Introduction
Neuroendocrine carcinomas (NEC) are a highly
heterogeneous group of malignant tumors, which originate from
neuroendocrine cells and account for approximately 1% of all
malignant tumors (1). In 1907,
Oberndorfer reported the first case of neuroendocrine neoplasm
(2). In recent years, an increasing
number of cases were reported. Previous studies have pointed out
that neuroendocrine neoplasm was more likely to occur in the
gastrointestinal tract (66%) and respiratory system (31%), such as
the colorectal and lungs (3),
rarely in gallbladder neuroendocrine. From data provided by
Surveillance Epidemiology and End Result (SEER) research,
gallbladder neuroendocrine carcinoma (GB-NEC) is extremely rare,
accounting for about 0.5% of all neuroendocrine neoplasms and 2.1%
of all gallbladder tumors (4). In
addition, GB-NEC was more aggressive and had a worse outcome than
adenocarcinomas of the gallbladder (5,6). Thus
far, most studies of the GB-NEC are case reports and small case
series. Therefore, there is still no consensus on the initiation
and development, molecular mechanisms, pathology and treatment of
GB-NEC.
Here we reported two cases of GB-NEC pathologically
diagnosed as mixed adenoneuroendocrine carcinoma (MANEC) and large
cell neuroendocrine carcinoma, respectively. These patients are
rarely diagnosed preoperatively because most patients will be
treated as standard gallbladder cancer. Both patients were treated
with a combination of surgery and chemotherapy. In addition, we
reviewed the relevant literature and described the progression of
the epidemiology, pathogenesis, pathology, clinical
characteristics, treatment of GB-NEC.
Case report
Case 1
The case was a 65-year-old male patient with no
family history of cancer and with a past medical history of
appendectomy and surgery for lumbar disc herniation. Suspected
gallbladder malignancy was found during physical examination. He
had no fever, abdominal pain, nausea, vomiting, weight loss and
other complaints. No abnormal clinical signs were detected by
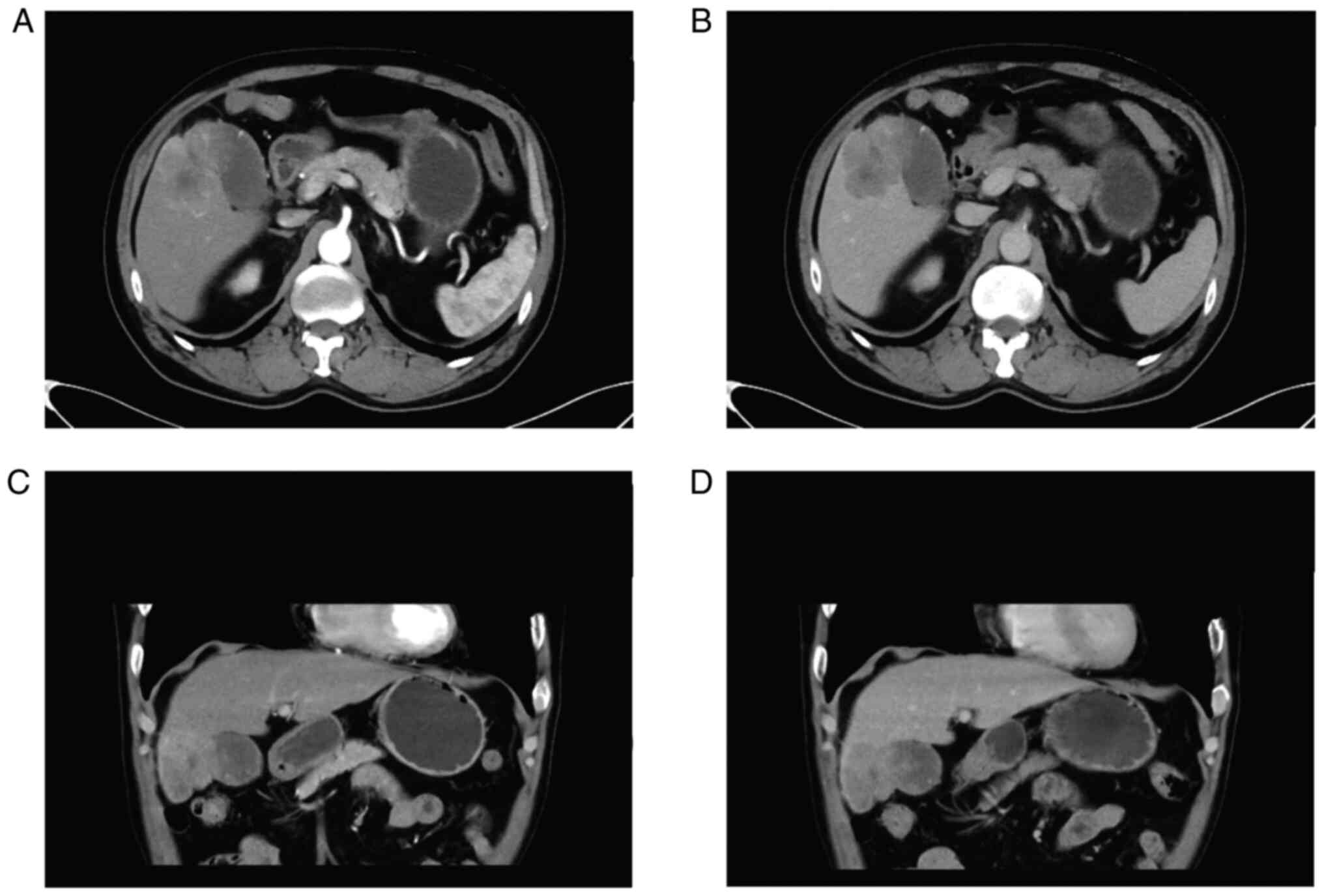
abdominal examination. Computed tomography (CT) scanning of upper
abdomen indicated soft tissue shadows could be seen at the bottom
of the gallbladder, invading the liver, and the boundary was
blurred. In addition, a cyst was found in the left lobe of liver.
Contrast-enhanced CT scanning indicated significant uneven
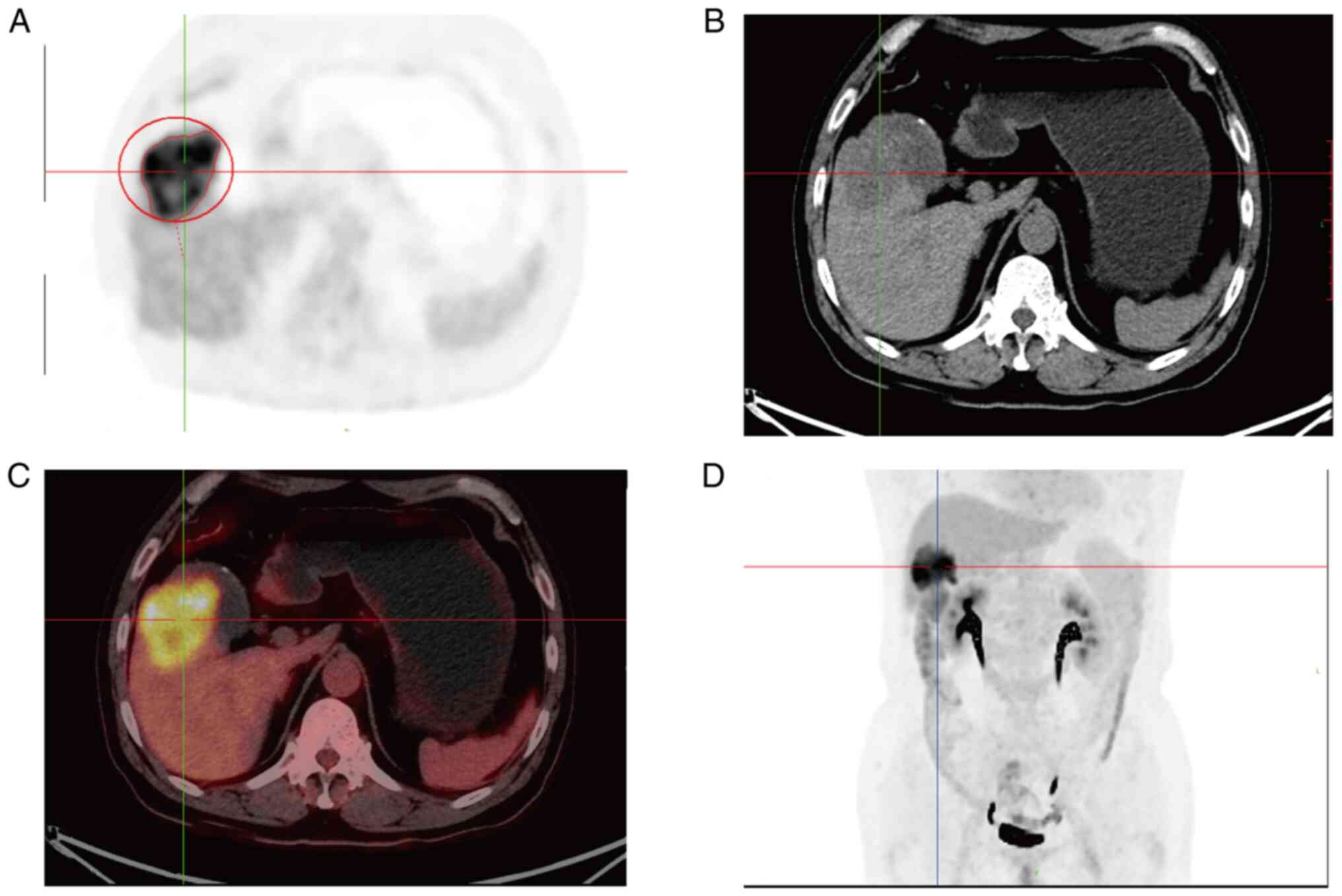
enhancement and it was about 60×45 mm in size (Fig. 1). Preoperative positron emission
tomography/computed tomography (PET/CT) showed irregular soft
tissue occupancy at the bottom of the gallbladder with abnormally
high metabolic activity and low-density occupancy in the lower
right anterior lobe with abnormally inhomogeneity high metabolic
activity (Fig. 2). Levels of tumor
markers were shown in Table I. All
these results suggested that gallbladder malignant lesions with the
right liver lobe involvement. CT provided no clear evidence of
distal metastases.
 | Table I.Tumor markers of the two cases. |
Table I.
Tumor markers of the two cases.
| Marker | Case 1 | Case 2 |
|---|
| CA-125 | 36.60 U/ml | 6.22 U/ml |
| CA-199 | <2.000 U/ml | 22.03 U/ml |
| CEA | Not applicable | 2.87 ng/ml |
| AFP | Not applicable | 2.66 IU/l |
After multidisciplinary team discussion,
preoperative examination was considered to be gallbladder malignant
tumor, which could be surgically removed. At the same time, in
order to reduce the risk of malignant tumor metastasis caused by
invasive biopsy, EUS-FNA or bile cytology was not performed, and
surgery was performed directly. During the operation, a solid mass
of 3×2 cm in size were found in the body of gallbladder,
infiltrating the liver. Cholecystectomy, the local resection of
right liver lobe and regional lymphadenectomy were performed.
Postoperative Pathology confirmed no tumor cell infiltration at the
incisor margin, R0 resection was performed, and metastatic
carcinoma was found in 1/9 (tubular adenocarcinoma) around the
gallbladder. It was also confirmed as MANEC with stage IIIB
(T3N1M0). Immunohistochemical stains were positive for
synaptophysin (Syn), chromogranin A (CgA), CD56, CK7 and CK19.
Furthermore, the Ki-67 indexes were over 50% in NEC tissue and 30%
in adenocarcinoma tissue (Fig. 3).
The patient had an uneventful recovery following the surgery and
received cisplatin-etoposide combination chemotherapy. The patient
was followed up for one year. The general condition was
satisfactory at present, and no tumor metastasis was observed.
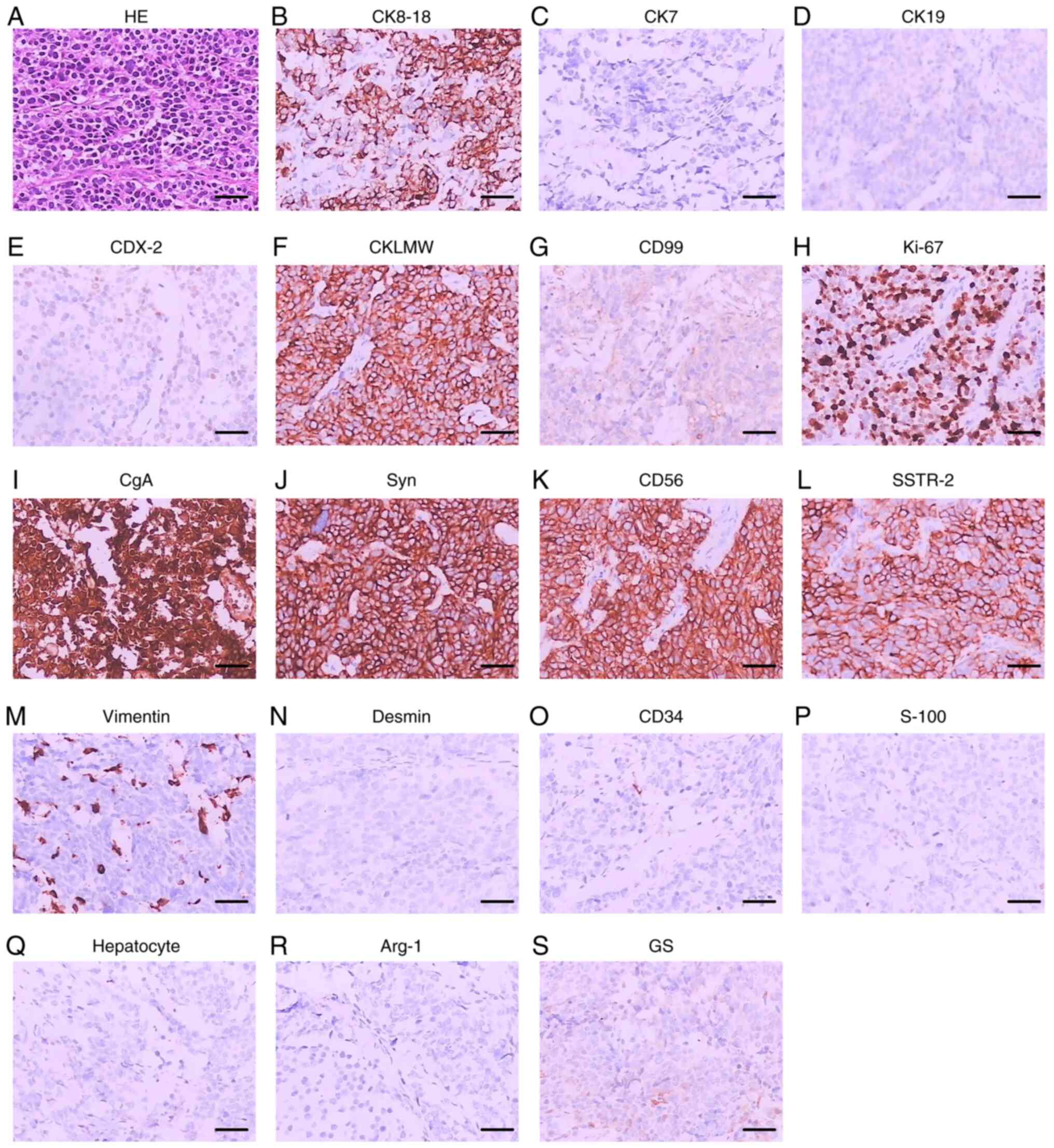 | Figure 3.HE staining and immunohistochemical
staining of case 1. (A) HE staining. (B) CK8-18. (C) CK7. (D) CK19.
(E) CDX-2. (F) CKLMW. (G) CD99. (H) Ki-67. (I) CgA. (J) Syn. (K)
CD56. (L) SSTR-2. (M) Vimentin. (N) Desmin. (O) CD34. (P) S-100.
(Q) Hepatocyte-specific antigen. (R) Arg-1. (S) GS. Scale bar, 5
µm. CK, cytokeratin; CDX, caudal-related homeobox transcription
factor; CKLMW, low molecular weight cytokeratin; CD, cluster of
differentiation; Ki-67, antigen KI67; CgA, chromogranin A; Syn,
synaptophysin; SSTR-2, somatostatin receptor type 2; S-100, soluble
protein-100; Arg-1, arginase-1; GS, glutamine synthetase. |
Case 2
The case was a 66-year-old male patient presented to
our hospital with paroxysmal upper abdomen pain for more than half
a year. The day before the visit, the patient presented a worsening
of upper abdomen pain accompanied by nausea and vomiting. Also, the
patient experienced loss of appetite and weight loss over 5 kg in
the past 6 months. Partial gastrectomy was performed 4 years ago
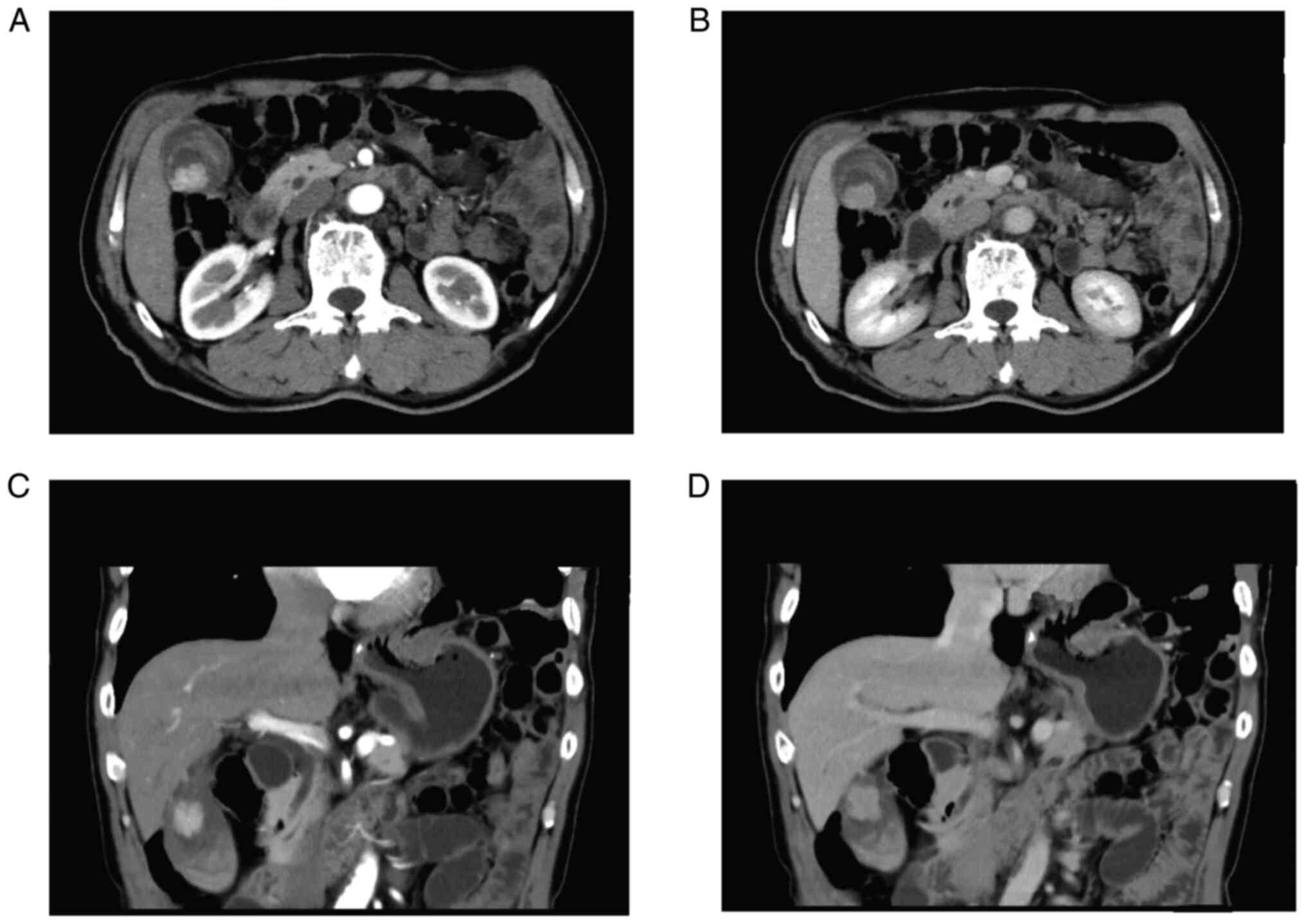
owing to esophageal carcinoma. Abdominal ultrasound suggested
thickening of the gallbladder wall and a liver cyst. CT scanning of
upper abdomen showed marked signal abnormality in the gallbladder
(Fig. 4). Levels of tumor markers
were showed in Table I. These
results indicated suspected gallbladder cancer.
After multidisciplinary team discussion, the patient
underwent surgical treatment. During the operation, a solid mass of
1.6×1.1×1 cm in size were found in gallbladder. Cut face showed a
solid and grayish tumor lesion. Intraoperative frozen pathology
showed that the tumor invaded the muscular layer but did not invade
the fibrous membrane. In order to reduce the risk of gallbladder
bed metastasis, cholecystectomy, hepatic segmental IVb + V
resection and regional lymph node resection were performed.
Postoperative pathology confirmed it as gallbladder large cell
neuroendocrine carcinoma (GB-LCNEC). Postoperative pathology
confirmed that no tumor cell infiltration was observed at the liver
resection margin and local lymphadenectomy, and R0 resection was
performed. Consequently, the patient was diagnosed with stage IIB
(T2bN0M0). Immunohistochemical stains were positive for CgA, CD56
and CK19. In addition, the Ki-67 indexes were over 80% (Fig. 5). The patient had an uneventful
recovery following the surgery and received cisplatin-etoposide
combination chemotherapy. The patient was followed up for 14
months. The general condition was satisfactory at present, and no
tumor metastasis was observed.
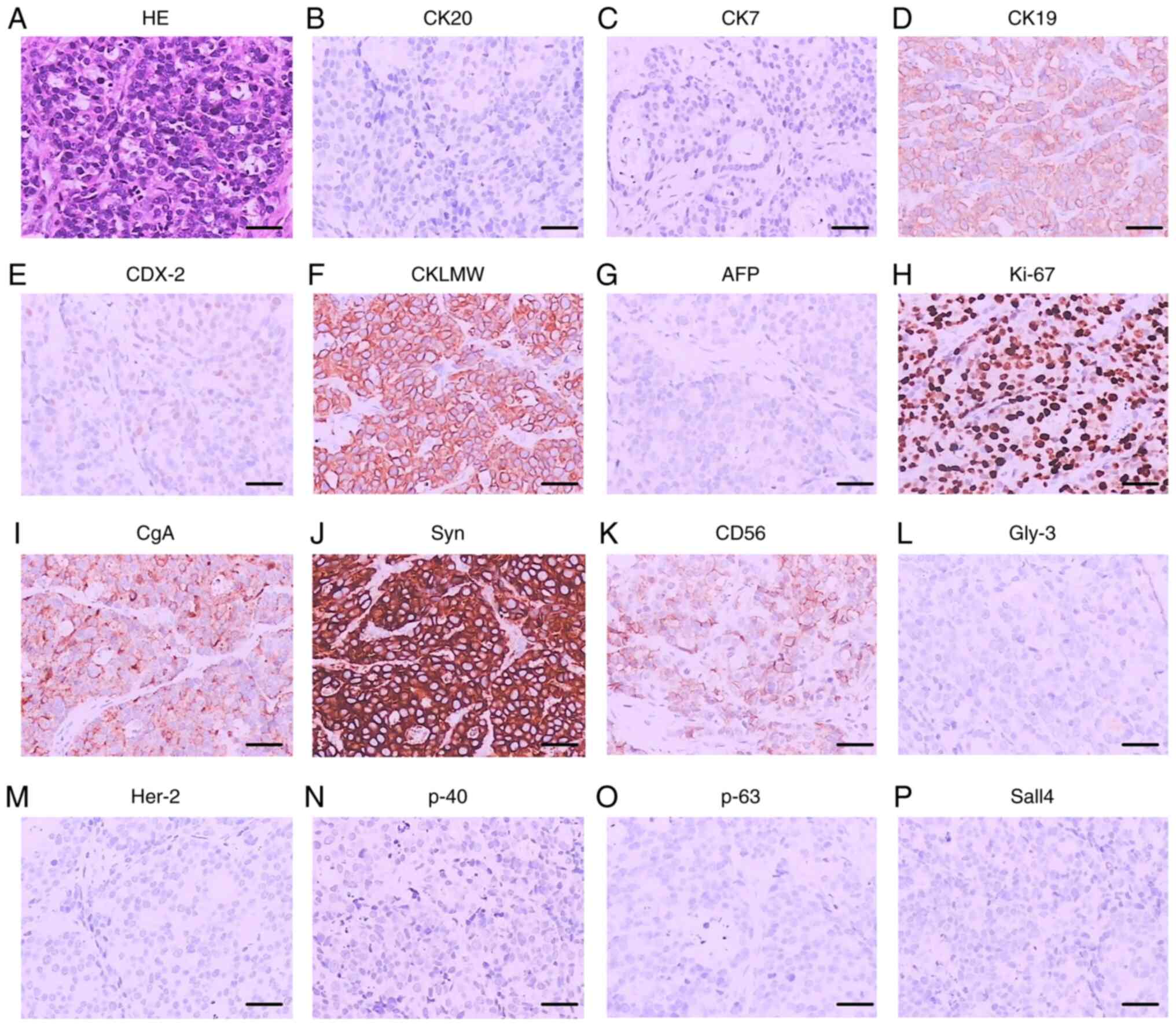 | Figure 5.HE staining and immunohistochemical
staining of case 2. (A) HE staining. (B) CK20. (C) CK7. (D) CK19.
(E) CDX-2. (F) CKLMW. (G) AFP. (H) Ki-67. (I) CgA. (J) Syn. (K)
CD56. (L) Gly-3. (M) Her-2. (N) p-40. (O) p-63. (P) Sall4. Scale
bar, 5 µm. CK, cytokeratin; CDX, caudal-related homeobox
transcription factor; CKLMW, low molecular weight cytokeratin; AFP,
α-fetoprotein; Ki-67, antigen KI67; CgA, chromogranin A; Syn,
synaptophysin; CD, cluster of differentiation; Gly-3, glypican-3;
Her-2, human epidermal growth factor receptor 2; Sall4, spalt like
transcription factor 4. |
Discussion
Neuroendocrine carcinomas (NECs) are a highly
heterogeneous group of malignant tumors, which originate from
neuroendocrine cells and account for approximately 1% of all
malignant tumors (1). The vast
majority of NEC were found in the gastrointestinal tract (66%),
followed by the lungs (31%), and were also found in tissues such as
ovaries and pancreas (3). Due to
the lack of specific clinical features and the insensitivity of
preoperative diagnosis, the majority of these patients will be
considered standard gallbladder cancer and eventually diagnosed as
GB-NEC by postoperative pathology. Based on the classification
criteria provided by WHO Classification of Tumors of the Digestive
System in 2010, neuroendocrine neoplasm (NEN) was classified into
three categories: the well-differentiated neuroendocrine tumors (G1
and G2) and poorly-differentiated neuroendocrine tumors (G3,
defined as NECs). According to the pathology, NECs could be
classified into small cell neuroendocrine carcinomas (SCNEC), large
cell neuroendocrine carcinomas (LCNEC) and MANEC (7). In this study, case 1 was confirmed as
MANEC, and case 2 was confirmed as LCNEC by post-surgical
pathologic diagnosis.
The etiology of GB-NEC remains still unclear.
Previous studies have proposed the following hypotheses: i)
Undifferentiated gallbladder stem cells differentiate into
neuroendocrine cells; ii) long-term chronic inflammatory
stimulation of the gallbladder mucosa leads to pathological
intestinal metaplasia, which in turn produces neuroendocrine cells
at the lesion site and develops into neuroendocrine carcinoma. iii)
In some cases, the gallbladder adenocarcinoma function switches to
a neuroendocrine one (8–10).
Recently, with the increasing cases of GN-NEC
reported, its molecular mechanism has received extensive attention.
Previous studies have pointed out that activation of epidermal
growth factor receptor (EGFR) can upregulate the expression of
downstream effector protein kinase B (PKB) and extracellular signal
regulate kinase (ECSRK) (10,11).
In addition, the expression level of the target protein of
rapamycin was positively correlated with the proliferation index of
the cells (6). These results
indicated high expressions of EGFR, ECSRK and the target protein of
rapamycin were linked to poor survival prognosis. Takizawa et
al (12) and Fujimasa et
al (13) reported loss of Rb1
and overexpression of were found in NECs of the colorectum and
esophagus. Lee and Sung reported a series of 34 GB-NEC cases
(14). Of the 34 GB-NEC patients,
25 (74%) showed loss of Rb1 and overexpression of p16. Although
these results have suggested that disruption of the Rb1-p16 pathway
might play a crucial role in GB-NEC, the detailed molecular
mechanism requires further exploration. Park et al pointed
out that BRAF mutations were present in about 9% of
gastroenteropancreatic neuroendocrine tumors, especially in
high-grade neuroendocrine carcinomas (15). Advanced colorectal NEC patients with
BRAFV600E mutation benefited from BRAF mutation-targeted
therapy (16). However, no
mutations of BRAFV600E were found in any of the 34
GB-NEC cases (14). Therefore, it
is still unclear whether BRAF mutation is related to GB-NEC, and
further research is needed. Another study has pointed out that ALK
rearrangement and immune microenvironment heterogeneity were
related to large cell neuroendocrine carcinoma of the lung, and its
correlation with GB-NEC still needs to be further explored
(17).
Traditional imaging methods, such as ultrasound, CT
and MRI, are not sensitive to the diagnosis of GB-NEC. Recent
studies have shown that the use of 68Ga-DOTANOC PET tracer in
PET/CT and PET/MRI is effective in the detection of primary NENs
and metastatic sites. At the same time, the staging and therapeutic
response evaluation of GB-NENs is a valuable tool (18,19).
The gold standard for diagnosis of GB-NEC is still
histopathological examination. Preoperative EUS-FNA or bile
cytology is a good diagnostic method for biliary malignancy, but
may be more suitable for patients with excessive tumor load,
difficult surgery, or distant metastasis. In this paper, two
patients with small tumor load, no distant metastasis, preoperative
evaluation can be radical resection, suitable for direct surgical
treatment. Neuron specific enolase (NSE), Syn, CgA and CD56 were
often positive in immunohistochemistry staining. High Ki-67 and
mitotic index may be predictors of poor prognosis (20). According to the immunohistochemical
characteristics, at least two of the three commonly used markers
SynA, CgA and CD56 should be widely expressed to confirm the
diagnosis of late MANEC (21,22).
In this study, Syn, CgA and CD56 were all positive staining in case
1 and CgA and CD56 were positive staining in case 2. Previous study
indicated CK7 and CK20 were positively expressed in gallbladder
adenocarcinoma and the expression rates of CK7 and CK20 in GB-NEC
were slightly lower (14,23). However, in our study, CK7 was only
positively expressed in adenocarcinoma tissue of case 1. CK7 and
CK20 were both negatively expressed in NEC tissue of case 2.
Interestingly, CK19 was positively expressed in both cases.
Overexpression of CD117 in colorectal NECs was reported in previous
studies. In addition, CD117 was positive in 25 (79%) of 34 GB-NEC
cases. Given the high frequencies of CD117 expression in NECs,
CD117 might potentially be applied as a novel NECs biomarker
(14,24,25).
Currently, surgical resection is still the primary
and most effective therapy for GB-NEC. Radical resection and
obtaining histologically negative surgical resection margins can
definitely prolong the survival time of patients (26–28).
For those advanced patients, cholecystectomy combined with partial
hepatectomy and lymph node dissection can significantly improve the
outcome (29). Patients not
suitable for surgery should be administered chemotherapy.
Postoperative chemoradiotherapy for highly aggressive GB-NEC
patients with early lymph node metastasis is an effective means to
prolong the survival time of patients. The recommended chemotherapy
includes streptozotocin, 5-fluorouracil, doxorubicin, cisplatin,
and etoposide (30). Due to the low
incidence of GB-NEC, there is still no standard chemotherapy
regimen to date. Relevant case reports suggest that gemcitabine,
docetaxel, or cisplatin in combination with cisplatin, sunitinib,
and docetaxel, respectively, could prolong survival time in
patients with neuroendocrine carcinoma of the gallbladder (31,32).
To date, there are still no targeted drugs in clinical practice.
Some studies have pointed out that the expression level of VEGF in
GB-NEC patients was increased, and sunitinib, a VEGF-targeting
inhibitor, could effectively prolong the progression-free survival
and overall survival of pancreatic neuroendocrine neoplasm
patients. The effectiveness of GB-NEC still needs further research.
Also, previous studies have shown that changes in the immune
microenvironment and ALK rearrangement are involved in the
development of lung large cell neuroendocrine carcinoma, and the
application of ALK inhibitors improved the prognosis of patients.
It might provide a new therapeutic option for the treatment of
GB-NEC (17,33,34).
Most GB-NEC patients are in an advanced stage of
disease at the time of presentation, and many have lost the chance
of radical surgery. Biopsy and pathologic examination can be
performed first to determine the tumor type, followed by
chemotherapy. Chemotherapy is essential for inoperable GB-NEC
patients and can improve patient survival (35). At present, cisplatin or carboplatin
plus etoposide is the main chemotherapy regimen for poorly
differentiated GB-NEC, and has achieved satisfactory results
(36). In addition, neoadjuvant
chemotherapy may help to reduce the tumor burden and provide the
opportunity for radical surgical resection (37). Another study compared the median
survival time of patients receiving surgical treatment alone and
postoperative combined adjuvant radiotherapy for 3.0 and 12.7
months, respectively, indicating that postoperative chemotherapy
can benefit patients' survival (38). GB-MANEC is rarer among gallbladder
neuroendocrine carcinomas (22).
The choice of chemotherapeutic regimens to be used lies on the
degree of MANEC differentiation and accordingly may follow a
‘treat-like-neuroendocrine tumor’ or a ‘treat-like-an
adenocarcinoma’ protocol as suggested by previous investigators
(39). Therefore, chemotherapy was
administered postoperatively and both patients survived. We will
continue to actively follow up.
At present, GB-NEC is still an aggressive malignancy
with poor prognosis. We reported two cases of GB-NECs and reviewed
the literature to deepen our understanding of GB-NEC. In future,
exploring the etiology and molecular mechanism of occurrence and
development of this disease will provide new strategies for its
treatment.
Acknowledgements
Not applicable.
Funding
This work was supported by the Key Research and Development
Project of Xingtai (grant no. 2020ZC273).
Availability of data and materials
All data generated or analyzed in this study are
included in this published article.
Authors' contributions
YPT, JTW, ZHL, JM, LCC and YW conceived and designed
the study. YL and YeZ provided study materials, collected data and
drafted the manuscript.. YL, ZGZ, DXL and HL performed surgery. WC,
ZKL, XX, YaZ, XCZ and LZ analyzed and interpreted the data. YL and
JTW confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The patients provided written informed consent to
participate.
Patient consent for publication
Written informed consent was obtained from the
patients for publication of the data and images in this case
report.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GB-NEC
|
gallbladder neuroendocrine
carcinoma
|
|
MANEC
|
mixed adeno-neuroendocrine
carcinoma
|
|
LCNEC
|
large cell neuroendocrine
carcinoma
|
|
NEC
|
neuroendocrine carcinoma
|
|
SEER
|
Surveillance Epidemiology and End
Result
|
|
CT
|
computed tomography
|
|
PET/CT
|
preoperative positron emission
tomography/computed tomography
|
|
Syn
|
synaptophysin
|
|
CgA
|
chromogranin A
|
|
MRCP
|
magnetic resonance
cholangiopancreatography
|
|
GB-LCNEC
|
gallbladder large cell neuroendocrine
carcinoma
|
|
SCNEC
|
small cell neuroendocrine
carcinoma
|
|
EGFR
|
epidermal growth factor receptor
|
|
PKB
|
protein kinase B
|
|
ECSRK
|
extracellular signal-regulated
kinase
|
References
|
1
|
Kumar K, Tariq H, Ahmed R, Chukwunonso C,
Niazi M and Ihimoyan A: Small-cell type, poorly differentiated
neuroendocrine carcinoma of the gallbladder: A case report and
review of the literature. Case Rep Oncol Med.
2019:89680342019.PubMed/NCBI
|
|
2
|
Oberndorfer S: Carcinoid tumors of the
small intestine. Frank Z. Pathol. 1:426–429. 1907.(In German).
|
|
3
|
Gustafsson BI, Kidd M and Modlin IM:
Neuroendocrine tumors of the diffuse neuroendocrine system. Curr
Opin Oncol. 20:1–12. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A and Evans
DB: One hundred years after ‘carcinoid’: epidemiology of and
prognostic factors for neuroendocrine tumors in 35,825 cases in the
United States. J Clin Oncol. 26:3063–3072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee KJ, Cho JH, Lee SH, Lee KH, Park BK,
Lee JK, Woo SM, Ryu JK, Lee JK, Kim YS, et al: Clinicopathological
characteristics of biliary neuroendocrine neoplasms: A multicenter
study. Scand J Gastroenterol. 52:437–441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yun SP, Shin N and Seo HI: Clinical
outcomes of small cell neuroendocrine carcinoma and adenocarcinoma
of the gallbladder. World J Gastroenterol. 21:269–275. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lüttges J: What's new? The 2010 WHO
classification for tumours of the pancreas. Pathologe. 32 (Suppl
2):S332–S336. 2011.(In German). View Article : Google Scholar
|
|
8
|
Adachi T, Haraguchi M, Irie J, Yoshimoto
T, Uehara R, Ito S, Tokai H, Noda K, Tada N, Hirabaru M, et al:
Gallbladder small cell carcinoma: A case report and literature
review. Surg Case Rep. 2:712016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duffy A, Capanu M, Abou-Alfa GK, Huitzil
D, Jarnagin W, Fong Y, D'Angelica M, Dematteo RP, Blumgart LH and
O'Reilly EM: Gallbladder cancer (GBC): 10-Year experience at
memorial sloan-kettering cancer centre (MSKCC). J Surg Oncol.
98:485–489. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Komori Y, Yada K, Ohta M, Uchida H,
Iwashita Y, Fukuzawa K, Kashima K, Yokoyama S, Inomata M and Kitano
S: Mammalian target of rapamycin signaling activation patterns in
pancreatic neuroendocrine tumors. J Hepatobiliary Pancreat Sci.
21:288–295. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Missiaglia E, Dalai I, Barbi S, Beghelli
S, Falconi M, della Peruta M, Piemonti L, Capurso G, Di Florio A,
delle Fave G, et al: Pancreatic endocrine tumors: Expression
profiling evidences a role for AKT-mTOR pathway. J Clin Oncol.
28:245–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takizawa N, Ohishi Y, Hirahashi M,
Takahashi S, Nakamura K, Tanaka M, Oki E, Takayanagi R and Oda Y:
Molecular characteristics of colorectal neuroendocrine carcinoma;
similarities with adenocarcinoma rather than neuroendocrine tumor.
Hum Pathol. 46:1890–1900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujimasa K, Ohike N, Norose T, Isobe T,
Kikuchi K, Otsuka K, Murakami M and Takimoto M: Frequent and
specific involvement of changes of the p16-RB pathway in esophageal
neuroendocrine carcinoma. Anticancer Res. 39:1927–1934. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee SM and Sung CO: Neuroendocrine
carcinomas of the gallbladder: A clinicopathologic and
immunohistochemical analysis of 34 resected cases. Am J Surg
Pathol. 44:1308–1321. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park C, Ha SY, Kim ST, Kim HC, Heo JS,
Park YS, Lauwers G, Lee J and Kim KM: Identification of the BRAF
V600E mutation in gastroenteropancreatic neuroendocrine tumors.
Oncotarget. 7:4024–4035. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burkart J, Owen D, Shah MH, Abdel-Misih
SRZ, Roychowdhury S, Wesolowski R, Haraldsdottir S, Reeser JW,
Samorodnitsky E, Smith A and Konda B: Targeting BRAF mutations in
high-grade neuroendocrine carcinoma of the colon. J Natl Compr Canc
Netw. 16:1035–1040. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tashiro T, Imamura K, Tomita Y, Tamanoi D,
Takaki A, Sugahara K, Sato R, Saruwatari K, Sakata S, Inaba M, et
al: Heterogeneous tumor-immune microenvironments between primary
and metastatic tumors in a patient with ALK rearrangement-positive
large cell neuroendocrine carcinoma. Int J Mol Sci. 21:97052020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumar A, Kumar B, Muthu GS and Mitra S:
68Ga-DOTANOC PET/CT detects a rare case of metastatic
neuroendocrine neoplasm of the gallbladder. Clin Nucl Med.
47:539–540. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berzaczy D, Giraudo C, Haug AR, Raderer M,
Senn D, Karanikas G, Weber M and Mayerhoefer ME: Whole-body
68Ga-DOTANOC PET/MRI versus 68Ga-DOTANOC PET/CT in patients with
neuroendocrine tumors: A prospective study in 28 patients. Clin
Nucl Med. 42:669–674. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eltawil KM, Gustafsson BI, Kidd M and
Modlin IM: Neuroendocrine tumors of the gallbladder: An evaluation
and reassessment of management strategy. J Clin Gastroenterol.
44:687–695. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu J, Nikiforova MN, Hodak SP, Yim JH, Cai
G, Walls A, Nikiforov YE and Seethala RR: Tumor-to-tumor metastases
to follicular variant of papillary thyroid carcinoma: Histologic,
immunohistochemical, and molecular studies of two unusual cases.
Endocr Pathol. 20:235–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Machairas N, Paspala A, Frountzas M,
Tsilimigras DI, Moris D, Ntomi V, Tsapralis D and Schizas D: Mixed
adenoneuroendocrine carcinoma (MANEC) of the gallbladder: A
systematic review of outcomes following surgical management. In
Vivo. 33:1721–1726. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kalekou H and Miliaras D: Cytokeratin 7
and 20 expression in gallbladder carcinoma. Pol J Pathol. 62:25–30.
2011.PubMed/NCBI
|
|
24
|
La Rosa S, Marando A, Furlan D, Sahnane N
and Capella C: Colorectal poorly differentiated neuroendocrine
carcinomas and mixed adenoneuroendocrine carcinomas: Insights into
the diagnostic immunophenotype, assessment of methylation profile,
and search for prognostic markers. Am J Surg Pathol. 36:601–611.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krug LM, Crapanzano JP, Azzoli CG, Miller
VA, Rizvi N, Gomez J, Kris MG, Pizzo B, Tyson L, Dunne M and Heelan
RT: Imatinib mesylate lacks activity in small cell lung carcinoma
expressing c-kit protein: A phase II clinical trial. Cancer.
103:2128–2131. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ayabe RI, Wach M, Ruff S, Martin S, Diggs
L, Wiemken T, Hinyard L, Davis JL, Luu C and Hernandez JM: Primary
gallbladder neuroendocrine tumors: Insights into a rare histology
using a large national database. Ann Surg Oncol. 26:3577–3585.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu W, Chen W, Chen J, Hong T, Li B, Qu Q
and He X: Neuroendocrine carcinoma of gallbladder: A case series
and literature review. Eur J Med Res. 24:82019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iype S, Mirza TA, Propper DJ, Bhattacharya
S, Feakins RM and Kocher HM: Neuroendocrine tumours of the
gallbladder: Three cases and a review of the literature. Postgrad
Med J. 85:213–218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niu C, Wang S, Guan Q, Ren X, Ji B and Liu
Y: Neuroendocrine tumors of the gallbladder. Oncol Lett.
19:3381–3388. 2020.PubMed/NCBI
|
|
30
|
Chiorean L, Bartos A, Pelau D, Iancu D,
Ciuleanu T, Buiga R, Oancea I, Mangrau A, Iancu C and Badea R:
Neuroendocrine tumor of gallbladder with liver and retroperitoneal
metastases and a good response to the chemotherapeutical treatment.
J Med Ultrason (2001). 42:271–276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Elahi F, Ahmadzadeh A, Yadollahzadeh M,
Hassanpour K and Babaei M: Neuroendocrine tumor of the gallbladder.
Arch Iran Med. 16:123–125. 2013.PubMed/NCBI
|
|
32
|
Okuyama Y, Fukui A, Enoki Y, Morishita H,
Yoshida N and Fujimoto S: A large cell neuroendocrine carcinoma of
the gall bladder: Diagnosis with 18FDG-PET/CT-guided biliary
cytology and treatment with combined chemotherapy achieved a
long-term stable condition. Jpn J Clin Oncol. 43:571–574. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Raymond E, Dahan L, Raoul JL, Bang YJ,
Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A,
et al: Sunitinib malate for the treatment of pancreatic
neuroendocrine tumors. N Engl J Med. 364:501–513. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yao JC, Shah MH, Ito T, Bohas CL, Wolin
EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG,
et al: Everolimus for advanced pancreatic neuroendocrine tumors. N
Engl J Med. 364:514–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yan S, Wang Y, Chen X, Zhang Y, Huang Z,
Zhao J, Zhou J, Li Z, Bi X, Luo Z, et al: Clinical analysis of 15
cases of gallbladder neuroendocrine carcinoma and comparison with
gallbladder adenocarcinoma using a propensity score matching.
Cancer Manag Res. 12:1437–1446. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu W, Chen W, He X, Qu Q, Hong T and Li
B: Cholecystectomy with gallbladder bed cautery might be sufficient
for T1bN0M0 neuroendocrine carcinoma of gallbladders: Cases report
and literature review. Medicine (Baltimore). 96:e87782017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kanetkar AV, Patkar S, Khobragade KH,
Ostwal V, Ramaswamy A and Goel M: Neuroendocrine carcinoma of
gallbladder: A step beyond palliative therapy, experience of 25
cases. J Gastrointest Cancer. 50:298–303. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen C, Wang L, Liu X, Zhang G, Zhao Y and
Geng Z: Gallbladder neuroendocrine carcinoma: Report of 10 cases
and comparision of clinicopathologic features with gallbladder
adenocarcinoma. Int J Clin Exp Pathol. 8:8218–8226. 2015.PubMed/NCBI
|
|
39
|
de Mestier L, Cros J, Neuzillet C, Hentic
O, Egal A, Muller N, Bouché O, Cadiot G, Ruszniewski P, Couvelard A
and Hammel P: Digestive system mixed
neuroendocrine-non-neuroendocrine neoplasms. Neuroendocrinology.
105:412–425. 2017. View Article : Google Scholar : PubMed/NCBI
|