Introduction
Anaplastic large cell lymphoma (ALCL) is a rare type of peripheral/mature T-cell lymphoma with high heterogeneity, accounting for ~15% of T cell lymphoma cases and 1–3% of all non-Hodgkin's lymphomas cases (1,2). According to the recent World Health Organization (WHO) classification, ALCL is detailly categorized into anaplastic lymphoma kinase (ALK) positive ALCL (ALK+ ALCL), ALK-negative ALCL (ALK− ALCL), primary cutaneous ALCL (pcALCL) and breast-implant associated ALCL (BIA-ALCL) (3). Among the above ALCL subtypes, ALK+ ALCL exhibits a relatively better prognosis compared with the other types, such as ALK− ALCL, with a 5-year overall survival (OS) rate of >80% (4). However, 30–40% of patients with ALK+ ALCL will develop a relapsed or refractory (R/R) disease to cytotoxic drugs, thus leading to relatively poor outcomes. Currently, the salvage treatment options for R/R ALK+ ALCL are not sufficient (5,6).
Arsenic trioxide (ATO), a Chinese traditional medicine, has attracted increasing attention globally due to its excellent anti-cancer effects (7,8). Currently, ATO is commonly used to treat several types of cancer, such as hepatocellular carcinoma, cervical cancer and leukemias (9–11). Regarding the effect of ATO on lymphoma treatment, it has been reported that ATO exerts its anti-tumor activity via several ways. Therefore, previous studies showed that ATO could induce lymphoma cell apoptosis via inactivating the Wnt/β-catenin and nuclear factor-κB (NF-κB) pathways (12,13), arresting lymphoma cell cycle (14), inhibiting mitochondrial activity and angiogenesis (14), modulating autophagy (15) and cooperating with other cytotoxic agents (16). Interestingly, another study revealed that ATO could inactivate ALK-fusion oncoprotein to repress ALK+ diffuse large B-cell lymphoma cell viability (17). More particularly, ATO could target the nucleophosmin-ALK fusion protein to promote the apoptosis of ALK+ ALCL cells (18). The above findings could provide evidence for uncovering the potency of ATO in treating R/R ALK+ ALCL. However, relevant data are still missing.
Therefore, the current study aimed to compare the efficacy and safety of ATO plus etoposide, solumedrol, high-dose cytarabine (ara-C) and cisplatin (ESHAP) chemotherapy with ESHAP chemotherapy alone for the treatment of patients with R/R ALK+ ALCL.
Materials and methods
Patients
A total of 24 patients with R/R ALK+ ALCL were enrolled between January 2018 and June 2022 in this prospective, cohort study. The inclusion criteria were as follows: i) Patients histologically diagnosed as ALCL; ii) confirmed as ALK+ ALCL by Ventana immunohistochemistry assay; iii) diagnosed as R/R ALCL patients, where refractory ALCL was defined as progression during the first-line treatment, and relapsed ALCL was defined as initially reaching CR, then disease occurred again confirmed by biopsy examination; iv) aged >18 years old; and v) treated with ESHAP alone or ATO plus ESHAP. The exclusion criteria were the following: i) Newly diagnosed ALCL patients; ii) patients with a prior history of hematopoietic stem cell transplantation; iii) with other malignant diseases; iv) without available clinical data for study analysis; and v) pregnant or breastfeeding female patients. Notably, the enrolled patients do not fit the stem cell transplantation in this study, therefore, no related transplantation information is available. The following ALCL patients were considered as not suitable for stem cell transplantation: i) Elderly patients with poor physical condition. ii) Donor mismatch. iii) Patients with high allergic constitution or severe allergic history. iv) Complicated with heart, lung, liver, kidney, and other important organ dysfunction even after treatment. v) Coagulation dysfunction such as hemophilia. vi) Serological test positive such as AIDS, syphilis and so on. vii) Chromosome or gene defects, immune function defects. viii) Patients unwilling to receive stem cell transplantation. The study was approved by the Ethics Committee of Tongji Hospital with approval number Ethical Review-KYSB-2018-139, Tongji University School of Medicine; and informed consent was obtained from all patients before the start of this prospective study. The enrolled patients were not involved in other clinical trial.
Data collection and treatment
The clinical characteristics of patients with R/R ALK+ ALCL were recorded after inclusion. The present study did not intervene in the treatment of patients. Patients were treated with ESHAP alone or ATO plus ESHAP based on patients' disease condition, benefit expectation, and patients' willingness. Generally, patients with higher disease state and acceptable physical condition (ECOG PS) tended to choose the ATO plus ESHAP treatment, others tended to choose ESHAP treatment. Patients treated with ESHAP alone were included in the ESHAP group, while those treated with ATO plus ESHAP chemotherapy in the ATO plus ESHAP group. Each treatment cycle lasted four weeks. Patients treated with the ESHAP regimen received etoposide (40 mg/m2; 1st-4th day), methylprednisolone (500 mg; 1st-5th day), ara-C (2 g/m2; 5th day) and cisplatin (25 mg/m2; 1st-4th day), as previously described (19). ATO (10 mg; 5 days/week) was administrated by intravenous injection (20). ATO has been approved to treat R/R lymphoma in China, which includes R/R ALCL.
Evaluation
Treatment response was evaluated after treatment based on the computed tomography scan results according to the Revised Response Criteria for Malignant Lymphoma (21). Additionally, the survival data of patients were also recorded. The last date of follow-up was November 30, 2022. Subsequently, event-free survival (EFS) and OS were calculated. Finally, the data on adverse events (AEs) were acquired and graded using the Common Terminology Criteria for Adverse Events v4.03.
Statistical analysis
All statistical analyses were performed using SPSS v22.0 software (IBM Corp.). GraphPad Prism v7.0 software (GraphPad Software, Inc.) was utilized for graph construction. The comparisons between the ESHAP and ATO plus ESHAP groups were performed using t-test, Wilcoxon rank sum test, χ2 test and Fisher's exact test. The association between the treatment regimen and EFS and OS was evaluated using Kaplan-Meier curves and analyzed with a log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
Patients' characteristics
Patients with R/R ALK+ ALCL in the ATO plus ESHAP group (n=11) exhibited an average age of 31.0±10.7 years, including 81.8% male and 18.2% female patients. Among all patients, 72.7% suffered from refractory disease, while 27.3% from relapsed disease. In terms of patients with R/R ALK+ ALCL in the ESHAP group (n=13), their average age was 32.8±12.8 years, including 69.2 and 30.8% male and female patients, respectively. The majority of the above patients (53.8%) were diagnosed with refractory disease, while the remaining 46.2% with relapsed disease. 10 (76.9%), 2 (15.4%), and 1 (7.7%) patient received first-line CHOP, EPOCH, and ESHAP in the ESHAP group, respectively; then 6 (54.5%), 4 (36.4%), and 1 (9.1%) patient received first-line CHOP, EPOCH, and ESHAP in the ATO plus ESHAP group, respectively; besides, no difference was observed regarding the first line treatment options between the two groups (P=0.470). No differences were observed in the patients' baseline characteristics between the ESHAP and ATO plus ESHAP groups (all P>0.05; Table I).
 |
Table I.
Clinical characteristics of patients with R/R ALK+ ALCL.
|
Table I.
Clinical characteristics of patients with R/R ALK+ ALCL.
| Items |
ESHAP group (n=13) |
ATO plus ESHAP group (n=11) |
P-value |
| Age (years), mean ± SD |
32.8±12.8 |
31.0±10.7 |
0.709 |
| Sex, no. (%) |
|
|
0.649 |
| Female |
4 (30.8) |
2 (18.2) |
|
| Male |
9 (69.2) |
9 (81.8) |
|
| Ann Arbor stage, no. (%) |
|
|
0.755 |
| I–II |
3 (23.1) |
3 (27.3) |
|
| III |
7 (53.8) |
4 (36.4) |
|
| IV |
3 (23.1) |
4 (36.4) |
|
| Disease status, no. (%) |
|
|
0.423 |
| Relapsed |
6 (46.2) |
3 (27.3) |
|
| Refractory |
7 (53.8) |
8 (72.7) |
|
| ECOG performance status, no. (%) |
|
|
0.188 |
| 0 |
3 (23.1) |
4 (36.4) |
|
| 1 |
7 (53.8) |
7 (63.6) |
|
| 2 |
3 (23.1) |
0 (0.0) |
|
| B symptoms, no. (%) |
|
|
1.000 |
| No |
4 (30.8) |
4 (36.4) |
|
| Yes |
9 (69.2) |
7 (63.6) |
|
| Previous chemotherapy, no. (%) |
|
|
1.000 |
| No |
0 (0.0) |
0 (0.0) |
|
| Yes |
13 (100.0) |
11 (100.0) |
|
| First-line chemotherapy regimen, no. (%) |
|
|
0.470 |
| CHOP |
10 (76.9) |
6 (54.5) |
|
| EPOCH |
2 (15.4) |
4 (36.4) |
|
| ESHAP |
1 (7.7) |
1 (9.1) |
|
| Previous radiation, no. (%) |
|
|
0.199 |
| No |
13 (100.0) |
9 (81.8) |
|
| Yes |
0 (0.0) |
2 (18.2) |
|
| Previous HSCT, no. (%) |
|
|
1.000 |
| No |
13 (100.0) |
11 (100.0) |
|
| Yes |
0 (0.0) |
0 (0.0) |
|
Treatment response
In detail, 72.7, 9.1, 0.0 and 18.2% of patients achieved complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD), respectively, in the ATO plus ESHAP group. Additionally, in the ESHAP group, 53.8, 15.4, 0.0 and 30.8% of patients achieved CR, PR, SD and PD, respectively (Fig. 1A). Furthermore, CR rate (72.7% vs. 53.8%; P=0.423) and objective response rate (ORR; 81.8% vs. 69.2%; P=0.649) were higher in the ATO plus ESHAP group compared with the ESHAP group. However, no statistically significant difference was observed (Fig. 1B and C).
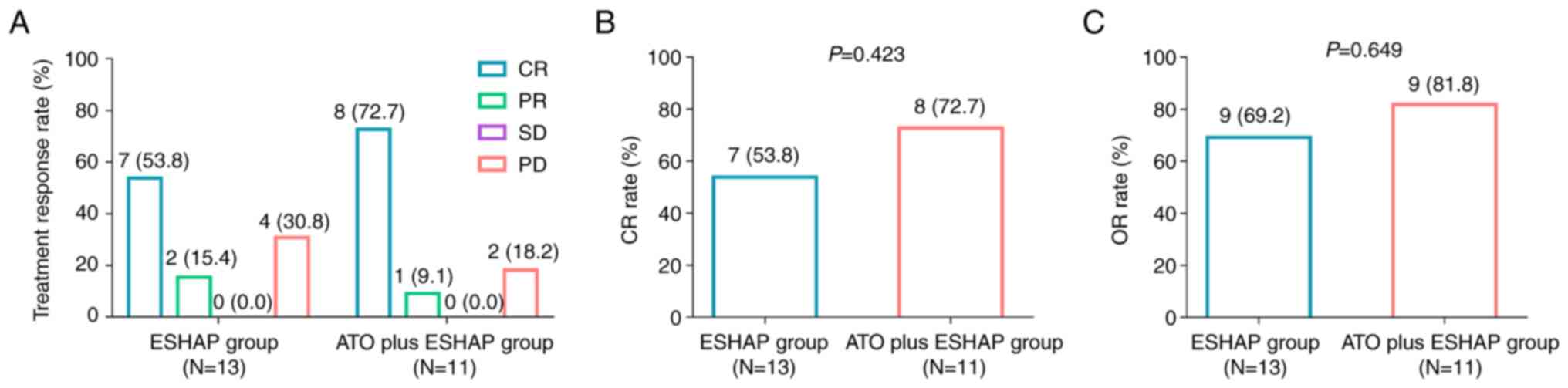 |
Figure 1.
Comparison of treatment response between groups. Comparison of (A) treatment response rate by Wilcoxon rank sum test, (B) CR rate and (C) OR rate by χ2 test between patients in the ESHAP group and those in the arsenic trioxide plus ESHAP group. ESHAP, etoposide, solumedrol, high-dose cytarabine and cisplatin; CR, complete response; OR, objective response; ATO, arsenic trioxide; PR, partial response; SD, stable disease; PD, progressive disease.
|
EFS and OS assessment
Encouragingly, EFS was prolonged in the ATO plus ESHAP group compared with the ESHAP group (P=0.047; Fig. 2A). More particularly, the 1-, 2- and 3-year accumulating EFS rates were 71.6, 59.7 and 59.7%, respectively, in the ATO plus ESHAP group. In the ESHAP group, the 1-, 2- and 3-year accumulating EFS rates were 46.2, 27.7 and 13.8%, respectively. Regarding OS rate, the results showed that it was higher in the ATO plus ESHAP group compared with the ESHAP group. However, there was not a statistically significant difference (P=0.261; Fig. 2B). Furthermore, the 1-, 2- and 3-year accumulating OS rates were 90.0, 77.1 and 77.1%, respectively, in the ATO plus ESHAP group, while the 1-, 2- and 3-year accumulating OS rates in the ESHAP group were 76.9, 59.8 and 59.8%, respectively.
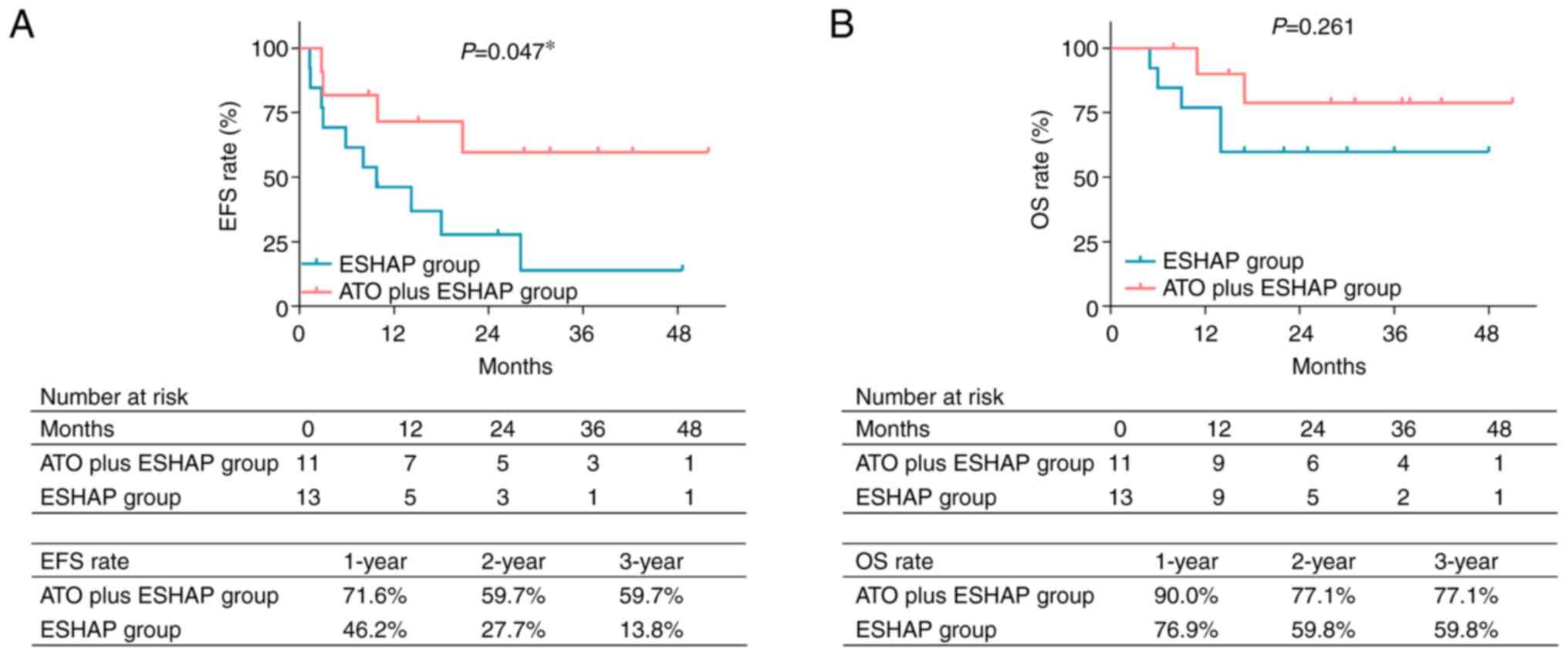 |
Figure 2.
Comparison of survival between groups. Comparison of (A) accumulating EFS rate and (B) accumulating OS rate between patients in the ESHAP group and those in the arsenic trioxide plus ESHAP group by log-rank test. *P<0.05. ESHAP, etoposide, solumedrol, high-dose cytarabine and cisplatin; EFS, event-free survival; ATO, arsenic trioxide; OS, overall survival.
|
Subgroup analyses
In patients with relapsed ALK+ ALCL, CR rate was higher in the ATO plus ESHAP group compared with the ESHAP group. However, there was not a statistically significant difference (100.0% vs. 66.7%; P=0.500; Fig. 3A). In addition, no difference was observed in ORR rate between both groups (100.0% vs. 100.0%; P=1.000; Fig. 3B). Consistently, no differences were obtained in the EFS (P=0.249) and OS (P=0.480) rates between the ATO plus ESHAP group and the ESHAP group (Fig. 3C and D). In patients with refractory ALK+ ALCL, CR (62.5% vs. 42.9%; P=0.619), ORR (75.0% vs. 42.9%; P=0.315), EFS (P=0.145) and OS (P=0.131) rates were more favorable in the ATO plus ESHAP group compared with the ESHAP group. However, this trend did not reach statistical significance (Fig. 3E-H).
 |
Figure 3.
Subgroup analysis of treatment response and survival. Comparison of (A) CR rate, (B) OR rate, (C) accumulating EFS rate and (D) accumulating OS rate between groups of patients with relapsed ALK+ ALCL. Comparison of (E) CR, (F) OR rate, (G) accumulating EFS and (H) accumulating OS rates between groups of patients with refractory ALK+ ALCL. CR and ORR were compared using χ2 test, accumulating EFS and OS rates were compared using log-rank test. CR, complete response; OR, objective response; EFS, event-free survival; OS, overall survival; ALK+ ALCL, anaplastic lymphoma kinase-positive anaplastic large cell lymphoma; ATO, arsenic trioxide.
|
Safety
Generally, the majority of AEs, including thrombocytopenia (81.8% vs. 46.2%; P=0.105), neutropenia (81.8% vs. 53.8%; P=0.211), fever (81.8% vs. 46.2%; P=0.105), anorexia (72.7% vs. 46.2%; P=0.240), nausea and vomiting (63.6% vs. 38.5%; P=0.219) and dyspnea (36.4% vs. 15.4%; P=0.182), were more prevalent in the ATO plus ESHAP group compared with the ESHAP group. However, no statistically significant differences were obtained (Table II). In terms of grade 3–4 AEs, thrombocytopenia (36.4% vs. 7.7%; P=0.142) and neutropenia (45.5% vs. 15.4%; P=0.182) were more commonly observed in the ATO plus ESHAP group compared with the ESHAP group.
 |
Table II.
Adverse events of patients with R/R ALK+ ALCL.
|
Table II.
Adverse events of patients with R/R ALK+ ALCL.
| |
ESHAP group (n=13) |
ATO plus ESHAP group (n=11) |
|
|
| |
|
|
|
|
| Items |
Total |
Grade 1–2 |
Grade 3–4 |
Total |
Grade 1–2 |
Grade 3–4 |
P1 value |
P2 value |
| Anemia, no. (%) |
11 (84.6) |
9 (69.2) |
2 (15.4) |
10 (90.9) |
6 (54.5) |
4 (36.4) |
1.000 |
0.357 |
| Leukopenia, no. (%) |
9 (69.2) |
7 (53.8) |
2 (15.4) |
10 (90.9) |
6 (54.5) |
4 (36.4) |
0.327 |
0.357 |
| Thrombocytopenia, no. (%) |
6 (46.2) |
5 (38.5) |
1 (7.7) |
9 (81.8) |
5 (45.5) |
4 (36.4) |
0.105 |
0.142 |
| Neutropenia, no. (%) |
7 (53.8) |
5 (38.5) |
2 (15.4) |
9 (81.8) |
4 (36.4) |
5 (45.5) |
0.211 |
0.182 |
| Fever, no. (%) |
6 (46.2) |
5 (38.5) |
1 (7.7) |
9 (81.8) |
7 (63.6) |
2 (18.2) |
0.105 |
0.576 |
| Fatigue, no. (%) |
7 (53.8) |
5 (38.5) |
2 (15.4) |
8 (72.7) |
5 (45.5) |
3 (27.3) |
0.423 |
0.630 |
| Anorexia, no. (%) |
6 (46.2) |
5 (38.5) |
1 (7.7) |
8 (72.7) |
7 (63.6) |
1 (9.1) |
0.240 |
1.000 |
| Nausea and vomiting, no. (%) |
5 (38.5) |
4 (30.8) |
1 (7.7) |
7 (63.6) |
5 (45.5) |
2 (18.2) |
0.219 |
0.576 |
| Stomatitis, no. (%) |
6 (46.2) |
5 (38.5) |
1 (7.7) |
6 (54.5) |
5 (45.5) |
1 (9.1) |
0.682 |
1.000 |
| Diarrhea, no. (%) |
4 (30.8) |
4 (30.8) |
0 (0.0) |
5 (45.5) |
4 (36.4) |
1 (9.1) |
0.675 |
0.458 |
| Elevated transaminase, no. (%) |
4 (30.8) |
4 (30.8) |
0 (0.0) |
5 (45.5) |
4 (36.4) |
1 (9.1) |
0.675 |
0.458 |
| Dyspnea, no. (%) |
2 (15.4) |
2 (15.4) |
0 (0.0) |
5 (45.5) |
4 (36.4) |
1 (9.1) |
0.182 |
0.458 |
| Elevated creatinine, no. (%) |
3 (23.1) |
2 (15.4) |
1 (7.7) |
4 (36.4) |
4 (36.4) |
0 (0.0) |
0.659 |
1.000 |
| Dizziness, no. (%) |
1 (7.7) |
1 (7.7) |
0 (0.0) |
2 (18.2) |
2 (18.2) |
0 (0.0) |
0.576 |
1.000 |
Discussion
It has been also reported that ATO may serve as maintenance therapy in patients with adult T-cell leukemia/lymphoma after induction therapy (22–25). However, the treatment response of ATO in patients with R/R ALK+ ALCL remains unclear. Herein, ATO plus ESHAP chemotherapy numerically improved both CR and ORR compared with ESHAP. However, these data did not reach statistical significance, partially due to the limited sample size. The higher numerical CR and ORR rates in patients treated with ATO plus ESHAP could be due the followings: Firstly, a previous study showed that ATO could inhibit the growth of ALK+ ALCL cells via degrading the ALK fusion oncoprotein (18). Secondly, ATO could directly induce cytotoxicity in ALK+ ALCL cells via regulating several signaling pathways, such as the NF-κB, p38/mitogen-activated protein kinase and wnt/β-catenin pathways (13,26). Finally, ATO and ESHAP could exert a synergistic effect on inhibiting ALK+ ALCL cells. However, further validation experiments are needed. Overall, the above findings indicated that ATO plus ESHAP exerted a better treatment efficacy in patients with R/R ALK+ ALCL compared with ESHAP. It could be explained that since ATO is considered to be appropriately applied in R/R ALCL patients ineligible to stem cell transplantation; then in order to match with this group, the control group also set this criteria (not ineligible to stem cell transplantation). Therefore, patients not receiving stem cell transplantation were recruited in the study.
Although the overall prognosis of patients with ALK+ ALCL is relatively good, with a 5-year OS rate of ~80%, the survival rate of patients with R/R ALK+ ALCL is not satisfactory (27). A previous study showed that in patients with R/R ALK+ ALCL, the median (95% confidence interval) progression-free survival (PFS) and OS were 3.8 (range, 0.7-14.8 months) and 13.6 (range, 0.7–89 months) months, respectively (6). Another study also demonstrated that the 2-year PFS and OS rate in patients with R/R ALK+ ALCL treated with chemotherapy was 45.0 and 78.9%, respectively (28). Therefore, the development of effective treatment approaches for patients with R/R ALK+ ALCL is of great importance. The results of the current study revealed that treatment of patients with R/R ALK+ ALCL with ATO plus ESHAP chemotherapy promoted EFS and increased OS compared with the ESHAP group. However, the difference in OS between the two groups did not reach statistical significance. The above findings could be due to the fact that ATO plus ESHAP could inhibit the survival of ALK+ ALCL cells via multiple ways, including degradation of AKL fusion protein and synergizing cytotoxicity, thus improving OS. Secondly, the sample size of the current study could not be large enough to reveal a statistically significant difference in OS between the above groups.
In terms of safety, it has been reported that the most common AEs of ATO are generally associated with hematological toxicities (20,23–25). Consistently, in the current study the data revealed that the main AEs of the two treatment approaches were also associated with hematological toxicity, such as anemia, leukopenia, thrombocytopenia and neutropenia. In addition, the incidence rate of total and grade 3–4 AEs were numerically higher in patients treated with ATO plus ESHAP compared with those treated with ESHAP. However, no statistical significance was observed. The above findings could be due to the cytotoxic activity of ATO to induce myelosuppression, thus enhancing the incidence of anemia, leukopenia, thrombocytopenia and neutropenia (29). Although ATO plus ESHAP could achieve a better treatment efficacy and survival in patients with R/R ALK+ ALCL, its administration should be carefully considered due to the high incidence of AEs, particularly those of grade 3–4. Therefore, clinicians should balance the survival gain and potential toxicity of ATO plus ESHAP when treating patients with R/R ALK+ ALCL.
However, the present study has some limitations that should be clarified. First, since R/R ALK+ ALCL is a relatively rare disease, the sample size of the current study is small. Therefore, the statistical power could be restricted. Secondly, the administration of ATO combined with other treatment options is not explored. Finally, the treatment efficacy of ATO plus ESHAP in patients with other types of ALCL, such as pcALCL and BIA-ALCL, is not investigated. Therefore, further studies are needed.
In conclusion, the current study demonstrated that treatment of patients with R/R ALK+ ALCL with ATO plus ESHAP chemotherapy exerted a superior treatment efficacy compared with ESHAP chemotherapy alone. However, further multicenter studies with larger sample sizes are needed to verify the above results. Furthermore, the potential toxicity of ATO plus ESHAP chemotherapy against the survival benefit should be balanced in clinical practice.
Acknowledgements
Not applicable.
Funding
This study was supported by Shanghai Municipal Health Commission Project (grant no. 201840100).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
AL and WZ were responsible for the conception of the present study. YDi acquired the clinical data. HL and YDo analyzed and interpreted the data. BX was responsible for statistical analysis. All authors drafted the work and revised it critically for important intellectual content. AL, WZ and YDi confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Tongji Hospital, Tongji University School of Medicine (approval no. Ethical Review-KYSB-2018-139) and written informed consent was obtained from all patients before the start of this prospective study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Zhang XR, Chien PN, Nam SY and Heo CY: Anaplastic large cell lymphoma: Molecular pathogenesis and treatment. Cancers (Basel). 14:16502022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leventaki V, Bhattacharyya S and Lim MS: Pathology and genetics of anaplastic large cell lymphoma. Semin Diagn Pathol. 37:57–71. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaseb H, Mukkamalla SKR and Rajasurya V: Anaplastic large cell lymphoma. StatPearls Treasure Island (FL): 2022
|
|
4
|
Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, Rimsza L, Pileri SA, Chhanabhai M, Gascoyne RD, et al: ALK-anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the international peripheral T-cell lymphoma project. Blood. 111:5496–5504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sibon D, Nguyen DP, Schmitz N, Suzuki R, Feldman AL, Gressin R, Lamant L, Weisenburger DD, Rosenwald A, Nakamura S, et al: ALK-positive anaplastic large-cell lymphoma in adults: An individual patient data pooled analysis of 263 patients. Haematologica. 104:e562–e565. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morel A, Brière J, Lamant L, Loschi M, Haioun C, Delarue R, Tournilhac O, Bachy E, Sonet A, Amorim S, et al: Long-term outcomes of adults with first-relapsed/refractory systemic anaplastic large-cell lymphoma in the pre-brentuximab vedotin era: A LYSA/SFGM-TC study. Eur J Cancer. 83:146–153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang QQ, Jiang Y and Naranmandura H: Therapeutic strategy of arsenic trioxide in the fight against cancers and other diseases. Metallomics. 12:326–336. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wahiduzzaman M, Ota A and Hosokawa Y: Novel mechanistic insights into the anti-cancer mode of arsenic trioxide. Curr Cancer Drug Targets. 20:115–129. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Z, Chen Q, Zhang W, Si G, Li J, Jiao D and Han X: Efficacy and safety of the arsenic trioxide/lipiodol emulsion in the transcatheter arterial chemoembolization combined with apatinib in the treatment of advanced hepatocellular carcinoma. Can J Gastroenterol Hepatol. 2021:55657932021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Pan D, Yang H, Huang J, He Z, Li H and Li D: Effects of arsenic trioxide combined with platinum drugs in treatment of cervical cancer: A protocol for systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 99:e229502020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kutny MA, Alonzo TA, Abla O, Rajpurkar M, Gerbing RB, Wang YC, Hirsch BA, Raimondi S, Kahwash S, Hardy KK, et al: Assessment of arsenic trioxide and all-trans retinoic acid for the treatment of pediatric acute promyelocytic leukemia: A report from the children's oncology group AAML1331 trial. JAMA Oncol. 8:79–87. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong L, Xu F and Chen F: Arsenic trioxide induces the apoptosis and decreases NF-κB expression in lymphoma cell lines. Oncol Lett. 16:6267–6274. 2018.PubMed/NCBI
|
|
13
|
Li XY, Li Y, Zhang L, Liu X, Feng L and Wang X: The antitumor effects of arsenic trioxide in mantle cell lymphoma via targeting Wnt/β-catenin pathway and DNA methyltransferase-1. Oncol Rep. 38:3114–3120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li HM, Long Y, Qing C, Yu M, Li ZH, Zhang XM, Li XJ, Chen YJ, Zhang YL and Liang Y: Arsenic trioxide induces apoptosis of Burkitt lymphoma cell lines through multiple apoptotic pathways and triggers antiangiogenesis. Oncol Res. 19:149–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li CL, Wei HL, Chen J, Wang B, Xie B, Fan LL and Li LJ: Arsenic trioxide induces autophagy and antitumor effects in Burkitt's lymphoma Raji cells. Oncol Rep. 32:1557–1563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kouhpaikar H, Sadeghian MH, Rafatpanah H, Kazemi M, Iranshahi M, Delbari Z, Khodadadi F, Ayatollahi H and Rassouli FB: Synergy between parthenolide and arsenic trioxide in adult T-cell leukemia/lymphoma cells in vitro. Iran J Basic Med Sci. 23:616–622. 2020.PubMed/NCBI
|
|
17
|
Yue LM, Chau D, Kwong YL and Tse E: Arsenic trioxide inhibits anaplastic lymphoma kinase (ALK)-positive diffuse large B-cell lymphoma through targeting ALK-fusion oncoprotein. Br J Haematol. 194:1085–1090. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Piao W, Chau D, Yue LM, Kwong YL and Tse E: Arsenic trioxide degrades NPM-ALK fusion protein and inhibits growth of ALK-positive anaplastic large cell lymphoma. Leukemia. 31:522–526. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park SH, Kim S, Ko OB, Koo JE, Lee D, Jeong YP, Huh J, Kim SB, Kim SW, Lee JL and Suh C: ESHAP salvage therapy for refractory and relapsed non-Hodgkin's lymphoma: A single center experience. Korean J Intern Med. 21:159–164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kchour G, Tarhini M, Kooshyar MM, El Hajj H, Wattel E, Mahmoudi M, Hatoum H, Rahimi H, Maleki M, Rafatpanah H, et al: Phase 2 study of the efficacy and safety of the combination of arsenic trioxide, interferon alpha, and zidovudine in newly diagnosed chronic adult T-cell leukemia/lymphoma (ATL). Blood. 113:6528–6532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, et al: Revised response criteria for malignant lymphoma. J Clin Oncol. 25:579–586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu HH, Hu J, Lo-Coco F and Jin J: The simpler, the better: Oral arsenic for acute promyelocytic leukemia. Blood. 134:597–605. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao H, Sun G, Kong D, Zhang Y, Shi W, Zhao M, Hong L and Qiao Z: A phase II study of arsenic trioxide in patients with relapsed or refractory malignant lymphoma. Med Oncol. 32:792015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hermine O, Dombret H, Poupon J, Arnulf B, Lefrère F, Rousselot P, Damaj G, Delarue R, Fermand JP, Brouet JC, et al: Phase II trial of arsenic trioxide and alpha interferon in patients with relapsed/refractory adult T-cell leukemia/lymphoma. Hematol J. 5:130–134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marçais A, Cook L, Witkover A, Asnafi V, Avettand-Fenoel V, Delarue R, Cheminant M, Sibon D, Frenzel L, de Thé H, et al: Arsenic trioxide (As2O3) as a maintenance therapy for adult T cell leukemia/lymphoma. Retrovirology. 17:52020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang J, Yao C, Liu Y, Yuan J, Wu L, Hosoi K, Yu S, Huang C, Wei H and Chen G: Arsenic trioxide induces expression of BCL-2 expression via NF-κB and p38 MAPK signaling pathways in BEAS-2B cells during apoptosis. Ecotoxicol Environ Saf. 222:1125312021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Daugrois C, Bessiere C, Dejean S, Anton-Leberre V, Commes T, Pyronnet S, Brousset P, Espinos E, Brugiere L, Meggetto F and Lamant L: Gene expression signature associated with clinical outcome in ALK-positive anaplastic large cell lymphoma. Cancers (Basel). 13:55232021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen MT, Fu XH, Huang H, Wang Z, Fang XJ, Yao YY, Ren QG, Chen ZG and Lin TY: Combination of crizotinib and chemotherapy in patients with relapsed or refractory anaplastic lymphoma kinase (ALK)-positive anaplastic large cell lymphoma (ALCL). Leuk Lymphoma. 62:571–580. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng X, Xiong X, Li Y, Feng C, Liu H, Wu P, Li C and Weng W: Encouraging early outcomes of treatment with arsenic trioxide combined with chemotherapy for alveolar rhabdomyosarcoma in children: 4 Case reports. Front Oncol. 11:7516232021. View Article : Google Scholar : PubMed/NCBI
|

















