Introduction
Osteosarcoma (OS) is the most common primary bone
tumor with an incidence of 0.3 cases/100,000 individuals/year
(1,2), with adolescents exhibiting a higher
incidence (3). OS is an aggressive
cancer characterized by early metastasis, high-grade malignancy,
and a poor prognosis (4,5). Treatment strategies for chemotherapy
and surgical surgery have enabled the disease-free survival rate to
significantly increase to 60% for localized OS (1). However, for patients with axial OS,
poor chemosensitivity, or metastatic OS, the prognosis is poor, and
the 5-year survival rate is 20–25% (1,6).
Over the past three decades, little progress has
been made in the treatment of OS (7). The most recent advances in OS
treatment came in the 1980s, when multi-agent chemotherapy was
shown to improve overall survival compared with surgery alone
(8). Therefore, there is a
necessity to investigate OS therapies to offer novel perspectives
on therapeutic management. Previous advances in immunotherapy have
highlighted new avenues for the treatment of cancer (9). A form of antitumor therapy known as
immunotherapy uses the host's own defense mechanisms to combat
cancer. Previously, immunotherapy has demonstrated outstanding
therapeutic effectiveness against a number of cancers (10). Cancer immunotherapy, which aims to
utilize the immune system to combat the tumor, has attracted wide
attention (11,12). Immune mechanisms including
cytokines, immune checkpoint inhibitors, tumor vaccines and
cellular immunotherapy may assist the host's defense against cancer
(13,14).
Numerous types of immune cells, including leucocytes
of a myeloid lineage, macrophages, helper T cells, cytotoxic T
cells, regulatory T cells, B cells, and dendritic cells, reside in
the tumor microenvironment (15).
Inter alia, in the tumor microenvironment, T-lymphocytes and
macrophages are the most common immune cells (16,17).
Previously, the research on utilizing macrophages
for combating tumors has opened a new window in the field of tumor
immunotherapy (18). Macrophages
are an important part of the innate immune system and play an
essential role in mediating the host's defense against infection
and cancer (19). Cancer
immunotherapy possesses considerable potential when
macrophage-mediated immunity is successfully activated (20). However, by producing a ‘do not eat
me’ signal using CD47 binding to signal regulatory protein α
(SIRPα) receptors on macrophages, tumor cells can prevent against
phagocytosis by macrophages (21).
CD47 checkpoint inhibitors have been preliminarily
shown to enhance macrophage-mediated phagocytosis of tumor cells,
but their effects have been limited (22). Additionally, as with other
checkpoint inhibitors, these antagonists need refinements to
improve their clinical value rate and objective remission rates
(23). Resolving these issues is
critical for paving the way for clinical trials on CD47-SIRPα based
immunotherapy. Previous tumor immunology research suggested that
the reduced effectiveness of CD47-SIRPα blockers may be due to the
presence of an immunosuppressive tumor microenvironment (24). Cancer cells in particular release
colony-stimulating factors, which polarize tumor-associated
macrophages (TAMs) toward the M2 phenotype (25,26).
M2 macrophages can recruit regulatory T cells to prevent T cell
immunological activation generated by CD47 blockage, as well as
enhancing tumor angiogenesis, which can contribute to tumor growth
and metastasis. M1 macrophages, conversely, play an important role
in innate host defense and tumor cell death by generating reactive
oxygen species (ROS), reactive nitrogen, and proinflammatory
cytokines (including interleukin-6 and tumor necrosis factor-α)
(27,28). Therefore, stimulating macrophages to
polarize towards the M1 phenotype, and increasing the proportion of
M1 relative to M2 macrophages in tumor tissues may significantly
improve the therapeutic effect of CD47 blockers.
Toll-like receptors (TLRs) are expressed in several
tissues and tumors and are important molecules in cancer evasion of
immune surveillance (29).
Activation of TLRs on macrophages can promote their immune
response, including secretion of pro-inflammatory cytokines and
enhancement of phagocytosis (30).
Therefore, local TLR activation is a popular antitumor
immunotherapy (31).
Lipopolysaccharides (LPS) are components of the outer wall of the
cell wall of Gram-negative bacteria and consist of lipids and
polysaccharides (32). LPS is also
the main ligand of TLR4, which can effectively activate macrophages
to transform towards an M1 phenotype to exert antitumor immune
effects (33).
In certain solid tumors, macrophages account for
half of the total tumor weight (34). The higher the content of
macrophages, the worse the prognosis of tumors, which indicates
that macrophages play a very important role in the occurrence and
development of tumors (35). Immune
targeting of macrophages to tumors has become an encouraging
therapeutic strategy. However, there are two major obstacles to
effective macrophage activation for antitumor immunotherapy:
Promoting M1 transformation of macrophages and anti-SIRPα-CD47
signaling. Therefore, it is hypothesized that simultaneously
stimulating macrophages to transform towards an M1 phenotype and
blocking the CD47-SIRP α signaling pathway can significantly
improve the antitumor effect of macrophages. In the present study,
the combined use of LPS and CD47mAb effectively activated
macrophages and significantly improved the anti-OS effect of
macrophages, providing novel avenues for tumor immunotherapy.
Materials and methods
Human specimens
In the present study, four OS specimens and one
normal bone specimen were obtained from the Department of
Orthopedics, The First Affiliated Hospital of Anhui Medical
University, from October 2019 to October 2021. All the OS specimens
used were puncture specimens of primary patients (two male and two
female; aged between 13 and 55), and the pathological results
showed that all of them were common OS. The main feature of common
OS is the direct formation of bone-like matrix by tumor cells
(36). Regrettably, these OS
specimens were not graded, which is a potential limitation to the
present study. Specimens were treated in accordance with the
guidelines in the Declaration of Helsinki (37). The present study was approved
(approval no. PJ2022-10-15) by the Clinical Medical Research Ethics
Committee of The First Affiliated Hospital of Anhui Medical
University (Fuyang, China).
Cell culture
All the cells (RAW264.7, K7M2, U2 OS, MG63) were
purchased from Wuhan Procell Life Technology Co., Ltd. Cells were
cultured in DMEM (Biological Industries) supplemented with 10% FBS
(Wisent Biotechnology), 100 U/ml penicillin (Beyotime Institute of
Biotechnology), and 100 µg/ml streptomycin (Beyotime Institute of
Biotechnology), at 37°C in a humidified incubator supplied with 5%
CO2. K7M2 OS cells and RAW264.7 macrophages were mainly
used for experiments in the present study. K7M2 cells instead of
the other OS cell lines were used in combination with RAW264.7
macrophages, because they are both of mouse origin.
Cell viability
Cell viability was measured using a Cell Counting
Kit-8 (CCK-8) assay. Briefly, K7M2 OS cells were seeded at a
density of 1×104 cells per well in 96-well plates in
quintuplicate and incubated with LPS (0.1 µg/ml) (MilliporeSigma),
CD47mAb (10 µg/ml) (Bio X Cell, clone MIAP301), or a combination of
both for 48 h. Subsequently, 10 µl of WST-8 reagent (Dalian Meilun
Biology Technology Co., Ltd.) was added to each well. After
incubation at 37°C for 1 h, the absorbance was measured with an
optical density of 450 nm using a Microplate Reader (Tecan Group,
Ltd.).
Cell proliferation analysis
For cell proliferation analysis, K7M2 cells were
pre-stained with 4 µM of the cell proliferation dye, CFSE
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. K7M2 cells and RAW264.7 cells were
co-cultured in 12-well double-compartment culture plates
(LABSELECT). These co-culture systems possess a membrane with a
pore diameter of 0.4 µm between the upper chamber and the lower
chamber of the culture plate, which does not allow cells to pass
through, but factors secreted by cells can pass through. A total of
2×105 K7M2 cells were added to the lower chamber and
1×106 RAW264.7 cells were added to the upper chamber.
After incubation with LPS (0.1 µg/ml) or CD47mAb (10 µg/ml) for 24
h, the K7M2 cells in the lower chamber were collected and washed
with PBS. Then the fluorescence intensity was detected by Flow
Cytometry (BD Biosciences).
Phagocytosis assay
To measure the phagocytic ability of macrophages
precisely, K7M2 and RAW264.7 cells were respectively
fluorescence-labeled with CFSE (Invitrogen; Thermo Fisher
Scientific, Inc.) and the Cell Proliferation Dye eFluor 670
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 10 min.
After washing with PBS, K7M2 cells were co-cultured with RAW264.7
cells at a ratio of 2:1 and incubated with 0.1 µg/ml LPS, 10 µg/ml
CD47mAb, or 0.1 µg/ml LPS + 10 µg/m CD47mAb for 48 h in a 12-well
plate. Subsequently, the cells were collected, washed with PBS
twice, and analyzed using a flow cytometer (Beckman Coulter, Inc.).
The same method was used to detect the results of the treatment
groups with or without 5 µM/l N-acetyl-cysteine (NAC; Beijing
Solarbio Science & Technology Co., Ltd.). The relative
phagocytosis index was calculated using FlowJo 10.8.1 (FlowJo LLC)
based on the proportion of CFSE and Dye 670 double positive
cells.
Laser confocal microscopy
To assess phagocytosis visually, K7M2 and RAW264.7
cells were co-cultured as aforementioned. The co-cultured cells
were added to a 24-well plate with round coverslips and cultured
for 48 h. Cells that had grown on the coverslips were fixed using
4% formaldehyde at room temperature for 30 min, and counterstained
using 10 µg/ml DAPI (Beijing Solarbio Science & Technology Co.,
Ltd.), for 10 min, followed by mounting in anti-fluorescence
quencher (Beijing Solarbio Science & Technology Co., Ltd.).
Finally, images were obtained by laser confocal microscopy (×63
magnification) (Leica GmbH).
Histopathological analysis
A total of four OS specimens and one normal bone
tissue specimen were collected and fixed with 4% paraformaldehyde
for 24 h. The paraffin-embedded dried tissues were sliced into 4-µm
thick slices. For histological analysis, tissue slices were stained
with hematoxylin and eosin, and images were captured using a light
microscope (×20 magnification).
Immunofluorescence staining
The antibodies used for immunofluorescence staining
were: CD47 Rabbit pAb (1:100; cat. no. 380870; ZENBIO), CD206
Rabbit mAb (1:100; cat. no. ET1702-04; HUABIO) or CD68 Mouse mAb
(1:100; cat. no. 250019; ZENBIO), and Goat Anti-Rabbit Alexa Fluor
488 secondary antibody (1:100; cat. no. M21012M; Abmart
Pharmaceutical Technology Co., Ltd.) or Goat Anti-Mouse Alexa Fluor
488 secondary antibody (1:100; cat. no. E-AB-1104; Elabscience
Biotechnology, Inc.). For immunohistochemistry, the
paraffin-embedded OS tissues and normal bone tissues were dewaxed
using dimethyl benzene for 10 min twice and rehydrated in a
descending alcohol series. Antigen retrieval was performed by
heating at 100°C in citrate buffer for 20 min and blocked using 5%
BSA (Bestbio; Nanjing Fengfeng Biomedical Technology Co., Ltd.) for
1 h. Then, the sections were incubated at 4°C overnight with the
primary antibody. The following day, the sections were incubated at
room temperature for 2 h with the secondary antibody. The nuclei
were counterstained with 10 µg/ml DAPI (Beyotime Institute of
Biotechnology) at room temperature for 10 min. After each step, the
sections were washed with PBS three times. Finally, the sections
were mounted in an anti-fluorescence quenching agent, and images
were obtained by fluorescence microscopy (×20 magnification).
ImageJ 1.53K (National Institutes of Health) was used to analyze
the fluorescence area of images. Immunocytochemistry was performed
as previously described (38).
Briefly, cells were plated in a 24-well plate, and after adhesion,
were fixed with 4% paraformaldehyde at room temperature for 30 min,
and immunofluorescence was performed as aforementioned. Images of
the cells were captured using a fluorescence microscope (×20
magnification).
Western blotting
Western blot analysis was performed as previously
described (39). Briefly, RIPA
lysis Buffer (Beyotime Institute of Biotechnology) was used to lyse
tumor cells and extract proteins. Lysates from each sample were
denatured and then loaded (30 µg/lane; protein concentration
determined by a DeNovix DS-11 spectrophotometer) onto 4–20%
SDS-gels, resolved using SDS-PAGE, transferred to PVDF membranes,
and blocked in non-fat milk at room temperature for 1.5 h, then
incubated with either NOS2 Rabbit pAb (1:200; cat. no. WL0992a;
Wanleibio Co., Ltd.) or rabbit anti-β-actin (1:1,000; cat. no.
AF7018; Affinity Biosciences) at 4°C overnight. The following day,
the membrane was incubated with horseradish peroxidase-conjugated
secondary antibody (1:10,000; cat. no. S0001; Affinity Biosciences)
at room temperature for 2 h. Finally, signals were visualized using
ECL super western blot detection reagent (Abmart Pharmaceutical
Technology Co., Ltd.). The relative expression of each protein was
calculated using ImageJ (version 1.53k; National Institutes of
Health) with β-actin as an internal reference.
Cell migration assay
Cell migration assays were performed using 24-well
Transwell plates with 8-µm pores (Corning, Inc.). A total of
2×105 K7M2 cells in 200 µl serum-free DMEM was added to
the upper chamber, and 600 µl supplemented media containing
1×105 RAW264.7 cells, or no cells as a control, was
added to the lower chamber. After 24 h of incubation with LPS (0.1
µg/ml) or CD47mAb (10 µg/ml), K7M2 cells were fixed using 4%
paraformaldehyde at room temperature for 30 min and stained using
0.1% crystal violet for 30 min at room temperature. Three visual
fields were randomly selected to count migratory cells under a
fluorescence microscope (×40 magnification).
ELISA
RAW264.7 cells were incubated with or without LPS
(0.1 µg/ml) or CD47mAb (10 µg/ml) for 24 h. The levels of TNF-α in
the cell culture supernatant of cells subjected to different
treatments were measured using a specific ELISA kit (cat. no.
m1002095-2; Shanghai Enzyme-linked Biotechnology Co., Ltd.)
according to the manufacturer's instructions.
Detection of NO
RAW264.7 cells were incubated with or without LPS
(0.1 µg/ml) or CD47mAb (10 µg/ml) for 24 h. NO was measured using a
commercial kit (cat. no. BC1470; Beijing Solarbio Science &
Technology Co., Ltd.) according to the manufacturer's protocol.
Absorbance was measured at an optical density of 550 nm using a
microplate reader.
Detection of CD86 and ROS levels
K7M2 cells and RAW264.7 cells were co-cultured as
aforementioned for the cell proliferation analysis, except with
2×106 RAW264.7 cells. After incubation with LPS (0.1
µg/ml) or CD47mAb (10 µg/ml) for 24 h, the macrophages in the lower
chamber were collected and washed with PBS (500 × g, 4°C, 5 min).
The washed cells were resuspended in 50 µl PBS and incubated with 1
µl CD86 flow antibody (cat. no. 564198; BD Biosciences) for 30 min
at 4°C, and subsequently washed with PBS. CD86 expression was
determined using flow cytometry. Cells were collected and ROS
levels were measured using an ROS detection kit (cat. no. S0033S;
Beyotime Institute of Biotechnology) according to the
manufacturer's protocol.
Apoptosis determination
In order to verify the apoptotic effect of LPS and
CD47mAb on OS cells, K7M2 OS cells were cultured with LPS or
CD47mAb in 12-well plates for 48 h. Then, K7M2 cells were collected
and washed with PBS (500 × g, 4°C, 5 min), and the proportion of
apoptosis was detected by Flow Cytometry (BD Biosciences). Next, to
verify the effect of polarized macrophages on the apoptosis of OS
cells, K7M2 cells and RAW264.7 cells were co-cultured in 12-well
double-compartment culture plates (LABSELECT). A total of
2×105 K7M2 cells were added to the lower chamber and
1×106 RAW264.7 cells were added to the upper chamber.
After incubation with LPS (0.1 µg/ml) or CD47mAb (10 µg/ml) for 24
h, the K7M2 cells in the lower chamber were collected and washed
with PBS. Apoptosis was measured using Annexin V-FITC (cat. no.
BB-4101-100T; BestBio; Nanjing Fengfeng Biomedical Technology Co.,
Ltd.) according to the manufacturer's protocol. The apoptosis data
was calculated using FlowJo 10.8.1 (FlowJo LLC).
Statistical analysis
All experiments were repeated at least three times,
and data are presented as the mean ± SD. The data in Fig. 1 were analyzed using one-way ANOVA,
followed by Dunnett's test. The data in Fig. 3E were analyzed using unpaired
Student's t-test. The data in Figs.
4H and S2D were analyzed using
one-way ANOVA, followed by LSD test. Other data were analyzed using
one-way ANOVA, followed by Tukey's post hoc test. All statistical
analysis was performed using GraphPad Prism 9.0.2 (Dotmatics).
P<0.05 was considered to indicate a statistically significant
difference.
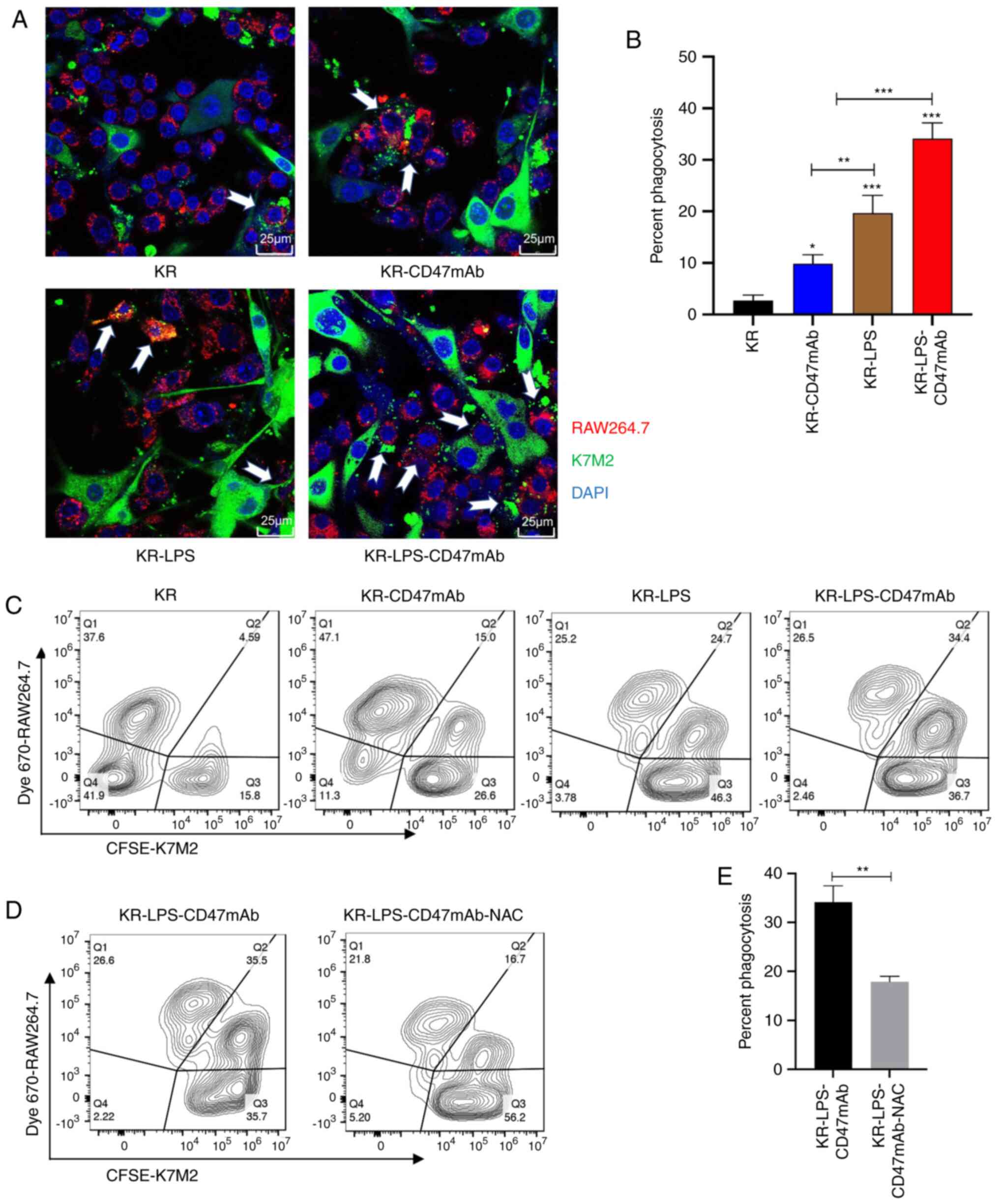 | Figure 3.Impact of LPS and CD47mAb on
phagocytosis. (A) CFSE-labeled K7M2 cells were co-cultured with Dye
670-labeled RAW264.7 cells and treated with LPS (0.1 µg/ml),
CD47mAb (10 µg/ml), or both. Phagocytosis was analyzed using a
laser confocal microscopy. White arrows indicate K7M2 cells
phagocytized and digested by macrophages (magnification, ×63) and
(B) corresponding quantitative phagocytosis. Phagocytosis was
calculated as follows: (number of macrophages with phagocytized
cancer cells/total number of macrophages) ×100%. (C) CFSE
pre-labeled K7M2 cells were co-cultured with Dye 670 pre-stained
RAW264.7 cells and incubated with LPS (0.1 µg/ml), CD47mAb (10
µg/ml), or both; phagocytosis (CFSE and Dye 670 positive cells) was
analyzed by flow cytometry. (D) The rate of phagocytosis following
treatment with the ROS scavenger NAC in the co-culture system was
detected using flow cytometry. (E) Quantification of phagocytosis
(CFSE and Dye 670 positive cells). Data are presented as the mean ±
SD of three independent repeats. *P<0.05, **P<0.01 and
***P<0.001 vs. KR or as otherwise indicated. K, K742; R,
RAW264.7; KR, K7M2 + RAW264.7; NAC, n-acetyl-cysteine; ROS,
reactive oxygen species; LPS, Lipopolysaccharide; CD47mAb, CD47
monoclonal antibody. |
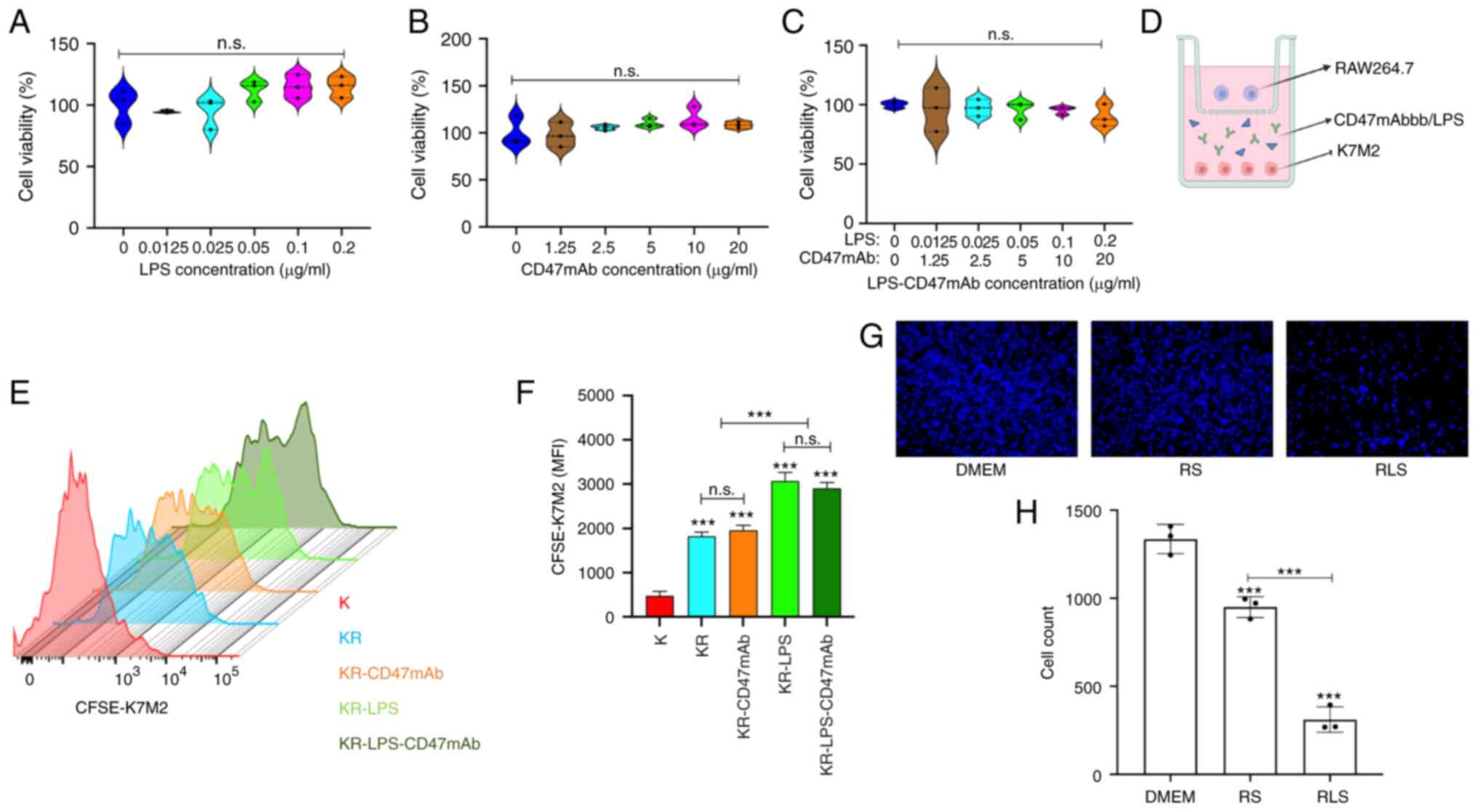 | Figure 4.LPS combined with CD47mAb inhibits
the proliferation of OS cells. (A-C) K7M2 cells were incubated with
LPS, CD47mAb, or both combined for 48 h and the cell viability was
analyzed using a Cell Counting Kit-8 assay. (D) Schematic diagram
of the K7M2 and RAW264.7 co-culture system. (E and F) CFSE
pre-stained K7M2 and RAW264.7 cells were co-cultured and
co-incubated with LPS, CD47mAb, or both for 24 h. The fluorescence
intensity of CFSE-K7M2 cells was detected by flow cytometry. The
greater the degree of proliferation, the weaker the fluorescence
intensity was. (G and H) RAW264.7 cells were incubated with or
without LPS for 24 h, after which cells were cultured in serum-free
DMEM for 24 h. The supernatant was collected and used to culture
K7M2 cells for 24 h. Subsequently, K7M2 cells were stained with
DAPI and images were captured. Magnification, ×10. DMEM group, K7M2
cells cultured with DMEM; RS, K7M2 cells cultured in RAW264.7 cell
culture supernatant; RLS, K7M2 cells cultured in RAW264.7 cell
culture supernatant treated with 0.1 µg/ml LPS. Data are presented
as the mean ± SD of three independent experiments. ***P<0.001
vs. K or as otherwise indicated. n.s., not significant; K, K7M2; R,
RAW264.7; KR, K7M2 + RAW264.7; LPS, lipopolysaccharide; OS,
osteosarcoma; CD47mAb, CD47 monoclonal antibody; MFI, Mean
Fluorescence Intensity. |
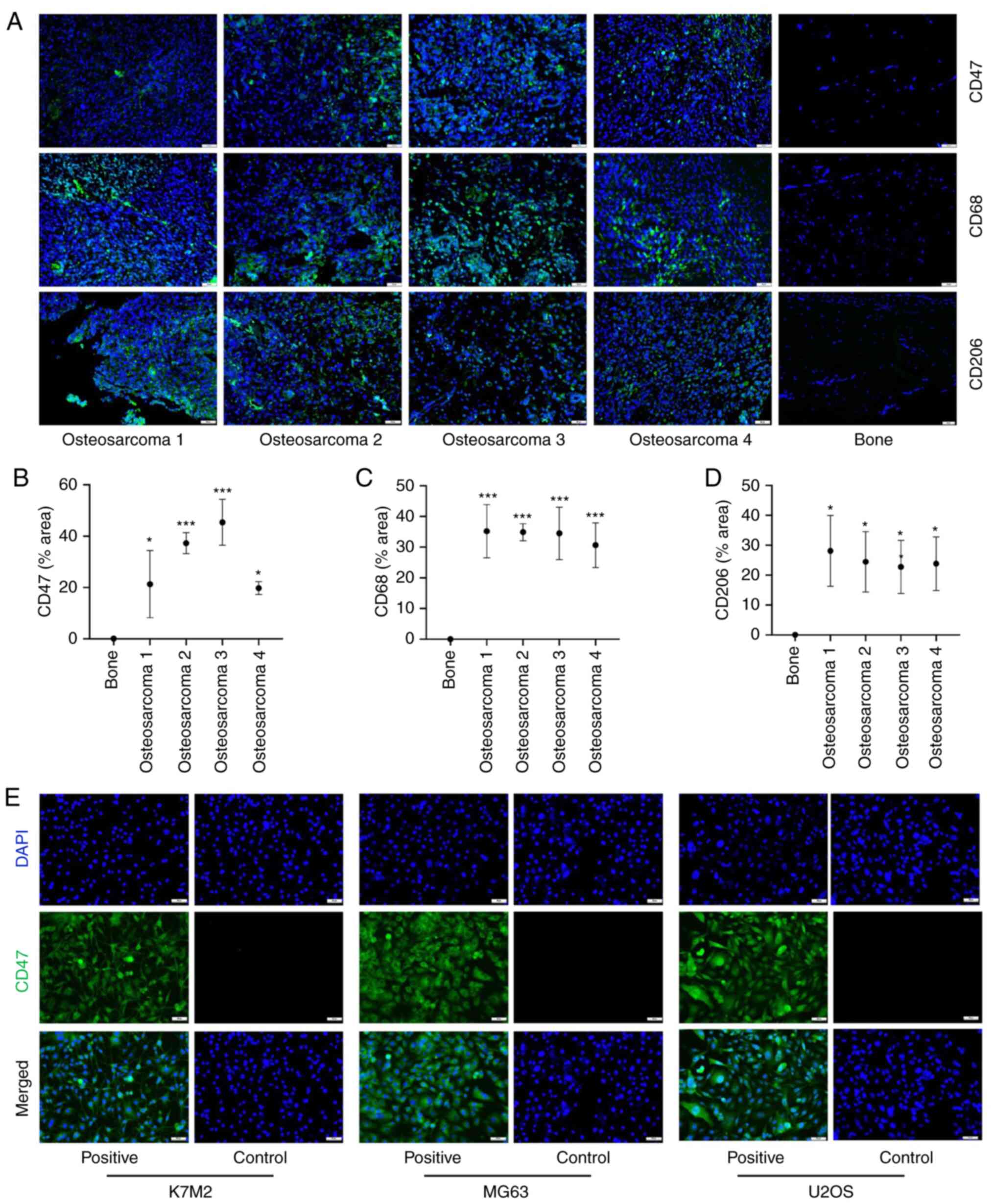
Results
CD47 and TAMs are found in human
OS
The expression of CD47 in OS is a prerequisite for
the use of CD47mAb as an immunotherapy in the treatment of OS.
Using immunofluorescence labeling, it was demonstrated that the
expression levels of CD47 in OS patient specimens were
significantly greater than that in normal bone tissues (Fig. 1A and B). At the same time,
immunofluorescence staining showed that the expression of the M2
TAMs markers, CD68 and CD206, in OS specimens were significantly
higher than those in normal bone tissues (Fig. 1A, C and D). This demonstrated that
M2 TAMs are abundantly present in OS. Taken with previous studies,
M2 TAMs will promote tumor development (28). However, due to the low incidence of
OS, specimen collection is difficult, thus only four OS samples
were studied. This may bring more errors to the experimental
results. More samples will be collected in the authors' future
research work. In OS tissues, tumor cells and various immune cells
are intertwined, and the components are unevenly distributed, thus
the immunofluorescence staining results of different samples are
quite different. Cell immunofluorescence labeling was also used to
validate the expression of CD47 in U-2 OS, MG63 and K7M2 OS cell
lines. The results revealed that the expression of CD47 is abundant
in U-2 OS, MG63, and K7M2 cell lines, using cell immunofluorescence
staining (Fig. 1E). The
pathological analysis of normal bone and OS tissues using H&E
staining is shown in Fig. S1.
Effect of LPS and CD47mAb on
macrophage activation
Different subtypes of macrophages express different
proteins and cytokines (40). To
verify the activation effect of LPS (0.1 µg/ml) and CD47mAb (10
µg/ml) on macrophages, the expression of M1 macrophage-related
proteins was analyzed in RAW264.7 cells that were co-cultured with
LPS, CD47mAb or both. The results of western blot analysis
demonstrated that LPS could increase the expression of NOS2 in
macrophages, but CD47mAb had no such effect (Fig. 2A and B). The supernatant from the
macrophage cultures was collected from the different treatment
groups, and the content of NO and TNF-α was measured; the results
(Fig. 2C and D) agreed with the
results of the western blot analysis. To further verify the effect
of LPS (0.1 µg/ml) on polarization and CD47mAb (10 µg/ml) on the
proportion of each macrophage phenotype in the macrophage-tumor
co-culture system, a co-culture chamber was used as shown in
Fig. 2E to separate tumor cells
from macrophages. After 24 h of culturing, RAW264.7 cells were
collected, and the expression of CD86, a surface marker of M1
macrophages, and ROS, an important product of macrophage
activation, was detected by flow cytometry. The results (Fig. 2F-I) also revealed that LPS could
effectively activate macrophages and transform them into the
antitumor M1 phenotype. These results suggested that LPS can
successfully polarize macrophages, but compared with LPS alone, the
combination of LPS and CD47mAb has no advantage in stimulating
macrophage polarization. Further experiments showed that CD47mAb
combined with LPS has a significant advantage in promoting the
phagocytosis of macrophages (Fig.
3). In the present study, the drug concentration of CD47mAb
used in this experiment refers to other relevant studies (41). The concentration of LPS was
determined by simple pre-experiment. The pre-experiment, including
Elisa and CCK-8 assays, showed that LPS (0.1 µg/ml) could
effectively stimulate macrophage polarization and without obvious
cytotoxicity (data not shown).
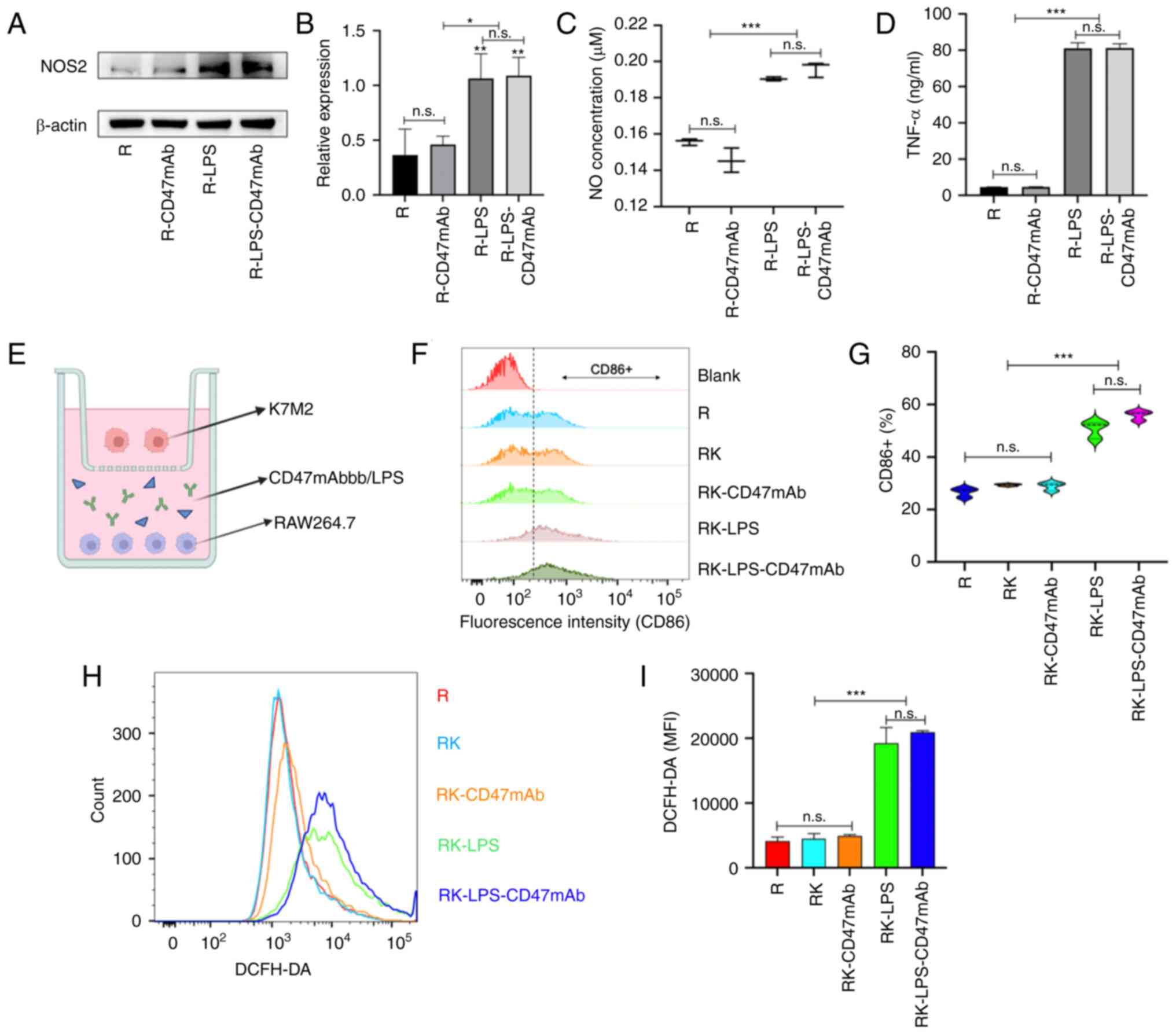 | Figure 2.Effect of LPS and CD47mAb on
macrophage activation. (A) Western blot analysis and (B)
densitometric analysis was used to confirm the expression of NOS2
in RAW264.7 cells treated as indicated. (C and D) NO and TNF-α
content in the supernatant of RAW264.7 treated as indicated. (E)
Co-culture model of the K7M2 and RAW264.7 cells. (F and G) Flow
cytometry was used to measure CD86 expression in the RAW264.7
cells. (H and I) The ROS content in RAW264.7 cells in the different
treatment groups was detected using flow cytometry. Data are
presented as the mean ± SD of three repeats. *P<0.05,
**P<0.01 and ***P<0.001. n.s., not significant; NO, nitric
oxide; TNF-α, tumor necrosis factor α; LPS, lipopolysaccharide;
CD47mAb, CD47 monoclonal antibody; ROS, reactive oxygen species;
MFI, mean fluorescence intensity; R, RAW264.7; K, K7M2; RK,
RAW264.7 + K7M2. |
Impact of LPS and CD47mAb on
phagocytosis
As a part of the immune system, the powerful
phagocytosis executed by macrophages plays an important role in
clearing tumorigenic cells (42).
In view of the polarization of macrophages induced by LPS and the
blocking effect of CD47mAb on the CD47-SIRPα signaling pathway, the
combined effect of both on tumor phagocytosis of macrophages was
investigated next. CFSE pre-stained K7M2 and Dye 670 pre-stained
RAW264.7 cells were co-cultured in a 2:1 ratio and incubated with
LPS, CD47mAb, or LPS and CD47mAb for 48 h. Then the phagocytic
function of macrophages was observed using laser confocal
microscopy. The results showed that the proportion of phagocytic
macrophages, when treated with the combination of LPS and CD47mAb,
was significantly higher than that in the control group and in the
group treated with LPS or CD47mAb alone (Fig. 3A and B). To obtain more accurate
data, the experiments were repeated and assessed using flow
cytometry. As demonstrated in Fig.
3C, the results of the flow cytometric analysis were consistent
with those of the confocal microscopy results. This suggested that
stimulating macrophage polarization and blocking the CD47-SIRPα
signaling pathway at the same time can more effectively overcome
tumor immune escape. ROS play a crucial function in controlling
macrophage phagocytosis (43).
Thus, a ROS scavenger, N-acetyl-cysteine, was used to scavenge the
ROS produced by the LPS-stimulated macrophages, and this resulted
in a significant decrease in the phagocytic ability of macrophages
(Fig. 3D). Thus, ROS plays a
crucial role in macrophage phagocytosis.
Combined effect of LPS and CD47mAb on
inhibition of OS growth
The anti-OS regimen of LPS combined with CD47mAb
effectively activated macrophages, and induced the release a large
number of cytotoxic factors including ROS, NO and TNF-α from
macrophages (Fig. 2). Therefore, it
was hypothesized that LPS combined with CD47mAb would both promote
the phagocytosis of OS cells by macrophages, and also inhibit the
proliferation of tumor cells by macrophages. First, CCK-8 assays
were used to detect the toxicity of LPS and CD47mAb on K7M2 cells
in the absence of macrophages. The results revealed that low
concentrations of LPS and CD47mAb had no significant effect on the
viability of K7M2 cells (Fig.
4A-C). Next, CFSE pre-stained K7M2 cells were co-cultured with
RAW264.7 cells in the co-culture chamber (Fig. 4D), and the co-cultured cells were
treated with LPS or CD47mAb for 24 h. Then, the fluorescence
intensity of CFSE-K7M2 was detected by flow cytometry. CFSE dye is
used to detect proliferation, and the fluorescence intensity of
CFSE-labeled cells is halved every time a stained cell is divided.
The fluorescence of K7M2 cells cultured alone was the weakest
compared with that of K742 cells co-cultured with macrophages
(Fig. 4E and F), indicating that
the number of divisions of K7M2 cells was higher in this group. In
the co-culture system, the fluorescence intensity of the groups
treated with LPS or LPS-CD47mAb was the highest (Fig. 4E and F), indicating that the
corresponding groups had the lowest number of divisions, whereas
CD47mAb did not have a notable effect on tumor cell division. This
result is consistent with the fact that LPS promotes the M1
polarization of macrophages. The results showed that macrophages
could inhibit the proliferation of tumor cells, and the inhibitory
effect of polarized macrophages on tumor proliferation was
significantly enhanced. To more intuitively observe the inhibition
of tumor proliferation by macrophages, K7M2 cells were cultured in
serum-free DMEM and RAW264.7 cell culture supernatant under
different treatment conditions. After 24 h, K7M2 cells were stained
with DAPI, and the results were observed using a fluorescence
microscope (Fig. 4G and H). In
addition, the results of apoptosis experiments demonstrated that
LPS could significantly promote the apoptosis of osteosarcoma cells
induced by macrophages (Fig.
S2).
Activated macrophages inhibit
migration of OS cells
OS is highly malignant and metastasizes early
(44). Macrophages are an important
part of the immune system, and it is of great significance to study
their role in tumor metastasis. The effects of LPS and CD47mAb on
the migratory ability of K7M2 cells were evaluated by Transwell
assay. The results showed that compared with the control group, LPS
and CD47mAb alone or combined did not significantly alter the
migratory ability of K7M2 cells (Fig.
5A and B). Next, macrophages were added to the lower chamber of
the Transwell insert to observe their ability to alter the
migration of K7M2 cells in the different treatment groups. As
demonstrated in Fig. 5C and D,
macrophages effectively inhibited the migratory ability of OS
cells. When LPS was added to the co-culture system, the migratory
ability of K7M2 cells decreased significantly, which indicated that
LPS could enhance the inhibition of tumor migration by macrophages,
but CD47mAb did not have this effect. This result is consistent
with the fact that LPS can promote the polarization of macrophages.
Therefore, the significant inhibitory effect of macrophages on
tumor migration when treated with a combination of LPS and CD47mAb
was primarily due to the effect of LPS on the polarization of
macrophages (Fig. 5C and D).
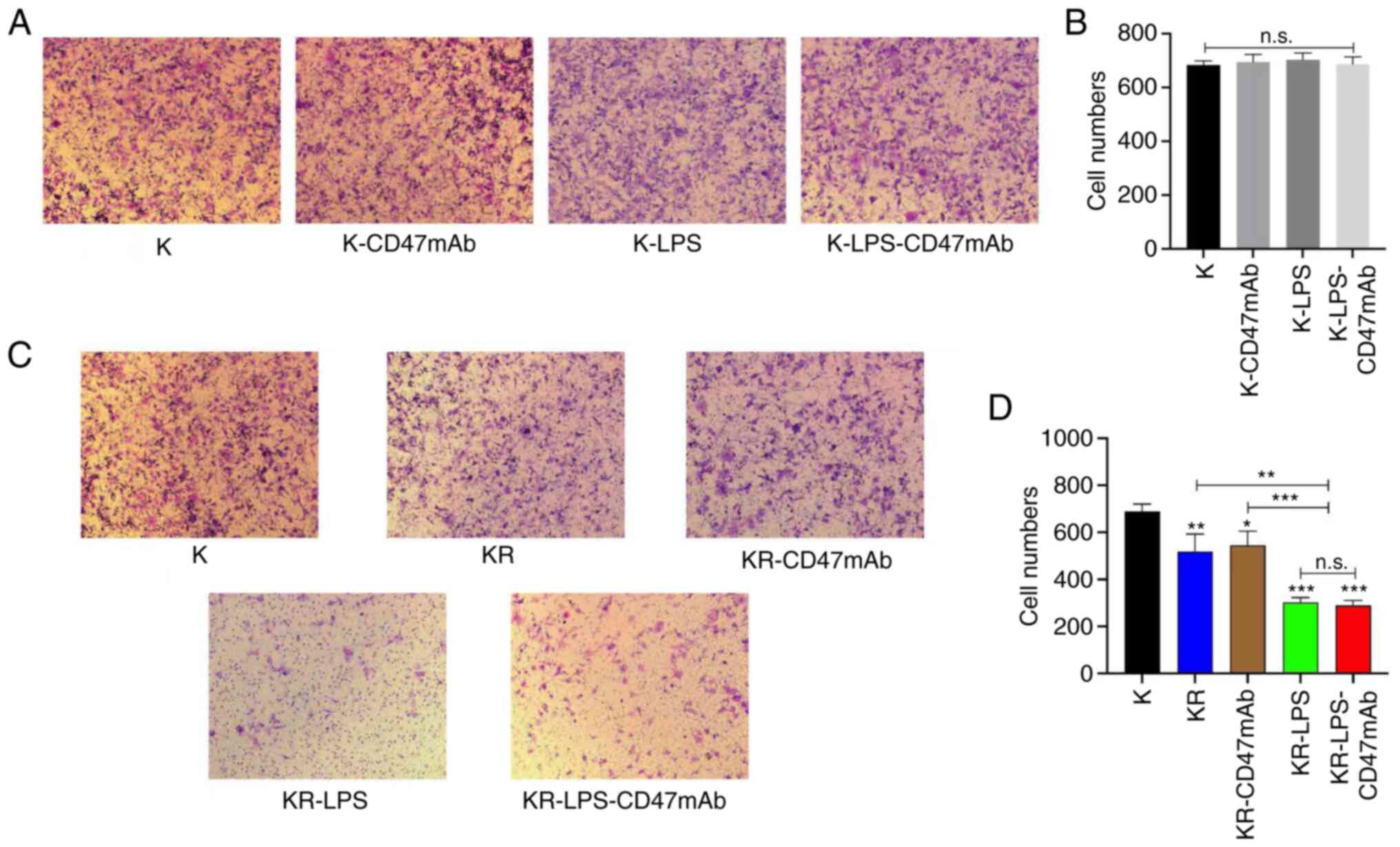 | Figure 5.Activated macrophages inhibit the
migration of OS. (A) The serum-free medium containing K7M2 was
added to the upper chamber of the Transwell inserts, and the lower
chamber was filled with supplemented media. After 24 h of
incubation with LPS or CD47mAb, migratory cells were stained and
observed. (B) Quantitative analysis of the Transwell migration
assays shown in panel A. (C) The serum-free media containing K7M2
was added to the upper chamber of the Transwell inserts, and the
lower chamber was filled with supplemented media. with or without
RAW264.7 cells; migratory cells were stained and observed.
Magnification, ×40. (D) Quantitative analysis of the Transwell
migration assay shown in panel C. Data are presented as the mean ±
SD of three independent experiments. *P<0.05, **P<0.01 and
***P<0.001 vs. K or as otherwise indicated. n.s., not
significant; K, K7M2; R, RAW264.7; KR, K7M2 + RAW264.7; LPS,
lipopolysaccharide; OS, osteosarcoma; CD47mAb, CD47 monoclonal
antibody. |
Discussion
There is mounting evidence that the immune system
may be commandeered to prevent carcinogenesis and eliminate tumor
cells (45,46). In cancer treatment, immunotherapy
using targeted checkpoint preparations has made notable progress
(47). CTLA4 antibodies, PD-1/PD-L1
antibodies, as well as CAR-T and TCR-T, have proven instrumental in
improving the management of cancer, and all of these utilize T
cells (48,49). Although immunotherapy based on T
cells has proven valuable, it has limitations, such as its muted
effect in solid tumors; most patients with PD-1/PDL-1 antibodies
used to treat solid tumors do not respond (50–53).
The poor effect of PD-1/PDL-1 antibody in solid tumors may be
related to the low expression of PDL-1 in solid tumors (54). In addition, the dense tumor
microenvironment of solid tumors limits the penetration of PDL-1
antibodies with high molecular weight into the tumor stroma
(55). Certain experiments have
shown that the drug conjugate of PDL-1 antibody can achieve the
purpose of chemically guided immunotherapy by destroying the tumor
matrix (56).
Thus, a novel avenue in the field of immunotherapy
is required to address this. Although the non-specific immune
response, represented by macrophages, has poor specificity, it has
a significantly wider coverage. CD47 is expressed on the surface of
tumor cells and its activation promotes tumor progression and tumor
immune escape. Blocking CD47 is considered to be another method of
immune checkpoint therapy (57).
CD47mAb combined with anti-GD2 has made notable progress in the
treatment of tumors (58). However,
the antitumor effect of CD47mAb alone is not significant (22,59).
In recent years, combinatorial immunotherapy has attracted
significant attention due to its additive or synergistic effects in
tumor therapy (60,61). The present study suggested that the
combination of macrophage activator LPS and CD47mAb can enhance the
activation of macrophages and exerts a stronger antitumor effect
than either used alone. A schematic illustration of LPS combined
with CD47mAb as a hypothetical method of immunotherapy in OS is
presented in Fig. 6.
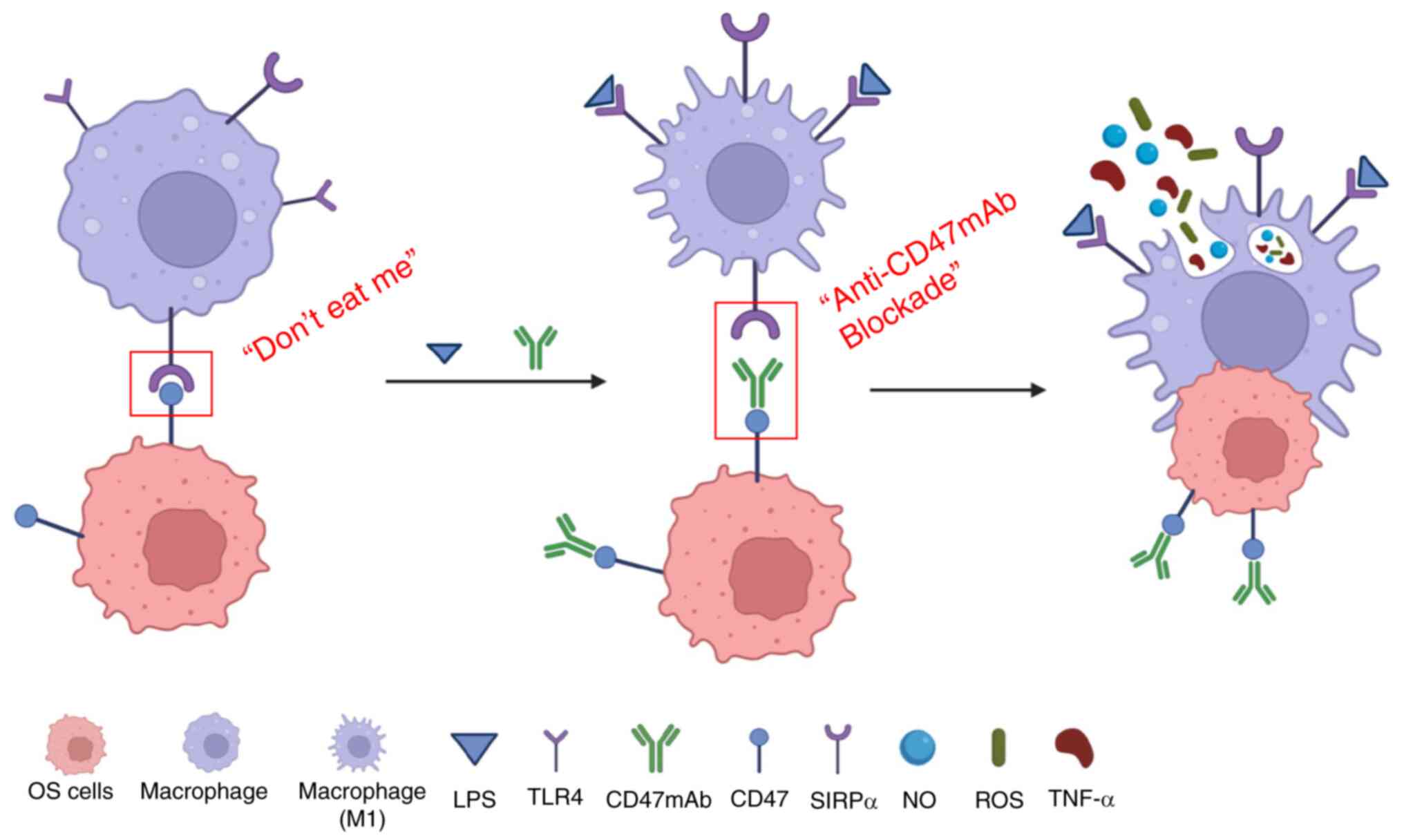 | Figure 6.Schematic illustration of LPS
combined with CD47mAb as a hypothetical method of immunotherapy in
OS. CD47 is present on the surface of OS cells and its binding to
SIRPα on macrophages inhibits phagocytosis by macrophages. Blocking
the interaction between CD47 and SIRPα with CD47mAb prevents the
inhibition of phagocytosis by macrophages, increasing phagocytosis
of the OS cells. LPS can bind to TLR4 receptors on macrophages and
promote macrophages to polarize towards an M1 phenotype. M1
macrophages exhibit a more potent phagocytotic ability and secrete
an increased quantity of antitumorigenic factors, including TNF-α,
NO and ROS. LPS and CD47mAb may thus have a significant synergistic
effect in tumor immunotherapy. OS, osteosarcoma; NO, nitric oxide;
TNF-α, tumor necrosis factor α; LPS, lipopolysaccharide; CD47mAb,
CD47 monoclonal antibody; ROS, reactive oxygen species; TLR4,
toll-like receptors 4; SIRPα, signal regulatory protein α. |
OS is a highly malignant tumor with a very low cure
rate and it poses a significant burden on patients and society
(4). Thus, there is an urgent need
of a more effective and economical treatment. The present results
demonstrated the efficacy of a novel combinatorial antitumor
immunotherapeutic approach using LPS and CD47mAb for the treatment
of OS. This research provides an effective and feasible innovative
idea for the treatment of OS. The results showed that low doses of
LPS and CD47mAb had almost no effect on the viability of OS. In a
co-culture system, LPS combined with CD47mAb significantly enhanced
the phagocytosis of macrophages on OS cells and reduced the
proliferation of OS cells. There are two primary causes underlying
this: First, LPS polarizes macrophages to an antitumor phenotype,
which exhibit increased tumor cytotoxicity and phagocytosis;
second, CD47mAb blocks the CD47-SIRPα signaling pathway by blocking
CD47 signal proteins on the surface of OS cells, thus further
enhancing the recognition and phagocytosis by macrophages to OS
cells. In the present study, it was identified that macrophages and
tumor cells come into contact with each other, and CD47mAb and LPS
play a joint role in promoting the phagocytosis of OS cells by
macrophages. Furthermore, it was revealed that macrophages inhibit
OS cells mainly through indirect actions, such as the release of
TNF-α, ROS and NO. Therefore, the combined use of LPS and CD47mAb
fully activated the antitumor effect of macrophages.
The combined effect of CD47mAb and LPS is mainly
shown in promoting the phagocytosis of macrophages. When CD47mAb is
used in combination with LPS, LPS is responsible for polarizing
macrophages and CD47mAb is responsible for blocking CD47-SIRPα
signaling pathway. It was also shown that whether LPS was combined
with CD47mAb or used alone, the anti-OS effect of macrophages was
higher than that of CD47mAb alone. This is because LPS can polarize
macrophages, promote macrophages to release various cytokines, and
then indirectly inhibit the activity of OS cells. However, CD47mAb
does not have this effect. This showed that the antitumor effect of
macrophages depends, to a large extent, on phenotypic
transformation; that is, M1 macrophages are the primary mediators
of tumor immunity. These results provide a favorable direction for
future research on tumor immunity.
In the present study, there were a large number of
M2 TAMs in the tumor tissues of patients with OS, consistent with a
previous study (62). As M2
macrophages promote tumor development, they cannot engulf tumor
cells, thus only M1 macrophages exhibit an antitumor effect
(27,40), and thus it is hypothesized that this
may be an important reason underlying the poor effect of CD47mAb
alone. Increasing the proportion of M1 macrophages in the tumor
tissues and reducing the proportion of M2 macrophages is an
effective method to increase the antitumor effects of macrophages.
To the best of our knowledge, although LPS has been used to
stimulate macrophage polarization in several studies, the present
study is the first to use LPS and CD47mAb together to treat OS.
Several studies have confirmed that stimulating
macrophages to polarize towards an M1 phenotype can inhibit tumor
growth (63,64). However, the immune escape exhibited
by tumors significantly limits the effects of M1 macrophages. In
the current study, LPS with CD47mab were used together to inhibit
tumor growth and effectively prevent the immune escape of tumor
cells. The actions of these two treatments complement each other
and promote the antitumor effects of M1 macrophages. In addition,
the present study did show that macrophages activated by LPS can
inhibit the migration of OS cells and promote their apoptosis. Of
course, in the cell migration assays, the apoptosis of OS cells
induced by macrophages will reduce the number of OS cells, which
has a potential impact on the results of the migration assays.
CD47mAb promotes the phagocytosis of tumor cells by
macrophages, and inhibition of CD47 can effectively enhance the
antitumor effects of macrophages by directly activating cytotoxic T
cells in immune-active mice (22,65).
Animal experiments were not performed in the present study, which
may be considered a limitation. In future studies, the efficacy of
LPS combined with CD47mAb in animal models will be determined as
well as its other roles in the tumor microenvironment.
In conclusion, the results of the present study
highlight the combined effect of simultaneously stimulating
macrophage polarization and blocking tumor immune escape pathways
in tumor immunotherapy. This new strategy of tumor immunotherapy
may serve as a reference for the development of clinical treatments
against OS, and may also provide novel immunotherapeutic approaches
for the management of several types of cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Major Project of Natural
Science Foundation of Anhui Province Colleges and Universities
(grant no. KJ2020ZD17).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH, PY and YQ conceived the study. PY, YQ and YL
performed the experiments and data analyses. PH and ZG contributed
to performing the experiments, data acquisition and data analysis.
YH, PY and YQ wrote the manuscript. YH, PY and YQ confirm the
authenticity of all the raw data. All authors revised the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved
(approval no. PJ2022-10-15) by the Clinical Medical Research Ethics
Committee of The First Affiliated Hospital of Anhui Medical
University (Hefei, China). Written informed consent for
participation was not required for the present study in accordance
with the national legislation and the institutional
requirements.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Casali PG, Bielack S, Abecassis N, Aro HT,
Bauer S, Biagini R, Bonvalot S, Boukovinas I, Bovee JVMG, Brennan
B, et al: Bone sarcomas: ESMO-PaedCan-EURACAN clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 29
(Suppl 4):iv79–iv95. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zoumpoulidou G, Alvarez-Mendoza C, Mancusi
C, Ahmed RM, Denman M, Steele CD, Tarabichi M, Roy E, Davies LR,
Manji J, et al: Therapeutic vulnerability to PARP1,2 inhibition in
RB1-mutant osteosarcoma. Nat Commun. 12:70642021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whelan J, McTiernan A, Cooper N, Wong YK,
Francis M, Vernon S and Strauss SJ: Incidence and survival of
malignant bone sarcomas in England 1979–2007. Int J Cancer.
131:E508–E517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ta HT, Dass CR, Choong PFM and Dunstan DE:
Osteosarcoma treatment: State of the art. Cancer Metastasis Rev.
28:247–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He P, Xu S, Guo Z, Yuan P, Liu Y, Chen Y,
Zhang T, Que Y and Hu Y: Pharmacodynamics and pharmacokinetics of
PLGA-based doxorubicin-loaded implants for tumor therapy. Drug
Deliv. 29:478–488. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Whelan JS and Davis LE: Osteosarcoma,
chondrosarcoma, and chordoma. J Clin Oncol. 36:188–193. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lagmay JP, Krailo MD, Dang H, Kim A,
Hawkins DS, Beaty O III, Widemann BC, Zwerdling T, Bomgaars L,
Langevin AM, et al: Outcome of patients with recurrent osteosarcoma
enrolled in seven phase II trials through children's cancer group,
pediatric oncology group, and children's oncology group: learning
from the past to move forward. J Clin Oncol. 34:3031–3038. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Link MP, Goorin AM, Miser AW, Green AA,
Pratt CB, Belasco JB, Pritchard J, Malpas JS, Baker AR, Kirkpatrick
JA, et al: The effect of adjuvant chemotherapy on relapse-free
survival in patients with osteosarcoma of the extremity. N Engl J
Med. 314:1600–1606. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Y: Cancer immunotherapy: Harnessing
the immune system to battle cancer. J Clin Invest. 125:3335–3337.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gattinoni L, Powell DJ Jr, Rosenberg SA
and Restifo NP: Adoptive immunotherapy for cancer: Building on
success. Nat Rev Immunol. 6:383–393. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mellman I, Coukos G and Dranoff G: Cancer
immunotherapy comes of age. Nature. 480:480–489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shionoya Y, Kanaseki T, Miyamoto S, Tokita
S, Hongo A, Kikuchi Y, Kochin V, Watanabe K, Horibe R, Saijo H, et
al: Loss of tapasin in human lung and colon cancer cells and escape
from tumor-associated antigen-specific CTL recognition.
Oncoimmunology. 6:e12744762017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Toor SM and Elkord E: Therapeutic
prospects of targeting myeloid-derived suppressor cells and immune
checkpoints in cancer. Immunol Cell Biol. 96:888–897. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salgado R, Denkert C, Demaria S, Sirtaine
N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL,
Penault-Llorca F, et al: The evaluation of tumor-infiltrating
lymphocytes (TILs) in breast cancer: Recommendations by an
international TILs working group 2014. Ann Oncol. 26:259–271. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mantovani A and Sica A: Macrophages,
innate immunity and cancer: Balance, tolerance, and diversity. Curr
Opin Immunol. 22:231–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fridman WH, Pagès F, Sautès-Fridman C and
Galon J: The immune contexture in human tumours: Impact on clinical
outcome. Nat Rev Cancer. 12:298–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rodell CB, Arlauckas SP, Cuccarese MF,
Garris CS, Li R, Ahmed MS, Kohler RH, Pittet MJ and Weissleder R:
TLR7/8-agonist-loaded nanoparticles promote the polarization of
tumour-associated macrophages to enhance cancer immunotherapy. Nat
Biomed Eng. 2:578–588. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Phanstiel DH, Van Bortle K, Spacek D, Hess
GT, Shamim MS, Machol I, Love MI, Aiden EL, Bassik MC and Snyder
MP: Static and dynamic DNA loops form AP-1-bound activation hubs
during macrophage development. Mol Cell. 67:1037–1048.e6. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Demaria O, Cornen S, Daëron M, Morel Y,
Medzhitov R and Vivier E: Publisher correction: Harnessing innate
immunity in cancer therapy. Nature. 576:E32019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chao MP, Weissman IL and Majeti R: The
CD47-SIRPα pathway in cancer immune evasion and potential
therapeutic implications. Curr Opin Immunol. 24:225–232. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu X, Pu Y, Cron K, Deng L, Kline J,
Frazier WA, Xu H, Peng H, Fu YX and Xu MM: CD47 blockade triggers T
cell-mediated destruction of immunogenic tumors. Nat Med.
21:1209–1215. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Advani R, Flinn I, Popplewell L, Forero A,
Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP,
et al: CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin's
lymphoma. N Engl J Med. 379:1711–1721. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Munn DH and Bronte V: Immune suppressive
mechanisms in the tumor microenvironment. Curr Opin Immunol.
39:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Noy R and Pollard JW: Tumor-associated
macrophages: From mechanisms to therapy. Immunity. 41:49–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Q, Wang C, Zhang X, Chen G, Hu Q, Li
H, Wang J, Wen D, Zhang Y, Lu Y, et al: In situ sprayed
bioresponsive immunotherapeutic gel for post-surgical cancer
treatment. Nat Nanotechnol. 14:89–97. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Komohara Y, Fujiwara Y, Ohnishi K and
Takeya M: Tumor-associated macrophages: Potential therapeutic
targets for anti-cancer therapy. Adv Drug Deliv Rev. 99:180–185.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gordon S and Martinez FO: Alternative
activation of macrophages: Mechanism and functions. Immunity.
32:593–604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang B, Zhao J, Li H, He KL, Chen Y, Chen
SH, Mayer L, Unkeless JC and Xiong H: Toll-like receptors on tumor
cells facilitate evasion of immune surveillance. Cancer Res.
65:5009–5014. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
De Schutter T, Andrei G, Topalis D,
Duraffour S, Mitera T, van den Oord J, Matthys P and Snoeck R:
Reduced tumorigenicity and pathogenicity of cervical carcinoma SiHa
cells selected for resistance to cidofovir. Mol Cancer. 12:1582013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grauer OM, Molling JW, Bennink E, Toonen
LW, Sutmuller RP, Nierkens S and Adema GJ: TLR ligands in the local
treatment of established intracerebral murine gliomas. J Immunol.
181:6720–6729. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hagelueken G, Clarke BR, Huang H,
Tuukkanen A, Danciu I, Svergun DI, Hussain R, Liu H, Whitfield C
and Naismith JH: A coiled-coil domain acts as a molecular ruler to
regulate O-antigen chain length in lipopolysaccharide. Nat Struct
Mol Biol. 22:50–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim J and Bae JS: Tumor-associated
macrophages and neutrophils in tumor microenvironment. Mediators
Inflamm. 2016:60581472016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH,
Wang XZ, Zhao YW and Wei YQ: Prognostic significance of
tumor-associated macrophages in solid tumor: A meta-analysis of the
literature. PLoS One. 7:e509462012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weilbaecher KN, Guise TA and McCauley LK:
Cancer to bone: A fatal attraction. Nat Rev Cancer. 11:411–425.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
World Medical Association, . World medical
association declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sui C, Wu Y, Zhang R, Zhang T, Zhang Y, Xi
J, Ding Y, Wen J and Hu Y: Rutin inhibits the progression of
osteoarthritis through CBS-mediated RhoA/ROCK signaling. DNA Cell
Biol. 41:617–630. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He P, Hu Y, Huang C, Wang X, Zhang H,
Zhang X, Dai H, Wang R and Gao Y: N-butanol extract of gastrodia
elata suppresses inflammatory responses in
lipopolysaccharide-stimulated macrophages and complete freund's
adjuvant-(CFA-) induced arthritis rats via inhibition of MAPK
signaling pathway. Evid Based Complement Alternat Med.
2020:16586182020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gordon S: Alternative activation of
macrophages. Nat Rev Immunol. 3:23–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mohanty S, Aghighi M, Yerneni K, Theruvath
JL and Daldrup-Link HE: Improving the efficacy of osteosarcoma
therapy: Combining drugs that turn cancer cell ‘don't eat me’
signals off and ‘eat me’ signals on. Mol Oncol. 13:2049–2061. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Feng M, Chen JY, Weissman-Tsukamoto R,
Volkmer JP, Ho PY, McKenna KM, Cheshier S, Zhang M, Guo N, Gip P,
et al: Macrophages eat cancer cells using their own calreticulin as
a guide: Roles of TLR and Btk. Proc Natl Acad Sci USA.
112:2145–2150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Singel KL and Segal BH: NOX2-dependent
regulation of inflammation. Clin Sci (Lond). 130:479–490. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tsuchiya H, Kanazawa Y, Abdel-Wanis ME,
Asada N, Abe S, Isu K, Sugita T and Tomita K: Effect of timing of
pulmonary metastases identification on prognosis of patients with
osteosarcoma: The Japanese musculoskeletal oncology group study. J
Clin Oncol. 20:3470–3477. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Palucka AK and Coussens LM: The basis of
oncoimmunology. Cell. 164:1233–1247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Raval RR, Sharabi AB, Walker AJ, Drake CG
and Sharma P: Tumor immunology and cancer immunotherapy: Summary of
the 2013 SITC primer. J Immunother Cancer. 2:142014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jiang X, Wang J, Deng X, Xiong F, Ge J,
Xiang B, Wu X, Ma J, Zhou M, Li X, et al: Role of the tumor
microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol
Cancer. 18:102019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Constantinidou A, Alifieris C and Trafalis
DT: Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): A
new era in cancer active immunotherapy. Pharmacol Ther. 194:84–106.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Quamine AE, Olsen MR, Cho MM and Capitini
CM: Approaches to enhance natural killer cell-based immunotherapy
for pediatric solid tumors. Cancers (Basel). 13:27962021.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang RF and Wang HY: Immune targets and
neoantigens for cancer immunotherapy and precision medicine. Cell
Res. 27:11–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Callea M, Albiges L, Gupta M, Cheng SC,
Genega EM, Fay AP, Song J, Carvo I, Bhatt RS, Atkins MB, et al:
Differential expression of PD-L1 between primary and metastatic
sites in clear-cell renal cell carcinoma. Cancer Immunol Res.
3:1158–1164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Humphries MP, McQuaid S, Craig SG, Bingham
V, Maxwell P, Maurya M, McLean F, Sampson J, Higgins P, Greene C,
et al: Critical appraisal of programmed death ligand 1 reflex
diagnostic testing: Current standards and future opportunities. J
Thorac Oncol. 14:45–53. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shin DS, Zaretsky JM, Escuin-Ordinas H,
Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W,
Sandoval S, Torrejon DY, et al: Primary resistance to PD-1 blockade
mediated by JAK1/2 mutations. Cancer Discov. 7:188–201. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Syn NL, Teng MWL, Mok TSK and Soo RA:
De-novo and acquired resistance to immune checkpoint targeting.
Lancet Oncol. 18:e731–e741. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sau S, Petrovici A, Alsaab HO, Bhise K and
Iyer AK: PDL-1 antibody drug conjugate for selective chemo-guided
immune modulation of cancer. Cancers (Basel). 11:2322019.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Vonderheide RH: CD47 blockade as another
immune checkpoint therapy for cancer. Nat Med. 21:1122–1123. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Theruvath J, Menard M, Smith BAH, Linde
MH, Coles GL, Dalton GN, Wu W, Kiru L, Delaidelli A, Sotillo E, et
al: Anti-GD2 synergizes with CD47 blockade to mediate tumor
eradication. Nat Med. 28:333–344. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Willingham SB, Volkmer JP, Gentles AJ,
Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin
R, Cohen JD, et al: The CD47-signal regulatory protein alpha
(SIRPa) interaction is a therapeutic target for human solid tumors.
Proc Natl Acad Sci USA. 109:6662–6667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Perry CJ, Muñoz-Rojas AR, Meeth KM,
Kellman LN, Amezquita RA, Thakral D, Du VY, Wang JX, Damsky W,
Kuhlmann AL, et al: Myeloid-targeted immunotherapies act in synergy
to induce inflammation and antitumor immunity. J Exp Med.
215:877–893. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wiehagen KR, Girgis NM, Yamada DH, Smith
AA, Chan SR, Grewal IS, Quigley M and Verona RI: Combination of
CD40 agonism and CSF-1R blockade reconditions tumor-associated
macrophages and drives potent antitumor immunity. Cancer Immunol
Res. 5:1109–1121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mohanty S, Yerneni K, Theruvath JL, Graef
CM, Nejadnik H, Lenkov O, Pisani L, Rosenberg J, Mitra S, Cordero
AS, et al: Nanoparticle enhanced MRI can monitor macrophage
response to CD47 mAb immunotherapy in osteosarcoma. Cell Death Dis.
10:362019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Luo Z, Li P, Deng J, Gao N, Zhang Y, Pan
H, Liu L, Wang C, Cai L and Ma Y: Cationic polypeptide
micelle-based antigen delivery system: A simple and robust adjuvant
to improve vaccine efficacy. J Control Release. 170:259–267. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bocanegra Gondan AI, Ruiz-de-Angulo A,
Zabaleta A, Gómez Blanco N, Cobaleda-Siles BM, García-Granda MJ,
Padro D, Llop J, Arnaiz B, Gato M, et al: Effective cancer
immunotherapy in mice by polyIC-imiquimod complexes and engineered
magnetic nanoparticles. Biomaterials. 170:95–115. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Soto-Pantoja DR, Terabe M, Ghosh A,
Ridnour LA, DeGraff WG, Wink DA, Berzofsky JA and Roberts DD: CD47
in the tumor microenvironment limits cooperation between antitumor
T-cell immunity and radiotherapy. Cancer Res. 74:6771–6783. 2014.
View Article : Google Scholar : PubMed/NCBI
|