Introduction
Endometrial cancer (EC) is the sixth most frequent
cancer type in women, and its incidence and mortality rates have
increased in recent years (1). From
1987 to 2014, the cases of EC increased by 75% globally and the
mortalities associated with EC increased by 300% (2,3). For
patients without distant metastasis, surgery is usually followed by
post-operative adjuvant therapy (4). Hormone therapy may be used to protect
patients with well-differentiated EC who wish to reproduce
(4). At present, there are few
effective anticancer drugs for EC; thus, it is urgent to develop
novel molecular targeted drugs for EC (5).
Cancer treatment has markedly changed in the past
decade with the development of tumor immunotherapy (6). EC responds well to immunotherapy
compared with other solid tumors owing to its unique immune
properties, such as a high tumor mutation load (7). In The Cancer Genome Atlas (TCGA), EC
is divided into four molecular subtypes: i) DNA Polymerase ε (POLE)
mutation, ii) microsatellite instability-high/mismatch repair
deficient (MSI-H/dMMR), iii) high copy number and iv) low copy
number. POLE mutation is associated with high-grade histology, but
prognosis for this type of EC is good (8). As this type of EC has a strong immune
response, it is the most suitable type for immunotherapy (9). Programmed death-ligand 1 (PD-L1) is
expressed in 92% of EC cases; it is indicative of advanced stage
and poor tumor differentiation. Patients with
chemotherapy-resistant, metastatic EC have high response rates to
programmed death-1 (PD-1) and PD-L1 inhibitors (10). The upregulated expression of CTLA-4,
LAG-3 and IDO in POLE tumors suggests potential in immunotherapy of
POLE tumors (11). Recently,
additional immune checkpoints have been identified such as TIGIT
and CD73, which will help to investigate the mechanism and efficacy
of immune checkpoint inhibitors (ICIs) in EC treatment (12,13).
ζ-Chain-associated protein kinase 70 (ZAP70) is a
tyrosine kinase that binds to the T-cell receptor (TCR) complex,
and acts on the TCR-mediated signaling pathway in thymocytes and
peripheral T cells (14). In
vivo, ITAM is dephosphorylated and ZAP70 is in a resting state
when the major histocompatibility complex-binding antigenic peptide
fragment (pMHC) of the pathogen or tumor is not recognized by the
TCR. When pMHC on a tumor or pathogen is recognized by the TCR,
tyrosine-protein kinase LCK on CD4 binds to ITAM, phosphorylating
ITAM, and ZAP70 is activated when it binds to phosphorylated ITAM
(15). Previous studies on ZAP70
have been restricted primarily to chronic lymphocytic leukemia
(CLL), with limited detailed research on solid tumors (16,17).
For example, high expression of ZAP70 in CLL is related to
non-mutated IgVH, malignant progression of the disease and poor
total survival rate (18). Since
the majority of ZAP70 is found in αβ and γδ T cells, natural killer
(NK) cells and a few immature B cells, the systemic side effects of
treatment targeting ZAP70 are manageable (19).
In the present study, transcriptome and clinical
data from patients with EC were downloaded from TCGA. According to
the results of weighted gene co-expression network analysis (WGCNA)
analysis, 14 key genes, including ZAP70, were identified. The
patients were divided into two groups according to the expression
levels of key genes (high vs. low), and the DEGs expressed in the
two groups were identified. The differences in gene function,
immune cell infiltration and ICI expression, as well as the
prognosis of patients, between the two groups were analyzed by
bioinformatics.
Materials and methods
Data sources and pre-processing
The normalized RNA expression data and clinical
characteristics (including age, status, FIGO stage and survival
time) of 587 patients with EC were downloaded from TCGA
(portal.gdc.cancer.gov) database (TCGA-UCEC) with the help of
TCGAbiolinks R package (2.18.0), which included 552 tumor and 35
normal samples (20). Transcriptome
data were transformed by log2(FPMK+1) (Fragments Per
Kilobase of exon per Million fragments mapped) (21).
DEGs between EC and normal
samples
DEGs were obtained by comparing htseq-counts data
between tumor specimens and normal specimens using the R package
DESeq2 (22). The threshold of
screening DEGs was set as |log2(foldchange)|>1 and
P<0.05.
Immune-related key modules and hub
genes screened by WGCNA
Immune-related key modules and hub genes were
screened using WGCNA (23). First,
a soft threshold was determined and the coexpression similarity was
raised to this threshold to calculate the adjacency. The soft
threshold was calculated by the criterion of approximate scale-free
topology. Second, the network structure was adjusted into
topological overlap matrix after the adjacency association was
determined, and genes with similar expression characteristics were
clustered hierarchically. Third, the dynamic tree cutting algorithm
was used to divide the modules, so that each module had ≥20 genes
and the similar modules were fused (24). Finally, after constructing the
network, immune-related modules and genes were obtained by
adjusting the criteria of mode-trait correlation, gene significance
(Pearson's correlation coefficient between genes and traits) and
module membership (Pearson's correlation coefficient between genes
and module feature genes). As a result, an immune-related key
module and hub genes were identified.
Functional enrichment analysis
Biological function analyses included Gene Ontology
(GO) (geneontology.org/), Kyoto Encyclopedia of Genes and Genomes
(KEGG) (genome.jp/kegg/) and Gene Set Enrichment Analysis (GSEA)
(25). KEGG analyzed the cells
metabolic pathways and functional pathways of gene enrichment. The
top 20 pathways with the lowest P-value were identified and
arranged according to their P-value (from smallest to largest) to
focus on the most statistically significant pathways. GSEA was used
to calculate the enrichment of known signaling pathways in specific
rankings (26).
Correlation analysis
The Search Tool for the Retrieval of Interacting
Genes/Proteins database was used to construct protein-protein
interaction (PPI) networks (27);
the confidence score was set to 0.700. Univariate Cox risk analysis
was performed on survival status and survival time using the
‘survival’ package, and key genes affecting the survival status of
patients with uterine corpus EC (UCEC) were identified (28). Spearman's correlations between gene
and gene, between genes and ESTIMATEScore, and between genes and
the single sample Gene Set Enrichment Analysis (ssGSEA) were
calculated using the R package corrplot (0.84)
(github.com/taiyun/corrplot). ESTIMATEScore is used to predict
tumor purity and the presence of infiltrating stromal cells and
immune cells in tumor tissue. This analysis estimates the ratio of
stromal cells and immune cells in malignant tumor tissue based on
gene expression data.
Consensus clustering
Patients with UCEC were divided into two groups
using the R package ConsensusClusterPlus (1.54.0), and DEGs were
obtained using the R package limma (29,30).
The patients with UCEC were divided into two groups by consensus
clustering based on transcriptome data of key genes in TCGA-UCEC
samples, aiming to cluster the samples with similar expression
levels of the key genes into one group.
Evaluation of the immune
microenvironment
The R package, Estimation (1.0.13), was used to
calculate and compare the StromalScore (stromal content),
ImmuneScore (degree of immune cell infiltration), ESTIMATEScore
(synthetic marks for stroma and immune) and TumorPurity (proportion
of cancer cells in the admixture) of patients with UCEC (31). The proportion of 22 (B cells naive,
B cells memory, Plasma cells, T cells CD8, T cells CD4 naive, T
cells CD4 memory resting, T cells CD4 memory activated, T cells
follicular helper, T cells regulatory (Tregs), T cells gamma delta,
NK cells resting, NK cells activated, Monocytes, Macrophages M0,
Macrophages M1, Macrophages M2, Dendritic cells resting, Dendritic
cells activated, Mast cells resting, Mast cells activated,
Eosinophils, Neutrophils) and 28 (Activated B cell, Activated CD4 T
cell, Activated CD8 T cell, Activated dendritic cell, CD56bright
natural killer cell, CD56dim natural killer cell, Central memory
CD4 T cell, Central memory CD8 T cell, Effector memory CD4 T cell,
Effector memory CD8 T cell, Eosinophil, Gamma delta T cell,
Immature B cell, Immature dendritic cell, Macrophage, Mast cell,
MDSC, Memory B cell, Monocyte, Natural killer cell, Natural killer
T cell, Neutrophil, Plasmacytoid dendritic cell, Regulatory T cell,
T follicular helper cell, Type 1 T helper cell, Type 17 T helper
cell, Type 2 T helper cell) immune cell types in each UCEC samples
were analyzed using the R package Cibersort (v1.03) and Gene Set
Variation Analysis (GSVA), respectively (32,33).
Verification of key gene
expression
To verify the expression of 14 key genes in EC and
normal endometrial tissue, the present study attempted to integrate
the normal endometrial samples from the Genotype-Tissue Expression
(GTEx) (http://commonfund.nih.gov/GTEx/) Project with EC
samples from TCGA database. A total of 101 normal endometrial
samples and 181 EC samples were obtained. The aforementioned 14 key
genes were searched on PubMed (pubmed.ncbi.nlm.nih.gov/) and the
Web of Science (https://www.webofscience.com/wos/) to look for genes
that have not been reported in EC or endometrium for further
study.
Patients
Tissue samples were collected from patients who
visited the Department of Obstetrics and Gynecology of General
Hospital of Northern Theater Command (Shenyang, China) between
January 2019 and December 2021. Tumoral tissue from 19 patients
with EC and normal endometrial tissue from 11 patients with
non-endometrial disease and non-cancer were collected and used to
detect the mRNA expression levels of ZAP70 by reverse
transcription-quantitative PCR (RT-qPCR). A comparison of age and
BMI between patients with EC and patients with non-endometrial
disease and non-cancer is presented in Table I. Paired EC and normal endometrial
tissues from 10 patients with EC were used for immunohistochemical
detection of ZAP70 protein expression. The clinicopathological
characteristics of all patients with EC were shown in Table II.
 | Table I.General characteristics of patients
with endometrial cancer and healthy control patients. |
Table I.
General characteristics of patients
with endometrial cancer and healthy control patients.
| Patient | Age, years (mean ±
SD) | BMI,
kg/m2 (mean ± SD) |
|---|
| Endometrial
cancer | 50.68±7.543 | 22.96±3.275 |
| Healthy
control | 53.18±5.930 | 23.45±2.826 |
| P-value | 0.3550 | 0.6833 |
 | Table II.Clinicopathological characteristics
of patients with endometrial cancer. |
Table II.
Clinicopathological characteristics
of patients with endometrial cancer.
| Clinicopathological
characteristic | Patients
(n=19) | Percentage |
|---|
| Age, years |
|
|
|
<50 | 7 | 36.80 |
|
≥50 | 12 | 63.20 |
| BMI,
kg/m2 |
|
|
|
<25 | 14 | 73.70 |
|
≥25 | 5 | 26.30 |
| History of estrogen
use |
|
|
|
Yes | 2 | 10.50 |
|
None | 17 | 89.50 |
| FIGO stage |
|
|
| I and
II | 12 | 63.20 |
| III and
IV | 7 | 36.80 |
| Survival
status |
|
|
|
Alive | 18 | 94.70 |
|
Deceased | 1 | 5.30 |
RT-qPCR
Tissue RNA from 19 patients with EC and 11 patients
with non-endometrial disease and non-cancer was extracted from
ground tissues using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). RNA was reverse transcribed into cDNA
using the PrimeScript™ RT Reagent Kit with gDNA Eraser (cat. no.
RR047A; Takara Bio, Inc.) according to the manufacturer's protocol.
cDNA was added to a 20-µl system containing SYBR Premix Ex Taq
(cat. no. RR820A; Takara Bio, Inc.). qPCR was performed using
QuantStudio™ 1 Real-Time PCR System (cat. no. A40425; Thermo
Scientific, Inc.), and the thermocycling conditions were set as: 1
cycle of pre-denaturation (95°C for 30 sec), 40 cycles of PCR
reaction (95°C 5 sec, 60°C 34 sec), and 1 cycle of solubilization
curve analysis (95°C 15 sec, 60°C 60 sec, 95°C 15 sec). ZAP70 and
GAPDH primer sequences are provided in Table III. The relative expression was
expressed by 2−∆∆Cq, and the data were normalized to
GAPDH (34). All experiments were
repeated three times.
 | Table III.Primer sequences used for reverse
transcription-quantitative PCR. |
Table III.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Primer sequence (5′
to 3′) |
|---|
| ZAP70 | F:
CAAGTTTGACACGCTCTGGC |
|
| R:
GTAGGGGCTCTCATACACGC |
| GAPDH | F:
CGGATTTGGTCGTATTGGG |
|
| R:
CTGGAAGATGGTGATGGGATT |
Immunohistochemistry and
immunofluorescence
Fresh tissues were fixed at 4°C with 4%
paraformaldehyde for 24 h and embedded in paraffin blocks. The
2-step plus Poly-HRP Anti Rabbit/Mouse IgG Detection System
immunohistochemistry kit (cat. no. E-IR-R211; Elabscience
Biotechnology, Inc.) was used to analyze tissue sections (4 µm).
The experiment was conducted according to the kit instructions. The
slides were incubated overnight at 4°C with rabbit anti-ZAP70
polyclonal antibody (1:200; E-AB-19063; Elabscience Biotechnology,
Inc.). Five visual fields were randomly selected for each section
under a 400× microscope for scoring, and the criteria were as
follows: 1. According to staining intensity, the sections were
divided into non-staining, light yellow, brown and brown, which
were recorded as 0, 1, 2 and 3 points in turn; 2. According to the
percentage of colored cells in the field of vision, the positive
cell rate was <10% : 1 point, 10–50% : 2 points, and >50% was
rated as 3 points. The product of the two scores gives the final
score. HEC-1-A (cat. no CL-0099; Procell Life Science &
Technology Co., Ltd.) and Ishikawa (cat. no. CL-0283; Procell Life
Science & Technology Co., Ltd.) cells were cultured in RPMI
1640 (cat. no SH30255.01; HyClone Biotechnology, Inc.) with 10%
fetal bovine serum (FBS) (cat. no 164210; Procell Life Science
& Technology Co., Ltd.). Cells were cultured under sterile
conditions at 37°C and 5% CO2. Cells were fixed on
coverslips, washed, permeabilized, sealed, and incubated with
rabbit anti-ZAP70 polyclonal antibody (1:200; E-AB-19063;
Elabscience Biotechnology, Inc.). Immunofluorescence Staining
Kit-Anti-Rabbit Alexa Fluor 647 (cat. no. P0180; Beyotime Institute
of Biotechnology) was used for testing the following day according
to the manufacturer's protocol. Slides were examined and images
captured under a fluorescence microscope (cat. no. 703548; Nikon
Corporation).
Statistical analysis
Data were analyzed with R software (4.0.2)
(https://www.r-project.org/), and
GraphPad Prism version 8.0 (Dotmatics) was used to evaluate
statistical difference. All hypothesis tests were two-sided, and
P<0.05 was considered to indicate a statistically significant
difference. Kaplan-Meier analysis was calculated using the R
package ‘glmnet’ and compared by log-rank analysis. Wilcoxon
rank-sum test was used to compare unpaired samples, whereas paired
samples of immunohistochemistry were compared using Wilcoxon
signed-rank test. Correlations were calculated with Spearman's
coefficient analysis.
Results
Differential expression analysis and
identification of immune hub genes by WGCNA
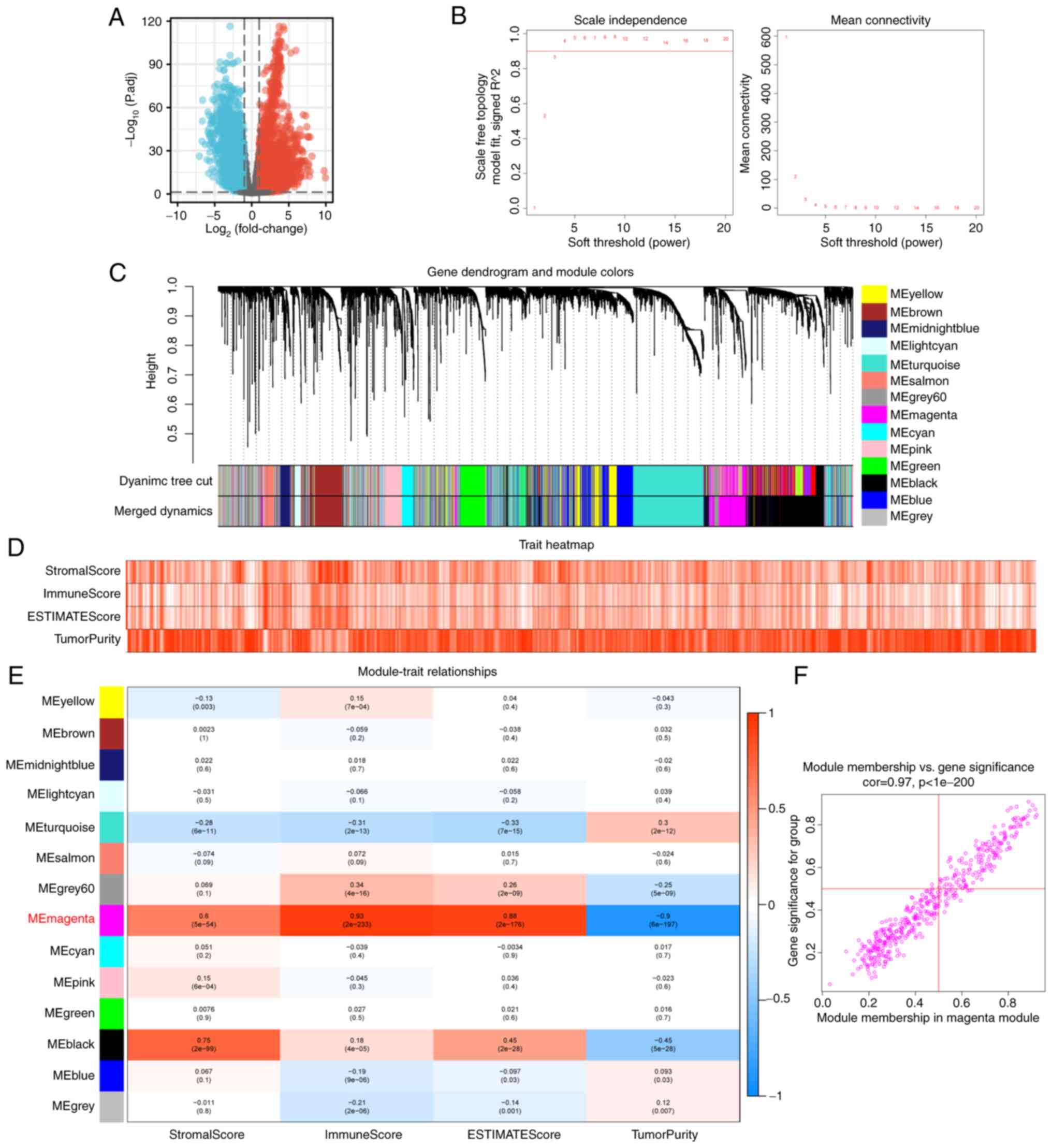
WGCNA was performed on 6,131 genes that differed
between EC and normal tissue from TCGA-UCEC (Fig. 1A). The soft threshold was set to 4
and the DEGs were clustered into 14 modules (Fig. 1B and C). Modules are highly
interrelated clusters of genes with similar ESTIMATEScores.
ESTIMATEScores of all samples are presented in the form of a heat
map in order to obtain the module with the strongest correlation
with immune score in the TCGA-UCEC sample (Fig. 1D). In the correlation heat map, ‘ME
magenta’ represented the strongest correlation module with
ImmuneScores (R=0.93; P<0.001; Fig.
1E). Finally, 108 hub genes which have high connectivity in the
magenta module were screened according to module membership (MM)
>0.5 and gene significance (GS) >0.5 (Fig. 1F). Module membership refers to the
correlation between each gene and the module characteristics of a
given module according to its gene expression profile. Gene
significance is a measure of the importance of a gene. The higher
the absolute value of GS of a gene, the greater its biological
significance. The cut-off values of MM and GS are customized
according to the distribution and number of hub genes in the
module.
Functional enrichment analysis and
identification of 108 hub genes
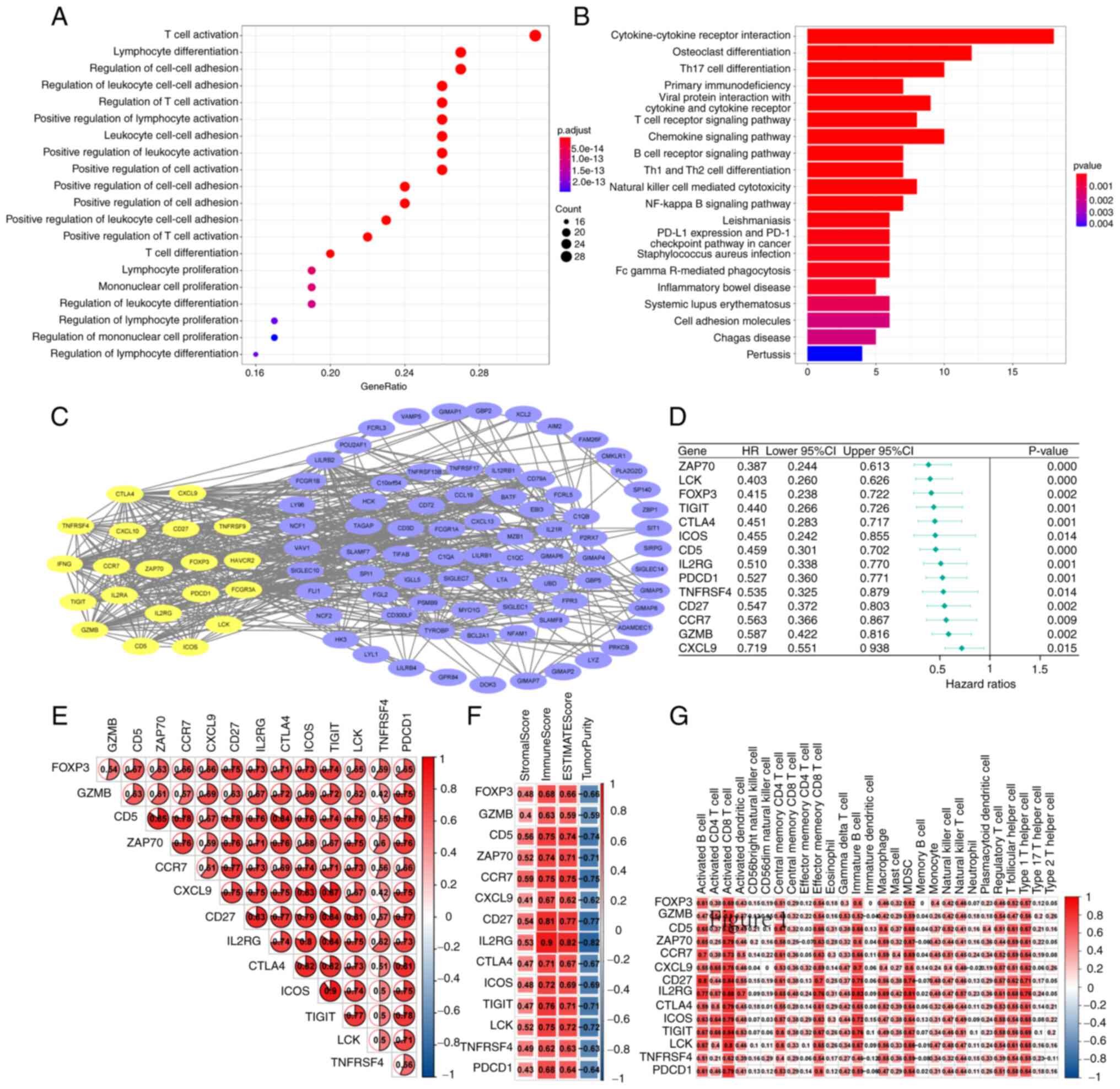
GO analysis of the 108 hub genes revealed that
several were enriched in ‘T cell activation’ and ‘lymphocyte
differentiation’ (P<0.001; Fig.
2A). Through KEGG enrichment, it was found that the top three
enrichment pathways of the 108 hub genes were ‘Cytokine-cytokine
receptor interaction’, ‘Osteoclast differentiation’ and ‘Th17 cell
differentiation’ (P<0.001; Fig.
2B). A PPI network was established, and 20 genes with closely
related functions (yellow) were identified (Fig. 2C). Cox proportional hazards model
found 14 genes that affect the survival outcome and survival time
of patients with UCEC (Fig. 2D);
ZAP70, LCK, FOXP3, TIGIT, CTLA4, ICOS, CD5, IL2RG, PDCD1, TNFRSF4,
CD27, CCR7, GZMB and CXCL9 were identified as key genes that may
affect the prognosis of patients with EC. Through gene-gene
Spearman's correlation analysis, a strong positive correlation
between these proteins encoded by key genes was found (Fig. 2E). Of these, ZAP70 has the strongest
correlation with CD5. Regarding the correlation between genes and
immune cell infiltration, the 14 key genes were positively
associated with ImmuneScore and negatively associated with
TumorPurity (Fig. 2F); of the key
genes, IL2RG had the strongest correlation with ImmuneScore
(Fig. 2F). ssGSEA show that, among
the 28 immune cell types, the 14 key genes mainly affected
activated CD8+ T cells, immature B cells and
Myeloid-derived suppressor cells (Fig.
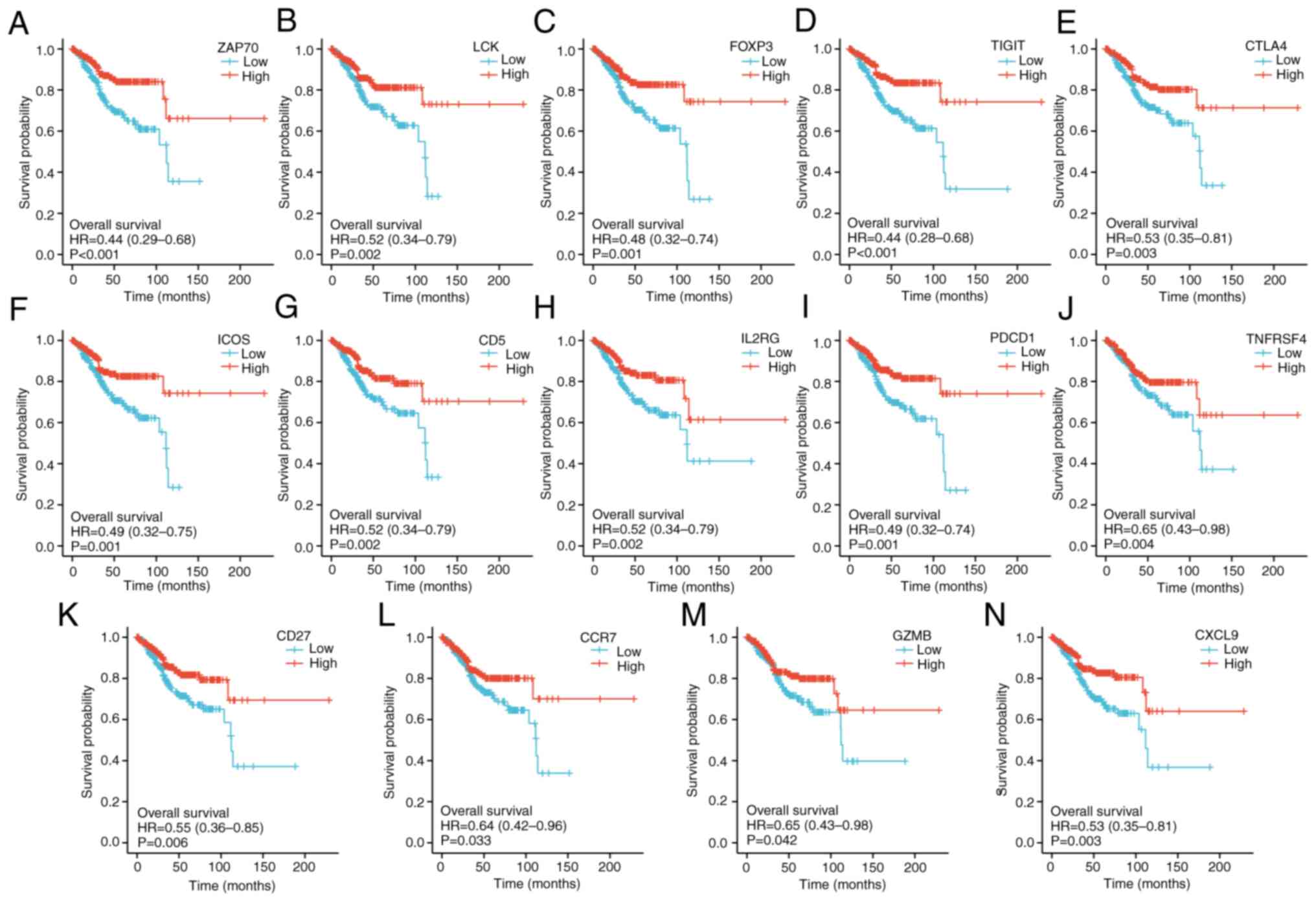
2G). To determine whether these key genes were associated with
a good prognosis in patients with UCEC, Kaplan-Meier analyses were
performed. The median expression of key genes in each TCGA-UCEC
sample was used as the cut-off point to separate the patients into
high and low group. The results showed that patients with UCEC with
higher expression of each key gene had a longer overall survival
time (Fig. 3).
Functional enrichment analysis of
DEGs
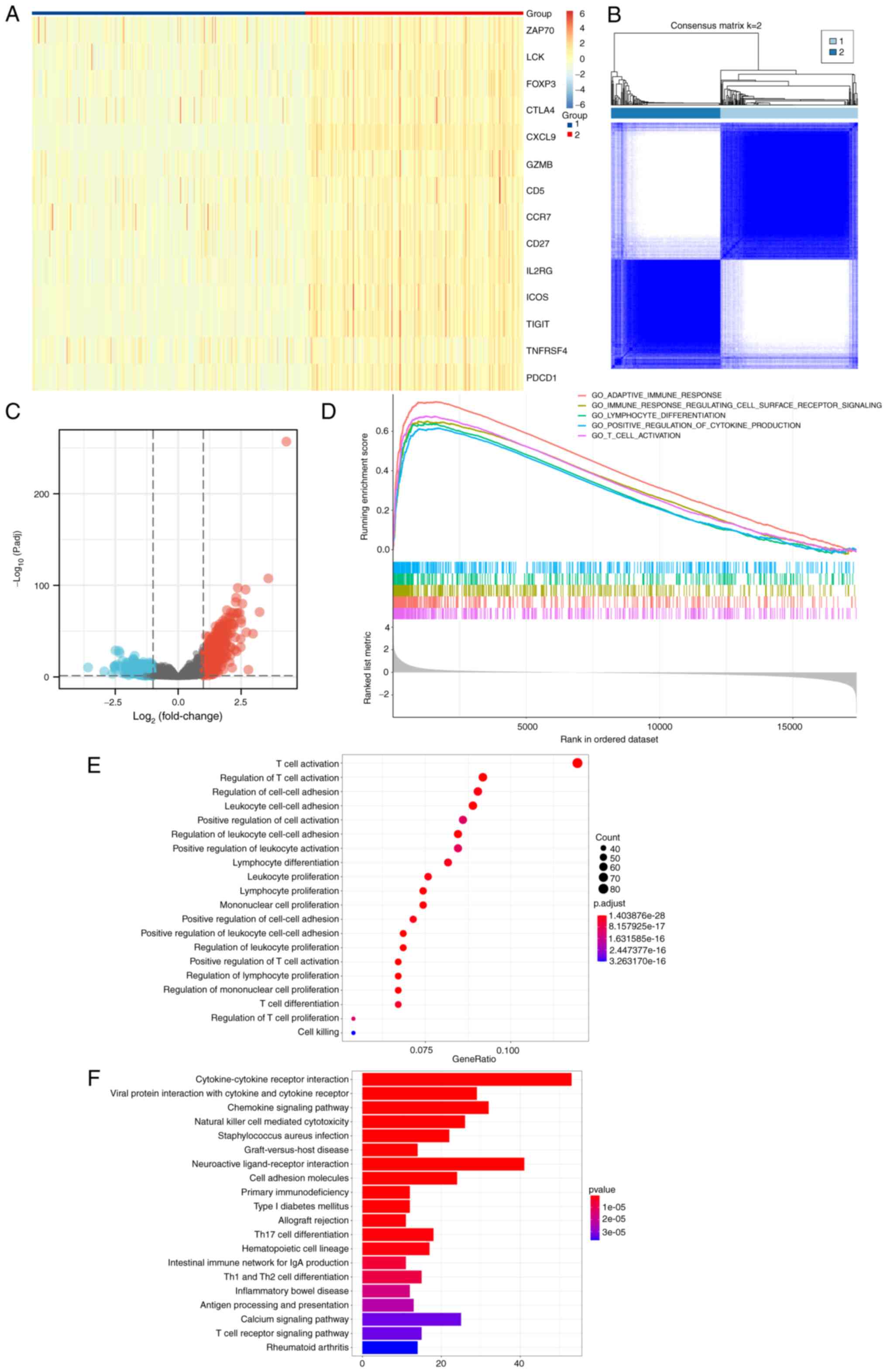
According to the results of consensus clustering,
expression of each key gene in group 2 was higher compared with
that in group 1 (Fig. 4A and B).
There were 763 DEGs with statistically significant differences
between patients of groups 1 and 2 (Fig. 4C). GSEA revealed that ‘adaptive
immune response’, ‘immune response regulating cell surface receptor
signaling’, ‘lymphocyte differentiation’, ‘positive regulation of
cytokine production’ and ‘T cell activation’ were enriched
(Fig. 4D). ‘T cell activation’,
‘regulation of T cell activation’, ‘regulation of cell-cell
adhesion’ in GO term enrichment analysis (Fig. 4E), and ‘Cytokine-cytokine receptor
interaction’, ‘Viral protein interaction with cytokine and cytokine
receptor’ and ‘Chemokine signaling pathway’ in KEGG pathway
enrichment analysis (Fig. 4F)
revealed the role of DEGs in tumor development, immunity and other
biological processes.
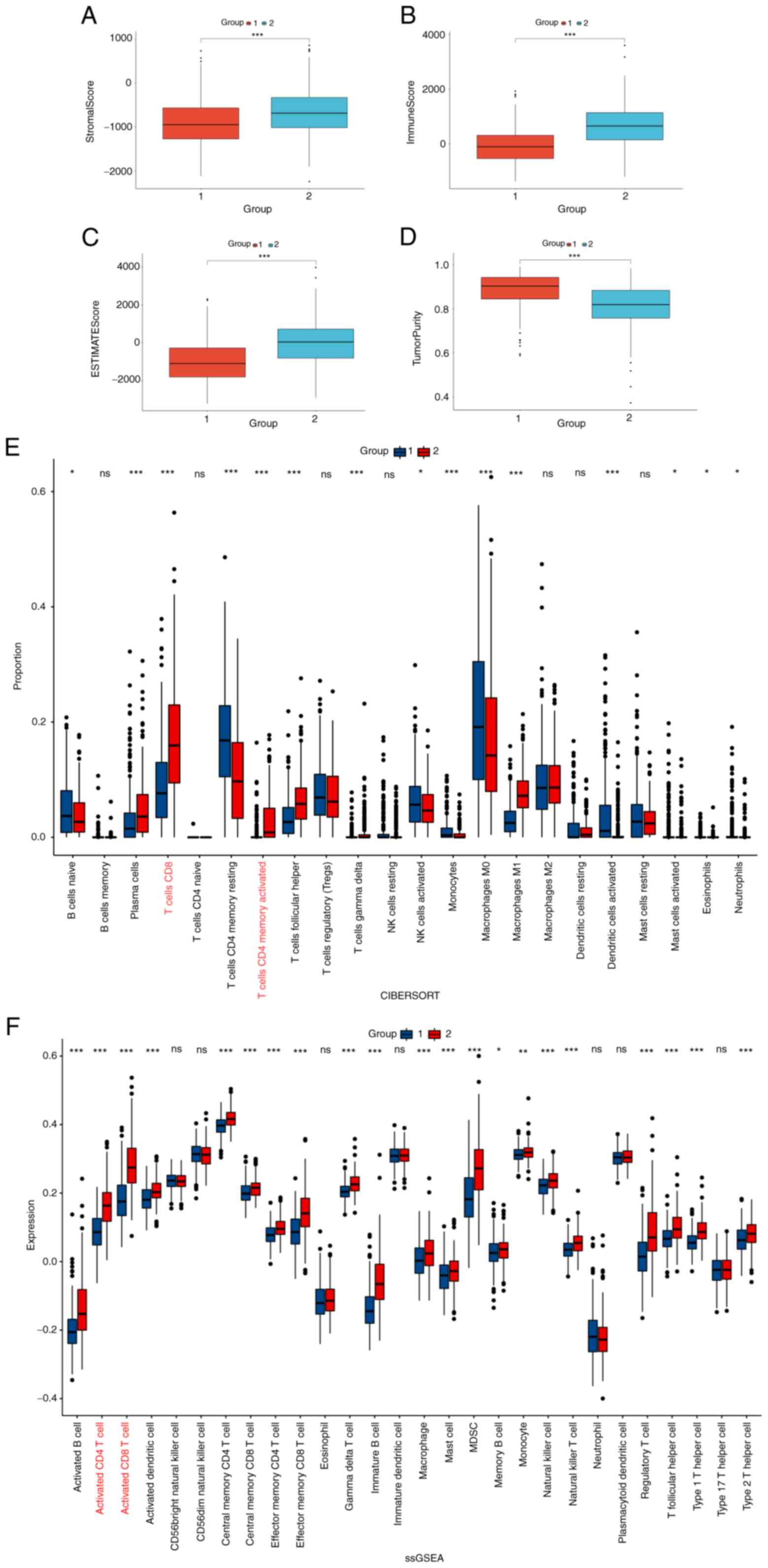
Difference in immune cell infiltration
and expression analysis of immune checkpoints
ESTIMATE, CIBERSORT and ssGSEA analyses were used to
determine the differences in the degree and composition of immune
cell infiltration between the two groups. The higher StromalScore
(Fig. 5A), ImmuneScore (Fig. 5B) and ESTIMATEScores (Fig. 5C), and lower TumorPurity (Fig. 5D) in group 2 compared with group 1
attested to a stronger degree of immune cell infiltration. The
percentages of activated CD4+ and CD8+ T
cells which are the main cells that play an antitumor role in group
2 were higher compared with group 1 in the 22 immune cell species
from CIBERSORT and in the 28 immune cell infiltration assays from
ssGSEA (Fig. 5E and F,
respectively). The expression levels of immune checkpoint markers
usually determine the sensitivity of patients to immunotherapy.
Notably, immune checkpoints markers PD1, PD-L1, PD-L2, CTLA-4,
CD80, CD86, LAG3, TIM3, TIGIT, OX40, GITR, 4-1BB, ICOS, CD40, CD27
and CD70 were upregulated in group 2 compared with their expression
levels in group 1 (Fig. 6).
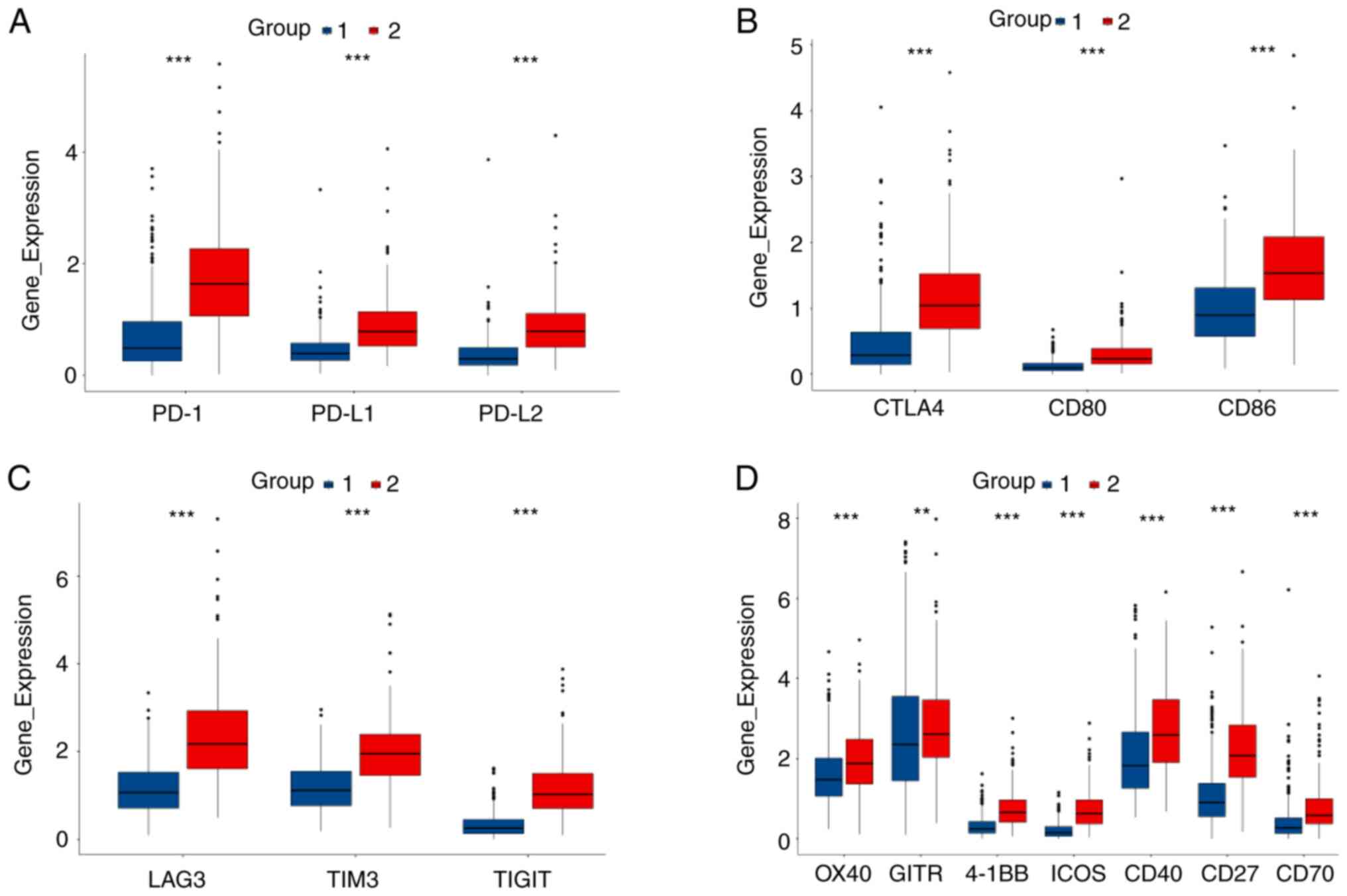 | Figure 6.Comparison of the expression levels
of immune checkpoint genes between two groups. (A) Comparison of
PD-1, PD-L1 and PD-L2 expression levels between two groups. (B)
Comparison of CTLA4, CD80 and CD86 expression levels between two
groups. (C) Comparison of LAG3, TIM3 and TIGIT expression levels
between two groups. (D) Comparison of OX40, GITR, 4-1BB, ICOS,
CD40, CD27 and CD70 expression levels between two groups. PD1,
programmed death 1; PD-L, programmed death ligand. **P<0.01 and
***P<0.001 |
Verification of expression levels of
ZAP70 in tissue and cells and its effect on prognosis
Next, the expression of 14 key genes were examined
in patients with UCEC from the GTEx-TCGA database. With the
exception of ZAP70 and CCR7 (Fig. S1A
and S1L), the expression of the other key genes was
significantly higher in EC tissues compared with expression in
normal tissues (Fig. S1). The
aforementioned 14 key genes were searched on PubMed and the Web of
Science. In addition to ZAP70, 13 other key genes were found to
have been reported in normal endometrium or EC (35–46).
Therefore, ZAP70 was selected for the subsequent experimental
verification because to the best of our knowledge, it has not been
reported in EC or endometrial tissue and thus represents a novel
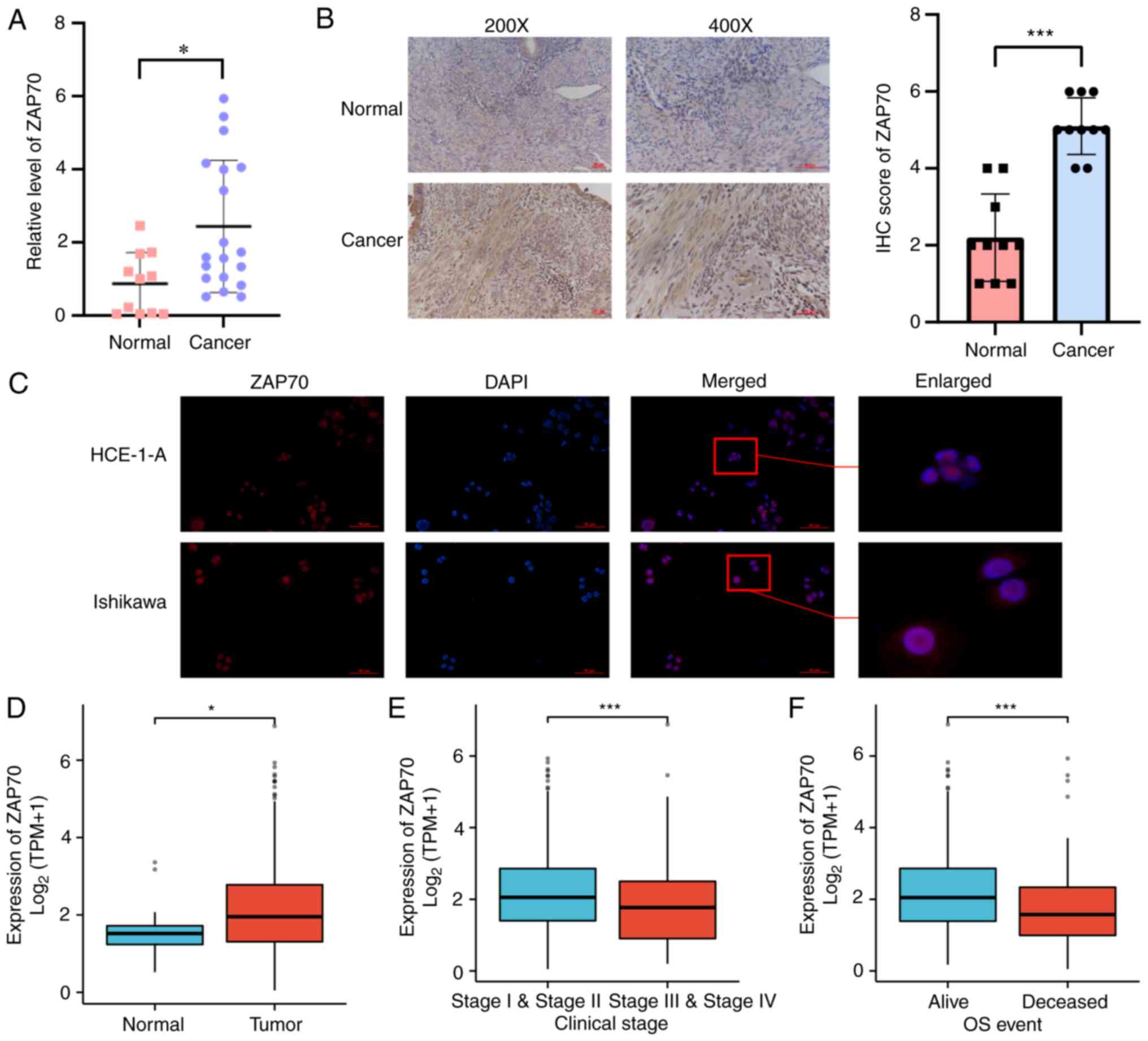
target. Subsequently, ZAP70 mRNA expression was examined in 19 EC
tissues and 11 healthy endometrial tissues. It was shown that ZAP70
mRNA expression in EC tissues was higher than that in healthy
endometrial tissues (Fig. 7A).
Next, ZAP70 protein expression was detected in 10 pairs of EC and
healthy endometrial tissues using immunohistochemistry. Consistent
with the mRNA expression levels, ZAP70 protein was 2.3 times more
expressed in EC than in matched healthy endometrial tissue
(Fig. 7B). This difference in
expression could be attributed to the improved breadth rather than
the accuracy of GTEx-TCGA data). The immunofluorescence assay data
showed that ZAP70 was mainly expressed in the nucleus of EC cells,
with limited expression in the cytoplasm (Fig. 7C). In TCGA database, the expression
level of ZAP70 in EC tissues was higher than that in normal
endometrial tissues (Fig. 7D).
ZAP70 was expressed lower in patients with FIGO stage III and IV EC
compared with those with FIGO stage I and II EC (Fig. 7E). ZAP70 expression was lower in
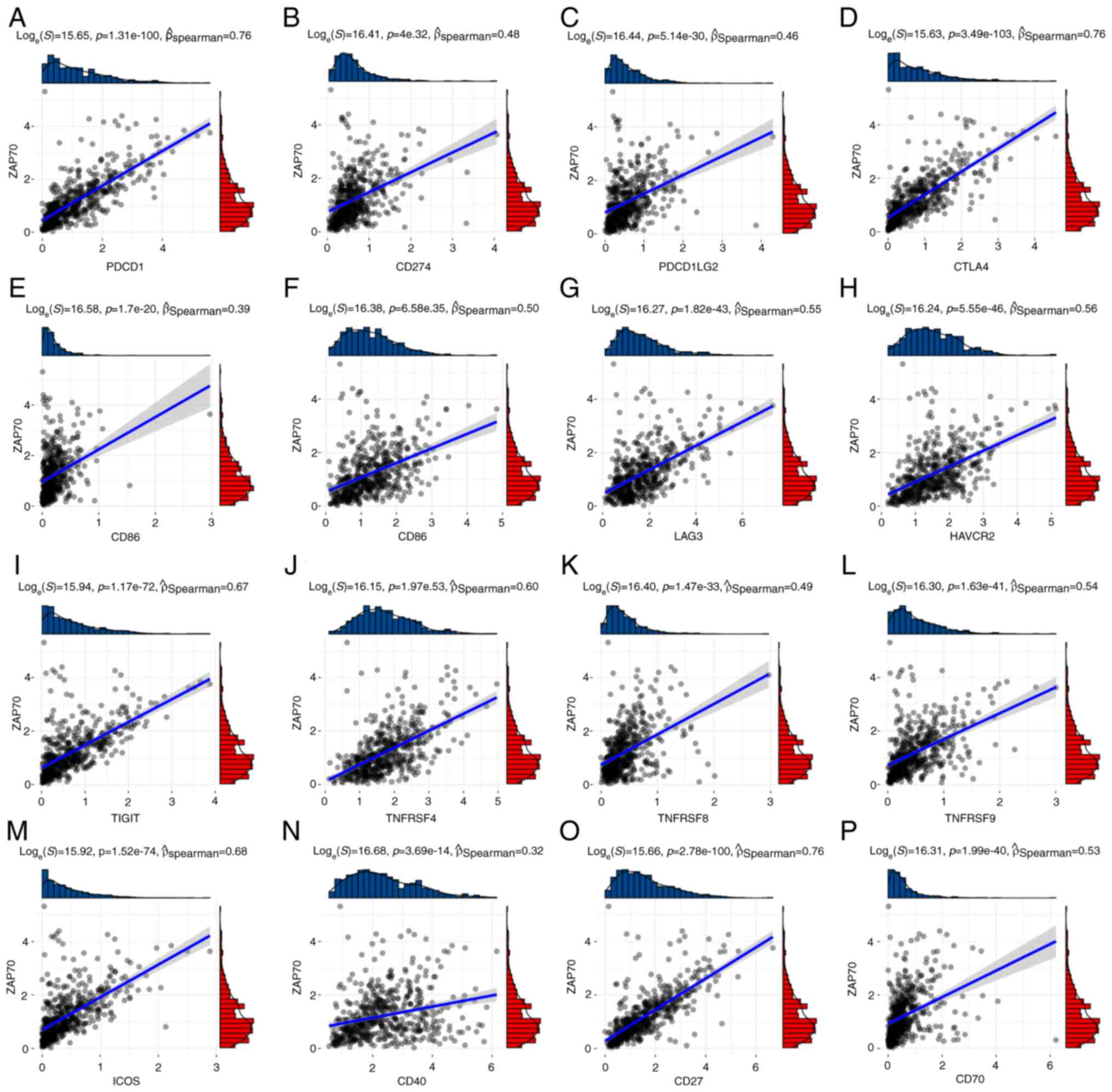
deceased patients than in those patients that were alive (Fig. 7F). In the correlation analysis
between ZAP70 and ICIs using TCGA-UCEC data, it was found that
ZAP70 was positively associated with 16 known immune checkpoints
(Fig. 8).
Discussion
The identification of immune checkpoints has
overturned the model of cancer treatment and ushered in a new era
of cancer treatment; however, only a small number of patients with
cancer benefit from immune checkpoint therapy (47). Anti-PD-1/PD-L1 have been widely
studied in MSI-H/dMMR EC, and their effects are encouraging. For
example, in the KEYNOTE-158 trial, 49 patients with MSI-H EC with
metastatic/relapsed disease were treated with pembrolizumab (PD-1
inhibitor), with an overall response rate (ORR) of 57%, a median
progression-free survival (PFS) of 25.7 months, and a complete
response in 16.3% of patients (48). In an efficacy study of durvalumab
(anti-PD-L1) in patients with advanced EC, the ORR in the
MMR-deficient group was 40% (n=35; 95%CI, 26–56), including 4 cases
of complete response and 10 cases of partial response (49). The emergence of additional
immunotherapeutic targets for advanced and refractory EC is
expected.
The current study involved WGCNA on 6,131 genes that
differed between cancer and normal tissues, and the magenta module,
which contained 108 hub genes, demonstrated the strongest
correlation with immune score. Through screening by PPI network and
univariate Cox risk analysis, 14 key genes including ZAP70 were
identified. ZAP70 is highly expressed in a wide variety of tumors
(16). In hematological tumors,
particularly CLL, ZAP70 enhances the phosphorylation of
ZAP70-positive CLL B cells Syk, BLNK and PLCγ, thereby promoting B
cell receptor (BCR)signal enhancement, cell proliferation and
migration to the tumor microenvironment (TME) (50,51).
In contrast to CLL, in solid tumors, high ZAP70 expression
indicates good prognosis and radiotherapy sensitivity (52). ZAP70 has been shown to be a
potential therapeutic target in the TME affecting prognosis in
laryngeal phosphorous cell carcinoma, breast cancer, prostate
cancer, cutaneous melanoma, bladder cancer and nasopharyngeal
cancer (53–58). In a novel chimeric antigen receptor
(CAR) strategy for targeting NK cells, activation of Syk/ZAP70
reversed HLA-G mediated immunosuppression and restored NK cell
lysis to ablate solid tumors (59).
In the present study, 108 immune-related DEGs were
examined and the functional enrichment pathways were associated
with T-cell activation and function in GO analysis. T cells are the
main component of TME, and numerous studies have been conducted on
targeted therapy using T cells (60,61).
Adoptive T-cell therapy (ACT) is the first and most effective
treatment to target the immune system. This therapy involves in
vitro amplification, screening and modification of autologous
lymphocytes reinjection to the patient to achieve T cell-mediated
tumor regression (62). Currently,
research is being carried out to promote the use of ACT for the
treatment of EC. To identify the factors influencing the prognosis
of patients with UCEC, the present study used the univariate Cox
risk analysis to identify 14 genes that may be protective factors:
ZAP70, LCK, FOXP3, TIGIT, CTLA4, ICOS, CD5, IL2RG, PDCD1, TNFRSF4,
CD27, CCR7, GZMB and CXCL9. Of these, ZAP70 had the highest
association with CD5, which has been demonstrated in previous
studies. Gladkikh et al (63) demonstrated that ZAP70 was
differentially expressed in normal B lymphocytes with CD5-high and
CD5-low expression, and low ZAP70 expression would lead to BCR
signal dysfunction, which confirmed its key role in altered BCR
signaling in B-CLL. When exploring the correlation between these
key genes and the immune microenvironment, it was demonstrated that
IL2RG was the most strongly associated gene with the ImmuneScore.
IL2RG is located on Xq13 of the X chromosome and its mutation
causes X-linked severe combined immunodeficiency (64). A defect in the IL2RG gene causes a
lack of T, NK and partially functional B cells, and this defect is
fatal (65). Two classical immune
checkpoints were observed in these key genes, PD-1 and CTLA4. In
2017, Le et al (66)
examined the sensitivity of patients with advanced dMMR cancer to
pembrolizumab treatment in 12 different tumor types. Of all the
tumor types, dMMR occurred most frequently in EC (17%), with an ORR
of 53% and a complete response rate of 21%, including 15 EC cases.
Oh and Chae (67) reported a case
of a patient with MMR-proficient, PD-L1-negative stage IV EC who
was treated with combined inhibition of PD-1 and CTLA-4. The
patient showed a marked response to the treatment shortly after
treatment, with multiple tumor metastases being reduced.
The present study performed consensus clustering
analysis based on the expression levels of 14 key genes in order to
divide patients with UCEC into two groups for comparison.
Functional enrichment analysis of DEGs in these two groups was
associated with T-cell-mediated immune pathways. In the KEGG
analysis, one of the pathways, ‘Neuroactive ligand-receptor
interaction’, was absent from the top three. However, there are ≥40
DEGs of the 763 DEGs involved in this pathway. Previous studies
have shown that the interaction between neuroactive ligands and
receptors, as a part of the TME, affects tumor progression and
invasion (68). Although there are
no clear studies showing that nerve signaling can affect the immune
microenvironment of patients with EC, the role of neuro-related
genes in the reclassification of EC has been previously suggested
(69). These findings indicated
that neuroactive ligand receptor interactions in the TME may be
used as a new research direction for immunotherapy of EC in the
future.
Following consensus clustering of patients with UCEC
in the present study, it was found that patients in group 2 with
higher expression of key genes had higher immune cell infiltration
status, particularly CD4+ and CD8+ T cells.
CD8+ T cells are the main effector cells of adaptive
immunity against cancer cells in the microenvironment. However,
during tumor development, CD8+ T cells differentiate
into a ‘dysfunctional’ state (70).
As these cells are less reactive to tumor antigens, cancer cells
cannot be recognized and eliminated (71). Activation of CD8+ T cells
and intranodal migration of memory T cells are critical for cancer
immunosurveillance, and these can be achieved by PD-1/PD-L1
inhibition or CAR T-cell therapy (72,73).
The key genes LCK and ZAP70 have a direct impact on CAR T-cell
therapy. Mestermann et al (74) showed that tyrosine kinase inhibitors
can interfere with LCK, thereby inhibiting the phosphorylation of
CD3ξ and ZAP70ξ. Inhibition of activated modules in the CAR
structures, such as CD28-CD3ξ or 4-1BB_CD3ξ, resulted in functional
inhibition of CD8+ and CD4+ CAR T cells. Based on these studies on
immune cell infiltration and immune checkpoints, it can be
speculated that patients with high expression of key genes, such as
ZAP70, may be more suitable for CAR T-cell therapy.
Finally, the expression and prognostic value of
ZAP70 in EC were detected by combining experimental and TCGA-UCEC
clinical data. First, high expression of ZAP70 mRNA and protein in
EC tissues was confirmed by RT-qPCR and immunohistochemistry,
respectively. However, the number of samples we used for this
result was low, which makes our conclusion have certain
limitations; thus, additional tissue samples are needed for further
research on the functions of ZAP70 in EC. ZAP70 was positively
associated with known ICIs, indicating that high expression of ICIs
may also exist in patients with EC who exhibit high expression of
ZAP70, thus potentially achieving an improved immunotherapeutic
effect. However, after analyzing the clinical data of TCGA-UCEC, it
was observed that high expression of ZAP70 was a factor of
favorable prognosis in patients with EC. High expression of ZAP70
was found in both early stage and surviving patients. Thus, it was
hypothesized that tumorigenesis may activate the immune state of
the patient, leading to the phosphorylation and activation of
ZAP70, which is a key factor affecting the TCR. High expression of
ZAP70 predicts that in patients, immune cells such as T cells and B
cells, are sensitive to pMHC on the tumor surface, which results in
a good prognosis. In the present study, ZAP70 expression in
patients with EC was positively associated with the infiltration of
activated CD8+ T cells. And the high intraepithelial
CD8+ T cell counts in the endometrium is associated with
longer PFS (75). These evidences
suggested that ZAP70 may be a good prognostic factor for patients
with EC. In the future, we will further study the expression and
function of ZAP70 in EC. The present study found that 14 key genes,
including ZAP70, may affect the immune microenvironment in patients
with EC. The results demonstrated the potential of ZAP70 as a
target for EC immunotherapy and may provide new possibilities for
immunotherapy in patients with EC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YMZ and YXY designed the project. YMZ and HOL
collected data. YMZ performed the experiments. YMZ analyzed the
data and wrote the manuscript. All authors read and approved the
final version of the manuscript. YMZ, HOL and YXY confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study has been approved by the Ethics
Committee of General Hospital of Northern Theater Command (approval
no. Y2023002), and the informed consent exemption application has
been approved by the Ethics Committee of General Hospital of
Northern Theater Command.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kalampokas E, Giannis G, Kalampokas T,
Papathanasiou AA, Mitsopoulou D, Tsironi E, Triantafyllidou O,
Gurumurthy M, Parkin DE, Cairns M and Vlahos NF: Current approaches
to the management of patients with endometrial cancer. Cancers
(Basel). 14:45002022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hamilton CA, Pothuri B, Arend RC, Backes
FJ, Gehrig PA, Soliman PT, Thompson JS, Urban RR and Burke WM:
Endometrial cancer: A society of gynecologic oncology
evidence-based review and recommendations. Gynecol Oncol.
160:817–826. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crosbie EJ, Kitson SJ, McAlpine JN,
Mukhopadhyay A, Powell ME and Singh N: Endometrial cancer. Lancet.
399:1412–1428. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Madariaga A, Coleman RL and González
Martín A: Novel therapies leading to a new landscape in gynecologic
tumors. Int J Gynecol Cancer. 33:321–322. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inoue F, Sone K, Toyohara Y, Tanimoto S,
Takahashi Y, Kusakabe M, Kukita A, Honjoh H, Nishijima A, Taguchi
A, et al: Histone arginine methyltransferase CARM1 selective
inhibitor TP-064 induces apoptosis in endometrial cancer. Biochem
Biophys Res Commun. 601:123–128. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pirš B, Škof E, Smrkolj V and Smrkolj Š:
Overview of immune checkpoint inhibitors in gynecological cancer
treatment. Cancers (Basel). 14:6312022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park JY, Lee JY, Lee YY, Shim SH, Suh DH
and Kim JW: Major clinical research advances in gynecologic cancer
in 2021. J Gynecol Oncol. 33:e432022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yanazume S, Iwakiri K, Kobayashi Y,
Kitazono I, Akahane T, Mizuno M, Togami S, Tanimoto A and Kobayashi
H: Cytopathological features associated with POLE mutation in
endometrial cancer. Cytopathology. Feb 2–2023.doi:
10.1111/cyt.13215 (Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mullen MM and Mutch DG: Endometrial tumor
immune response: Predictive biomarker of response to immunotherapy.
Clin Cancer Res. 25:2366–2368. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rousset-Rouviere S, Rochigneux P, Chrétien
AS, Fattori S, Gorvel L, Provansal M, Lambaudie E, Olive D and
Sabatier R: Endometrial carcinoma: Immune microenvironment and
emerging treatments in immuno-oncology. Biomedicines. 9:6322021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Talhouk A, Derocher H, Schmidt P, Leung S,
Milne K, Gilks CB, Anglesio MS, Nelson BH and McAlpine JN:
Molecular subtype not immune response drives outcomes in
endometrial carcinoma. Clin Cancer Res. 25:2537–2548. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Burke KP, Patterson DG, Liang D and Sharpe
AH: Immune checkpoint receptors in autoimmunity. Curr Opin Immunol.
80:1022832023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Izawa M, Tanaka N, Murakami T, Anno T,
Teranishi Y, Takamatsu K, Mikami S, Kakimi K, Imamura T, Matsumoto
K and Oya M: Single-cell phenotyping of CD73 expression reveals the
diversity of the tumor immune microenvironment and reflects the
prognosis of bladder cancer. Lab Invest. 103:1000402023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ashouri JF, Lo WL, Nguyen TTT, Shen L and
Weiss A: TZAP70, too little, too much can lead to autoimmunity.
Immunol Rev. 307:145–160. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chakraborty AK and Weiss A: Insights into
the initiation of TCR signaling. Nat Immunol. 15:798–807. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leveille E, Chan LN, Mirza AS, Kume K and
Müschen M: SYK and ZAP70 kinases in autoimmunity and lymphoid
malignancies. Cell Signal. 94:1103312022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Linley AJ and Slupsky JR: Poor prognosis
is ZAP70′ed into focus in CLL. Blood. 137:3586–3587. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rosenwald A, Alizadeh AA, Widhopf G, Simon
R, Davis RE, Yu X, Yang L, Pickeral OK, Rassenti LZ, Powell J, et
al: Relation of gene expression phenotype to immunoglobulin
mutation genotype in B cell chronic lymphocytic leukemia. J Exp
Med. 194:1639–1647. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Au-Yeung BB, Levin SE, Zhang C, Hsu LY,
Cheng DA, Killeen N, Shokat KM and Weiss A: A genetically selective
inhibitor demonstrates a function for the kinase Zap70 in
regulatory T cells independent of its catalytic activity. Nat
Immunol. 11:1085–1092. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Colaprico A, Silva TC, Olsen C, Garofano
L, Cava C, Garolini D, Sabedot TS, Malta TM, Pagnotta SM,
Castiglioni I, et al: TCGAbiolinks: An R/Bioconductor package for
integrative analysis of TCGA data. Nucleic Acids Res. 44:e712016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan G, An Y, Xu B, Wang N, Sun X and Sun
M: Potential Impact of ALKBH5 and YTHDF1 on Tumor Immunity in Colon
Adenocarcinoma. Front Oncol. 11:6704902021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Langfelder P and Horvath S: WGCNA: An R
package for weighted correlation network analysis. BMC
Bioinformatics. 9:5592008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Langfelder P and Horvath S: Eigengene
networks for studying the relationships between co-expression
modules. BMC Syst Biol. 1:542007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reimand J, Isserlin R, Voisin V, Kucera M,
Tannus-Lopes C, Rostamianfar A, Wadi L, Meyer M, Wong J, Xu C, et
al: Pathway enrichment analysis and visualization of omics data
using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat Protoc.
14:482–517. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Szklarczyk D, Gable AL, Lyon D, Junge A,
Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork
P, et al: STRING v11: Protein-protein association networks with
increased coverage, supporting functional discovery in genome-wide
experimental datasets. Nucleic Acids Res. 47:D607–D613. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tibshirani R: The lasso method for
variable selection in the Cox model. Stat Med. 16:385–395. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wilkerson MD and Hayes DN:
ConsensusClusterPlus: A class discovery tool with confidence
assessments and item tracking. Bioinformatics. 26:1572–1573. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoshihara K, Shahmoradgoli M, Martínez E,
Vegesna R, Kim H, Torres-Garcia W, Treviño V, Shen H, Laird PW,
Levine DA, et al: Inferring tumour purity and stromal and immune
cell admixture from expression data. Nat Commun. 4:26122013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Newman AM, Liu CL, Green MR, Gentles AJ,
Feng W, Xu Y, Hoang CD, Diehn M and Alizadeh AA: Robust enumeration
of cell subsets from tissue expression profiles. Nat Methods.
12:453–457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hänzelmann S, Castelo R and Guinney J:
GSVA: Gene set variation analysis for microarray and RNA-seq data.
BMC Bioinformatics. 14:72013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Greenwood JD, Minhas K, di Santo JP,
Makita M, Kiso Y and Croy BA: Ultrastructural studies of
implantation sites from mice deficient in uterine natural killer
cells. Placenta. 21:693–702. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kolben T, Mannewitz M, Perleberg C,
Schnell K, Anz D, Hahn L, Meister S, Schmoeckel E, Burges A,
Czogalla B, et al: Presence of regulatory T-cells in endometrial
cancer predicts poorer overall survival and promotes progression of
tumor cells. Cell Oncol (Dordr). 45:1171–1185. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Degos C, Heinemann M, Barrou J, Boucherit
N, Lambaudie E, Savina A, Gorvel L and Olive D: Endometrial tumor
microenvironment alters human NK cell recruitment, and resident NK
cell phenotype and function. Front Immunol. 10:8772019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang XQ, Zhou WJ, Luo XZ, Tao Y and Li DJ:
Synergistic effect of regulatory T cells and proinflammatory
cytokines in angiogenesis in the endometriotic milieu. Hum Reprod.
32:1304–1317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu Z, Zhang J, Zhang Q, Wei S, Shi R, Zhao
R, An L, Grose R, Feng D and Wang H: Single-cell sequencing reveals
the heterogeneity and intratumoral crosstalk in human endometrial
cancer. Cell Prolif. 55:e132492022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Antsiferova YS, Sotnikova NY, Posiseeva LV
and Shor AL: Changes in the T-helper cytokine profile and in
lymphocyte activation at the systemic and local levels in women
with endometriosis. Fertil Steril. 84:1705–1711. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hofmann AP, Gerber SA and Croy BA: Uterine
natural killer cells pace early development of mouse decidua
basalis. Mol Hum Reprod. 20:66–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Heikkinen J, Möttönen M, Alanen A and
Lassila O: Phenotypic characterization of regulatory T cells in the
human decidua. Clin Exp Immunol. 136:373–378. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fu B, Li X, Sun R, Tong X, Ling B, Tian Z
and Wei H: Natural killer cells promote immune tolerance by
regulating inflammatory TH17 cells at the human maternal-fetal
interface. Proc Natl Acad Sci USA. 110:E231–E240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Diao R, Wei W, Zhao J, Tian F, Cai X and
Duan YG: CCL19/CCR7 contributes to the pathogenesis of
endometriosis via PI3K/Akt pathway by regulating the proliferation
and invasion of ESCs. Am J Reprod Immunol. 782017.doi:
10.1111/aji.12744 (Epub ahead of print).
|
|
45
|
Mei J, Zhou WJ, Zhu XY, Lu H, Wu K, Yang
HL, Fu Q, Wei CY, Chang KK, Jin LP, et al: Suppression of autophagy
and HCK signaling promotes PTGS2high FCGR3-NK cell differentiation
triggered by ectopic endometrial stromal cells. Autophagy.
14:1376–1397. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Agostinis C, Masat E, Bossi F, Ricci G,
Menegazzi R, Lombardelli L, Zito G, Mangogna A, Degan M, Gattei V,
et al: Transcriptomics and immunological analyses reveal a
pro-angiogenic and anti-inflammatory phenotype for decidual
endothelial cells. Int J Mol Sci. 20:16042019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hollebecque A, Chung HC, de Miguel MJ,
Italiano A, Machiels JP, Lin CC, Dhani NC, Peeters M, Moreno V, Su
WC, et al: Safety and antitumor activity of α-PD-L1 antibody as
monotherapy or in combination with α-TIM-3 antibody in patients
with microsatellite instability-high/mismatch repair-deficient
tumors. Clin Cancer Res. 27:6393–6404. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Marabelle A, Le DT, Ascierto PA, Di
Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M,
Penel N, Hansen AR, et al: Efficacy of pembrolizumab in patients
with noncolorectal high microsatellite instability/mismatch
repair-deficient cancer: Results from the phase II KEYNOTE-158
study. J Clin Oncol. 38:1–10. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Antill Y, Kok PS, Robledo K, Yip S,
Cummins M, Smith D, Spurdle A, Barnes E, Lee YC, Friedlander M, et
al: Clinical activity of durvalumab for patients with advanced
mismatch repair-deficient and repair-proficient endometrial cancer.
A nonrandomized phase 2 clinical trial. J Immunother Cancer.
9:e0022552021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen L, Widhopf G, Huynh L, Rassenti L,
Rai KR, Weiss A and Kipps TJ: Expression of ZAP-70 is associated
with increased B-cell receptor signaling in chronic lymphocytic
leukemia. Blood. 100:4609–4614. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen L, Apgar J, Huynh L, Dicker F,
Giago-McGahan T, Rassenti L, Weiss A and Kipps TJ: ZAP-70 directly
enhances IgM signaling in chronic lymphocytic leukemia. Blood.
105:2036–2041. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu Y, Wang Y, Yang J, Bi Y and Wang H:
ZAP-70 in chronic lymphocytic leukemia: A meta-analysis. Clin Chim
Acta. 483:82–88. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sun M, Chen S and Fu M: Model
establishment of prognostic-related immune genes in laryngeal
squamous cell carcinoma. Medicine (Baltimore). 100:e242632021.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu J and Zhang J: T-cell receptors
provide potential prognostic signatures for breast cancer. Cell
Biol Int. 45:1220–1230. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sun X, Wang L, Li H, Jin C, Yu Y, Hou L,
Liu X, Yu Y, Yan R and Xue F: Identification of microenvironment
related potential biomarkers of biochemical recurrence at 3 years
after prostatectomy in prostate adenocarcinoma. Aging (Albany NY).
13:16024–16042. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Brito C, Costa-Silva B, Barral DC and Pojo
M: Unraveling the relevance of ARL GTpases in cutaneous melanoma
prognosis through integrated bioinformatics analysis. Int J Mol
Sci. 22:92602021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kang Z, Li W, Yu YH, Che M, Yang ML, Len
JJ, Wu YR and Yang JF: Identification of Immune-related genes
associated with bladder cancer based on immunological
characteristics and their correlation with the prognosis. Front
Genet. 12:7635902021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lyu M, Yi X, Huang Z, Chen Y, Ai Z, Liang
Y, Feng Q and Xiang Z: A transcriptomic analysis based on aberrant
methylation levels revealed potential novel therapeutic targets for
nasopharyngeal carcinoma. Ann Transl Med. 10:472022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jan CI, Huang SW, Canoll P, Bruce JN, Lin
YC, Pan CM, Lu HM, Chiu SC and Cho DY: Targeting human leukocyte
antigen G with chimeric antigen receptors of natural killer cells
convert immunosuppression to ablate solid tumors. J Immunother
Cancer. 9:e0030502021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yin X, He L and Guo Z: T-cell exhaustion
in CAR-T-cell therapy and strategies to overcome it. Immunology.
Mar 20–2023.doi: 10.1111/imm.13642 (Epub ahead of print).
View Article : Google Scholar
|
|
61
|
Rowan AG, Ponnusamy K, Ren H, Taylor GP,
Cook LBM and Karadimitris A: CAR-iNKT cells targeting clonal TCRVβ
chains as a precise strategy to treat T cell lymphoma. Front
Immunol. 14:11186812023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Brentjens RJ, Latouche JB, Santos E, Marti
F, Gong MC, Lyddane C, King PD, Larson S, Weiss M, Rivière I and
Sadelain M: Eradication of systemic B-cell tumors by genetically
targeted human T lymphocytes co-stimulated by CD80 and
interleukin-15. Nat Med. 9:279–286. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gladkikh AA, Potashnikova DM, Tatarskiy V
Jr, Yastrebova M, Khamidullina A, Barteneva N and Vorobjev I:
Comparison of the mRNA expression profile of B-cell receptor
components in normal CD5-high B-lymphocytes and chronic lymphocytic
leukemia: A key role of ZAP70. Cancer Med. 6:2984–2997. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lin JX and Leonard WJ: The common cytokine
Receptor γ chain family of cytokines. Cold Spring Harb Perspect
Biol. 10:a0284492018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Blanco E, Izotova N, Booth C and Thrasher
AJ: Immune reconstitution after gene therapy approaches in patients
with X-Linked severe combined immunodeficiency disease. Front
Immunol. 11:6086532020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Oh MS and Chae YK: Deep and durable
response with combination CTLA-4 and PD-1 blockade in mismatch
repair (MMR)-proficient endometrial cancer. J Immunother. 42:51–54.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Faulkner S, Jobling P, March B, Jiang CC
and Hondermarck H: Tumor neurobiology and the war of nerves in
cancer. Cancer Discov. 9:702–710. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chen F, Qin T, Zhang Y, Wei L, Dang Y, Liu
P and Jin W: Reclassification of endometrial cancer and
identification of key genes based on neural-related genes. Front
Oncol. 12:9514372022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Blake MK, O'Connell P and Aldhamen YA:
Fundamentals to therapeutics: Epigenetic modulation of CD8+ T Cell
exhaustion in the tumor microenvironment. Front Cell Dev Biol.
10:10821952022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Philip M and Schietinger A: CD8+ T cell
differentiation and dysfunction in cancer. Nat Rev Immunol.
22:209–223. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Duckworth BC, Qin RZ and Groom JR: Spatial
determinates of effector and memory CD8+ T cell fates. Immunol Rev.
306:76–92. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Nelson MA, Ngamcherdtrakul W, Luoh SW and
Yantasee W: Prognostic and therapeutic role of tumor-infiltrating
lymphocyte subtypes in breast cancer. Cancer Metastasis Rev.
40:519–536. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mestermann K, Giavridis T, Weber J, Rydzek
J, Frenz S, Nerreter T, Mades A, Sadelain M, Einsele H and Hudecek
M: The tyrosine kinase inhibitor dasatinib acts as a pharmacologic
on/off switch for CAR T cells. Sci Transl Med. 11:eaau59072019.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Brunner A, Hinterholzer S, Riss P, Heinze
G and Brustmann H: Immunoexpression of B7-H3 in endometrial cancer:
Relation to tumor T-cell infiltration and prognosis. Gynecol Oncol.
124:105–111. 2012. View Article : Google Scholar : PubMed/NCBI
|