compare three unpaired groups, and unpaired
Student's t-tests were used to compare two unpaired groups,
respectively. All experiments were independently repeated at least
three times. P<0.05 was considered significant.
Results
LAT1-positive cells in HNSCC have
strong spheroid-forming and invasive potential
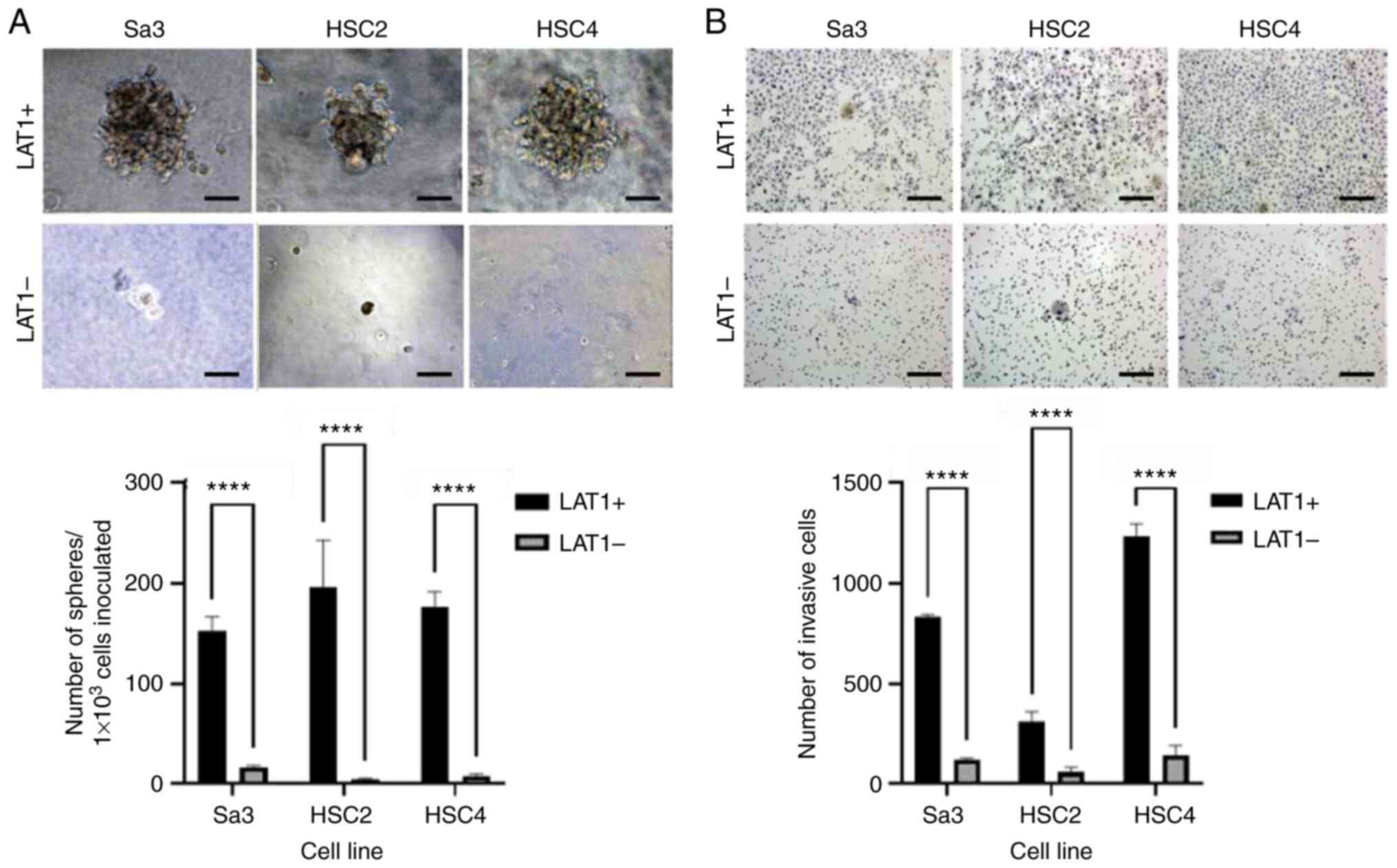
Three HNSCC cell lines, that is, Sa3, HSC2, and
HSC4, were separated into LAT1-positive and -negative cells using a
flow cytometer. The LAT1-positive cells had strong spheroid
formation ability, whereas the LAT1-negative cells could hardly
form spheroids (Fig. 1A).
LAT1-positive cells from the three different cell lines also
exhibited enhanced invasive ability (Fig. 1B).
Patients with LAT1-positive HNSCC have
a poor prognosis and are refractory to chemoradiotherapy
According to TNM Classification of Malignant
Tumours, 8th ed., Union for International Cancer Control (27), 78.0% of patients were at advanced
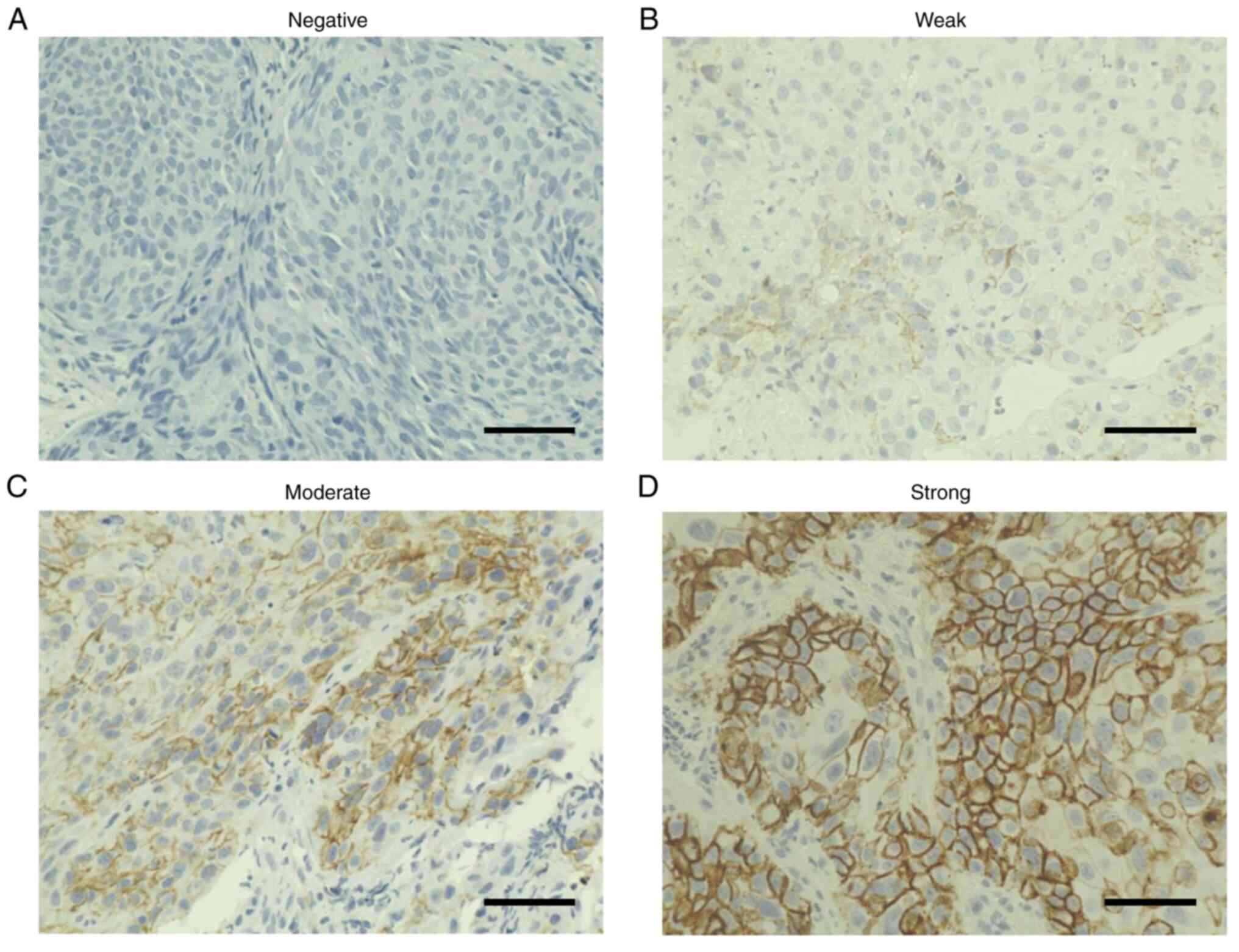
stages (Table I). The intensity of
LAT1 immunostaining of biopsy specimens was determined by skilled
pathologists to be negative (Fig.
2A), weak (Fig. 2B), moderate
(Fig. 2C), or strong (Fig. 2D). Specimens with negative and weak
immunostaining were classified into the Low group, whereas
specimens with moderate and strong immunostaining were classified
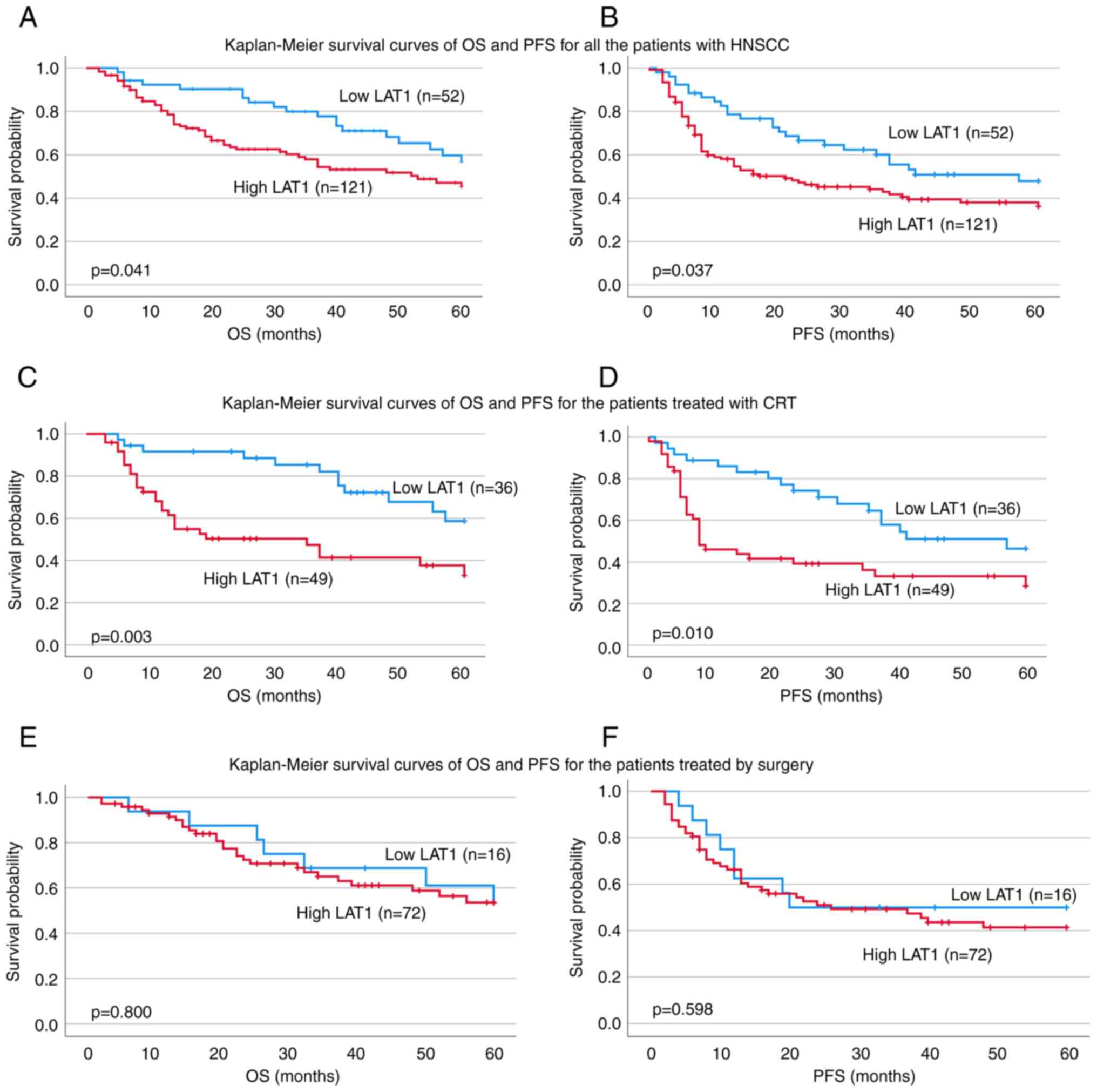
into the High group. Of the 173 patients (Table I), 52 were in the Low group and 121
were in the High group. The 5-year OS was 56.8% in the Low group,
whereas it was 45.3% in the High group (P=0.041) (Fig. 3A); moreover, PFS was 47.9% in the
Low group and 36.2% in the High group (P=0.037) (Fig. 3B). Furthermore, multivariate
analysis revealed that LAT1 was an independent prognostic factor
for OS (Table IIA) and PFS
(Table IIB) [OS, P=0.045, HR:
1.710, 95% confidence interval (95% CI): 1.013-2.887; PFS, P=0.037,
HR: 1.749, 95% CI: 1.013-2.887].
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Value |
|---|
| Mean age ± SD,
years | 65.4±10.6 |
| Sex, n
(male/female) | 143/30 |
| T category n
(%) |
|
|
T1 | 16 (9.2) |
|
T2 | 70 (40.5) |
|
T3 | 37 (21.4) |
|
T4 | 50 (28.9) |
| N category, n
(%) |
|
|
N0 | 49 (28.3) |
|
N1 | 17 (9.8) |
|
N2 | 103 (59.5) |
|
N3 | 4 (2.4) |
| M category, n
(%) |
|
|
M0 | 173 (100.0) |
| Stage, n (%) |
|
|
I | 11 (6.4) |
|
II | 27 (15.6) |
|
III | 19 (11.0) |
|
IV | 116 (67.0) |
| Tumor sites, n
(%) |
|
|
Tongue | 32 (18.5) |
|
Nasopharynx | 9 (5.2) |
|
Oropharynx | 55 (31.8) |
|
Hypopharynx | 63 (36.4) |
|
Gingiva | 14 (8.1) |
 | Table II.Univariate and multivariate analyses
of OS and PFS. |
Table II.
Univariate and multivariate analyses
of OS and PFS.
| A, 5-year OS |
|---|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (<65 vs.
>65 years) | 1.537 | 0.963-2.455 | 0.072 | 1.827 | 1.133-2.946 | 0.013a |
| Sex (female vs.
male) | 1.356 | 0.696-2.640 | 0.371 | 1.422 | 0.724-2.796 | 0.307 |
| T category (T1-T2
vs. T3-T4) | 1.954 | 1.221-3.128 | 0.005b | 1.691 | 1.032-2.769 | 0.037a |
| N category (N0 vs.
N1-N3) | 1.845 | 1.046-3.253 | 0.034a | 1.666 | 0.904-3.068 | 0.102 |
| Stage (I–II vs.
III–IV) | 1.918 | 1.010-3.642 | 0.046a | NA | NA | NA |
| LAT1 (low vs.
high) | 1.710 | 1.013-2.887 | 0.045a | 1.749 | 1.033-2.961 | 0.037a |
|
| B, 5-year
PFS |
|
|
| Univariate
analysis | Multivariate
analysis |
|
|
|
|
|
Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| Age (<65 vs.
>65 years) | 1.046 | 0.700-1.564 | 0.825 | 1.200 | 0.792-1.816 | 0.390 |
| Sex (female vs.
male) | 1.434 | 0.783-2.627 | 0.243 | 1.488 | 0.807-2.743 | 0.203 |
| T category (T1-T2
vs. T3-T4) | 1.503 | 1.003-2.254 | 0.048a | 1.388 | 0.908-2.123 | 0.130 |
| N category (N0 vs.
N1-N3) | 1.509 | 0.937-2.430 | 0.090 | 1.366 | 0.818-2.282 | 0.233 |
| Stage (I–II vs.
III–IV) | 1.434 | 0.849-2.422 | 0.178 | NA | NA | NA |
| LAT1 (low vs.
high) | 1.610 | 1.019-2.543 | 0.041a | 1.640 | 1.035-2.597 | 0.035a |
In total, 85 of the 173 patients were treated with
chemoradiation, including 36 patients in the Low group and 49
patients in the High group (Table
III). The 5-year OS for patients treated with chemoradiation
was 58.7% in the Low group and 33.0% in the High group (P=0.003)
(Fig. 3C). The PFS for the Low
group was found to be better than the prognosis for the High group
(46.4% in the Low group vs. 28.4% in the High group, P=0.001)
(Fig. 3D). The multivariate
analysis showed that LAT1 was an independent prognostic factor for
OS and PFS in patients who underwent chemoradiotherapy (OS:
P=0.008, HR: 2.697, 95% CI: 1.292-5.464; PFS: P=0.017, HR: 2.124,
95% CI: 1.147-3.933) (Table IV).
Surgical treatment was available for 88 patients (Table V), but there were no significant
differences in terms of OS (53.5% for Low and 53.6% for High)
(Fig. 3E) or PFS (50.0% for Low and
41.4% for High) (Fig. 3F),
(Table VI).
 | Table III.Characteristics of patients treated
by chemoradiotherapy. |
Table III.
Characteristics of patients treated
by chemoradiotherapy.
| Characteristic | Value |
|---|
| Mean age ± SD,
years | 65.6±9.1 |
| Sex, n
(male/female) | 75/10 |
| T category, n
(%) |
|
|
T1 | 6 (7.1) |
|
T2 | 35 (41.2) |
|
T3 | 21 (24.7) |
|
T4 | 23 (27.0) |
| N category, n
(%) |
|
|
N0 | 13 (15.3) |
|
N1 | 12 (14.1) |
|
N2 | 56 (65.9) |
|
N3 | 4 (4.7) |
| M category, n
(%) |
|
|
M0 | 85 (100.0) |
| Stage, n (%) |
|
|
I | 2 (2.4) |
|
II | 7 (8.2) |
|
III | 12 (14.1) |
|
IV | 64 (75.3) |
| Tumor sites, n
(%) |
|
|
Tongue | 2 (2.4) |
|
Nasopharynx | 9 (10.6) |
|
Oropharynx | 40 (47.0) |
|
Hypopharynx | 32 (37.6) |
|
Gingiva | 2 (2.4) |
 | Table IV.Univariate and multivariate analyses
of OS and PFS. |
Table IV.
Univariate and multivariate analyses
of OS and PFS.
| A, 5-year OS |
|---|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (<65 vs.
>65 years) | 1.988 | 1.008-3.920 | 0.047a | 1.707 | 0.852-3.418 | 0.131 |
| Sex (female vs.
male) | 1.865 | 0.574-6.053 | 0.300 | 2.157 | 0.639-7.283 | 0.216 |
| T category (T1-T2
vs. T3-T4) | 2.798 | 1.438-5.443 | 0.002b | 2.351 | 1.168-4.730 | 0.017a |
| N category (N0 vs.
N1-N3) | 1.624 | 0.635-4.156 | 0.311 | 0.896 | 0.314-2.560 | 0.838 |
| Stage (I–II vs.
III–IV) | 3.360 | 0.808-13.963 | 0.095 | NA | NA | NA |
| LAT1 (low vs.
high) | 2.695 | 1.361-5.336 | 0.004b | 2.657 | 1.292-5.464 | 0.008b |
|
| B, 5-year
PFS |
|
|
| Univariate
analysis | Multivariate
analysis |
|
|
|
|
|
Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| Age (<65 vs.
>65 years) | 1.331 | 0.744-2.382 | 0.336 | 1.160 | 0.634-2.123 | 0.630 |
| Sex (female vs.
male) | 2.542 | 0.789-8.188 | 0.118 | 2.772 | 0.845-9.091 | 0.092 |
| T category (T1-T2
vs. T3-T4) | 1.925 | 1.080-3.430 | 0.026a | 1.767 | 0.954-3.273 | 0.070 |
| N category (N0 vs.
N1-N3) | 1.587 | 0.674-3.734 | 0.290 | 0.974 | 0.388-2.441 | 0.955 |
| Stage (I–II vs.
III–IV) | 2.550 | 0.792-8.215 | 0.117 | NA | NA | NA |
| LAT1 (low vs.
high) | 2.120 | 1.170-3.842 | 0.013a | 2.124 | 1.147-3.933 | 0.017a |
 | Table V.Characteristics of patients treated
by surgery. |
Table V.
Characteristics of patients treated
by surgery.
| Characteristic | Value |
|---|
| Mean age ± SD,
years | 65.2±11.9 |
| Sex, n
(male/female) | 68/20 |
| T category, n
(%) |
|
|
T1 | 10 (11.4) |
|
T2 | 35 (39.8) |
|
T3 | 16 (18.2) |
|
T4 | 27 (30.6) |
| N category, n
(%) |
|
|
N0 | 36 (40.9) |
|
N1 | 5 (5.7) |
|
N2 | 47 (53.4) |
|
N3 | 0 (0.0) |
| M category, n
(%) |
|
|
M0 | 88 (100.0) |
| Stage, n (%) |
|
|
I | 9 (10.2) |
|
II | 20 (22.7) |
|
III | 7 (8.0) |
|
IV | 52 (59.1) |
| Tumor sites, n
(%) |
|
|
Tongue | 30 (34.1) |
|
Nasopharynx | 0 (0.0) |
|
Oropharynx | 15 (17.0) |
|
Hypopharynx | 31 (35.3) |
|
Gingiva | 12 (13.6) |
 | Table VI.Univariate and multivariate analyses
of OS and PFS. |
Table VI.
Univariate and multivariate analyses
of OS and PFS.
| A, 5-year OS |
|---|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (<65 vs.
>65 years) | 1.150 | 0.587-2.257 | 0.683 | 1.501 | 0.728-3.093 | 0.271 |
| Sex (female vs.
male) | 1.026 | 0.446-2.357 | 0.952 | 1.032 | 0.439-2.424 | 0.943 |
| T category (T1-T2
vs. T3-T4) | 1.314 | 0.667-2.586 | 0.430 | 1.036 | 0.483-2.225 | 0.927 |
| N category (N0 vs.
N1-N3) | 1.897 | 0.906-3.973 | 0.089 | 2.133 | 0.913-4.984 | 0.080 |
| Stage (I–II vs.
III–IV) | 1.447 | 0.675-3.103 | 0.342 | NA | NA | NA |
| LAT1 (low vs.
high) | 1.113 | 0.484-2.561 | 0.801 | 1.102 | 0.474-2.564 | 0.822 |
|
| B, 5-year
PFS |
|
|
| Univariate
analysis | Multivariate
analysis |
|
|
|
|
|
Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| Age (<65 vs.
>65 years) | 0.807 | 0.454-1.435 | 0.466 | 0.941 | 0.497-1.780 | 0.851 |
| Sex (female vs.
male) | 1.038 | 0.501-2.148 | 0.920 | 1.005 | 0.475-2.127 | 0.989 |
| T category (T1-T2
vs. T3-T4) | 1.164 | 0.656-2.064 | 0.604 | 1.001 | 0.527-1.901 | 0.998 |
| N category (N0 vs.
N1-N3) | 1.474 | 0.806-2.696 | 0.208 | 1.427 | 0.707-2.880 | 0.322 |
| Stage (I–II vs.
III–IV) | 1.139 | 0.609-2.128 | 0.684 | NA | NA | NA |
| LAT1 (low vs.
high) | 1.224 | 0.572-2.620 | 0.603 | 1.180 | 0.545-2.555 | 0.674 |
Radioresistant cells have an expanded
LAT1-positive fraction and enhanced malignant potential
Because of the poor prognosis of LAT1-positive
patients and their resistance to radiotherapy, we examined the
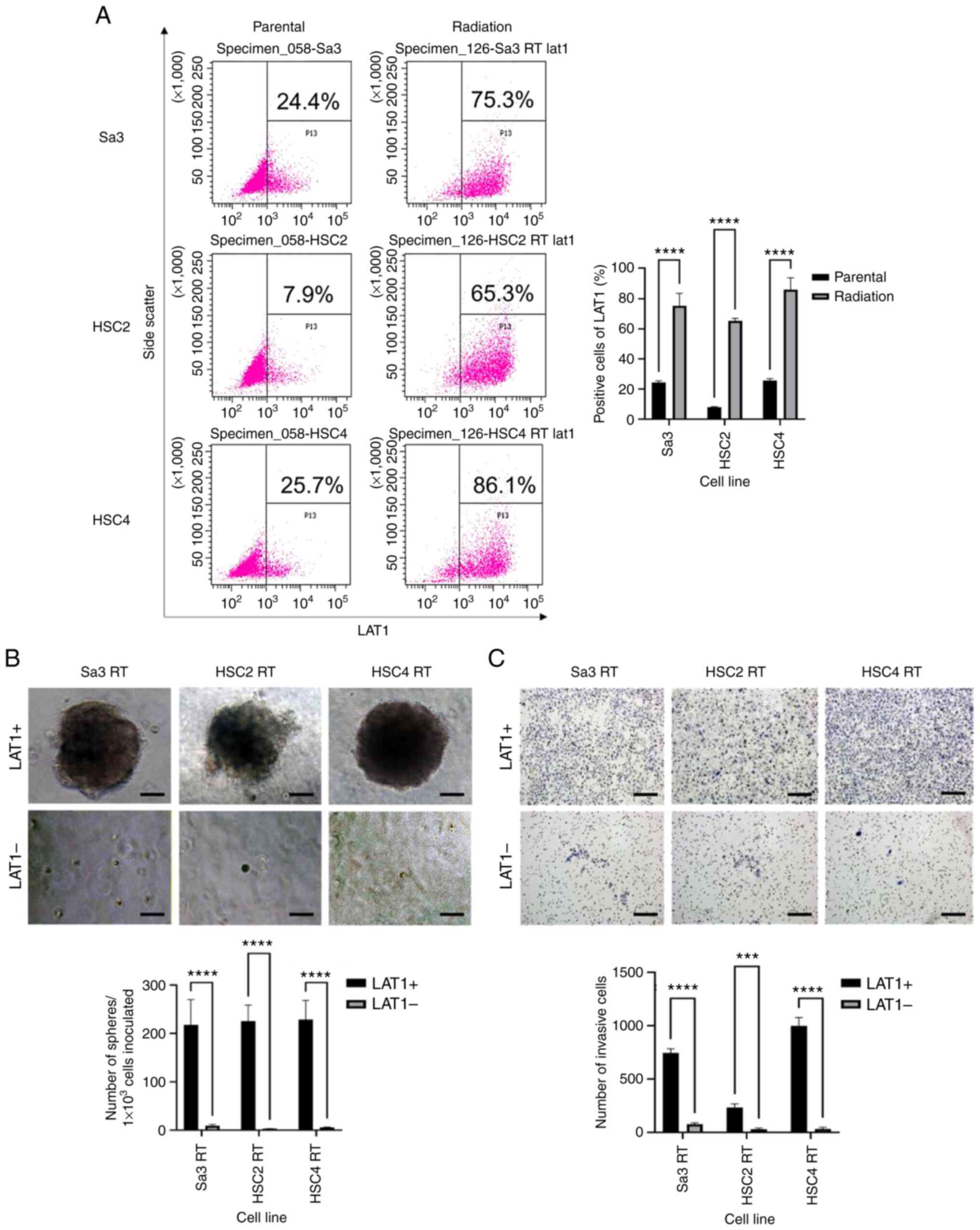
expression of LAT1 in three cell lines, Sa3, HSC2, and HSC4, after
irradiation with 60 Gy. The LAT1-positive fraction increased from
10–20% before irradiation to 60–80% after irradiation (Fig. 4A). After irradiation with 60 Gy,
cells were separated into LAT1-positive and LAT1-negative cells
using flow cytometry and cultured in serum-free semifluid medium.
The LAT1-positive cells exhibited enhanced spheroid-forming and
invasive abilities (Fig. 4B and C),
indicating that radioresistant cells had high malignant
potential.
LAT1 inhibition reduces the
LAT1-positive fraction of normal and radioresistant cells and
reduces the malignant potential
JPH203 was added to RPMI 1640 and incubated for 1
day, after which the LAT1-positive fractions were compared.
Treatment with JPH203 reduced the fraction of LAT1-positive cells
from 10–20% to 2–5% (Fig. 5A). In
radioresistant cells (irradiated as described above), JPH203
reduced the LAT1-positive fraction from 80 to 20–40% (Fig. 5B). Thus, JPH203 reduced the
LAT1-positive fraction in both the parental and radioresistant
cells.
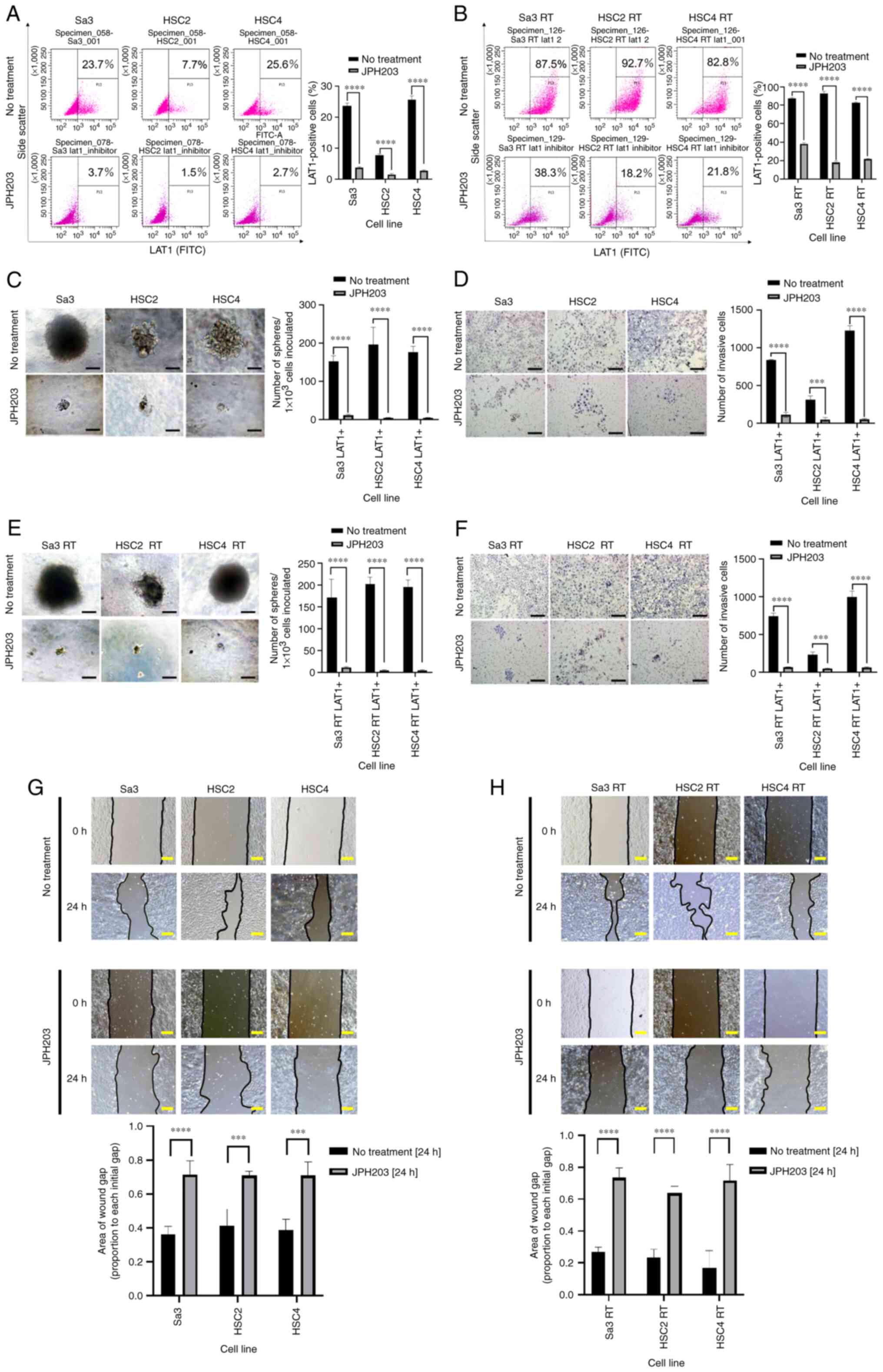 | Figure 5.(A and B) Reduction in LAT1-positive
cells in response to JPH203. Cells were incubated with JPH203 in
RPMI 1640 medium for 24 h, and LAT1 expression was examined by flow
cytometry. (A) In the parental cell lines, the LAT1-positive
fraction decreased from 10–20% to 2–5%. (B) In the radioresistant
cell lines, the LAT1-positive fraction decreased from 60–80% to
20–40%. (C-F) Inhibition of spheroid formation and invasiveness by
JPH203 in both the LAT1-positive parental cells and radioresistant
cells. (C) Spheroid formation and (D) invasion of the parental cell
lines were examined after adding JPH203 to the culture medium.
Spheroid formation and invasion of LAT1-positive cells were
significantly suppressed. In the radioresistant cells, JPH203 was
added to RPMI 1640 medium, and spheroid formation and invasion were
examined. (E) Spheroid-formation and (F) invasion were
significantly suppressed, indicating that JPH203 was effective
against radioresistant cells. (G and H) In the presence of JPH203,
wound healing was retarded in both parental and radioresistant
cells compared with their corresponding untreated groups. Cells
were grown to 90% confluency in a 60-mm dish, and a 500-µm-straight
line was drawn using a 200-µl pipet tip to perform a 24-h wound
healing assay. (C, E, and G) Scale bar, 100 µm. (D and F) Scale
bar, 200 µm. (H) Scale bar, 120 µm. ***P<0.001, ****P<0.0001.
LAT1, L-type amino acid transporter 1; LAT1+, LAT1-positive cell;
RT, radiotolerant. |
Parental and radioresistant cells were separated
into LAT1-positive and LAT1-negative cells and cultured in
serum-free semifluid medium supplemented with JPH203. After JPH203
treatment, the spheroid formation ability was not significantly
different between LAT1-positive and LAT1-negative cells (Fig. 5C and D) Similarly, no differences in
invasive ability were detected between LAT1-positive and
LAT1-negative cells after JPH203 treatment in both normal and
radioresistant cells (Fig. 5E and
F).
JPH203 inhibits the migratory ability
of normal and radioresistant cells
Wound healing assays were performed in both parental
and radioresistant cells. JPH203 was effective in inhibiting the
migration in both the parental (Fig.
5G) and radioresistant cells (Fig.
5H).
Discussion
We demonstrated that LAT1 is strongly involved in
sphere formation, invasion, and migration in HNSCC. Furthermore,
patients with LAT1-positive specimens had a worse prognosis and
were more resistant to chemoradiotherapy compared to patients with
low LAT1 expression. JPH203, a LAT1 inhibitor, suppressed sphere
formation, invasion, and migration in radioresistant cells.
We have previously reported that CD98hc is a marker
for cancer stem cells in HNSCC (24), and similar reports have been
published by other investigators (28,29).
Since CD98hc binds to amino acid transporters in the light chain,
LAT1-positive cells may have cancer stem cell characteristics.
LAT1-positive cells can form spheres in serum-free semifluid
medium, which is a characteristic of cancer stem cells (30). Although other stem cell markers,
such as Oct3/4, Nanog, and SOX2, need to be investigated, LAT1 may
be an important therapeutic target because it induces
chemoradiotherapy resistance.
The mTOR signaling pathway plays an important role
in invasion and migration. Amino acids, including leucine or amino
acid prodrugs, are transported into cells by LAT1 and cause
activation of mTORC1, resulting in enhanced invasion and migration
(31). In our study, the enhanced
invasion and migration of LAT1-positive cells may result from the
activation of mTOR signaling.
According to the LAT1 immunostaining of HNSCC
patient biopsies, high LAT1 expression was associated with poor
prognosis and chemoradiotherapy resistance. In a previous report,
high LAT1 expression was associated with an extremely poor
prognosis in resected tongue cancer (32). However, in our study, no significant
differences were detected in the LAT1 expression groups after
surgical treatment. The lack of differences may be due to the
staging based on clinical imaging diagnosis rather than
pathological indicators and grouping head and neck cancers
together. We believe that the ability to predict chemoradiotherapy
resistance at the biopsy stage based on LAT1 expression is a
significant finding of this study.
About half of HNSCC patients relapse after
chemoradiotherapy or surgery, and immune checkpoint inhibitors have
achieved some success. However, their efficacy is limited, and
HNSCC remains a disease with a poor prognosis (33). Therefore, JPH203, a LAT1 inhibitor,
is expected to be a new therapeutic agent. The expression of LAT1
increased from 10–20% to 60–80% after irradiation. Sphere
formation, invasion, and migration are enhanced in LAT1-positive
cells, even in radioresistant cell lines. The high fraction of
LAT1-positive cells in resistant cell lines indicates high
malignancy (34). Recurrent tumors
may need to utilize more amino acids to survive and proliferate;
however, the mechanism must be clarified.
JPH203 suppressed sphere formation, invasion, and
migration in both the parental and radioresistant cells. After the
addition of JPH203, the LAT1-positive cell fraction was noted to
decrease to 2–5% in the parental cells and significantly decreased
to 20–40% in the radioresistant cells. The expression of LAT1 was
also suppressed by BCH, which is an inhibitor of LAT1 and LAT2.
However, the expression of LAT1 is upregulated by feedback with
prolonged exposure to JPH203 (35).
In this study, the results were obtained after 24 h. The long-term
expression of LAT1 requires further investigation.
In HNSCC, LAT1-positive cells are highly malignant
and capable of sphere formation, invasion, and migration. Targeting
these cells will improve the prognosis of HNSCC. Furthermore, LAT1
expression at the biopsy stage can be used to determine
radiosensitivity. This will play an important role in designing
tailor-made treatment strategies. For instance, patients with high
LAT1 expression can undergo surgery first, whereas patients with
low LAT1 expression can undergo chemoradiation first. JPH203
concomitant radiation therapy may be an alternative to
platinum-based agents. JPH203 may also be an effective treatment
for recurrent tumors that have become radioresistant, and the
availability of other options, in addition to nivolumab and
pembrolizumab, will improve the prognosis of HNSCC patients. In
addition to treatment, 18F-FAMT, a LAT1-selective amino
acid PET, has been found to be effective in cancer diagnosis
(36). In the HNSCC field, the
function of LAT1 needs to be clarified urgently and actively
applied in the future.
In conclusion, LAT1-positive cells in HNSCC are
those with enhanced spheroid formation, invasion, and migration, as
well as those in radioresistant cell lines. Immunostaining of HNSCC
patient specimens showed that LAT1 is an independent prognostic
factor and resistant to chemoradiotherapy. JPH203, a LAT1
inhibitor, could strongly suppress spheroid formation, invasion,
and migration of LAT1 positive cells. Therefore, JPH203 should also
be used in the field of HNSCC, as LAT1 is a prognostic factor and
can be used to predict therapeutic efficacy.
Acknowledgements
The authors would like to thank Mr. Yusuke Ono
(Department of Molecular and Tumour Pathology, Akita University
Graduate School of Medicine, Akita, Japan) and Ms. Reiko Ito
(Department of Molecular and Tumour Pathology, Akita University
Graduate School of Medicine, Akita, Japan) for their technical
assistance, and Ms. Eriko Kumagai (Department of Molecular and
Tumour Pathology, Akita University Graduate School of Medicine,
Akita, Japan) for her secretarial work.
Funding
The present study was supported by JSPS KAKENHI (grant nos.
18K09311 and 19K07497).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YK and YO designed the outline of the study. HS, YK,
MM, HH, SS, TY, MS and AI conducted the experiments and data
analyses. YK, SH and YO confirmed the authenticity of all raw data.
YK and YO interpreted the data and wrote the draft. YO revised the
draft before the submission. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients. All procedures used in this research were approved by the
Ethical Committee of Akita University Hospital (approval no. 2532;
Akita, Japan). The study was performed according to the Declaration
of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
HR
|
hazard ratio
|
|
LAT1
|
L-type amino acid transporter 1
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pignon JP, Le Maître A, Maillard E and
Bourhis J; MACH-NC Collaborative Group, : Meta-analysis of
chemotherapy in head and neck cancer (MACH-NC): An update on 93
randomised trials and 17,346 patients. Radiother Oncol. 92:4–14.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Begg AC: Predicting recurrence after
radiotherapy in head and neck cancer. Semin Radiat Oncol.
22:108–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Department of Veterans Affairs Laryngeal
Cancer Study Group, . Wolf GT, Fisher SG, Hong WK, Hillman R,
Spaulding M, Laramore GE, Endicott JW, McClatchey K and Henderson
WG: Induction chemotherapy plus radiation compared with surgery
plus radiation in patients with advanced laryngeal cancer. N Engl J
Med. 324:1685–1690. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forastiere AA, Goepfert H, Maor M, Pajak
TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et
al: Concurrent chemotherapy and radiotherapy for organ preservation
in advanced laryngeal cancer. N Engl J Med. 349:2091–2098. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bernier J, Domenge C, Ozsahin M,
Matuszewska K, Lefèbvre JL, Greiner RH, Giralt J, Maingon P,
Rolland F, Bolla M, et al: Postoperative irradiation with or
without concomitant chemotherapy for locally advanced head and neck
cancer. N Engl J Med. 350:1945–1952. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Corvò R: Evidence-based radiation oncology
in head and neck squamous cell carcinoma. Radiother Oncol.
85:156–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Solomon B, Young RJ, Bressel M, Urban D,
Hendry S, Thai A, Angel C, Haddad A, Kowanetz M, Fua T, et al:
Prognostic significance of PD-L1+ and CD8+
immune cells in HPV+ oropharyngeal squamous cell
carcinoma. Cancer Immunol Res. 6:295–304. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dovedi SJ, Adlard AL, Lipowska-Bhalla G,
Mckenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M,
Stewart R, et al: Acquired resistance to fractionated radiotherapy
can be overcome by concurrent PD-L1 blockade. Cancer Res.
74:5458–5468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanai Y, Segawa H, Miyamoto Ki, Uchino H,
Takeda E and Endou H: Expression cloning and characterization of a
transporter for large neutral amino acids activated by the heavy
chain of 4F2 antigen (CD98). J Biol Chem. 273:23629–23632. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Babu E, Kanai Y, Chairoungdua A, Kim DK,
Iribe Y, Tangtrongsup S, Jutabha P, Li Y, Ahmed N, Sakamoto S, et
al: Identification of a novel system L amino acid transporter
structurally distinct from heterodimeric amino acid transporters. J
Biol Chem. 278:43838–43845. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Utsunomiya-Tate N, Endou H and Kanai Y:
Cloning and functional characterization of a system ASC-like
Na+-dependent neutral amino acid transporter. J Biol Chem.
271:14883–14890. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sloan JL and Mager S: Cloning and
functional expression of a human Na(+) and Cl(−)-dependent neutral
and cationic amino acid transporter B(0+). J Biol Chem.
274:23740–23745. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sato H, Tamba M, Ishii T and Bannai S:
Cloning and expression of a plasma membrane cystine/glutamate
exchange transporter composed of two distinct proteins. J Biol
Chem. 274:11455–11458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee Y, Wiriyasermkul P, Jin C, Quan L,
Ohgaki R, Okuda S, Kusakizako T, Nishizawa T, Oda K, Ishitani R, et
al: Cryo-EM structure of the human L-type amino acid transporter 1
in complex with glycoprotein CD98hc. Nat Struct Mol Biol.
26:510–517. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nawashiro H, Otani N, Shinomiya N, Fukui
S, Ooigawa H, Shima K, Matsuo H, Kanai Y and Endou H: L-type amino
acid transporter 1 as a potential molecular target in human
astrocytic tumors. Int J Cancer. 119:484–492. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaira K, Oriuchi N, Imai H, Shimizu K,
Yanagitani N, Sunaga N, Hisada T, Tanaka S, Ishizuka T, Kanai Y, et
al: Prognostic significance of L-type amino acid transporter 1
expression in resectable stage I–III nonsmall cell lung cancer. Br
J Cancer. 98:742–748. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaira K, Oriuchi N, Shimizu K, Ishikita T,
Higuchi T, Imai H, Yanagitani N, Sunaga N, Hisada T, Ishizuka T, et
al: Evaluation of thoracic tumors with (18)F-FMT and (18)F-FDG
PET-CT: A clinicopathological study. Int J Cancer. 124:1152–1160.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakata T, Ferdous G, Tsuruta T, Satoh T,
Baba S, Muto T, Ueno A, Kanai Y, Endou H and Okayasu I: L-type
amino-acid transporter 1 as a novel biomarker for high-grade
malignancy in prostate cancer. Pathol Int. 59:7–18. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Furuya M, Horiguchi J, Nakajima H, Kanai Y
and Oyama T: Correlation of L-type amino acid transporter 1 and
CD98 expression with triple negative breast cancer prognosis.
Cancer Sci. 103:382–389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaira K, Sunose Y, Arakawa K, Ogawa T,
Sunaga N, Shimizu K, Tominaga H, Oriuchi N, Itoh H, Nagamori S, et
al: Prognostic significance of L-type amino-acid transporter 1
expression in surgically resected pancreatic cancer. Br J Cancer.
107:632–638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oda K, Hosoda N, Endo H, Saito K,
Tsujihara K, Yamamura M, Sakata T, Anzai N, Wempe MF, Kanai Y and
Endou H: L-type amino acid transporter 1 inhibitors inhibit tumor
cell growth. Cancer Sci. 101:173–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawasaki Y, Omori Y, Suzuki S and Yamada
T: CD98hc as a marker of radiotherapy-resistant cancer stem cells
in head and neck squamous cell carcinoma. Arch Med Sci. 2020.
View Article : Google Scholar
|
|
25
|
Rietbergen MM, Martens-De Kemp SR,
Bloemena E, Witte BI, Brink A, Baatenburg de Jong RJ, Leemans CR,
Braakhuis BJ and Brakenhoff RH: Cancer stem cell enrichment marker
CD98: A prognostic factor for survival in patients with human
papillomavirus-positive oropharyngeal cancer. Eur J Cancer.
50:765–773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi DW, Kim DK, Kanai Y, Wempe MF, Endou
H and Kim JK: JPH203, a selective L-type amino acid transporter 1
inhibitor, induces mitochondria-dependent apoptosis in Saos2 human
osteosarcoma cells. Korean J Physiol Pharmacol. 21:599–607. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM classification of malignant tumours. 8th edition.
Wiley Blackwell; Oxford: pp. 17–54. 2017
|
|
28
|
Digomann D, Kurth I, Tyutyunnykova A, Chen
O, Löck S, Gorodetska I, Peitzsch C, Skvortsova II, Negro G,
Aschenbrenner B, et al: The CD98 heavy chain is a marker and
regulator of head and neck squamous cell carcinoma
radiosensitivity. Clin Cancer Res. 25:3152–3163. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martens-De Kemp SR, Brink A, Stigter-Van
Walsum M, Damen JM, Rustenburg F, Wu T, van Wieringen WN,
Schuurhuis GJ, Braakhuis BJ, Slijper M and Brakenhoff RH: CD98
marks a subpopulation of head and neck squamous cell carcinoma
cells with stem cell properties. Stem Cell Res. 10:477–488. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kawasaki Y, Omori Y, Li Q, Nishikawa Y,
Yoshioka T, Yoshida M, Ishikawa K and Enomoto K: Cytoplasmic
accumulation of connexin32 expands cancer stem cell population in
human HuH7 hepatoma cells by enhancing its self-renewal. Int J
Cancer. 128:51–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo W, Zhang H, Zhang Y, Liang P, Wang X,
Ma J, Tan D, Tan Y, Song J, Ji P and Zhao T: L-type amino acid
transporter 1 promotes proliferation and invasion of human
chorionic trophoblast and choriocarcinoma cells through mTORC1. Am
J Transl Res. 12:6665–6681. 2020.PubMed/NCBI
|
|
32
|
Toyoda M, Kaira K, Ohshima Y, Ishioka NS,
Shino M, Sakakura K, Takayasu Y, Takahashi K, Tominaga H, Oriuchi
N, et al: Prognostic significance of amino-acid transporter
expression (LAT1, ASCT2, and xCT) in surgically resected tongue
cancer. Br J Cancer. 110:2506–2513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
So YK, Byeon SJ, Ku BM, Ko YH, Ahn MJ, Son
YI and Chung MK: An increase of CD8+ T cell infiltration
following recurrence is a good prognosticator in HNSCC. Sci Rep.
10:200592020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kawasaki Y, Omori Y and Yamada T:
Increased expression of CD44v9, a cancer stem cell marker, in head
and neck squamous cell carcinoma cells after irradiation. Int J
Cancer Oncol. 4:225–230. 2017.
|
|
35
|
Cormerais Y, Pagnuzzi-Boncompagni M,
Schrötter S, Giuliano S, Tambutté E, Endou H, Wempe MF, Pagès G,
Pouysségur J and Picco V: Inhibition of the amino-acid transporter
LAT1 demonstrates anti-neoplastic activity in medulloblastoma. J
Cell Mol Med. 23:2711–2718. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wiriyasermkul P, Nagamori S, Tominaga H,
Oriuchi N, Kaira K, Nakao H, Kitashoji T, Ohgaki R, Tanaka H, Endou
H, et al: Transport of 3-fluoro-L-α-methyl-tyrosine by
tumor-upregulated L-type amino acid transporter 1: A cause of the
tumor uptake in PET. J Nucl Med. 53:1253–1261. 2012. View Article : Google Scholar : PubMed/NCBI
|