Introduction
According to the latest studies, non-Hodgkin
lymphoma (NHL) is the most common hematological malignancy
worldwide, accounting for ~3% of cancer incidence and mortality
(1). CD20+ B-cell NHL
includes diffuse large B-cell lymphoma (DLBCL), follicular lymphoma
(FL), mantle cell lymphoma (MCL) and marginal zone lymphoma (MZL),
accounting for 80% of all NHL cases (2). Despite newly developed therapies and
protocols (such as the next-generation anti-CD20 monoclonal
antibody obinutuzumab, Bruton's tyrosine kinase inhibitors and
chimeric antigen receptor T-cell immunotherapy), rituximab-based
regimens such as the combination of rituximab, cyclophosphamide,
epirubicin, vincristine and prednisone (R-CHOP) are still the
standard first-line therapeutic strategy for treating DLBCL
worldwide (3).
Rituximab is a chimeric human/mouse monoclonal
antibody that directly targets the CD20 antigen present on the
surface of normal and malignant B lymphocytes. Currently, the
anti-lymphoma mechanisms of rituximab have been ascribed to
antibody-dependent, cell-mediated cytotoxicity and
complement-dependent cytotoxicity through the binding of the
fragment antigen-binding domain of rituximab to the CD20 antigen
(4). Furthermore, R-CHOP regimens
improve rates of progression-free survival (PFS) and overall
survival (OS) in patients with DLBCL (5). However, the infusion-related reactions
(IRRs) of rituximab, which occur particularly after the first
infusion, with an incidence of 30–50%, have become a major clinical
problem for hematologists and patients with DLBCL (6,7). The
main symptoms of IRRs vary from mild to life threatening, the
latter of which results in discontinued rituximab treatment and,
therefore, affects clinical outcome (8). Thus, further exploration of how to
reduce the incidence of IRRs to rituximab to improve the quality of
life for patients with DLBCL is needed.
Rituximab has also been commonly administered to
patients with autoimmune disorders, such as systemic lupus
erythematosus (SLE) and rheumatoid arthritis (RA). Notably, the IRR
rate of rituximab was higher in patients with B-cell NHL
(25.0-35.9%) compared with patients with SLE and RA (9.4-17.5%;
P<0.0001) (9,10). Compared with patients with B-cell
NHL, patients with SLE and RA are invariably administered
concomitant steroid treatment, such as prednisone, prior to
rituximab treatment, which might, in part, explain the
immunosuppressive status and the resulting lower incidence of
rituximab IRRs (11). Inspired by
these previous results, a prospective, randomized and controlled
study for patients with DLBCL was conducted, which aimed to compare
the incidence of rituximab IRRs between standard R-CHOP-like
regimens and a prednisone premedication-modified R-CHOP-like
protocol.
Materials and methods
Patient selection
Patients with newly-diagnosed DLBCL (age range,
18–70 years; n=88) were enrolled between January 2019 and August
2022 in the study at Jiujiang University Affiliated Hospital
(Jiujiang, China), Ruichang People's Hospital (Ruichang, China) and
Lushan People's Hospital (Lushan, China). Inclusion criteria were
as follows: i) All patients were ≥18 years old at admission; ii)
all the pathological diagnoses of DLBCL were confirmed in the
Pathology Department of Jiujiang University Affiliated Hospital;
and iii) all patients consented to participate in the study and
signed an informed consent form. Exclusion criteria were as
follows: i) The patient had a history of rituximab treatment prior
to enrollment in the study; ii) the patient refused to participate
in the study; and iii) the patient was initially diagnosed with
central nervous system DLBCL or HIV-related DLBCL.
The clinicopathological features of all patients at
diagnosis were recorded (Table I),
including age, sex, Eastern Cooperative Oncology Group (ECOG)
performance status (12), bulky
lesion (i.e., the longest diameter of nodal masses >10 cm in
size), number of extra-nodal sites, serum level of lactate
dehydrogenase, B symptoms, Lugano stage (version 2014) (13) and age-adjusted International
Prognostic Index (i.e. low-, moderate- and high-risk).
 | Table I.Baseline clinicopathological features
of patients in the control (n=44) and pretreatment (n=44)
groups. |
Table I.
Baseline clinicopathological features
of patients in the control (n=44) and pretreatment (n=44)
groups.
| Clinicopathological
feature | Control group | Treatment group | P-value |
|---|
| Age,
yearsa | 48.5±6.75 | 45.9±5.92 | 0.0581b |
| Sex, n (%) | | | 0.1355c |
| Male | 18 (40.9) | 25 (56.8) |
|
|
Female | 26 (59.1) | 19 (43.2) |
|
| ECOG score, n
(%) | | | 0.1347c |
| 0-1 | 20 (45.5) | 27 (61.4) |
|
| 2-4 | 24 (54.5) | 17 (38.6) |
|
| Bulky lesion, n
(%) | | | 0.5135c |
|
Present | 16 (36.4) | 19 (43.2) |
|
|
Absent | 28 (63.6) | 25 (56.8) |
|
| B symptoms, n
(%) | | | 0.3754c |
|
Present | 14 (31.8) | 18 (40.9) |
|
|
Absent | 30 (68.2) | 26 (59.1) |
|
| Number of extra-nodal
sites, n (%) |
|
| 0.2373c |
| ≤2 | 10 (22.7) | 15 (34.1) |
|
|
>2 | 34 (77.3) | 29 (65.9) |
|
| LDH, n (%) | | | 0.3858c |
|
Normal | 16 (36.3) | 20 (45.5) |
|
|
Elevated | 28 (63.6) | 24 (54.5) |
|
| Lugano stage, n
(%) | | | 0.3102d |
| I | 7 (15.9) | 9 (20.5) |
|
| II | 8 (18.2) | 11 (25.0) |
|
|
III | 25 (56.8) | 21 (47.7) |
|
| IV | 4 (9.1) | 3 (6.8) |
|
| aaIPI, n (%) | | | 0.2804d |
|
Low-risk | 5 (11.4) | 9 (20.5) |
|
|
Intermediate-high risk | 30 (68.2) | 28 (63.6) |
|
| High
risk | 9 (20.5) | 7 (15.9) |
|
Cohort size calculation and
randomization
The cohort size was assessed based on the minimum
requirement of a 25% reduction in the incidence of rituximab IRRs;
that is 40% of standard regimen and 15% of modified protocol. The
relative parameters were set as 80% power, 10% α error (based on a
one-sided test) and 10% estimated loss rate. According to the
calculation formula of cohort size in clinical trials (PASS version
15.0; www.ncss.com), the anticipated total
cohort size included 88 cases, with 44 cases per group (14). According to the random number table
method, the 88 patients with DLBCL were divided into two groups
with balanced randomization (1:1).
Therapeutic regimens
In the control group, standard R-CHOP-like regimens
were administered as follows: 375 mg/m2 Rituximab (Roche
Diagnostics), intravenously on day 1; 750 mg/m2
cyclophosphamide (Jiangsu Hengrui Pharmaceuticals Co., Ltd.),
intravenously on day 2; 60 mg/m2 epirubicin (Zhejiang
Hisun Chemical Co., Ltd.) or 30 mg/m2 pegylated
liposomal doxorubicin (CSPC Pharmaceutical Group Ltd.),
intravenously on day 2; 1.4 mg/m2 vincristine (maximum
dose of 2 mg; Zhejiang Hisun Chemical Co., Ltd.), intravenously on
day 2; and 100 mg/day prednisone (Zhejiang Xianju Pharmaceutical
Co., Ltd.), orally on days 1–5.
The treatment group received the modified
R-CHOP-like regimen as follows: 100 mg/day Prednisone, orally on
days 1–5; 375 mg/m2 rituximab, intravenously on day 4;
750 mg/m2 cyclophosphamide, intravenously on day 5; 60
mg/m2 epirubicin or 30 mg/m2 pegylated
liposomal doxorubicin, intravenously on day 5; and 1.4
mg/m2 vincristine (maximum dose of 2 mg), intravenously
on day 5.
For both groups, the treatment cycle was repeated
every 21 days and the total number of cycles was 6–8 cycles
according to each patient's condition. All patients in the control
and treatment groups were given 25 mg diphenhydramine
intramuscularly and 25 mg indometacin orally 30 min prior to
rituximab treatment. Rituximab was mixed with 500 ml normal saline
and infused intravenously with an initial rate of 50 mg/h,
increasing gradually by 50 mg/h every 30 min until it reached 400
mg/h.
If no IRRs occurred during the 1st and 2nd cycle,
the initial rate of rituximab infusion was 100 mg/h during the 3rd
and all subsequent cycles, then increased by 100 mg/h every 30 min
up to 400 mg/h for 6–8 cycles.
Incidence of IRR grade to rituximab
and chemotherapeutic adverse events
The primary endpoint of the study was the incidence
of IRRs to rituximab from the 1st to the 4th cycle. IRRs to
rituximab and chemotherapeutic adverse events were graded according
to the National Cancer Institute Common Terminology Criteria for
Adverse Events (version 4.0) (15).
All the episodes of adverse events were recorded in detail, such as
vomiting, nausea, leukopenia, granulocytopenia, alopecia,
thrombocytopenia and cardiotoxicity.
Clinical outcome evaluation
The secondary endpoint was clinical efficacy,
including the short- and long-term outcomes, which were evaluated
after every two cycles of treatment according to the 2014 Lugano
classification (16). The
short-term outcomes included complete remission (CR), partial
remission (PR), stable disease and progressive disease (PD).
Overall response rate (ORR) included CR and PR. The long-term
outcomes comprised PFS and OS; PFS was calculated from the start of
treatment until disease progression (i.e. any appearance of a new
lesion or enlargement of the remitted targeted lesion), and OS was
defined from the date of treatment until death or the end of
follow-up. The last follow-up was completed in November 2022.
Statistical analysis
All statistical analyses were performed using SPSS
(version 19.0; IBM Corp.) and GraphPad Prism (version 7.0;
Dotmatics). Continuous variables are presented as the mean ±
standard deviation, analyzed by unpaired Student's t-test.
Categorical variables are presented as counts and percentages, and
were assessed by χ2 or Fisher's exact test. Rank data
were analyzed using ridit analysis. Survival data (PFS and OS) were
analyzed using the Kaplan-Meier method, and the log-rank test was
used to compare the survival outcomes of the two groups. A
one-sided test was used in the study, and P<0.05 was considered
to indicate a statistically significant difference.
Results
Clinical features
A total of 88 patients with DLBCL were included in
this study. As shown in Table I,
there were no marked differences in baseline clinicopathological
features between the control and the treatment groups
(P>0.05).
IRR incidence of rituximab
As presented in Table
II, the total incidence of IRRs to rituximab in the treatment
group was significantly lower compared with that in the control
group (15.9% vs. 43.2%; P=0.0051). In addition, there was a
significant difference in incidence between the different grades of
IRRs by ridit analysis (P=0.0053). The vast majority of actual IRRs
to rituximab were grade I and II in both groups. Grade III or
higher IRRs to rituximab were only reported in 9.1% (4/44) and 2.3%
(1/44) of patients in the control and treatment groups,
respectively. These results strongly indicated that the prednisone
premedication modification decreased the total incidence of IRRs to
rituximab, but did not affect the incidence of different grades of
IRRs to rituximab.
 | Table II.IRR incidence of rituximab in the two
groups (n=44 patients/group). |
Table II.
IRR incidence of rituximab in the two
groups (n=44 patients/group).
| IRR grade | Control group, n
(%) | Treatment group, n
(%) | P-value |
|---|
| 0a | 25 (56.8) | 37 (84.1) | 0.0053b |
| I | 5 (11.4) | 2 (4.5) |
|
| II | 10 (22.7) | 4 (9.1) |
|
| III | 3 (6.8) | 1 (2.3) |
|
| IV | 1 (2.3) | 0 (0.0) |
|
| V | 0 (0.0) | 0 (0.0) |
|
| Total
incidence | 19 (43.2) | 7 (15.9) | 0.0051c |
In the present study, 26 out of the 88 total
patients (29.5%) experienced >1 IRR episode. As shown in
Table III, the incidence of IRRs
to rituximab in the treatment group was significantly lower
compared with that of the control group in the 1st (15.9 vs. 43.2%;
P=0.0051) and 2nd (6.8 vs. 27.3%; P=0.0107) cycle; however, no
significant differences were identified in the 3rd and 4th
cycle.
 | Table III.Occurrence cycles of rituximab IRRs
between the two groups (n=44 patients/group). |
Table III.
Occurrence cycles of rituximab IRRs
between the two groups (n=44 patients/group).
|
| Number of IRRs |
|
|---|
|
|
|
|
|---|
| Cycle | Control group, n
(%) | Treatment group, n
(%) | P-value |
|---|
| 1st | 19 (43.2) | 7 (15.9) | 0.0051a |
| 2nd | 12 (27.3) | 3 (6.8) | 0.0107a |
| 3rd | 5 (11.4) | 0 (0.0) |
|
| 4th | 1 (2.3) | 0 (0.0) |
|
Clinical outcomes
The clinical outcomes of both groups were compared.
As indicated in Table IV, no
significant differences in ORRs were observed between the control
and the treatment group by either ridit analysis or χ2
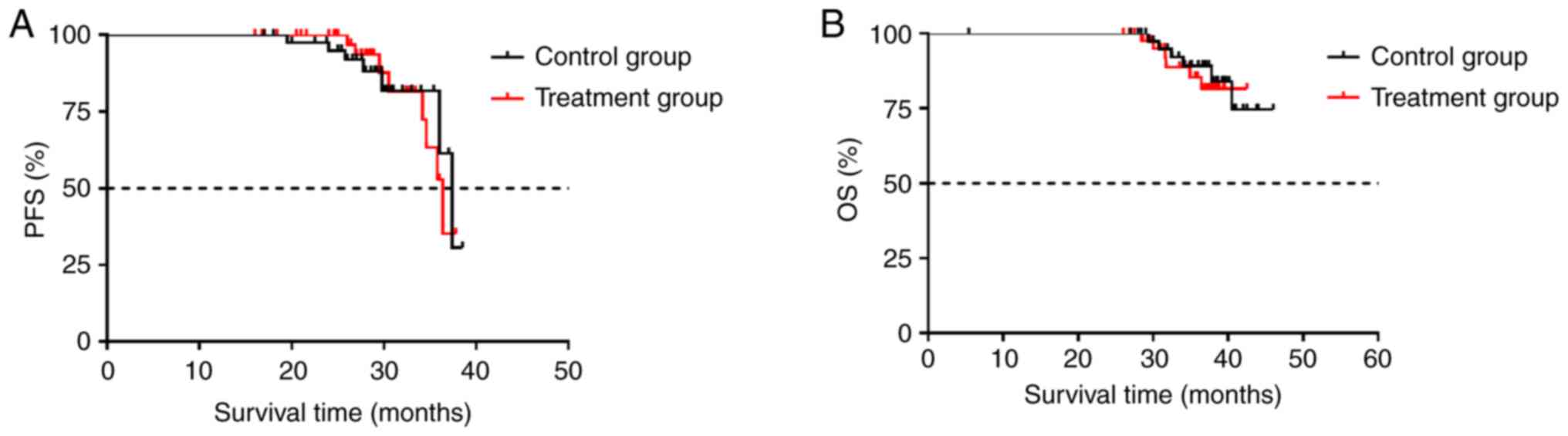
test. As shown in Fig. 1, the
median PFS (mPFS) was 37.4 and 36.4 months in the control and
treatment group, respectively (P=0.5244). Additionally, although
median OS (mOS) was not reached within the follow up of 47 months,
no significant difference in OS was found between the two groups
(P=0.5878). These results strongly suggested that the prednisone
premedication did not influence the clinical outcomes in patients
with DLBLC.
 | Table IV.Clinical outcomes in the two groups
(n=44 patients/group). |
Table IV.
Clinical outcomes in the two groups
(n=44 patients/group).
| Clinical
efficacy | Control group, n
(%) | Treatment group, n
(%) | P-value |
|---|
| CR | 12 (27.3) | 14 (31.8) | 0.9383a |
| PR | 29 (65.9) | 25 (56.8) |
|
| SD | 2 (4.5) | 3 (6.8) |
|
| PD | 1 (2.3) | 2 (4.5) |
|
| ORR | 41 (93.2) | 39 (88.6) | 0.7133b |
Adverse events
As indicated in Table
V, the common adverse events included hematological and
non-hematological toxicities. The majority of adverse events were
of grade I and II. The grade ≥III toxicities mainly included
vomiting and nausea (<20%), leukopenia and granulocytopenia
(<20%), alopecia (<25%), thrombocytopenia (<10%) and
cardiotoxicity (<5%). The main hematological and
non-hematological toxicities were manageable with dose adjustment
and supportive care. No deaths associated with adverse events were
reported. Notably, there was no significant difference in adverse
event incidence between the two groups, with the exception of IRRs
to rituximab (Table II).
 | Table V.Treatment-related adverse effects in
the two groups (n=44 patients/group). |
Table V.
Treatment-related adverse effects in
the two groups (n=44 patients/group).
|
| Control group, n
(%) | Treatment group, n
(%) |
|
|---|
|
|
|
|
|
|---|
| Adverse effect | All grades | Grade ≥III | All grades | Grade ≥III |
P-valuea |
|---|
| Vomiting and
nausea | 36 (81.8) | 8 (18.2) | 33 (75.0) | 6 (13.6) | 0.437 |
| Leukopenia and
granulocytopenia | 32 (72.7) | 7 (15.9) | 34 (77.3) | 8 (18.2) | 0.6225 |
|
Thrombocytopenia | 25 (56.8) | 4 (9.1) | 23 (52.2) | 3 (6.8) | 0.6686 |
| Cardiotoxicity | 14 (31.8) | 2 (4.5) | 12 (27.2) | 1 (2.3) | 0.6403 |
| Alopecia | 35 (79.5) | 10 (22.7) | 37 (84.1) | 9 (20.5) | 0.5804 |
| Hemorrhagic
cystitis | 4 (9.1) | 0 (0.0) | 2 (4.5) | 0 (0.0) | 0.6723 |
Discussion
Rituximab-based R-CHOP regimens have been broadly
administered in patients with DLBCL in China. The issue of IRRs to
rituximab has become a major obstacle that cannot be ignored. At
present, the mechanisms of IRRs have been mainly attributed to
rituximab-induced cytokine release syndrome, which involves the
release of TNF-α, IL-1 and IL-6 in the blood (17). Rising levels of cytokines trigger a
series of symptoms of inflammatory response, including fever,
chills, rigors, rash, headache, hypotension, breathlessness,
bronchospasm, nausea, vomiting and even allergic shock (8,18).
Additionally, some factors were found to be associated with a risk
of IRRs to rituximab. Laudati et al (19) demonstrated that female patients
exhibited a higher incidence of IRRs to rituximab compared with
male patients with CD20+ B-cell NHL (34.1 vs. 21.1%; P=0.04). Cho
et al (6) reported that B
symptoms were independently related to IRRs to rituximab [hazard
ratio (HR), 1.850; P=0.036], whereas bone marrow (BM) infiltration
was independently linked to re-IRRs (>2 repeated experiences) to
rituximab (HR, 4.904; P=0.029). Similarly, Ohata et al
(20) showed that BM involvement
was a distinctive risk factor for IRRs to rituximab in patients
with CD20+ B-cell NHL. The results indicated that both B
symptoms and BM infiltration were the high-risk indicators for IRRs
to rituximab in patients with DLBCL.
According to a patient survey, 34.0-49.5% of those
with B-cell NHL, including DLBCL, experienced IRRs at least once
during treatment with rituximab (6,7,11,20).
Generally, the majority of IRRs to rituximab were of mild to
moderate degree, whereas ~10% of patients with B-cell NHL
experienced severe or life-threatening IRRs, which greatly affected
the patients' compliance and treatment continuity (8). Tsutsumi et al (21) established a risk-stratified
rituximab protocol for patients with B-cell NHL to minimize IRRs to
rituximab. During the 1st cycle of rituximab infusion, the patients
in the low- and moderate-risk groups received standard infusions of
25–200 mg/h (total of 4.3 h). The patients in the high-risk group
received longer infusions of 25–100 mg/h (total of 6.8 h). The
researchers found that the overall incidence of IRRs was 28% and
all IRRs were grade ≤II. No severe IRRs of grade ≥III were
reported. Notably, only 1% all of patients developed IRRs in the
2nd cycle of rituximab infusion, and no IRR episodes occurred in
the 3rd cycle. For some patients with B-cell neoplasia and
hypersensitivity to rituximab, a 12-step rituximab desensitization
protocol was performed to successfully assist 10 patients in
completing the scheduled immunochemotherapy (22). Also, another 16-step rituximab
desensitization method was carried out to successfully minimize the
IRRs to rituximab in 2 patients with MZL and 1 with RA (23). Thus, the question of how to decrease
the occurrence of IRRs to rituximab is still a major clinical issue
for hematology and oncology practitioners.
At present, rituximab has been recommended as the
main therapeutic agent in autoimmune disorders, including
thrombotic thrombocytopenic purpura, SLE, RA and nephrotic syndrome
(24–27). A retrospective, multicenter study
compared the incidence of IRRs to rituximab in patients with B-cell
lymphoproliferative disorders, such as DLBCL, FL, MCL and chronic
lymphocytic leukemia, and in patients with autoimmune disorders,
such as SLE and RA. Notably, the rate of IRRs was significantly
higher in the former group (25.0-35.9%) compared with the rate in
the latter group (9.4-17.5%; P<0.001) (11). The history of concomitant steroid
use before rituximab treatment has been mainly ascribed to the
lower incidence of IRRs to rituximab in autoimmune disorders
(28). In the study by Laudati
et al (19), a low incidence
of IRRs was found in patients with B-cell NHL treated with
dexamethasone premedication in comparison with no dexamethasone
pretreatment (19.1 vs. 36.7%; P=0.005). Hence, the use of steroid
pretreatment prompted the design of a novel prednisone
premedication modification of the R-CHOP-like protocol in the
present study consisting of oral prednisone for 3 days prior to
rituximab administration for patients with DLBCL in the treatment
group and standard R-CHOP-like regimen in the control group.
In the present study, it was revealed that the total
incidence of IRRs to rituximab was significantly lower in the
treatment group compared with that in the control group, and that
most of the IRRs were of grade ≤II in both groups. Notably, a
significant difference in incidence was observed between the
different grades of IRRs. These results suggested that the
prednisone premedication modification decreased the total incidence
and the grade incidence of IRRs to rituximab. The underlying
mechanism may be partly ascribed to lower tumor load resulting from
prednisone-pretreatment for 3 days, which was presented clinically
by the alleviation of B symptoms and shrinkage of superficial
lymphadenopathy in most of the patients from the treatment group.
To some degree, the usage of prednisone in advance may prevent the
occurrence of tumor lysis syndrome, in particular for some patients
with DLBCL with bulky lesions, which in turn decreases the
incidence of IRRs to rituximab.
Results from the present study confirmed that the
strategy of steroid premedication could reduce the risk of IRRs to
rituximab, which was consistent with previous study results
(7,11,19).
Notably, unlike other studies, the innovative point of the current
study was to modify the sequence of prednisone and rituximab
treatment in the R-CHOP-like protocol without any additional
steroid pretreatment. In the present study, a total of 29.5%
patients experienced >1 IRR episode. Importantly, the incidence
of IRRs to rituximab in the treatment group decreased significantly
compared with that in the control group in the 1st and the 2nd
cycle; however, no marked differences were observed in the 3rd and
4th cycles for both groups. These results were in line with
previous study results (6,21), highlighting the necessity and
importance of monitoring IRRs to rituximab in the first two courses
of immunochemotherapy for patients with DLBCL. Clinically, the
incidence of IRRs to rituximab gradually reduced over the
therapeutic courses and with the decrease of tumor burden in
patients with DLBCL. There was no significant association of IRRs
to rituximab with clinical outcomes in patients with DLBLC, which
was in agreement with previous study results (6). Finally, the hematological and
non-hematological toxicities, most of grade I or II, were similar
in the two groups in the present study.
In conclusion, the novel strategy of prednisone
pretreatment in the R-CHOP-like regimen in the present study
significantly reduced the total incidence of IRRs to rituximab in
patients with DLBCL. There is a necessity and importance to closely
monitor IRRs to rituximab in the first three cycles of
immunochemotherapy for patients with DLBCL. Furthermore, IRRs to
rituximab did not affect the clinical efficacy of the R-CHOP-like
treatment. Therefore, the novel protocol may be considered a
promising method to minimize the incidence of IRRs to rituximab in
patients with DLBCL. Owing to the small sample size of the present
study, it would be necessary to increase the cohort size and to
explore the novel prednisone-premedication regimen for other types
of B-cell NHL.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JD conceptualized and wrote the manuscript, and
generated the random allocation sequence. ZL collected and analyzed
the data, and assigned participants to interventions. HG and XJ
provided the data, and enrolled and treated the patients. JD and ZL
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was performed in accordance with The
Declaration of Helsinki. All patients signed written informed
consent to participate in the study. This study was approved by the
Medical Ethical Committee of Jiujiang University Affiliated
Hospital (Jiujiang, China) on January 6, 2019 (approval no.
jjuhmer-a-2019-01), the Medical Ethical Committee of Ruichang
People's Hospital on January 8, 2019 (Ruichang, China; approval no.
rchmer-2019-01) and the Medical Ethical Committee of Lushan
People's Hospital on January 7, 2019 (Lushan, China; approval no.
lshmer-2019-02).
Patient consent for publication
All patients signed written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thandra KC, Barsouk A, Saginala K, Padala
SA, Barsouk A and Rawla P: Epidemiology of non-Hodgkin's lymphoma.
Med Sci (Basel). 9:52021.PubMed/NCBI
|
|
3
|
Pasvolsky O, Rozental A, Raanani P,
Gafter-Gvili A and Gurion R: R-CHOP compared to R-CHOP + X for
newly diagnosed diffuse large B-cell lymphoma: A systematic review
and meta-analysis. Acta Oncol. 60:744–749. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zou L, Song G, Gu S, Kong L, Sun S, Yang L
and Cho WC: Mechanism and treatment of rituximab resistance in
diffuse large bcell lymphoma. Curr Cancer Drug Targets. 19:681–687.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Witlox WJA, Grimm SE, Riemsma R, Armstrong
N, Ryder S, Duffy S, Carrera VH, Posadzki P, Worthy G, Pouwels
XGLV, et al: Lenalidomide with rituximab for previously treated
follicular lymphoma and marginal zone lymphoma: An evidence review
group perspective of a NICE single technology appraisal.
Pharmacoeconomics. 39:171–180. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho KM, Keam B, Ha H, Kim M, Jung JW, Song
WJ, Kim TM, Jeon YK, Kang HR, Kim DW, et al: Clinical significance
of rituximab infusion-related reaction in diffuse large B-cell
lymphoma patients receiving R-CHOP. Korean J Intern Med.
34:885–893. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jung JW, Kang HR, Lee SH and Cho SH: The
incidence and risk factors of infusion-related reactions to
rituximab for treating B cell malignancies in a single tertiary
hospital. Oncology. 86:127–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paul F and Cartron G: Infusion-related
reactions to rituximab: Frequency, mechanisms and predictors.
Expert Rev Clin Immunol. 15:383–389. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dao KH and Bermas BL: Systemic lupus
erythematosus management in pregnancy. Int J Womens Health.
14:199–211. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taylan A: Rituximab therapy in
pericarditis associated with rheumatoid arthritis. Rheumatol Int.
42:1843–1847. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
D'Arena G, Simeon V, Laurenti L,
Cimminiello M, Innocenti I, Gilio M, Padula A, Vigliotti ML, De
Lorenzo S, Loseto G, et al: Adverse drug reactions after
intravenous rituximab infusion are more common in hematologic
malignancies than in autoimmune disorders and can be predicted by
the combination of few clinical and laboratory parameters: Results
from a retrospective, multicenter study of 374 patients. Leuk
Lymphoma. 58:2633–2641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simcock R and Wright J: Beyond performance
status. Clin Oncol (R Coll Radiol). 32:553–561. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu J, Wei J, Jiang X, Yu W, Tong H, Jin
J, Yan S and Xu W: Prognostic effects of clinical parameters,
genetic abnormalities, and subtypes in primary gastric diffuse
large B-cell lymphoma: A cohort analysis of 146 patients. Leuk
Lymphoma. 63:3362–3369. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang YY and Sun RH: Application of PASS in
sample size estimation of non-inferiority, equivalence and
superiority design in clinical trials. Zhonghua Liu Xing Bing Xue
Za Zhi. 37:741–744. 2016.(In Chinese). PubMed/NCBI
|
|
15
|
Gong Y, Luo L, Wang L, Chen J, Chen F, Ma
Y, Xu Z, Sun Y, Luo L, Shi C and Li X: Association of MTHFR and
ABCB1 polymorphisms with MTX-induced mucositis in Chinese
paediatric patients with acute lymphoblastic leukaemia, lymphoma or
osteosarcoma-A retrospective cohort study. J Clin Pharm Ther.
46:1557–1563. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheson BD, Fisher RI, Barrington SF,
Cavalli F, Schwartz LH, Zucca E, Lister TA; Alliance, Australasian
Leukaemia and Lymphoma Group and Eastern Cooperative Oncology
Group, ; et al: Recommendations for initial evaluation, staging,
and response assessment of Hodgkin and non-Hodgkin lymphoma: The
Lugano classification. J Clin Oncol. 32:3059–3068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moore JE, Bloom PC, Chu CC, Bruno JE,
Herne CA, Baran AM, Quataert SA, Mosmann TR, Taylor RP, Wallace DS,
et al: Rituximab induced cytokine release with high serum IP-10
(CXCL10) concentrations is associated with infusion reactions. Leuk
Res. 129:1070722023.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fouda GE and Bavbek S: Rituximab
hypersensitivity: From clinical presentation to management. Front
Pharmacol. 11:5728632020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Laudati C, Clark C, Knezevic A, Zhang Z
and Barton-Burke M: Hypersensitivity reactions: Priming practice
change to reduce incidence in first-dose rituximab treatment. Clin
J Oncol Nurs. 22:407–414. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohata S, Takenaka K, Sugiyama D and
Sugimoto T: Bone marrow infiltration is a distinctive risk factor
for rituximab infusion-related reactions in CD20-positive B-cell
non-Hodgkin lymphoma. Adv Hematol. 2022:36887272022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsutsumi D, Hayama T, Miura K, Uchiike A,
Tsuboi S, Otsuka S, Hatta Y and Kishikawa Y: A novel rituximab
administration protocol to minimize infusion-related adverse
reactions in patients with B-cell lymphoma. Int J Clin Pharm.
44:366–373. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Novelli S, Soto L, Caballero A, Moreno ME,
Lara MJ, Bayo D, Quintas A, Jimeno P, Zamora MI, Bigorra T, et al:
Assessment of confirmed clinical hypersensitivity to rituximab in
patients affected with B-cell neoplasia. Adv Hematol.
2020:42315612020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang BC and Castells MC: Rituximab
hypersensitivity and desensitization: A personalized approach to
treat cancer and connective tissue diseases. Ann Allergy Asthma
Immunol. 123:11–15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watanabe A, Shiseki M, Oishi M, Kobayashi
M, Oshima S, Osanai S, Ryuzaki M, Izuka Y, Tanaka N, Ishiyama M, et
al: Successful rituximab treatment in thrombotic thrombocytopenic
purpura patients complicated by other autoimmune disorders: Two
case reports. Intern Med. 60:2859–2862. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Onuora S: Systemic lupus erythematosus:
Rituximab improves SLE disease activity. Nat Rev Rheumatol.
14:622018. View Article : Google Scholar
|
|
26
|
Assadi F, Mazaheri M and Sadeghi-Bodj S:
Randomized controlled trial to compare safety and efficacy of
mycophenolate vs cyclosporine after rituximab in children with
steroid-resistant nephrotic syndrome. Pharmacotherapy. 42:690–696.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smolen JS, Cohen SB, Tony HP, Scheinberg
M, Kivitz A, Balanescu A, Gomez-Reino J, Cen L, Poetzl J, Shisha T
and Kollins D: Efficacy and safety of Sandoz biosimilar rituximab
for active rheumatoid arthritis: 52-week results from the
randomized controlled ASSIST-RA trial. Rheumatology (Oxford).
60:256–262. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gilaberte RS and Isenberg D: Differences
in the development of adverse infusion reactions to rituximab in
patients with systemic lupus erythematosus, rheumatoid arthritis
and non-Hodgkin's lymphoma-enigma variations. Front Med (Lausanne).
9:8828912022. View Article : Google Scholar : PubMed/NCBI
|