Introduction
Natural killer (NK) cells are powerful cytotoxic
lymphocytes that play a vital role in the innate immune response by
eliminating abnormal cells without relying on specific antigens
(1,2). The function and specificity of NK
cells are determined by the binding of activating and inhibitory
receptors that bind to various ligands on the surface of the target
cells (2,3). NK cells are specifically sensitive to
cancer or transformed cells that exhibit reduced or absent
expression of major histocompatibility complex (MHC) class I
molecules. By contrast, NK cells have low sensitivity to cancer
cells with high expression of MHC class I molecules (2,4). Thus,
a stronger additional activation signal is needed to overcome this
shortcoming of NK cells.
Ionizing radiation (IR) gives rise to systemic
antitumor immune responses as well as local antitumor effects by
expressing a variety of immunomodulatory molecules that recruit and
stimulate immune cells, such as macrophages, and dendritic, T, and
NK cells (5,6). IR induces the expression of various
immune stimulatory molecules, such as MHC class I molecules, NK
group 2D (NKG2D) ligands, co-stimulatory molecules, Fas/CD95,
vascular cell adhesion molecule-1 and intercellular adhesion
molecule-1 (5–8). In particular, NKG2D ligands are
important key factors for increasing the sensitivity of NK cells to
cancer cells. Radiation-induced NKG2D ligands showed different
expression patterns for a variety of cancer cells (9–11).
NK cells express low-affinity Fc immunoglobulin G
(IgG) receptor (FcγRIII/CD16), which triggers antibody-dependent
cellular cytotoxicity (ADCC). ADCC is one of the major immune
effector mechanisms responsible for the efficacy of antibody-based
cancer therapies (12).
EGFR is overexpressed in various types of malignant
cells present in colorectal, head and neck, lung, and pancreatic
cancer; such types of cancer have a poor prognosis (13–16).
Cetuximab (Erbitux) is a chimeric IgG1 monoclonal antibody (mAb)
that binds to EGFR and has been approved by the Food and Drug
Administration for treating patients with metastatic colorectal
cancer (17). However, the
treatment efficacy of patients with metastatic colorectal cancer
was limited when cetuximab alone was used (18). Thus, the efficacy of cetuximab could
be enhanced by NK-mediated immunotherapy to provoke ADCC in
antibody-coated target cells.
NK cells have been investigated in a variety of
therapeutic strategies for the management of cancer (19,20).
However, it is necessary to obtain a sufficient number of cells
with high purity for the therapeutic use of NK cells. Previous
studies have reported methods for large-scale NK cell expansion
using various cancer cell-derived feeder cells that activate NK
cells through cell-to-cell contact (21–25).
Our previous study reported a method for large-scale NK cell
expansion by combining an anti-CD16 mAb and autologous PBMCs
irradiated with 25 Gy (26). This
method provided an appropriate environment for activating and
expanding NK cells, and effectively inhibited the proliferation of
T cells. In the present study, a novel method to enhance the
expression levels of various activating ligands that stimulated the
sensitivity of NK cells to PBMCs was developed to more effectively
expand NK cells. This new method expands high-purity activated NK
cells >10,000-fold and is accompanied by limited contamination
from other cells.
Several studies on the combination of targeted
antibody therapy and NK cells have been reported (27–29),
but the combination treatment using radiation and antibody therapy
together with NK cells has not been studied to the best of the
authors' knowledge. The present study investigated the effects of
these combinations on SW480 and HT-29 human colorectal cancer cells
using expanded NK cells. Radiotherapy and targeted antibody therapy
were simultaneously applied to highly cytotoxic NK cells expanded
in vitro to treat the target cancer more effectively. Taken
together, it was demonstrated that NK cells were efficiently
expanded in vitro by activated/irradiated PBMCs, and this
multimodal approach more effectively eliminated target cancer
cells.
Materials and methods
Human cancer cell lines
Two human colorectal cancer cell lines, SW480 (cat.
no. CCL-288) and HT29 (cat. no. HTB-38) were obtained from American
Type Culture Collection and characterized by STR profiling. All
cell lines were cultured in RPMI 1640 medium (Welgene, Inc.)
supplemented with 10% FBS (Biowest) and antibiotic-antimycotic
(Thermo Fisher Scientific, Inc.) and maintained at 37°C in a
humidified atmosphere supplied with 5% CO2 air.
Generation of activated and irradiated
PBMCs
Experiments using human blood were approved
(approval no. D-2002-032-002) by the Institutional Review Board
(IRB) of Dongnam Institute of Radiological & Medical Sciences
(Jangan-eup, South Korea), and written informed consent was
obtained from all donors prior to participation in the present
study. Peripheral blood mononuclear cells (PBMCs) were collected
from healthy donors, and the buffy coat layer was separated by
density gradient centrifugation using Lymphoprep™ reagent,
according to the manufacturer's protocol (Stemcell Technologies,
Inc.; cat. no. 07801). The buffy coat layer was harvested and
washed 3 times with normal saline (Dai Han Pharm. Co., Ltd.). The
isolated PBMCs were incubated for ≥30 min with or without 500 ng/ml
anti-human CD3 (GMP CD3 pure; Miltenyi Biotec GmbH; cat. no.
170-076-116) mAb, 1,000 U/ml recombinant human (rh) IFN-γ (R&D
Systems, Inc.; cat. no. 285-GMP-100), and 1,000 U/ml rhIL-2
(Proleukin; Novartis). Activated PBMCs were washed 3 times with
normal saline, and 1 ml NK culture medium was added. The cells were
irradiated with or without a dose of 25 Gy in a blood irradiator
(Eckert & Ziegler), and cultured for 0, 24, 48, and 72 h. The
cells were incubated with antibodies against human CD48-fluorescein
isothiocyanate (FITC; 1:50; BD Pharmigen; BD Biosciences; cat. no.
555759), CD112-phycoerythrin (PE; 1:50; BD Pharmigen; BD
Biosciences; cat. no. 551057), CD155-PE (1:50; R&D Systems,
Inc.; cat. no. FAB25301P) and NKG2D ligands [including MHC class I
polypeptide-related sequence A (MICA)-PE (1:50; R&D Systems
Inc.; cat. no. FAB1300P), MHC class I polypeptide-related sequence
B (MICB)-PE (1:50; R&D Systems Inc.; cat. no. FAB1599P), UL16
binding protein (ULBP)-1-PE (1:50; R&D Systems Inc.; cat. no.
FAB1380P), ULBP-2/5/6-PE (1:50; R&D Systems Inc.; cat. no.
FAB1298P) and ULBP-3-PE (1:50; R&D Systems Inc.; cat. no.
FAB1517P)] for 20 min in the dark at room temperature. The cells
were also incubated with each isotype control immunoglobulin
(Ig)M-FITC (1:50; BD Pharmigen; BD Biosciences; cat. no. 555583),
IgG1-PE (1:50; BD Pharmigen; BD Biosciences; cat. no. 555749, 1:50;
R&D Systems Inc.; cat. no. IC002P), IgG2b-PE (1:50; R&D
Systems Inc.; cat. no. IC0041P), and IgG2a-PE (1:50; R&D
Systems Inc.; cat. no. IC003P) for 20 min in the dark at room
temperature and evaluated by flow cytometry (FCM) on an FC 500
system (Beckman Coulter, Inc.). Data were analyzed using CXP
version 2.2 software (Beckman Coulter, Inc.).
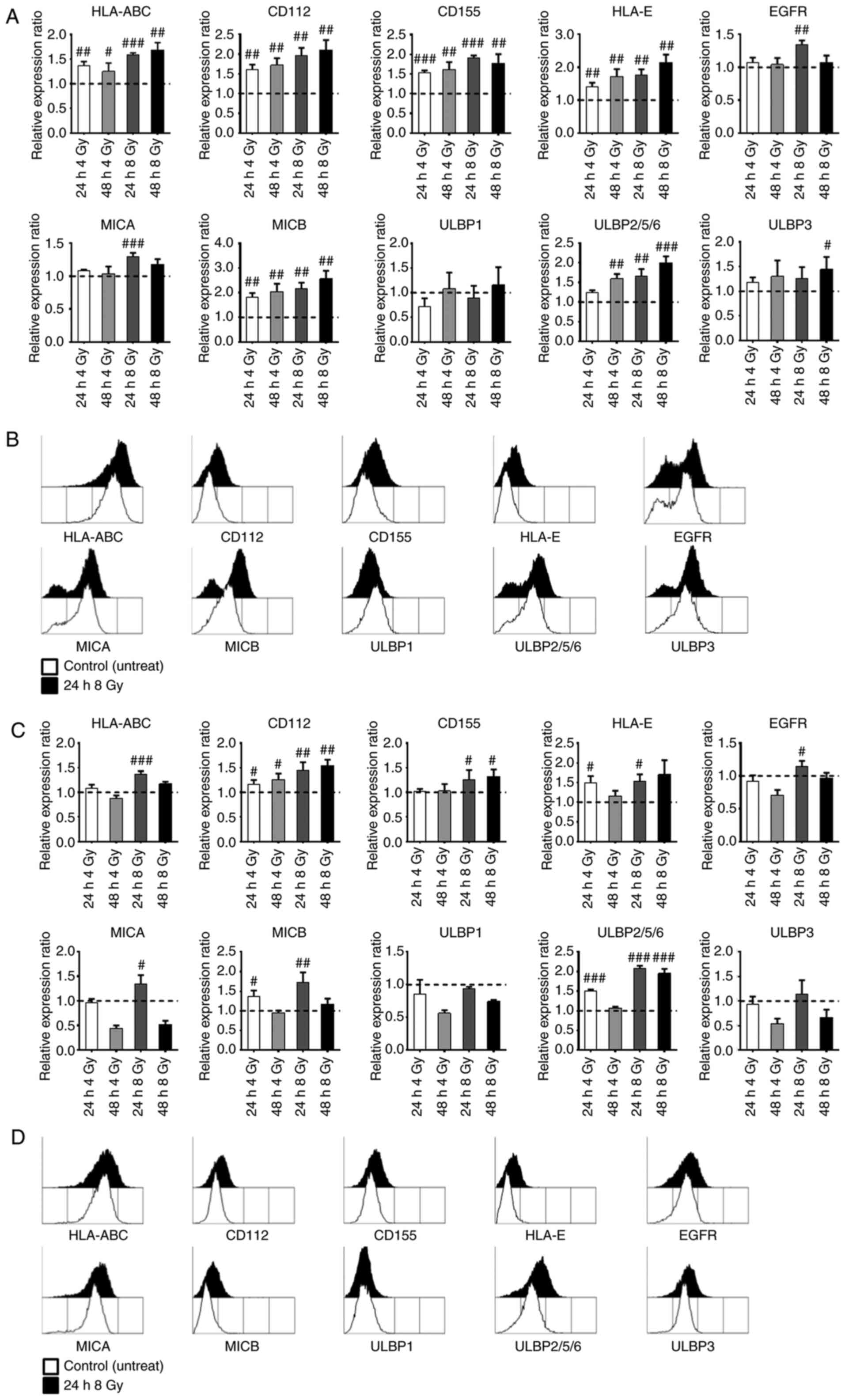
Analysis of the expression levels of
NKG2D ligands, MHC class I, and EGFR
SW480 and HT-29 cells were cultured in RPMI 1640
complete medium and maintained at 37°C in a humidified incubator
supplied with 5% CO2 air. The cells were harvested at 0,
24, or 48 h after irradiation at 4 or 8 Gy. The cells were
incubated with anti-human EGFR (10 µg/ml; Thermo Fisher Scientific,
Inc.; cat. no. MA5-13070), human leukocyte antigen (HLA)-ABC-PE
(1:50; BD Pharmigen, PD Biosciences; cat. no. 555553), HLA-E-PE
(1:50; BD Pharmigen; BD Biosciences; cat. no. 566921) and NKG2D
ligands (MICA; 2.5 µg/ml; R&D Systems Inc.; cat. no. MAB1300,
MICB; 2.5 µg/ml; R&D Systems Inc.; cat. no. MAB1599, ULBP-1;
2.5 µg/ml; R&D Systems Inc.; cat. no. MAB1380), ULBP-2/5/6 (2.5
µg/ml; R&D Systems Inc.; cat. no. MAB1298), or ULBP-3; 2.5
µg/ml; R&D Systems Inc.; cat. no. MAB1517) antibodies for 20
min in the dark at room temperature. EGFR and NKG2D ligand
detection was performed with a secondary PE-goat anti-mouse IgG
(1:500; multiple adsorption; BD Pharmigen; BD Biosciences; cat. no.
550589). The cells were also incubated with each isotype control
IgG1-unconjugated (10 µg/ml; Thermo Fisher Scientific, Inc.; cat.
no. 14-4714-82), IgG1-PE (1:50; BD Pharmigen; BD Biosciences; cat.
no. 555749), IgG2B-unconjugated (2.5 µg/ml; R&D Systems Inc.;
cat. no. MAB0041), and IgG2A-unconjugated (2.5 µg/ml; R&D
Systems Inc.; cat. no. MAB003) for 20 min in the dark at room
temperature and were analyzed by FCM.
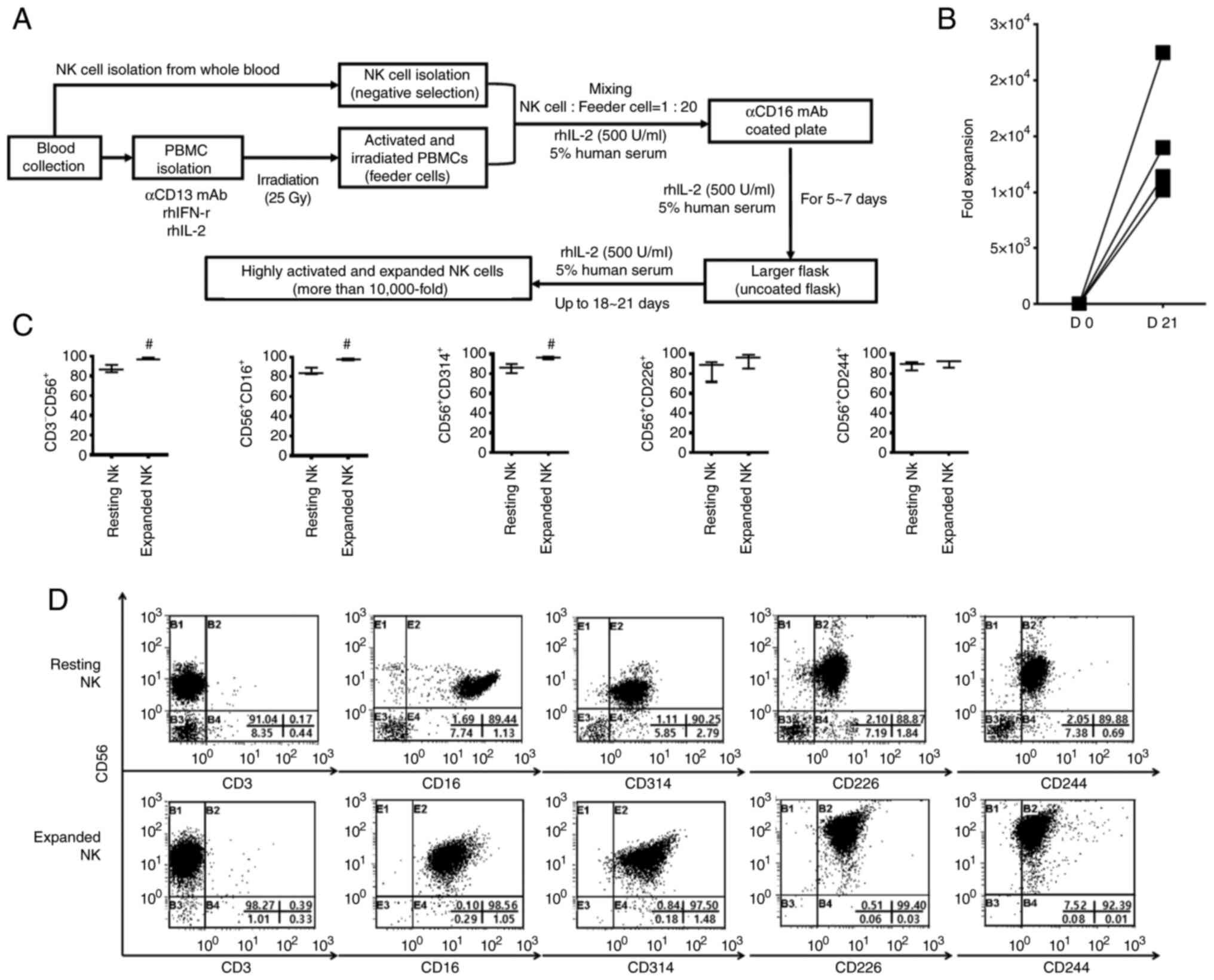
NK cell isolation and expansion
NK cells were isolated from whole blood by negative
selection (untouched cell isolation) using the EasySep™
Direct Human NK Cell Isolation Kit (Stemcell Technologies, Inc.)
according to the manufacturer's instructions. Alternatively, NK
cells could be isolated from various kits (NK Cell Isolation kit,
Miltenyi Biotec GmbH; cat. no. 130-092-657) based on untouched cell
isolation. The purity of the isolated NK cells was evaluated by FCM
using anti-human CD3-FITC (1:100; Beckman Coulter, Inc.; cat. no.
A07746) and CD56-PE-cyanine 5 (1:100; Beckman Coulter, Inc.; cat.
no. A07789) mAbs. The cells were also incubated with each isotype
control IgG1-FITC (1:100; Beckman Coulter, Inc.; cat. no. A07795),
and IgG1-PC5 (1:100; Beckman Coulter, Inc.; cat. no. A07798) for 20
min in the dark at room temperature. Isolated NK cells
(1×105 cells/ml) alongside activated and irradiated
autologous PBMCs (2×106 cells/ml) were co-cultured in
coated plates with 5 µg/ml anti-human CD16 mAb (eBioscience; Thermo
Fisher Scientific, Inc.; cat. no. 16-0167-82). NK cells were
cultured in the presence of 500 IU/ml rhIL-2 and 5% human serum
(Biowest) in CellGenix Serum-free Good Manufacturing PracticeMedia
(CellGenix; Sartorius; cat. no. 20801-0500). On days 7–8, the cells
were transferred to a larger culture flask containing Lymphocyte
Growth Medium 3 (Lonza Group Ltd.; cat. no. CC-3211) containing 500
IU/ml rhIL-2 and 5% human serum. Fresh culture medium was added
every 2 to 3 days for 18–21 days. NK cells were manufactured under
GMP conditions.
NK cell phenotype analysis
NK cells were incubated with anti-human CD3-FITC
(1:100; Beckman Coulter, Inc.; cat. no. A07746), anti-human CD16-PE
(1:100; Beckman Coulter, Inc.; cat. no. A07766), anti-human
CD56-PE-cyanine5 (1:100; Beckman Coulter, Inc.; cat. no. A07789),
anti-human CD314-PE (1:100; Beckman Coulter, Inc.; cat. no.
A08934), anti-human CD226 [DNAX accessory molecule (DNAM)-1]-FITC
(1:50; BD Pharmigen; BD Biosciences; cat. no. 559788), or
anti-human CD244 (2B4)-FITC (1:50; BD Pharmigen; BD Biosciences;
cat. no. 550815) for 20 min in the dark at room temperature. The
cells were also incubated with each isotype control: IgG1-FITC
(1:100; Beckman Coulter, Inc.; cat. no. A07795), IgG1-PE (1:100;
Beckman Coulter, Inc.; cat. no. A07796), IgG1-PE-cyanine5 (1:100;
Beckman Coulter, Inc.; cat. no. A07798), IgG1-FITC (1:50; BD
Pharmigen; BD Biosciences; cat. no. 555748), and IgG2a-FITC (1:50;
BD Pharmigen; BD Biosciences; cat. no. 555573) for 20 min in the
dark at room temperature and evaluated by FCM.
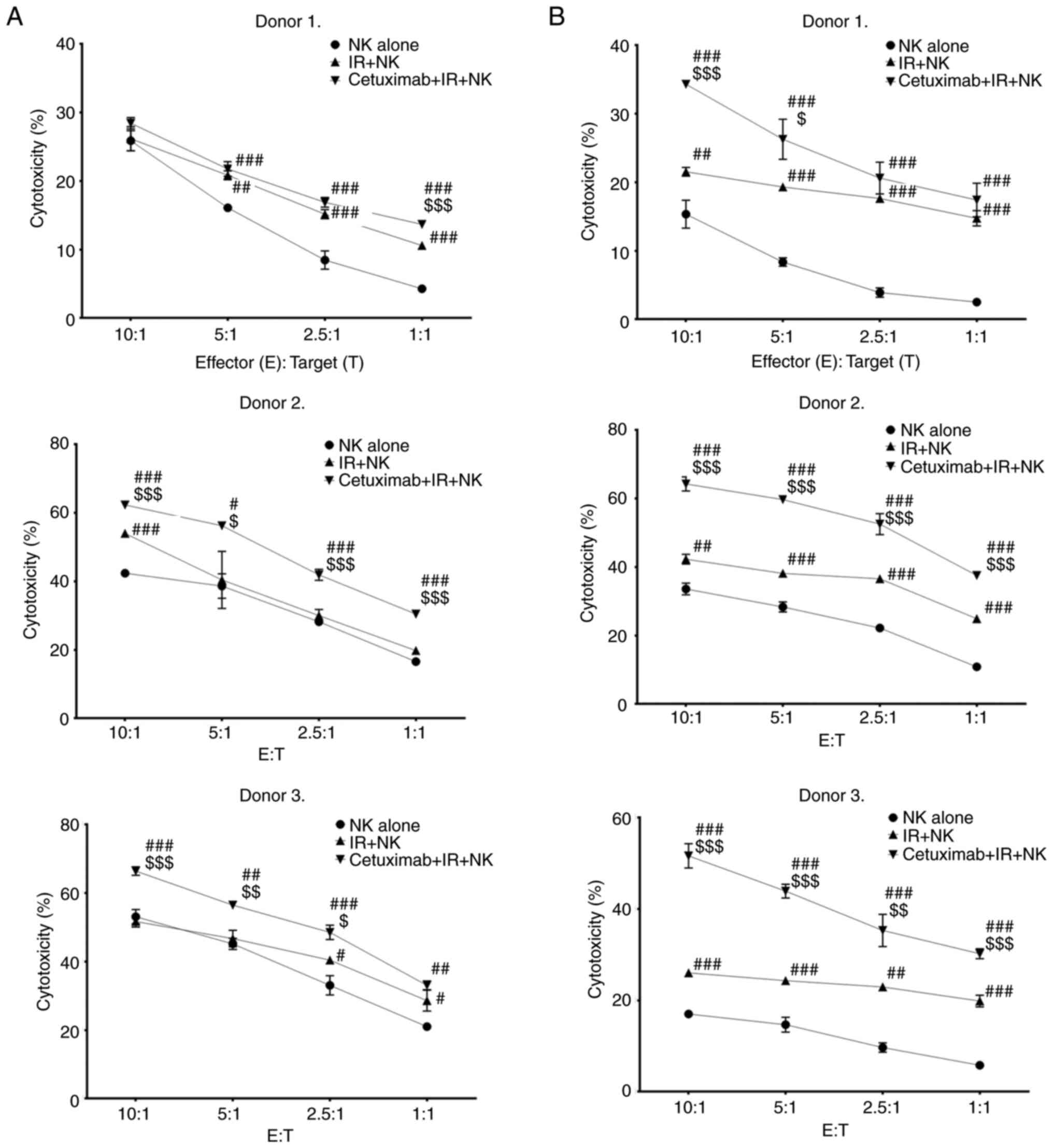
NK cell-mediated cytotoxicity
assay
SW480 and HT-29 cells (target cells) were seeded at
5×105 cells/dish 2 days before the cytotoxicity
experiments. One day after seeding, the cells were irradiated with
or without a dose of 8 Gy in a blood irradiator and cultured for 24
h. After 24 h, the cells were co-cultured with or without 10 µg/ml
cetuximab (Merck KGaA; Erbitux injection 5 mg/ml) for 30 min at
37°C. The cells were washed with PBS three times and stained with
carboxyfluorescein succinimidyl ester (CFSE)-FITC (eBioscience;
Thermo Fisher Scientific, Inc.; cat. no. 65-0850-84) at a final
concentration of 5 µM for 10 min at 37°C. After labeling, the
reaction was stopped with FBS, and the cells were washed 3 times
with normal saline. NK and CFSE-labeled target cells were seeded
into round-bottomed 96-well plates with different
effector-to-target cell number ratios (10:1, 5:1, 2.5:1 and 1:1)
and incubated for 4 h. Subsequently, the cells were transferred to
a round bottom 5-ml tube. Propidium iodide (MilliporeSigma) was
added to a final concentration of 2 µg/ml for dead cell DNA
labeling, and the dead cells were then measured by FCM. The
following groups were established: NK alone (NK + SW480 or HT-29);
IR + NK (NK + irradiated SW480 or HT-29); and cetuximab + IR + NK
(NK + irradiated SW480 or HT-29 + cetuximab).
Statistical analysis
Statistical analysis was performed in SPSS version
18.0 (IBM Corp.) and GraphPad Prism version 6.0 (GraphPad Software,
Inc.). Data are presented as the mean ± standard deviation of 3
repeats. A paired Student's t-test was used to compare the
expression of molecules on the cell surface before and after
irradiation or activation plus irradiation. For comparisons between
the two groups, an unpaired Student's t-test was used, and a
one-way ANOVA followed by a Tukey's post hoc test was used to
compare multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
A combination of anti-CD3 mAb, IFN-r,
and IL-2 enhances radiation-induced activating ligand expression in
human PBMCs
Our previous study developed a large-scale NK cell
expansion method using irradiated autologous PBMCs (26). In the present study, PBMCs isolated
from healthy donors were activated by treatment with anti-CD3 mAb,
IFN-r, and IL-2, and then irradiated with 25 Gy. Radiation alone
and activated/irradiated PBMCs were cultured for 0, 24, 48, or 72
h, and then the expression of NKG2D, 2B4, and DNAM-1 ligands were
analyzed by FCM at each time point. The cell surface expression
levels were quantified using median fluorescence intensities
(MFIs). Relative expression ratios were calculated by dividing the
MFI of the 24, 48, and 72 h samples by that of the untreated PBMCs
(0 h). As shown in Fig. 1, the
various activating ligands that stimulated the sensitivity of NK
cells showed different patterns depending on the donor and time.
Irradiated PBMCs exhibited significantly increased expression
levels of MICA, MICB, ULBP1, ULBP2/5/6, ULBP3, and CD155 compared
with those of untreated PBMCs. Activated/irradiated PBMCs also
exhibited significantly increased expression of MICA, MICB, ULBP1,
ULBP2/5/6, CD48, and CD155 compared with those of untreated PBMCs.
In particular, the expression levels of MICA, MICB, ULBP1,
ULBP2/5/6, and CD155 on activated and irradiated PBMCs were
significantly increased compared with those of PBMCs subjected to
radiation alone. Therefore, radiation altered the expression levels
of activating ligands in human PBMCs, and the combination of
anti-CD3 mAb, IFN-r, and IL-2 further promoted these
alterations.
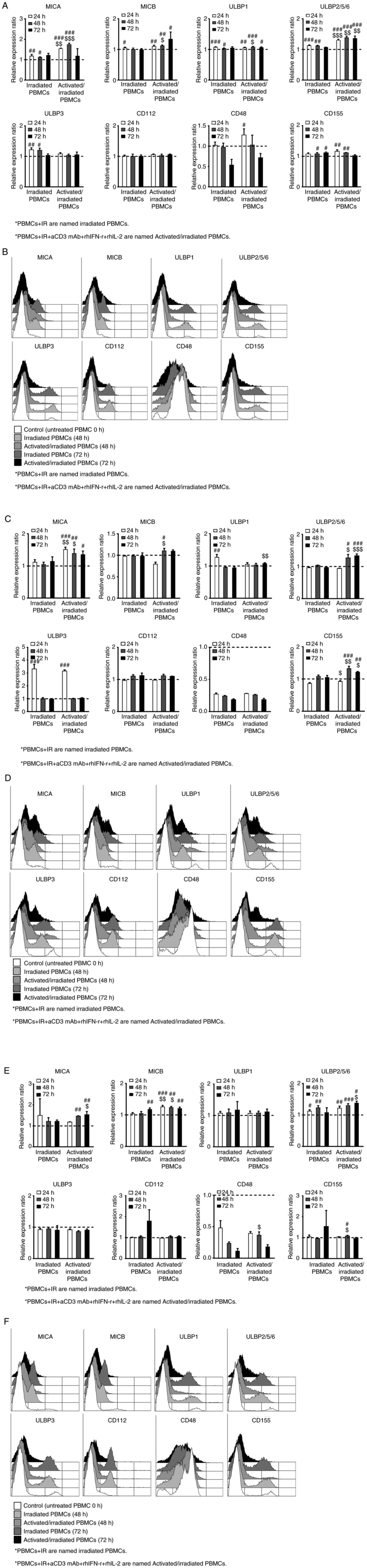 | Figure 1.Combination of anti-CD3 mAb, IFN-r,
and IL-2 with radiation further promotes the expression levels of
activating ligands in human PBMCs. PBMCs were cultured with or
without anti-CD3 mAb, IFN-r, and IL-2 and then irradiated with 25
Gy. The cells were cultured for 0, 24, 48, or 72 h. CD48, CD112,
CD155, and natural killer group 2D ligands (MICA, MICB, ULBP-1,
ULBP-2/5/6, and ULBP-3) were analyzed by flow cytometry. (A, C, and
E) Ratios of MFIs obtained from irradiated PBMCs and
activated/irradiated PBMCs. Relative expression ratios were
calculated by dividing the MFI of irradiated PBMCs (24, 48, and 72
h) or activated/irradiated PBMCs (24, 48, and 72 h) by that of
untreated PBMCs (0 h). (B, D, and F) Representative flow cytometry
histograms of each donor [white, untreated PBMCs (0 h); bright
gray, irradiated PBMCs (48 h); gray, activated/irradiated PBMCs (48
h); dark gray, irradiated PBMCs (72 h); black, activated/irradiated
PBMCs (72 h)]. The data are presented as the mean ± SD of 3 donors.
Statistical significance was determined using paired and unpaired
Student's t-test. #P<0.05, ##P<0.005,
###P<0.0005 [untreated PBMCs (0 h) vs. 24, 48, or 72
h irradiated or activated/irradiated PBMCs]. $P<0.05,
$$P<0.005, $$$P<0.0005 (24, 48, and 72
h irradiated PBMCs vs. 24, 48, and 72 h activated/irradiated
PBMCs). mAb, monoclonal antibody; r, recombinant; PBMCs, peripheral
blood mononuclear cells; MFI, median fluorescence intensity; MIC,
MHC class I polypeptide-related sequence; ULBP, UL16 binding
protein. |
Activated and irradiated PBMCs
potently induce the expansion of NK cells
To determine the expansion efficiency of NK cells by
activated and irradiated PBMCs, NK cells isolated from the whole
blood of healthy donors were expanded using activated and
irradiated autologous PBMCs. As shown in Fig. 2, isolated NK cells expanded
effectively in vitro during the culture period and expanded
by >10,000-fold at 3 weeks (Fig. 2A
and B). In particular, T cell contamination, which can induce
graft-vs.-host disease, was hardly observed in the expanded NK
cells finally obtained (Fig. 2C and
D). In addition, the expanded NK cells showed high expression
of various activating receptors (Fig.
2C and D). These results indicated that the combination of
anti-CD3 mAb, IFN-r, and IL-2 with radiation potently promoted the
expansion of NK cells by inducing the expression of various
activating ligands of PBMCs.
Radiation increases the expression
levels of various ligands associated with NK cell sensitivity in
human colorectal cancer cells
The expression levels of various activating and
inhibitory ligands were evaluated in SW480 and HT-29 human
colorectal cancer cells following radiation. SW480 and HT-29 cells
were harvested at 0, 24, or 48 h after irradiation at 4 or 8 Gy,
and analyzed using FCM. The cell surface expression levels were
quantified using MFIs. Relative expression ratios were calculated
by dividing the 24 and 48 h samples' MFI by the untreated samples'
MFI. As shown in Fig. 3, the
HLA-ABC and HLA-E expression levels were increased by radiation in
both SW480 (Fig. 3A and B) and
HT-29 (Fig. 3C and D) cells. The
EGFR expression level was increased only at 24 h after 8 Gy
radiation, and then it decreased in both SW480 and HT-29 cells. The
expression levels of CD112, CD155, MICA, MICB, and ULBP2/5/6 were
significantly increased by radiation in both SW480 and HT-29 cells.
In particular, the expression levels of MICA, MICB, and ULBP2/5/6
showed a higher increase at 8 Gy of radiation in both SW480 and
HT-29 cells, and there were more significant differences at 24 h
after 8 Gy radiation in HT-29 cells. Therefore, these results
indicated that radiation strongly increased the expression of
various ligands that modulate the sensitivity of NK cells in SW480
and HT-29 colorectal cancer cells.
Combination treatment using radiation
and cetuximab along with expanded NK cells efficiently enhances
cytotoxic activity against human colorectal cancer cells
The cytotoxic activity of expanded NK cells in the
presence or absence of cetuximab and/or radiation was confirmed in
SW480 and HT-29 human colorectal cancer cells. Expanded NK cells
were co-cultured with the irradiated and/or cetuximab-coated human
colorectal cancer cells at various ratios for 4 h. As shown in
Fig. 4, despite the high expression
of MHC class I of SW480 and HT-29 cells (Fig. 4), the expanded NK cells effectively
lysed these colorectal cancer cells, and this antitumor cytotoxic
activity was significantly enhanced by combination treatment of
radiation. The combination of expanded NK cells and radiation
showed higher cytotoxic activity compared with that of NK alone in
HT-29 cells (Fig. 4B). However, the
cytotoxic activity of the combination of expanded NK cells and
radiation against SW480 (Fig. 4A)
cells was relatively lower than that of HT-29 cells. This result
may be due to the higher expression levels of MHC class I (HLA-A,
-B, -C, and -E) molecules in SW480 cells by irradiation compared
with that of HT-29 cells. Importantly, the combination treatment of
radiation and cetuximab with expanded NK cells showed the strongest
antitumor cytotoxic activity among all the treatments for SW480 and
HT-29 cells. Taken together, these results indicated that expanded
NK cells were capable of effectively removing human colorectal
cancer cells, and the antitumor cytotoxic activity of expanded NK
cells was further enhanced by cetuximab-mediated ADCC and
radiation-induced activating ligands.
Discussion
Numerous studies have used irradiated cancer cells
as feeder cells for NK cell expansion. However, since these methods
may cause unforeseen complications due to the cancer cells used,
their safety must be thoroughly verified before clinical
application. Furthermore, the final product obtained by these
methods is typically highly contaminated with unwanted T cells
(21–25). In a previous study, our group
reported a new method of large-scale expansion of potent NK cells
(26). This method used an
anti-CD16 mAb and irradiated autologous PBMCs without the use of
cancer cell-derived feeder cells for the expansion of NK cells. In
the present study, a novel method to more effectively expand NK
cells was developed. PBMCs isolated from whole blood were activated
by treatment with anti-CD3 mAb, IFN-r, and IL-2, and then exposed
to radiation. The activated and irradiated PBMCs further increased
the expression levels of various activating ligands that increased
the sensitivity of NK cells compared with PBMCs irradiated with
radiation alone. A previous study reported that activation of T
cells resulted in the expression of multiple NKG2D ligands (MICA,
ULBP1, ULBP2, and ULBP3) through an Ataxia-telangiectasia mutated
(ATM)/Ataxia-telangiectasia and Rad3-related (ATR)-dependent
mechanism (30). Also, INF-r
increased the expression of MIC molecules on monocytes (31). This method expanded high-purity
activated NK cells by ≥10,000-fold with little contamination of T
cells. The expanded NK cells exhibited upregulated expression of
various activating receptors and promoted the secretion of
cytotoxic granules.
Colorectal cancer is the third leading cause of
cancer and the second leading cause of cancer-associated mortality,
and its annual incidence is gradually increasing worldwide
(32). Surgery is the most
effective treatment option for patients with early-stage colorectal
cancer, but numerous patients are diagnosed in either metastatic or
recurrent states, which are inoperable at the time of initial
diagnosis. Recently, an EGFR-targeted antibody (cetuximab) was sued
to treat colorectal cancer in addition to conventional treatments,
such as chemotherapy and radiotherapy; however, it is not
recommended due to its low therapeutic effect when used alone
(17,18). Therefore, our group developed a
novel treatment approach using a combination of expanded NK cells
with radiotherapy to overcome the shortcomings of this targeted
antibody therapy and enhance its effects.
The present study investigated the combined effect
of cetuximab and radiation with expanded NK cells using SW480 and
HT-29 human colorectal cancer cells. These colorectal cancer cells
are not only highly resistant to cetuximab monotherapy but also
resistant to NK cells due to their higher HLA-E expression levels
compared with those of other human colorectal cancer cells such as
COLO320, Caco-2, and SW620 (29,33). A
synergistic antitumor effect was observed when expanded NK cells
were used in combination with cetuximab and radiation.
Radiation increased the expression levels of various
ligands that modulate NK cell sensitivity in SW480 and HT-29
colorectal cancer cells. In particular, the expression levels of
activating ligands such as DNAM-1 and NKG2D ligands were further
increased by radiation. Also, the current study investigated the
effect of radiation on EGFR expression in SW480 and HT-29 cells;
however, further studies are needed to fully understand the effect
of radiation on EGFR expression. The cytotoxic activity of the
expanded NK cells may have been synergistically enhanced, as
radiation increases the sensitivity of NK cells to cancer cells,
and EGFR-targeted antibody (cetuximab) induces ADCC. Irradiation of
cancer cells resulted in a variety of biological changes that
increased the responsiveness of NK cells (6). In particular, radiation played an
important role in recruiting NK cells to the tumor site, thus
providing various activation signals (6,34).
Importantly, the DNA damage response by radiation increased the
expression of DNAM-1 and NKG2D ligands, which stimulated the
sensitivity of NK cells to cancer cells by activating the ATM/ATR
signaling pathway, leading to enhanced cancer cell killing effect
(9,35,36).
ADCC is a key factor in the clinical efficacy of
therapeutic antibodies (12). A
significant correlation between ADCC and clinical response to
treatment with therapeutic antibodies in various patients with
cancer was previously reported (27–29).
Furthermore, it was demonstrated that antibody-resistant cancer
cells were effectively eliminated by ADCC. The susceptibility to
ADCC was similar in both antibody-sensitive and antibody-resistant
cells (37). Therefore, the
administration of expanded NK cells may further enhance the
clinical efficacy of therapeutic antibodies by inducing potent
ADCC.
NK cells recognize their targets independent of
human leukocyte antigen, which greatly reduces the risk of
graft-vs.-host disease (38). These
characteristics of NK cells provide unique advantages for
allogeneic therapeutic applications (39). Previous studies have shown that the
adoptive transfer of NK cells following ex vivo activation
and expansion is safe and well-tolerated in various cancer patients
(39–43), with fever and fatigue being the most
commonly reported side effects. Therefore, similar side effects are
expected in colorectal cancer patients receiving adoptive NK cell
transfer.
Soft tissue sarcoma (STS) is correlated with the
expression of programmed cell death-1 (PD-1), PD ligand-1 (PD-L1),
New York esophageal squamous cell carcinoma-1 (NY-ESO-1), and
melanoma-associated antigen-A4 (MAGE-A4), which may indicate a poor
prognosis (44). Activated NK cells
express PD-1, and PD-1/PD-L1 blockade can increase NK cell
production of IFN-γ and CD107a, as well as the anti-tumor effect of
NK cells (45). Combining NK cells
with immune checkpoint inhibitors (anti-PD-1 or anti-PD-L1 mAb) may
effectively inhibit STS. While NK cells do not directly recognize
NY-ESO-1 and MAGE-A4 antigens like T cells, they can still inhibit
STS by binding to ligands expressed in the tumors (46). Combining specific antibodies that
bind to NY-ESO-1 or MAGE-A4 with NK cells may have ADCC and direct
effects on NK cells.
One potential limitation to consider is the lack of
a normal cell line for comparison. Without a normal cell line, it
may be challenging to determine the extent to which the observed
effects are specific to the experimental conditions used. Another
limitation to consider is the lack of in vivo validating
experimental data. While in vitro studies can provide
valuable insights into cellular processes, they may not fully
reflect the complexity of physiological systems in vivo.
However, in this study, the efficacy of in vitro activated
and expanded NK cells was confirmed using two types of colorectal
cancer cell lines. In future studies, the effectiveness of in
vitro activated and expanded NK cells will be verified using a
variety of colorectal cancer cell lines, and their efficacy and
safety in vivo will also be determined using severe combined
immunodeficiency disease (SCID) mice. Through these efforts, we aim
to provide direction for future research and address the
limitations of in vitro studies.
In summary, a novel method to efficiently expand
high-purity NK cells with a potent antitumor activity using
activated and irradiated autologous PBMCs was developed in the
present study. Combination treatment of radiotherapy and cetuximab
together with the expanded NK cells was demonstrated to be an
effective method to inhibit human colorectal cancer cells.
Therefore, this multimodal approach may help to design strategies
for eradicating cancer cells in the clinical setting of NK
cell-based immunotherapy. A combination of conventional treatments
and/or targeted anticancer agents with NK cell-based immunotherapy
could be a promising strategy to enhance the immune response and
overcome the limitations of current treatments for colorectal
cancer. This approach could increase survival rates and improve the
quality of life of patients, particularly in patients with
advanced-stage cancer.
Acknowledgements
The authors would like to thank Dr Chul Won Choi
(Department of Radiation Oncology, Dongnam Institute of
Radiological & Medical Sciences, Busan, South Korea) and Dr
Sang Youn Hwang (Department of Radiation Oncology, Dongnam
Institute of Radiological & Medical Sciences, Busan, South
Korea) for their medical knowledge assistance.
Funding
The present study was supported by the Dongnam Institute of
Radiological & Medical Sciences grant funded by the Korean
government (grant no. 50593-2022).
Availability of data and materials
Raw data were generated at Dongnam Institute of
Radiological & Medical Sciences. Derived data supporting the
findings of the present study are available from the corresponding
author on reasonable request.
Authors' contributions
YSP and JHB designed the study. EKK, HRL and WCS
designed the experimental methods, performed the experiments and
wrote the manuscript. GYP performed the data analysis. WCS and YSP
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Experiments using human blood were approved
(approval no. D-2002-032-002) by the IRB of Dongnam Institute of
Radiological & Medical Sciences (Jangan-eup, South Korea), and
written informed consent was obtained from all the donors before
involvement in the study.
Patient consent for publication
Written informed consent was obtained from the
donors for publication of the data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Trinchieri G: Biology of natural killer
cells. Adv Immunol. 47:187–376. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lanier LL: NK cell recognition. Annu Rev
Immunol. 23:225–274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caligiuri MA: Human natural killer cells.
Blood. 112:461–469. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Long EO: Regulation of immune responses
through inhibitory receptors. Annu Rev Immunol. 17:875–904. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Formenti SC and Demaria S: Systemic
effects of local radiotherapy. Lancet Oncol. 10:718–726. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park B, Yee C and Lee KM: The effect of
radiation on the immune response to cancers. Int J Mol Sci.
15:927–943. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Friedman EJ: Immune modulation by ionizing
radiation and its implications for cancer immunotherapy. Curr Pharm
Des. 8:1765–1780. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Demaria S and Formenti SC: Role of T
lymphocytes in tumor response to radiotherapy. Front Oncol.
2:952012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim JY, Son YO, Park SW, Bae JH, Chung JS,
Kim HH, Chung BS, Kim SH and Kang CD: Increase of NKG2D ligands and
sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat
shock and ionizing radiation. Exp Mol Med. 38:474–484. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
González S, López-Soto A, Suarez-Alvarez
B, López-Vázquez A and López-Larrea C: NKG2D ligands: key targets
of the immune response. Trends Immunol. 29:397–403. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ames E, Canter RJ, Grossenbacher SK, Mac
S, Smith RC, Monjazeb AM, Chen M and Murphy WJ: Enhanced targeting
of stem-like solid tumor cells with radiation and natural killer
cells. Oncoimmunology. 4:e10362122015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sulica A, Morel P, Metes D and Herberman
RB: Ig-binding receptors on human NK cells as effector and
regulatory surface molecules. Int Rev Immunol. 20:371–414. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schlessinger J: Cell signaling by receptor
tyrosine kinases. Cell. 103:211–225. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mendelsohn J and Baselga J: The EGF
receptor family as targets for cancer therapy. Oncogene.
19:6550–6565. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hynes NE and Lane HA: ERBB receptors and
cancer: The complexity of targeted inhibitors. Nat Rev Cancer.
5:341–354. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nicholson RI, Gee JM and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37 (Suppl 4):S9–S15. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Graham J, Muhsin M and Kirkpatrick P:
Cetuximab. Nat Rev Drug Discov. 3:549–550. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sotelo Lezama MJ, Sastre Valera J and
Diaz-Rubio Garcia E: Impact of cetuximab in current treatment of
metastatic colorectal cancer. Expert Opin Biol Ther. 14:387–399.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fang F, Xiao W and Tian Z: NK cell-based
immunotherapy for cancer. Semin Immunol. 31:37–54. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng M, Chen Y, Xiao W, Sun R and Tian Z:
NK cell-based immunotherapy for malignant diseases. Cell Mol
Immunol. 10:230–252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lim SA, Kim TJ, Lee JE, Sonn CH, Kim K,
Kim J, Choi JG, Choi IK, Yun CO, Kim JH, et al: Ex vivo expansion
of highly cytotoxic human NK cells by cocultivation with irradiated
tumor cells for adoptive immunotherapy. Cancer Res. 73:2598–2607.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gong W, Xiao W, Hu M, Weng X, Qian L, Pan
X and Ji M: Ex vivo expansion of natural killer cells with high
cytotoxicity by K562 cells modified to co-express major
histocompatibility complex class I chain-related protein A, 4-1BB
ligand, and interleukin-15. Tissue Antigens. 76:467–475. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujisaki H, Kakuda H, Shimasaki N, Imai C,
Ma J, Lockey T, Eldridge P, Leung WH and Campana D: Expansion of
highly cytotoxic human natural killer cells for cancer cell
therapy. Cancer Res. 69:4010–4017. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Denman CJ, Senyukov VV, Somanchi SS,
Phatarpekar PV, Kopp LM, Johnson JL, Singh H, Hurton L, Maiti SN,
Huls MH, et al: Membrane-bound IL-21 promotes sustained ex vivo
proliferation of human natural killer cells. PLoS One.
7:e302642012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Berg M, Lundqvist A, McCoy P Jr, Samsel L,
Fan Y, Tawab A and Childs R: Clinical-grade ex vivo-expanded human
natural killer cells up-regulate activating receptors and death
receptor ligands and have enhanced cytolytic activity against tumor
cells. Cytotherapy. 11:341–355. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee HR, Son CH, Koh EK, Bae JH, Kang CD,
Yang K and Park YS: Expansion of cytotoxic natural killer cells
using irradiated autologous peripheral blood mononuclear cells and
anti-CD16 antibody. Sci Rep. 7:110752017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beano A, Signorino E, Evangelista A, Brusa
D, Mistrangelo M, Polimeni MA, Spadi R, Donadio M, Ciuffreda L and
Matera L: Correlation between NK function and response to
trastuzumab in metastatic breast cancer patients. J Transl Med.
6:252008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cartron G, Dacheux L, Salles G,
Solal-Celigny P, Bardos P, Colombat P and Watier H: Therapeutic
activity of humanized anti-CD20 monoclonal antibody and
polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood.
99:754–758. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Veluchamy JP, Spanholtz J, Tordoir M,
Thijssen VL, Heideman DA, Verheul HM, de Gruijl TD and van der
Vliet HJ: Combination of NK cells and cetuximab to enhance
anti-tumor responses in RAS mutant metastatic colorectal cancer.
PLoS One. 11:e01578302016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cerboni C, Zingoni A, Cippitelli M,
Piccoli M, Frati L and Santoni A: Antigen-activated human T
lymphocytes express cell-surface NKG2D ligands via an
ATM/ATR-dependent mechanism and become susceptible to autologous
NK-cell lysis. Blood. 110:606–615. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang H, Ruan Z, Wang Y, Han J, Fu X, Zhao
T, Yang D, Xu W, Yang Z, Wang L, et al: MHC class I chain-related
molecules induced on monocytes by IFN-gamma promote NK cell
activation. Mol Immunol. 45:1548–1556. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Veluchamy JP, Lopez-Lastra S, Spanholtz J,
Bohme F, Kok N, Heideman DA, Verheul HM, Di Santo JP, de Gruijl TD
and van der Vliet HJ: In vivo efficacy of umbilical cord blood stem
cell-derived NK cells in the treatment of metastatic colorectal
cancer. Front Immunol. 8:872017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Canter RJ, Grossenbacher SK, Foltz JA,
Sturgill IR, Park JS, Luna JI, Kent MS, Culp WTN, Chen M, Modiano
JF, et al: Radiotherapy enhances natural killer cell cytotoxicity
and localization in pre-clinical canine sarcomas and first-in-dog
clinical trial. J Immunother Cancer. 5:982017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gasser S, Orsulic S, Brown EJ and Raulet
DH: The DNA damage pathway regulates innate immune system ligands
of the NKG2D receptor. Nature. 436:1186–1190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cerboni C, Fionda C, Soriani A, Zingoni A,
Doria M, Cippitelli M and Santoni A: The DNA damage response: A
common pathway in the regulation of NKG2D and DNAM-1 ligand
expression in normal, infected, and cancer cells. Front Immunol.
4:5082014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kute TE, Savage L, Stehle JR Jr,
Kim-Shapiro JW, Blanks MJ, Wood J and Vaughn JP: Breast tumor cells
isolated from in vitro resistance to trastuzumab remain sensitive
to trastuzumab anti-tumor effects in vivo and to ADCC killing.
Cancer Immunol Immunother. 58:1887–1896. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Malmberg KJ, Carlsten M, Björklund A,
Sohlberg E, Bryceson YT and Ljunggren HG: Natural killer
cell-mediated immunosurveillance of human cancer. Semin Immunol.
31:20–29. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang Y, Lim O, Kim TM, Ahn YO, Choi H,
Chung H, Min B, Her JH, Cho SY, Keam B, et al: Phase I study of
random healthy donor-derived allogeneic natural killer cell therapy
in patients with malignant lymphoma or advanced solid tumors.
Cancer Immunol Res. 4:215–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ishikawa E, Tsuboi K, Saijo K, Harada H,
Takano S, Nose T and Ohno T: Autologous natural killer cell therapy
for human recurrent malignant glioma. Anticancer Res. 24:1861–1871.
2004.PubMed/NCBI
|
|
41
|
Sakamoto N, Ishikawa T, Kokura S, Okayama
T, Oka K, Ideno M, Sakai F, Kato A, Tanabe M, Enoki T, et al: Phase
I clinical trial of autologous NK cell therapy using novel
expansion method in patients with advanced digestive cancer. J
Transl Med. 13:2772015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Masuyama J, Murakami T, Iwamoto S and
Fujita S: Ex vivo expansion of natural killer cells from human
peripheral blood mononuclear cells co-stimulated with anti-CD3 and
anti-CD52 monoclonal antibodies. Cytotherapy. 18:80–90. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ishikawa T, Okayama T, Sakamoto N, Ideno
M, Oka K, Enoki T, Mineno J, Yoshida N, Katada K, Kamada K, et al:
Phase I clinical trial of adoptive transfer of expanded natural
killer cells in combination with IgG1 antibody in patients with
gastric or colorectal cancer. Int J Cancer. 142:2599–2609. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hashimoto K, Nishimura S, Ito T, Kakinoki
R and Akagi M: Immunohistochemical expression and
clinicopathological assessment of PD-1, PD-L1, NY-ESO-1, and
MAGE-A4 expression in highly aggressive soft tissue sarcomas. Eur J
Histochem. 66:33932022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu Y, Cheng Y, Xu Y, Wang Z, Du X, Li C,
Peng J, Gao L, Liang X and Ma C: Increased expression of programmed
cell death protein 1 on NK cells inhibits NK-cell-mediated
anti-tumor function and indicates poor prognosis in digestive
cancers. Oncogene. 36:6143–6153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Boerman GH, van Ostaijen-ten Dam MM, Kraal
KC, Santos SJ, Ball LM, Lankester AC, Schilham MW, Egeler RM and
van Tol MJ: Role of NKG2D, DNAM-1 and natural cytotoxicity
receptors in cytotoxicity toward rhabdomyosarcoma cell lines
mediated by resting and IL-15-activated human natural killer cells.
Cancer Immunol Immunother. 64:573–583. 2015. View Article : Google Scholar : PubMed/NCBI
|