Discovery and identification of CTHRC1
Collagen triple helix repeat containing 1 (CTHRC1)
was first identified in 2005 in a screen for differentially
expressed genes between normal and balloon-wounded rat arteries
(1). The CTHRC1-related gene was
subsequently discovered in two distant cnidarian species, the
jellyfish Nematostella vectensis and the hydrozoan Clytia
hemisphaerica, both of which contained multiple copies of the
gene. In addition, an attempt was made to trace the origin of
CTHRC1 by studying the C-terminal domains of CTHR and CTHRC1, but
this remains unclear (2). Located
at chromosome position 8q22.3, the CTHRC1 gene encodes a 28-kDa
secretory glycosylated protein (1,3).
The evolutionarily conserved structure of CTHRC1 has
been elucidated among several species, with no homologous proteins
discovered (1). It is composed of a
leucine-rich hydrophobic signal peptide at the N-terminus, a short
12 repeat Gly-X-Y collagen motif responsible for triple helix
formation, and a number of highly conserved amino acids at the
C-terminus (1,4).
In vitro detection of CTHRC1 using antibodies
specific to the corresponding domain of recombinant CTHRC1
expressed in Escherichia coli resulted in the detection of
five lower molecule weight forms of CTHRC1, ranging between 10 and
30 kDa, of which only one form was not cleaved by plasminase
(5). The abundance of processed
CTHRC1 implies that CTHRC1 may appear in a differentially modified
form. In dedifferentiated smooth muscle cells (SMCs), monomeric
CTHRC1 was found to bind with cytoplasmic proteins, suggesting a
potential functional regulation of CTHRC1 (5). Plasmin was reported to cleave a
putative propeptide of CTHRC1 to generate an N-terminally truncated
molecule capable of regulating procollagen synthesis (5). N-glycosidase is capable of cleaving
CTHRC1 into minor fragments, which are the active form of CTHRC1
involved in some signaling transductions (6). As the name may suggest, CTHRC1 is
susceptible to cleavage by collagenases.
Expression pattern of CTHRC1
Immunohistochemical studies on cell types from a
number of organs have shown the localized expression of CTHRC1 in
the cytoplasm (7–11). One particular study has suggested
that CTHRC1 is membrane-anchored (3).
Comprehensive expression analysis revealed that
CTHRC1 was present in a number of tissues and organs of embryonic
and postnatal mice (3). CTHRC1 was
also found to be expressed in differentiated SMCs of arterioles,
large veins, the uterus and gastrointestinal tract, as well as in
myoepithelial cells, neurons, Purkinje cells and parafollicular
cells of the thyroid gland (5). In
particular, CTHRC1 has also been found in calcified atherosclerotic
plaques in a rat model of carotid balloon catheter-induced injury
(1).
In addition to endogenous detection in normal
tissues, the presence of CTHRC1 has been observed in a number of
malignancies. The expression levels of CTHRC1 in 24 different tumor
tissues were analyzed by the UALCAN database, and the transcription
level in all 24 tumor tissues was abnormally elevated compared with
that in normal tissues (8).
Additionally, CTHRC1 is typically expressed at
epithelial-mesenchymal interfaces, such as the epidermis and
dermis, the epithelium of the basal cornea, airway and esophagus,
and the choroid plexus and meninges (12).
A number of studies have demonstrated CTHRC1
expression in a variety of human solid tumors; however, a previous
study has suggested that cells from a pancreatic tumor did not
express CTHRC1 (8,13). The aforementioned study also noted
that expression of CTHRC1 was only present in the stroma
surrounding tumor cells, which includes vessel and skeletal muscle
cells, as well as fibroblasts. There are four explanations for this
paradox: i) Endogenous CTHRC1 has been revealed to exist in a
number of molecular weight forms, meaning it may exist in different
forms with a variety of sequences after some modification; hence,
each modified CTHRC1 protein may contain a different epitope map;
ii) the combination of CTHRC1 with undetermined cytoplasmic
proteins may prevent the exposure of specific epitopes on CTHRC1,
although when, where and how this binding occurs remains unknown;
and iii) the specificity of anti-CTHRC1 antibodies may vary between
institutions, possibly leading to different epitope
recognition.
Regulation of the expression of CTHRC1
Transforming growth factor-β (TGF-β)
and bone morphogenetic protein 4 (BMP-4) are involved in regulating
the expression of CTHRC1
The mRNA expression levels of CTHRC1 have been shown
to be gradually increased under TGF-β1 and BMP-4 stimulation
(1). Sequence analysis of the
CTHRC1 promoter region revealed a Smad protein-binding site, which
is a downstream member of the TGF-β1- and BMP-4-mediated pathways
(14). In keloid fibroblasts,
CTHRC1 expression was revealed to be upregulated in a
concentration-dependent manner by TGF-β1 (15). CTHRC1 and phosphos-Smad 3 form a
negative feedback loop in the TGF-β pathway. CTHRC1 is specifically
induced by TGF-β1 by phosphorylating Smad3 and binding to the H
promoter, which in turn accelerates the degradation of
phosphorylated Smad3 (16).
Dolichyl-phosphate
N-acetylglucosaminephosphotransferase (DPAGT1)
DPAGT1 is an N-glycosylation gene that functions as
the primary regulator of the metabolic pathway in protein
N-glycosylation (17). The
amplification of CTHRC1 is positively associated with its own
hyperglycosylation, which is modulated by DPAGT1. In oral squamous
cell carcinoma (OSCC), DPAGT1 and CTHRC1 were shown to be
upregulated simultaneously, and the inhibition of DPAGT1 by small
interfering RNA led to decreased CTHRC1 expression (18). Partial inhibition of DPAGT1
expression in OSCC cells downregulated CTHRC1 abundance by reducing
its half-life (18).
Hepatitis B virus (HBV) infection
In hepatocytes, HBV was shown to stimulate CTHRC1
mRNA and protein levels in a time- and dose-dependent manner
(19). Two sequences were found to
be involved in HBV-activated CTHRC1 expression by constructing
full-length promoters or mutants of CTHRC1, regulated by nuclear
factor-κB (NF-κB) and cAMP response element binding protein
(19). Overexpression of CTHRC1 in
turn promoted HBV replication (20).
Proto-oncogene, Wnt-3a
Wnt3a is a member of a diverse family of secreted
lipid-modified signaling glycoproteins that act as ligands to
activate the Wnt/β-catenin pathway (21). In OSCC, Wnt3a was shown to markedly
increase the mRNA transcript and protein levels of CTHRC1, and
increased recruitment and binding of β-catenin to the T-cell factor
sites at the CTHRC1 promoter contributed to this observation
(18).
MicroRNAs (miRNAs/miRs)
miRNAs are associated with individual life
activities, such as cell growth, tissue differentiation, and tumor
transformation and metastasis. miR-30c is an important member of
the miR-30 family, and its expression was found to be significantly
lower in breast cancer (BC) compared with in normal tissue
(22). Notably, the expression of
miR-30c has been revealed to be negatively associated with CTHRC1,
and miR-30c may inhibit CTHRC1 activation of the GSK-3β/β-catenin
signaling process, reduce β-catenin protein in the nucleus and
inhibit BC cell proliferation, invasion and migration (23). In BC, miR-30e can negatively
regulate CTHRC1 (24). In gastric
cancer, overexpression of let-7b, a miRNA belonging to the let-7
family has been shown to reduce the levels of CTHRC1 protein.
Conversely, in cells treated with a let-7b inhibitor, CTHRC1
expression was higher compared with that in control cells (25). It was reported that let-7b
suppressed the activity of CTHRC1 through binding position 20–26 in
the 3′-untranslated region (3′-UTR) of CTHRC1 mRNA (25). In melanoma, both miR-134 and CTHRC1
have been reported to be highly expressed, but there is a negative
interaction between the two: miR-134 can directly target the 3′-UTR
of CTHRC1 to downregulate CTHRC1, thereby limiting the migration
and invasion ability of melanoma cells (26). The same regulation was discovered
for miR-509-3p, which can bind to the potential binding site of
CTHRC1 to reduce the expression of CTHRC1. miR-509-3p can also
increase the levels of a-catenin and E-cadherin, and reduce
mesenchymal markers such as waveform and fibronectin levels to
inhibit melanoma metastasis and invasion (27). In human ovarian cancer, miR-30b-3p,
through targeting of the CTHRC1 3′-UTR, can increase E-cadherin and
β-catenin expression, and inhibit the epithelial-mesenchymal
transition (EMT) process in ovarian cancer cells to suppress tumor
invasion and metastasis (28). In
prostate cancer, miR-30e-5p can also play a role in regulating the
CTHRC1/EMT axis to inhibit the proliferation and invasion of PCa
cells by targeting the CTHRC1 3′-UTR (29).
Participation in multiple signaling
pathways
CTHRC1 and TGF-β signaling
TGF-β signaling has been shown to be highly active
at locations where CTHRC1 is transiently overexpressed, implying a
linkage between the two (1). In a
transgenic mouse animal model, increased phosphorylation of Smad2/3
in the downstream TGF-β signaling pathway stimulated the expression
of CTHRC1 mRNA and protein. Conversely, overexpression of CTHRC1
significantly inhibited TGF-β signaling transduction by reducing
phospho-Smad2/3 activity (1,30).
Therefore, it is possible that CTHRC1 may regulate the TGF-β
signaling cascade through a negative feedback loop. Similarly, an
experiment with polyvinylalcohol sponge found that during the
middle stage of wound healing, CTHRC1 can recruit additional M2
macrophages by activating the TGF-β/Smad pathway to promote wound
healing. TGF-β expression may be downregulated by CTHRC1 during the
later remodeling stage of wound healing (31) (Fig.
1A). These results are not inconsistent with each other,
suggesting that TGF-β and CTHRC1 interact to maintain tissue
morphology and homeostasis.
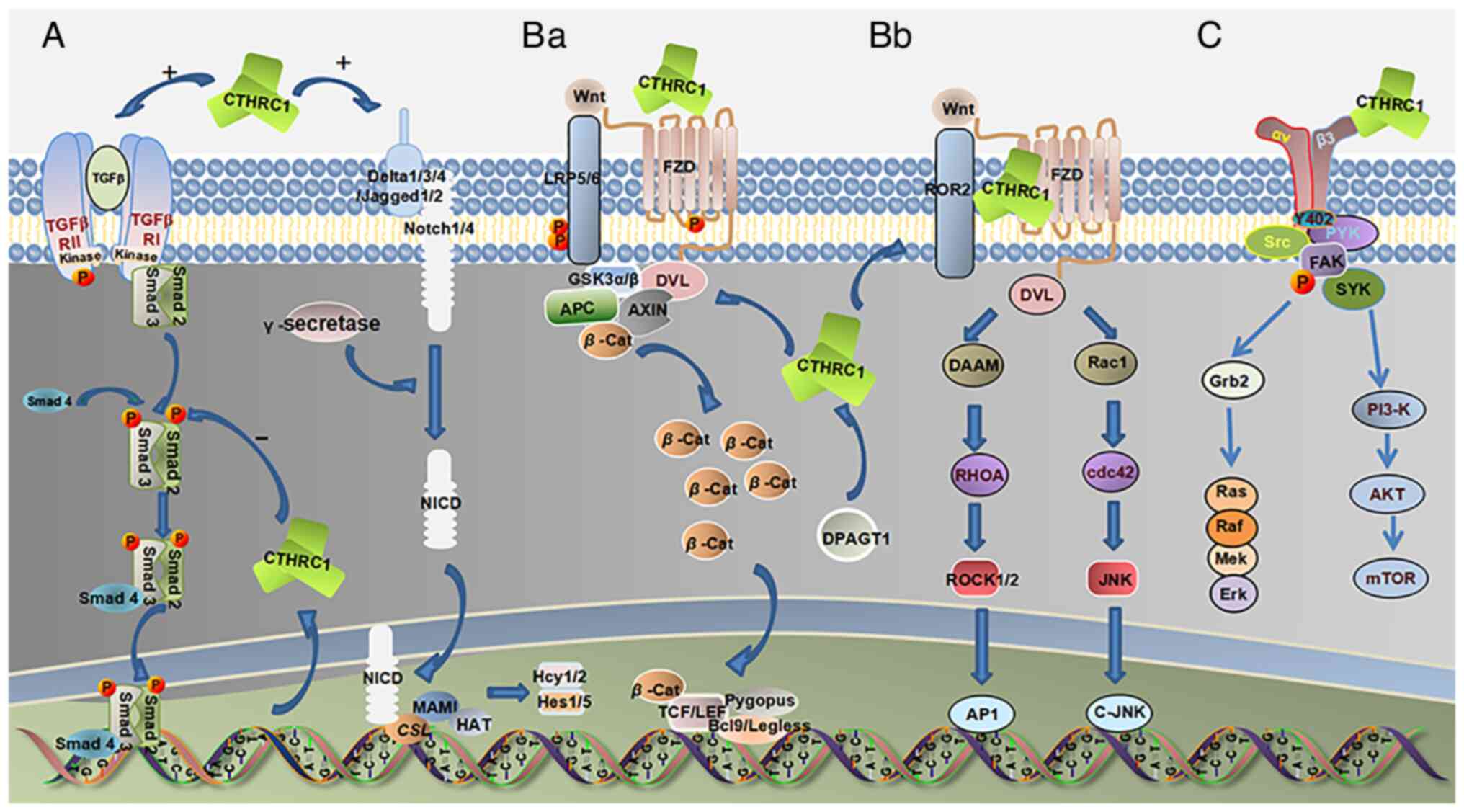 | Figure 1.(A) In wound healing, CTHRC1 can
recruit additional M2 macrophages to the wound site to promote skin
repair and activate the TGF-β and Notch pathways. DPAGT1 can
regulate the activation of CTHRC1, and CTHRC1 activates the TGF-β
pathway; simultaneously, there is a key negative feedback loop. The
increased phosphorylation level of Smad2/3 in the TGF-β downstream
signaling pathway stimulates the expression of CTHRC1. However,
overexpression of CTHRC1 in turn suppresses Smad2/3
phosphorylation. WNT signaling includes the WNT/β-catenin classical
pathway and the β-catenin atypical pathway. (Ba) In the
WNT/β-catenin classical pathway, CTHRC1 can activate this pathway
and significantly increase β-catenin stability and transcriptional
activity. (Bb) In non-classical WNT/PCP signaling, CTHRC1 can
promote and stabilize the formation of the CTHRC1-Wnt-FZD/Ror2
complex, thereby selectively activating the WNT/PCP pathway, whose
downstream molecules include the GTPase family, such as Rac1, RhoA
and JNK. CTHRC1 can enhance RhoA and Rac1 activities, and increase
JNK phosphorylation, and DPAGT1 can regulate the activation of
CTHRC1 on the Wnt/PCP pathway. (C) Upregulation of CTHRC1 activates
Src-FAK signaling, which is associated with cancer progression and
metastasis. |
CTHRC1 and Wnt/planar cell polarity
(Wnt/PCP) signaling pathway
CTHRC1 has been shown to promote and stabilize
formation of the CTHRC1-Wnt-Fzd/Ror2 complex, thus selectively
activating the Wnt/PCP pathway (Fig.
1Bb). A highly conserved 200-amino acid region in the
C-terminal domain of CTHRC1 was discovered to be responsible for
this biological selection (32).
DPAGT1 has been demonstrated to regulate the activation effect of
CTHRC1 on the Wnt/PCP pathway; the interaction between Wnt protein
and CTHRC1 was revealed to be more intensive and the downstream
effector of the Wnt/PCP pathway was more abundant in
DPAGT1-transfected cells (18). The
Wnt/PCP signaling pathways affect the development of various types
of cancer. In gastrointestinal stromal tumors (GISTs), CTHRC1 may
activate human umbilical vein endothelial cells (HUVECs) in the
Wnt/PCP signaling pathway to promote ERK phosphorylation and JNK to
promote tumor angiogenesis (33).
Western blotting showed that overexpression of CTHRC1 in cervical
cancer tissue and HeLa cells increased Wnt5a, Ror2 and p-c-Jun,
thereby activating the Wnt/PCP signaling pathway and promoting
tumor invasion and metastasis (34).
CTHRC1 and Wnt/β-catenin signaling
pathway
The relationship between CTHRC1 and the
Wnt/β-catenin pathway has been shown to be cell type-dependent. In
placental trophoblastic cells and cancer-associated fibroblasts,
CTHRC1 can function as an activator by significantly increasing the
stability and transcriptional activity of β-catenin, a hallmark of
Wnt/β-catenin signaling activation (Fig. 1Ba) (35). However, in GISTs, CTHRC1 functions
as an inhibitor by promoting the degradation of β-catenin (35–37).
The mechanism by which CTHRC1 influences the Wnt/β-catenin pathway
in an opposing way in different cell types needs to be further
explored.
CTHRC1 in Notch and other signaling
pathways
The Notch signaling pathway allows communication
between two adjacent cells and triggers a variety of downstream
responses to maintain a stable cell state. A number of studies have
shown that the Notch pathway is activated in wound macrophages
(31,38). It may also be involved in tissue
repair processes, and CTHRC1 may promote wound healing by
activating it and the TGF-β pathway (31). Furthermore, the Notch signaling
pathway is expressed in tumor cells, but it remains to be
investigated whether CTHRC1 can participate in the development and
progression of tumors through activation of the Notch signaling
pathway.
It is well known that the proto-oncogene
tyrosine-protein kinase Src, focal adhesion kinase (FAK),
mitogen-activated protein kinase kinase (MEK) and ERK are key
molecules in their respective signaling pathways and participate in
tumorigenesis in various types of cancer (39–41).
Overexpression of CTHRC1 has been shown to activate these pathways
by increasing their phosphorylation level in numerous studies
(Fig. 1C) (39,41–43).
However, the detailed roles of CTHRC1 in these pathways have yet to
be elucidated.
Biological function of CTHRC1
Arterial remodeling
Constitutive expression of CTHRC1 can significantly
reduce adventitial collagen deposition, neointimal lesion formation
and intimal SMC dedifferentiation in the repair process of injured
arteries, suggesting that CTHRC1 may perform an important role in
vascular remodeling (30). In
vitro experiments suggested that overexpression of CTHRC1
inhibited collagen extracellular matrix (ECM) deposition in the
adventitia of injured arteries by reducing the mRNA synthesis of α1
and α2 chains of collagen type I (1). In vivo experiments further
confirmed that the adventitia of the carotid artery was 50% thinner
in CTHRC1 transgenic mice than in wild-type littermates (30). In addition, an association between
TGF-β and CTHRC1 has been reported. TGF-β plays an important role
in promoting angiogenesis during arterial remodeling by activating
Smad2/3 complex, upregulating collagen synthesis, and increasing
collagen deposition and SMC proliferation (4). TGF-β stimulates upregulation of CTHRC1
expression; however, by reducing Smad2/3 phosphorylation, CTHRC1
inhibits the expression of the TGF-β target genes type I and type
III collagen, thereby inhibiting collagen deposition, enhancing
cell migration and promoting vascular repair (1,16).
Regulation in dystrophic muscle
diseases
CTHRC1 has been shown to be a marker for the
severity of disease progression in Duchenne muscular dystrophy
(DMD) and congenital muscular dystrophy (CMD) (44). Gene expression of CTHRC1 was
upregulated in DMD and CMD transgenic mice as well as in biopsy
specimens of patients with DMD (44). The presence of CTHRC1 was associated
with the deposition of type I collagen, and the collagen fibers
adjacent to collagen and CTHRC1 were smaller, suggesting that
CTHRC1 controlled collagen synthesis in DMD and CMD (44).
Regulation of metabolism
CTHRC1 has been detected in the plasma of CTHRC1
transgenic mice, exhibiting a half-life of ~2.5 h, which suggests
it could be secreted into the circulatory system (45). CTHRC1 aids in regulating glucose and
energy metabolism in the liver and skeletal muscle of animal
models, suggesting that hepatocytes and myocytes may express CTHRC1
receptors (45). Moreover,
constitutive expression of CTHRC1 was observed in some areas of the
brain of rats and pigs, such as the chromophobe cells and
colloid-filled follicles in the anterior pituitary lobe (45). Since no specific hormone has been
reported to localize in chromophobe cells of the anterior pituitary
lobe thus far, whether CTHRC1 is the first discovered hormone
secreted from this site needs to be verified (45).
CTHRC1 can promote bone formation
A number of signaling pathways mediate bone
homeostasis, including TGF-β and BMP, in which CTHRC1 is also
involved. The regulation of bone formation and maintenance by
CTHRC1 suggests that it interacts with TGF-β and BMP-Smad signaling
(46). In vitro and in
vivo studies have suggested that CTHRC1 is a positive regulator
of bone formation, and can promote the differentiation and
mineralization of bone progenitor cells, and the proliferation and
differentiation of osteoblasts, thereby inducing high bone mass
(46). The autonomy of CTHRC1 in
promoting osteogenic differentiation depends on cell type, and
CTHRC1 has been demonstrated to have an autonomous function in
skull osteoblasts in vitro but not in bone marrow-derived
mesenchymal stem cells (39). The
identity of CTHRC1-producing cells remains controversial. CTHRC1
has been shown to serve a role in the coupling process of bone
resorption to formation as a coupling factor of osteoclast
secretion that regulates bone remodeling. However, studies have
shown that CTHRC1 is not derived from osteoclasts but instead from
osteoblasts and osteocytes, and can inhibit the formation of
osteoclasts and their activity (39,47).
Role in the peripheral nervous
system
Yamamoto et al (6) found that mice with complete CTHRC1
knockout showed no significant phenotypic abnormalities and
remained fertile. The PCP homolog, Vangl2, was additionally
introduced in the experiments. Concurrently, it was revealed that
CTHRC1LacZ Vangl2Lp/− mouse embryos exhibited
a weak PCP phenotype, while CTHRC1LacZ/LacZ;
Vangl2Lp/+ mouse embryos showed complete closure of the
neural tube, suggesting that CTHRC1 affects neural tube opening by
modulating the PCP signaling pathway. However, no significant
abnormal PCP pathway or neural tube closure was observed in mouse
embryos with complete CTHRC1 knockout without enhanced PCP
mutations, suggesting the presence of functional redundant genes;
however, their true nature remains unknown and should be
investigated. Furthermore, CTHRC1 has been shown to be active in
peripheral nerve cells. Schwann cells serve an important role in
peripheral nerve repair, and can guide axon growth through
self-demyelination and directional differentiation (48). In Schwann cells, CTHRC1 is
upregulated in axonal interactions. Upregulation of CTHRC1
stimulates Schwann cell proliferation and delays myelination
through in vitro loss of function and in vivo gain of
function (49). After sciatic nerve
injury, the expression of CTHRC1 has been reported to be increased
in Schwann cells overexpressing long non-coding RNA NONMMUG014387,
which may promote the proliferation of Schwann cells by regulating
the Wnt/PCP pathway (50). After
peripheral nerve injury, most miRNAs are downregulated, and the
downregulated expression of miR-9 is accompanied by upregulated
expression of CTHRC1. A luciferase assay demonstrated that
upregulated miR-9 could significantly downregulate CTHRC1
expression by directly targeting the CTHRC1 3′-UTR and reducing
Schwann cell inhibition (51).
These results suggested that CTHRC1 may be a novel myelination
regulator in the peripheral nervous system (49–51).
Participation in the progression of solid
tumors in humans
Melanoma
Melanoma is a type of skin cancer caused by
malignant tumors of melanocytes. CTHRC1 was found to be expressed
in invasive melanomas; however, in benign moles or noninvasive
stage melanomas, it was not present, suggesting it may be
associated with the invasion and metastasis of melanoma (14). It has been reported that CTHRC1 is
involved in the tumorigenesis of melanoma mediated by miR-134-25 or
miR-155-26 and miR-509-3p (26,27,52).
In miR-509-3p-mediated melanoma, it was found that overexpressed
CTHRC1 reduced the protein levels of α-catenin and E-cadherin, and
enhanced the levels of vimentin and fibronectin, thus promoting the
metastasis and invasion of melanoma (27).
BC
Upregulation of CTHRC1 was previously shown in BC,
and its expression level was significantly associated with lymph
node classification, histological grade and pathological
Tumor-Node-Metastasis (pTNM) stage (23,36,53).
It has been reported that CTHRC1 promotes BC cell invasion and
metastasis by activating the Wnt/β-catenin signaling pathway
between EMTs (23,36). CTHRC1 expression may also be
associated with the prognosis of patients; in patients with
elevated expression of CTHRC1, shorter overall survival (OS) and
relapse-free survival times were observed, leading CTHRC1 to be
considered an independent prognostic indicator for patients with BC
(23).
Colorectal cancer (CRC)
CTHRC1 has been indicated as a useful biomarker for
CRC, as increased mRNA expression levels have been detected in CRC
tissues compared with in normal tissues (54,55).
CTHRC1 can affect the EMT process by inducing TGF-β signal
transduction, thus enhancing the migration and invasion of CRC
cells, and its high expression is associated with poor prognosis of
patients with CRC (54,55). It has also been found that CTHRC1,
which promotes liver metastasis of CRC, comes from CRC cells rather
than hepatic stellate cells in patients with CRC exhibiting liver
metastasis (55). It promotes tumor
proliferation mainly through the TGF-β signaling pathway (55). Meanwhile, in vivo studies in
mice showed that the combination of anti-CTHRC1 monoclonal antibody
and anti-PD-1 monoclonal antibody reduced liver metastasis in CRC.
Therefore, CTHRC1 may be a therapeutic target for CRC liver
metastasis (55).
Gastric cancer (GC)
An increased level of CTHRC1 has been discovered in
GC (56,57). HIF-1α and CXCR4 perform important
roles in GC metastasis, and the expression of CTHRC1 has been
reported to increase the expression of activated HIF-1α/CXCR4
signaling pathways, which may result in GC cell migration and
invasion (56). Upregulation of
CTHRC1 in GC has been shown to be significantly associated with
pTNM stage, tumor differentiation, depth of tumor invasion, lymph
node metastasis, recurrence, vascular/lymphatic invasion, tumor
size and peritoneal seeding (7,58).
CTHRC1 expression may also be related to the prognosis of patients
with GC, with higher CTHRC1 expression associated with a lower
survival rate of patients with GC (57,58).
Furthermore, a patient prognosis model based on CTHRC1 has been
developed for stomach adenocarcinoma (59).
Pancreatic cancer (PC)
Reverse transcription-PCR (RT-PCR) and
immunohistochemical analysis revealed that CTHRC1 mRNA and protein
levels were significantly increased in pancreatic ductal
adenocarcinoma compared with in normal pancreatic ductal epithelium
(42). Gene knock-in-and-out
analysis suggested that high expression of CTHRC1 induced apparent
metastatic spread to several secondary organs in PC, whereas
abolishing expression of CTHRC1 predominantly decreased primary
tumor progression and distant metastasis (42). By activating Src and ERK signaling
pathways, CTHRC1 can induce cytoskeletal recombination, lamellar
pseudopodia formation and sticky point increase in cell turnover in
an autocrine manner, and enhance the motility and adhesion of PC
cells (42). CTHRC1 can also
activate the ERK/AP-1 signaling pathway to promote Ang-2 secretion,
upregulate the Tie2 receptor ligand Ang-2 and lead to recruitment
of Tie2-expressing monocytes in tumor tissues to induce tumor
angiogenesis (42,43).
Hepatocellular carcinoma (HCC)
CTHRC1 expression in HCC tissues has been shown to
be significantly higher than in adjacent tissues, and the
expression level of CTHRC1 was associated with tumor size, extent
of vascular invasion, TNM stage and Barcelona clinic liver cancer
stage (60,61). CTHRC1 also performs a role in the
precancerous microenvironment of HCC (62). Studies have shown that CTHRC1 is an
independent factor affecting OS in HCC. The establishment of a HCC
survival prediction model showed that CTHRC1 was negatively
associated with patient prognosis, and was determined to be a key
factor in predicting HCC OS (61,63).
Patients with HCC with higher CTHRC1 expression had lower 10-year
OS and disease-free survival rates (64).
GIST
Immunohistochemical staining of GISTs have shown
that they exhibit increased CTHRC1 expression and microvascular
density (33). HUVEC migration and
invasion are increased in GISTs, suggesting that the overexpression
of CTHRC1 could promote tumor angiogenesis (33). RT-PCR, western blotting and
immunohistochemical analysis revealed that CTHRC1 expression was
closely associated with the risk grade of National Institutes of
Health classification and prognosis of GIST (37). Kaplan-Meier curve analysis showed
that CTHRC1 expression was negatively associated with the OS and
disease-free status (DFS) of patients with GIST (37).
Non-small-cell lung cancer
(NSCLC)
Proteomic analysis of multiple studies has shown
that CTHRC1 protein and mRNA levels are significantly overexpressed
in NSCLC tissues and cell lines compared with the levels in
adjacent non-cancerous tissues and normal lung epithelial cells
(65,66). CTHRC1 can activate c-Jun/MMP7,
c-Jun/MMP9 and NF-κB/MMP9 signaling to enhance the invasive ability
of NSCLC (66). Upregulation of
CTHRC1 has been reported to be significantly associated with
differentiation, TNM stage, lymph node status and smoking status,
and to strengthen the invasive ability, increase the colony
formation and increase the migration of NSCLC cells (65). In addition, the expression of CTHRC1
may be considered an independent prognostic factor for both OS and
DFS in NSCLC. A previous study showed that the diagnostic value of
CTHRC1 was only observed in patients with NSCLC with a history of
cigarette smoking, suggesting that CTHRC1 may interact with
tobacco-based compounds in the development of NSCLC (67).
Prostate cancer (PrCa)
Although CTHRC1 has been found to perform an
important role in proliferation and metastasis in a number of
tumors, its role in PrCa has not been fully elucidated. It has been
reported that CTHRC1 is significantly expressed in PrCa and plays
an important role in the tumor microenvironment, promoting the
proliferation and migration of PrCa cells and affecting the
prognosis and treatment of PrCa, and CTHRC1 has also been shown to
be related to the recurrence rate and survival rate of cancer
following treatment (29,68). In the early detection of PrCa by
ELISA, CTHRC1 has been shown to be a potential diagnostic marker
for PrCa, but further studies are needed to determine its clinical
utility as a diagnostic marker (69).
Conclusions
Cell specificity of CTHRC1
functioning
CTHRC1 has been reported to inhibit TGF-β signaling
in SMCs, whereas this function has not been detected in endothelial
cells (30). Overexpression of
CTHRC1 can reduce the mRNA expression levels of collagen type I and
decrease collagen deposition in SMCs, but not in osteoblasts
(1,30). Notably, CTHRC1 promoted the
migratory ability of SMCs in a wounded artery, but the function was
reversed in Schwann cells (49). In
addition, CTHRC1 can activate canonical Wnt/β-catenin signaling in
NSCLC, whereas it inhibits it in GIST (66). Knockdown of CTHRC1 had no effect on
the proliferation rate of PrCa cells, but significantly inhibited
the proliferation and survival of HCC cells (3,42).
Based on these findings, it is reasonable to assume that CTHRC1
functions with cellular specificity. It was previously reported
that CTHRC1 is a hormone that may regulate cell function in an
autocrine manner (42,45,49),
which suggest that the types of membrane-anchored receptors
specific to CTHRC1 may vary across cell types and cause differences
in signal transduction, ultimately leading to distinctive
outcomes.
Possible mechanism by which CTHRC1
regulates the migration and invasion of solid tumors
CTHRC1 has been reported to be associated with
multiple signaling cascades, suggesting that it performs a
functional role in the physical and biological behavior of cells.
However, CTHRC1 also promotes the carcinogenesis of solid tumors,
as outlined in a number of studies. The present review sought to
extract some possible mechanisms for how CTHRC1 is involved in
carcinogenesis. First, CTHRC1 was found to control collagen type I
deposition and thus regulate the formation of the ECM through
crosstalk with the TGF-β signaling pathway. The ECM has been shown
to be the basic skeleton of the tumor microenvironment, which
performs a pivotal role in promoting cancer cell survival, invasion
and metastasis (70,71). The suggestion that CTHRC1 regulates
the tumor microenvironment is strengthened by the phenomenon that
CTHRC1 expression is largely present in the interstitial region of
tumor cells, such as stromal cells, vessels and fibroblasts, which
are key components of the surrounding tumor environment. Second,
activation of the Wnt/PCP pathway by CTHRC1 contributes to the
migratory and invasive nature of tumors. The PCP signaling pathway
regulates actin polymerization and cytoskeletal reorganization,
which enables it to alter cellular morphology and increase cellular
motility (72–74). Third, loss of intercellular
adhesiveness, EMT, disruption of the basement membrane and
degradation of the ECM are the four phases influencing the mobility
and invasiveness of cancer cells (75). RT-PCR and immunoblotting indicated
that some EMT-associated molecules, such as MMP9 and vimentin, were
increased, whereas EMT inhibitory proteins, such as E-cadherin,
were decreased in a cell line that highly expressed CTHRC1.
Conversely, knockdown of CTHRC1 in this cell line markedly reversed
the effect (27,28,66).
Fourth, overexpression of CTHRC1 was shown to increase the
phosphorylation level of the proto-oncogene tyrosine-protein
kinases Src, FAK, MEK and ERK, which are key transmitters in
promoting the migratory and invasive ability of numerous types of
cancer (37,39,42).
The existence of a CTHRC1-related
regulatory feedback loop
The expression sites of CTHRC1 on a cellular level
were found to overlap considerably with those of TGF-β family
members and interstitial collagens (12), which suggests a close relationship
between CTHRC1 and TGF-β and ECM formation, as well as the pathway
by which CTHRC1 performs its role. As aforementioned, a negative
feedback regulatory loop possibly exists between CTHRC1 and TGF-β.
Here, this review provides a hypothesis for how this feedback loop
may work, although it is likely that the real network between these
two would be much more complex. During the reparative process of
tissue injury, TGF-β not only induces the production of collagen
and ECM deposition through the activation of Smad-dependent
signaling, but also simultaneously stimulates the expression of
CTHRC1. With the negative feedback loop mechanism, the activity of
TGF-β signaling transduction is accurately controlled by elevated
levels of CTHRC1 in itself so that injured tissue can avoid excess
repair; thus, the tissue normality is preserved. Nevertheless,
in vivo, the expression of CTHRC1 may be delicately
controlled by a variety of genetic signaling pathways rather than a
single factor, which may also contribute to a series of
pathological statuses, for example, luminal constriction during
artery remodeling after injury and the occurrence of keloids during
wound healing of the skin.
A study regarding the involvement of CTHRC1 in the
development of Barrett's esophagus and esophageal adenocarcinoma
revealed that CTHRC1 is expressed in tissue repair processes.
However, when CTHRC1 mutations occur, CTHRC1 loses its ability to
form secondary structures and helix-loop-helix structures;
therefore, collagen deposition, fibrosis and TGF-β/WNT signaling
pathway involvement are disrupted so that the esophagus cannot be
correctly repaired. This promotes gastroesophageal reflux disease,
which may lead to an increased risk of developing esophageal
adenocarcinoma (76). The negative
feedback loop implies that the dynamic balance between TGF-β and
CTHRC1 is a physiological behavior important in maintaining the
morphology and homeostasis of tissue organisms.
Furthermore, in HBV-associated HCC, overexpression
of CTHRC1 enhanced phospho-AKT in a concentration-dependent manner
(19). AKT has been recognized as a
key factor in the PI3K/AKT signaling cascade for regulating the
activation of NF-κB (77,78). NF-κB has also been reported to
elevate the expression of CTHRC1 (19). Therefore, in contrast to the
relationship between CTHRC1 and the TGF-β signaling cascade, it is
assumed that a positive feedback regulatory loop might be present
among CTHRC1, AKT and NF-κB for promoting the carcinogenesis of
HCC; however the mechanism needs to be further explored.
In summary, CTHRC1 not only participates in the
physical and biological activity of normal cells, but also the
carcinogenesis of various human solid tumors, dependent on the
crosstalk with multiple cell signal transduction pathways. This may
allow for the development of new therapies to treat related
diseases by targeting CTHRC1.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
QZ and YJL completed the writing and revision of the
first draft, and JD, JL and XPT all participated in the revision of
the article. All authors read and approved the final version of the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pyagay P, Heroult M, Wang Q, Lehnert W,
Belden J, Liaw L, Friesel RE and Lindner V: Collagen triple helix
repeat containing 1, a novel secreted protein in injured and
diseased arteries, inhibits collagen expression and promotes cell
migration. Circ Res. 96:261–268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leclère L, Nir TS, Bazarsky M, Braitbard
M, Schneidman-Duhovny D and Gat U: Dynamic evolution of the Cthrc1
genes, a newly defined collagen-like family. Genome Biol Evol.
12:3957–3970. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tameda M, Sugimoto K, Shiraki K, Yamamoto
N, Okamoto R, Usui M, Ito M, Takei Y, Nobori T, Kojima T, et al:
Collagen triple helix repeat containing 1 is overexpressed in
hepatocellular carcinoma and promotes cell proliferation and
motility. Int J Oncol. 45:541–548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
LeClair R and Lindner V: The role of
collagen triple helix repeat containing 1 in injured arteries,
collagen expression, and transforming growth factor beta signaling.
Trends Cardiovasc Med. 17:202–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leclair RJ, Wang Q, Benson MA, Prudovsky I
and Lindner V: Intracellular localization of Cthrc1 characterizes
differentiated smooth muscle. Arterioscler Thromb Vasc Biol.
28:1332–1338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamamoto S, Nishimura O, Misaki K, Nishita
M, Minami Y, Yonemura S, Tarui H and Sasaki H: Cthrc1 selectively
activates the planar cell polarity pathway of Wnt signaling by
stabilizing the Wnt-receptor complex. Dev Cell. 15:23–36. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu Z, Xu J, Li L, Ye W, Chen B, Zeng J
and Huang Z: Comprehensive analysis reveals CTHRC1, SERPINE1, VCAN
and UPK1B as the novel prognostic markers in gastric cancer. Transl
Cancer Res. 9:4093–4110. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sial N, Ahmad M, Hussain MS, Iqbal MJ,
Hameed Y, Khan M, Abbas M, Asif R, Rehman JU, Atif M, et al: CTHRC1
expression is a novel shared diagnostic and prognostic biomarker of
survival in six different human cancer subtypes. Sci Rep.
11:198732021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsukui T, Sun KH, Wetter JB,
Wilson-Kanamori JR, Hazelwood LA, Henderson NC, Adams TS, Schupp
JC, Poli SD, Rosas IO, et al: Collagen-producing lung cell atlas
identifies multiple subsets with distinct localization and
relevance to fibrosis. Nat Commun. 11:19202020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ruiz-Villalba A, Romero JP, Hernández SC,
Vilas-Zornoza A, Fortelny N, Castro-Labrador L, San Martin-Uriz P,
Lorenzo-Vivas E, García-Olloqui P, Palacio M, et al: Single-cell
RNA sequencing analysis reveals a crucial Role for CTHRC1 (collagen
triple helix repeat containing 1) cardiac fibroblasts after
myocardial infarction. Circulation. 142:1831–1847. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou F, Shen D, Xiong Y, Cheng S, Xu H,
Wang G, Qian K, Ju L and Zhang X: CTHRC1 is a prognostic biomarker
and correlated with immune infiltrates in kidney renal papillary
cell carcinoma and kidney renal clear cell carcinoma. Front Oncol.
10:5708192021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Durmus T, LeClair RJ, Park KS, Terzic A,
Yoon JK and Lindner V: Expression analysis of the novel gene
collagen triple helix repeat containing-1 (Cthrc1). Gene Expr
Patterns. 6:935–940. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duarte CW, Stohn JP, Wang Q, Emery IF,
Prueser A and Lindner V: Elevated plasma levels of the pituitary
hormone Cthrc1 in individuals with red hair but not in patients
with solid tumors. PLoS One. 9:e1004492014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang L, Dai DL, Su M, Martinka M, Li G and
Zhou Y: Aberrant expression of collagen triple helix repeat
containing 1 in human solid cancers. Clin Cancer Res. 12:3716–3722.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Cao J, Li M, Yu Y, Yang Y, Xiao X,
Wu Z, Wang L, Tu Y and Chen H: Collagen triple helix repeat
containing-1 inhibits transforming growth factor-b1-induced
collagen type I expression in keloid. Br J Dermatol. 164:1030–1036.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bian Z, Miao Q, Zhong W, Zhang H, Wang Q,
Peng Y, Chen X, Guo C, Shen L, Yang F, et al: Treatment of
cholestatic fibrosis by altering gene expression of Cthrc1:
Implications for autoimmune and non-autoimmune liver disease. J
Autoimmun. 63:76–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mendelsohn RD, Helmerhorst EJ, Cipollo JF
and Kukuruzinska MA: A hypomorphic allele of the first
N-glycosylation gene, ALG7, causes mitochondrial defects in yeast.
Biochim Biophys Acta. 1723:33–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu G, Sengupta PK, Jamal B, Yang HY,
Bouchie MP, Lindner V, Varelas X and Kukuruzinska MA:
N-glycosylation induces the CTHRC1 protein and drives oral cancer
cell migration. J Biol Chem. 288:20217–20227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang R, Cao Y, Bai L, Zhu C, Li R, He H,
Liu Y, Wu K, Liu F and Wu J: The collagen triple helix repeat
containing 1 facilitates hepatitis B virus-associated
hepatocellular carcinoma progression by regulating multiple
cellular factors and signal cascades. Mol Carcinog. 54:1554–1566.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bai L, Zhang W, Tan L, Yang H, Ge M, Zhu
C, Zhang R, Cao Y, Chen J, Luo Z, et al: Hepatitis B virus hijacks
CTHRC1 to evade host immunity and maintain replication. J Mol Cell
Biol. 7:543–556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cadigan KM and Nusse R: Wnt signaling: A
common theme in animal development. Genes Dev. 11:3286–3305. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han W, Cui H, Liang J and Su X: Role of
MicroRNA-30c in cancer progression. J Cancer. 11:2593–2601. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lai YH, Chen J, Wang XP, Wu YQ, Peng HT,
Lin XH and Wang WJ: Collagen triple helix repeat containing-1
negatively regulated by microRNA-30c promotes cell proliferation
and metastasis and indicates poor prognosis in breast cancer. J Exp
Clin Cancer Res. 36:922017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xi C, Wang J, Sun H, Zhang X and Kang H:
RETRACTED: Loss of microRNA-30e induced by extracellular vesicles
from cancer-associated fibroblasts promotes breast cancer
progression by binding to CTHRC1. Exp Mol Pathol. 118:1045862021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu J, Feng J, Zhi X, Tang J, Li Z, Xu Y,
Yang L, Hu Z and Xu Z: Let-7b inhibits cell proliferation,
migration, and invasion through targeting Cthrc1 in gastric cancer.
Tumour Biol. 36:3221–3229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Fu Y, Gao Y, Li H, Ma L, Shu C, Li N
and Ma C: microRNA-134 inhibits melanoma growth and metastasis by
negatively regulating collagen triple helix repeat containing-1
(CTHRC1). Int J Clin Exp Pathol. 11:4319–4330. 2018.PubMed/NCBI
|
|
27
|
Yuan K, Sun Y and Ji Y: miR-509-3p
suppresses migration, invasion, and epithelial-mesenchymal
transition in melanoma cells by targeting collagen triple helix
repeat containing 1. Balkan Med J. 38:177–182. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Zhou J, Wang J, Chen X, Zhu Y and
Chen Y: Mir-30b-3p affects the migration and invasion function of
ovarian cancer cells by targeting the CTHRC1 gene. Biol Res.
53:102020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma Z, Chao F, Wang S, Song Z, Zhuo Z,
Zhang J, Xu G and Chen G: CTHRC1 affects malignant tumor cell
behavior and is regulated by miR-30e-5p in human prostate cancer.
Biochem Biophys Res Commun. 525:418–424. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
LeClair RJ, Durmus T, Wang Q, Pyagay P,
Terzic A and Lindner V: Cthrc1 is a novel inhibitor of transforming
growth factor-beta signaling and neointimal lesion formation. Circ
Res. 100:826–833. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qin S, Zheng JH, Xia ZH, Qian J, Deng CL
and Yang SL: CTHRC1 promotes wound repair by increasing M2
macrophages via regulating the TGF-β and notch pathways. Biomed
Pharmacother. 113:1085942019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kelley MW: Leading Wnt down a PCP path:
Cthrc1 acts as a coreceptor in the Wnt-PCP pathway. Dev Cell.
15:7–8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fu SW, Chen HY, Lin XL, Yang L and Ge ZZ:
Collagen triple helix repeat containing 1 promotes tumor
angiogenesis in gastrointestinal stromal tumors. Oncol Lett.
14:7499–7505. 2017.PubMed/NCBI
|
|
34
|
Zheng M, Zhou Q, Liu X, Wang C and Liu G:
CTHRC1 overexpression promotes cervical carcinoma progression by
activating the Wnt/PCP signaling pathway. Oncol Rep. 41:1531–1538.
2019.PubMed/NCBI
|
|
35
|
Li Y, Xing BX, Wang YH, Yu S, Zhao H, Lv
QQ and Lu CX: CTHRC1 promotes growth, migration and invasion of
trophoblasts via reciprocal Wnt/β-catenin regulation. J Cell Commun
Signal. 16:63–74. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li H, Liu W, Zhang X and Wang Y:
Cancer-associated fibroblast-secreted collagen triple helix repeat
containing-1 promotes breast cancer cell migration, invasiveness
and epithelial-mesenchymal transition by activating the
Wnt/β-catenin pathway. Oncol Lett. 22:8142021.PubMed/NCBI
|
|
37
|
Ma MZ, Zhuang C, Yang XM, Zhang ZZ, Ma H,
Zhang WM, You H, Qin W, Gu J, Yang S, et al: CTHRC1 acts as a
prognostic factor and promotes invasiveness of gastrointestinal
stromal tumors by activating Wnt/PCP-Rho signaling. Neoplasia.
16:265–278. 278.e1–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kimball AS, Joshi AD, Boniakowski AE,
Schaller M, Chung J, Allen R, Bermick J, Carson WF IV, Henke PK,
Maillard I, et al: Notch regulates macrophage-mediated inflammation
in diabetic wound healing. Front Immunol. 8:6352017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang C, Li Z, Shao F, Yang X, Feng X, Shi
S, Gao Y and He J: High expression of Collagen Triple Helix Repeat
Containing 1 (CTHRC1) facilitates progression of oesophageal
squamous cell carcinoma through MAPK/MEK/ERK/FRA-1 activation. J
Exp Clin Cancer Res. 36:842017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu X, Bian Y, Wen X, Wang M, Li Y and Wan
X: Collagen triple helix repeat containing 1 promotes endometrial
cancer cell migration by activating the focal adhesion kinase
signaling pathway. Exp Ther Med. 20:1405–1414. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo B, Yan H, Li L, Yin K, Ji F and Zhang
S: Collagen triple helix repeat containing 1 (CTHRC1) activates
Integrin β3/FAK signaling and promotes metastasis in ovarian
cancer. J Ovarian Res. 10:692017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Park EH, Kim S, Jo JY, Kim SJ, Hwang Y,
Kim JM, Song SY, Lee DK and Koh SS: Collagen triple helix repeat
containing-1 promotes pancreatic cancer progression by regulating
migration and adhesion of tumor cells. Carcinogenesis. 34:694–702.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee J, Song J, Kwon ES, Jo S, Kang MK, Kim
YJ, Hwang Y, Bae H, Kang TH, Chang S, et al: CTHRC1 promotes
angiogenesis by recruiting Tie2-expressing monocytes to pancreatic
tumors. Exp Mol Med. 48:e2612016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Spector I, Zilberstein Y, Lavy A, Genin O,
Barzilai-Tutsch H, Bodanovsky A, Halevy O and Pines M: The
involvement of collagen triple helix repeat containing 1 in
muscular dystrophies. Am J Pathol. 182:905–916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stohn JP, Perreault NG, Wang Q, Liaw L and
Lindner V: Cthrc1, a novel circulating hormone regulating
metabolism. PLoS One. 7:e471422012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kimura H, Kwan KM, Zhang Z, Deng JM,
Darnay BG, Behringer RR, Nakamura T, de Crombrugghe B and Akiyama
H: Cthrc1 is a positive regulator of osteoblastic bone formation.
PLoS One. 3:e31742008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Takeshita S, Fumoto T, Matsuoka K, Park
KA, Aburatani H, Kato S, Ito M and Ikeda K: Osteoclast-secreted
CTHRC1 in the coupling of bone resorption to formation. J Clin
Invest. 123:3914–3924. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Parrinello S, Napoli I, Ribeiro S,
Wingfield Digby P, Fedorova M, Parkinson DB, Doddrell RD, Nakayama
M, Adams RH and Lloyd AC: EphB signaling directs peripheral nerve
regeneration through Sox2-dependent Schwann cell sorting. Cell.
143:145–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Apra C, Richard L, Coulpier F, Blugeon C,
Gilardi-Hebenstreit P, Vallat JM, Lindner V, Charnay P and Decker
L: Cthrc1 is a negative regulator of myelination in Schwann cells.
Glia. 60:393–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pan B, Shi ZJ, Yan JY, Li JH and Feng SQ:
Long non-coding RNA NONMMUG014387 promotes Schwann cell
proliferation after peripheral nerve injury. Neural Regen Res.
12:2084–2091. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou S, Gao R, Hu W, Qian T, Wang N, Ding
G, Ding F, Yu B and Gu X: MiR-9 inhibits Schwann cell migration by
targeting Cthrc1 following sciatic nerve injury. J Cell Sci.
127:967–976. 2014.PubMed/NCBI
|
|
52
|
Li Y, Zhang Y, Ma C, Wang S, Li N, Wang J,
Ma G and Zhang L: Overexpression of CTHRC1 in human melanoma
promotes tumorigenesis targeted by miRNA155. Int J Clin Exp Pathol.
10:8199–8210. 2017.PubMed/NCBI
|
|
53
|
Kim JH, Baek TH, Yim HS, Kim KH, Jeong SH,
Kang HB, Oh SS, Lee HG, Kim JW and Kim KD: Collagen triple helix
repeat containing-1 (CTHRC1) expression in invasive ductal
carcinoma of the breast: the impact on prognosis and correlation to
clinicopathologic features. Pathol Oncol Res. 19:731–737. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ni S, Ren F, Xu M, Tan C, Weng W, Huang Z,
Sheng W and Huang D: CTHRC1 overexpression predicts poor survival
and enhances epithelial-mesenchymal transition in colorectal
cancer. Cancer Med. 7:5643–5654. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang XL, Hu LP, Yang Q, Qin WT, Wang X,
Xu CJ, Tian GA, Yang XM, Yao LL, Zhu L, et al: CTHRC1 promotes
liver metastasis by reshaping infiltrated macrophages through
physical interactions with TGF-β receptors in colorectal cancer.
Oncogene. 40:3959–3973. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ding X, Huang R, Zhong Y, Cui N, Wang Y,
Weng J, Chen L and Zang M: CTHRC1 promotes gastric cancer
metastasis via HIF-1α/CXCR4 signaling pathway. Biomed Pharmacother.
123:1097422020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wei G, Dong Y, He Z, Qiu H, Wu Y and Chen
Y: Identification of hub genes and construction of an
mRNA-miRNA-lncRNA network of gastric carcinoma using integrated
bioinformatics analysis. PLoS One. 16:e02617282021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gu L, Liu L, Zhong L, Bai Y, Sui H, Wei X,
Zhang W, Huang P, Gao D, Kong Y and Lou G: Cthrc1 overexpression is
an independent prognostic marker in gastric cancer. Hum Pathol.
45:1031–1038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang T, Wen W, Liu H, Zhang J, Zhang X and
Wang Y: Development and validation of a novel prognosis prediction
model for patients with stomach adenocarcinoma. Front Med
(Lausanne). 8:7934012021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen G, Wang D, Zhao X, Cao J, Zhao Y,
Wang F, Bai J, Luo D and Li L: miR-155-5p modulates malignant
behaviors of hepatocellular carcinoma by directly targeting CTHRC1
and indirectly regulating GSK-3β-involved Wnt/β-catenin signaling.
Cancer Cell Int. 17:1182017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhou H, Su L, Liu C, Li B, Li H, Xie Y and
Sun D: CTHRC1 may serve as a prognostic biomarker for
hepatocellular carcinoma. Onco Targets Ther. 12:7823–7831. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li J, Wang Y, Ma M, Jiang S, Zhang X,
Zhang Y, Yang X, Xu C, Tian G, Li Q, et al: Autocrine CTHRC1
activates hepatic stellate cells and promotes liver fibrosis by
activating TGF-β signaling. EBioMedicine. 40:43–55. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xu YJ, He MK, Liu S, Huang LC, Bu XY, Kan
A and Shi M: Construction of a single nucleotide variant
score-related gene-based prognostic model in hepatocellular
carcinoma: Analysis of multi-independent databases and validation
in vitro. Cancer Cell Int. 21:6102021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Peng D, Wei C, Zhang X, Li S, Liang H,
Zheng X, Jiang S and Han L: Pan-cancer analysis combined with
experiments predicts CTHRC1 as a therapeutic target for human
cancers. Cancer Cell Int. 21:5662021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ke Z, He W, Lai Y, Guo X, Chen S, Li S,
Wang Y and Wang L: Overexpression of collagen triple helix repeat
containing 1 (CTHRC1) is associated with tumour aggressiveness and
poor prognosis in human non-small cell lung cancer. Oncotarget.
5:9410–9424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
He W, Zhang H, Wang Y, Zhou Y, Luo Y, Cui
Y, Jiang N, Jiang W, Wang H, Xu D, et al: CTHRC1 induces non-small
cell lung cancer (NSCLC) invasion through upregulating MMP-7/MMP-9.
BMC Cancer. 18:4002018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu X, Liu B, Cui Y, Wang F, Sun H and Lv
F: Collagen triple helix repeat containing 1 (Cthrc1) is an
independently prognostic biomarker of non-small cell lung cancers
with cigarette smoke. Tumour Biol. 35:11677–11683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhou Q, Xiong W, Zhou X, Gao RS, Lin QF,
Liu HY, Li JN and Tian XF: CTHRC1 and PD-1/PD-L1 expression
predicts tumor recurrence in prostate cancer. Mol Med Rep.
20:4244–4252. 2019.PubMed/NCBI
|
|
69
|
Bacolod MD and Barany F: A unified
transcriptional, pharmacogenomic, and gene dependency approach to
decipher the biology, diagnostic markers, and therapeutic targets
associated with prostate cancer metastasis. Cancers (Basel).
13:51582021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Neophytou CM, Panagi M, Stylianopoulos T
and Papageorgis P: The role of tumor microenvironment in cancer
metastasis: Molecular mechanisms and therapeutic opportunities.
Cancers (Basel). 13:20532021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cox TR: The matrix in cancer. Nat Rev
Cancer. 21:217–238. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang Y: Wnt/Planar cell polarity
signaling: A new paradigm for cancer therapy. Mol Cancer Ther.
8:2103–2109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wansleeben C and Meijlink F: The planar
cell polarity pathway in vertebrate development. Dev Dyn.
240:616–626. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Babayeva S, Zilber Y and Torban E: Planar
cell polarity pathway regulates actin rearrangement, cell shape,
motility, and nephrin distribution in podocytes. Am J Physiol Renal
Physiol. 300:F549–F560. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Orloff M, Peterson C, He X, Ganapathi S,
Heald B, Yang YR, Bebek G, Romigh T, Song JH, Wu W, et al: Germline
mutations in MSR1, ASCC1, and CTHRC1 in patients with Barrett
esophagus and esophageal adenocarcinoma. JAMA. 306:410–419. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Buhrmann C, Mobasheri A, Busch F, Aldinger
C, Stahlmann R, Montaseri A and Shakibaei M: Curcumin modulates
nuclear factor kappaB (NF-kappaB)-mediated inflammation in human
tenocytes in vitro: Role of the phosphatidylinositol 3-kinase/Akt
pathway. J Biol Chem. 286:28556–28566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Caporali S, Levati L, Graziani G, Muzi A,
Atzori MG, Bonmassar E, Palmieri G, Ascierto PA and D'Atri S: NF-κB
is activated in response to temozolomide in an AKT-dependent manner
and confers protection against the growth suppressive effect of the
drug. J Transl Med. 10:2522012. View Article : Google Scholar : PubMed/NCBI
|















