Introduction
As the most common primary retroperitoneal
malignancy, primary retroperitoneal liposarcoma (PRPLS) originates
from the adipose tissue in the retroperitoneal space. Although
PRPLS accounts for <0.1% of all malignant tumors, it has hidden
clinical symptoms and rapid progress (1–3).
Therefore, most PRPLS tumors are huge and have a complex
relationship with adjacent organs, making the operation difficult
and frequently requiring combined organ resection. The features of
multicentric origin lead to a high local recurrence rate of PRPLS
and most patients with PRPLS have a history of repeated surgery
during the disease course. Although the tumor resection rate is
gradually increased due to the continuous improvement of surgical
technology and methods, the 5-year local recurrence rate of PRPLS
is still up to 20–75% and this is also the main cause of death for
PRPLS cases (3–5). Identifying the risk factors for
neoplasm recurrence and carrying out targeted prevention and
treatment are the focus and difficulties of current clinical
research.
To date, the mechanisms of PRPLS recurrence have
remained largely elusive and the following factors are considered
to have a role (6–8). First, the huge tumor volume and dense
adhesion lead to the disappearance of the anatomical space between
the surrounding structures with the tumor capsule and this change
may increase the operative difficulty and result in an increased
probability of residual tumor tissue or capsule. Furthermore, the
tumor's invasion of internal organs, blood vessels or nerves may
make the complete resection of PRPLS difficult. In addition, PRPLS
is similar to normal adipose tissue and lobulated retroperitoneal
liposarcoma (RPLS) is easy to be considered as multiple tumors and
resected in pieces, which may lead to residual tumor tissue.
Since PRPLS is rare in the clinic, related clinical
studies are lacking (9–12). Wu et al (9) revealed that pathological subtype and
histological grade were associated with local recurrence, and
histological grade could be used as an independent marker. In the
study by Yan et al (10),
increased intraoperative bleeding and poor tumor classification
were proved to be associated with a poor prognosis of PRPLS.
Furthermore, Sun et al (11)
found that age, recurrence, tumor site and tumor necrosis were
useful markers of RPLS prognosis. However, published studies were
designed for both primary and local recurrent RPLS, and the short-
and long-term recurrence of tumors were not carefully distinguished
(12,13). Prognostic factor analysis of
short-term (≤1 year) recurrence (STR) and nomogram construction for
PRPLS were both lacking. Therefore, the present study was performed
to explore the predictors of STR and construct a novel nomogram of
local recurrence-free survival (LRFS) for surgically resected
PRPLS.
Materials and methods
Study participants
Patients with PRPLS who underwent radical operation
at the First Medical Center of Chinese People's Liberation Army
(PLA) General Hospital (Beijing, China) were included in this
retrospective observational study. Relevant clinical data were
collected using an electronic medical record (EMR) system. The
inclusion criteria were as follows: i) Primary tumor with radical
surgery (R0 resection) at our unit; ii) tumor originated from the
retroperitoneal soft tissue and postoperative pathology confirmed
liposarcoma; and iii) hospitalization period from January 2008 to
December 2021. The exclusion criteria were as follows: i) Recurrent
cases; ii) patients who did not undergo surgery, underwent
palliative surgery (R2 resection) or with positive postoperative
margin (R1 resection); iii) patients who died from surgical
complications or other causes; and iv) cases lost to follow-up or
refused to participate. This study was approved by the Medical
Ethics Committee of the First Medical Center of the Chinese PLA
General Hospital.
Data collection and outcome
evaluation
The following case data were collected from the EMR
system: Sex, age, body mass index (BMI), preoperative
neutrophil/lymphocyte ratio (NLR), abdominal operation history,
clinical symptoms, tumor resection method, combined organ excision,
operative time, intraoperative bleeding, application of
intraperitoneal chemotherapy drug, transfer to intensive care unit
(ICU), tumor diameter, multiple primary tumors, tumor shape, tumor
capsule, histological subtype and tumor necrosis. The case data
were acquired in three categories: Demographic characteristics,
surgical characteristics and pathological characteristics. The
preoperative clinical symptoms observed in the present study
included abdominal pain and distension, gastrointestinal
obstruction, back pain and lower limb paresthesia, which were
caused by tumor compression or invasion. The sampling time to
determine the preoperative NLR was 2–3 days prior to the surgery.
Combined organ resection was selected if the tumor had invaded
surrounding organs and piecemeal resection was considered only when
complete resection was not feasible. A negative resection margin
was defined as R0 resection and procedures with a positive
postoperative margin were considered an R1 resection. R2 resection
(palliative) was considered if there was any residual tumor
observed during an operation. The intraperitoneal chemotherapy drug
used in this study was mainly implantable sustained-release
fluorouracil, which was placed in the abdominal cavity prior to
closure. The tumor diameter was expressed as the largest tumor
diameter after the postoperative assessment.
Postoperative follow-up
In the present study, patients had a follow-up every
3–4 months in the first 2 years after the surgery and every 6
months thereafter. During the follow-up period, routine physical
examination and abdominopelvic magnetic resonance imaging or
computerized tomography were performed to evaluate the recurrence
of RPLS. Based on the interval from operation to neoplasm
recurrence, the included PRPLS cases were divided into an STR (≤1
year) group and non-STR (>1 year) group. LRFS was defined as the
period from radical operation to local recurrence.
Statistical analysis
SPSS software (version 26.0; IBM Corporation) and R
software (version 4.2.2) were used for the statistical analyses.
Categorical data were expressed as n (%) and compared using the
two-sided χ2 test. The median (interquartile range, IQR)
was used to illustrate continuous variables and comparison among
groups was performed using the Mann-Whitney U-test. In addition,
receiver operating characteristic (ROC) curves of continuous
outcomes were drawn and dichotomous outcomes were obtained based on
cut-off values. Subsequently, binary logistic regression analysis
and Cox proportional hazard regression analysis were conducted to
determine the predictors of STR and LRFS, respectively. Variables
with P<0.15 in the univariate analysis were included in the
multivariate analysis and variables with P<0.05 in the
multivariate analysis were considered independent predictors. LRFS
rates were estimated based on the Kaplan-Meier method and were
compared between groups by the log-rank test. A nomogram was
constructed using the independent predictors, aiming to predict 1-,
3- and 5-year LRFS of surgically resected PRPLS.
Results
Patient selection
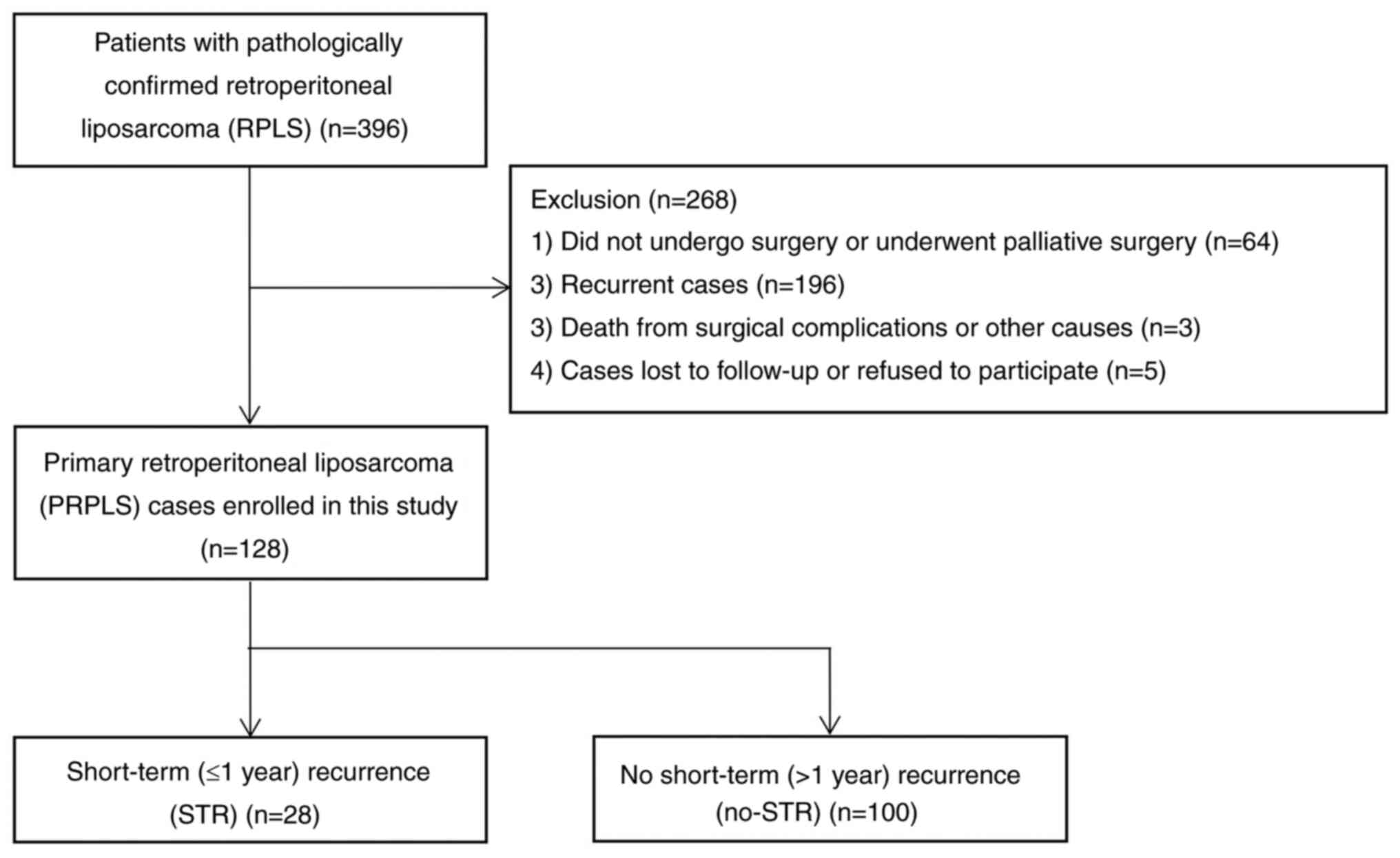
Initially, 396 patients with pathologically
confirmed RPLS were retrieved using the EMR system. Of these, 64
did not undergo radical surgery, 196 were recurrent cases, three
died from surgical complications or other causes and five were lost
to follow-up. After excluding these patients, the data from the
remaining 128 patients were finally included in the present
analysis. At a median follow-up time of 30.0 (IQR, 14.3-67.5)
months, 94 patients (73.4%) had tumor recurrence and 28 (21.9%)
experienced STR (Fig. 1). The 1-,
3- and 5-year LRFS rates were 78.1, 47.3 and 35.5%,
respectively.
Logistic regression analysis for
STR
According to the interval from surgery to neoplasm
recurrence, the 128 PRPLS cases were divided into the STR (n=28)
and non-STR (n=100) group. The demographic, surgical and
pathological characteristics of the two groups were compared and
statistically significant differences were found in preoperative
NLR (P=0.040), clinical symptoms (P=0.012), resection method
(P=0.034), operative time (P=0.015), intraoperative blood loss
(P=0.002), transfer to ICU (P=0.003), tumor capsule (P=0.001),
histological subtype (P=0.006) and tumor necrosis (P<0.001)
(Table I). In addition, ROC curves
of continuous outcomes were drawn and dichotomous outcomes were
obtained based on cut-off values, including age (≥55 or <55
years), BMI (≥23 or <23 kg/m2), preoperative NLR
(≥2.38, or <2.38), operative time (≥260 or <260 min),
intraoperative blood loss (≥1,200 or <1,200 ml) and tumor
diameter (≥20 or <20 cm) (Fig.
S1). Of these converted variables, age (≥55 vs. <55 years;
P=0.015), preoperative NLR (≥2.38 vs. <2.38; P=0.005), operative
time (≥260 vs. <260 min; P<0.001) and intraoperative blood
loss (≥1,200 vs. <1,200 ml; P=0.001) were associated with STR.
Subsequently, the variables of age (≥55 vs. <55 years),
preoperative NLR (≥2.38 vs. <2.38), clinical symptoms, resection
method, operative time (≥260 vs. <260 min), intraoperative blood
loss (≥1,200 vs. <1,200 ml), transfer to ICU, intact tumor
capsule, histological subtype and tumor necrosis were further
included in a multivariate logistic regression analysis. The
multivariate analysis revealed that age ≥55 years [odds ratio
(OR)=5.607, P=0.010], operative time ≥260 min (OR=9.716, P=0.005)
and tumor necrosis (OR=3.781, P=0.037) were independent risk
factors of STR (Table II). In
addition, the above three variables were included in a multivariate
logistic regression model for further analysis and the re-analysis
also indicated that age ≥55 years (OR=5.421, P=0.003), operative
time ≥260 min (OR=10.524, P<0.001) and tumor necrosis (OR=7.231,
P<0.001) were independent risk factors (Table III).
 | Table I.Characteristics of included primary
retroperitoneal liposarcoma cases in STR group and non-STR
group. |
Table I.
Characteristics of included primary
retroperitoneal liposarcoma cases in STR group and non-STR
group.
| Variable | Total (n=128) | STR group (n=28) | Non-STR group
(n=100) | P-value |
|---|
| Sex |
|
|
| 0.840 |
| Male | 71 (55.5) | 16 (57.1) | 55 (55.0) |
|
|
Female | 57 (44.5) | 12 (42.9) | 45 (45.0) |
|
| Age, years | 54 (48, 64) | 59 (51, 65) | 53 (47, 62) | 0.076 |
| BMI,
kg/m2 | 23.55 (21.49,
25.24) | 23.55 (21.08,
24.66) | 23.55 (21.57,
25.44) | 0.614 |
| Preoperative NLR | 2.99 (1.95,
3.58) | 3.57 (2.56,
3.74) | 2.72 (1.74,
3.58) | 0.040 |
| Previous abdominal
surgery |
|
|
| 0.697 |
| Yes | 31 (24.2) | 6 (21.4) | 25 (25.0) |
|
| No | 97 (75.8) | 22 (78.6) | 75 (75.0) |
|
| Clinical
symptoms |
|
|
| 0.012 |
| Yes | 74 (57.8) | 22 (78.6) | 52 (52.0) |
|
| No | 54 (42.2) | 6 (21.4) | 48 (48.0) |
|
| Resection method |
|
|
| 0.034 |
|
Piecemeal | 9 (7.0) | 5 (17.9) | 4 (4.0) |
|
|
Complete | 119 (93.0) | 23 (82.1) | 96 (96.0) |
|
| Combined organ
excision |
|
|
| 0.082 |
|
Yes | 73 (57.0) | 20 (71.4) | 53 (53.0) |
|
| No | 55 (43.0) | 8 (28.6) | 47 (47.0) |
|
| Operative time,
min | 184 (140, 240) | 209 (163, 280) | 178 (136, 235) | 0.015 |
| Intraoperative
blood loss, ml | 475 (200,
1000) | 900 (313,
1800) | 400 (200, 875) | 0.002 |
| Intraperitoneal
chemotherapy drug application |
|
|
| 0.810 |
|
Yes | 62 (48.4) | 13 (46.4) | 49 (49.0) |
|
| No | 66 (51.6) | 15 (53.6) | 51 (51.0) |
|
| Transfer to
ICU |
|
|
| 0.003 |
|
Yes | 25 (19.5) | 11 (39.3) | 14 (14.0) |
|
| No | 103 (80.5) | 17 (60.7) | 86 (86.0) |
|
| Pathological
characteristics |
|
|
|
|
| Tumor diameter,
cm | 25.0 (18.6,
32.0) | 25.8 (19.0,
32.8) | 25 (18.6, 32) | 0.723 |
| Multiple primary
tumors |
|
|
| 0.338 |
|
Yes | 24 (18.8) | 7 (25.0) | 17 (17.0) |
|
| No | 104 (81.2) | 21 (75.0) | 83 (83.0) |
|
| Tumor shape |
|
|
| 0.908 |
|
Irregular | 40 (31.3) | 9 (32.1) | 31 (31.0) |
|
|
Regular | 88 (68.7) | 19 (67.9) | 69 (69.0) |
|
| Tumor capsule |
|
|
| 0.001 |
|
Intact | 98 (76.6) | 15 (53.6) | 83 (83.0) |
|
|
Broken | 30 (23.4) | 13 (46.4) | 17 (17.0) |
|
| Histological
subtype |
|
|
| 0.006 |
|
Well-differentiated | 45 (35.2) | 5 (17.9) | 40 (40.0) |
|
|
De-differentiated | 18 (14.1) | 9 (32.1) | 9 (9.0) |
|
| Other
subtypes | 65 (50.7) | 14 (50.0) | 51 (51.0) |
|
| Tumor necrosis |
|
|
| <0.001 |
|
Yes | 36 (28.1) | 16 (57.1) | 20 (20.0) |
|
| No | 92 (71.9) | 12 (42.9) | 80 (80.0) |
|
 | Table II.Multivariate analysis of predictors
for short-term (≤1 year) recurrence. |
Table II.
Multivariate analysis of predictors
for short-term (≤1 year) recurrence.
| Variable | β coefficient | OR (95% CI) | P-value |
|---|
| Age ≥55 years | 1.724 | 5.607
(1.517-20.726) | 0.010 |
| Preoperative NLR
≥2.38 | 1.416 | 4.121
(0.889-19.096) | 0.070 |
| Clinical
symptoms | 1.000 | 2.717
(0.760-9.714) | 0.124 |
| Complete
resection | −0.812 | 0.444
(0.066-3.007) | 0.406 |
| Combined organ
excision | −0.308 | 0.735
(0.214-2.528) | 0.625 |
| Operative time ≥260
min | 2.274 | 9.716
(1.975-47.791) | 0.005 |
| Intraoperative
blood loss ≥1,200 ml | −0.284 | 0.753
(0.162-3.492) | 0.717 |
| Transfer to
ICU | 0.577 | 1.781
(0.349-9.095) | 0.488 |
| Intact tumor
capsule | −0.761 | 0467
(0.124-1.764) | 0.262 |
| Histological
subtype |
|
| 0.088 |
|
De-differentiated vs.
well-differentiated | 1.706 | 5.506
(0.833-36.376) | 0.077 |
| Other
subtypes vs. well-differentiated | 0.054 | 1.055
(0.229-4.871) | 0.945 |
| Tumor necrosis | 1.330 | 3.781
(1.087-13.156) | 0.037 |
 | Table III.Further multivariate analysis for
short-term (≤1 year) recurrence. |
Table III.
Further multivariate analysis for
short-term (≤1 year) recurrence.
| Variable | β coefficient | OR (95% CI) | P-value |
|---|
| Age ≥55 years | 1.690 | 5.421
(1.768-16.616) | 0.003 |
| Operative time ≥260
min | 2.354 | 10.524
(3.131-35.374) | <0.001 |
| Tumor necrosis | 1.978 | 7.231
(2.526-20.696) | <0.001 |
Cox regression analysis and nomogram
construction for LRFS
Univariate and multivariate Cox proportional hazard
regression models were constructed to identify the predictors of
LRFS. Univariate analysis indicated that clinical symptoms [hazard
ratio (HR)=1.947, P=0.002], complete resection (HR=0.239,
P<0.001), operative time (HR=1.006, P<0.001), intraoperative
blood loss (HR=1.001, P<0.001), transfer to ICU (HR=1.947,
P=0.009), tumor capsule (HR=0.594, P=0.029),
histological subtype (P=0.003) and tumor necrosis (HR=1.647,
P=0.028) were associated with LRFS. Variables with P<0.15 in the
univariate analysis were included in the multivariate analysis.
Multivariate Cox regression analysis revealed that clinical
symptoms (HR=1.746, P=0.017), complete resection (HR=0.370,
P=0.021) and de-differentiated vs. well-differentiated
histological subtype (HR=1.975, P=0.048) were independent
predictors of LRFS (Table IV). In
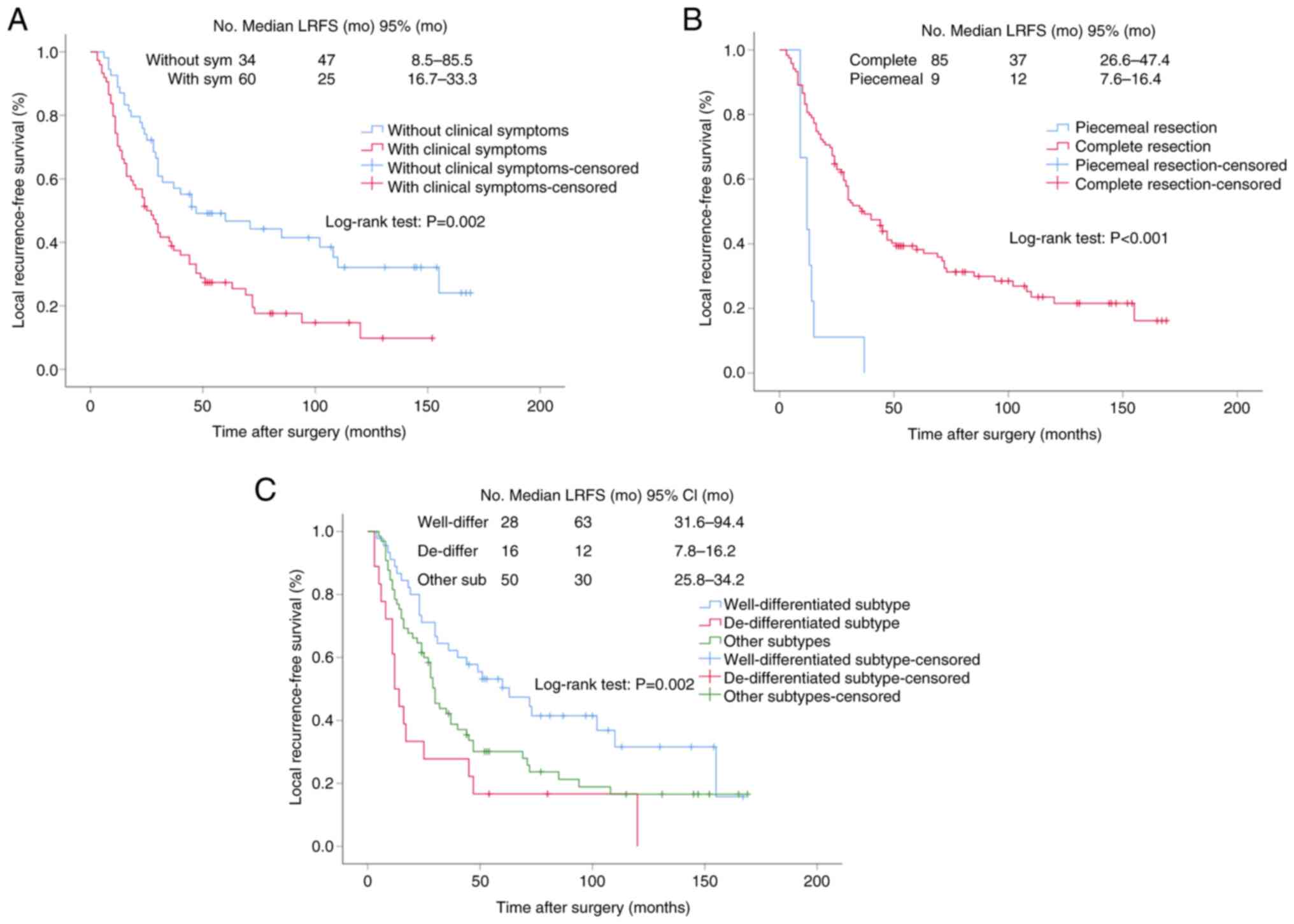
addition, Kaplan-Meier curves of LRFS for clinical symptoms,
resection method and histological subtype were drawn, and the
curves also showed that clinical symptoms (P=0.002),
resection method (P<0.001) and histological subtype
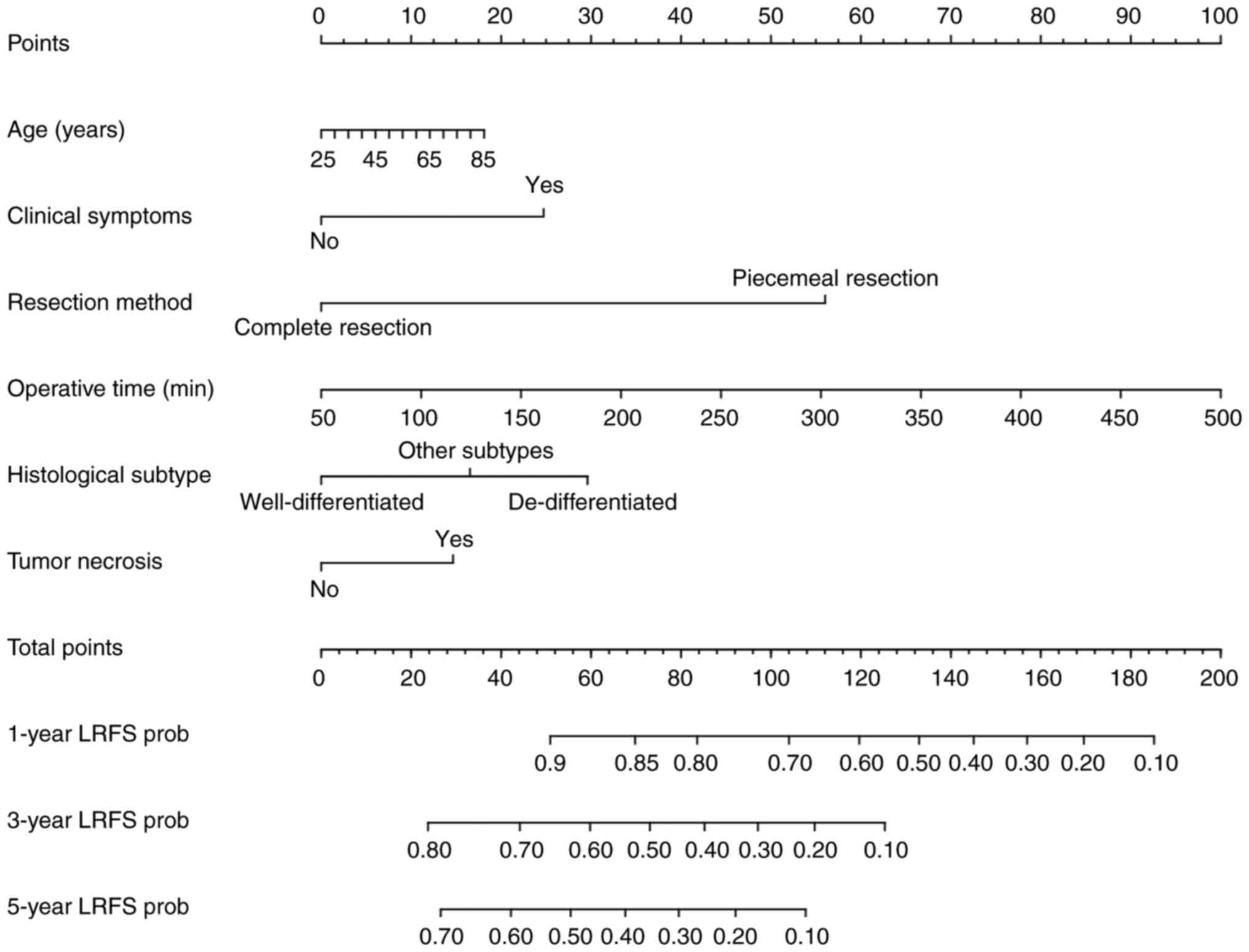
(P=0.002) were important factors affecting LRFS (Fig. 2). Subsequently, a nomogram was
constructed using age, clinical symptoms, resection method,
operative time, histological subtype and tumor necrosis to predict
the 1-, 3- and 5-year LRFS of surgical resected PRPLS (Fig. 3). The prediction model's concordance
index (C-index) was 0.701, suggesting a good discriminative
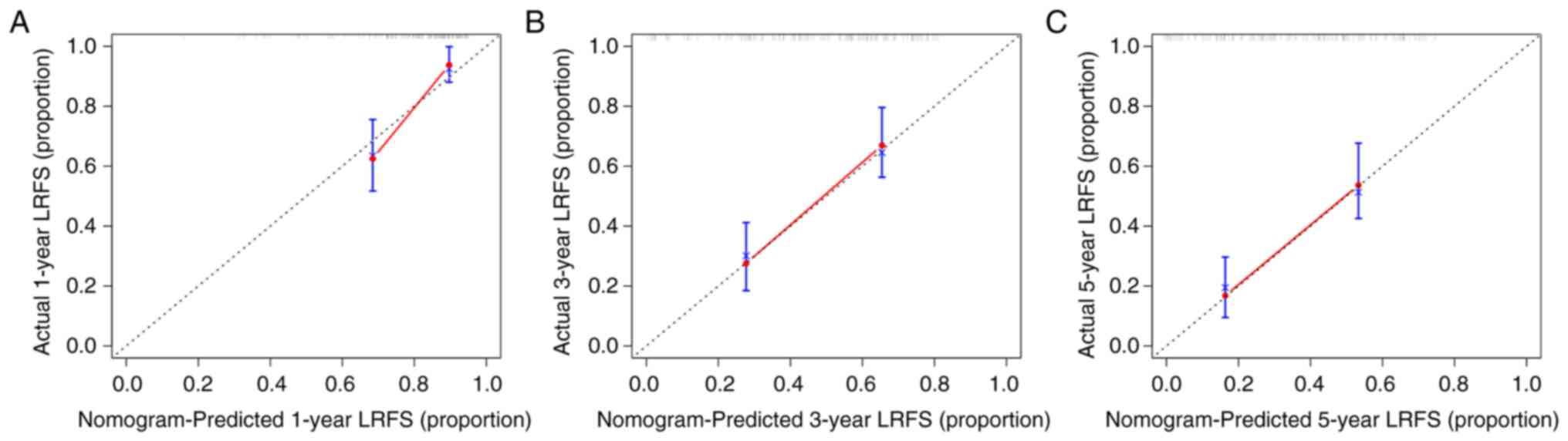
capability of the nomogram. The calibration plots for the LRFS
probability at 1, 3 and 5 years also indicated that the nomogram
had a good calibration (Fig.
4).
 | Table IV.Univariate and multivariate analyses
of predictors for local recurrence-free survival. |
Table IV.
Univariate and multivariate analyses
of predictors for local recurrence-free survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex (male) | 1.138 | 0.754-1.719 | 0.538 |
|
|
|
| Age | 1.009 | 0.990-1.028 | 0.356 |
|
|
|
| Preoperative
NLR | 1.030 | 0.986-1.076 | 0.184 |
|
|
|
| BMI
(kg/m2) | 0.954 | 0.896-1.017 | 0.147 | 0.955 | 0.891-1.024 | 0.196 |
| Previous abdominal
surgery | 1.007 | 0.628-1.614 | 0.979 |
|
|
|
| Clinical
symptoms | 1.947 | 1.267-2.994 | 0.002 | 1.746 | 1.105-2.760 | 0.017 |
| Complete
resection | 0.239 | 0.116-0.492 | <0.001 | 0.370 | 0.159-0.861 | 0.021 |
| Combined organ
excision | 1.402 | 0.924-2.127 | 0.112 | 0.703 | 0.431-1.148 | 0.159 |
| Operative time | 1.006 | 1.003-1.009 | <0.001 | 1.004 | 1.000-1.007 | 0.059 |
| Intraoperative
blood loss | 1.001 | 1.000-1.001 | <0.001 | 1.000 | 1.000-1.001 | 0.095 |
| Intraperitoneal
chemotherapy drug application | 0.936 | 0.624-1.405 | 0.749 |
|
|
|
| Transfer to
ICU | 1.947 | 1.178-3.220 | 0.009 | 1.026 | 0.567-1.856 | 0.933 |
| Tumor diameter | 1.013 | 0.994-1.033 | 0.171 |
|
|
|
| Multiple primary
tumors | 1.589 | 0.966-2.614 | 0.068 | 1.379 | 0.796-2.390 | 0.252 |
| Tumor shape | 1.458 | 0.949-2.240 | 0.085 | 0.998 | 0.594-1.677 | 0.993 |
| Tumor capsule | 0.594 | 0.372-0.949 | 0.029 | 0.865 | 0.476-1.572 | 0.635 |
| Histological
subtype |
|
| 0.003 |
|
| 0.126 |
| De-differentiated
vs. well-differentiated | 2.888 | 1.555-5.363 | 0.001 | 1.975 | 1.006-3.875 | 0.048 |
| Other subtypes vs.
well-differentiated | 1.638 | 1.029-2.608 | 0.037 | 1.423 | 0.875-2.314 | 0.155 |
| Tumor necrosis | 1.647 | 1.055-2.573 | 0.028 | 1.253 | 0.761-2.064 | 0.375 |
Discussion
As a rare soft tissue sarcoma, PRPLS has a poor
prognosis and poses a serious threat to human health. Owing to the
unclear effects of radiotherapy and chemotherapy on RPLS, the
application of adjuvant therapy is still controversial. Therefore,
none of the cases included in the present study received
radiotherapy or chemotherapy prior to or after surgery. The
therapeutic effect and application time of radiotherapy and
chemotherapy on RPLS still require to be further explored. At
present, surgical resection remains the method of choice for PRPLS
cases with indications to obtain potential cure opportunities
(5–7,14,15).
In the present study, the tumor was completely resected under the
condition of conforming to the standard of safe resection margin
(8). However, the 1-, 3- and 5-year
LRFS rates of PRPLS cases were still as low as 78.1, 47.3 and
35.5%, respectively. For PRPLS cases who experienced recurrence
after surgery and had surgical indications, a second operation
should be conducted (7). Owing to
the previous lack of risk factor analysis and a nomogram for PRPLS
recurrence, the present study was performed to identify the
predictors and construct the nomogram to facilitate targeted
prevention of recurrence. After excluding the interaction between
variables, multivariate analyses indicated that operative time was
an important predictor for both STR and LRFS. In the present study,
therefore, operative time was used to construct the nomogram. As
retroperitoneal malignancies are clinically rare, a limited number
of PRPLS cases were included in the present study. Dividing the
included cases into modeling and validation sets may have reduced
the accuracy of the predictive model. Thus, the nomogram was not
validated by an external patient series, limiting its value. The
prediction model's C-index and calibration plots indicated that the
nomogram established in the present study had a good
calibration.
Previous research has found a correlation between
age and survival time for patients with PRPLS who underwent radical
surgery, but a correlation between age and postoperative recurrence
has not been reported (2,11,16).
In this analysis, age ≥55 years was proved to be an independent
risk factor for STR. Decreased immune function, aging organs and
disordered anatomy accompanied by increased age may contribute to
this phenomenon. Tumor necrosis may be caused by the rapid growth
and chronic ischemic injury of solid tumors, which may reflect the
degree of tumor malignancy and hypoxia in the tumor. Therefore,
tumor necrosis is significantly correlated with the prognosis for
numerous common tumor types. In general, a large extent of tumor
necrosis and a low degree of differentiation indicate a high degree
of malignancy, which may lead to a higher recurrence rate and
unfavorable prognosis (10,11,17).
Prolonged surgical duration was another important
predictor for 1-year recurrence. Huge tumor volume, dense adhesion
and tumor invasion of surrounding tissues and organs bring great
difficulties to the radical operation, thus further prolonging the
operation time, increasing the chance of residual tumor and tumor
cells disseminating and spreading (18,19).
In addition, the huge tumor may compress and invade the internal
organs, resulting in non-specific clinical symptoms, such as
abdominal pain and distension, gastrointestinal obstruction, back
pain and lower limb paresthesia (17). Piecemeal resection was considered
only when complete resection was impossible to complete and
piecemeal resection also increased the risk of intraoperative
bleeding and tumor cell dissemination (12,20).
Furthermore, prolonged operative time increases the exposure time,
possibility of injury and degree of edema in tissues, leading to an
increased risk of intraoperative bleeding and transfer to ICU. As a
consequence, the clinical symptoms, resection method,
intraoperative blood loss and transfer to the ICU were related to
the operative duration and may affect PRPLS recurrence.
Tumor-related inflammation may induce the tumor itself or
surrounding cells to express various molecules, thus forming a
micro-environment that may promote tumor progression (21,22).
As a common marker of the serologic inflammatory response, elevated
preoperative NLR was also found to be associated with STR in this
study.
Histological subtypes, including
well-differentiated, de-differentiated, mixed, mucinous and
pleomorphic subtype, was also an important predictor for PRPLS
recurrence (10,23). According to previous literature
evidence, the prognosis of different histological subtypes
exhibited marked variation and well-differentiated PRPLS had a
lower local recurrence rate and a significantly prolonged the
recurrence interval as compared to other subtypes (8,12). The
present study also indicated that the incidence of tumor recurrence
was significantly lower in the well-differentiated group and the
de-differentiated histological subtype was able to be used as an
independent risk factor of LRFS for PRPLS cases. De-differentiated
PRPLS frequently has an incomplete tumor capsule and irregular
tumor shape, which makes the boundary between the tumor and normal
tissue difficult to identify, thus prolonging the operation time,
increasing intraoperative bleeding and hampering the completion of
radical resection (10,19,23,24).
Therefore, ensuring the integrity of the tumor
resected by the first operation was particularly important and the
tumor with its surrounding tissue should be excised as whole as
possible to ensure a negative margin (25). Furthermore, intraoperative
pathological examination is recommended to confirm the histological
subtypes and the condition of tumor necrosis. For de-differentiated
PRPLS cases with tumor necrosis, careful operation and examination,
and appropriate expansion of tumor resection are requisite to avoid
residual tumor tissue. Furthermore, a shortened review interval and
increased review number after the operation are also required, so
as to detect the STR of tumors.
Although the present study was the first to explore
prognostic factors of STR and construct a novel nomogram of LRFS
for surgically resected PRPLS, it had certain limitations. First,
the analysis was performed utilizing a retrospective database from
a single center, affecting the quality of evidence. Furthermore,
case data with a large time span may have been one of the sources
of information bias. In addition, the small sample size caused by
the low incidence also affected the reliability of the analysis
results to a certain extent. In the future, multicenter prospective
studies with large samples and long-term follow-up are required to
further validate and complement the results of the present
analysis.
In conclusion, age ≥55 years, operative time ≥260
min and tumor necrosis were identified as independent risk factors
of STR for surgically resected PRPLS. Clinical symptoms, piecemeal
resection and de-differentiated histological subtype may be used as
independent predictors of LRFS. Based on the above variables, a
nomogram with good calibration was constructed to predict the 1-,
3- and 5-year LRFS for surgically resected PRPLS.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZY and PL conceived and designed the study and
drafted the manuscript. ZY, XZ and SZ participated in writing the
manuscript, as well as analyzing and interpreting the data. JG and
NL collected and analyzed the data and produced the tables and
figures. ZY and PL confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the Chinese PLA General Hospital (Beijing, China).
This study was undertaken according to the provisions of the
Declaration of Helsinki. The requirement for written informed
consent was waived due to the retrospective nature of the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bagaria SP, Gabriel E and Mann GN:
Multiply recurrent retroperitoneal liposarcoma. J Surg Oncol.
117:62–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Y, Wu G, Zhang Y, Yang W, Wang X, Duan
L, Niu L, Chen J, Zhou W, Liu J, et al: Development and validation
of a prognostic model to predict the prognosis of patients with
retroperitoneal liposarcoma: A large international population-based
cohort study. Front Oncol. 12:8578272022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muratori F, Frenos F, Bettini L, Matera D,
Mondanelli N, Scorianz M, Cuomo P, Scoccianti G, Beltrami G, Greto
D, et al: Liposarcoma: Clinico-pathological analysis, prognostic
factors and survival in a series of 307 patients treated at a
single institution. J Orthop Sci. 23:1038–1044. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salerno KE and Baldini EH: Role of
radiation therapy in retroperitoneal sarcoma. J Natl Compr Canc
Netw. 20:845–849. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Molina G, Hull MA, Chen YL, DeLaney TF, De
Amorim Bernstein K, Choy E, Cote G, Harmon DC, Mullen JT and Haynes
AB: Preoperative radiation therapy combined with radical surgical
resection is associated with a lower rate of local recurrence when
treating unifocal, primary retroperitoneal liposarcoma. J Surg
Oncol. 114:814–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lewis JJ, Leung D, Woodruff JM and Brennan
MF: Retroperitoneal soft-tissue sarcoma: Analysis of 500 patients
treated and followed at a single institution. Ann Surg.
228:355–365. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neuhaus SJ, Barry P, Clark MA, Hayes AJ,
Fisher C and Thomas JM: Surgical management of primary and
recurrent retroperitoneal liposarcoma. Br J Surg. 92:246–252. 2015.
View Article : Google Scholar
|
|
8
|
Singer S, Antonescu CR, Riedel E and
Brennan MF: Histologic subtype and margin of resection predict
pattern of recurrence and survival for retroperitoneal liposarcoma.
Ann Surg. 238:358–371. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu YX, Liu JY, Liu JJ, Yan P, Tang B, Cui
YH, Zhao YL, Shi Y, Hao YX, Yu PW and Qian F: A retrospective,
single-center cohort study on 65 patients with primary
retroperitoneal liposarcoma. Oncol Lett. 15:1799–1810.
2018.PubMed/NCBI
|
|
10
|
Yan Y, Xia S, Teng D, Hu S, Li S, Wang Y,
Du X and Li R: Resection outcomes for primary and local recurrent
retroperitoneal liposarcoma patients. Ann Transl Med. 8:14502020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun P, Ma R, Liu G, Wang L, Chang H and Li
Y: Pathological prognostic factors of retroperitoneal liposarcoma:
Comprehensive clinicopathological analysis of 124 cases. Ann Transl
Med. 9:5742021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xue G, Wang Z, Li C, Lv A, Tian X, Wu J,
Qiu H and Hao C: A novel nomogram for predicting local
recurrence-free survival after surgical resection for
retroperitoneal liposarcoma from a Chinese tertiary cancer center.
Int J Clin Oncol. 26:145–153. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sánchez-Hidalgo JM, Rufián-Peña S,
Durán-Martínez M, Arjona-Sánchez Á, Salcedo-Leal I, Lopez-Cillero P
and Briceño-Delgado J: Risk factors of early recurrence in
retroperitoneal liposarcoma. Cir Esp (Engl Ed). 96:568–576. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gronchi A, Miah AB, Dei Tos AP, Abecassis
N, Bajpai J, Bauer S, Biagini R, Bielack S, Blay JY, Bolle S, et
al: Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS
clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 32:1348–1365. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nassif EF, Cope B, Traweek R, Witt RG,
Erstad DJ, Scally CP, Thirasastr P, Zarzour MA, Ludwig J, Benjamin
R, et al: Real-world use of palbociclib monotherapy in
retroperitoneal liposarcomas at a large volume sarcoma center. Int
J Cancer. 150:2012–2024. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhuang A, Wu Q, Tong H, Zhang Y and Lu W:
Development and validation of a nomogram for predicting
recurrence-free survival of surgical resected retroperitoneal
liposarcoma. Cancer Manag Res. 13:6633–6639. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao J, Liu J, Chen M, Liu W and He X:
Diagnosis and prognosis of retroperitoneal liposarcoma: A single
Asian center cohort of 57 cases. J Oncol. 2021:75940272021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spolverato G, Chiminazzo V, Lorenzoni G,
Fiore M, Radaelli S, Sanfilippo R, Sangalli C, Barisella M,
Callegaro D and Gronchi A: Oncological outcomes after major
vascular resections for primary retroperitoneal liposarcoma. Eur J
Surg Oncol. 47:3004–3010. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Littau MJ, Kulshrestha S, Bunn C, Agnew S,
Sweigert P, Luchette FA and Baker MS: The importance of the margin
of resection and radiotherapy in retroperitoneal liposarcoma. Am J
Surg. 221:554–560. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miao CL, Zhang LL, Tseng WW, Qiu FB, Lu
WQ, Dai YG, Rao XS, Li WJ, Zhang GK, Chen J, et al: A better
overall survival (OS) for total (ipsilateral) retroperitoneal
lipectomy than standard complete resection in patients with
retroperitoneal liposarcoma: A comparative multi-institutional
study. Ann Transl Med. 10:7852022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Naszai M, Kurjan A and Maughan TS: The
prognostic utility of pre-treatment neutrophil-to-lymphocyte-ratio
(NLR) in colorectal cancer: A systematic review and meta-analysis.
Cancer Med. 10:5983–5997. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guthrie GJK, Charles KA, Roxburgh CSD,
Horgan PG, McMillan DC and Clarke SJ: The systemic
inflammation-based neutrophil-lymphocyte ratio: Experience in
patients with cancer. Crit Rev Oncol Hematol. 88:218–230. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dehner CA, Hagemann IS and Chrisinger JSA:
Retroperitoneal dedifferentiated liposarcoma. Am J Clin Pathol.
156:920–925. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu W, Zou B, Tang M, Li X, Huang M, Chen
W and Miao C: Prediction of intraoperative bleeding and blood
transfusion in patients with recurrent retroperitoneal liposarcoma:
A retrospective study. Ann Transl Med. 10:9862022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishii K, Yokoyama Y, Nishida Y, Koike H,
Yamada S, Kodera Y, Sassa N, Gotoh M and Nagino M: Characteristics
of primary and repeated recurrent retroperitoneal liposarcoma:
Outcomes after aggressive surgeries at a single institution. Jpn J
Clin Oncol. 50:1412–1418. 2020. View Article : Google Scholar : PubMed/NCBI
|