Introduction
Lipomatous tumours represent a category of neoplasms
with a broad spectrum and clinical behaviour (1). Liposarcomas are the most common
malignant tumours of soft tissue of mesenchymal origin (2,3). They
can be located in any part of the body with fatty tissue (4).
Several histological types have been described and
their classification has changed over the last two decades, with
new clinical entities appearing. The importance of diagnosis after
histology is relevant to predict tumour behaviour and
prognosis.
From the first description by Rudolf Virchow in 1857
of a tumour originating from adipose tissue with mixed features,
which he called ‘myxoma lipomatodes lesion’, to the current concept
of liposarcoma, several classifications have emerged (5–7)
(Table I). Currently, the fifth WHO
classification of Tumors of Soft Tissue and Bone, published in
2020, establishes atypical lipomatous tumour as a tumour of
intermediate grade of malignancy and well-differentiated
liposarcoma (WDL) with its variants (lipoma-like, sclerosing and
inflammatory), dedifferentiated liposarcoma (DDL), myxoid
liposarcoma (MLP) and pleomorphic liposarcoma (PLP) as malignant
adipocytic tumours. It also introduces two histological subtypes
not described in the previous classifications: atypical spindle
cell/pleomorphic lipomatous tumour (ASC) and pleomorphic myxoid
liposarcoma (MP) (8,9). While ASC originates as a superficial
lipomatous mass predominantly in the extremities with a low
recurrence rate, distant metastasis as well as dedifferentiation
phenomena, MP is characterised by large lesions predominantly in
young patients, located in the mediastinum with a highly aggressive
character (high local recurrence, distant metastatic capacity with
affinity for lung and bone and low survival rate) (10–12).
 | Table I.Evolution of the WHO classification
of liposarcomas. |
Table I.
Evolution of the WHO classification
of liposarcomas.
| 1994 | 2002 | 2013 | 2020 |
|---|
|
| Intermediate
aggressiveness |
| Intermediate
aggressiveness |
|
Well-differentiated |
Well-differentiated |
Well-differentiated | Well-differentiated
/atypical |
| liposarcoma | liposarcoma | liposarcoma | lipomatous tumour
(WDL/ALT) |
| Adipocyte
lipoma-like |
|
| Malignant
adipocytic tumours |
| Sclerosing | Malignant
adipocytic tumours |
| Adipocyte
lipoma-like |
| Inflammatory |
|
| Inflammatory |
| Myxoid
liposarcoma | Myxoid
liposarcoma | Myxoid
liposarcoma | Myxoid
liposarcoma |
| Round cell
liposarcoma |
|
| Sclerosing |
| Pleomorphic
liposarcoma | Pleomorphic
liposarcoma | Pleomorphic
liposarcoma | Pleomorphic
liposarcoma |
|
Dedifferentiated |
Dedifferentiated |
Dedifferentiated | Dedifferentiated
liposarcoma |
| liposarcoma | liposarcoma | liposarcoma | Atypical spindle
cella |
| |
|
| Pleomorphic myxoid
liposarcomaa |
WDL/ATL together with DDL represent the most
frequent types of liposarcoma. WDL/ALT accounts for 40% of all
liposarcomas (3,13). The terms WDL and ALT are used
interchangeably to refer to tumours with identical histology but
different anatomical location. According to the WHO classification
of these lesions, ALT will be used for those liposarcomas located
in the extremities or superficial trunk while WDL would be reserved
for those located in the retroperitoneum, mediastinum or
paratesticular (14).
We present a series of patients operated on in our
centre, carrying out a descriptive and analytical statistical
analysis with the aim of studying the main prognostic factors of
these tumours with respect to recurrence and survival.
Materials and methods
Retrospective observational study
All patients operated on at the Hospital
Universitario Príncipe de Asturias de Alcalá de Henares in Alcalá
de Henares, Madrid, Spain, during the period from October 2000 to
January 2020 were collected.
Due to changes in the WHO classification of bone and
soft tissue tumours, the Anatomical Pathology Department was asked
to review the tissues and their classification according to the
fifth WHO classification.
The inclusion criteria were: final histological
diagnosis of liposarcoma (any of its variants), resected disease
with curative intent and patients over 18 years of age. Patients
with a previous history of liposarcoma and those with soft tissue
lesions in which immunohistochemical or molecular studies were
negative for liposarcoma were excluded. In addition, other soft
tissue tumours such as solitary fibrous tumour, soft tissue
sarcomas, gastrointestinal stromal tumours or lipomas were
excluded.
The diagnosis of liposarcoma was determined by the
Department of Pathology, through the microscopic and macroscopic
study of the submitted specimen. To distinguish between the
different histological subtypes (WDL/ALT, DDL and MLP) the
determination of murine double minute-2 (MDM2) and cyclin-dependent
kinase 4 (CDK4) was performed. The amplification of MDM2 and CDK4
was based on fluorescence in situ hybridization (FISH)
analysis (15). Prior to 2016, we
did not have this amplification technique in our centre, so it has
only been determined in the cases of establishing the differential
diagnosis of the histological subtype from that year on in the
study patients. The determination of the Ki-67 cell replacement
index was performed by immunohistochemistry, using MIB-1 monoclonal
assays, specific for the Ki67 nuclear protein (16). To carry out the evaluation of the
immunohistochemical expression of Ki-67, three random fields of
representative sections of each lesion were selected. The positive
cell count was performed using a ×400 magnification microscope
objective. After, all visualized brown nuclear staining was
interpreted as positive immunohistochemical expression for Ki67.
The total cells of each cell population and the number of stained
cells were counted, in order to obtain the total percentage of
stained cells per cell population and a total percentage of the
expression of each marker of the analyzed specimen.
Variables
Epidemiological variables (age, sex, comorbidities),
location of the lesion, form of presentation, diagnosis, tumour
size, histological subtype, degree of differentiation, as well as
those related to the surgical intervention (average length of stay,
associated surgery, recurrence, type of recurrence, relapse,
presence of distant metastasis or type of surgical resection) or
type of adjuvant treatment were collected. All variables were
collected in a Microsoft Excel 2020® spreadsheet.
The odds ratio (OR) was calculated to describe the
risk the recurrence from histology tumour or type of surgery
(R0/R1/R2 resection). The OR determines an estimate (with
confidence interval) for the relationships between dichotomic
variables. The significance level used to calculate the confidence
level was 0.05 (alpha level), which indicates a confidence level of
95%. Fisher's test was used to study whether there was an
association between two qualitative variables.
In the case of categorical variables, the proportion
of each category with respect to the total number of patients was
calculated. For qualitative variables, the distribution of
phenomena was studied, while for quantitative variables, the mean
and standard deviation were studied.
Survival (calculated in months) of the patients
included in the study was estimated using the Kaplan-Meier method.
It was performed both for patients with a histological diagnosis of
WDL/ALT based on their location (retroperitoneal vs.
non-retroperitoneal), to compare patients with histology other than
WDL/ALT (non-WDL/ALT, which includes DDL, PLP and MLP) depending on
its location (retroperitoneal or non-retroperitoneal) and to
compare survival regardless of the histological subtype of
liposarcoma, establishing location as a variable (retroperitoneal
and non-retroperitoneal).
Ethical approval
The present study was approved by the Ethics
Committee of the Fundación para la Investigación del Hospital
Universitario Príncipe de Asturias (protocol number: OE 49/2020) on
23rd February 2021, with a favourable opinion, exempting the
informed consent of the patients included as it was a retrospective
study.
Results
Patients
Fifty-two patients (17 females (59.3±13.7) and 35
males (57.1±16.7 years) diagnosed with liposarcoma in the described
period were studied. The overall mean age was 57.2±15.9 years.
In the study we decided to divide patients into two
groups according to location (group A (retroperitoneal location)
and group B (non-retroperitoneal location, dependent on superficial
fatty tissue).
Group A (retroperitoneal location) consisted of 16
patients (30.7%). Within group B, the most frequent locations were:
lower limb (22 patients; 42.3%), upper limb (5 patients; 9.6%),
dorsal (2 patients; 3.8%), inguinal (4 patients; 7.7%), head and
neck (2 patients; 3.8%) and perianal (1 patient; 1.9%).
Retroperitoneal location
Group A consisted of 16 patients (mean age 60.6±13.3
years), divided into 6 males (mean age 61.7±16.1 years) and 10
females (mean age 60±12.3 years). The clinical characteristics in
relation to presentation, diagnosis, tumour size, degree of
differentiation and histology are shown in Table II. In all patients the diagnosis
was made by CT scan with intravenous contrast. In only 2 patients,
MRI was performed as an adjunct (12.5%).
 | Table II.Clinical features of patients with
retroperitoneal and non-retroperitoneal liposarcomas. |
Table II.
Clinical features of patients with
retroperitoneal and non-retroperitoneal liposarcomas.
| Patient | Sex | Age, years | Location | Clinical
presentation | Group | Diagnosis | Size, cm | Histology |
|---|
| Patient 1 | Female | 45 |
Retroperitoneal | Tumour | Group A | CT | 22×16 | WDL/ALT |
| Patient 2 | Male | 69 | Upper limb | Tumour | Group B | US | 7×6×3 | WDL/ALT |
| Patient 3 | Male | 33 | Upper limb | Tumour | Group B | CT + US | 7×8 | MLP |
| Patient 4 | Male | 53 | Upper limb | Tumour | Group B | CT | 6 | PLP |
| Patient 5 | Female | 47 | Upper limb | Local pain | Group B | MRI | 9X6 | WDL/ALT |
| Patient 6 | Male | 63 | Lower limb | Tumour | Group B | US | 10×8×5 | WDL/ALT |
| Patient 7 | Female | 47 | Back | Tumour | Group B | US | 7.5×3.2×3.7 | WDL/ALT |
| Patient 8 | Male | 49 | Back | Tumour | Group B | US | 14×6.5×2 | WDL/ALT |
| Patient 9 | Female | 54 | Lower limb | Tumour | Group B | US | 3×3×1.5 | WDL/ALT |
| Patient 10 | Male | 43 | Lower limb | Tumour | Group B | US | 6×4×3.5 | WDL/ALT |
| Patient 11 | Female | 41 | Upper limb | Tumour | Group B | US | 7×3.5×3 | WDL/ALT |
| Patient 12 | Male | 82 | Lower limb | Tumour | Group B | US | 10 | WDL/ALT |
| Patient 13 | Male | 69 | Lower limb | Tumour | Group B | CT+MRI | 11×9×8 | MLP |
| Patient 14 | Male | 81 | Lower limb | Tumour | Group B | CT | 15×10×8 | WDL/ALT |
| Patient 15 | Female | 57 |
Retroperitoneal | Tumour | Group A | CT | 15×9 | MLP |
| Patient 16 | Female | 28 | Lower limb | Tumour | Group B | US + MRI | 20×10×15 | WDL/ALT |
| Patient 17 | Male | 22 | Lower limb | Tumour | Group B | MRI | 8×5×2 | MLP |
| Patient 18 | Female | 50 | Lower limb | Tumour | Group B | CT + MRI | 10×5.5 | MLP |
| Patient 19 | Female | 63 | Lower limb | Tumour | Group B | MRI | 13×6×2 | MLP |
| Patient 20 | Female | 71 | Lower limb | Tumour | Group B | MRI | 20×13×6 | WDL/ALT |
| Patient 21 | Male | 54 | Lower limb | Tumour | Group B | US + MRI | 18×10×10 | WDL/ALT |
| Patient 22 | Male | 40 | Lower limb | Tumour | Group B | US + MRI | 19×11×8 | MLP |
| Patient 23 | Female | 49 | Lower limb | Tumour | Group B | US + MRI | 12.5×8.5×7 | WDL/ALT |
| Patient 24 | Female | 65 | Lower limb | Tumour | Group B | CT | 11×6×3 | WDL/ALT |
| Patient 25 | Female | 84 | Lower limb | Tumour | Group B | MRI | 21×17×7 | MLP |
| Patient 26 | Male | 41 | Lower limb | Tumour | Group B | US | 11×5 | DDL |
| Patient 27 | Female | 61 | Lower limb | Tumour | Group B | US | 4×1.2×1 | PLP |
| Patient 28 | Female | 69 | Lower limb | Tumour | Group B | MRI | 12.4×10.3 | PLP |
| Patient 29 | Male | 58 | Lower limb | Tumour | Group B | MRI | 24×19×3 | WDL/ALT |
| Patient 30 | Female | 68 | Lower limb | Tumour | Group B | US | 7×6×4 | WDL/ALT |
| Patient 31 | Female | 58 | Lower limb | Local pain | Group B | MRI | 11×9×5 | WDL/ALT |
| Patient 32 | Female | 86 | Lower limb | Tumour | Group B | MRI | 23×12×16 | WDL/ALT |
| Patient 33 | Female | 61 | Lower limb | Tumour | Group B | MRI | 9×4 | WDL/ALT |
| Patient 34 | Male | 71 | Perianal | Tumour | Group B | MRI | 8×6×3 | WDL/ALT |
| Patient 35 | Male | 33 | Lower limb | Tumour | Group B | MRI | 8×3×1 | MLP |
| Patient 36 | Male | 58 | Cervical | Tumour | Group B | US | 3.3×2.5×2 | MLP |
| Patient 37 | Male | 62 | Cervical | Tumour | Group B | US | 5×5×3 | WDL/ALT |
| Patient 38 | Female | 72 |
Retroperitoneal | Abdominal pain | Group A | CT + US | 20×13×10 | MLP |
| Patient 39 | Female | 56 |
Retroperitoneal | Tumour | Group A | CT | 28×25×15 | MLP |
| Patient 40 | Female | 49 |
Retroperitoneal | Tumour | Group A | CT | 24.5×16×6 | WDL/ALT |
| Patient 41 | Female | 76 |
Retroperitoneal | Tumour | Group A | CT | 9×6.5×7 | MLP |
| Patient 42 | Male | 82 |
Retroperitoneal | Incidental | Group A | CT | 4×2×2 | WDL/ALT |
| Patient 43 | Male | 45 |
Retroperitoneal | Tumour | Group A | CT | 14×13×4 | WDL/ALT |
| Patient 44 | Male | 58 |
Retroperitoneal | Abdominal pain | Group A | CT + MRI | 16×11×13 | WDL/ALT |
| Patient 45 | Male | 46 |
Retroperitoneal | Tumour | Group A | CT | 33×20×15 | PLP |
| Patient 46 | Male | 80 |
Retroperitoneal | Ascites | Group A | CT | 25×18×12 | DDL |
| Patient 47 | Female | 64 |
Retroperitoneal | Anaemia | Group A | CT | 21×18×13 | DDL |
| Patient 48 | Male | 59 |
Retroperitoneal | Tumour | Group A | CT | 8×7×7.5 | WDL/ALT |
| Patient 49 | Female | 42 |
Retroperitoneal | Asthenia | Group A | CT + US | 20×12×8 | DDL |
| Patient 50 | Female | 76 |
Retroperitoneal | Abdominal pain | Group A | CT + US | 12×10×3 | DDL |
| Patient 51 | Female | 63 |
Retroperitoneal | Abdominal pain | Group A | CT + MRI | 18×15×12 | DDL |
| Patient 52 | Male | 76 | Testicular | Tumour | Group B | US | 3×3×2 | DDL |
Histopathological study revealed 6 atypical/well
differentiated liposarcomas (WDL/ATL), 37.5%, 5 dedifferentiated
(DDL), 31.2%, 4 myxoid liposarcomas (MPL) (25%) and 1 pleomorphic
liposarcoma (PLP) (6.2%).
Regarding histology, we observed that the mean age
of presentation for WDL/ATL was 56.3±14 years (67% men), DDL was
65±14.8 years (20% men), PLP 46 years (100% male) and MLP of
65.25±10.2 years (100% female).
Three patients died during follow-up (18.7%) related
to disease progression. Surgery was a complete resection with
unaffected surgical margins (R0) in 9 patients (56.2%) and with
microscopic involvement (R1) in 7 patients (43.7%). No surgical
resections with macroscopically affected margins (R2) were
described. The mean length of stay was 12.62±6.3 days.
In 87.5% (14 patients), surgery required at least
one visceral resection due to tumour involvement. A colectomy
(right or sigmoidectomy) was associated in 9 patients (56.2%), 1
nephrectomy (6.2%), 1 orchiectomy (6.2%), 1 adrenalectomy (6.2%)
and 2 splenectomies (12.5%).
Overall survival was 61.4±57.2 months. Regarding
histological type survival was 71.4±56.5 months (WDL/ATL), 22.7±7.5
months (DDL), 100.1±78.2 months (MLP) and 41.3 months (PLP).
Six patients had recurrence (3 WDL/ATL and 3 LPM)
after surgery (37.5%), 3 of them died during follow-up. The overall
disease-free interval was 29.8±12 months. A disease-free interval
of 36.1±13.8 months was observed for WDL/ATL and 23.5±7.3 months
for MLP (Fig. 1).
The OR was calculated as a function of recurrence in
relation to histology (OR (WDL/ATL) 1.3 (95% CI P=0.736) and OR
(MLP) 2 (95% CI P=0.441). The OR for recurrence was 1.5 (95% CI
P=0.02) for R1 vs. R0 resection.
All patients in whom recurrence was described, it
was detected locally in the peritoneum where the original tumour
was located. Only 1 patient showed pulmonary metastasis. Three
patients received adjuvant treatment with systemic chemotherapy
(first-line adriamycin-based regimens). Only one patient received
intraperitoneal hyperthermic chemotherapy with doxorubucin in
conjunction with cytoreduction surgery. Of the two patients with
local recurrence, one underwent salvage surgery and is currently
free of disease, while the other patient was not considered for
further treatment due to advanced age.
Non-retroperitoneal location
Group B consisted of 36 patients (mean age 57.2±15.9
years), divided into 17 females (mean age 58.9±14.8 years) and 19
males (53.6±17.1 years). The characteristics of each liposarcoma
(diagnostic presentation, size, grade and histology) are listed in
Table II.
In 16 patients MRI was sufficient to approximate the
diagnosis and to study the relationship with neighbouring
structures. In 6 patients, CT was performed, while ultrasound was
performed in 9 patients as the only imaging test. Only 9 patients
underwent surgery without imaging.
Histopathological study revealed 22
atypical/well-differentiated liposarcomas (WDL/ATL), 61%, 2
dedifferentiated (DDL), 5.5%, 9 myxoid liposarcomas (MLP) (25%) and
3 pleomorphic liposarcomas (PLP) (8.3%). Histological markers
(MDM-2, CK4, Ki67) were obtained from only 20 patients (Table III).
 | Table III.Histological markers (MDM-2, CK4,
Ki67) of liposarcomas. |
Table III.
Histological markers (MDM-2, CK4,
Ki67) of liposarcomas.
| Patient | MD M2 | CD K4 | Sex | Age, years | Location | Group | Grade (FNC
LCC) | Histological
subtype | Ki67 |
|---|
| Patient 7 | (+) | (−) | Female | 47 | Back | Group B | 1 | WDL/ALT | 1% |
| Patient 8 | (+) | (−) | Male | 49 | Back | Group B | 1 | WDL/ALT | Not performed |
| Patient 11 | (−) | (−) | Female | 41 | Upper Limb | Group B | 1 | WDL/ALT | Not performed |
| Patient 14 | (−) | (+) | Male | 81 | Lower Limb | Group B | 2 | WDL/ALT | 5-10 |
| Patient 20 | (−) | (+) | Male | 71 | Perianal | Group B | 1 | WDL/ALT | <1% |
| Patient 21 | (+) | (+) | Female | 54 | Lower Limb | Group B | 1 | WDL/ALT | <1% |
| Patient 23 | (−) | (+) | Female | 49 | Lower Limb | Group B | 1 | WDL/ALT | <1% |
| Patient 27 | (+) | (+) | Female | 61 | Lower Limb | Group B | 1 | WDL/ALT | 5% |
| Patient 29 | (+) | (+) | Male | 58 |
Retroperitoneal | Group A | 2 | WDL/ALT | 20-25% |
| Patient 30 | (−) | (+) | Female | 68 | Lower Limb | Group B | 1 | WDL/ALT | <1% |
| Patient 32 | (−) | (+) | Female | 86 | Lower Limb | Group B | 1 | WDL/ALT | <1% |
| Patient 37 | (+) | (+) | Male | 62 | Retrocervical | Group B | 1 | WDL/ALT | <1% |
| Patient 45 | (+) | (+) | Male | 46 |
Retroperitoneal | Group A | 2 | PLP | Not performed |
| Patient 46 | (+) | (+) | Male | 80 |
Retroperitoneal | Group A | 3 | DDL | 70% |
| Patient 47 | (+) | (+) | Female | 64 |
Retroperitoneal | Group A | 2 | DDL | 12-16% |
| Patient 48 | (+) | (+) | Male | 59 |
Retroperitoneal | Group A | 1 | WDL/ALT | 6-9% |
| Patient 49 | (+) | (+) | Female | 42 |
Retroperitoneal | Group A | 2 | DDL | 20% |
| Patient 50 | (+) | (+) | Female | 76 |
Retroperitoneal | Group A | 1 | DDL | Not performed |
| Patient 51 | (+) | (+) | Female | 63 |
Retroperitoneal | Group A | 2 | DDL | 0.02% |
| Patient 52 | (+) | (+) | Male | 76 | Testicular | Group B | 2 | DDL | Not performed |
The mean age of presentation for WDL/ATL was 59.4±14
years (45% men), DDL was 58.5±24.7 years (100% men), PLP 61±8 years
(33% male) and MLP of 50.22±20.4 years (100% male).
Surgery was a complete resection with unaffected
surgical margins (R0) in 22 patients (61.1%), with microscopic
involvement (R1) in 12 patients (33.3%) and with macroscopic
involvement (R2) in 2 patients (5.5%). In all patients in whom
surgery was not an R0, margins of the surgical site were widened
except in two patients (given their advanced age, 84 and 86 years
respectively) and in 3 others in whom, due to the tumour location,
complementary postoperative radiotherapy was decided.
The mean length of stay was 2±3.2 days. Only two
patients died during follow-up in relation to progression of their
oncological disease (1 patient with a history of MLP and 1 patient
with PLP
The overall survival of the patients described in
group B was 87.9±65.2 months. Regarding histological type survival
was 62.9±45.9 months (WDL/ATL), 48.3±35.1 months (DDL), 146.0±78.7
months (MLP) and 123.4±38.6 months (PLP). Overall survival was
assessed using Kaplan-Meier curves. Survival was analysed by
comparing the influence of the location (retroperitoneal vs.
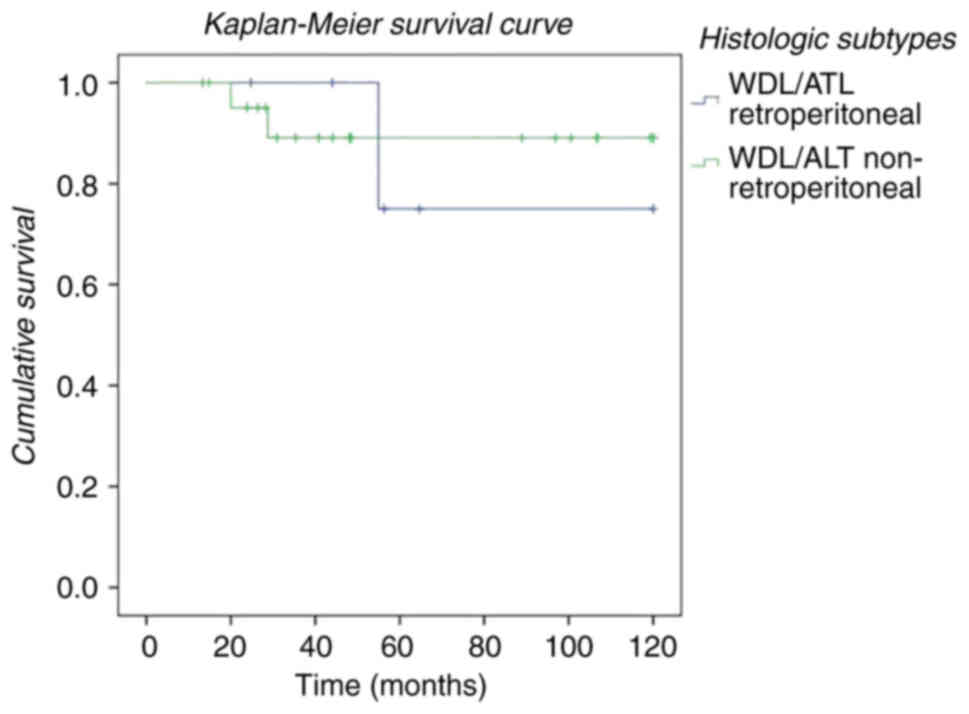
non-retroperitoneal) of the WDL/ATL liposarcomas in our series
(Fig. 2). Improved survival was
observed in patients with a non-retroperitoneal location. We also
analysed the survival of the two groups in relation to their
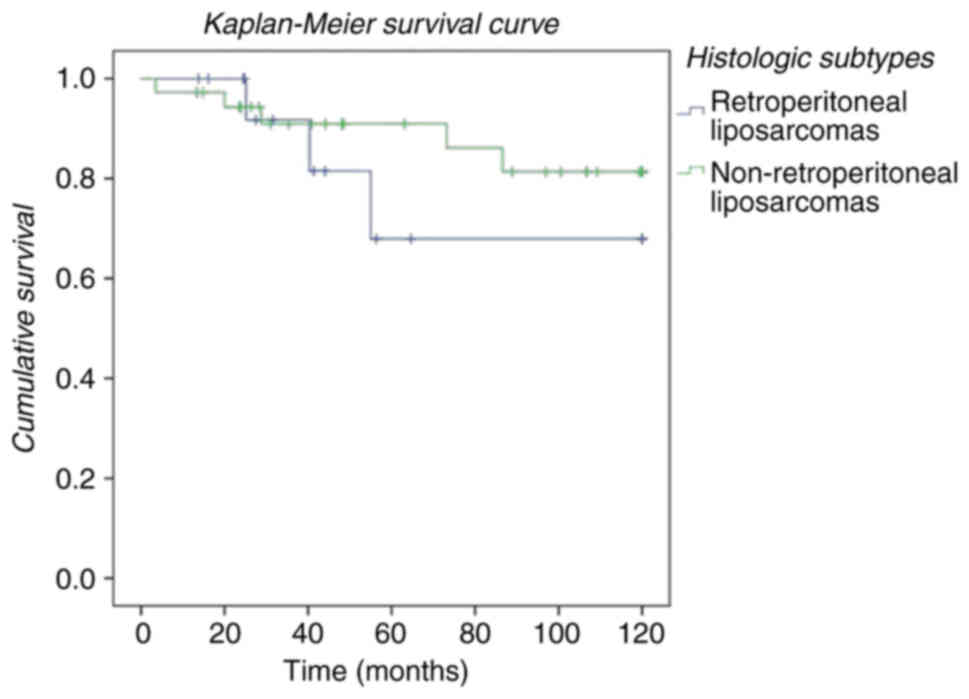
location (retroperitoneal vs. non-retroperitoneal), regardless of
histological type (Fig. 3). We
observed that patients operated on with a diagnosis of liposarcoma
located at the retroperitoneal level had a lower survival than
those whose location was extraperitoneal, regardless of
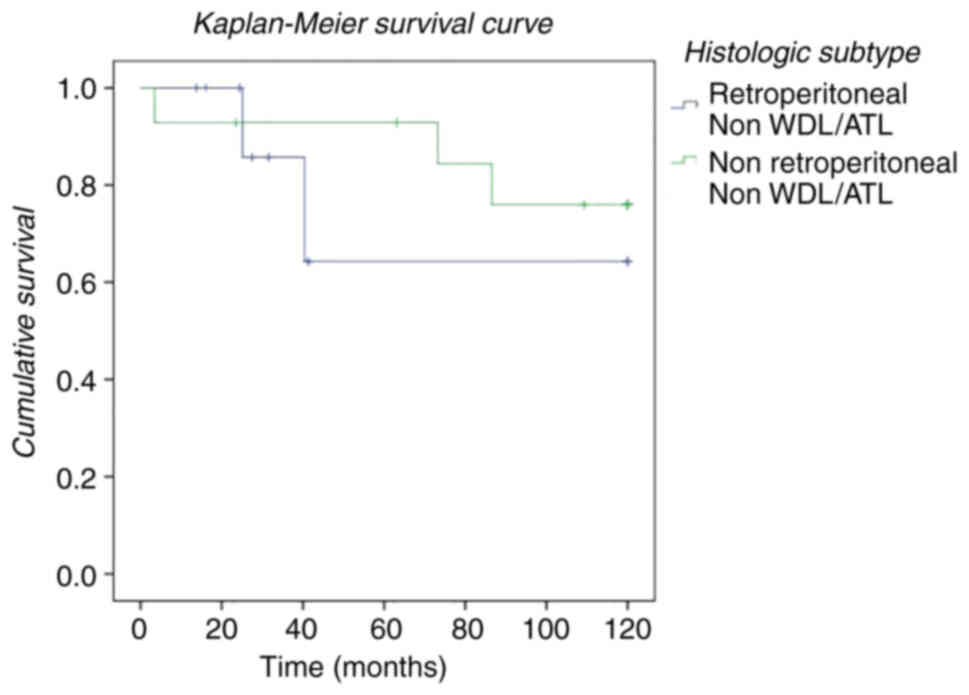
histological subtype. Finally, we studied the influence of location
(retroperitoneal vs. non-retroperitoneal) without taking WDL/ATL
histology into account, defining a group (non-WDL/ATL) made up of
DDL, PLP and MLP histologies (Fig.
4). In our study, we found that survival was lower in those
patients who underwent surgery with a diagnosis of liposarcoma
located at the retroperitoneal level in relation to the DDL, MLP
and PLP subtypes compared to extraperitoneal location.
During follow-up only 2 recurrences with two deaths
were described. The recurrence interval in these patients was
100.4±72.7 months. One patient was treated with postoperative
radiotherapy and the other patient was treated with chemotherapy
(several lines of treatment; adriamycin, trabectadine and
ifosfamide), with progression of the disease at the pulmonary level
and death of both patients. In relation to recurrence, the relative
risk was analysed according to histological type: OR (MLP): 7.73
(P=0.225) and OR (PLP): 21 (95% CI P=0.07) as well as the type of
surgical resection: OR (R1): 1.8 (95% CI P=0.77) and OR (R2): 69
(95% CI P=0.001).
Discussion
Liposarcoma is the most common mesenchymal
malignancy of soft tissue. They can be located in any part of the
body where there is fatty tissue (17). They all have lipoblasts
(hyperchromatic cells with indented nucleoli and vacuolated
cytoplasm) that can complete adipogenesis like their predecessor
the adipocyte (18).
Genetic and molecular alterations in liposarcomas
have been described. The most frequently described alterations are
amplifications in the 12q 13–15 region that involve the MDM2 and
CDK4 genes and that have implications not only for establishing the
diagnosis of malignancy but also for the prognosis of this
tumours.
Each type of liposarcoma is associated with its own
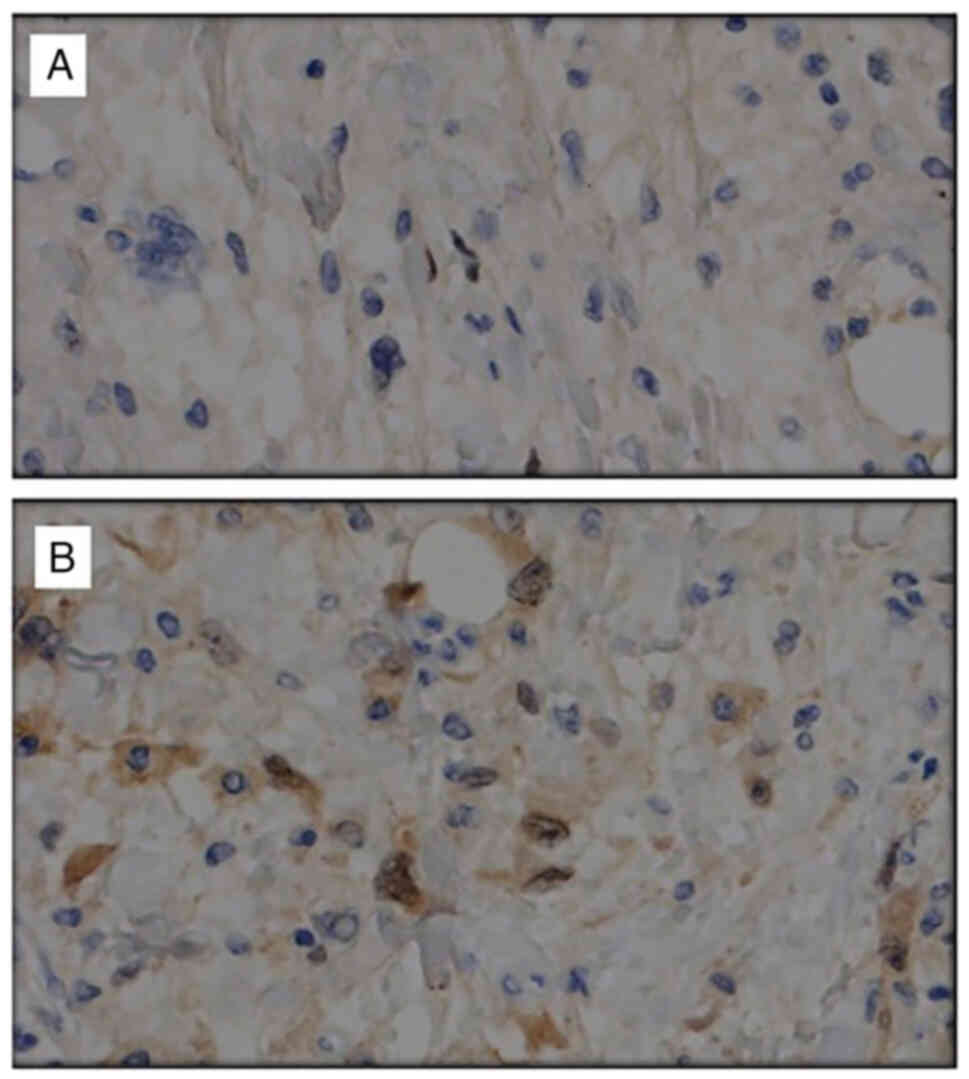
genetic mutation and histopathological findings (19). WDL and DDL are associated with a
high level of 12q.13.15 amplifications as well as MDM2 and CDK4
positivity (3) (Fig. 1). (DDL also has amplifications of
6q23 and 1p32), while the myxoid type lacks these in favour of
expressing FUS/EWSR1-DDIT3) (8). In
our series, the possibility to perform MDM2 and CDK4 determination
became available in 2016, so it was only obtained in 20 patients
(Table III). These
immunohistochemical techniques serve to establish the differential
diagnosis between the different types of LPS. Thus, co-expression
of MDM2 and CDK4 is very common in DDL. In our series, all DDL that
underwent immunohistochemistry against MDM2 and CDK4 were positive.
However, only 38% expressed both proteins in WDL (20,21).
DDL can arise spontaneously or be the result of
malignant transformation of a pre-existing WDL/ALT. It accounts for
18% of all liposarcomas and is up to 5 times more frequent in the
retroperitoneum than in the extremities. In our series, we found a
greater number of cases of DDL in the peritoneum (31%) compared to
the extraperitoneal location (5.5%). This could be explained by the
fact that undifferentiated liposarcoma (DDL) is a subtype of
high-grade liposarcoma, which progresses from a previous
well-differentiated liposarcoma (WDL/ATL) and this presents a
higher frequency of retroperitoneal location. On the other hand, we
have observed in our series a higher number of extraperitoneal
WDL/ATL (61%) compared to retroperitoneal location (37.5%). This
could be explained by the small number of cases in our series or by
the fact that it is the most frequent extraperitoneal
histology.
Unlike WDL/ALT, which has a local recurrence of less
than 50%, no distant metastases and close to 100% survival, DDL has
a higher potential for distant metastases (15–20%) with a
predominance in the lung, recurrence rates of 40–80% and 5-year
survival of 30% (1,18). While DDL and WDL/ALT occur in the
sixth and seventh decade of life, MLP (<20% of all liposarcomas)
is typical of younger patients (fourth and fifth decade of life),
with no sex predominance and extremity location. In contrast to
other liposarcomas, they have a good response to treatment with
chemotherapy and radiotherapy. Finally, PLP (5–15% of all
liposarcomas) occurs in older patients (seventh decade of life),
predominantly in men and mainly located in the extremities
(1,5).
In our series we have observed a similar
distribution with respect to the age of presentation of WDL/ATL and
DDL and location. MLP was found in older patients (sixth and
seventh decade of life) whereas non retroperitoneal PLP were
founded in seventh decade of life with female gender
predisposition.
The form of presentation of these tumours is
directly related to their size and location. They may present as
slowly and progressively growing masses of adipose tissue
(sometimes painful), while in other cases they may be an incidental
finding after an imaging test, as occurs when they are located in
the retroperitoneum. Symptoms such as abdominal pain, early
satiety, neurological or obstructive symptoms due to compression
(14).
The differential diagnosis is made both with other
benign soft tissue tumours (spindle cell lipoma, inflammatory
myofibroblastic tumour or even with lipomas with areas of necrosis
after trauma) and with malignant tumours such as carcinomas of the
gastrointestinal tract, gastrointestinal stromal tumours (GIST) or
even with solitary fibrous tumour (3,14).
For diagnosis, many authors consider
thoraco-abdomino-pelvic CT to be the gold standard, both to
determine the characteristics of the tumour and to determine the
presence of distant metastasis or its relationship with
neighbouring structures. According to Kim and Munk, the degree of
differentiation of the liposarcoma can be estimated after the CT
scan. Low grade liposarcomas present as radiolucent masses while
intermediate grade liposarcomas are associated with the presence of
septa. High-grade liposarcomas present as heterogeneous, dense
masses with contrast uptake (22,23).
MRI is reserved for assessing neurovascular invasion or muscle
involvement in these lesions, presenting as a hypointense signal on
T1 and hyperintense on T2 (1,14). All
retroperitoneal tumours were examined with CT scan in order to
check the relationship with neighbouring organs. A little cases
were studied by MRI. In case non retroperitoneal tumours, CT scan
were not necessary and ultrasound and MRI were preferred (Table II).
In general, there is no lymphatic involvement at the
time of diagnosis. Treatment is mainly surgical. However, there is
no consensus on the most appropriate margin of resection for
WDL/ALT of the trunk and extremities, differentiating between a
marginal excision (excision of the tumour along its pseudocapsule)
and a wide excision (wide excision of tissue that includes a margin
of at least 1 to 2 cm of tissue or tumour-affected tissue)
(13). Although recurrence
described in the literature is higher after marginal excision
(11.9% vs. 3.3%), there are insufficient studies that have
demonstrated an increased mortality associated with recurrence. On
the contrary, other authors have shown that the free margin has an
impact on survival in retroperitoneal liposarcomas (19). Although it seems logical to think
that an R2 resection has a higher recurrence rate than an R0 or R1
resection, authors such as Keung et al describe in their
work that patients with affected margins are not significantly
associated with worse local recurrence, although it was associated
with a higher rate of distant metastases and a lower disease-free
interval (24). In our series, we
did find a significant association between resection margin
involvement and risk of recurrence in both series.
In contrast to patients diagnosed with
non-retroperitoneal WDL/ATL, patients with retroperitoneal WDL/ATL
had a shorter survival (blue line, Fig.
2). We compare both groups (retroperitoneal and
non-retroperitoneal), and patients with non-retroperitoneal
liposarcomas had a better overall survivor and disease free
interval (Fig. 3). Finally, despite
the fact that the survival of patients with DDL, MLP, or PLP
histology is lower than patients with WDL/ATL, we observed that
survival in this group was higher in patients with extraperitoneal
location (Fig. 4).
Other manuscripts describe an overall survival of up
to 70% after R0 or R1 resection compared to those patients
undergoing R2 (16%) (4). In our
study, patients who underwent surgery for liposarcoma in a
non-retroperitoneal location (R1 resection) had 100% survival
compared to those who underwent R2 resection (0% survival). At the
retroperitoneal level, the survival of patients who underwent R0
resection was also 100%, while those who underwent R1 resection had
71.4% survival.
In our study, we decided to divide the patients into
two groups according to tumour location (retroperitoneal and
non-retroperitoneal). Although it is well known that prognosis is
directly related to complete resection with free margins in all
subtypes, location can be a variable to take into account in those
cases in which adjuvant or neoadjuvant treatment is required. For
example, abdominal involvement may be treated by cytoreduction
surgery and HIPEC and include excision of nearby organs depending
on tumour infiltration. On the other hand, liposarcomas located in
the extremities have better delimitation and better response to
radiotherapy, with less morbidity. In addition, in recent years, a
type of treatment consisting of intra-arterial infusion of
chemotherapy has obtained good results with a decrease in the
number of amputations (25).
The role of radiotherapy and chemotherapy (adjuvant
or neoadjuvant) is currently controversial. According to the
European Society for Medical Oncology (ESMO), neoadjuvant
chemotherapy is an alternative for tumours that are initially
unresectable (26).
Anthracycline-based chemotherapy schedules (such as doxorubucin at
doses of 75 mg/m2 have shown better responses without a
significant impact on survival in selected patients. In addition,
MLP has a high sensitivity to chemotherapy along with a high
response rate to these regimens in contrast to WDL/ALT and DDL
which are chemoresistant. Cytoreductive surgery with
intraperitoneal chemotherapy administration (HIPEC) has also been
employed in selected patients, associated with significant toxicity
and limited clinical benefit (27–29).
In our series, one patient underwent cytoreduction surgery and
HIPEC after peritoneal recurrence of WDL/ALT with no recurrence
during follow-up to date.
Finally, preoperative radiotherapy has been shown to
have a relevant role in potentially resectable patients, who do not
require urgent surgery, using a lower dose of radiation with a
consequent lower toxicity than that which would be used after the
operation.
The current WHO classification for bone and soft
tissue tumours has recently been updated in 2020 by introducing two
more histological types with different characteristics. Total 52
cases of malignant adipocytic tumours collected during 2000–2020
were analysed with the new WHO classification updated 2020. The
involvement of the resection margins together with the histological
type myxoid liposarcoma was the main indicator in our series.
Treatment is mainly surgical, and the use of
radiotherapy and chemotherapy is currently controversial. In our
study we have found differences in relation to the survival of each
histological subtype and its location, finding greater survival in
DDL, LPM and LPP located at the extraperitoneal level.
Resectability (R0) was not influenced by liposarcoma location.
In addition, in our study we have observed that the
retroperitoneal location negatively influences the prognosis,
probably in relation to the involvement of the surgical margins and
the need to extend the surgery to neighbouring organs due to local
infiltration.
Acknowledgements
Not applicable.
Funding
This research was coordinated by ProA Capital, Halekulani S.L.,
MJR. co-financed by the European Development Regional Fund ‘A way
to achieve Europe’, as well as P2022/BMD-7321 (Comunidad de
Madrid).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FMM, BMG, AQV, LDG, ABM, CVM, EOM, CDC, MDA, FGMN,
AGC, MAM and MAO contributed to the design of the study. FMM was a
major contributor in the writing of the manuscript. BMG and MDA
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Fundación para la Investigación del Hospital Universitario
Príncipe de Asturias (protocol code: OE 49/2020).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
WHO
|
World Health Organization
|
|
WDL/ATL
|
atypical/well-differentiated
liposarcoma
|
|
DDL
|
dedifferentiated liposarcoma
|
|
MLP
|
myxoid liposarcoma
|
|
PLP
|
pleomorphic liposarcoma
|
|
MRI
|
magnetic resonance imaging
|
|
CT
|
computed tomography
|
|
MDM2
|
murine double minute-2
|
References
|
1
|
Johnson CN, Ha AS, Chen E and Davidson D:
Lipomatous soft-tissue tumors. J Am Acad Orthop Surg. 26:779–788.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Waters R, Horvai A, Greipp P, John I,
Demicco EG, Dickson BC, Tanas MR, Larsen BT, Ud Din N, Creytens DH,
et al: Atypical lipomatous tumour/well-differentiated liposarcoma
and de-differentiated liposarcoma in patients aged ≤40 years: A
study of 116 patients. Histopathology. 75:833–842. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thway K: Well-differentiated liposarcoma
and dedifferentiated liposarcoma: An updated review. Semin Diagn
Pathol. 36:112–121. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu C, Ma Z, Zhang H, Yu J and Chen S:
Giant retroperitoneal liposarcoma with a maximum diameter of 37 cm:
A case report and review of literature. Ann Transl Med. 8:12482020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang L, Luo R, Xiong Z, Xu J and Fang D:
Pleomorphic liposarcoma: An analysis of 6 case reports and
literature review. Medicine (Baltimore). 97:e99862018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Enzinger FM and Winslow DJ: Liposarcoma. A
study of 103 cases. Virchows Arch Pathol Anat Physiol Klin Med.
335:367–388. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mankin HJ, Mankin KP and Harmon DC:
Liposarcoma: A soft tissue tumor with many presentations.
Musculoskelet Surg. 98:171–177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Creytens D: What's new in adipocytic
neoplasia? Virchows Arch. 476:29–39. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mashima E, Sawada Y and Nakamura M: Recent
advancement in atypical lipomatous tumor research. Int J Mol Sci.
22:9942021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Creytens D, Mentzel T, Ferdinande L, van
Gorp J, Van Dorpe J and Flucke U: Atypical mitoses are present in
otherwise classical pleomorphic lipomas-reply. Hum Pathol.
81:300–302. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fletcher CD: The evolving classification
of soft tissue tumours-an update based on the new 2013 WHO
classification. Histopathology. 64:2–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kallen ME and Hornick JL: The 2020 WHO
Classification: What's New in Soft tissue tumor pathology? Am J
Surg Pathol. 45:e1–e23. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi KY, Jost E, Mack L and
Bouchard-Fortier A: Surgical management of truncal and extremities
atypical lipomatous tumors/well-differentiated liposarcoma: A
systematic review of the literature. Am J Surg. 219:823–827. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vijay A and Ram L: Retroperitoneal
liposarcoma: A comprehensive review. Am J Clin Oncol. 38:213–219.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gyllborg D, Langseth CM, Qian X, Choi E,
Salas SM, Hilscher MM, Lein ES and Nilsson M: Hybridization-based
in situ sequencing (HybISS) for spatially resolved transcriptomics
in human and mouse brain tissue. Nucleic Acids Res. 48:e1122020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ortega MA, Saez MA, Fraile-Martínez O,
Asúnsolo Á, Pekarek L, Bravo C, Coca S, Sainz F, Mon MÁ, Buján J
and García-Honduvilla N: Increased angiogenesis and
lymphangiogenesis in the placental villi of women with chronic
venous disease during pregnancy. Int J Mol Sci. 21:24872020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guadagno E, Peltrini R, Stasio L,
Fiorentino F, Bucci L, Terracciano L and Insabato L: A challenging
diagnosis of mesenchymal neoplasm of the colon: Colonic
dedifferentiated liposarcoma with lymph node metastases-a case
report and review of the literature. Int J Colorectal Dis.
34:1809–1814. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bagaria SP, Gabriel E and Mann GN:
Multiply recurrent retroperitoneal liposarcoma. J Surg Oncol.
117:62–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Binh MB, Sastre-Garau X, Guillou L, de
Pinieux G, Terrier P, Lagace R, Aurias A, Hostein I and Coindre JM:
MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing
well-differentiated and dedifferentiated liposarcoma subtypes: A
comparative analysis of 559 soft tissue neoplasms with genetic
data. Am J Surg Pathol. 29:1340–1347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McCormick Y, Cols D, Mentzel T, Beham A
and Fletcher CD: Dedifferentiated liposarcoma. Clinicopathologic
analysis of 32 cases suggesting a better prognostic subgroup among
pleomorphic sarcomas. Am J Surg Pathol. 18:1213–1223. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim T, Murakami T, Oi H, Tsuda K,
Matsushita M, Tomoda K, Fukuda H and Nakamura H: CT and MR imaging
of abdominal liposarcoma. AJR Am J Roentgenol. 166:829–833. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Munk PL, Lee MJ, Janzen DL, Connell DG,
Logan PM, Poon PY and Bainbridge TC: Lipoma and liposarcoma:
Evaluation using CT and MR imaging. AJR Am J Roentgenol.
169:589–594. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Keung EZ, Hornick JL, Bertagnolli MM,
Baldini EH and Raut CP: Predictors of outcomes in patients with
primary retroperitoneal dedifferentiated liposarcoma undergoing
surgery. J Am Coll Surg. 218:206–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jakob J, Tunn PU, Hayes AJ, Pilz LR, Nowak
K and Hohenberger P: Oncological outcome of primary non-metastatic
soft tissue sarcoma treated by neoadjuvant isolated limb perfusion
and tumor resection. J Surg Oncol. 109:786–790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Artigas Raventós V: Mesenchymal
tumors-sarcoma: A new AEC work group. Cir Esp (Engl Ed).
96:527–528. 2018.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baratti D, Pennacchioli E, Kusamura S,
Fiore M, Balestra MR, Colombo C, Mingrone E, Gronchi A and Deraco
M: Peritoneal sarcomatosis: Is there a subset of patients who may
benefit from cytoreductive surgery and hyperthermic intraperitoneal
chemotherapy? Ann Surg Oncol. 17:3220–3228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baumgartner JM, Ahrendt SA, Pingpank JF,
Holtzman MP, Ramalingam L, Jones HL, Zureikat AH, Zeh HJ III,
Bartlett DL and Choudry HA: Aggressive locoregional management of
recurrent peritoneal sarcomatosis. J Surg Oncol. 107:329–334. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bonvalot S, Cavalcanti A, Le Pechoux C,
Terrier P, Vanel D, Blay JY, Le Cesne A and Elias D: Randomized
trial of cytoreduction followed by intraperitoneal chemotherapy
versus cytoreduction alone in patients with peritoneal
sarcomatosis. Eur J Surg Oncol. 31:917–923. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lim SJ, Cormier JN, Feig BW, Mansfield PF,
Benjamin RS, Griffin JR, Chase JL, Pisters PW, Pollock RE and Hunt
KK: Toxicity and outcomes associated with surgical cytoreduction
and hyperthermic intraperitoneal chemotherapy (HIPEC) for patients
with sarcomatosis. Ann Surg Oncol. 14:2309–2318. 2007. View Article : Google Scholar : PubMed/NCBI
|