Introduction
Inflammatory myofibroblastic tumors (IMTs) are rare
mesenchymal neoplasms with myofibroblastic differentiation. IMTs
can occur at any age, although the disease has a predilection for
children, adolescents and young adults (1). IMTs typically arise in the abdominal
cavity, retroperitoneum, pelvis, lung, and head and neck; they
occur less commonly in the gastrointestinal tract, pancreas,
bladder and uterus (2). The
preferred option of treatment for IMTs of the urinary bladder is
surgical resection; however, treatment options are limited for
patients with advanced disease and/or unresectable cases (3).
~50% of IMTs harbor anaplastic lymphoma kinase (ALK)
gene rearrangements, resulting in constitutive gene activation and
cytoplasmic ALK protein expression (4). A targeted therapeutic approach against
ALK fusion has been proposed as a novel and promising option for
therapy. A patient with a RANBP2-ALK-positive IMT was found to have
a partial response to the ALK tyrosine kinase inhibitor (TKI),
crizotinib (5). In addition, a
neoadjuvant therapy for a patient with FN1-ALK fusion treated with
the ALK TKI lorlatinib exhibited a reduction in tumor size.
The majority of urinary tract IMTs with an ALK gene
rearrangement harbor a fibronectin 1 (FN1)-ALK gene fusion
(6). In the present case report,
another case of an IMT of the urinary bladder with FN1-ALK fusion
was presented. In the current case, the FN1-ALK fusion involved ALK
exon 19 and FN1 exon 23. By contrast, the majority of IMTs with ALK
fusion at other organs involve ALK exon 20, whereas ALK fusions
involving exon 18 or 19 have been reported only in genitourinary
IMTs with a 5′-gene fusion partner FN1 (3,6–9).
Together with a review of the clinicopathological characteristics
of such cases, it is suggested that the presence of FN1-ALK fusions
with ALK exon 18 or 19 rearrangements may be specific to a subset
of IMTs arising in the urinary bladder.
Case report
A 45-year-old woman was referred to the Chungbuk
National University Hospital institute with a 1-week history of
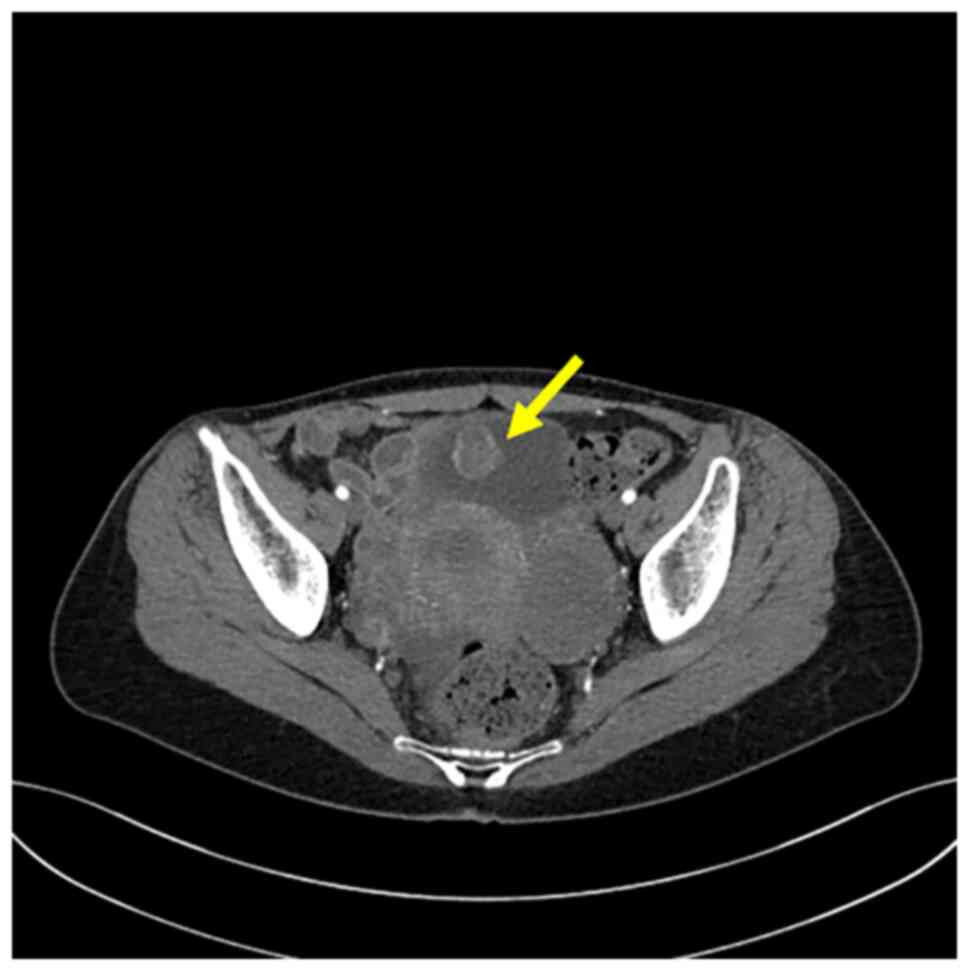
gross hematuria. Computed tomography (CT) revealed the presence of
a 2.2×2 cm, heterogeneously enhancing round mass located in the
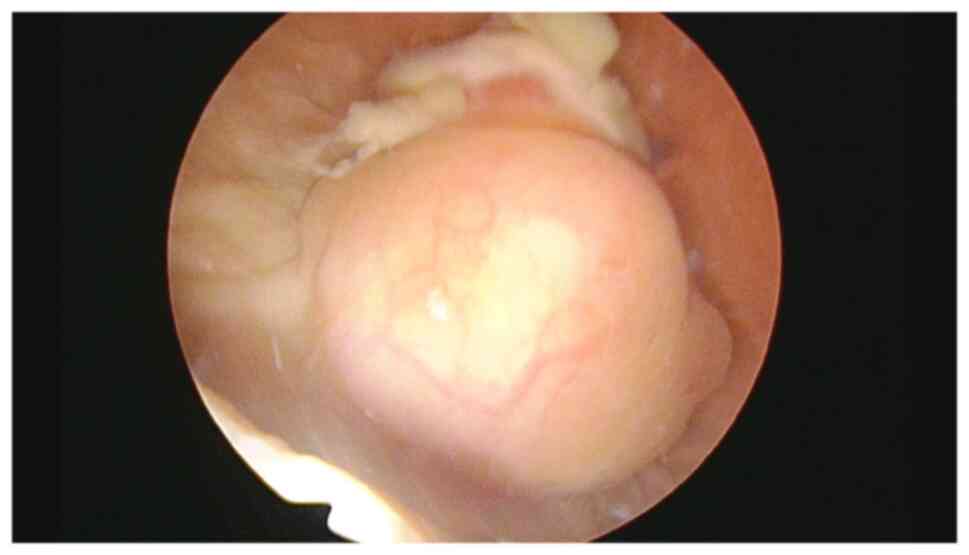
anterior/superior wall of the urinary bladder (Fig. 1). Cystoscopy identified it as a
solid erythematous mass (Fig. 2).
The patient subsequently underwent transurethral resection of the
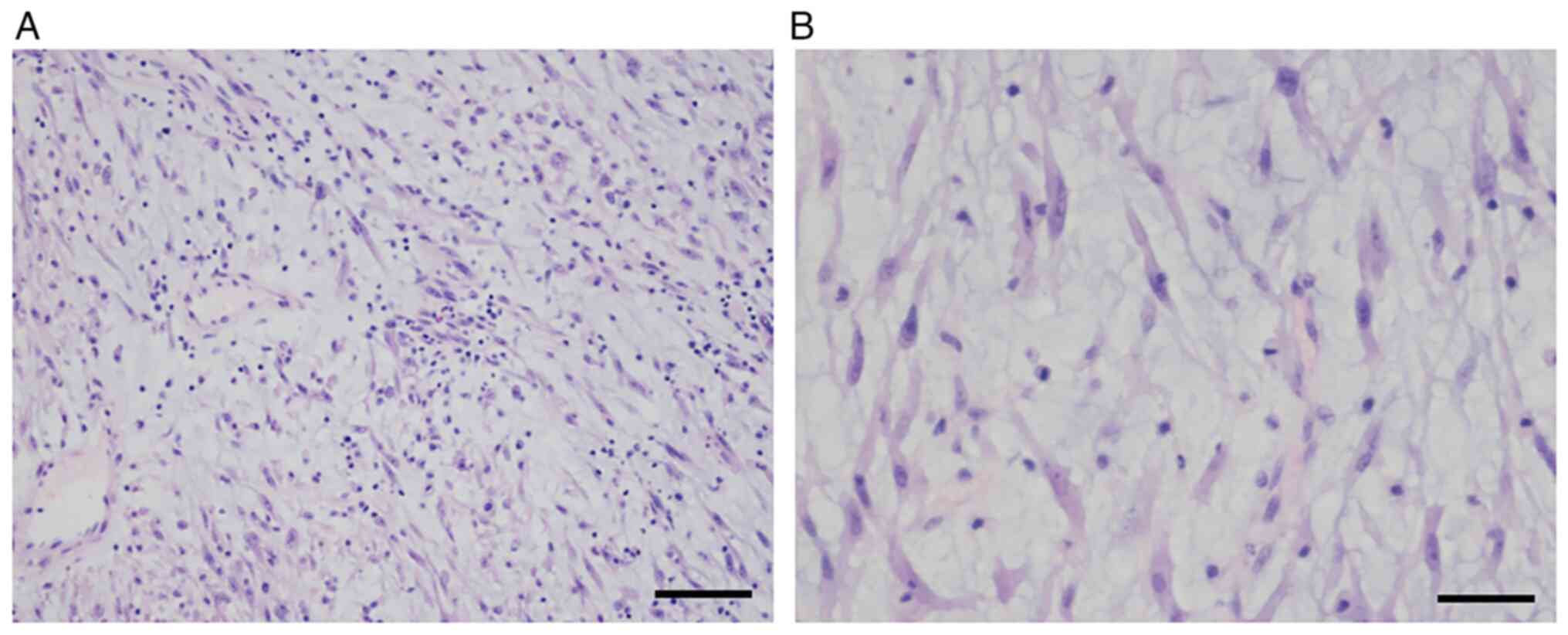
lesion in May 2021. Microscopically, the mass was found to comprise
stellate and spindled myofibroblastic cells in loose fascicles,
with a myxoid background and a mixed inflammatory infiltrate
comprising neutrophils, eosinophils and lymphocytes (Fig. 3A). The tumor cells had a pale
eosinophilic cytoplasm, with long tapering processes. The nuclei
were found to be elongated, ovoid or round, with a bland
appearance, and contained fine chromatin and small-to-inconspicuous
nucleoli (Fig. 3B). Mitotic figures
were present (3–4 per 10 high-power fields), along with focal
necrosis. The formalin-fixed, paraffin-embedded tumor specimens
were sectioned (4-µm thickness) and then stained. Fully automated
immunostaining was performed using a BenchMark XT autostainer
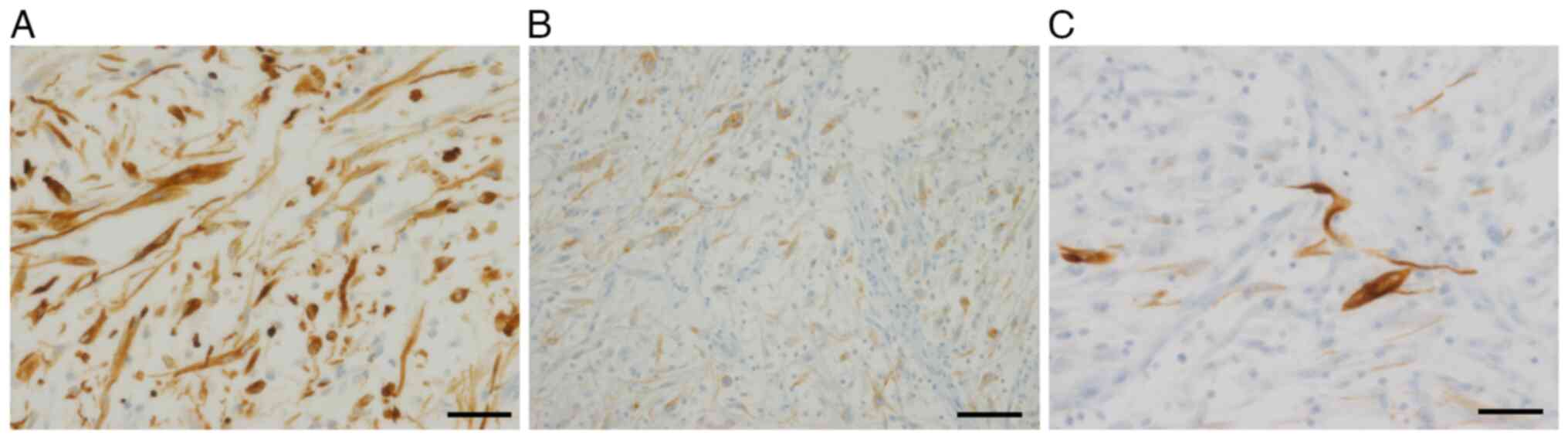
(Ventana Medical Systems, Inc.) Immunohistochemical analysis
revealed that the tumor cells were positive for vimentin (cat. no.
790-2917, ready-to-use; Ventana Medical Systems, Inc.), cytokeratin
AE1/AE3 (cat. no. PA0909; ready-to-use; Leica Biosystems) and ALK
(cat. no. CMC20421040; 1:50; Cell Marque; MilliporeSigma), with
cytoplasmic staining and focal-positive staining for desmin (cat.
no. CMC24321040; 1:50; Cell Marque; MilliporeSigma) (Fig. 4A-C), but negative for EMA (cat. no.
790-4463; ready-to-use; Ventana Medical Systems, Inc.), S-100 (cat.
no. CMC33021060; 1:200; Cell Marque; MilliporeSigma) and α-SMA
(cat. no. PA0943; 1:150; Leica Biosystems Nussloch GmbH), leading
to a suspicion of IMT. Therefore, targeted next-generation
sequencing was subsequently performed. RNA was extracted using the
RecoverAll™ Total Nucleic Acid Isolation Kit (Thermo Fisher
Scientific, Inc.). RNA was reverse transcribed using an Ion Torrent
NGS Reverse Transcription Kit (Thermo Fisher Scientific, Inc.).
Automated library preparation was performed using Oncomine™
Comprehensive Assay Plus, RNA, Chef-Ready panel (Thermo Fisher
Scientific, Inc.) and an Ion AmpliSeq™ Kit for Chef DL8 (Thermo
Fisher Scientific, Inc.). Templating and sequencing were performed
using an Ion 550™ Chip Kit (cat. no. A34537; Thermo Fisher
Scientific, Inc.), using IonS5 XL (Thermo Fisher Scientific, Inc.)
and Ion Chef instrument systems (Thermo Fisher Scientific, Inc.).
All procedures were performed per the manufacturer's protocols.
Next-generation sequencing succeeded in identifying FN1-ALK fusion
between exon 23 of FN1 and exon 19 of ALK. To date, the patient has
undergone outpatient follow-up for 18 months, with no signs of
tumor recurrence.
Discussion
IMT of the urinary bladder is a rare tumor that
follows an indolent clinical course. IMTs are often associated with
ALK gene rearrangement, and the FN1 gene is the most common fusion
partner in the urinary bladder (6,10).
Including the present case, 13 cases of urinary bladder IMTs with
FN1-ALK fusion have been reported (3,6,7,9,11).
It should be noted that seven cases reported that were by Acosta
et al (6) were designated by
these authors as pseudo-sarcomatous myofibroblastic proliferation
(PMP), a separate entity from IMTs; however, these tumors are
currently classified as IMTs by the WHO Classification of Tumors of
the Urinary System and Male Genital Organs (5th edition) (12), which details that IMTs and PMP share
histological features and a myofibroblastic immunohistochemical
phenotype, even though no definite gene fusion is detected in the
case of PMP. The clinicopathological characteristics of this
subtype were investigated [with the exception of the seven cases
reported by Acosta et al (6)
that featured no detailed clinical data] (Table I). Of the remaining six cases, the
neoplasm was detected in two children and four young-to-middle aged
adults; the majority of the patients were female. The most common
clinical symptom identified was hematuria. Patients with large
tumors underwent neoadjuvant treatment and partial cystectomy,
whereas the others underwent transurethral resection. All three
patients with available data concerning prognosis lived
uneventfully. ALK immunohistochemistry was positive in all cases.
Notably, immunostaining for α-SMA was positive in most bladder IMTs
(13), but negative in the present
case.
 | Table I.Clinical features of previously
reported cases of a primary retroperitoneal mucinous cystic
neoplasm with borderline malignancy. |
Table I.
Clinical features of previously
reported cases of a primary retroperitoneal mucinous cystic
neoplasm with borderline malignancy.
| First uthor,
year | Age, years | Sex | Size, cm | Clinical
symptoms | Treatment | Outcome | Fusion | ALK IHC | (Refs.) |
|---|
| Lovly et al,
2014 | 8 | F | 3 | ND | ND | ND | Exon 23 of FN1 Intron
18 of ALK |
| (3) |
| Lovly et al,
2014 | 26 | F | 3 | ND | ND | ND | Exon 23 of FN1 | Positive | (3) |
|
|
|
|
|
|
|
| Intron 18 of ALK |
|
|
| Ouchi et al,
2015 | 12 | M | ND | Gross hematuria | Neoadjuvant treatment
(meloxicam) + partial | NED, 12 months | Exon 20 of FN1 | Positive | (7) |
|
|
|
|
|
| cystectomy |
| Exon 19 of ALK |
|
|
| Bertz et al,
2020 | 64 | F | ND | ND | TUR-B | ND | ND | Positive | (11) |
| Reinhart et
al, 2020 | 43 | F | 7 | Dysuria and
macrohematuria | Neoadjuvant therapy
(crizotinib → lorlatinib) | NED, 12 months | Exon 36 of FN1 | Positive | (9) |
|
|
|
|
|
| + partial
cystectomy |
| Exon 19 of ALK |
|
|
| Present case | 45 | F | 2.2×2 | Gross hematuria | TUR-B | NED, 18 months | Exon 23 of FN1 | Positive |
|
|
|
|
|
|
|
|
| Exon 19 of ALK |
|
|
Overall, the recurrence rate for IMTs is ~20%,
although it is markedly higher for abdominal and pelvic IMTs (up to
85%), and lower for IMTs of the lung (<2%) and bladder (<4%)
(2,14–16).
Metastasis occurred in <5% of the IMT cases (2,16). The
clinical behavior of IMT, particularly that of the urinary bladder,
was found to be indolent; therefore, the treatment of choice is
surgical resection, and adjuvant chemotherapy is not usually
necessary. However, neoadjuvant therapy may be required to reduce
the size of the tumor prior to surgery.
The current RNA sequencing results revealed the
presence of FN1-ALK fusion. In cases of IMT of the urinary bladder,
the most common fusion partner is FN1 (6). IMTs of other organs harbor ALK fusions
with various fusion partners, most commonly TPM3 and CLTC (3). Other partners of ALK include HNRNPA1
(17), TPM4 (18) and ATIC (19). Gene fusions involving other kinases,
including ROS1 and PDGFRB, have been reported in small subsets of
IMT (10). To date, FN1 has been
identified as a fusion partner for ALK almost exclusively in
genitourinary IMTs [i.e., the reported 13 bladder IMTs (including
our case) and two uterine IMTs] (3,6–9,11).
All 13 bladder IMTs were found to be immunopositive for ALK
(3,6,7,9,11).
FN1 is located at chromosome 2q35, and encodes
fibronectin, a glycoprotein widely distributed in plasma and the
extracellular matrix. In vitro, fibronectin is required for
transforming growth factor-β-induced myofibroblastic
differentiation (20). As in most
other ALK fusion genes, the oncogenic activity of the fusion gene
is probably due to the strong promoter function of FN1, which
facilitates activation of ALK via homodimerization of the fusion
protein (21).
In the present case study, the FN1-ALK fusion
involved ALK exon 19 and FN1 exon 23. The majority of previously
reported ALK fusions have featured a common breakpoint between
exons 19 and 20 (22). Thus, the
ALK fusions have always been found to include exons 20–29, which
code for the cytoplasmic ALK tyrosine kinase domain (23), whereas in genitourinary IMTs, all
the reported cases with the FN1-ALK fusion have involved ALK exon
18 or 19, with the exception of the cases that lacked data
(3,6–9).
Therefore, the transmembrane domain of the ALK protein encoded by
exons 19–20 was retained in genitourinary IMTs with FN1-ALK fusion,
resulting in membrane/cytoplasmic localization of ALK (22,24).
Tumors with FN1-ALK fusion have shown different
treatment responses, depending on the type of ALK inhibitors used
to treat the patient. Reinhart et al (9) reported that, in IMTs of the urinary
bladder with FN1-ALK fusion, neoadjuvant treatment with the
first-generation ALK inhibitor crizotinib showed no tumor response,
whereas, by contrast, treatment with the next-generation ALK
inhibitor lorlatinib led to a rapid and deep response. In uterine
leiomyosarcoma with the FN1-ALK fusion, the tumor continued to
progress when the patient was administered therapies including
crizotinib, but a remarkable patient response resulted from
administering the second-generation ALK inhibitors, alectinib and
lorlatinib (25). In neither case
were resistance mutations against the first generation TKI,
crizotinib, identified. In an in vitro study by Childress
et al (24), tumor cells
expressing FN1-ALK were found to have a significantly higher
sensitivity to lorlatinib compared with crizotinib.
It is unclear why the FN1-ALK fusion has different
sensitivities, depending on the type of ALK inhibitor used for the
therapy. Childress et al (24) showed that FN1-ALK forms
significantly more foci and more colonies in soft agar compared
with the positive control (note that full-length ALK receptor
retains the ALK transmembrane domain harboring the active ALK
mutation). Therefore, in addition to the unique properties of
retaining the transmembrane domain in the FN1-ALK fusion, the
5′-fusion partner FN1 itself may affect the biochemical and
cellular properties of the ALK fusion protein, including its kinase
activity, protein stability, transformative potential and response
to ALK TKIs.
In conclusion, a rare case of an IMT with FN1-ALK
fusion in the urinary bladder was reported in the present case
study. The presence of FN1-ALK fusions with ALK with exon 18 or 19
rearrangement may be specific to a subset of IMTs, wherein ALK is
inhibited upon treatment with second-generation ALK inhibitors, but
not with crizotinib. This could be of importance both in the
neoadjuvant and in the palliative settings, particularly in terms
of negating the need for radical surgery. Therefore, the detection
of a distinct FN1-ALK fusion not only assists the diagnosis of
IMTs, but also helps to determine the most appropriate course of
treatment. However, since only one case was reported, the current
conclusions are limited and may not represent all patients.
Considering the rarity of this condition, gathering data from all
cases of urinary bladder IMTs with FN1-ALK fusion will help to
confirm the molecular characteristics and the efficacy of ALK TKIs
in the treatment for IMTs.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data analyzed during the current study is
available at https://www.ncbi.nlm.nih.gov/sra/PRJNA935024.
Authors' contributions
SMS and HCL made substantial contributions to the
conception and design of the work, and drafted and revised the
manuscript. CGW and OJL interpreted the pathological data. YJK
analyzed the patient data. SMS and CGW confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study adhered to the guidelines
established by the Declaration of Helsinki and was approved
(approval no. 2022-11-018) by the Institutional Review Board of
Chungbuk National University Hospital (Cheongju, Korea).
Patient consent for publication
Written informed consent for the publication of
anonymous case information was provided by the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gleason BC and Hornick JL: Inflammatory
myofibroblastic tumours: Where are we now? J Clin Pathol.
61:428–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coffin CM, Hornick JL and Fletcher CD:
Inflammatory myofibroblastic tumor: Comparison of
clinicopathologic, histologic, and immunohistochemical features
including ALK expression in atypical and aggressive cases. Am J
Surg Pathol. 31:509–520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lovly CM, Gupta A, Lipson D, Otto G,
Brennan T, Chung CT, Borinstein SC, Ross JS, Stephens PJ, Miller VA
and Coffin CM: Inflammatory myofibroblastic tumors harbor multiple
potentially actionable kinase fusions. Cancer Discov. 4:889–895.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coffin CM, Patel A, Perkins S,
Elenitoba-Johnson KS, Perlman E and Griffin CA: ALK1 and p80
expression and chromosomal rearrangements involving 2p23 in
inflammatory myofibroblastic tumor. Mod Pathol. 14:569–576. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Butrynski JE, D'Adamo DR, Hornick JL, Dal
Cin P, Antonescu CR, Jhanwar SC, Ladanyi M, Capelletti M, Rodig SJ,
Ramaiya N, et al: Crizotinib in ALK-rearranged inflammatory
myofibroblastic tumor. N Engl J Med. 363:1727–1733. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Acosta AM, Demicco EG, Dal Cin P, Hirsch
MS, Fletcher CDM and Jo VY: Pseudosarcomatous myofibroblastic
proliferations of the urinary bladder are neoplasms characterized
by recurrent FN1-ALK fusions. Mod Pathol. 34:469–477. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ouchi K, Miyachi M, Tsuma Y, Tsuchiya K,
Iehara T, Konishi E, Yanagisawa A and Hosoi H: FN1: A novel fusion
partner of ALK in an inflammatory myofibroblastic tumor. Pediatr
Blood Cancer. 62:909–911. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haimes JD, Stewart CJR, Kudlow BA, Culver
BP, Meng B, Koay E, Whitehouse A, Cope N, Lee JC, Ng T, et al:
Uterine inflammatory myofibroblastic tumors frequently harbor ALK
fusions with IGFBP5 and THBS1. Am J Surg Pathol. 41:773–780. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reinhart S, Trachsel Y, Fritz C, Wagner U,
Bode-Lesniewska B, John H and Pless M: Inflammatory myofibroblastic
tumor of the bladder with FN1-ALK gene fusion: Different response
to ALK inhibition. Urology. 146:32–35. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Antonescu CR, Suurmeijer AJ, Zhang L, Sung
YS, Jungbluth AA, Travis WD, Al-Ahmadie H, Fletcher CD and Alaggio
R: Molecular characterization of inflammatory myofibroblastic
tumors with frequent ALK and ROS1 gene fusions and rare novel RET
rearrangement. Am J Surg Pathol. 39:957–967. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bertz S, Stohr R, Gaisa NT, Wullich B,
Hartmann A and Agaimy A: TERT promoter mutation analysis as a
surrogate to morphology and immunohistochemistry in problematic
spindle cell lesions of the urinary bladder. Histopathology.
77:949–962. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
WHO Classification of Tumours Editorial
Board, . Urinary and male genital tumours. International Agency for
Research on Cancer. 8. 5th edition. WHO Classification of tumours
series; Lyon: 2022
|
|
13
|
Montgomery EA, Shuster DD, Burkart AL,
Esteban JM, Sgrignoli A, Elwood L, Vaughn DJ, Griffin CA and
Epstein JI: Inflammatory myofibroblastic tumors of the urinary
tract: A clinicopathologic study of 46 cases, including a malignant
example inflammatory fibrosarcoma and a subset associated with
high-grade urothelial carcinoma. Am J Surg Pathol. 30:1502–1512.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coffin CM, Watterson J, Priest JR and
Dehner LP: Extrapulmonary inflammatory myofibroblastic tumor
(inflammatory pseudotumor). A clinicopathologic and
immunohistochemical study of 84 cases. Am J Surg Pathol.
19:859–872. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cook JR, Dehner LP, Collins MH, Ma Z,
Morris SW, Coffin CM and Hill DA: Anaplastic lymphoma kinase (ALK)
expression in the inflammatory myofibroblastic tumor: A comparative
immunohistochemical study. Am J Surg Pathol. 25:1364–1371. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Teoh JY, Chan NH, Cheung HY, Hou SS and Ng
CF: Inflammatory myofibroblastic tumors of the urinary bladder: A
systematic review. Urology. 84:503–508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Inamura K, Kobayashi M, Nagano H, Sugiura
Y, Ogawa M, Masuda H, Yonese J and Ishikawa Y: A novel fusion of
HNRNPA1-ALK in inflammatory myofibroblastic tumor of urinary
bladder. Hum Pathol. 69:96–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hisaoka M, Shimajiri S, Matsuki Y,
Meis-Kindblom JM, Kindblom LG, Li XQ, Wang J and Hashimoto H:
Inflammatory myofibroblastic tumor with predominant anaplastic
lymphoma kinase-positive cells lacking a myofibroblastic phenotype.
Pathol Int. 53:376–381. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Debiec-Rychter M, Marynen P, Hagemeijer A
and Pauwels P: ALK-ATIC fusion in urinary bladder inflammatory
myofibroblastic tumor. Genes Chromosomes Cancer. 38:187–190. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lygoe KA, Wall I, Stephens P and Lewis MP:
Role of vitronectin and fibronectin receptors in oral mucosal and
dermal myofibroblast differentiation. Biol Cell. 99:601–614. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mano H: ALKoma: A cancer subtype with a
shared target. Cancer Discov. 2:495–502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ren H, Tan ZP, Zhu X, Crosby K, Haack H,
Ren JM, Beausoleil S, Moritz A, Innocenti G, Rush J, et al:
Identification of anaplastic lymphoma kinase as a potential
therapeutic target in ovarian cancer. Cancer Res. 72:3312–3323.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marino-Enriquez A and Dal Cin P: ALK as a
paradigm of oncogenic promiscuity: Different mechanisms of
activation and different fusion partners drive tumors of different
lineages. Cancer Genet. 206:357–373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Childress MA, Himmelberg SM, Chen H, Deng
W, Davies MA and Lovly CM: ALK Fusion partners impact response to
ALK Inhibition: Differential effects on sensitivity, cellular
phenotypes, and biochemical properties. Mol Cancer Res.
16:1724–1736. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Testa S, Million L, Longacre T and Bui N:
Uterine leiomyosarcoma with FN1-Anaplastic lymphoma kinase fusion
responsive to alectinib and lorlatinib. Case Rep Oncol. 14:812–819.
2021. View Article : Google Scholar : PubMed/NCBI
|